Abstract
BACKGROUND
The influence of age on balance of benefit vs. potential harm of blood pressure (BP)-lowering therapy for elderly hypertensives is unclear. We evaluated the modifying effects of age on BP lowering for various adverse outcomes in hypertensive patients older than 60 years without specified comorbidities.
METHODS
All relevant randomized controlled trials (RCTs) were systematically identified. Coronary heart disease, stroke, heart failure (HF), cardiovascular death, major adverse cardiovascular events (MACE), renal failure (RF), and all-cause death were assessed. Meta-regression analysis was used to explore the relationship between achieved systolic BP (SBP) and the risk of adverse events. Random-effects meta-analysis was used to pool the estimates.
RESULTS
Our study included 18 RCTs (n = 53,993). Meta-regression analysis showed a lower achieved SBP related with a lower risk of stroke and cardiovascular death, but an increased risk of RF. The regression slopes were comparable between populations stratifying by age 75 years. In subgroup analysis, the relative risks of a more aggressive BP lowering strategy were similar between patients aged older or less than 75 years for all outcomes except for RF (P for interaction = 0.02). Compared to treatment with final achieved SBP 140–150 mm Hg, a lower achieved SBP (<140 mm Hg) was significantly associated with decreased risk of stroke (relative risk = 0.68; 95% confidence interval = 0.55–0.85), HF (0.77; 0.60–0.99), cardiovascular death (0.68; 0.52–0.89), and MACE (0.83; 0.69–0.99).
CONCLUSIONS
To treat hypertension in the elderly, age had trivial effect modification on most outcomes, except for renal failure. Close monitoring of renal function may be warranted in the management of elderly hypertension.
Keywords: adverse vascular events, blood pressure, death, elderly hypertension, effect modification, hypertension, meta-analysis
INTRODUCTION
Throughout middle and old age, blood pressure (BP) is strongly and directly related to vascular and all-cause mortality, with increasing absolute risk with age.1 The elderly now outnumber the young for the first time in recorded history, with the number of people aged 65 or older being projected to grow from an estimated 524 million in 2010 to nearly 1.5 billion in 2050.2 More importantly, the hypertension is very common in the older population; for example, among US adults ≥60 years of age, the prevalence of hypertension was 67.2% from 2011 to 2014.3 Taken together, these observations suggest that the management of hypertension in the elderly is an important aspect of public health.
The recently announced 2017 ACC/AHA hypertension clinical practice guideline recommends a SBP target of <130 mm Hg for elderly hypertensives, similar to that for the other populations.4 However, other guidelines suggest a higher SBP target for the elderly, especially octogenarians.5–7 The recommendation of a higher BP treatment target for the elderly has been considered prudent mindful of the fact that increasing BP may be a physiological adaptation to aging and the aged maybe more susceptible to multiple co-morbidities and treatment-related side effects. However, the effect of age on balance of benefit vs. potential harm of BP-lowering therapy, especially for the elderly, has never been comprehensively investigated. We thus conducted the present systematic review to investigate the impact of age in modifying the benefit or harm of antihypertensive treatment for elderly hypertensives. The results of our meta-analysis may contribute to the debate about whether a common BP goal irrespective of age is a more rational recommendation.
METHODS
The prespecified protocol for this systematic review and meta-analysis was registered in PROSPERO (CRD42017056876), and the study closely adhered to the PRISMA guidelines8 (Supplementary Table 1).
Data sources and literature search
We systematically searched MEDLINE and the Cochrane Library databases from inception to 12 December 2016, with keywords and the Medical Subject Headings (MeSH) terms related to the PICOS elements to identify all relevant studies (Supplementary Methods). Further articles were retrieved by re-running the search algorithm before the final analyses. We also manually checked the reference lists of reviews, meta-analyses, and original publications for additional studies. No language restrictions were applied on any of these searches.
Study selection
Based on a previous suggestion,9 we only included randomized controlled trials (RCTs) that compared intensive BP control with less intensive BP control in hypertensive adults aged 60 years and older at trial entry in this systematic review. Any classes of antihypertensive agents could be used for BP management, and there were no restrictions on BP targets. The exclusion criteria were (i) studies focusing on a specified population with concomitant diseases, such as diabetes mellitus, stroke, heart failure, or coronary artery disease; (ii) studies with a follow-up time of less than 1 year; and (iii) studies in which there was a between-group difference of less than 2 mm Hg in achieved SBP. Reports from subgroups or substudies of the main trial that satisfied these eligibility criteria were also included in this analysis. One researcher (C.J.H.) performed the procedure of selecting the studies, which were further rechecked by a second researcher (H.M.C.) for accuracy.
Data extraction and quality assessment
Relevant data extracted from each eligible trial were collected using a spreadsheet, and included study and participant characteristics, baseline and achieved BP levels, the BP-lowering regimens used, and outcome events. Two researchers (C.J.H. and H.M.C.) independently performed the data extraction and judged the methodological quality of the included trials using the Cochrane Collaboration’s tool for assessing the risk of bias10 and the Grading of Recommendations Assessment, Development and Evaluation (GRADE) system for rating the quality of evidence.11 Any discrepancies were resolved through discussion.
Outcomes
Our outcomes of interest were coronary heart disease (including fatal and nonfatal myocardial infarction and sudden cardiac death), stroke (including fatal and nonfatal events, except for transient ischemic attacks), heart failure (including fatal and nonfatal events), cardiovascular death, major adverse cardiovascular events (MACE; the composite of nonfatal myocardial infarction, nonfatal stroke, nonfatal heart failure, and cardiovascular death), renal failure (including fatal and nonfatal events of doubling of serum creatinine concentration or end-stage renal disease requiring dialysis or transplantation (Supplementary Table 2)), and all-cause death. We also investigated the development of cognitive decline (defined as a reduction in cognitive function from baseline) and dementia (diagnosed according to standard clinical criteria) as well as treatment-related side effects (including falls, fractures, syncope, hypotension, and electrolyte imbalance). All adverse outcomes were binary. Supplementary Tables 3 and 4 summarize the outcomes examined for BP-lowering treatment.
Data synthesis and analysis
In this meta-analysis, we used aggregated data and performed a quantitative synthesis of the findings from the included studies. The relative risk (RR), calculated from the number of events and participants in each group, for every outcome was used as the measure of the effect of the intervention. If the direct outcome data were not available, we estimated them using the summary statistic and its confidence interval (CI). Assuming that the subjects in each study were drawn from different populations that led to variations in the effect size, we primarily used the DerSimonian and Laird random-effects model with the Mantel–Haenszel weighting scheme to obtain the pooled estimates of the effects of the interventions and 95% CIs.12 The results from a fixed-effects model were used when the number of studies was small and low heterogeneity was present. Heterogeneity among studies was assessed using both Cochran’s Q and Higgins’s I2 statistics.10 Publication bias was detected using funnel plots and Egger’s regression asymmetry test.13 The trim and fill method was conducted to assess the effect of publication bias.14,15
We performed meta-regression analysis using a mixed-effects model to explore the relationships between achieved SBP levels and the risk of events. A potential curvilinear association was further examined by using restricted cubic spline function with three knots at the 25th, 50th, and 75th percentiles of achieved SBP distribution. The P value for nonlinearity was calculated by testing the null hypothesis of zero for the coefficient of the second spline. To investigate the effect modification by age on the relationship between antihypertensive treatment and adverse vascular outcomes, we first used meta-regression to explore the treatment effect variance that was potentially caused by baseline mean ages of the included trials and subsequently performed subgroup analysis by mean ages of the trials which were divided into two groups with <75 or ≥75 years old. In addition, we assessed the effectiveness of intensive (<140 mm Hg) vs. standard (140–150 mm Hg) SBP control for the prevention of adverse outcomes. We also evaluated the possible modulating effect of frailty on the impact of antihypertensive agents. Statistical significance was defined as a two-tailed P value of less than 0.05. All analyses were performed using R software (version 3.1.3, R Foundation for Statistical Computing), Review Manager (version 5.3, Cochrane Collaboration), and the Comprehensive Meta-Analysis software package (version 2.2.064, Biostat, Englewood, NJ).
RESULTS
After performing the search strategy and excluding duplicate publications, we identified 1,346 potentially relevant articles. After screening the titles and abstracts, we further retrieved the full-text of each relevant study and performed a detailed evaluation of 33 articles. Of these, 23 articles from 18 trials met our inclusion criteria and were deemed eligible for the meta-analysis. Supplementary Figure 1 summarizes the literature selection process.
Study characteristics and quality assessment
The characteristics of the included trials and participants are shown in Table 1. Of the 18 trials, one (the pilot study for the Hypertension in the Very Elderly Trial, HYVET pilot) had a three-arm design with two comparisons. In total, the 18 RCTs enrolled 53,993 elderly hypertensive patients with a mean age of 74 years. The mean length of follow-up was 3.3 years (range 1.1–5.8 years), and there were more female participants in most of the trials than males. Assessment of the methodologic quality of these trials showed that potential bias resulted from inadequate sequence generation and concealment of allocation and lack of blinding due to an open-label or single-blind design (Supplementary Figures 2 and 3). Funnel plots and Egger’s test for MACE indicated an asymmetric distribution (Egger’s test P = 0.02) with larger treatment effects in the smaller studies (Supplementary Figure 4). Through stratifying the treatment effects by sample size, the separate funnels for each subgroup were symmetrical (Egger’s test P ≥ 0.20) (Supplementary Figure 5A). In addition, as shown by the trim and fill analysis results (Supplementary Figure 5B), our study conclusion was not affected by potential publication bias.
Table 1.
Characteristics of the included trials and participants
| Comparison | SBP, mm Hg (Int/Std) | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Trial | Year | Region | Participants, n (Int/Std) | Intensive | Standard | Follow-up, years | Mean age, years | Male, % | Comorbidities, % | Baseline | Achieved | Outcome |
| ASCOT-BPLA16 | 2011 | Europe | 8,137 (4,042/4,095) | CCB ± ACEi (target BP < 140/90 mm Hg) | BB ± diuretic | 5.5 (median) | 71.1 | 73.5 | CHD, 0; CVA, 15.5; HF, 0; DM, 28.4 | 168.4/167.8 | 137.3/139.7 | CHD, stroke, HF, CV death, MACE, all- cause death |
| EWPHE17 | 1985 | Europe | 840 (416/424) | Diuretic | Placebo | 4.7 | 72.0 | 30.2 | CVD, 35.6; HF, 0 | 183/182 | 148/167 | CHD, stroke, HF, CV death, MACE, RF, all- cause death |
| FEVER18,19 | 2011 | China | 3,179 (1,631/1,548) | CCB | Placebo | 3.3 | 69.5 | 65.5 | CVD, 27.8; DM, 13.4 | 156.3/156.3 | 139.7/145.5 | Stroke, CV death, all-cause death |
| HEP20 | 1986 | UK | 884 (419/465) | BB ± diuretic | No treatment | 4.4 | 68.8 | 30.9 | HF, 0; DM, 0 | 196.7/196.1 | 162.7/180.1 | CHD, stroke, HF, CV death, all- cause death |
| HYVET pilot21 | 2003 | Europe | 852 (426/426) | Diuretic (target BP < 150/80 mm Hg) | No treatment | 1.1 | 83.8 | 36.9 | CHD, 2.9; CVA, 4.7 | 181.5/181 | 152/174 | Stroke, CV death, all-cause death |
| 857 (431/426) | ACEi (target BP < 150/80 mm Hg) | No treatment | 1.1 | 83.7 | 36.3 | CHD, 3.3; CVA, 4.7 | 181.9/181 | 151/174 | Stroke, CV death, all-cause death | |||
| HYVET22 | 2008 | Multinational | 3,845 (1,933/1,912) | Diuretic ± ACEi (target BP < 150/80 mm Hg) | Placebo | 2.1 | 83.6 | 39.5 | CVD, 11.8; CHD, 3.1; CVA, 6.8; HF, 2.9; DM, 6.8 | 173/173 | 143.5/158.5 | CHD, stroke, HF, CV death, MACE, all- cause death |
| HYVET-COG23 | 2008 | 3,336 (1,687/1649) | 2.2 | 83.5 | 39.5 | CVD, 11.3; CVA, 6.5 | 173.1/172.9 | 143.5/158.3 | Cognitive decline, dementia | |||
| JATOS24 | 2008 | Japan | 4,418 (2,212/2,206) | SBP < 140 mm Hg | SBP 140– 159 mm Hg | 2 | 73.6 | 38.9 | CHD, 3.0; CVA, 4.3; HF, 0; CKD, 9.9; DM, 11.8 | 171.6/171.5 | 135.9/145.6 | CHD, stroke, HF, CV death, MACE, RF, all- cause death |
| MRC-225,26 | 1992 | UK | 4,396 (2,183/2,213) | Diuretic or BB (target SBP ≤ 150 or ≤160 mm Hg) | Placebo | 5.8 | 70.3 | 41.8 | HF, 0; CKD, 0; DM, 0 | 184.7/184.5 | 151.5/165 | CHD, stroke, CV death, MACE, all-cause death |
| SCOPE27,28 | 2003 | Multinational | 4,937 (2,477/2,460) | ARB | Placebo | 3.7 | 76.4 | 35.5 | CHD, 4.6; CVA, 3.9; DM, 12.1 | 166/166.5 | 145.2/148.5 | CHD, stroke, CV death, MACE, all-cause death, cognitive decline, dementia |
| SHEP pilot29 | 1989 | USA | 551 (443/108) | Diuretic (target SBP < 160 mm Hg or > 20 mm Hg below baseline level) | Placebo | 2.8 | 72.1 | 37 | NR | 172/172 | 142/157 | CHD, stroke, HF, CV death, MACE, all- cause death |
| SHEP30–32 | 1991, 2001 | USA | 4,736 (2,365/2,371) | Diuretic ± BB (target SBP < 160 mm Hg or > 20 mm Hg below baseline level) | Placebo | 4.5 | 71.5 | 43.2 | CHD, 4.9; CVA, 1.4; HF, 0.3; CKD, 0; DM, 10.1 | 170.5/170.1 | 144/155.1 | CHD, stroke, HF, CV death, MACE, RF, all- cause death, cognitive declinea, dementia |
| SPRINT-SENIOR33 | 2016 | USA | 2,636 (1,317/1,319) | SBP < 120 mm Hg | SBP < 140 mm Hg | 3.14 (median) | 79.9 | 62.1 | CVD, 24.5; HF, 0; CKD, 44; DM, 0 | 141.6/141.6 | 123.4/134.8 | CHD, stroke, HF, CV death, MACE, RF, all- cause death |
| STONE34 | 1996 | China | 1,632 (817/815) | CCB (target BP 140– 159/<90 mm Hg) | Placebo | 2.5 | 66.4 | 46.9 | CVD, 0; CHD, 0; CVA, 0; HF, 0; CKD, 0; DM, 0 | 168.5/168.6 | 146.85/156.29 | CHD, stroke, HF, CV death, MACE, all- cause death |
| STOP- Hypertension35 | 1991 | Sweden | 1,627 (812/815) | BB or diuretic | Placebo | 2.1 | 75.7 | 37.4 | NR | 195/195 | 167/186 | CHD, stroke, HF, CV death, MACE, all- cause death |
| Syst-China36,37 | 1998 | China | 2,394 (1,253/1,141) | CCB ± ACEi and/ or diuretic (target SBP < 150 mm Hg) | Placebo | 3 (median) | 66.5 | 64.4 | CVD, 11.2; CHD, 9.4; CVA, 1.4; DM, 4.1 | 170.7/170.2 | 150.7/159.3 | CHD, stroke, HF, CV death, MACE, RF, all- cause death |
| Syst-Eur38,39 | 1997 | Europe | 4,695 (2,398/2,297) | CCB ± ACEi and/ or diuretic (target SBP < 150 mm Hg) | Placebo | 2 (median) | 70.3 | 33.2 | CVD, 29.9; CHD, 3.5; CVA, 1.2; HF, 0; DM, 10.5 | 173.8/173.9 | 150.8/160.9 | CHD, stroke, HF, CV death, MACE, RF, all- cause death |
| 2002 | 2,902 (1,485/1,417) | 3.9 (median) | 68 (median) | 33.9 | CVD, 26.3; CVA, 1.3; HF, 0 | 173.5/173.4b | 149.1/156.1 | Dementia | ||||
| VALISH40 | 2010 | Japan | 3,079 (1,545/1,534) | SBP < 140 mm Hg | SBP 140– 149 mm Hg | 2.85 | 76.1 | 37.5 | CHD, 5.0; CVA, 6.6; HF, 1.7; CKD, 1.4; DM, 13.0 | 169.5/169.6 | 136.6/142 | CHD, stroke, CV death, MACE, RF, all-cause death |
| Wei et al.41 | 2013 | China | 724 (363/361) | BP < 140/90 mm Hg | BP < 150/90 mm Hg | 4 | 76.6 | 66.3 | CHD, 7.5; CVA, 6.6; HF, 0; CKD, 0; DM, 23.3 | 158.8/160.3 | 135.7/149.7 | CHD, stroke, HF, CV death, MACE, all- cause death |
Abbreviations: ACEi, angiotensin-converting enzyme inhibitor; ARB, angiotensin-receptor blocker; BB, beta-blocker; ASCOT-BPLA, Anglo-Scandinavian Cardiac Outcomes Trial—Blood Pressure Lowering Arm; CCB, calcium channel blocker; CHD, coronary heart disease; CKD, chronic kidney disease; CV, cardiovascular; CVA, cerebrovascular accident; CVD, cardiovascular disease; DM, type 2 diabetes; EWPHE, European Working Party on High Blood Pressure in the Elderly; FEVER, Felodipine Event Reduction; HEP, Hypertension in the Elderly in Primary Care; HF, heart failure; HYVET, Hypertension in the Very Elderly Trial; HYVET-COG, Hypertension in the Very Elderly Trial cognitive function assessment; Int, intervention; JATOS, Japanese Trial to Assess Optimal Systolic Blood Pressure in Elderly Hypertensive Patients; MACE, major adverse cardiovascular events; MRC, Medical Research Council; NR, not reported; RF, renal failure; SBP, systolic blood pressure; SCOPE, Study on Cognition and Prognosis in the Elderly; SHEP, Systolic Hypertension in the Elderly Program; SPRINT, Systolic Blood Pressure Intervention Trial; Std, standard; STONE, Shanghai Trial of Nifedipine in the Elderly; STOP-Hypertension, Swedish Trial in Old Patients with Hypertension; Syst-China, Systolic Hypertension in China; Syst-Eur, Systolic Hypertension in Europe; VALISH, Valsartan in Elderly Isolated Systolic Hypertension.
aThere were 1,368 and 1,317 participants in the intensive and standard groups, respectively, for the investigation of cognitive decline, and data on baseline and achieved SBP were not reported.32,42
bBaseline SBP data for Syst-Eur was from the report in 1998.43
Achieved blood pressure level and risk of adverse vascular outcomes
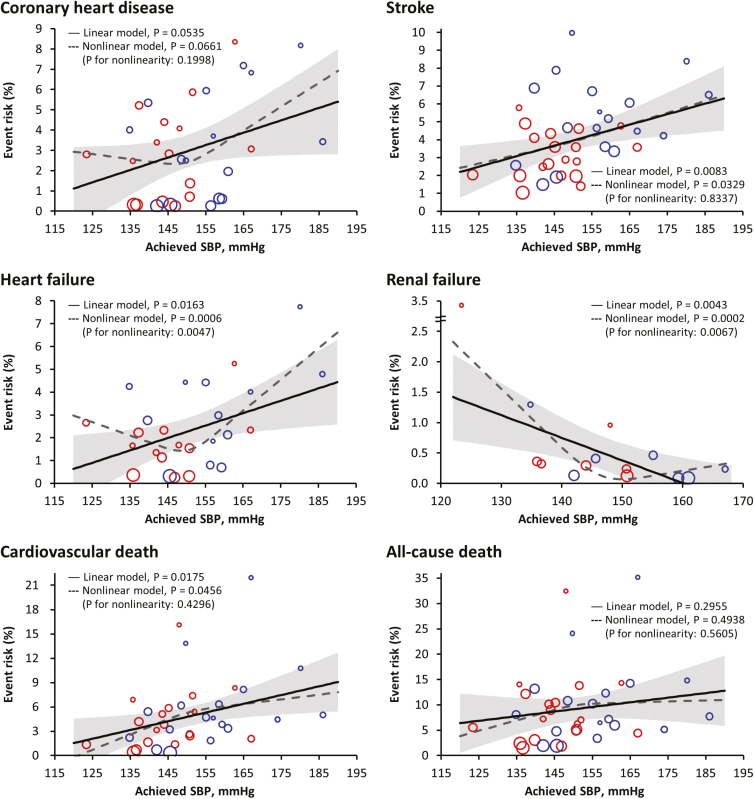
We conducted meta-regression analyses to elucidate relationships between achieved BP levels and the development of adverse vascular events. The lower final achieved SBP was associated with a lower risk for coronary heart disease (P = 0.0535), stroke (P = 0.0083), heart failure (P = 0.0163), and cardiovascular death (P = 0.0175), but associated with a higher risk for renal failure (P = 0.0043) in a linear manner (Figure 1). In the multivariable meta-regression analysis, age remained significantly associated only with stroke (P = 0.0455) after accounting for final achieved SBP; however, consistent results of the associations of achieved SBP with stroke were still observed (P = 0.0068; data not shown).
Figure 1.
Relationship between achieved systolic blood pressure (SBP) and cumulative risk of adverse outcomes. Meta-regression analyses were performed on the data from combining the intensive (colored in red) and standard (colored in blue) control groups. The regression fit (solid line) and 95% confidence interval (gray shaded area) for linear model and the regression fit (dash line) for nonlinear model are shown. The size of the circle represents the weight of each trial and is inversely proportional to the standard error of the effect estimate.
By fitting regression models with restricted cubic splines, we did not find any evidence of significant departure from linearity for these adverse outcomes except for heart failure (P for nonlinearity = 0.0047) and renal failure (P for nonlinearity = 0.0067). After excluding the SPRINT-SENIOR trial that was a key contributor to this nonlinear relation, the significance of nonlinearity was not detected (P for nonlinearity = 0.0960 for heart failure and P for nonlinearity = 0.8150 for renal failure; data not shown).
Effect of antihypertensive treatment modified by age
For hypertensive adults ≥60 years of age, the effectiveness of antihypertensive treatment for the prevention of primary cardio- and cerebrovascular events and death was evident. However, it is unclear whether the treatment effect within this elderly population is influenced by age. Linear meta-regression showed that the risk of renal failure conferred by antihypertensive treatment tended to increase with baseline mean age of the study (β = 0.0919, 95% CI = −0.0129 to 0.1967, P = 0.0858) (Table 2).
Table 2.
Meta-regression analysis for the relationship of the effect of antihypertensive treatment with baseline mean age
| Outcomes | Studies, n | β (95% CI)a | P value |
|---|---|---|---|
| Coronary heart disease | 16 | −0.0036 (−0.0376 to 0.0305) | 0.8370 |
| Stroke | 19 | 0.0080 (−0.0114 to 0.0274) | 0.4194 |
| Heart failure | 13 | −0.0277 (−0.0629 to 0.0076) | 0.1237 |
| Cardiovascular death | 19 | 0.0099 (−0.0092 to 0.0289) | 0.3112 |
| MACE | 15 | 0.0026 (−0.0178 to 0.0230) | 0.8033 |
| Renal failure | 7 | 0.0919 (−0.0129 to 0.1967) | 0.0858 |
| All-cause death | 19 | 0.0044 (−0.0142 to 0.0231) | 0.6411 |
| Cognitive decline | 3 | 0.0161 (−0.0171 to 0.0493) | 0.3414 |
| Dementia | 4 | 0.0308 (−0.0214 to 0.0831) | 0.2472 |
Abbreviations: CI, confidence interval; MACE, major adverse cardiovascular events.
aThe regression coefficient is the change in the risk of different outcomes for each 1-year older baseline age.
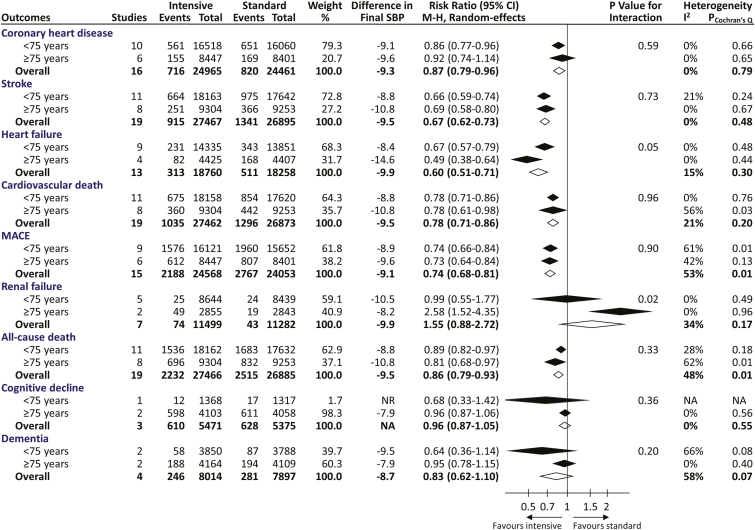
We subsequently performed subgroup analyses to compare the overall benefits and harms of BP-lowering therapy across two prespecified age groups (<75 and ≥75 years) for various adverse outcomes (Figure 2 and Supplementary Figure 6). There were no significant differences in the treatment effects on coronary heart disease, stroke, cardiovascular death, MACE and all-cause death (all P for interaction > 0.05), with pooled risk reductions by 13%–33% (P ≤ 0.005). Nevertheless, a significant effect modification by age was identified for renal failure (P for interaction = 0.02) and a borderline significant effect modification was noted for heart failure (P for interaction = 0.05), indicating that the treatment effect was better for hypertensive adults ≥75 years of age in reducing heart failure but worse in renal failure as compared with those <75 years of age. Additionally, our results showed that the incidence rates of cognitive decline and dementia were not significantly affected by BP-lowering treatment regardless of age groups, but the studies appear to lack power for these outcomes.
Figure 2.
Effects of blood pressure-lowering treatment on various adverse outcomes, stratified by baseline mean ages of the included trials. Difference in final SBP indicates achieved SBP difference between intensive and standard control groups and was estimated by averaging the means of every trial weighted by the number of participants. P value for testing heterogeneity of the risk ratios across age groups is provided. Diamond represents the pooled estimate of relative risks and its 95% confidence interval. MACE, major adverse cardiovascular events; NA, not applicable; NR, not reported
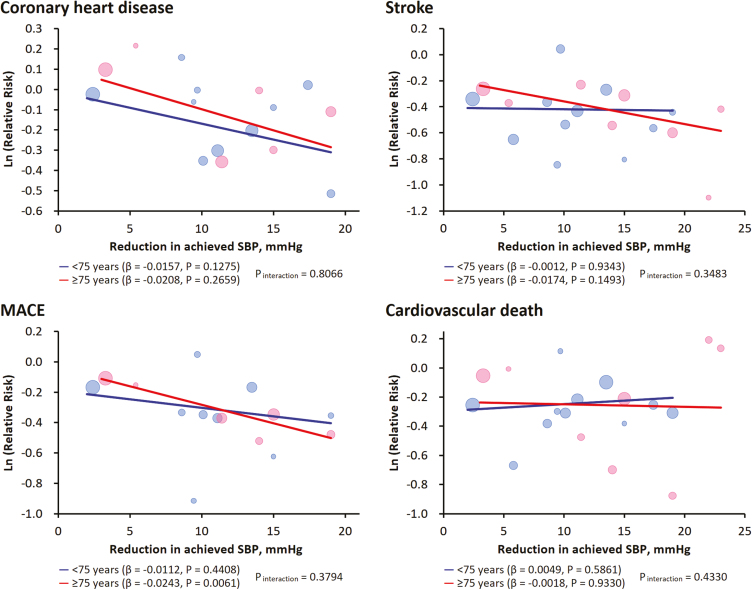
We furthermore conducted stratified meta-regression analyses to delineate the pattern of treatment effect against the extent of achieved SBP reduction across age groups. As shown in Figure 3 and Supplementary Figure 7, the treatment-related benefit from SBP reduction in older hypertensive adults (≥75 years of age) seems comparable to that in younger adults (<75 years of age) for coronary heart disease, stroke, cardiovascular death, and MACE as well as heart failure and all-cause death (all P for interaction > 0.34).
Figure 3.
Meta-regression analyses of treatment effect in relation to achieved systolic blood pressure (SBP) reduction for various adverse outcomes, stratified by baseline mean ages of the included trials. The regression fits for two age groups are shown. The size of the circle represents the weight of each trial and is inversely proportional to the standard error of the effect estimate. Ln, natural logarithm; MACE, major adverse cardiovascular events.
Effects of intensive lowering of bp on adverse vascular outcomes
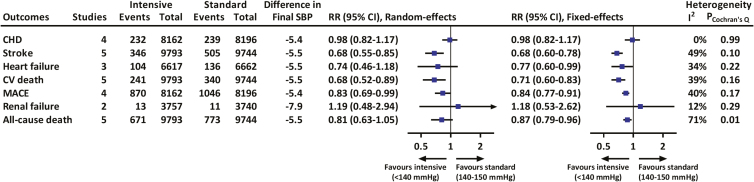
We used final achieved BP as the surrogate of BP targets to evaluate the guideline recommendation for the management of hypertension. The results showed that treatment achieving a final SBP <140 mm Hg significantly decreased the risk of stroke (RR = 0.68, 95% CI = 0.55 to 0.85), heart failure (0.77, 0.60 to 0.99), cardiovascular death (0.68, 0.52 to 0.89), and MACE (0.83, 0.69 to 0.99) compared with an achieved SBP of 140–150 mm Hg (Figure 4). There were insufficient data to evaluate the effects of more intensive (<130 mm Hg) vs. standard (<140 mm Hg) SBP control on cardiovascular disease because only one study (SPRINT-SENIOR) has reported achieving these lower levels of BP.33
Figure 4.
Effects of intensive vs. standard blood pressure-lowering treatment on various adverse outcomes in trials with an achieved SBP of <140 vs. 140–150 mm Hg. Difference in final SBP indicates achieved SBP difference between intensive and standard control groups and was estimated by averaging the means of every trial weighted by the number of participants. Boxes and horizontal lines denote pooled relative risk and 95% confidence interval. CHD, coronary heart disease; CV, cardiovascular; MACE, major adverse cardiovascular events; RR, relative risk; SBP, systolic blood pressure.
The overall quality of evidence was moderate to high for these vascular outcomes, in which lower quality evidence was rated by reason of inconsistency and imprecision (Supplementary Table 5). Eleven fewer cardiovascular death (95% CI from 4 fewer to 17 fewer) could be prevented per 1,000 elderly subjects with a final achieved SBP <140 mm Hg than 140–150 mm Hg.
Adverse side effects of antihypertensive treatment and physical function
Very few studies reported the possible side effects relating to antihypertensive treatment including falls, fractures, syncope, hypotension, and electrolyte abnormality (Supplementary Table 4). Thus, we used a composite measure for this outcome by summing these adverse events. Pooled results suggested no significantly increased risk of the reported side effects (Supplementary Figure 8A). Furthermore, the cumulative risk was not correlated with final achieved SBP and the risk ratio was not correlated with the achieved SBP difference between intensive and standard control groups (Supplementary Figure 8B). This evidence was rated to be low quality (data not shown) and no publication bias was found (Egger’s test P = 0.51, data not shown).
We also examined whether physical function of the elderly hypertensive patients could modify the effects of BP treatment. Analyses from two trials33,44 showed no heterogeneity in the risk reduction of cardiovascular events or all-cause death associated with antihypertensive treatment across various levels of frailty (P for interaction ≥ 0.63) (Supplementary Figures 9 and 10).
DISCUSSION
In this systematic review and meta-analysis of RCTs (18 trials, 53,993 patients) which included hypertensive patients aged 60 years or older without specified comorbidities, a more aggressive BP lowering therapy, as shown in the meta-regression analysis, significantly reduced the risk of stroke and cardiovascular mortality, but increased the risk of renal failure in elderly hypertensive patients. The body of evidence suggests that age caused trivial effect modification on most outcomes relating to BP lowering treatment, except for renal failure. Compared with an achieved SBP of 140–150 mm Hg, a treatment level of <140 mm Hg was associated with a significant reduction in the risk of stroke, heart failure, cardiovascular mortality, and MACE, and an insignificant increase in the risk of renal failure. Pooled statistics from few studies suggested comparable risks of treatment related side effects between intensive and less intensive treatment strategies. In subjects aged 60 years or older, the principal phenotype of hypertension is isolated systolic hypertension (SBP ≥140 mm Hg and diastolic BP <90 mm Hg).45 As such, the treatment considerations for SBP apparently outweigh those for diastolic BP in elderly hypertensive patients. We therefore focused our analysis on SBP treatment goals.
Our study represents the most comprehensive meta-analysis to date to specifically investigate the effect modification by age on various important clinical outcomes for the old aged population. As shown in our meta-regression and subgroup analyses, the effect modification by age on vascular events is insignificant, except for renal failure. Therefore, while a more aggressive BP reduction for elderly hypertensives could reduce their risk of stroke, cardiovascular death, and vascular events, close monitoring their renal function may be required.
We performed meta-regression models with restricted cubic splines to evaluate the possibility of nonlinear relationships between achieved SBP levels and the risk of adverse vascular outcomes. The results showed that a nonlinear relation originally detected in heart failure and renal failure seemed to disappear after excluding the SPRINT-SENIOR trial from further analysis, but a linear association remained (P = 0.0004 for heart failure and P = 0.0826 for renal failure, data not shown).
One particular strength of our study is that we only included data from randomized controlled trials because a previous large observation study reporting a terminal decline of SBP in the final 2 years of life suggests that nonrandomized epidemiological associations of low SBP with higher mortality may be accounted for by reverse causation,9 which may help explain the discrepancy between clinical trial results and nonrandomized studies, and it is inadvisable to base blood pressure treatment recommendations on nonrandomized data for effectiveness outcomes.
Recently, the SPRINT-SENIOR trial evaluated a more aggressive strategy with a systolic BP target of <120 mm Hg vs. a target of <140 mm Hg in a subgroup of patients aged over 75 years, and found a significant reduction in fatal and nonfatal major cardiovascular events and all-cause mortality without a significant increased risk in serious adverse events.33 The SPRINT trial so far is the only one study that the achieved final SBP is less than 130 mm Hg. To evaluate the influence of these BP levels on vascular outcomes, we conducted a sensitivity analysis by excluding the SPRINT-SENIOR trial (data not shown), and the results were still in agreement with the original analysis. Therefore, a common SBP goal for the elderly and general population remains a reasonable recommendation.
The findings of our study are generally consistent with those of previous systematic reviews.46–48 In a meta-analysis including 31 trials and 190,606 participants, there were no differences between younger (<65 years) and older (≥65 years) adults with regards the effects of lowering BP on the relative risk of major cardiovascular events.46 Although that study did not specifically investigate the effect modification by age, it indirectly supports our study conclusion that a common BP target may be reasonable for the hypertensive population regardless of age. Another recent systematic review and meta-analysis suggested that treatment to at least the current guideline standards for BP (<150/90 mm Hg) substantially improved health outcomes in older adults, however, there was less consistent evidence that lower BP targets are beneficial for high-risk patients.47 Although it was a comprehensive and rigorous systematic review, there are several important differences from our study. First, they included studies based on a mean age of >60 years and included studies with younger subjects not regarded as elderly. In contrast, we identified and included studies based on the inclusion criteria of subjects aged 60 years or older. Second, their review included studies with comorbidities such as previous stroke (SPS3) and diabetes (Advance and Accord). However, all international hypertension guidelines recommend specific BP targets according to specific comorbidities, while our study focused on elderly populations without specific comorbidities. Lastly, their review did not specifically investigate the effects modification by age and the optimal BP targets and provided analysis of 140/85 mm Hg vs. other levels, which does not answer the research question proposed in our study. In a recently published meta-analysis which included only four “high-quality” RCTs (JATOS,24 SPRINT-SENIOR,33 VALISH,40 Wei et al.41), the authors suggested that intensive BP control (systolic BP <140 mm Hg) decreased the risk of MACEs including cardiovascular mortality and heart failure.48 In addition, this intensive BP control was associated with a borderline reduced risk of stroke compared with standard treatment (RR = 0.80, 95% CI = 0.61 to 1.05). In contrast, our study demonstrated that intensive treatment with an achieved SBP level of <140 mm Hg, as compared with 140–150 mm Hg, was associated with a significant reduced risk of stroke, heart failure, cardiovascular mortality, and MACE (Figure 4). With the inclusion of more eligible studies in our systematic review, our study has more power and may more faithfully represent the current body of evidence.
Given the higher baseline absolute risk of the elderly population and the similar risk reduction from BP lowering therapy to that in the younger population,46 the number needed to treat to avoid one clinical outcome is much lower for the elderly population than that for a younger population. Taken together, a common treatment target for the elderly hypertensive population without comorbidities may be considered.
Study limitations
There are several limitations to this meta-analysis. First, the included trials comprise two types of studies involving the comparison of antihypertensive drugs or BP targets, therefore our results could not be used to definitely determine the optimal BP target of elderly hypertensives. Second, similar to other meta-analyses, the absence of primary data and the selective reporting of primary studies may have confounded our study results. Third, there may be considerable variability in the definitions of renal failure, cognitive decline, dementia, and adverse side effects across the included studies. Fourth, the generalizability of these findings to higher-risk population is constrained. Fifth, despite the comprehensive literature search, we may have failed to identify some eligible published or unpublished studies. However, with consistent findings as those reported in previous meta-analyses,46–48 our study conclusions may not be altered substantially even if unidentified studies exist. A final important caveat is the complex issue of frailty, which may occur at any age but is much more common with advanced age. Most studies have excluded patients who were institutionalized, for example, in care homes and were not living independently. Moreover, elderly patients who volunteer for trials are less likely to be frail and unwell and receive more intensive monitoring and clinical support during the trial, than they might otherwise receive during routine care. Furthermore, such patients are probably less likely to develop adverse effects of treatment. It is therefore unknown if our conclusions would apply to the frail and dependent elderly population. Further research in such patients is urgently needed.
CONCLUSIONS
Evidence from RCTs supports the use of antihypertensive agents in lowering blood pressure for the independent elderly population without comorbidities such as diabetes, chronic kidney disease, chronic heart failure, coronary artery disease, and stroke. In such patients, there appears to be no substantial effect modification by age on the outcomes relating to BP lowering, except possibly for renal failure. Intensive SBP control to <140 mm Hg was associated with a significant reduction in the risk of most adverse outcomes in the elderly population compared with a BP level of 140–150 mm Hg. While the current body of evidence supports the same SBP target for elderly hypertensive patients as that for the general population, close monitoring their renal function in the management of hypertension may be advisable.
Supplementary Material
DISCLOSURE
C.-E.C. reports personal fees from Astrazeneca, MSD, Boehringer-Ingelheim, Pfizer, Nova Nordick, and Daiichi Sankyo, outside the submitted work. B.W. reports personal fees from Novartis, Boehringer Ingelheim, and Merck Sharpe and Dohme, outside the submitted work. K.K. reports grants from Teijin Pharma Limited, Omron Helthcare Co., Ltd., FUKUDA DENSHI, Bayer Yakuhin Ltd., A &D Co., Ltd., Daiichi Sankyo Company, Limited, Mochida Pharmaceutical Co., Ltd, EA Pharma, Otsuka Pharmaceutical Co., Ltd., Boehringer Ingelheim Japan, Inc., Mitsubishi Tanabe Pharma Corporation, Medtronic Japan Co., Ltd., and Takeda Pharmaceutical Company Limited; reports personal fees from Takeda Pharmaceutical Company Limited, Daiichi Sankyo Company, Limited, and Omron Healthcare Co., Ltd., outside the submitted work. C.-H.C. reports personal fees from TAKEDA Pharmaceuticals International, Boehringer Ingelheim Pharmaceuticals, Inc., Novartis Pharmaceuticals Corporation, Daiichi Sankyo, Inc., and Pfizer Inc., outside the submitted work. C.-J.H., S.-H.S., T.-D.W., and H.-M.C. declare no competing interests.
FUNDING
This work was supported in part by grants from the Research and Development Contract NO1-AG-1-2118, the Ministry of Health and Welfare (MOHW104-TDU-B-211-113-003, MOHW106-TDU-B-211-113001), and Taipei Veterans General Hospital-National Yang-Ming University Excellent Physician Scientists Cultivation Program (no. 105-Y-B-056). The funders of the study had no role in the study design, data collection, data analysis, data interpretation, or writing of the report. C.-J.H. and H.-M.C. had full access to all the data in the study, and H.-M.C. had final responsibility for the decision to submit for publication.
AUTHOR CONTRIBUTIONS
All authors conceived the concept and design of the study. C.-J.H. and H.-M.C. contributed to the acquisition of data. C.-J.H. did the statistical analyses. All authors were involved in the analysis and interpretation of data. C.-J.H. and H.-M.C. drafted the manuscript. All authors contributed to the critical revision of the manuscript for important intellectual content. All authors have read and approved the final version. C.-J.H. and H.-M.C. take responsibility for the integrity of the data and the accuracy of the analyses. H.-M.C. was the study supervision.
REFERENCES
- 1. Lewington S, Clarke R, Qizilbash N, Peto R, Collins R; Prospective Studies Collaboration. Age-specific relevance of usual blood pressure to vascular mortality: a meta-analysis of individual data for one million adults in 61 prospective studies. Lancet 2002; 360:1903–1913. [DOI] [PubMed] [Google Scholar]
- 2. World Health Organization. Global Health and Aging (NIH publication no. 11–7737). In: National Institutes of Health UDoHaHS (ed), World Health Organization: Geneva, 2011. [Google Scholar]
- 3. Benjamin EJ, Blaha MJ, Chiuve SE, Cushman M, Das SR, Deo R, de Ferranti SD, Floyd J, Fornage M, Gillespie C, Isasi CR, Jiménez MC, Jordan LC, Judd SE, Lackland D, Lichtman JH, Lisabeth L, Liu S, Longenecker CT, Mackey RH, Matsushita K, Mozaffarian D, Mussolino ME, Nasir K, Neumar RW, Palaniappan L, Pandey DK, Thiagarajan RR, Reeves MJ, Ritchey M, Rodriguez CJ, Roth GA, Rosamond WD, Sasson C, Towfighi A, Tsao CW, Turner MB, Virani SS, Voeks JH, Willey JZ, Wilkins JT, Wu JH, Alger HM, Wong SS, Muntner P; American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Heart disease and stroke statistics—2017 update: a report from the American Heart Association. Circulation 2017; 135:e146–e603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Whelton PK, Carey RM, Aronow WS, Casey DE Jr, Collins KJ, Dennison Himmelfarb C, DePalma SM, Gidding S, Jamerson KA, Jones DW, MacLaughlin EJ, Muntner P, Ovbiagele B, Smith SC Jr, Spencer CC, Stafford RS, Taler SJ, Thomas RJ, Williams KA Sr, Williamson JD, Wright JT Jr. 2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA guideline for the prevention, detection, evaluation, and management of high blood pressure in adults: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. J Am Coll Cardiol 2018; 71:e127–e248. [DOI] [PubMed] [Google Scholar]
- 5. Task Force Members. 2013 ESH/ESC guidelines for the management of arterial hypertension: the task force for the management of arterial hypertension of the European Society of Hypertension (ESH) and of the European Society of Cardiology (ESC). Eur Heart J 2013; 34: 2159–2219. [DOI] [PubMed] [Google Scholar]
- 6. James PA, Oparil S, Carter BL, Cushman WC, Dennison-Himmelfarb C, Handler J, Lackland DT, LeFevre ML, MacKenzie TD, Ogedegbe O, Smith SC Jr, Svetkey LP, Taler SJ, Townsend RR, Wright JT Jr, Narva AS, Ortiz E. 2014 evidence-based guideline for the management of high blood pressure in adults: report from the panel members appointed to the Eighth Joint National Committee (JNC 8). JAMA 2014; 311:507–520. [DOI] [PubMed] [Google Scholar]
- 7. Weber MA, Schiffrin EL, White WB, Mann S, Lindholm LH, Kenerson JG, Flack JM, Carter BL, Materson BJ, Ram CV, Cohen DL, Cadet JC, Jean-Charles RR, Taler S, Kountz D, Townsend R, Chalmers J, Ramirez AJ, Bakris GL, Wang J, Schutte AE, Bisognano JD, Touyz RM, Sica D, Harrap SB. Clinical practice guidelines for the management of hypertension in the community a statement by the American Society of Hypertension and the International Society of Hypertension. J Hypertens 2014; 32:3–15. [DOI] [PubMed] [Google Scholar]
- 8. Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gøtzsche PC, Ioannidis JP, Clarke M, Devereaux PJ, Kleijnen J, Moher D. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate healthcare interventions: explanation and elaboration. BMJ 2009; 339:b2700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Ravindrarajah R, Hazra NC, Hamada S, Charlton J, Jackson SHD, Dregan A, Gulliford MC. Systolic blood pressure trajectory, frailty, and all-cause mortality >80 years of age: cohort study using electronic health records. Circulation 2017; 135:2357–2368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Higgins JPT, Green S.. Cochrane Handbook for Systematic Reviews of Interventions. Wiley: Hoboken, NJ, 2011. [Google Scholar]
- 11. Atkins D, Best D, Briss PA, Eccles M, Falck-Ytter Y, Flottorp S, Guyatt GH, Harbour RT, Haugh MC, Henry D, Hill S, Jaeschke R, Leng G, Liberati A, Magrini N, Mason J, Middleton P, Mrukowicz J, O’Connell D, Oxman AD, Phillips B, Schünemann HJ, Edejer T, Varonen H, Vist GE, Williams JW Jr, Zaza S; GRADE Working Group. Grading quality of evidence and strength of recommendations. BMJ 2004; 328:1490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Borenstein M, Hedges LV, Higgins JP, Rothstein HR. A basic introduction to fixed-effect and random-effects models for meta-analysis. Res Synth Methods 2010; 1:97–111. [DOI] [PubMed] [Google Scholar]
- 13. Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ 1997; 315:629–634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Duval S, Tweedie R. A nonparametric “trim and fill” method of accounting for publication bias in meta-analysis. J Am Stat Assoc 2000; 95:89–98. [Google Scholar]
- 15. Duval S, Tweedie R. Trim and fill: a simple funnel-plot-based method of testing and adjusting for publication bias in meta-analysis. Biometrics 2000; 56:455–463. [DOI] [PubMed] [Google Scholar]
- 16. Collier DJ, Poulter NR, Dahlöf B, Sever PS, Wedel H, Buch J, Caulfield MJ; ASCOT Investigators. Impact of amlodipine-based therapy among older and younger patients in the Anglo-Scandinavian Cardiac Outcomes Trial-Blood Pressure Lowering Arm (ASCOT-BPLA). J Hypertens 2011; 29:583–591. [DOI] [PubMed] [Google Scholar]
- 17. Amery A, Birkenhäger W, Brixko P, Bulpitt C, Clement D, Deruyttere M, De Schaepdryver A, Dollery C, Fagard R, Forette F. Mortality and morbidity results from the European Working Party on High Blood Pressure in the Elderly trial. Lancet 1985; 1:1349–1354. [DOI] [PubMed] [Google Scholar]
- 18. Zhang Y, Zhang X, Liu L, Zanchetti A; FEVER Study Group. Effects of individual risk factors on the residual risk of cardiovascular events in a population of treated Chinese patients with hypertension: data from the Felodipine Event Reduction (FEVER) study. J Hypertens 2010; 28:2016–2025. [DOI] [PubMed] [Google Scholar]
- 19. Zhang Y, Zhang X, Liu L, Zanchetti A; FEVER Study Group. Is a systolic blood pressure target <140 mmHg indicated in all hypertensives? Subgroup analyses of findings from the randomized FEVER trial. Eur Heart J 2011; 32:1500–1508. [DOI] [PubMed] [Google Scholar]
- 20. Coope J, Warrender TS. Randomised trial of treatment of hypertension in elderly patients in primary care. BMJ (Clinical Research Ed) 1986; 293:1145–1151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Bulpitt CJ, Beckett NS, Cooke J, Dumitrascu DL, Gil-Extremera B, Nachev C, Nunes M, Peters R, Staessen JA, Thijs L; Hypertension in the Very Elderly Trial Working Group. Results of the pilot study for the Hypertension in the Very Elderly Trial. J Hypertens 2003; 21:2409–2417. [DOI] [PubMed] [Google Scholar]
- 22. Beckett NS, Peters R, Fletcher AE, Staessen JA, Liu L, Dumitrascu D, Stoyanovsky V, Antikainen RL, Nikitin Y, Anderson C, Belhani A, Forette F, Rajkumar C, Thijs L, Banya W, Bulpitt CJ; HYVET Study Group. Treatment of hypertension in patients 80 years of age or older. N Engl J Med 2008; 358:1887–1898. [DOI] [PubMed] [Google Scholar]
- 23. Peters R, Beckett N, Forette F, Tuomilehto J, Clarke R, Ritchie C, Waldman A, Walton I, Poulter R, Ma S, Comsa M, Burch L, Fletcher A, Bulpitt C; HYVET Investigators. Incident dementia and blood pressure lowering in the Hypertension in the Very Elderly Trial cognitive function assessment (HYVET-COG): a double-blind, placebo controlled trial. Lancet Neurol 2008; 7:683–689. [DOI] [PubMed] [Google Scholar]
- 24. Group JS. Principal results of the Japanese trial to assess optimal systolic blood pressure in elderly hypertensive patients (JATOS). Hypertens Res 2008; 31:2115–2127. [DOI] [PubMed] [Google Scholar]
- 25. Medical Research Council trial of treatment of hypertension in older adults: principal results. MRC Working Party. BMJ (Clinical Research Ed) 1992; 304:405–412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Carr MJ, Bao Y, Pan J, Cruickshank K, McNamee R. The predictive ability of blood pressure in elderly trial patients. J Hypertens 2012; 30:1725–1733. [DOI] [PubMed] [Google Scholar]
- 27. Lithell H, Hansson L, Skoog I, Elmfeldt D, Hofman A, Olofsson B, Trenkwalder P, Zanchetti A; SCOPE Study Group. The Study on Cognition and Prognosis in the Elderly (SCOPE): principal results of a randomized double-blind intervention trial. J Hypertens 2003; 21:875–886. [DOI] [PubMed] [Google Scholar]
- 28. Trenkwalder P, Elmfeldt D, Hofman A, Lithell H, Olofsson B, Papademetriou V, Skoog I, Zanchetti A; Study on COgnition and Prognosis in the Elderly (SCOPE). The Study on COgnition and Prognosis in the Elderly (SCOPE)—major CV events and stroke in subgroups of patients. Blood Press 2005; 14:31–37. [DOI] [PubMed] [Google Scholar]
- 29. Perry HM Jr, Smith WM, McDonald RH, Black D, Cutler JA, Furberg CD, Greenlick MR, Kuller LH, Schnaper HW, Schoenberger JA. Morbidity and mortality in the Systolic Hypertension in the Elderly Program (SHEP) pilot study. Stroke 1989; 20:4–13. [DOI] [PubMed] [Google Scholar]
- 30. Prevention of stroke by antihypertensive drug treatment in older persons with isolated systolic hypertension. Final results of the Systolic Hypertension in the Elderly Program (SHEP). SHEP Cooperative Research Group. JAMA 1991; 265:3255–3264. [PubMed] [Google Scholar]
- 31. Kostis JB, Davis BR, Cutler J, Grimm RH Jr, Berge KG, Cohen JD, Lacy CR, Perry HM Jr, Blaufox MD, Wassertheil-Smoller S, Black HR, Schron E, Berkson DM, Curb JD, Smith WM, McDonald R, Applegate WB. Prevention of heart failure by antihypertensive drug treatment in older persons with isolated systolic hypertension. SHEP Cooperative Research Group. JAMA 1997; 278:212–216. [PubMed] [Google Scholar]
- 32. Di Bari M, Pahor M, Franse LV, Shorr RI, Wan JY, Ferrucci L, Somes GW, Applegate WB. Dementia and disability outcomes in large hypertension trials: lessons learned from the systolic hypertension in the elderly program (SHEP) trial. Am J Epidemiol 2001; 153:72–78. [DOI] [PubMed] [Google Scholar]
- 33. Williamson JD, Supiano MA, Applegate WB, Berlowitz DR, Campbell RC, Chertow GM, Fine LJ, Haley WE, Hawfield AT, Ix JH, Kitzman DW, Kostis JB, Krousel-Wood MA, Launer LJ, Oparil S, Rodriguez CJ, Roumie CL, Shorr RI, Sink KM, Wadley VG, Whelton PK, Whittle J, Woolard NF, Wright JT Jr, Pajewski NM; SPRINT Research Group. Intensive vs standard blood pressure control and cardiovascular disease outcomes in adults aged ≥75 years: a randomized clinical trial. JAMA 2016; 315:2673–2682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Gong L, Zhang W, Zhu Y, Zhu J, Kong D, Pagé V, Ghadirian P, LeLorier J, Hamet P. Shanghai trial of nifedipine in the elderly (STONE). J Hypertens 1996; 14:1237–1245. [DOI] [PubMed] [Google Scholar]
- 35. Dahlöf B, Lindholm LH, Hansson L, Scherstén B, Ekbom T, Wester PO. Morbidity and mortality in the Swedish Trial in Old Patients with Hypertension (STOP-Hypertension). Lancet 1991; 338:1281–1285. [DOI] [PubMed] [Google Scholar]
- 36. Liu L, Wang JG, Gong L, Liu G, Staessen JA. Comparison of active treatment and placebo in older Chinese patients with isolated systolic hypertension. Systolic Hypertension in China (Syst-China) Collaborative Group. J Hypertens 1998; 16:1823–1829. [DOI] [PubMed] [Google Scholar]
- 37. Wang JG, Staessen JA, Gong L, Liu L. Chinese trial on isolated systolic hypertension in the elderly. Systolic Hypertension in China (Syst-China) Collaborative Group. Arch Intern Med 2000; 160:211–220. [DOI] [PubMed] [Google Scholar]
- 38. Staessen JA, Fagard R, Thijs L, Celis H, Arabidze GG, Birkenhäger WH, Bulpitt CJ, de Leeuw PW, Dollery CT, Fletcher AE, Forette F, Leonetti G, Nachev C, O’Brien ET, Rosenfeld J, Rodicio JL, Tuomilehto J, Zanchetti A. Randomised double-blind comparison of placebo and active treatment for older patients with isolated systolic hypertension. The Systolic Hypertension in Europe (Syst-Eur) Trial Investigators. Lancet 1997; 350:757–764. [DOI] [PubMed] [Google Scholar]
- 39. Forette F, Seux ML, Staessen JA, Thijs L, Babarskiene MR, Babeanu S, Bossini A, Fagard R, Gil-Extremera B, Laks T, Kobalava Z, Sarti C, Tuomilehto J, Vanhanen H, Webster J, Yodfat Y, Birkenhäger WH; Systolic Hypertension in Europe Investigators. The prevention of dementia with antihypertensive treatment: new evidence from the Systolic Hypertension in Europe (Syst-Eur) study. Arch Intern Med 2002; 162:2046–2052. [DOI] [PubMed] [Google Scholar]
- 40. Ogihara T, Saruta T, Rakugi H, Matsuoka H, Shimamoto K, Shimada K, Imai Y, Kikuchi K, Ito S, Eto T, Kimura G, Imaizumi T, Takishita S, Ueshima H; Valsartan in Elderly Isolated Systolic Hypertension Study Group. Target blood pressure for treatment of isolated systolic hypertension in the elderly: valsartan in elderly isolated systolic hypertension study. Hypertension 2010; 56:196–202. [DOI] [PubMed] [Google Scholar]
- 41. Wei Y, Jin Z, Shen G, Zhao X, Yang W, Zhong Y, Wang J. Effects of intensive antihypertensive treatment on Chinese hypertensive patients older than 70 years. J Clin Hypertens (Greenwich) 2013; 15:420–427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Parsons C, Murad MH, Andersen S, Mookadam F, Labonte H. The effect of antihypertensive treatment on the incidence of stroke and cognitive decline in the elderly: a meta-analysis. Future Cardiol 2016; 12:237–248. [DOI] [PubMed] [Google Scholar]
- 43. Forette F, Seux ML, Staessen JA, Thijs L, Birkenhäger WH, Babarskiene MR, Babeanu S, Bossini A, Gil-Extremera B, Girerd X, Laks T, Lilov E, Moisseyev V, Tuomilehto J, Vanhanen H, Webster J, Yodfat Y, Fagard R. Prevention of dementia in randomised double-blind placebo-controlled Systolic Hypertension in Europe (Syst-Eur) trial. Lancet 1998; 352:1347–1351. [DOI] [PubMed] [Google Scholar]
- 44. Warwick J, Falaschetti E, Rockwood K, Mitnitski A, Thijs L, Beckett N, Bulpitt C, Peters R. No evidence that frailty modifies the positive impact of antihypertensive treatment in very elderly people: an investigation of the impact of frailty upon treatment effect in the HYpertension in the Very Elderly Trial (HYVET) study, a double-blind, placebo-controlled study of antihypertensives in people with hypertension aged 80 and over. BMC Med 2015; 13:78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Franklin SS, Jacobs MJ, Wong ND, L’Italien GJ, Lapuerta P. Predominance of isolated systolic hypertension among middle-aged and elderly US hypertensives: analysis based on National Health and Nutrition Examination Survey (NHANES) III. Hypertension 2001; 37:869–874. [DOI] [PubMed] [Google Scholar]
- 46. Turnbull F, Neal B, Ninomiya T, Algert C, Arima H, Barzi F, Bulpitt C, Chalmers J, Fagard R, Gleason A, Heritier S, Li N, Perkovic V, Woodward M, MacMahon S; Blood Pressure Lowering Treatment Trialists’ Collaboration. Effects of different regimens to lower blood pressure on major cardiovascular events in older and younger adults: meta-analysis of randomised trials. BMJ 2008; 336:1121–1123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Weiss J, Freeman M, Low A, Fu R, Kerfoot A, Paynter R, Motu’apuaka M, Kondo K, Kansagara D. Benefits and harms of intensive blood pressure treatment in adults aged 60 years or older: a systematic review and meta-analysis. Ann Intern Med 2017; 166:419–429. [DOI] [PubMed] [Google Scholar]
- 48. Bavishi C, Bangalore S, Messerli FH. Outcomes of intensive blood pressure lowering in older hypertensive patients. J Am Coll Cardiol 2017; 69:486–493. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.