Abstract
Background.
Transcatheter aortic valve replacement (TAVR) and transcatheter mitral valve replacement expose operators to radiation. These procedures differ primarily in whether they are performed via a transfemoral (TF) or an alternative access (AA) approach. This study compared operator radiation exposure during transcatheter valve implantation when performed via a TF vs an AA approach, when performed in a catheterization lab vs a hybrid operating room (OR), and investigated the potential benefit of disposable shielding.
Methods.
Dosimeters were worn during TAVR-TF (n = 50) and TAVR-AA (n = 31) procedures by operators. All TAVR-AA procedures were performed in a hybrid OR and TF procedures were performed in either catheterization labs (n = 16) or a hybrid OR (n = 34). Disposable radiation shielding pads (RADPAD; Worldwide Innovations and Technologies, Inc, Kansas City) or a placebo were added in a randomized, blinded fashion.
Results.
Team radiation exposure was higher after TAVR-AA vs TAVR-TF (median 15.1 mRad [interquartile range: IQR 8.6, 32.4] vs 5.5 mRad [IQR 2.4, 9.8], P < .001). TAVR-TF procedures required the same amount of fluoroscopy time regardless of where they were performed (20.3 ± 7.4 min in hybrid OR vs 19.0 ± 6.4 min in catheterization lab, P = .55). However, radiation exposure for TAVR-TF remained higher when performed in a hybrid OR (median 9.0 mRad [IQR 4.5, 11.9] vs 2.2 mRad [IQR 1.3, 2.8], P < .001). Radiation exposure was greatest for TAVR-AA (median 15.1 mRad [IQR 8.6, 32.4]). The use of RADPAD did not decrease radiation exposure (median 9.0 mRad [IQR 4.5, 14.7] vs 9.4 mRad [IQR 2.8, 19.5], P = .82).
Conclusions.
Procedures performed in the hybrid OR were associated with higher operator radiation exposure. In comparison with the TF approach, AA cases had the highest levels of operator radiation. This is particularly important in cases of transcatheter mitral valve replacement that can only be done via an AA approach. The use of disposable radiation shielding in this series did not attenuate operator radiation exposure. Radiation shielding within hybrid ORs should be scrutinized in an effort to remain on par with that found within catheterization labs.
As the number of transcatheter valve replacement procedures grow, there remains a strong concern for increased radiation exposure to both patient and operator. Transcatheter aortic valve replacement (TAVR) was the first widely adopted catheter valve procedure, and, more recently, transcatheter mitral valve replacement (TMVR) has emerged within the field of cardiac structural interventions. Catheter-based structural valve replacement is routinely performed via transfemoral access (TF), although for some cases of TAVR and presently for most cases of TMVR, an alternative access (AA) approach involving incisions about the chest are required. Structural heart procedures are complex procedures, often requiring longer procedural and fluoroscopy times as compared to routine angiography and single vessel intervention.1 Several studies have demonstrated that these procedures are associated with increased effective radiation dose exposure to both proceduralists and patients.2,3
Various factors determine operator radiation exposure during transcatheter valve replacement, including operator position, duration of the procedure, and the level of radiation shielding.4 It is greatly influenced by technical, procedural, and patient factors. This includes fluoroscopy time, field of view, table height, radiation source to patient and patient to image intensifier distance, and patient size. Radiation exposure is inversely proportional to the square of the distance from the radiation source. The smaller the distance between the operator’s body and the area where (scattered) x-rays originate, the higher the dose of radiation exposure. Additionally, the higher the distance to the image intensifier, the higher the scatter radiation would be that can potentially be absorbed by the operators. Lastly, thicker body masses (eg, obese patients) require higher radiation doses.5
Ionizing radiation causes physical and chemical damage to the cell and can have deterministic and stochastic effects. Deterministic effects encompass predictable tissue reactions that occur once a certain threshold of exposure is reached—for example, skin erythema, hair loss, and cataracts.6–8 Alternatively, stochastic effects refer to the potential for future harm to tissue, including carcinogenesis and genetic mutation, which may occur at random without reaching a threshold dose level.9
As percutaneous structural heart programs are increasingly established around the country, hospitals and providers often struggle to create procedural spaces that provide both superior imaging and adequate access for surgical incisions, while maintaining a high level of safety for both patient and provider within a radiation-rich environment. Although radiation safety is simply translated as the practice of utilizing as little radiation as possible for adequate imaging while optimizing radiation shielding, this is a relatively new concept for many cardiac surgeons—many of whom are unfamiliar with radiation environments and are newcomers to the concepts of radiation safety. It remains debatable whether TAVR/TMVR procedures are best performed within a traditional cardiac catheterization lab vs a hybrid surgical space. Optimizing these procedural spaces for catheter-based valve procedures and scrutinizing radiation shielding options, both fixed and disposable, remain challenges for many institutions.10
This study compared operator radiation exposure during TAVR when performed via a TF vs an AA approach, when performed in a catheterization lab vs a hybrid operating room (OR), and further investigated the potential additional benefit of disposable radiation shielding on operator radiation exposure.
Patients and Methods
This study was approved by the Washington University School of Medicine institutional review board. Informed consent and permission for release of information were obtained from all patients.
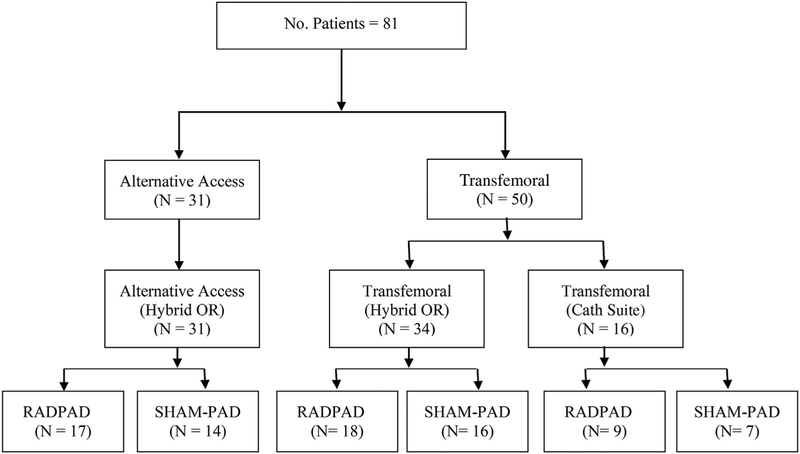
Dosimetry measurements were taken from primary and secondary operators during TAVR cases done with the Edwards Sapien XT and Sapien 3 valves (Edwards Life-sciences, Irvine, CA). Dosimetry data was obtained on 81 procedures (n = 50 TF, n = 31 AA) (Figure 1). All AA cases performed in a hybrid OR. In contrast, transfemoral TAVR cases were done in both cardiac catheterization rooms (n = 16) as well as the hybrid OR (n = 34). All cardiac catheterization rooms contained standard under-the-table as well as overhead shielding, a feature not present in the hybrid OR. The catheterization suite was equipped with a Philips AlluraClarity x-ray system (Philips, Amsterdam, Netherlands) and the hybrid OR utilized a Siemens Axiom Artis dTA system (Siemens, Munich, Germany).
Figure 1.
Patient distribution. (Cath, catheterization; No., number; OR, operating room; RADPAD, disposable radiation shielding pad; SHAM-PAD, placebo pad.)
All cases were randomized to be performed with either a disposable radiation shielding pad (RADPAD; Worldwide Innovations and Technologies, Inc, Kansas City MO) or a placebo (SHAM-PAD) in a blinded fashion. The disposable shielding and the placebo pads were identical in appearance and deidentified by the manufacturer to blind operators to the type of pad being used for each procedure. For alternative access cases, the disposable RADPAD (model 5102A-O) or SHAM-PAD was placed on both sides of the patient. These shields measured 14 × 17 inches and were without fenestration. For TAVR-TF procedures, a disposable femoral access shield (RADPAD, model 5300A-O) or SHAM-PAD, measuring 14.5 × 16.5 inches, with a single fenestration for obtaining access, was placed on the right side of the patient. Both the RADPAD and SHAM-PAD were typically not in the fluoroscopy field of view. The SHAM-PAD was also radiopaque to allow for true blinding during the procedure.
Radiation readings were taken at the torso of first and second position operators using MYDOSE mini dosimeters, model PDM-127B-SH (Hitachi Aloka Medical Ltd, Tokyo, Japan) with readouts provided in millirads (mRad). These readings were subsequently indexed to procedural fluoroscopy time (mRad/min) and reported as a median value with corresponding first and third quartiles (median [first quartile, third quartile]). The maximum operator dose for each case was recorded. In addition, the total radiation exposure for the first and second operators was used to quantify team exposure. Team exposure was measured to more accurately reflect the total radiation exposure for providers and negate some of the variability in operator positioning that occurs by default throughout the case (operators switching from primary to secondary position or vice versa). In order to account for potential differences in fluoroscopy time between cases, team radiation exposure was also indexed to fluoroscopy time (mRad/min). The dosimetry data were not normally distributed; therefore, comparisons were performed using the Mann-Whitney U-test and summarized by the median value with accompanying first and third quartiles (median [first quartile, third quartile]). All statistical analyses were performed using SAS 9.4 (SAS Institute, Cary, NC).
Results
RADPAD vs Sham Shield (SHAM-PAD)
The RADPAD made no difference in either maximum individual or team radiation exposure across both the alternative access and transfemoral approaches (Table 1). Even after indexing by fluoroscopy time, the RADPAD did not attenuate team radiation exposure (0.5 [0.3, 1.3] mRad/min in RADPAD group vs 0.6 [0.1, 1.6] mRad/min in SHAM-PAD group, P = .73).
Table 1.
Comparison of RADPAD and Sham Shield (SHAM-PAD)
| Variable | RADPAD (n = 44) | SHAM-PAD (n = 37) | P Value |
|---|---|---|---|
| Fluoroscopy time, min | 16.6 ± 6.6 | 16.5 ± 7.7 | .97 |
| Individual radiation, mRAD | 5.4 (2.7, 9.2) | 6.3 (2.1, 14.7) | .75 |
| Team radiation, mRad | 9.0 (4.5, 14.7) | 9.4 (2.8, 19.5) | .82 |
| Team radiation per flouroscopy time, mRad/min | 0.5 (0.3, 1.3) | 0.6 (0.1, 1.6) | .73 |
Values are presented as mean ± SD or median (quartile 1, quartile 3). P value determined by Mann-Whitney U-test.
Transfemoral vs Alternative Access
TAVR-TF procedures required greater amounts of fluoroscopy when compared with TAVR-AA procedures (19.8 ± 7.0 min vs 11.3 ± 3.1 min, P < .001) (Table 2). Despite the increased amount of fluoroscopy utilized for TAVR-TF procedures, TAVR-TF was associated with less maximum individual and team radiation exposure. TAVR-AA was associated with increased radiation exposure. The association between alternative access procedures and radiation exposure remained even when indexed by fluoroscopy time (team radiation per fluoroscopy time for TF 0.3 [0.1, 0.5] mRad/min vs 1.7 [1.1, 3.2] mRad/min in AA, P < .001).
Table 2.
Comparison of Transfemoral and Alternative Access
| Variable | Transfemoral (n = 50) | Alternative Access (n = 31) | P Value |
|---|---|---|---|
| Fluoroscopy time, min | 19.8 ± 7.0 | 11.3 ± 3.1 | <.001 |
| Individual radiation, mRAD | 3.4 (1.8, 6.8) | 9.4 (6.4, 21.3) | <.001 |
| Team radiation, mRad | 5.5 (2.4, 9.8) | 15.1 (8.6, 32.4) | <.001 |
| Team radiation per flouroscopy time, mRad/min | 0.3 (0.1, 0.5) | 1.7 (1.1, 3.2) | <.001 |
Values are presented as mean ± SD or median (quartile 1, quartile 3). P value determined by Mann-Whitney U-test.
Catheterization Laboratory vs Hybrid Operating Room
TAVR-TF procedures were performed utilizing the same amounts of fluoroscopy regardless of where the procedure was performed (20.3 ±7.4 min in the hybrid OR vs 19.0 ± 6.4 min in the catheterization lab, P = .55). Despite the similar fluoroscopy use, TAVR-TF procedures performed in the hybrid OR were associated with significantly higher individual (5.1 [2.9, 9.2] mRad vs 1.5 [0.9, 2.1] mRad, P < .001) and team (9.0 [4.5, 11.9] mRad vs 2.2 [1.3, 2.8] mRad, P < .001) radiation exposure (Table 3).
Table 3.
Comparison of Transfemoral Transcatheter Aortic Valve Replacement: Hybrid Operating Room and Catheterization Suite
| Variable | Hybrid Operating Room (n = 34) | Catheterization Suite (n = 16) | P Value |
|---|---|---|---|
| Fluoroscopy time, min | 20.3 ± 7.4 | 19.0 ± 6.4 | .55 |
| Individual radiation, mRAD | 5.1 (2.9, 9.2) | 1.5 (0.9, 2.1) | <.001 |
| Team radiation, mRad | 9.0 (4.5, 11.9) | 2.2 (1.3, 2.8) | <.001 |
| Team radiation per fluoroscopy time, mRad/min | 0.4 (0.3, 0.7) | 0.1 (0.1, 0.2) | <.001 |
Values are presented as mean ± SD or median (quartile 1, quartile 3). P value determined by Mann-Whitney U-test.
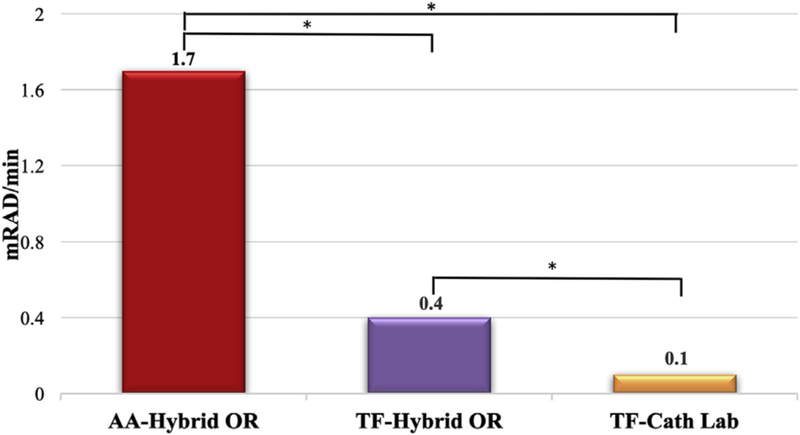
In comparing radiation exposure across access routes and by procedural space, TAVR-AA cases (all performed in the hybrid OR) were found to have the highest levels of operator radiation exposure (1.7 [1.1, 3.2] mRad/min). Radiation exposure was next highest for TAVR-TF cases performed in the hybrid OR (0.4 [0.3, 0.7] mRad/min) and the lowest radiation exposure for operators among all TAVR cases was found in TAVR-TF cases performed in the catheterization lab (0.1 [0.1, 0.2] mRad/min) (Figure 2).
Figure 2.
Median team radiation exposure for transcatheter aortic valve replacement performed via alternative access in the hybrid operating room (AA-Hybrid OR), transfemoral access in the hybrid operating room (TF-Hybrid OR), and transfemoral access in the cardiac catheterization suite (TF-Cath Lab). * P < .001.
Comment
A principal finding of this investigation was that TAVR procedures performed in hybrid OR and those done via an AA approach were associated with significantly more radiation exposure to the procedural team when compared with those performed in catheterization laboratory and via TF approach. This finding is likely explained by operator positioning that dictates operators to be in a more cranial position for alternative access procedures and closer to the radiation source when compared with TF procedures.4 As subsequent radiation exposure is inversely proportional to the square of the distance from the radiation source, this results in significantly greater operator radiation exposure despite the reduced amount of fluoroscopy used in alternative access procedures (transapical, transaortic, or subclavian) when compared with TF.4 Although transapical TAVR has specifically been demonstrated to require less fluoroscopy than other approaches and does reduce patient radiation exposure,3,11 these benefits do not appear to translate towards the proceduralists, given the findings of this study. TAVR-AA at our site accounts for less than 10% to 15% of all TAVRs performed,11 but the significantly greater radiation exposure associated to providers with these procedures must be recognized. According to our study and based on the radiation dosage associated with transfemoral procedures, a team performing 100 transfemoral procedures in the cardiac catheterization lab over a year would have the equivalent radiation exposure to that of 1 low-dose computed tomography scan of the chest. That same team performing the same number of procedures in the hybrid OR would have the affective exposure of having received 4 low-dose computed tomography scans of the chest. The findings of this study have direct implications not only for TAVR-AAs, but also for the recently introduced TMVR procedures that are performed in hybrid OR settings and primarily through an AA approach.
In this study, the inferior radiation protection afforded operators within the hybrid OR is derived from analysis of the TAVR-TF cohort. TAVR-TF procedures performed in the hybrid OR were associated with significantly higher individual and team radiation when compared with the same TF procedure performed in the cardiac catheterization laboratory (Figure 2). Although hybrid ORs at most institutions are designed to accommodate a variety of cardiac and vascular procedures with multiple operator positions, the versatility of this environment is a potential weakness of these spaces, often limiting additional shielding options (eg, overhead and under-table shielding) and increasing operator exposure to scatter radiation.4 It should be recognized that the imaging equipment within our hybrid OR, at the time of this study, was of an earlier generation than the newer “low-dose” x-ray systems found in our recently renovated catheterization labs.12,13 Taken as a whole, for hybrid spaces to remain successful, they must be designed to facilitate specific procedures, maximizing built-in shielding and, importantly, be routinely upgraded as advances in imaging systems are developed. Our findings should not undermine the potential advantages of a hybrid OR over a catheterization laboratory—which include ease of emergent surgical conversion in case of severe complications (eg, annular rupture), ease of converting to open vascular access, and lower infection rates due to the more aseptic environment—but should emphasize on how hybrid ORs should strive to mimic radiation safety practice and shielding commonly seen within catheterization labs. The findings of this study led hospital leadership at our institution to immediately upgrade shielding within our hybrid space and recommend the installation of a new “low-dose” imaging system.
Radiation dose should be kept As Low As Reasonably Achievable (the ALARA principle). Radiation dose parameters including fluoroscopy time, accumulated air kerma and air kerma area product should be monitored throughout the procedure and recorded. There are many ways in which radiation exposure can be reduced during cardiac procedures. These include using good beam geometry, reducing fluoroscopy pulse rate, maintaining a minimal patient to image intensifier distance, and keeping the radiation source (x-ray tube) as far away as possible. Additionally, effective management of radiation exposures by proper use of equipment, adequate training of fluoroscopic operators, and frequent quality control can contribute to an overall reduction in patient and team exposures.
In this study, RADPAD provided no significant protection to lower individual operator and team radiation exposure for either the AA or TF approaches. Although this is contrary to previously reported procedures,14–18 the explanations for this may be multifaceted. In contrast to a positive study for the use of RADPAD by Sharma and colleages,18 this study assessed radiation exposure as a team, rather than that of the single lead operator. Most importantly, this study was conducted in a randomized, blinded fashion, eliminating as much as possible biased behavior with regard to fluoroscopy use, which can clearly affect study outcomes. Improvements in RADPAD utilization or construction, particularly for TAVR-AA cases, as well as a larger, blinded, randomized comparison, are likely necessary before the routine use of disposable scatter pads can be supported.
There are several limitations to this study. This was a small, single-center study of radiation protection in TAVR procedures. Importantly, the optimal shielding location for the RADPAD is unknown. The individual variation for radiation exposure was high, suggesting the variability in individual operators’ adherence to best radiation safety practices. To mitigate such variability, operator exposure was represented as team radiation in order to minimize this variation. Moreover, the dosimeters were worn by the primary and secondary operators at the level of their torso. We were not able to discern what region of the operator’s body was most exposed to the image intensifier (eg, upper body vs lower body). Additionally, given the randomized nature of this study, we assumed that the number of cutdowns performed were equivalent in TF cases done in the catheterization lab vs hybrid OR. In this study, we did not account for the use of femoral cutdowns, which are known to reduce overall total fluoroscopy time and radiation exposure.
In conclusion, this report demonstrated increased operator radiation exposure for AA cases and higher radiation exposure for all cases done in a hybrid OR compared with the cardiac catheterization suite. Additionally, the RADPAD failed to lower individual or team radiation exposure. The findings of this investigation carry implications for any procedure that are performed in hybrid procedural spaces and certainly for those that require an AA approach. It behooves proceduralists to critically scrutinize radiation safety within their own hybrid OR spaces and operators must be aware of potential limitations of fixed and disposable shielding options in reducing radiation exposure during catheter-based valve procedures.
Footnotes
Presented at the Ninety-sixth Annual Meeting of the American Association for Thoracic Surgery, Baltimore, MD, May 14–18, 2016.
References
- 1.Boland JE, Wang LW, Love BJ, Wynne DG, Muller DW. Radiation dose during percutaneous treatment of structural heart disease. Heart Lung Circ. 2014;23:1075–1083. [DOI] [PubMed] [Google Scholar]
- 2.Signorotto P, del Vecchio A, Montorfano M, et al. Dosimetric data and radiation risk analysis for new procedures in interventional cardiology. Radiat Prot Dosimetry. 2010;142:201–208. [DOI] [PubMed] [Google Scholar]
- 3.Daneault B, Balter S, Kodali SK, et al. Patient radiation exposure during transcatheter aortic valve replacement procedures. EuroIntervention. 2012;8:679–684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sauren LD, van Garsse L, van Ommen V, Kemerink GJ. Occupational radiation dose during transcatheter aortic valve implantation. Catheter Cardiovasc Interv. 2011;78:770–776. [DOI] [PubMed] [Google Scholar]
- 5.Okamoto K, Ito J, Sakai K, Yoshimura S. The principle of digital subtraction angiography and radiological protection. Interv Neuroradiol. 2000;6:25–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Balter S, Hopewell JW, Miller DL, Wagner LK, Zelefsky MJ. Fluoroscopically guided interventional procedures: a review of radiation effects on patients’ skin and hair. Radiology. 2010;254:326–341. [DOI] [PubMed] [Google Scholar]
- 7.Vlietstra RE, Wagner LK, Koenig T, Mettler F. Radiation burns as a severe complication of fluoroscopically guided cardio-logical interventions. J Interv Cardiol. 2004;17:131–142. [DOI] [PubMed] [Google Scholar]
- 8.Koenig TR, Wolff D, Mettler FA, Wagner LK. Skin injuries from fluoroscopically guided procedures: part 1, characteristics of radiation injury. AJR Am J Roentgenol. 2001;177:3–11. [DOI] [PubMed] [Google Scholar]
- 9.Venneri L, Rossi F, Botto N, et al. Cancer risk from professional exposure in staff working in cardiac catheterization laboratory: insights from the National Research Council’s Biological Effects of Ionizing Radiation VII Report. Am Heart J. 2009;157:118–124. [DOI] [PubMed] [Google Scholar]
- 10.Fetterly KA, Mathew V, Lennon R, Bell MR, Holmes DR Jr, Rihal CS. Radiation dose reduction in the invasive cardiovascular laboratory: implementing a culture and philosophy of radiation safety. JACC Cardiovasc Interv. 2012;5:866–873. [DOI] [PubMed] [Google Scholar]
- 11.Henn MC, Percival T, Zajarias A, et al. Learning alternative access approaches for transcatheter aortic valve replacement: implications for new transcatheter aortic valve replacement centers. Ann Thorac Surg. 2017;103:1399–1405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Stangenberg L, Shuja F, van der Bom IMJ, et al. Modern fixed imaging systems reduce radiation exposure to patients and providers. Vasc Endovascular Surg. 2018;52:52–58. [DOI] [PubMed] [Google Scholar]
- 13.Sullivan PM, Harrison D, Badran S, Takao CM, Ing FF. Reduction in radiation dose in a pediatric cardiac catheterization lab using the Philips AlluraClarity X-ray System. Pediatr Cardiol. 2017;38:1583–1591. [DOI] [PubMed] [Google Scholar]
- 14.Brambilla M, Occhetta E, Ronconi M, Plebani L, Carriero A, Marino P. Reducing operator radiation exposure during cardiac resynchronization therapy. Europace. 2010;12:1769–1773. [DOI] [PubMed] [Google Scholar]
- 15.Germano JJ, Day G, Gregorious D, Natarajan V, Cohen T. A novel radiation protection drape reduces radiation exposure during fluoroscopy guided electrophysiology procedures. J Invasive Cardiol. 2005;17:469–472. [PubMed] [Google Scholar]
- 16.Politi L, Biondi-Zoccai G, Nocetti L, et al. Reduction of scatter radiation during transradial percutaneous coronary angiography: a randomized trial using a lead-free radiation shield. Catheter Cardiovasc Interv. 2012;79:97–102. [DOI] [PubMed] [Google Scholar]
- 17.Murphy JC, Darragh K, Walsh SJ, Hanratty CG. Efficacy of the RADPAD protective drape during real world complex percutaneous coronary intervention procedures. Am J Cardiol. 2011;108:1408–1410. [DOI] [PubMed] [Google Scholar]
- 18.Sharma D, Ramsewak A, Manoharan G, Spence MS. Efficacy of RADPADÒ protection drape in reducing radiation exposure to the primary operator during Transcatheter Aortic Valve Implantation (TAVI). Minerva Cardioangiol. 2016;64:41–46. [PubMed] [Google Scholar]