Abstract
Objective: Symptoms of ADHD are expected to be more difficult to treat in patients with a combination of ADHD and autism spectrum disorder (ASD) as opposed to only ADHD. Little evidence is available on the influence of ASD on the effects of pharmacotherapy in adults with ADHD. This study addresses this gap. Method: 60 adults with ADHD and comorbid ASD were selected from an outpatient clinic and compared with 226 adults from the same clinic with only ADHD. Similar treatment regimens were received. Results: Significant decreases in symptoms of ADHD were found in both groups. A diagnosis of ASD did not affect the reduction in symptoms of ADHD. No significant group differences in side effects or vital signs were found. Conclusion: Results show that medication for ADHD can effectively and safely be prescribed to patients with ADHD and comorbid ASD. Suggestions for future research are discussed.
Keywords: ADHD, autism spectrum disorders, comorbidity, adult ADHD treatment, pharmacotherapy
Autism spectrum disorder (ASD) and ADHD frequently co-occur (Leyfer et al., 2006; Reiersen & Todd, 2008; Simonoff et al., 2008). The Diagnostic and Statistical Manual of Mental Disorders (4th ed., text rev.; DSM-IV-TR; American Psychiatric Association [APA], 2000) nevertheless prohibited the comorbid diagnosis of ADHD and ASD. Given the significant comorbidity and overlap in the symptoms of ADHD and ASD, however, the newer Diagnostic and Statistical Manual of Mental Disorders (5th ed.; DSM-5; APA, 2013) now allows comorbid diagnosis (Luteijn et al., 2000). The reported comorbidity rate of ASD for ADHD in adulthood varies from 14% to 78% (Gargaro, Rinehart, Bradshaw, Tonge, & Sheppard, 2011; Leyfer et al., 2006; Sinzig, Walter, & Doepfner, 2009). Conversely, ADHD has been found to be the second most common comorbid disorder diagnosed in adults with ASD, after social anxiety disorder (Simonoff et al., 2008).
Persistence Into Adulthood
ADHD and ASD have their onsets in childhood (Hartman, Geurts, Franke, Buitelaar, & Rommelse, 2016; Jensen & Steinhausen, 2015). The worldwide prevalence of ADHD in adulthood is estimated to be 2.8% (Fayyad et al., 2017; Michielsen et al., 2012). No data are available on the occurrence of ASD in adults in the Netherlands. In England, approximately 1% of the population is diagnosed with ASD and the rate has not been found to decrease with age (Brugha et al., 2011).
Treatment
In the Netherlands, the recommended pharmacological treatment for ADHD is methylphenidate (MPH), dexamphetamine (DEX), atomoxetine (ATX), or bupropion (Dutch Psychiatric Association, 2015). Pharmacological treatment is also recommended for ADHD and comorbid ASD by the most recent treatment guideline (National Institute for Health and Care Excellence, 2018). However, little evidence is available on the safety and effectiveness of ADHD pharmacotherapy in this population of patients. In the Dutch treatment guideline for ASD, it is stated no evidence is available on the safety and effectiveness of using ADHD pharmacotherapy in adults with ASD (Kan et al., 2013).
Extensive research on pharmacotherapy has, however, been conducted among children with ADHD and comorbid ASD (Cortese, Castelnau, Morcillo, Roux, & Bonnet-Brilhault, 2012; Di Martino, Melis, Cianchetti, & Zuddas, 2004; Findling, 2005; Ghuman et al., 2009; Hazell, 2007; Jahromi et al., 2009; Nickels et al., 2008; Pearson et al., 2013; Posey et al., 2007; Roy, Dillo, Bessling, Emrich, & Ohlmeier, 2009; Stigler, Desmond, Posey, Wiegand, & McDougle, 2004). The outcomes generally show some effectiveness of MPH in the treatment of hyperactivity in ASD (Sturman, Deckx, & van Driel, 2017). Unfortunately, many of the children with ASD experienced side effects of MPH, causing discontinuation of the medication. One study reported a dropout rate of 18% (Research Units on Pediatric Psychopharmacology Autism Network, 2005).
There is no evidence available for the use of DEX or bupropion in patients with ADHD and comorbid ASD. While evidence is modest for the use of ATX relative to psychostimulants, improvement in ADHD behavior as a result of ATX has been reported (Aman et al., 2014; Arnold et al., 2006; Harfterkamp et al., 2012). The side effects of using ATX have been well tolerated (Tumuluru et al., 2017).
Modafinil is another psychostimulant used to treat symptoms of ADHD. It is not recommended as pharmacological treatment for ADHD, only to be used when other means turn out to be insufficiently effective (Dutch Psychiatric Association, 2015; Kan et al., 2013; Kooij, 2009).
Aim
As illustrated by the mentioned research on MPH in children, it is possible that the pharmacological effects of MPH might be different for patients with ASD than for patients with only ADHD (Research Units on Pediatric Psychopharmacology Autism Network, 2005). More research on the effectiveness and side effects of pharmacological treatment for ADHD in a group of patients with comorbid ASD is thus needed to make the choice of treatment less dependent on the personal experience of the psychiatrist and more evidence based (Kan et al., 2013).
In retrospect, the effectiveness and side effects of pharmacotherapy were compared for 60 adults with ADHD and ASD, on one hand, and 226 adults with only ADHD, on the other hand. The treatment consisted of MPH, DEX, ATX, bupropion, or modafinil. To our knowledge, this is the first study of the treatment of ADHD in an adult ASD population.
Research Questions
Research Question 1: Do adults with ADHD and comorbid ASD experience less effectiveness of pharmacological treatment for ADHD than adults with only ADHD?
Research Question 2: Do adults with ADHD and comorbid ASD experience different or more severe side effects of pharmacological treatment for ADHD than adults with only ADHD, as measured in side-effect scores, blood pressure, heart rate, and weight?
Method
Participants
Participants were selected from a population being treated at the Radboudumc adult outpatient psychiatric department sometime between January 2013 and May 2018. The selection process is depicted in Figure 1. Data were drawn from the hospital administrative database using the diagnostic treatment codes of ADHD (314.00 and 314.01) and ASD (299.00 and 299.80). This search returned a total of 1,408 patients with ADHD, with 19.6% also having a diagnosis of ASD (n = 276, see Figure 1). Patients were then selected for having confirmed diagnoses of ADHD and ASD according to the Diagnostic and Statistical Manual of Mental Disorders (4th ed.; DSM-IV; APA, 1994) or—since January 2017—the DSM-5 criteria. This was, where available, established using the Diagnostic Interview for ADHD in Adults (DIVA; Kooij & Francken, 2009) and—starting in the second half of 2016—the ‘Nederlands Interview ten behoeve van Diagnostiek Autismespectrumstoornis bij volwassenen’ (NIDA, translation: Dutch Interview for Diagnosis of Autism Spectrum Disorder in adults, Vuijk, 2014). All of the selected case records were then required to meet the following criteria: (a) received treatment according to the ADHD guidelines (Dutch Psychiatric Association, 2015; National Institute for Health and Care Excellence, 2008) and (b) had at least two extractable, complete symptom score lists available for analysis with (1) the first one recorded at the onset of treatment (T1) and (2) the second one recorded at the end of the clinical titration process (T2). The largest reason for exclusion for further analysis was absence of at least two recorded symptom lists. The population for analysis consisted of 286 ADHD patients, with 21.0% (n = 60) also having a diagnosis of ASD (see Figure 1). Table 1 provides an overview of the patient characteristics.
Figure 1.
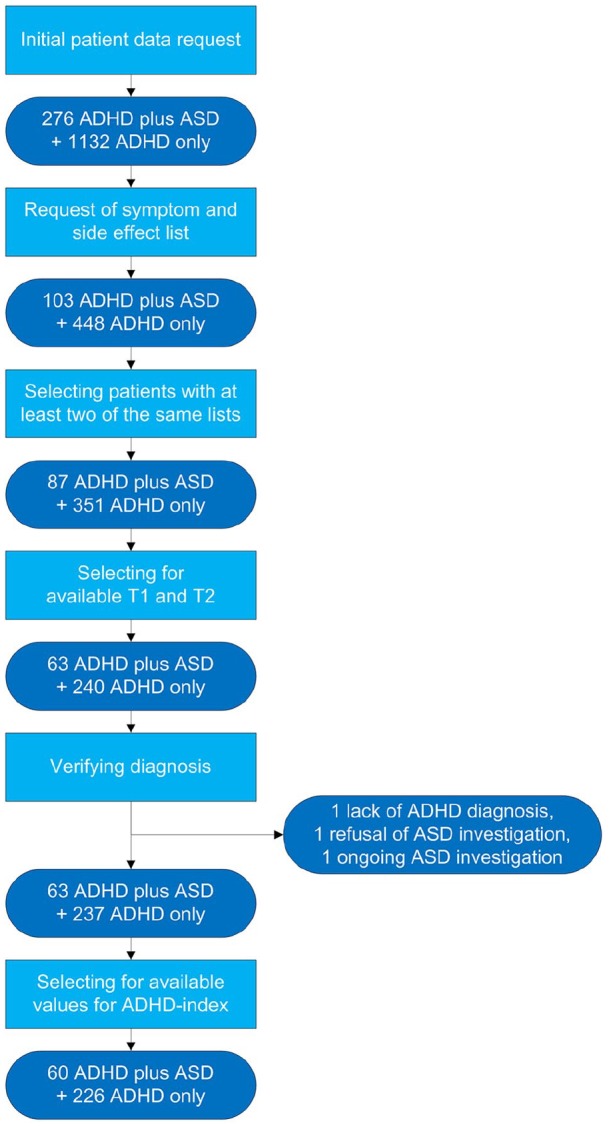
Patient selection process.
Note. ASD = autism spectrum disorder.
Table 1.
Baseline characteristics.
| ADHD plus ASD | ADHD only | p | |||
|---|---|---|---|---|---|
| Gender | |||||
| Male | 43 | 71.70% | 119 | 52.70% | .008* |
| Age (years) | 35.6 ± 11.2 | 35.1 ± 11.7 | .794 | ||
| ADHD subtype | |||||
| ADHD-C | 24 | 40.00% | 94 | 41.60% | .684 |
| ADHD-I | 28 | 46.70% | 110 | 48.70% | |
| ADHD-H | 2 | 3.30% | 9 | 4.00% | |
| ADHD-NOS | 6 | 10.00% | 13 | 5.80% | |
| CAARS:S-S ADHD-index | 20.4 ± 4.5 | 19.9 ± 4.8 | .451 | ||
| CAARS: S-S total score | 42.9 ± 10.0 | 42.6 ± 10.1 | .810 | ||
| Inattention/Memory | 9.7 ± 2.7 | 9.7 ± 2.9 | .850 | ||
| Hyperactivity/Restlessness | 7.5 ± 3.1 | 8.4 ± 2.9 | .029* | ||
| Impulsivity/Emotional Lability | 6.9 ± 2.8 | 6.0 ± 2.9 | .050* | ||
| Problems With Self-Concept | 7.7 ± 3.2 | 7.4 ± 3.7 | .613 | ||
| 13-item side effect score | 8.6 ± 4.6 | (n = 44) | 7.2 ± 5.3 | (n = 165) | .116 |
| 20-item side effect score | 11.9 ± 7.4 | (n = 22) | 9.5 ± 6.1 | (n = 80) | .142 |
| Sleeping disorder | 1.21 ± 0.98 | (n = 24) | 1.04 ± 0.99 | (n = 96) | .427 |
| Decreased appetitea | 0.42 ± 0.69 | (n = 45) | 0.36 ± 0.68 | (n = 169) | .467 |
| Weight lossa | 0.07 ± 0.25 | (n = 45) | 0.22 ± 0.55 | (n = 171) | .091 |
| Headachea | 0.73 ± 0.84 | (n = 45) | 0.72 ± 0.89 | (n = 170) | .772 |
| Palpitationsa | 0.20 ± 0.40 | (n = 45) | 0.35 ± 0.62 | (n = 170) | .213 |
| Nervousnessa | 1.04 ± 0.80 | (n = 45) | 1.02 ± 0.84 | (n = 171) | .809 |
| Dizzynessa | 0.56 ± 0.72 | (n = 45) | 0.36 ± 0.61 | (n = 170) | .058 |
| Agitationa | 1.78 ± 0.88 | (n = 45) | 1.26 ± 0.99 | (n = 171) | .001* |
| Anxietya | 0.82 ± 0.83 | (n = 45) | 0.74 ± 0.93 | (n = 170) | .325 |
| Gastrointestinal complaintsa | 0.62 ± 0.91 | (n = 45) | 0.51 ± 0.82 | (n = 171) | .520 |
| Dry moutha | 0.60 ± 0.89 | (n = 45) | 0.39 ± 0.68 | (n = 171) | .189 |
| Tics/involuntary movementsa | 0.43 ± 0.66 | (n = 44) | 0.27 ± 0.62 | (n = 171) | .043* |
| Euphoria/unusual happinessa | 0.36 ± 0.71 | (n = 45) | 0.29 ± 0.55 | (n = 171) | .888 |
| Sadness/unhappinessa | 1.09 ± 0.97 | (n = 45) | 0.80 ± 0.84 | (n = 171) | .073 |
| Sweating | 1.13 ± 1.08 | (n = 24) | 0.55 ± 0.88 | (n = 96) | .007* |
| Tense jaw muscles | 0.58 ± 0.97 | (n = 24) | 0.48 ± 0.85 | (n = 96) | .623 |
| Hair loss | 0.08 ± 0.28 | (n = 24) | 0.23 ± 0.55 | (n = 95) | .239 |
| Itching | 0.33 ± 0.64 | (n = 24) | 0.25 ± 0.60 | (n = 95) | .489 |
| Rash | 0.57 ± 0.90 | (n = 23) | 0.27 ± 0.68 | (n = 84) | .132 |
| Sexual complaints | 0.31 ± 0.75 | (n = 13) | 0.30 ± 0.74 | (n = 54) | .951 |
| Heart rate (bpm) | 70 ± 13 | (n = 39) | 71 ± 12 | (n = 122) | .551 |
| Systolic blood pressure (mmHg) | 126 ± 16 | (n = 41) | 125 ± 15 | (n = 123) | .565 |
| Diastolic blood pressure (mmHg) | 81 ± 12 | (n = 41) | 79 ± 9 | (n = 123) | .246 |
| Weight (kg) | 88.7 ± 25.1 | (n = 36) | 77.8 ± 15.8 | (n = 117) | .002* |
| Total | N = 60 | N = 226 | N = 286 | ||
Note. The number of cases with a 20-item score is a subset of the number with a 13-item score, that is, all those with 20-item scores also have 13-item scores (because the 13-item list is a subset of the 20 items). ASD = autism spectrum disorder; ADHD-C = ADHD of the combined type; ADHD-I = ADHD of the inattentive type; ADHD-H = ADHD of the hyperactive type; ADHD-NOS = ADHD not otherwise specified; CAARS = Conners’ ADHD Rating Scale: Self Report–Short Version.
Side effects included in the original 13-item score.
p < .05.
Data
ADHD symptoms were scored using the Conners’ ADHD Rating Scale: Self Report–Short Version (CAARS: S-S; Conners, Erhardt, & Sparrow, 1999). This is standard practice at the Radboudumc. The CAARS is a widely accepted ADHD scoring instrument, and one of the few providing information on different symptom domains through sub-scores (Rosler et al., 2006). It has also been shown to outperform several other ADHD screening instruments (Dakwar et al., 2012). Main outcome was measured by the ADHD-index of the CAARS, which is a subset of items of the CAARS and is used as an indicator of ADHD severity in clinical practice.
Side effects of ADHD medication were measured using either a 13-item or 20-item checklist with 4-point Likert-type scales for item response. The 13-item list was initially used in the Radboudumc. This was later expanded to 20 items, adopted for use in June 2016, and is now the standard for assessment at the Radboudumc.
Changes in heart rate, blood pressure, and body weight as a result of ADHD medication were also assessed. Data were evaluated from two moments in time: at the onset of treatment (T1) and at the end of the clinical titration process (T2). The differences for these two points in time were then compared for the two groups of patients receiving medication.
Analyses
The effectiveness of treatment was estimated by examining the decreases in the symptoms of ADHD as measured by the CAARS: S-S ADHD-index. The significance of any within-group decreases was evaluated using paired-sample t tests. A three-way factorial analysis of covariance (ANCOVA) was conducted to determine a statistically significant difference between comorbid ASD diagnosis, ADHD subtype, and gender on the ADHD-index, controlling for age. ASD diagnosis included two levels (yes or no). ADHD subtype included four levels (combined type, inattentive type, hyperactive type, and not-otherwise-specified type). Gender included two levels (male or female). When significant main or interaction effects were found for the ADHD-index, the CAARS sub-scores were further analyzed to look for main or interaction effects. ADHD subtype was controlled for in these analyses, as patients with high levels of hyperactivity/impulsivity were found to respond differently to MPH than patients with low levels (Beery, Quay, & Pelham, 2017).
The side effects of treatment were estimated by examining the absolute differences between T1 and T2 for the individual item scores on the 13-item or (where available) 20-item lists. The absolute increases or decreases in the item scores were compared within the groups of patients with ADHD and comorbid ASD or only ADHD using signed-rank tests. Between-group differences were assessed using Mann–Whitney tests. The relative differences in heart rate, blood pressure, and body weight as a result of ADHD medication were assessed using t tests for independent samples.
Results
Demographic and Baseline Characteristics
A significant gender difference was found for the two groups of patients: 47% of the only ADHD group was female versus 28% of the ADHD plus ASD group, χ²(1, N = 286) = 6.978; p = .008. The mean ages for the two groups of patients and distribution of ADHD subtypes within the two groups did not differ.
At baseline, the ADHD plus ASD group showed lower scores on the Hyperactivity/Restlessness subscale of the CAARS than the ADHD-only group. The group of patients with only ADHD showed lower scores on the Impulsivity/Emotional Lability subscale than the ADHD plus ASD group. The ADHD-index and total score for the CAARS: S-S were similar. The ADHD-only group was less affected by agitation, tics/involuntary movements, and sweating at baseline than the ADHD plus ASD group, but the total side effect score at baseline did not differ for the two groups of patients. After Bonferroni correction, only the baseline difference in agitation remained significant (p < .0025). No significant baseline differences were found in heart rate and blood pressure. Baseline body weight was, however, significantly higher in the ADHD plus ASD group compared with the ADHD-only group. See Table 1 for further details.
Effectiveness
As depicted in Figure 2, a paired-sample t test showed a mean reduction of 24.6% on the ADHD-index for the ADHD plus ASD group. This reduction was significant and represents a large effect, t(59) = −7.561; p = .000; r = .70. A reduction of 31.3% was found for the ADHD-only group, which was also significant and represents a large effect, t(225) = −16.495; p = .000; r = .74. In the ANCOVA, the covariate age did not significantly relate to reductions in the ADHD-index, F(1, 270) = 1.655; p = .199; r = .08. There was no significant effect of gender on ADHD symptom reduction, F(1, 270) = .014; p = .907; = .000, and there was no significant effect of ADHD subtype on ADHD symptom reduction, F(3,270) = .128; p = .944; = .001. After control for the variables of age, gender, and ADHD subtype, a comorbid diagnosis of ASD also did not significantly affect ADHD symptom reduction, F(1, 270) = .017; p = .896; = .000.
Figure 2.
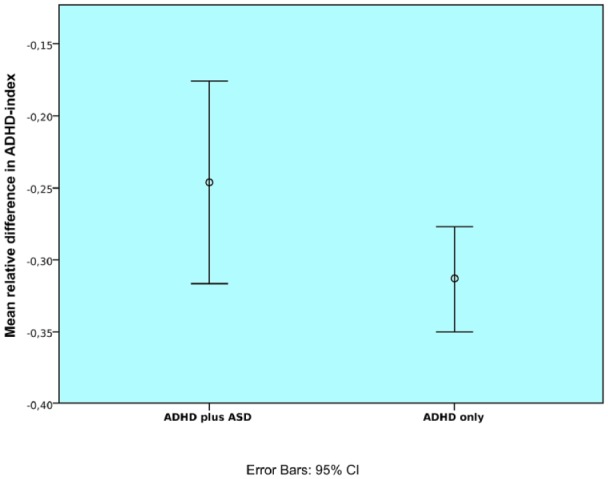
Mean relative difference for the ADHD symptom index at T1 and T2. Mean reduction of 24.6% in the ADHD plus ASD group and 31.3% in the ADHD-only group.
Note. ASD = autism spectrum disorder; CI = confidence interval.
Side Effects
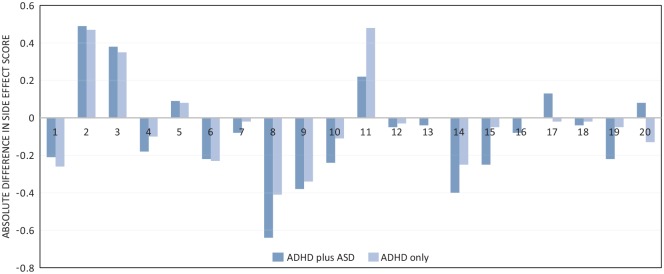
As can be seen in Table 2 and Figure 3, the signed-rank tests revealed significant increases in the ADHD plus ASD group for the side effects of decreased appetite and weight loss. Significant decreases were found in this group for the side effects of agitation, anxiety, and sadness/unhappiness.
Table 2.
Comparison of Individual Side Effects.
| Side effect | ADHD plus ASD |
ADHD only |
Comparison |
||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| n | Minimum | Maximum | M | Z | Sig. | r | n | Minimum | Maximum | M | Z | Sig. | r | U | Z | p | r | ||
| 1 | Sleeping disorder | 24 | −2 | 2 | −.21 | −0.99 | .404 | −.20 | 96 | −3 | 3 | −.26 | −2.30 | .021* | −.23 | 1,145.0 | −.048 | .963 | .00 |
| 2 | Decreased appetitea | 45 | −2 | 3 | .49 | −2.68 | .007* | −.40 | 169 | −3 | 3 | .47 | −5.46 | .000* | −.42 | 3,697.5 | −.301 | .764 | −.02 |
| 3 | Weight lossa | 45 | −1 | 3 | .38 | −3.09 | .001* | −.46 | 171 | −3 | 3 | .35 | −4.56 | .000* | −.35 | 3,674.5 | −.518 | .606 | −.04 |
| 4 | Headachea | 45 | −2 | 1 | −.18 | −1.38 | .179 | −.21 | 170 | −2 | 2 | −.10 | −1.67 | .096 | −.13 | 3,605.5 | −.649 | .515 | −.04 |
| 5 | Palpitationsa | 45 | −1 | 2 | .09 | −1.00 | .460 | −.15 | 170 | −3 | 2 | .08 | −1.55 | .135 | −.12 | 3,787.0 | −.119 | .912 | −.01 |
| 6 | Nervousnessa | 45 | −3 | 2 | −.22 | −1.41 | .171 | −.21 | 171 | −3 | 2 | −.23 | −3.10 | .002* | −.24 | 3,808.0 | −.112 | .910 | −.01 |
| 7 | Dizzynessa | 48 | −2 | 2 | −.08 | 0.00 | 1.000 | .00 | 173 | −2 | 2 | −.02 | −0.57 | .600 | −.04 | 3,962.5 | −.647 | .536 | −.04 |
| 8 | Agitationa | 45 | −2 | 2 | −.64 | −3.78 | .000* | −.56 | 171 | −3 | 3 | −.41 | −4.66 | .000* | −.36 | 3,303.0 | −1.535 | .125 | −.10 |
| 9 | Anxietya | 45 | −2 | 1 | −.38 | −3.15 | .002* | −.47 | 170 | −3 | 1 | −.34 | −4.70 | .000* | −.36 | 3,497.0 | −.989 | .325 | −.07 |
| 10 | Gastrointestinal complaintsa | 45 | −2 | 1 | −.24 | −1.95 | .052 | −.29 | 171 | −3 | 3 | −.11 | −1.81 | .071 | −.14 | 3,536.5 | −.958 | .340 | −.07 |
| 11 | Dry moutha | 45 | −2 | 2 | .22 | −1.86 | .086 | −.28 | 171 | −2 | 3 | .48 | −5.88 | .000* | −.45 | 3,316.0 | −1.533 | .125 | −.10 |
| 12 | Tics/involuntary movementsa | 44 | −1 | 1 | −.05 | −0.54 | .791 | −.08 | 171 | −3 | 3 | −.03 | −0.47 | .645 | −.04 | 3,597.0 | −.582 | .607 | −.04 |
| 13 | Euphoria/unusual happinessa | 45 | −3 | 2 | −.04 | −0.33 | .760 | −.05 | 171 | −2 | 2 | .00 | −0.01 | 1.000 | .00 | 3,785.0 | −.211 | .869 | −.01 |
| 14 | Sadness/unhappinessa | 45 | −3 | 2 | −.40 | −2.62 | .009* | −.39 | 171 | −3 | 2 | −.25 | −3.40 | .001* | −.26 | 3,565.0 | −.825 | .411 | −.06 |
| 15 | Sweating | 24 | −3 | 2 | −.25 | −0.97 | .396 | −.20 | 96 | −3 | 2 | −.05 | −0.50 | .637 | −.05 | 1,048.0 | −.747 | .457 | −.07 |
| 16 | Tense jaw muscles | 24 | −2 | 1 | −.08 | −0.82 | .750 | −.17 | 96 | −2 | 2 | .00 | −0.13 | .912 | −.01 | 1,065.5 | −.664 | .491 | −.06 |
| 17 | Hair loss | 24 | −1 | 2 | .13 | −1.13 | .500 | −.23 | 95 | −2 | 3 | −.02 | −0.48 | .702 | −.05 | 1,019.5 | −1.202 | .228 | −.11 |
| 18 | Itching | 24 | −1 | 1 | −.04 | −0.33 | 1.000 | −.07 | 95 | −2 | 3 | −.02 | −0.44 | .656 | −.04 | 1,126.0 | −.118 | .973 | −.01 |
| 19 | Rash | 23 | −2 | 2 | −.22 | −1.12 | .363 | −.23 | 84 | −3 | 2 | −.05 | −0.58 | .612 | −.06 | 840.5 | −1.250 | .185 | −.12 |
| 20 | Sexual complaints | 13 | 0 | 1 | .08 | −1.67 | .188 | −.46 | 54 | −3 | 3 | −.13 | −1.06 | .326 | −.14 | 296.0 | −1.305 | .230 | −.16 |
Note. ADHD plus ASD/ADHD only = within group difference before and after treatment, analysis through signed-rank test; comparison = between group difference, analysis through Mann–Whitney test. ASD = autism spectrum disorder; M = mean increase of the side-effect score; sig. = exact two-tailed significance. aSide effects included in the original 13-item score.
p < .05.
Figure 3.
Mean absolute difference between T1 and T2 side-effect scores within groups for 20 individual items.
Note. See Table 2 for side effects corresponding to the numbers on horizontal axis. ASD = autism spectrum disorder.
In the ADHD-only group, significant increases were found for the side effects of decreased appetite, weight loss, and dry mouth. Significant decreases were found for sleeping disorder, nervousness, agitation, anxiety, and sadness/unhappiness.
The results of the Mann–Whitney tests, as summarized in Table 2, show no significant differences between the groups for the changes in side effects. That is, the side effects increased or decreased similarly across the two groups of patients.
Analysis of subgroups of patients identified on the basis of the type of medication that was taken revealed significant differences for the patients taking DEX. The subgroup of patients with ADHD plus ASD and taking DEX experienced a mean decrease of 0.40 on the score for headache while the subgroup of patients with only ADHD experienced a mean decrease of 0.02 for headache (z = −2.12; p = .034; r = −.09). This difference did not remain significant after Bonferroni correction (p > .0025).
Heart Rate, Blood Pressure, and Body Weight
The t-test results for independent samples showed the changes in heart rate, blood pressure, and body weight to not differ significantly across the two groups of patients with ADHD. A trend was found for a group difference in increases of heart rate (+11.62% vs. +5.44%), but this did not reach statistical significance (p = .053). See Table 3 for further details.
Table 3.
Differences in Vital Signs and Body Weight Compared Between T1 and T2.
| ADHD plus ASD |
ADHD only |
Comparison |
|||||||
|---|---|---|---|---|---|---|---|---|---|
| n | M | SD | n | M | SD | t | p | r | |
| Heart rate (bpm) | 39 | +11.62% | 18.93% | 122 | +5.44% | 16.72% | −1.946 | .053 | .15 |
| SBP (mmHg) | 41 | +0.42% | 12.66% | 123 | −0.66% | 8.81% | −.607 | .545 | .05 |
| DBP (mmHg) | 41 | +0.23% | 11.46% | 123 | +1.20% | 11.39% | .469 | .640 | .04 |
| MAP (mmHg) | 41 | +0.19% | 10.82% | 123 | +0.25% | 9.08% | .038 | .970 | .00 |
| Body weight (kg) | 36 | −2.14% | 4.13% | 117 | −1.98% | 3.93% | .212 | .832 | .02 |
Note. Comparison using t tests for independent samples. ASD = autism spectrum disorder; SBP = systolic blood pressure; DBP = diastolic blood pressure; MAP = mean arterial pressure; M = mean relative increase.
Time to Establishment, Type of Treatment, and Dosage
The time until establishment of optimal pharmacological treatment (i.e., the elapsed time between T1 and T2) did not differ significantly for the ADHD plus ASD group and the ADHD-only group (141 and 120 days, respectively), t(284) = −1.142; p = .254; r = .00.
No significant group difference was found for the distribution of medication types within the groups (Fisher’s exact test = 5.656; p = .115). See Table 4 for the distributions.
Table 4.
Medication Type.
| ADHD plus ASD |
ADHD only |
Total | |||
|---|---|---|---|---|---|
| n | % | n | % | ||
| MPH | 26 | 43.3 | 123 | 54.4 | 149 |
| DEX | 25 | 41.7 | 84 | 37.2 | 109 |
| Bupr | 7 | 11.7 | 18 | 8.0 | 25 |
| Othera | 2 | 3.3 | 1 | 0.4 | 3 |
| Total | 60 | 226 | 286 | ||
Note. ASD = autism spectrum disorder; MPH = methylphenidate; DEX = dexamphetamine; bupr = bupropion.
Two patients using atomoxetine in the ADHD plus ASD group and one using modafinil in the ADHD-only group.
Finally, the ADHD plus ASD group used daily MPH doses ranging from 10 to 90 mg, daily DEX doses ranging from 5 to 38 mg, and daily bupropion doses ranging from 300 to 450 mg. The ADHD-only group used daily MPH doses ranging from 5 to 100 mg, DEX doses ranging from 2.5 to 40 mg, and bupropion doses ranging from 150 to 450 mg. On average, the ADHD plus ASD group used a significantly higher daily dosage of bupropion compared with the ADHD-only group (see Table 5).
Table 5.
Daily Dosage (mg/day) of Medication at T2 (Clinically Ascertained Optimal Medication Dose).
| ADHD plus ASD |
ADHD only |
Comparison |
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| n | M | SD | Minimum | Maximum | n | M | SD | Minimum | Maximum | t | p | r | |
| MPH | 26 | 41.4 | 21.0 | 10.0 | 90.0 | 123 | 40.0 | 18.2 | 5.0 | 100.0 | −0.332 | .740 | .03 |
| DEX | 25 | 18.4 | 8.6 | 5.0 | 38.0 | 84 | 16.5 | 8.4 | 2.5 | 40.0 | −0.966 | .336 | .09 |
| Bupr | 7 | 364.3 | 80.2 | 300.0 | 450.0 | 18 | 258.3 | 112.8 | 150.0 | 450.0 | −2.260 | .034* | .43 |
Note. Comparison using t tests for independent samples. ASD = autism spectrum disorder; M = mean daily dose; MPH = methylphenidate; DEX = dexamphetamine; bupr = bupropion.
p < .05.
Discussion
The aim of this study was to document the effectiveness and side effects of pharmacotherapy for ADHD in adults with ASD. The results show the pharmacological treatment of ADHD in adult patients with ASD to not be less effective than the pharmacological treatment of ADHD in patients with only ADHD. Both groups were found to experience side effects to a similar extent, while changes in heart rate, blood pressure, and weight during the course of medication did not differ for the two groups.
ADHD affects children with a male to female (M:F) ratio of approximately 3:1 while similar rates of persistence into adulthood have been found for ADHD (Fayyad et al., 2017; Willcutt, 2012). A recent meta-analysis reported a pooled odds ratio (OR) of 1.23 for persistence of ADHD into adulthood for females, which did not differ significantly from the figure of persistence for males (Caye et al., 2016). A relatively high number of females were represented in the present study, producing an M:F ratio of 2.5:1 in the ADHD plus ASD group and 1:1 in the ADHD-only group. According to the research by Arnett, Pennington, Willcutt, DeFries, and Olson (2015), males have more severe symptoms of ADHD than females on average and greater variance in their symptom scores as well. When gender was accounted for in the multivariate analyses we conducted, no effect of gender was found for symptom reduction. This shows our results to be externally valid despite the overrepresentation of females in the selected patient population. A lower representation of females was found in the ADHD plus ASD group than in the only ADHD group, which is not surprising because—even more so than for ADHD—ASD is more prevalent in males than in females with a ratio of 4.3:1 (Fombonne, 2003).
A significant group difference was found for the occurrence of headache among users of DEX. The difference was small, however, and the scores showed decreases as opposed to increases in headache during DEX use in both of the ADHD patient groups. The clinical relevance of this finding is thus limited.
A trend toward a larger increase in heart rate was found for the ADHD plus ASD group but did not reach statistical significance. The small observed difference and small effect size limit the clinical relevance of this finding.
The higher daily dosage of bupropion in the ADHD plus ASD group compared to the ADHD-only group was not accompanied by group differences in side effects. For other types of medication, no differences in dosage were seen between the groups. It is thus possible that ASD patients may require a higher dose of bupropion to attain similar changes in ADHD symptoms as for patients with only ADHD. The clinical relevance of this possibility is nevertheless difficult to judge due to the relatively small number of patients taking bupropion in the present sample (n = 25).
To our knowledge, we were the first to study the pharmacotherapy of ADHD in adults with ASD. A major strength of the present research is that a representative sample of patients from daily clinical practice was studied, and a variety of ADHD subtypes and medication types therefore considered in addition to gender. The design of the study allowed for the inclusion of a large number of patients and did not require research funding. A longer mean period of time between treatment measurements (T1 and T2) was also employed than is usually the case in randomized controlled trials examining the effectiveness and side effects of ADHD medication.
Due to the retrospective nature of the study, the analyses only included information on patients whose pharmacological treatment was sufficiently documented. Due to the selection process, 79.3% of cases were excluded from the current analysis and lack of sufficient documentation was the largest reason for this exclusion (see Figure 1). The reasons for insufficient documentation of pharmacological treatment were heterogeneous and difficult to trace. Based on available data, it was impossible to tell if any differences in this selection process occurred based on compliance, disease severity, or number of side effects. The proportion of patients with ADHD and ASD found for the final selection of patients (21.0%) did not differ significantly from that for the initial selection of patients (19.6%), χ²(1, n = 1,694) = 0.283; p = .595. The part of the selection process which would be expected to generate the largest amount of bias due to discontinuation of medication for any reason would be the selection of patients who have two recorded lists at respectively T1 and T2, as is required for analysis (20.8% of which has ASD), compared with the group of patients who had either only one recorded list, or multiple lists, but no proper T1 and T2 endpoints (16.1% of which has ASD). Although this part of the selection is the most likely to be influenced, no significant differences were found for the occurrence of ASD between these groups, χ²(1, n = 551) = 1.9511; p = .1624. Both comparisons show failures to adequately document treatment occurred similarly in both groups and did therefore not induce a clear bias. The reported proportions are also consistent with those reported in other relevant research and thus representative (Gargaro et al., 2011; Leyfer et al., 2006; Sinzig et al., 2009).
For the analysis, values were used from the latest available measurement point in the clinical titration process to determine changes in effectiveness and side effects during the course of treatment. Most of the patients were verifiably put on medication and discharged with medication, but for some of the patients no more symptom scores were recorded after a given point in time or contact with the clinic was lost. In these cases, the latest available measurement point might not always have been the optimal medication dose. These cases were included in the current analysis because it is still possible to infer from these data the effectiveness and side effects relative to a certain medication dose, irregardless of this being the optimal dose or not.
Numerous statistical tests were performed, which increased the chances of type I errors occurring (i.e., finding positive results where there are none). Given the already small number of significant results found, however, it is unlikely that this has led to overestimated findings. Even without correction for multiple testing, similar results were found for the two groups of patients with ADHD we studied.
As mentioned in the introduction to our study, treatment with MPH has been reported to significantly and clinically reduce hyperactivity in children with ASD (Sturman et al., 2017). It has been hypothesized that this finding could be extrapolated to an adult population (Kooij, 2009). This assumption found support in the present study, which showed pharmacological treatment of adults with diagnoses of ADHD and ASD to be just as successful as the pharmacological treatment of adults with only ADHD.
The increased occurrence of side effects reported previously for the pharmacological treatment of children with ADHD and ASD is not supported by the present data for adults (Research Units on Pediatric Psychopharmacology Autism Network, 2005). We only have limited information on those patients who discontinued treatment, which could obviously be due to unwanted side effects. But our data are consistent with those of Santosh, Baird, Pityaratstian, Tavare, and Gringras (2006) who generally found no differences in symptoms and side effects when MPH was taken by children and adolescents with ADHD plus ASD versus those with only ADHD.
Although ATX is the second choice of treatment after MPH or DEX according to guidelines (Dutch Psychiatric Association, 2015; National Institute for Health and Care Excellence, 2018), the current study found bupropion to be prescribed more often than ATX. This is due to financial considerations in the Netherlands where bupropion is fully covered by basic insurance policies and ATX is not (Consumentenbond, 2018; Zorginstituut Nederland, 2018). To our knowledge, we are the first to study DEX and bupropion use in patients with ADHD and ASD. Available reviews suggested that these were effective for the treatment of an adult ADHD population (Castells, Ramos-Quiroga, Bosch, Nogueira, & Casas, 2011; Verbeeck, Bekkering, Van den Noortgate, & Kramers, 2017). The present results show the distribution of the different types of medication for the treatment of a group of patients with ADHD and ASD to be similar to that for a group of patients with only ADHD and generally similar levels of treatment effectiveness to be experienced by the two groups.
Conclusion
The results of the present study appear to suggest that medication for ADHD can effectively and safely be prescribed to patients with ADHD and ASD and thus support for current guidelines (Kan et al., 2013; National Institute for Health and Care Excellence, 2018). The current guidelines recommend following the same treatment regimen as for patients with only ADHD when treating patients with ADHD and comorbid ASD. In contrast to the recommendation of the Dutch guideline to “start low and go slow” (Kan et al., 2013), the present findings suggest that patients with ADHD and ASD do not require lower optimal doses, more time to reach optimal dosage, or experience more side effects of medication when compared to patients without ASD. In other words: According to these results no additional prescription precaution appears to be required.
The above conclusion must be drawn with caution, given the retrospective nature of the data on which it is based. Limited information was available on those patients who discontinued medication. Although no bias seems to have occurred in the selection of patients between the two groups, unknown factors may have affected these results. In the future, a randomized controlled trial should be conducted to evaluate the effectiveness and possible side effects of pharmacological treatment for ADHD in patients with ASD more reliably.
Author Biographies
J.J. Muit graduated in 2018 as MD and now works in the Department of Psychiatry at the Radboud University Medical Center Nijmegen, The Netherlands. In 2019, he will start his residency in Psychiatry at the same insitution.
N. Bothof recently completed his residency in Psychiatry at the Radboud University Medical Center, and now is Psychiatrist at the mental health organization GGZ Oost Brabant.
C.C. Kan is Psychiatrist in the Department of Psychiatry at the Radboud University Medical Center. He is specialized in neurodevelopmental disorders and the chairman of CASS18+, a network of mental health workers that aims to improve care for adults with autism spectrum disorder in the Netherlands. He authored the 2013 guideline for autism spectrum disorder in adults.
Footnotes
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
ORCID iD: J. J. Muit  https://orcid.org/0000-0002-0353-2905
https://orcid.org/0000-0002-0353-2905
References
- Aman M. G., Smith T., Arnold L. E., Corbett-Dick P., Tumuluru R., Hollway J. A., . . . Handen B. (2014). A review of atomoxetine effects in young people with developmental disabilities. Research in Developmental Disabilities, 35, 1412-1424. doi: 10.1016/j.ridd.2014.03.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- American Psychiatric Association. (1994). Diagnostic and statistical manual of mental disorders (4th ed.). Washington, DC: Author. [Google Scholar]
- American Psychiatric Association. (2000). Diagnostic and statistical manual of mental disorders (4th ed., text rev.). Washington, DC: Author. [Google Scholar]
- American Psychiatric Association. (2013). Diagnostic and statistical manual of mental disorders (5th ed.). Arlington, VA: American Psychiatric Publishing. [Google Scholar]
- Arnett A. B., Pennington B. F., Willcutt E. G., DeFries J. C., Olson R. K. (2015). Sex differences in ADHD symptom severity. Journal of Child Psychology and Psychiatry, 56, 632-639. doi: 10.1111/jcpp.12337 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnold L. E., Aman M. G., Cook A. M., Witwer A. N., Hall K. L., Thompson S., Ramadan Y. (2006). Atomoxetine for hyperactivity in autism spectrum disorders: Placebo-controlled crossover pilot trial. Journal of the American Academy of Child and Adolescent Psychiatry, 45, 1196-1205. doi: 10.1097/01.chi.0000231976.28719.2a [DOI] [PubMed] [Google Scholar]
- Beery S. H., Quay H. C., Pelham W. E., Jr. (2017). Differential response to methylphenidate in inattentive and combined subtype ADHD. Journal of Attention Disorders, 21, 62-70. doi: 10.1177/1087054712469256 [DOI] [PubMed] [Google Scholar]
- Brugha T. S., McManus S., Bankart J., Scott F., Purdon S., Smith J., . . . Meltzer H. (2011). Epidemiology of autism spectrum disorders in adults in the community in England. Archives of General Psychiatry, 68, 459-465. doi: 10.1001/archgenpsychiatry.2011.38 [DOI] [PubMed] [Google Scholar]
- Castells X., Ramos-Quiroga J. A., Bosch R., Nogueira M., Casas M. (2011). Amphetamines for attention deficit hyperactivity disorder (ADHD) in adults. The Cochrane Database of Systematic Reviews, 15, CD007813. doi: 10.1002/14651858.CD007813.pub2 [DOI] [PubMed] [Google Scholar]
- Caye A., Spadini A. V., Karam R. G., Grevet E. H., Rovaris D. L., Bau C. H., . . . Kieling C. (2016). Predictors of persistence of ADHD into adulthood: A systematic review of the literature and meta-analysis. European Child & Adolescent Psychiatry, 25, 1151-1159. doi: 10.1007/s00787-016-0831-8 [DOI] [PubMed] [Google Scholar]
- Conners C. K., Erhardt D., Sparrow E. (1999). Conners’ Adult ADHD Rating Scales (CAARS): Technical manual. North Tonawanda, NY: Multi-Health Systems. [Google Scholar]
- Consumentenbond. (2018). Vergoedingen ADHD medicijnen (translation: Financial compensation ADHD medication). Retrieved from https://www.consumentenbond.nl/zorgverzekering/vergoedingen/adhd-medicijnen
- Cortese S., Castelnau P., Morcillo C., Roux S., Bonnet-Brilhault F. (2012). Psychostimulants for ADHD-like symptoms in individuals with autism spectrum disorders. Expert Review of Neurotherapeutics, 12, 461-473. doi: 10.1586/ern.12.23 [DOI] [PubMed] [Google Scholar]
- Dakwar E., Mahony A., Pavlicova M., Glass A., Brooks D., Mariani J. J., Levin F. R. (2012). The utility of attention-deficit/hyperactivity disorder screening instruments in individuals seeking treatment for substance use disorders. The Journal of Clinical Psychiatry, 73, e1372-e1378. doi: 10.4088/JCP.12m07895 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Martino A., Melis G., Cianchetti C., Zuddas A. (2004). Methylphenidate for pervasive developmental disorders: Safety and efficacy of acute single dose test and ongoing therapy: An open-pilot study. Journal of Child and Adolescent Psychopharmacology, 14, 207-218. doi: 10.1089/1044546041649011 [DOI] [PubMed] [Google Scholar]
- Dutch Psychiatric Association. (2015). Richtlijn ADHD bij volwassenen, fase 1 diagnostiek en medicamenteuze behandeling (translation: Guideline ADHD in adults: phase 1 diagnostics and pharmacological treatment). Utrecht, The Netherlands: Dutch Psychiatric Association. [Google Scholar]
- Fayyad J., Sampson N. A., Hwang I., Adamowski T., Aguilar-Gaxiola S., Al-Hamzawi A., . . . WHO World Mental Health Survey Collaborators. (2017). The descriptive epidemiology of DSM-IV Adult ADHD in the World Health Organization World Mental Health Surveys. Attention Deficit and Hyperactivity Disorders, 9(1), 47-65. doi: 10.1007/s12402-016-0208-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Findling R. L. (2005). Pharmacologic treatment of behavioral symptoms in autism and pervasive developmental disorders. The Journal of Clinical Psychiatry, 66(Suppl. 10), 26-31. [PubMed] [Google Scholar]
- Fombonne E. (2003). Epidemiological surveys of autism and other pervasive developmental disorders: An update. Journal of Autism and Developmental Disorders 33, 365-382. [DOI] [PubMed] [Google Scholar]
- Gargaro B. A., Rinehart N. J., Bradshaw J. L., Tonge B. J., Sheppard D. M. (2011). Autism and ADHD: How far have we come in the comorbidity debate? Neuroscience & Biobehavioral Reviews, 35, 1081-1088. doi: 10.1016/j.neubiorev.2010.11.002 [DOI] [PubMed] [Google Scholar]
- Ghuman J. K., Aman M. G., Lecavalier L., Riddle M. A., Gelenberg A., Wright R., . . . Fort C. (2009). Randomized, placebo-controlled, crossover study of methylphenidate for attention-deficit/hyperactivity disorder symptoms in preschoolers with developmental disorders. Journal of Child and Adolescent Psychopharmacology, 19, 329-339. doi: 10.1089/cap.2008.0137 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harfterkamp M., van de Loo-Neus G., Minderaa R. B., van der Gaag R. J., Escobar R., Schacht A., . . . Hoekstra P. J. (2012). A randomized double-blind study of atomoxetine versus placebo for attention-deficit/hyperactivity disorder symptoms in children with autism spectrum disorder. Journal of the American Academy of Child and Adolescent Psychiatry, 51, 733-741. doi: 10.1016/j.jaac.2012.04.011 [DOI] [PubMed] [Google Scholar]
- Hartman C. A., Geurts H. M., Franke B., Buitelaar J. K., Rommelse N. N. J. (2016). Changing ASD-ADHD symptom co-occurrence across the lifespan with adolescence as crucial time window: Illustrating the need to go beyond childhood. Neuroscience & Biobehavioral Reviews, 71, 529-541. doi: 10.1016/j.neubiorev.2016.09.003 [DOI] [PubMed] [Google Scholar]
- Hazell P. (2007). Drug therapy for attention-deficit/hyperactivity disorder-like symptoms in autistic disorder. Journal of Paediatrics and Child Health, 43(1-2), 19-24. doi: 10.1111/j.1440-1754.2007.00995.x [DOI] [PubMed] [Google Scholar]
- Jahromi L. B., Kasari C. L., McCracken J. T., Lee L. S., Aman M. G., McDougle C. J., . . . Posey D. J. (2009). Positive effects of methylphenidate on social communication and self-regulation in children with pervasive developmental disorders and hyperactivity. Journal of Autism and Developmental Disorders, 39, 395-404. doi: 10.1007/s10803-008-0636-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jensen C. M., Steinhausen H. C. (2015). Comorbid mental disorders in children and adolescents with attention-deficit/hyperactivity disorder in a large nationwide study. ADHD Attention Deficit and Hyperactivity Disorders, 7(1), 27-38. doi: 10.1007/s12402-014-0142-1 [DOI] [PubMed] [Google Scholar]
- Kan C. C., Geurts H. M., Van den Bosch K., Forceville E. J. M., Van Manen J., Schuurman C. H., Van Duin D. (2013). Multidisciplinaire richtlijn diagnostiek en behandeling van autismespectrumstoornissen bij volwassenen. (translation: Multidisciplinary guideline diagnostics and treatment of autism spectrum disorder in adults) Utrecht, The Netherlands: de Tijdstroom. [Google Scholar]
- Kooij J. J. S. (2009). ADHD bij volwassenen: Diagnostiek en behandeling. (translation: ADHD in adults: Diagnostics and treatment) Amsterdam, The Netherlands: Pearson Assessment and Information. [Google Scholar]
- Kooij J. J. S., Francken M. H. (2009). Diagnostisch Interview Voor ADHD bij volwassenen (DIVA 2.0) (translation: Diagnostic Interview for ADHD in Adults). The Hague, The Netherlands: Kenniscentrum ADHD Bij Volwassenen. [Google Scholar]
- Leyfer O. T., Folstein S. E., Bacalman S., Davis N. O., Dinh E., Morgan J., . . . Lainhart J. E. (2006). Comorbid psychiatric disorders in children with autism: Interview development and rates of disorders. Journal of Autism and Developmental Disorders, 36, 849-861. doi: 10.1007/s10803-006-0123-0 [DOI] [PubMed] [Google Scholar]
- Luteijn E. F., Serra M., Jackson S., Steenhuis M. P., Althaus M., Volkmar F., Minderaa R. (2000). How unspecified are disorders of children with a pervasive developmental disorder not otherwise specified? A study of social problems in children with PDD-NOS and ADHD. European Child & Adolescent Psychiatry, 9, 168-179. [DOI] [PubMed] [Google Scholar]
- Michielsen M., Semeijn E., Comijs H. C., van de Ven P., Beekman A. T., Deeg D. J., Kooij J. J. (2012). Prevalence of attention-deficit hyperactivity disorder in older adults in The Netherlands. British Journal of Psychiatry, 201, 298-305. doi: 10.1192/bjp.bp.111.101196 [DOI] [PubMed] [Google Scholar]
- National Institute for Health and Care Excellence. (2008). Attention deficit hyperactivity disorder: Diagnosis and management. London, England: Author. [PubMed] [Google Scholar]
- National Institute for Health and Care Excellence. (2018). Attention deficit hyperactivity disorder: Diagnosis and management. London, England: Author. [PubMed] [Google Scholar]
- Nickels K., Katusic S. K., Colligan R. C., Weaver A. L., Voigt R. G., Barbaresi W. J. (2008). Stimulant medication treatment of target behaviors in children with autism: A population-based study. Journal of Developmental and Behavioral Pediatrics, 29(2), 75-81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearson D. A., Santos C. W., Aman M. G., Arnold L. E., Casat C. D., Mansour R., . . . Cleveland L. A. (2013). Effects of extended release methylphenidate treatment on ratings of attention-deficit/hyperactivity disorder (ADHD) and associated behavior in children with autism spectrum disorders and ADHD symptoms. Journal of Child and Adolescent Psychopharmacology, 23, 337-351. doi: 10.1089/cap.2012.0096 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Posey D. J., Aman M. G., McCracken J. T., Scahill L., Tierney E., Arnold L. E., . . . McDougle C. J. (2007). Positive effects of methylphenidate on inattention and hyperactivity in pervasive developmental disorders: An analysis of secondary measures. Biological Psychiatry, 61, 538-544. doi: 10.1016/j.biopsych.2006.09.028 [DOI] [PubMed] [Google Scholar]
- Reiersen A. M., Todd R. D. (2008). Co-occurrence of ADHD and autism spectrum disorders: Phenomenology and treatment. Expert Review of Neurotherapeutics, 8, 657-669. doi: 10.1586/14737175.8.4.657 [DOI] [PubMed] [Google Scholar]
- Research Units on Pediatric Psychopharmacology Autism Network. (2005). Randomized, controlled, crossover trial of methylphenidate in pervasive developmental disorders with hyperactivity. Archives of General Psychiatry, 62, 1266-1274. doi: 10.1001/archpsyc.62.11.1266 [DOI] [PubMed] [Google Scholar]
- Rosler M., Retz W., Thome J., Schneider M., Stieglitz R. D., Falkai P. (2006). Psychopathological rating scales for diagnostic use in adults with attention-deficit/hyperactivity disorder (ADHD). European Archives of Psychiatry and Clinical Neuroscience, 256(Suppl. 1), i3-11. doi: 10.1007/s00406-006-1001-7 [DOI] [PubMed] [Google Scholar]
- Roy M., Dillo W., Bessling S., Emrich H. M., Ohlmeier M. D. (2009). Effective methylphenidate treatment of an adult Aspergers Syndrome and a comorbid ADHD: A clinical investigation with fMRI. Journal of Attention Disorders, 12, 381-385. doi: 10.1177/1087054708320436 [DOI] [PubMed] [Google Scholar]
- Santosh P. J., Baird G., Pityaratstian N., Tavare E., Gringras P. (2006). Impact of comorbid autism spectrum disorders on stimulant response in children with attention deficit hyperactivity disorder: A retrospective and prospective effectiveness study. Child: Care, Health and Development, 32, 575-583. doi: 10.1111/j.1365-2214.2006.00631.x [DOI] [PubMed] [Google Scholar]
- Simonoff E., Pickles A., Charman T., Chandler S., Loucas T., Baird G. (2008). Psychiatric disorders in children with autism spectrum disorders: prevalence, comorbidity, and associated factors in a population-derived sample. Journal of the American Academy of Child and Adolescent Psychiatry, 47, 921-929. doi: 10.1097/CHI.0b013e318179964f [DOI] [PubMed] [Google Scholar]
- Sinzig J., Walter D., Doepfner M. (2009). Attention deficit/hyperactivity disorder in children and adolescents with autism spectrum disorder: Symptom or syndrome? Journal of Attention Disorders, 13, 117-126. doi: 10.1177/1087054708326261 [DOI] [PubMed] [Google Scholar]
- Stigler K. A., Desmond L. A., Posey D. J., Wiegand R. E., McDougle C. J. (2004). A naturalistic retrospective analysis of psychostimulants in pervasive developmental disorders. Journal of Child and Adolescent Psychopharmacology, 14, 49-56. doi: 10.1089/104454604773840481 [DOI] [PubMed] [Google Scholar]
- Sturman N., Deckx L., van Driel M. L. (2017). Methylphenidate for children and adolescents with autism spectrum disorder. The Cochrane Database of Systematic Reviews, 11, CD011144. doi: 10.1002/14651858.CD011144.pub2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tumuluru R. V., Corbett-Dick P., Aman M. G., Smith T., Arnold L. E., Pan X., . . . Handen B. L. (2017). Adverse events of atomoxetine in a double-blind placebo-controlled study in children with autism. Journal of Child and Adolescent Psychopharmacology, 27, 708-714. doi: 10.1089/cap.2016.0187 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verbeeck W., Bekkering G. E., Van den Noortgate W., Kramers C. (2017). Bupropion for attention deficit hyperactivity disorder (ADHD) in adults. The Cochrane Database of Systematic Reviews, 10, CD009504. doi: 10.1002/14651858.CD009504.pub2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vuijk R. (2014). Nederlands Interview ten behoeve van Diagnostiek Autismespectrumstoornis bij volwassenen (NIDA, translation: Dutch Interview for Diagnosis of Autism Spectrum Disorder in adults; ). Retrieved from https://www.autismenetwerkzhz.nl/media/30084/20141001-2014-NIDA-volwasssenen-RVuijk-.pdf [Google Scholar]
- Willcutt E. G. (2012). The prevalence of DSM-IV attention-deficit/hyperactivity disorder: A meta-analytic review. Neurotherapeutics, 9, 490-499. doi: 10.1007/s13311-012-0135-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zorginstituut Nederland. (2018). Medicijnkosten (translation: Cost of medication). Retrieved from https://www.medicijnkosten.nl/