Abstract
Early differentiation between different types of inflammatory arthritis and subsequent initiation of modern treatments can improve patient outcomes by reducing disease activity and preventing joint damage. Routine clinical evaluation, laboratory testing, and radiographs are typically sufficient for differentiating between inflammatory and predominantly degenerative arthritis (e.g., osteoarthritis). However, in some patients with inflammatory arthritis, these techniques fail to accurately identify the type of early-stage disease. Further evaluation by ultrasound imaging can delineate the inflammatory arthritis phenotype present. Ultrasound is a noninvasive, cost-effective method that enables the evaluation of several joints at the same time, including functional assessments. Further, ultrasound can visualize pathophysiological changes such as synovitis, tenosynovitis, enthesitis, bone erosions, and crystal deposits at a subclinical level, which makes it an effective technique to identify and differentiate most common types of inflammatory arthritis. Limitations associated with ultrasound imaging should be considered for its use in the differentiation and diagnosis of inflammatory arthritides.
Keywords: Ultrasound, Inflammatory arthritis, Synovitis, Enthesitis, Bone erosions, Imaging
Introduction
The development and progression of inflammatory arthritis depends on both environmental and genetic factors and can affect an estimated 115 to 271 people per 100,000 adults [1, 2]. Symptoms of joint, tendon, or entheseal inflammation can be either short lived or persistent. If inflammation continues, permanent skeletal damage can occur, leading to morbidity and disability [3]. The advent of the modern treatment armamentarium and treat-to-target strategies now makes rapid evaluation and accurate diagnosis in patients with inflammatory arthritis important. Specifically, early treatment with targeted therapies can alter long-term outcomes by minimizing disease activity, preventing joint damage and disability, and improving patients’ quality of life [2, 4–6].
Routine clinical evaluations that consist of a thorough history and physical examination, laboratory testing, and plain radiography can often establish the presence of arthritis. However, it can sometimes be challenging to differentiate between inflammatory and degenerative causes of arthritis, especially when clinical signs are sparse and serologies are negative. Initially, it is important to determine if a patient has inflammatory arthritis or a predominantly degenerative arthritis such as osteoarthritis. Subsequently, the patient should be evaluated to determine the type of inflammatory arthritis if inflammation is the suspected cause of joint pain. Common inflammatory joint disorders in adults include crystal-induced arthritis, rheumatoid arthritis (RA), and spondyloarthritis (SpA) including psoriatic arthritis (PsA), reactive arthritis, enteropathic arthritis, and ankylosing spondylitis (AS) [2]. Additionally, inflammatory arthritis or bursitis in older patients (≥ 50 years of age) may be a result of polymyalgia rheumatica (PMR) or crystalline arthropathies [7–9]. Common symptoms of inflammatory arthritis may include joint swelling, erythema, morning stiffness longer than 0.5–1 h, and radiographic evidence of bone loss around joints [10, 11]. The number of joints involved, the type of joints involved (e.g., small vs large), and the pattern of joint involvement (e.g., symmetrical vs asymmetrical) can also be similar between arthritides [1, 12–14]. Further, unique disease manifestations, such as enthesitis and dactylitis in obese patients, can be clinically challenging to detect [15]. Additionally, serologies may fail to conclusively differentiate between these diseases and elevation of acute-phase reactants is nonspecific [16–19].
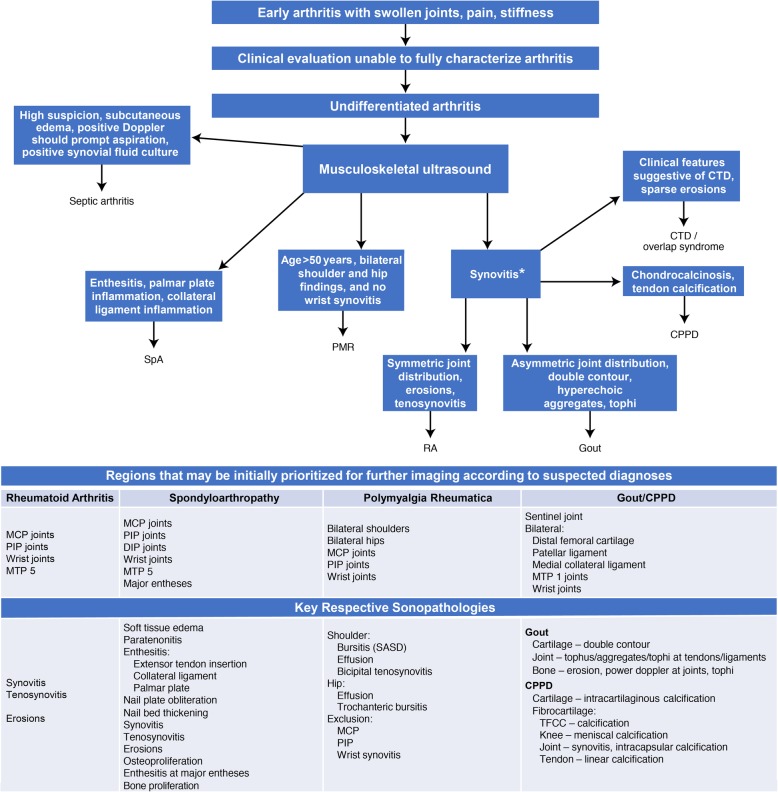
During early disease and in patients with milder symptoms, in whom clinical findings are not definitive, imaging is needed to accurately differentiate between different types of inflammatory arthritis. European League Against Rheumatism (EULAR) recommendations for the management of early arthritis are guided by an overarching principle that “a definitive diagnosis in a patient with early arthritis should only be made after a careful history taking and clinical examination, which should also guide laboratory testing and additional procedures” [20]. In our opinion, imaging, just like clinical examination, needs to be considered in the context of clinical presentation, with possible differential diagnoses taking demographic characteristics into account (Fig. 1). In current practice, ultrasound, magnetic resonance imaging (MRI) techniques, and, to a lesser extent, computed tomography (CT) are generally regarded as superior to conventional radiographs for identifying and differentiating early signs of inflammatory arthritis [21]. While MRI and CT are useful, CT is limited by exposure to ionizing radiation and MRI is limited by high cost and limited availability. Ultrasound, a nonionizing imaging technique, is often preferred because many musculoskeletal structures can be examined, it can be performed at the point-of-care, and it can be used on patients for whom MRI is contraindicated. In patients with inflammatory arthritis, ultrasound can detect important clues such as subclinical synovitis, asymptomatic entheseal inflammation, bone erosions, and crystal deposits, which could otherwise be missed in physical examinations [4, 22–28]. The importance of ultrasound has also been highlighted by its inclusion in the two most recent EULAR/American College of Rheumatology (ACR) classification criteria for PMR and gout [29, 30].
Fig. 1.
Use of ultrasound in diagnostic decision making. This algorithm was developed by the authors and was not based on a clinical study. Note: *Osteoarthritis can cause synovitis but is excluded from this algorithm. CPPD, calcium pyrophosphate deposition; CTD, connective tissue disorder; MCP, metacarpophalangeal; MTP, metatarsophalangeal, PIP, proximal interphalangeal; PMR, polymyalgia rheumatica; RA, rheumatoid arthritis; SASD, subacromial-subdeltoid; SpA, spondyloarthritis; TFCC, triangular fibrocartilage complex
Ultrasound can be used to generate high-resolution images of joints, tendons, entheses, synovia, cartilage, bursae, bony cortex, nails, and soft-tissue vascularity. Structures can be imaged in a dynamic, multiplanar fashion, allowing for visualization of synovial changes, joint effusions, tendon tears, and bone erosions [24, 31, 32]. Musculoskeletal ultrasound can be used to differentiate trauma-related injuries that can initially mimic arthritis, including muscle lesions, occult fractures, and tendon rupture or subluxation [28]. Power Doppler ultrasound (PDUS) imaging is used to assess soft tissue and nerve lesions, tissue vascularization, and hyperemia of synovial structures, tendons, and entheses [4, 33, 34]. The inability of ultrasound to penetrate bone surface and, hence, visualize bone structures is an important limitation to bear in mind, which may be addressed using correlative plain X-rays. Additionally, only a small number of studies have examined how ultrasound should be integrated to the process of diagnosis for inflammatory arthritis.
This manuscript provides an in-depth review of how ultrasound—a portable, convenient, noninvasive, and cost-effective imaging technique—can be used in the differential diagnosis of early inflammatory arthritis phenotypes (Table 1) and also assesses any important limitations of the technique. The authors also propose an algorithm (Fig. 1) that may enable working through a differential diagnosis both clinically and by prioritizing anatomical targets.
Table 1.
Ultrasound features used in differentiation of early inflammatory arthritis
| Rheumatoid arthritis | Spondyloarthritis | Crystal arthropathies | Polymyalgia rheumatica | Septic arthritis |
|---|---|---|---|---|
|
• Joint effusion, synovial proliferation, synovial pannus, and hyperemia in typical RA distribution • Tenosynovial effusions, synovial hypertrophy, and hyperemia • Cortical bone erosions and cartilage lesions • Multijoint assessments confirming typical distribution of involvement |
• Enthesitis characterized by tendon/ligament hypoechogenicity and thickening, calcification, bone erosions, intralesional focal calcification or fibrous tissue, and abnormal vascularization at enthesis insertion on power Doppler ultrasound • Cortical bone erosions and enthesophytes (heterogeneous to RA) • Synovitis and tenosynovitis • Confounding factors: age, BMI |
• Tophaceous deposits: • Cartilage: double contour sign (gout) • Periarticular: heterogeneous collection in soft tissue, “snowstorm” appearance sometimes with anechoic rim • Tendons and ligaments: intratendinous tophi and ovoid-shaped microdeposits with hyperechoic densities • Cortical bone erosions • CPPD deposits: • Hyaline cartilage: hyperechoic, within the layer of cartilage • Fibrocartilage: hyperechoic, rounded or amorphous deposits • Basic calcium phosphate: • Hyperechoic foci with variable acoustic shadowing • Hyperemia on Doppler |
• Bilateral subacromial/subdeltoid bursitis • Biceps long-head tenosynovitis • Trochanteric bursitis • Synovitis • Hip effusion • Less common findings include enthesitis, glenohumeral effusions, flexor tenosynovitis, and peripheral synovitis • Should not have hand- or wrist-joint synovitis |
• Joint effusion, sometimes with hyperechogenicity and heterogeneity • Increased peri-synovial vascularity with color Doppler • Ultrasound can guide joint aspiration • Clinical suspicion has the highest priority |
BMI body mass index, CPPD calcium pyrophosphate dehydrate, RA rheumatoid arthritis
Ultrasonographic evaluation in suspected inflammatory arthritis
Synovitis and tenosynovitis
Among the key features in diagnosing inflammatory arthritis is the presence of synovitis as well as the distribution of joints involved. In mild or early-onset inflammatory arthritis, it may be difficult to discern clinical synovitis. Similarly, mild tenosynovitis may not be clinically apparent. Synovitis and tenosynovitis are common features of early RA and SpA (Fig. 2a–d). Synovitis is characterized on grayscale ultrasound by intra-articular tissue that is abnormally thickened, hypoechoic or anechoic (relative to subdermal fat), nondisplaceable, and poorly compressible [26]. As synovial proliferation progresses, articular cartilage becomes disrupted, and erosions can be observed at the osteochondral junction [4].
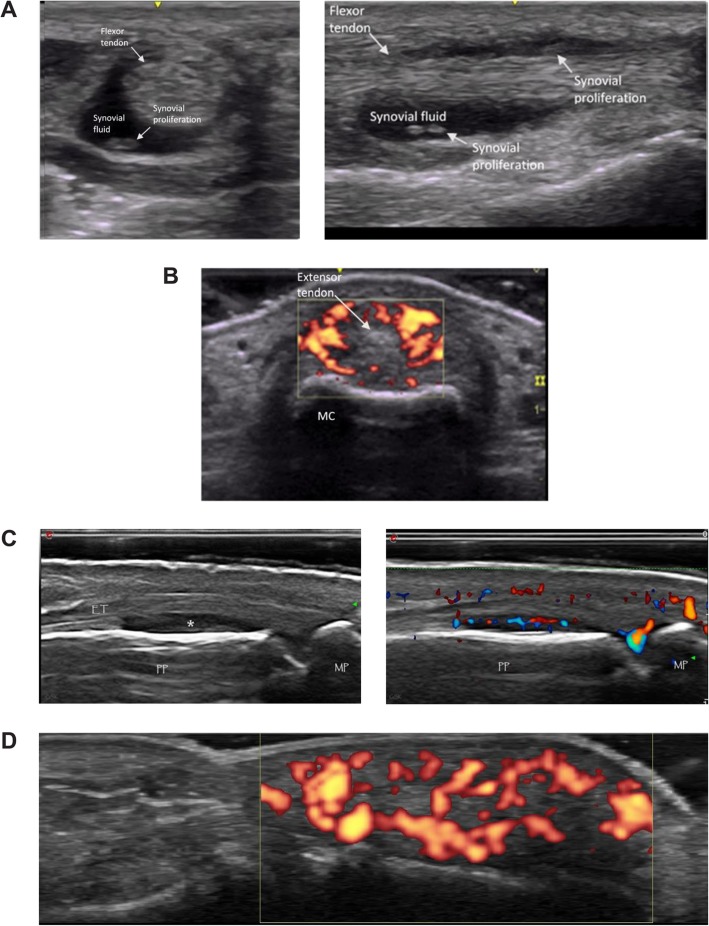
Fig. 2.
Ultrasound imaging of synovitis and tenosynovitis. a Flexor tenosynovitis in transverse (left) and longitudinal (right) views. b Metacarpophalangeal joint paratenonitis, dorsal aspect of second metacarpophalangeal joint. MC, metacarpal. c Dorsal proximal interphalangeal B-mode (left) and power Doppler (right) images indicating synovitis in the recess (asterisk). PP, proximal phalanx; MP, middle phalanx; ET, extensor digitorum tendon. d Positive power Doppler signal of finger pulp
Tenosynovitis is characterized by hypoechoic or anechoic thickened tissue with or without fluid in the tendon sheath [26] and is not a specific lesion. Presence of synovial hypertrophy should prompt the use of PDUS or color Doppler to establish vascularity and, hence, inflammation of the tissue. The degree of Doppler sensitivity of the user’s equipment should be known to avoid false negative testing. Doppler imaging findings need to be taken into context with the overall clinical picture, and the operator should recognize the pitfalls of false positive and false negative results. Doppler sensitivity can be gauged by the degree of vascularity of the distal finger pulp (Fig. 2d), with Doppler signal in more than one third of the finger indicating a reasonable sensitivity of the machine and settings. Thus, sonographic signs of synovitis should include both synovial hypertrophy and vascularity.
The value of ultrasound in identifying subclinical synovitis has been demonstrated by finding synovitis in asymptomatic joints of patients with early oligoarthritis that led to the reclassification of oligoarthritis as polyarthritis for many patients [4, 22, 23]. In patients with arthralgia not diagnosed with inflammatory arthritis, the absence of ultrasound-detected synovitis is associated with a high (89%) negative predictive value for the development of inflammatory arthritis over 1 year [35].
Features of RA that can be visualized on ultrasound include rheumatoid nodules and synovial cysts, as well as common secondary complications, such as median nerve entrapment in the carpal tunnel [36]. Additionally, the distribution of joint involvement may help differentiate RA from PsA as, for example, synovitis of the distal interphalangeal joints is characteristic of PsA rather than RA [33]. Synovial hypertrophy in the finger joints of patients with RA can be particularly well characterized with ultrasound by comprehensively examining palmar and dorsal aspects of proximal interphalangeal and metacarpophalangeal joints. In RA, synovial hypertrophy is most often detected at the dorsal metacarpophalangeal joints and palmar aspect of the proximal interphalangeal joints [37]. However, if the diagnosis is in question, then both dorsal and palmar aspects should be examined to evaluate signs of tendonitis and palmar plate enthesitis. MRI studies of patients with dactylitis have shown increased signal at the palmar plate and there is some discussion that this may be a form of enthesitis [38]. In a study of patients with early PsA and RA, Zabotti et al. [39] found that synovitis was observed more frequently in patients with RA. In patients with early PsA, periarticular soft-tissue edema, metacarpophalangeal joint peri-extensor tenonitis, and proximal interphalangeal joint extensor tendon enthesitis were found more often [39]. Palmar plate inflammation (Fig. 3a), digital enthesitis (Fig. 3b), and collateral ligament enthesitis may also help differentiate PsA from RA. Diffuse extensor paratenonitis and flexor tenosynovitis (Fig. 2b) is also observed in patients with PsA dactylitis.
Fig. 3.
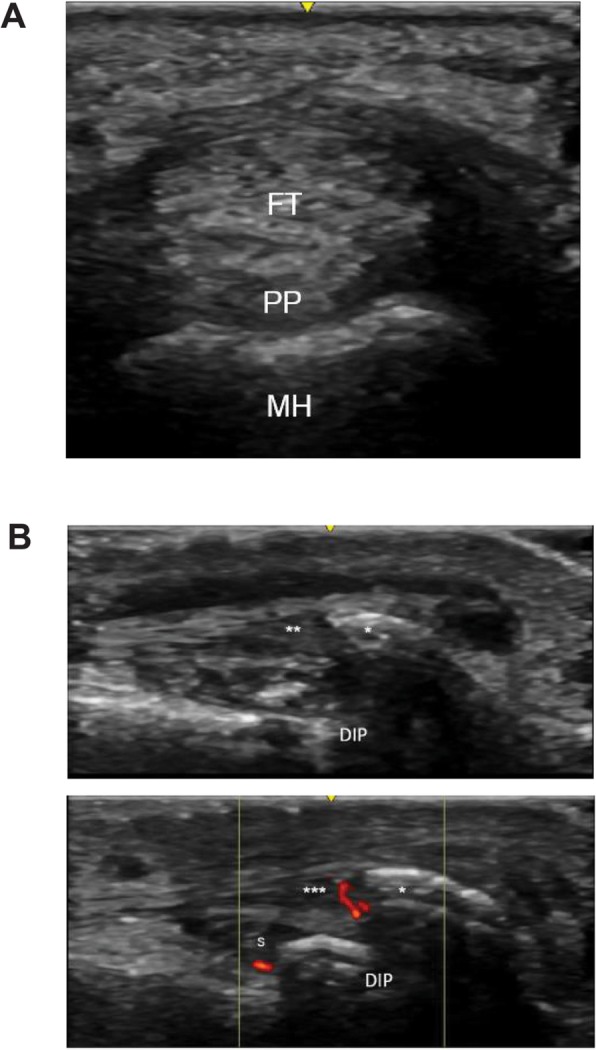
Ultrasound findings for differentiation of psoriatic arthritis from rheumatoid arthritis. a Short-axis view of palmar plate inflammation. FT, flexor tendon; MH, metacarpal head; PP, palmar plate. b Dorsal long view of enthesitis of the extensor tendon from a distal interphalangeal joint in a patient with psoriatic arthritis. DIP, distal interphalangeal; S, DIP synovitis; asterisk (*), enthesophyte; double asterisks (**), extensor tendon demonstrating thickening, hypoechogenicity, and loss of fibrillar architecture; triple asterisks (***), extensor tendon with insertional Doppler
These features can serve as additional differentiating factors between early SpA and RA [24, 39]. Paratenonitis (defined as the lack of a sheath on the extensor tendon above the metacarpophalangeal joint with accompanying inflammatory changes to the extensor tendon consisting of increased thickness, loss of fibrillar architecture, and increased power Doppler signal) may also be found in established RA [39, 40]. Flexor tenosynovitis is strongly associated with dactylitis, which occurs in 32 to 48% of patients with PsA [41], and along with joint synovitis, flexor tenosynovitis is among the most commonly reported features of dactylitic digits. Other reported dactylitic tissue changes visible by ultrasound include soft-tissue thickening or edema, osteoproliferation, and sesamoid abnormalities [42]. Although these sonographic features have been well documented in patients with clinically obvious dactylitis, their presence in a patient with early inflammatory arthritis may help differentiate early PsA from RA.
Imaging findings need to be correlated with clinical presentation and suspected differential diagnoses. For example, synovitis can be the result of lupus, gout, or osteoarthritis [27, 43–45], but imaging findings can narrow the differential diagnoses considerably. An important limitation is the awareness of findings in apparently normal populations. Recently, studies have demonstrated the prevalence of ultrasound-detected joint inflammatory abnormalities (synovial effusion and/or synovial hypertrophy) in the hands and feet of healthy individuals. In a study of 207 healthy individuals, 6621 joints were analyzed and 9% had at least 1 ultrasound abnormality [46]. However, B-mode findings with PDUS score of > 2 only occurred in a minority of patients. Further, because this was a cross-sectional study, it is not clear if these individuals had onset of early inflammatory arthritis. Thus, care needs to be exercised in interpreting imaging findings in patients with minimal symptoms and should be considered in the overall clinical context.
Enthesitis
Enthesitis is a hallmark clinical feature of SpA, especially PsA, and is observed less frequently in other inflammatory arthritides, such as RA. Entheseal inflammation is often asymptomatic and may be overlooked on clinical examination [24]. For example, Balint and colleagues [47] found that in a study of 35 patients with SpA, clinical examination identified enthesitis in 22% (75/348) of sites compared with 56% (195/348) of sites on ultrasound examination. Ultrasound examination has also been used to demonstrate that nail disease in PsA and psoriasis is associated with distal interphalangeal enthesopathy [48].
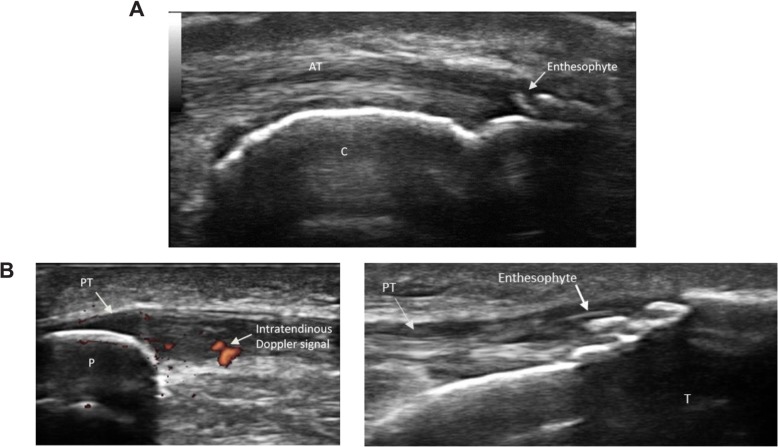
Sonography can depict not only echotexture changes (such as loss of fibrillar echotexture and tendon thickening) at the enthesis but also peri-entheseal Doppler signal. It can also demonstrate pathologic changes in the adjacent tissues, such as proximal tendinopathy, bone erosions, and bursitis. In many cases, ultrasound can be used to visualize subclinical enthesitis that cannot be detected with physical examination [49]. Inactive or chronic enthesitis may manifest as tendon thickening, bulky enthesophytes, intratendinous calcification, and bone erosions [26, 32]. Further, in PsA, the severity of sonographic enthesitis is associated with peripheral and axial joint damage [50]. Entheseal ultrasound assessment should include longitudinal and transverse scans with tendons in both neutral and flexed positions [25]. A flexed position may provide better visualization of grayscale abnormalities, but may create tension that diminishes a Doppler signal [51]. Although there is some controversy about which entheses should be evaluated by ultrasound when a diagnosis of SpA is suspected, inclusion of the Achilles tendon and selection of the knee (quadriceps and patellar) and plantar fascia entheses are typically recommended (Fig. 4) [52]. Assessment of Achilles entheses, however, should be approached with caution as age, body mass index, and regular physical exercise have all been associated with structural damage on ultrasound in PsA [53].
Fig. 4.
Ultrasound imaging of enthesitis. a Achilles enthesophyte in a patient with spondyloarthritis. AT, Achilles tendon; C, calcaneus. b Patellar enthesitis in a patient with psoriatic arthritis. Left, Doppler with abnormal intratendinous signal; right, enthesophyte. P, patella; PT, patellar tendon/ligament; T, tibia
A hallmark of inflammatory peripheral enthesitis seen with PDUS is vascularization at cortical bone insertion. The Outcome Measures in Rheumatology (OMERACT) ultrasound subgroup proposes the inclusion of enthesitis as part of an outcome measure only when the visualized signal is within 2 mm of the bony cortex [54]. Other groups, such as Group for Research and Assessment of Psoriasis and Psoriatic Arthritis (GRAPPA) ultrasound committee, have proposed scoring separately in proximal and distal entheses as well as bursa and to test the relative specificity of site-specific Doppler signals in subjects with and without enthesitis [55]. This will enable a data-driven approach to establishing the most sensitive and specific combination of findings associated with a diagnosis of spondyloarthritis at the patient level. Regardless, detection of any vascularized entheses by PDUS is a sensitive and specific characteristic for diagnosis of SpA [56]. Nearby structures should also be evaluated because adjacent bursitis and tendon calcification are commonly observed by ultrasound at sites of enthesitis [52, 57].
While not used in routine clinical practice, ultrasound enthesitis scoring systems have been studied as tools for diagnostic classification of SpA [57, 58]. The most common scoring systems are the Glasgow Ultrasound Enthesitis Scoring System (GUESS) and the MAdrid Sonographic Enthesitis Index (MASEI) [15, 25]. GUESS combines grayscale ultrasound evaluations of 5 lower-limb entheseal sites, while MASEI combines grayscale and PDUS evaluations of 6 upper- and lower-extremity entheseal sites [25]. In a cross-sectional study that evaluated 25 patients with SpA and 29 matched controls, de Miguel and colleagues [58] found that a MASEI score of ≥ 18 could be used with specificity of 82.8% as a cutoff to differentiate between cases of SpA and healthy controls. In a separate study of 113 patients with early SpA and 57 matched controls, de Miguel and colleagues found that a MASEI cutoff score of ≥ 20 had specificity of 89.5% [59]. However, age and body mass index are significantly correlated with GUESS and MASEI scores, and degenerative or mechanical abnormalities in weight-bearing joints may be incorrectly identified as inflammatory arthritis, especially in obese patients for whom excess weight puts added mechanical stress on lower limb entheses [15, 60, 61]. Recent literature has focused on examination of hand entheses to differentiate between early RA and PsA. Zabotti et al. [39] found that extensor tendon tenonitis, extensor slip enthesitis, and periarticular edema were useful in differentiating PsA from RA. However, extensor slip abnormalities can also be seen in patients with osteoarthritis [62] and RA [40].
Bone erosions
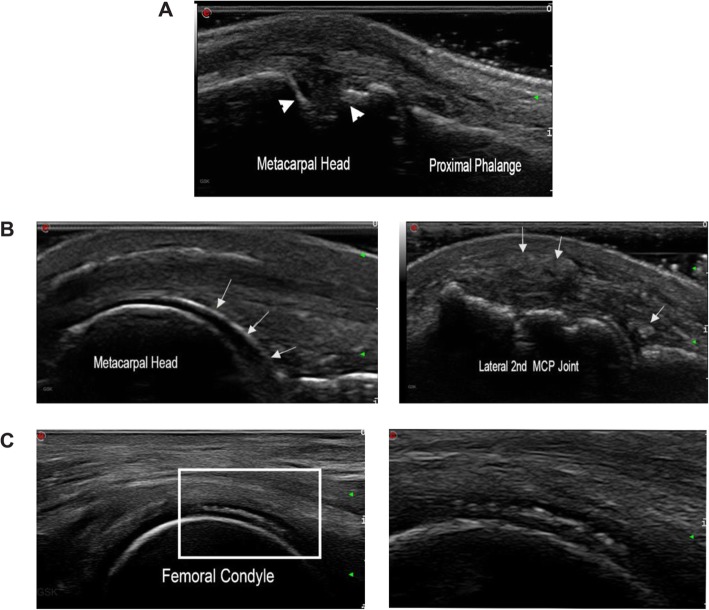
Bone erosion is an important hallmark of both RA and SpA that can be identified with ultrasound (Fig. 5) based on intra-articular discontinuity of the bone surface that is visible in two perpendicular planes [4, 26]. Ultrasound can be used to accurately identify cortical irregularities of at least 2 mm in width as breaks in the bone surface associated with inflammatory arthritis [63]. Ultrasonic detection of bone erosions is more feasible in hand and toe joints than in bones with poor ultrasound windows, such as carpal and tarsal bones [4]. In a recent study, joint erosions were predominantly found in patients with RA (91.4%), followed by gout (90.0%), PsA (75.0%), osteoarthritis (70.0%), and finally healthy volunteers (33.3%) [64]. Although the mere presence of ultrasound-detected erosions may not be specific for RA, larger erosions at the second and fifth metacarpophalangeal joints, fifth metatarsophalangeal joint, and distal ulna may sway the diagnosis towards RA [64]. Further, in patients with RA whose PDUS synovitis activity and clinical disease activity are well controlled, the detection of bone erosion with ultrasound after discontinuation of biologic disease-modifying antirheumatic drugs may be a risk factor for relapse [65].
Fig. 5.
Ultrasound imaging of bone erosions and crystal deposits. a Transverse view of second metacarpophalangeal joint in a patient with rheumatoid arthritis; arrowheads denote bone erosion. b Left, chondrosynovial urate deposition at the second metacarpophalangeal joint (arrows); right, at the same joint, intra- and peri-articular tophaceous deposits seen as heterogeneous collections (arrows). c Left, calcium pyrophosphate crystal deposition seen sandwiched within the cartilage; right, magnified view of the white rectangular area on the left
Distinguishing between bone erosions from physiologic cortical breaks that are not caused by inflammation is important. These false-positive ultrasound findings are typically a result of small lesions (< 2 mm) or lesions found at the palmar grooves of the metacarpal heads and phalangeal bases, where nutrient blood vessels pass through vascular bone channels and enter the bone marrow. Another source of false-positive findings are pseudo-erosions formed by osteophytes in forceps-like arrangements, which are common in patients with PsA and can make it difficult to visualize the cortical bone surface [63].
Crystal deposits
Ultrasound can uniquely demonstrate the differential chondrosynovial deposition of urate in comparison to intra-cartilaginous chondrocalcinosis (Fig. 5) [4, 27, 28].
Tophi within soft tissues and tendons can be identified as heterogeneous collections with hyperechoic dots, and frequently with anechoic rims [4, 66]. These may be clinically undetectable and yet cause significant symptoms when involved in a flareup. Careful examination of the symptomatic areas may help in detecting these deposits and thus assist in diagnosing a patient with episodic arthralgia. In calcium pyrophosphate dehydrate crystal deposition disease, tendon calcifications can also be observed as well as classical chondrocalcinosis in multiple joints [4, 67]. OMERACT ultrasound definitions of calcium pyrophosphate dehydrate crystal deposition disease provided reliable results in the hyaline cartilage and fibrocartilage of the knee—the most frequently involved site in the disease—however, the definitions were not as reliable at other anatomical sites [68]. In a subsequent study which evaluated a wider range of anatomical locations, OMERACT ultrasound definitions of calcium pyrophosphate dehydrate crystal deposition disease were reliable for the triangular fibrocartilage of the wrist and the acromioclavicular joint [69]. As with other imaging modalities, the presence of chondrocalcinosis does not imply calcium pyrophosphate dehydrate crystal deposition disease and careful clinical correlation needs to occur. Ultrasound plays an active role in many procedures, including guided needle placement for the location and safe aspiration of synovial fluid to obtain a definitive crystal analysis [70]. Further, in addition to helping diagnose crystal deposits, ultrasound is sensitive to changes in gout and can be used to monitor tophus burden [71].
Considerations in older patients
In older patients presenting with shoulder and hip pain, a diagnosis of PMR should be considered. Ultrasound features that are suggestive of a differential diagnosis of PMR include bilateral subacromial and subdeltoid bursitis, long biceps tendon tenosynovitis, trochanteric bursitis, and glenohumeral and hip effusion [7, 28, 72].
In developing the 2012 ACR/EULAR classification criteria for PMR, evaluation of scoring criteria in 125 patients with new-onset PMR and 169 controls showed that adding ultrasound measures to the scoring system increased specificity for discriminating PMR from other mimicking conditions such as elderly-onset RA (EORA) from 78 to 81% [29]. A subsequent systematic literature review by Sakellariou and colleagues [73] found that bilateral shoulder bursitis on ultrasound had the highest specificity of any individual finding for diagnosis of PMR. The absence of synovial proliferation at the hand or wrist on ultrasound is also suggestive of PMR rather than EORA [28]. Negative serologic testing for rheumatoid factor or anticitrullinated protein antibodies can also help rule out a diagnosis of EORA [7].
Conclusions
With increasing availability of biologic therapies that target specific disease pathogenesis, it is more important than ever for clinicians to be able to differentiate between different types of inflammatory arthritis. Subsequent differentiation of the specific phenotype of inflammatory arthritis present can be complicated by ambiguity in the clinical picture and laboratory findings not allowing for a clear diagnosis. Consequently, imaging—especially ultrasound—is now an essential part of early inflammatory arthritis diagnosis and differentiation, and its inclusion in two ACR/EULAR classification criteria highlights its importance [29, 30] despite the limited number of studies that have examined how ultrasound should be integrated to the diagnostic process for inflammatory arthritis. Furthermore, detection of subclinical deposits of tophi is often an epiphany in patients with episodic seronegative arthralgia. Thus, ultrasound has become a valuable tool in the hands of an experienced clinician in evaluating patients with arthralgia who have sparse clinical signs.
Acknowledgments
Technical assistance with editing and styling of the manuscript for submission was provided by Oxford PharmaGenesis Inc., Newtown, PA, USA.
Abbreviations
- ACR
American College of Rheumatology
- AS
Ankylosing spondylitis
- CT
Computed tomography
- EORA
Elderly-onset RA
- EULAR
European League Against Rheumatism
- GRAPPA
Group for Research and Assessment of Psoriasis and Psoriatic Arthritis
- GUESS
Glasgow Ultrasound Enthesitis Scoring System
- MASEI
MAdrid Sonographic Enthesitis Index
- MRI
Magnetic resonance imaging
- OMERACT
Outcome Measures in Rheumatology
- PDUS
Power Doppler ultrasound
- PMR
Polymyalgia rheumatica
- PsA
Psoriatic arthritis
- RA
Rheumatoid arthritis
- SpA
Spondyloarthritis
Authors’ contributions
GSK, CB, and AD all contributed to development of the concept for this review, substantively revised multiple drafts of this review, approved the submitted version of this review, and agreed to be personally accountable for their contribution to this review.
Funding
Technical assistance with editing and styling of the manuscript for submission was funded by Novartis Pharmaceuticals Corporation. The authors received no financial support or other form of compensation related to the development of this manuscript. The sponsor had no other role in the preparation, content, or decision to submit this manuscript.
Availability of data and materials
Data sharing is not applicable to this article as no datasets were generated or analyzed during the current study.
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
GSK: Consultant Novartis.
CB: Speaker/consultant for AbbVie, Novartis, Regeneron, Sanofi, and Genzyme.
AD: Received research grants from AbbVie, Eli Lilly, GSK, Janssen, Novartis, Pfizer, and UCB; and has served on the advisory boards of AbbVie, Amgen, BMS, Eli Lilly, Janssen, Novartis, Pfizer, and UCB.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Mc Ardle A, Flatley B, Pennington SR, FitzGerald O. Early biomarkers of joint damage in rheumatoid and psoriatic arthritis. Arthritis Res Ther. 2015;17:141. doi: 10.1186/s13075-015-0652-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hazes JM, Luime JJ. The epidemiology of early inflammatory arthritis. Nat Rev Rheumatol. 2011;7:381–390. doi: 10.1038/nrrheum.2011.78. [DOI] [PubMed] [Google Scholar]
- 3.Merola JF, Espinoza LR, Fleischmann R. Distinguishing rheumatoid arthritis from psoriatic arthritis. RMD Open. 2018;4:e000656. doi: 10.1136/rmdopen-2018-000656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Epis O, Paoletti F, d'Errico T, Favalli E, Garau P, Mancarella L, et al. Ultrasonography in the diagnosis and management of patients with inflammatory arthritides. Eur J Intern Med. 2014;25:103–111. doi: 10.1016/j.ejim.2013.08.700. [DOI] [PubMed] [Google Scholar]
- 5.Finckh A, Liang MH, van Herckenrode CM, de Pablo P. Long-term impact of early treatment on radiographic progression in rheumatoid arthritis: a meta-analysis. Arthritis Rheum. 2006;55:864–872. doi: 10.1002/art.22353. [DOI] [PubMed] [Google Scholar]
- 6.Gladman DD, Thavaneswaran A, Chandran V, Cook RJ. Do patients with psoriatic arthritis who present early fare better than those presenting later in the disease? Ann Rheum Dis. 2011;70:2152–2154. doi: 10.1136/ard.2011.150938. [DOI] [PubMed] [Google Scholar]
- 7.Codreanu C, Enache L. Is ultrasound changing the way we understand rheumatology? Including ultrasound examination in the classification criteria of polymyalgia rheumatica and gout. Med Ultrason. 2015;17:97–103. doi: 10.11152/mu.2013.2066.171.ccle. [DOI] [PubMed] [Google Scholar]
- 8.Richette P, Bardin T, Doherty M. An update on the epidemiology of calcium pyrophosphate dihydrate crystal deposition disease. Rheumatology (Oxford) 2009;48:711–715. doi: 10.1093/rheumatology/kep081. [DOI] [PubMed] [Google Scholar]
- 9.Zhu Y, Pandya BJ, Choi HK. Prevalence of gout and hyperuricemia in the US general population: the National Health and nutrition examination survey 2007-2008. Arthritis Rheum. 2011;63:3136–3141. doi: 10.1002/art.30520. [DOI] [PubMed] [Google Scholar]
- 10.Mease PJ, Garg A, Helliwell PS, Park JJ, Gladman DD. Development of criteria to distinguish inflammatory from noninflammatory arthritis, enthesitis, dactylitis, and spondylitis: a report from the GRAPPA 2013 annual meeting. J Rheumatol. 2014;41:1249–1251. doi: 10.3899/jrheum.140182. [DOI] [PubMed] [Google Scholar]
- 11.Cohen JM, Husni ME, Qureshi AA, Merola JF. Psoriatic arthritis: it's as easy as “PSA”. J Am Acad Dermatol. 2015;72:905–906. doi: 10.1016/j.jaad.2014.12.008. [DOI] [PubMed] [Google Scholar]
- 12.Helliwell PS, Porter G, Taylor WJ, CASPAR Study Group Polyarticular psoriatic arthritis is more like oligoarticular psoriatic arthritis, than rheumatoid arthritis. Ann Rheum Dis. 2007;66:113–117. doi: 10.1136/ard.2006.054288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Veale DJ, Fearon U. What makes psoriatic and rheumatoid arthritis so different? RMD Open. 2015;1:e000025. doi: 10.1136/rmdopen-2014-000025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gladman DD. Clinical features and diagnostic considerations in psoriatic arthritis. Rheum Dis Clin N Am. 2015;41:569–579. doi: 10.1016/j.rdc.2015.07.003. [DOI] [PubMed] [Google Scholar]
- 15.Eder L, Jayakar J, Thavaneswaran A, Haddad A, Chandran V, Salonen D, et al. Is the MAdrid Sonographic Enthesitis Index useful for differentiating psoriatic arthritis from psoriasis alone and healthy controls? J Rheumatol. 2014;41:466–472. doi: 10.3899/jrheum.130949. [DOI] [PubMed] [Google Scholar]
- 16.Punzi L, Podswiadek M, Oliviero F, Lonigro A, Modesti V, Ramonda R, et al. Laboratory findings in psoriatic arthritis. Reumatismo. 2007;59(Suppl 1):52–55. doi: 10.4081/reumatismo.2007.1s.52. [DOI] [PubMed] [Google Scholar]
- 17.Landry A, Docherty P, Ouellette S, Cartier LJ. Causes and outcomes of markedly elevated C-reactive protein levels. Can Fam Physician. 2017;63:e316–e323. [PMC free article] [PubMed] [Google Scholar]
- 18.Suresh E. Diagnosis and management of gout: a rational approach. Postgrad Med J. 2005;81:572–579. doi: 10.1136/pgmj.2004.030692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Caylor TL, Perkins A. Recognition and management of polymyalgia rheumatica and giant cell arteritis. Am Fam Physician. 2013;88:676–684. [PubMed] [Google Scholar]
- 20.Combe B, Landewe R, Daien CI, Hua C, Aletaha D, Álvaro-Gracia JM, et al. 2016 update of the EULAR recommendations for the management of early arthritis. Ann Rheum Dis. 2017;76:948–959. doi: 10.1136/annrheumdis-2016-210602. [DOI] [PubMed] [Google Scholar]
- 21.Forney MC, Winalski CS, Schils JP. Magnetic resonance imaging of inflammatory arthropathies of peripheral joints. Top Magn Reson Imaging. 2011;22:45–59. doi: 10.1097/RMR.0b013e31825c008d. [DOI] [PubMed] [Google Scholar]
- 22.Freeston JE, Coates LC, Nam JL, Moverley AR, Hensor EM, Wakefield RJ, et al. Is there subclinical synovitis in early psoriatic arthritis? A clinical comparison with gray-scale and power Doppler ultrasound. Arthritis Care Res (Hoboken) 2014;66:432–439. doi: 10.1002/acr.22158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wakefield RJ, Green MJ, Marzo-Ortega H, Conaghan PG, Gibbon WW, McGonagle D, et al. Should oligoarthritis be reclassified? Ultrasound reveals a high prevalence of subclinical disease. Ann Rheum Dis. 2004;63:382–385. doi: 10.1136/ard.2003.007062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Anandarajah A. Imaging in psoriatic arthritis. Clin Rev Allergy Immunol. 2013;44:157–165. doi: 10.1007/s12016-012-8304-4. [DOI] [PubMed] [Google Scholar]
- 25.Eder L, Barzilai M, Peled N, Gladman DD, Zisman D. The use of ultrasound for the assessment of enthesitis in patients with spondyloarthritis. Clin Radiol. 2013;68:219–223. doi: 10.1016/j.crad.2012.07.018. [DOI] [PubMed] [Google Scholar]
- 26.Wakefield RJ, Balint PV, Szkudlarek M, Filippucci E, Backhaus M, D'Agostino MA, et al. Musculoskeletal ultrasound including definitions for ultrasonographic pathology. J Rheumatol. 2005;32:2485–2487. [PubMed] [Google Scholar]
- 27.Fodor D, Nestorova R, Vlad V, Micu M. The place of musculoskeletal ultrasonography in gout diagnosis. Med Ultrason. 2014;16:336–344. doi: 10.11152/mu.201.3.2066.164.df1. [DOI] [PubMed] [Google Scholar]
- 28.Patil P, Dasgupta B. Role of diagnostic ultrasound in the assessment of musculoskeletal diseases. Ther Adv Musculoskelet Dis. 2012;4:341–355. doi: 10.1177/1759720X12442112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Dasgupta B, Cimmino MA, Kremers HM, Schmidt WA, Schirmer M, Salvarani C, et al. 2012 Provisional classification criteria for polymyalgia rheumatica: a European League Against Rheumatism/American College of Rheumatology collaborative initiative. Arthritis Rheum. 2012;64:943–954. doi: 10.1002/art.34356. [DOI] [PubMed] [Google Scholar]
- 30.Neogi T, Jansen TL, Dalbeth N, Fransen J, Schumacher HR, Berendsen D, et al. 2015 gout classification criteria: an American College of Rheumatology/European league against rheumatism collaborative initiative. Arthritis Rheumatol. 2015;67:2557–2568. doi: 10.1002/art.39254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Brown AK. Using ultrasonography to facilitate best practice in diagnosis and management of RA. Nat Rev Rheumatol. 2009;5:698–706. doi: 10.1038/nrrheum.2009.227. [DOI] [PubMed] [Google Scholar]
- 32.Terslev L, Naredo E, Iagnocco A, Balint PV, Wakefield RJ, Aegerter P, et al. Defining enthesitis in spondyloarthritis by ultrasound: results of a Delphi process and of a reliability reading exercise. Arthritis Care Res (Hoboken). 2014;66:741–748. doi: 10.1002/acr.22191. [DOI] [PubMed] [Google Scholar]
- 33.Poggenborg RP, Terslev L, Pedersen SJ, Østergaard M. Recent advances in imaging in psoriatic arthritis. Ther Adv Musculoskelet Dis. 2011;3:43–53. doi: 10.1177/1759720X10394031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Schirmer M, Duftner C, Schmidt WA, Dejaco C. Ultrasonography in inflammatory rheumatic disease: an overview. Nat Rev Rheumatol. 2011;7:479–488. doi: 10.1038/nrrheum.2011.95. [DOI] [PubMed] [Google Scholar]
- 35.van der Ven M, van der Veer-Meerkerk M, Ten Cate DF, Rasappu N, Kok MR, Csakvari D, et al. Absence of ultrasound inflammation in patients presenting with arthralgia rules out the development of arthritis. Arthritis Res Ther. 2017;19:202. doi: 10.1186/s13075-017-1405-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ekiz T, Ozturk GT, Yalcin S, Kinikli G, Ozcakar L. Ultrasonographic diagnosis of posterior interosseous nerve entrapment due to ganglion cyst in a patient with rheumatoid arthritis. Rheumatology (Oxford) 2014;53:2208. doi: 10.1093/rheumatology/keu342. [DOI] [PubMed] [Google Scholar]
- 37.Kaeley GS, Nishio MJ, Goyal JR, MacCarter DK, Wells AF, Chen S, et al. Changes in ultrasonographic vascularity upon initiation of adalimumab combination therapy in rheumatoid arthritis patients with an inadequate response to methotrexate. Arthritis Rheumatol. 2016;68:2584–2592. doi: 10.1002/art.39751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kaeley GS, Eder L, Aydin SZ, Gutierrez M, Bakewell C. Dactylitis: a hallmark of psoriatic arthritis. Semin Arthritis Rheum. 2018;48:263–273. doi: 10.1016/j.semarthrit.2018.02.002. [DOI] [PubMed] [Google Scholar]
- 39.Zabotti A, Salvin S, Quartuccio L, De Vita S. Differentiation between early rheumatoid and early psoriatic arthritis by the ultrasonographic study of the synovio-entheseal complex of the small joints of the hands. Clin Exp Rheumatol. 2016;34:459–465. [PubMed] [Google Scholar]
- 40.Ramrattan LA, Kaeley GS. Sonographic characteristics of extensor tendon abnormalities and relationship with joint disease activity in rheumatoid arthritis: a pilot study. J Ultrasound Med. 2017;36:985–992. doi: 10.7863/ultra.16.05024. [DOI] [PubMed] [Google Scholar]
- 41.Liu JT, Yeh HM, Liu SY, Chen KT. Psoriatic arthritis: epidemiology, diagnosis, and treatment. World J Orthop. 2014;5:537–543. doi: 10.5312/wjo.v5.i4.537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bakewell CJ, Olivieri I, Aydin SZ, Dejaco C, Ikeda K, Gutierrez M, et al. Ultrasound and magnetic resonance imaging in the evaluation of psoriatic dactylitis: status and perspectives. J Rheumatol. 2013;40:1951–1957. doi: 10.3899/jrheum.130643. [DOI] [PubMed] [Google Scholar]
- 43.Huh YH, Lee G, Song WH, Koh JT, Ryu JH. Crosstalk between FLS and chondrocytes is regulated by HIF-2α-mediated cytokines in arthritis. Exp Mol Med. 2015;47:e197. doi: 10.1038/emm.2015.88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Suzuki T. Power Doppler ultrasonographic assessment of the ankle in patients with inflammatory rheumatic diseases. World J Orthop. 2014;5:574–584. doi: 10.5312/wjo.v5.i5.574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Tang CY, Fung B. The last defence? Surgical aspects of gouty arthritis of hand and wrist. Hong Kong Med J. 2011;17:480–486. [PubMed] [Google Scholar]
- 46.Padovano I, Costantino F, Breban M, D'Agostino MA. Prevalence of ultrasound synovial inflammatory findings in healthy subjects. Ann Rheum Dis. 2016;75:1819–1823. doi: 10.1136/annrheumdis-2015-208103. [DOI] [PubMed] [Google Scholar]
- 47.Balint PV, Kane D, Wilson H, McInnes IB, Sturrock RD. Ultrasonography of entheseal insertions in the lower limb in spondyloarthropathy. Ann Rheum Dis. 2002;61:905–910. doi: 10.1136/ard.61.10.905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Acosta-Felquer ML, Ruta S, Rosa J, Marin J, Ferreyra-Garrot L, Galimberti ML, et al. Ultrasound entheseal abnormalities at the distal interphalangeal joints and clinical nail involvement in patients with psoriasis and psoriatic arthritis, supporting the nail-enthesitis theory. Semin Arthritis Rheum. 2017;47:338–342. doi: 10.1016/j.semarthrit.2017.05.002. [DOI] [PubMed] [Google Scholar]
- 49.Kristensen S, Christensen JH, Schmidt EB, Olesen JL, Johansen MB, Arvesen KB, et al. Assessment of enthesitis in patients with psoriatic arthritis using clinical examination and ultrasound. Muscles Ligaments Tendons J. 2016;6:241–247. doi: 10.32098/mltj.02.2016.12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Polachek A, Cook R, Chandran V, Gladman DD, Eder L. The association between sonographic enthesitis and radiographic damage in psoriatic arthritis. Arthritis Res Ther. 2017;19:189. doi: 10.1186/s13075-017-1399-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Gutierrez M, Filippucci E, Grassi W, Rosemffet M. Intratendinous power Doppler changes related to patient position in seronegative spondyloarthritis. J Rheumatol. 2010;37:1057–1059. doi: 10.3899/jrheum.090900. [DOI] [PubMed] [Google Scholar]
- 52.Kaeley GS, D'Agostino MA, Grassi W, Ostergaard M, Olivieri I. GRAPPA 2011: proceedings from the ultrasound imaging module. J Rheumatol. 2012;39:2211–2213. doi: 10.3899/jrheum.120826. [DOI] [PubMed] [Google Scholar]
- 53.Michelsen B, Diamantopoulos AP, Soldal DM, Hammer HB, Kavanaugh A, Haugeberg G. Achilles enthesitis defined by ultrasound is not associated with clinical enthesitis in patients with psoriatic arthritis. RMD Open. 2017;3:e000486. doi: 10.1136/rmdopen-2017-000486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Balint PV, Terslev L, Aegerter P, Bruyn GAW, Chary-Valckenaere I, Gandjbakhch F, et al. Reliability of a consensus-based ultrasound definition and scoring for enthesitis in spondyloarthritis and psoriatic arthritis: an OMERACT US initiative. Ann Rheum Dis. 2018;77:1730–1735. doi: 10.1136/annrheumdis-2018-213609. [DOI] [PubMed] [Google Scholar]
- 55.Tom S, Zhong Y, Cook R, Aydin SZ, Kaeley G, Eder L. Development of a preliminary ultrasonographic enthesitis score in psoriatic arthritis - GRAPPA Ultrasound Working Group. J Rheumatol. 2019;46:384–390. doi: 10.3899/jrheum.171465. [DOI] [PubMed] [Google Scholar]
- 56.D'Agostino MA, Olivieri I. Enthesitis. Best Pract Res Clin Rheumatol. 2006;20:473–486. doi: 10.1016/j.berh.2006.03.007. [DOI] [PubMed] [Google Scholar]
- 57.Sakellariou G, Iagnocco A, Delle Sedie A, Riente L, Filippucci E, Montecucco C. Ultrasonographic evaluation of entheses in patients with spondyloarthritis: a systematic literature review. Clin Exp Rheumatol. 2014;32:969–978. [PubMed] [Google Scholar]
- 58.de Miguel E, Cobo T, Muñoz-Fernández S, Naredo E, Usón J, Acebes JC, et al. Validity of enthesis ultrasound assessment in spondyloarthropathy. Ann Rheum Dis. 2009;68:169–174. doi: 10.1136/ard.2007.084251. [DOI] [PubMed] [Google Scholar]
- 59.de Miguel E, Muñoz-Fernández S, Castillo C, Cobo-Ibáñez T, Martín-Mola E. Diagnostic accuracy of enthesis ultrasound in the diagnosis of early spondyloarthritis. Ann Rheum Dis. 2011;70:434–439. doi: 10.1136/ard.2010.134965. [DOI] [PubMed] [Google Scholar]
- 60.Gisondi P, Tinazzi I, El-Dalati G, Gallo M, Biasi D, Barbara LM, et al. Lower limb enthesopathy in patients with psoriasis without clinical signs of arthropathy: a hospital-based case-control study. Ann Rheum Dis. 2008;67:26–30. doi: 10.1136/ard.2007.075101. [DOI] [PubMed] [Google Scholar]
- 61.Yumusakhuylu Y, Kasapoglu-Gunal E, Murat S, Kurum E, Keskin H, Icagasioglu A, et al. A preliminary study showing that ultrasonography cannot differentiate between psoriatic arthritis and nodal osteoarthritis based on enthesopathy scores. Rheumatology (Oxford) 2016;55:1703–1704. doi: 10.1093/rheumatology/kew218. [DOI] [PubMed] [Google Scholar]
- 62.Tan AL, Toumi H, Benjamin M, Grainger AJ, Tanner SF, Emery P, et al. Combined high-resolution magnetic resonance imaging and histological examination to explore the role of ligaments and tendons in the phenotypic expression of early hand osteoarthritis. Ann Rheum Dis. 2006;65:1267–1272. doi: 10.1136/ard.2005.050112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Finzel S, Ohrndorf S, Englbrecht M, Stach C, Messerschmidt J, Schett G, et al. A detailed comparative study of high-resolution ultrasound and micro-computed tomography for detection of arthritic bone erosions. Arthritis Rheum. 2011;63:1231–1236. doi: 10.1002/art.30285. [DOI] [PubMed] [Google Scholar]
- 64.Zayat AS, Ellegaard K, Conaghan PG, Terslev L, Hensor EM, Freeston JE, et al. The specificity of ultrasound-detected bone erosions for rheumatoid arthritis. Ann Rheum Dis. 2015;74:897–903. doi: 10.1136/annrheumdis-2013-204864. [DOI] [PubMed] [Google Scholar]
- 65.Kawashiri SY, Fujikawa K, Nishino A, Okada A, Aramaki T, Shimizu T, et al. Ultrasound-detected bone erosion is a relapse risk factor after discontinuation of biologic disease-modifying antirheumatic drugs in patients with rheumatoid arthritis whose ultrasound power Doppler synovitis activity and clinical disease activity are well controlled. Arthritis Res Ther. 2017;19:108. doi: 10.1186/s13075-017-1320-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Nalbant S, Corominas H, Hsu B, Chen LX, Schumacher HR, Kitumnuaypong T. Ultrasonography for assessment of subcutaneous nodules. J Rheumatol. 2003;30:1191–1195. [PubMed] [Google Scholar]
- 67.Grassi W, Meenagh G, Pascual E, Filippucci E. “crystal clear”-sonographic assessment of gout and calcium pyrophosphate deposition disease. Semin Arthritis Rheum. 2006;36:197–202. doi: 10.1016/j.semarthrit.2006.08.001. [DOI] [PubMed] [Google Scholar]
- 68.Filippou G, Scirè CA, Damjanov N, Adinolfi A, Carrara G, Picerno V, et al. Definition and reliability assessment of elementary ultrasonographic findings in calcium pyrophosphate deposition disease: a study by the OMERACT calcium pyrophosphate deposition disease ultrasound subtask force. J Rheumatol. 2017;44:1744–1749. doi: 10.3899/jrheum.161057. [DOI] [PubMed] [Google Scholar]
- 69.Filippou G, Scirè CA, Adinolfi A, Damjanov NS, Carrara G, Bruyn GAW, et al. Identification of calcium pyrophosphate deposition disease (CPPD) by ultrasound: reliability of the OMERACT definitions in an extended set of joints-an international multiobserver study by the OMERACT calcium pyrophosphate deposition disease ultrasound subtask force. Ann Rheum Dis. 2018;77:1194–1199. doi: 10.1136/annrheumdis-2018-213217. [DOI] [PubMed] [Google Scholar]
- 70.Grassi W, Gutierrez M. Psoriatic arthritis: need for ultrasound in everyday clinical practice. J Rheumatol Suppl. 2012;89:39–43. doi: 10.3899/jrheum.120241. [DOI] [PubMed] [Google Scholar]
- 71.Peiteado D, Villalba A, Martin-Mola E, Balsa A, De Miguel E. Ultrasound sensitivity to changes in gout: a longitudinal study after two years of treatment. Clin Exp Rheumatol. 2017;35:746–751. [PubMed] [Google Scholar]
- 72.Rutigliano IM, Scirocco C, Ceccarelli F, Finucci A, Iagnocco A. Musculoskeletal ultrasound in the evaluation of polymyalgia Rheumatica. Med Ultrason. 2015;17:361–366. doi: 10.11152/mu.2013.2066.173.aig. [DOI] [PubMed] [Google Scholar]
- 73.Sakellariou G, Iagnocco A, Riente L, Ceccarelli F, Carli L, Di Geso L, et al. Ultrasound imaging for the rheumatologist XLIII. Ultrasonographic evaluation of shoulders and hips in patients with polymyalgia rheumatica: a systematic literature review. Clin Exp Rheumatol. 2013;31:1–7. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data sharing is not applicable to this article as no datasets were generated or analyzed during the current study.