Abstract
Rationale and Objective:
National guidelines recommend delivering a continuous renal replacement therapy (CRRT) dose of 20–25 mL/kg/hr. However, practice patterns nationwide are highly variable and inconsistent prescribing may lead to errors in medication dosing as well as increase rates of electrolyte and acid-base abnormalities. We describe an initiative to standardize CRRT practice patterns and reduce dosing variability.
Study Design:
Quality improvement study
Setting and Participants:
Adult patients treated with CRRT at the University of Colorado Hospital (UCH) between January 2016 and October 2017.
Quality Improvement Activities:
An assessment of the magnitude of the variability in CRRT dosing and the following specific interventions were implemented over the course of one year: 1) modification of the electronic medical record (EMR) to include calculated average 24 hour dose in real-time, 2) modification of the CRRT procedure note to include comments on dosing, 3) modification of the CRRT order set to display calculations, 4) yearly educational sessions for renal fellows outlining CRRT-specific dosing targets.
Outcomes:
The primary outcome was weekly percentage of CRRT treatments with an average delivered daily dose of 20–25 mL/kg/hr. Process and balancing outcomes included CRRT flowsheet accuracy, documentation of rates of delivered dose, and nursing satisfaction.
Analytical Approach:
Rates of weekly CRRT dosing in compliance with national guidelines were determined and used to create run charts showing compliance rates before and after the QI interventions.
Results:
Among 837 treatments prior to the intervention, 279 (33%) daily CRRT sessions achieved an average dose of 20–25 mL/kg/hr. Following implementation of interventions, 631 out of 952 treatments (66%) achieved this goal. Week-to-week variation in dosing was significantly reduced.
Limitations:
A single-center study generating data that may not be generalizable to institutions with different CRRT nursing models or different EMR systems.
Conclusions:
Changes to the EMR and documentation templates as well as education of CRRT providers about dosing were associated with a doubling of the rate of appropriate CRRT dosing and reduction in dosing variability.
Keywords: Quality Improvement, continuous renal replacement therapy (CRRT), dialysis dose, intensive care unit (ICU)
Plain Language Summary:
Continuous renal replacement therapy (CRRT) is a form of continuous dialysis used in critically ill patients, often in the intensive care unit. National guidelines recommend that CRRT be prescribed at a dose of 20–25 mL/kg/hr, however, prescription practices are variable. Inconsistent prescribing patterns among providers may lead to errors in dosing of medications such as antibiotics, and increase rates of blood chemistry abnormalities. This study describes a quality improvement initiative to increase compliance with national guidelines for CRRT dosing. Over the course of one year, multiple interventions to improve compliance were implemented, including modifications to the electronic medical record, modifications to the procedure note and CRRT order templates, and education of providers regarding proper prescribing practices. Following these interventions, the rate of administration of CRRT using dosing consistent with national guidelines doubled from 33% to 66%.
Introduction
Acute kidney injury (AKI) is a common and widely recognized source of morbidity and mortality in hospitalized patients. It is reported in up to 20% of all hospitalized patients1 and in 30–50% of hospitalized patients admitted to the intensive care unit (ICU)2, and is associated with increased rates of in-hospital mortality3, 4 even in mild disease5–7. The in-hospital mortality of patients with severe AKI requiring renal replacement therapy (RRT) is estimated at approximately 33% overall8 and can be >50% in patients in the ICU9, 10. RRT is the only FDA approved therapy for AKI, and continuous RRT (CRRT) is generally the preferred modality in the ICU because it is associated with less hemodynamic instability than intermittent therapy11.
A major component of the CRRT prescription is dose, which is based on urea clearance. In CRRT, where dialysate or replacement fluid flow rates are relatively low, urea clearance is a function of effluent flow rate (ultrafiltration plus therapy fluid flow rates)12. Kidney Disease-Improving Global Outcomes (KDIGO) recommended in their 2012 clinical practice guidelines that patients treated with CRRT receive an effluent flow rate of 20–25 mL/kg/hr, and gave the recommendation a 1A rating13. In 2013, another major body, the National Kidney Foundation–Kidney Disease Outcomes Quality Initiative (NKF-KDOQI), published their commentary of the KDIGO AKI guidelines, and endorsed the recommendation for a CRRT dose of 20–25 mL/kg/hr14. Both underdosing and overdosing patients on CRRT may increase the risk of adverse events including major electrolyte abnormalities and inappropriate drug dosing, among others. Suboptimal care can lead to increased length of ICU stays, increased time to renal recovery, and increased cost.
Despite clear national guidelines, CRRT dosing remains highly variable. Nationwide survey results indicate that fewer than 50% of practitioners target a specific dose, and only 15% of patients have regular dose assessments15. Indeed, preliminary data at University of Colorado Hospital (UCH) showed that average daily delivered dose of CRRT after accounting for machine downtime was not routinely documented in the medical record. Review of the CRRT program at the University of Colorado Hospital (UCH) also showed that prescription patterns varied widely among nephrology faculty.
Our goal in undertaking this quality initiative was to standardize CRRT treatments at UCH. The specific aim of our interventions was to increase the percentage of daily CRRT sessions within the target range of 20–25 mL/kg/hr to at least 65% within one year.
Methods
Setting
The University of Colorado Hospital is a large, quaternary referral center that performs 1,200 to 1,500 CRRT treatments annually. CRRT at UCH in performed by the ICU nurse alone without collaboration with dialysis nurses16. There are approximately 600 ICU nurses, and CRRT education consists of 2 training sessions conducting by the Medical ICU nurse educator, followed by 2–3 orientation shifts with an experienced ICU nurse. Annual retraining is offered but not required. Determinations on the need for CRRT are made at UCH solely by the nephrology consulting service, which consists of one renal fellow and one attending.
The study was funded by a University of Colorado Clinical Effectiveness and Patient Safety Grant for Residents and Fellows (CEPS-RF) award. The project was reviewed by the Colorado Multiple Institutional Review Board (COMIRB), which deemed it exempt quality improvement research. No consent was required or obtained. The project was also approved by clinical leadership within the UCH renal division and the UCH intensive care units.
Baseline Magnitude Assessment
Charts were retrospectively reviewed from January through October of 2016. The average dose of CRRT delivered for each treatment day was recorded, and the weekly percentage of treatments with an average dose of 20–25 mL/kg/hr was determined.
Interventions
Primary stakeholders were identified, and a CRRT advisory group was created which included representatives from nephrology staff, critical care primary physicians, ICU nurses, nurse managers and educators, pharmacists, and IT support. Identified barriers to adequate CRRT dosing included difficulty in determining the dose of therapy actually delivered accounting for time off circuit, inaccuracy and inconsistency in data collection and data entry into the EMR, and difficulties in communication between the physicians and nurses operating the CRRT machines. Another barrier identified was that the CRRT order set and procedure template note did not include dosing information. Based on the magnitude assessment and discussion with primary stakeholders, the following interventions were pursued: 1) Modification of the EMR to automatically calculate average delivered CRRT dose over 24 hours, 2) training of ICU nurses to standardize data input, 3) creation and distribution of a provider protocol document to nephrology staff and fellows to standardize CRRT delivery, 4) modification of the CRRT procedure note to include calculated average doses, 5) Modification of the CRRT order set to provide formulas for dose calculation, and 6) presentation of results at quarterly division QI meetings.
CRRT flowsheet details
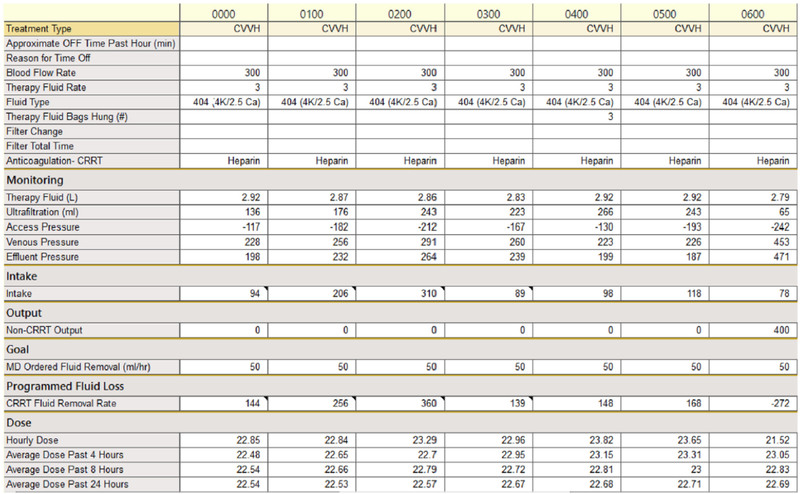
An example of the CRRT flowsheet is given in Figure 1. Data is entered hourly by the ICU nurse, who records the modality, blood flow rate, prescribed fluid rate, prescribed net ultrafiltration rate, fluid composition, anticoagulation type, actual therapy fluid delivered, actual ultrafiltration, and machine pressures. The hourly dose is calculated based on the total effluent for that hour divided by the patient’s most recent recorded weight (weights were recorded at admission and daily). Because UCH uses 100% pre-filter CVVH, an adjustment factor for pre-filter dilution was applied when CVVH was used. The 24-hour dose was calculated by taking the average of the 24 doses calculated over the preceding 24 hours. Of note, if fewer than 24 hourly doses were available, the program would average the hourly doses over the number of hours that had been completed. For instance, at 12 hours into therapy, the “Average Dose Past 24 Hours” line would actually display the average over 12 hours, not 24. If the patient was off CRRT, which could happen due to operating room trips, radiology procedures, or circuit clotting, an effluent rate of “0” was entered, which calculated a dose of 0 for that hour, and thereby accounted for down time during the day.
Figure 1.
An example of the CRRT dosing flowsheet following the quality improvement intervention.
The daily value for a treatment session was based on the average 24 hour dose as calculated at 7 AM, which is the time of the morning shift change for UCH ICU nurses. When initiating CRRT, a minimum of 12 hours were required to count as the first treatment session. For patients started before 1900, the first 7 AM dose was counted as the day 1 dose value. For patients started after 1900, the second 7 AM dose was counted as the day 1 dose value. This rule also applied if CRRT was discontinued by the renal team, and later needed to be restarted. When a patient was taken off CRRT, such as to transition to hemodialysis, the days of therapy were counted up to the 7 AM value preceding discontinuation.
Measures
The primary objective measure was percentage of treatments per week in which the average daily dose of CRRT was 20–25 ml/kg/hr. Patients who died or were taken off CRRT within 12 hours of treatment initiation were excluded. Secondary measures included mortality, number of CRRT treatments, ICU length of stay, and hospital length of stay. The average CRRT dose and compliance rates specifically on the first day of treatment were also recorded.
The main process measure was accuracy of data entry. At UCH, CRRT flowsheet data is entered hourly into the EMR by ICU nurses. The data was reviewed by the QI team, and the number of hours without an error in data entry was recorded. The most common error was incomplete entry of data, specifically when entering an effluent flow rate of “0” during time off CRRT (for the purposes of determining the primary outcome, when this data point was missing, it was added in after the fact, and doses were recalculated). In order to be counted as complete, documentation was required to contain data on the modality, blood flow rate, actual therapy fluid rate, and actual ultrafiltration, which are the components required to calculate the actual dose.
Another process measure was the percentage of nephrology attending notes that address the previous day’s CRRT dose. The QI team reviewed nephrology procedure notes daily to determine the percentage of notes that included the patient’s average CRRT dose over the previous day. In order to be counted, the note needed to contain the dose, as well as a comment regarding whether the dose was in the desired range or not, and if not, whether the dose would be adjusted.
The main balancing measure was time spent by nurses on CRRT charting. We anticipated that an undesirable effect of our interventions could be an increase in nursing time spent charting CRRT data, which could increase nursing dissatisfaction and detract from other important duties. Nurses were randomly surveyed at the ends of their shift, and specifically asked if they felt their charting time had increased, and if so, by how much.
Implementation
Changes to the CRRT flowsheet and order set in the EMR were applied in October and November of 2016, respectively. ICU nurse training, which included educational sessions as well as daily review of data input with feedback, was implemented from October 2016 to February 2017. The long duration of training was due to the large number of ICU nurses employed at UCH (>600 nurses). Accuracy of data input was recorded as above, and nurses were given daily feedback regarding inaccuracies. Training was continued until data was entered accurately >95% of the time on average.
In February 2017, we began collecting data on the percentage of treatments at goal, as well as data regarding the frequency with which nephrology procedure notes indicated the average dose delivered to the patient over 24 hours. Provider notes rarely contained dosing information, so in addition to interventions within the ordering system, changes were made to the CRRT procedure note template. Dosing data was automatically populated into the note, and a brief comment was required from the provider regarding whether dose would be increased, decreased, or remain unchanged.
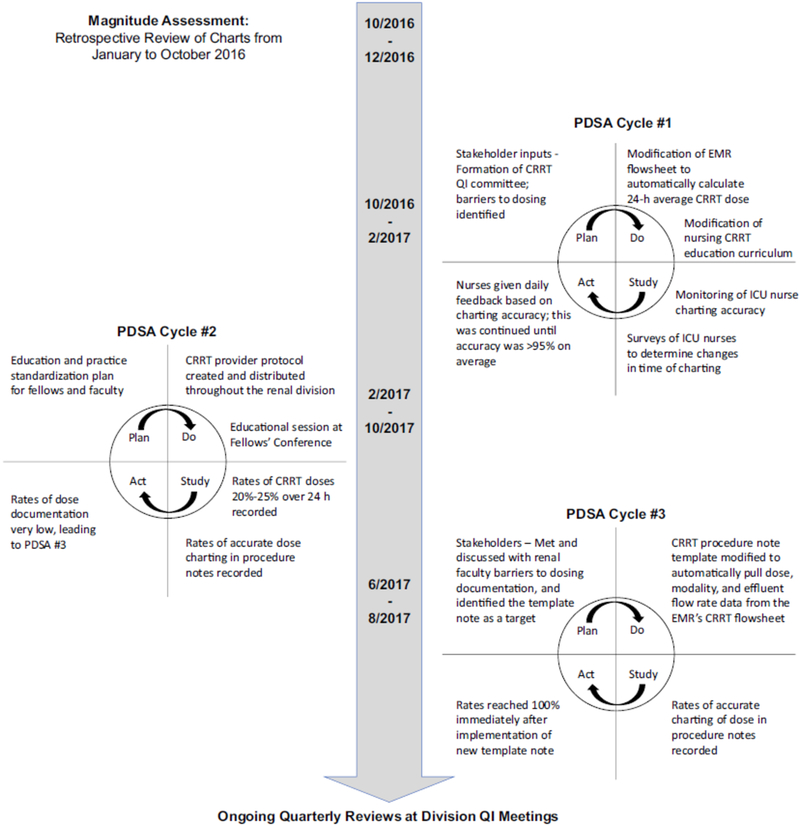
Formal data collection continued from February to October 2017. Figure 2 outlines the flow of the trial.
Figure 2.
Timeline of major Quality Initiative interventions.
Statistical Analysis
Mean ± standard deviation or counts and percentages were used to describe the distribution of continuous and categorical variables, respectively. In Table 1, t-test was used for comparison of continuous variables and chi-square was used for comparison of categorical variables. Using the generalized estimating equation (GEE) framework, we compared the mean doses for the pre-intervention and post-intervention periods. Following the construction of histograms for dose stratified by period, we determined that the distribution was right-skewed. As a result, we employed the log link function, which estimates the mean ratio between periods (as opposed to the mean difference in normal linear regression). Subject ID was our repeated measure, in order to account for correlation of observations from the same subject. The run chart was constructed by plotting weekly compliance values over time. All statistical tests were considered to be significant at a 2-sided p < 0.05. All analyses were performed using SAS software version 9.4 (SAS Inc., Cary, NC).
Table 1.
Demographics, clinical characteristics, and secondary outcomes in patients before and after implementation of QI interventions.
| Prior to Intervention N = 125 | After Intervention N = 122 | p-value | |
|---|---|---|---|
| Characteristics | |||
| Age, mean (SD) | 55.7 (14.9) | 54.1 (16.2) | 0.40 |
| Female Gender | 58 (46.4) | 47 (38.5) | 0.21 |
| 0.22 | |||
| 75 (60.0) | 86 (62.3) | ||
| 10 (8.0) | 20 (14.5) | ||
| 27 (21.6) | 22 (15.9) | ||
| Unknown | 13 (10.4) | 10 (7.2) | |
| Cause of AKI* | |||
| Sepsis | 46 (36.8) | 45 (36.9) | 0.99 |
| Post-Operative | 32 (25.6) | 34 (27.9) | 0.69 |
| Liver Disease | 20 (16.0) | 22 (18.0) | 0.67 |
| Cardiogenic Shock | 28 (22.4) | 29 (23.8) | 0.80 |
| Cardiorenal | 8 (6.4) | 11 (9.0) | 0.44 |
| Other† | 31 (24.8) | 27 (22.1) | 0.62 |
| ESRD | 22 (17.6) | 13 (10.7) | 0.12 |
| 0.96 | |||
| 1 (0.8) | 2 (1.6) | ||
| 17 (13.5) | 17 (13.9) | ||
| 14 (11.1) | 16 (13.1) | ||
| 57 (45.2) | 51 (41.8) | ||
| 4 (3.2) | 2 (1.6) | ||
| 16 (12.7) | 17 (13.9) | ||
| Multiple ICU locations | 16 (12.7) | 17 (13.9) | |
| Outcomes | |||
| Mortality | 75 (60.0) | 54 (44.3) | 0.01 |
| CRRT Days, mean (SD) | 8.1 (22.9) | 8.7 (8.9) | 0.77 |
| Hospital LOS, mean (SD) | 23.3 (35.0) | 30.0 (34.5) | 0.13 |
| ICU LOS, mean (SD) | 20.0 (35.1) | 19.4 (17.2) | 0.87 |
Numbers given are figures and percents, unless otherwise specified.
In most cases, the etiology of AKI was multifactorial, so the categories are not mutually exclusive. This is why the percentages add to above 100%, and why separate p-values were calculated for each category.
Other consists of nephrotoxin induced, rhabdomyolysis-induced, malignancy associated, and unknown etiology.
Results
Baseline data –
Dosing data was collected from January to October 2016 (prior to implementation). 915 treatment sessions were identified. 78 treatment sessions were excluded because the delivered dose could not be ascertained based on the data available, leaving 837 to analyze. The average dose was 26.1 mL/kg/hr, with a standard deviation of 7.4. A total of 279 doses (33%) were between 20 and 25 mL/kg/hr. 173 (21%) were below 20 mL/kg/hr, and 385 (46%) were >25 mL/kg/hr. Within the group with an above-goal dose, 187 (22%) treatments were above goal at >30 mL/kg/hr, and 21 (2%) were extremely above goal at >50 mL/kg/hr.
Demographic Data –
As shown in Table 1, the CRRT populations at UCH before and after the intervention implementations were similar in terms of age, gender, and race. The etiology of AKI was also similar, with sepsis the leading cause of AKI requiring CRRT. The ICU locations of treatment were also comparable, with the medical ICU accounting for the largest percentage of treatments. The indications for CRRT were collected in the post-intervention group, and results are shown in Table S1. Patients nearly universally had KDIGO stage 2 or 3 AKI, and oliguria/anuria and volume overload were the most common reasons for CRRT initiation.
Primary Objective Outcome –
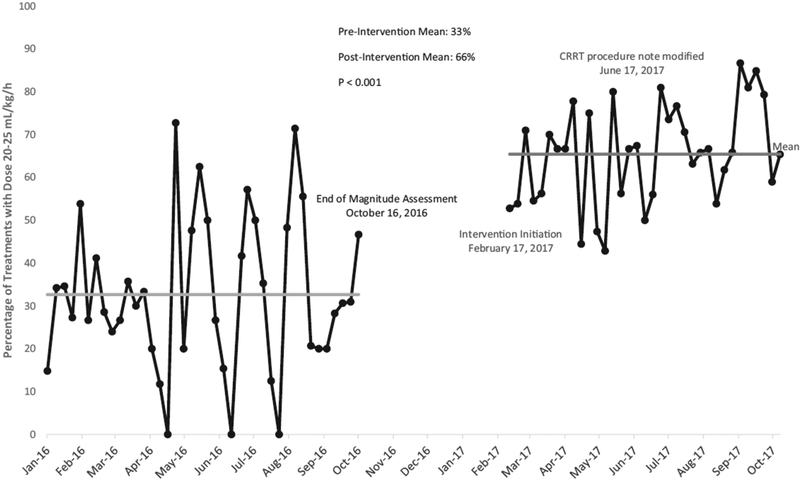
Data was collected between February and October 2017, and included 952 treatments. A comparison of outcomes before and after implementation of interventions is shown in Table 2. The number of treatments that achieved the goal dose was 631 (66%). We noted a significant decline in the number of under-dose treatments with 12% (n=113) receiving doses <20 mL/kg/hr. There was a similar decline in the number of over-dosed treatments to 22% (n=209) of patients receiving doses >25 mL/kg/hr. Only 37 out of the 952 treatments (4%) were above goal at >30/mL/kg. Notably, there were no treatments with an extremely above goal dose of >50 ml/kg/hr. A run chart showing percentage in range per week is given in Figure 3, which shows an increase in compliance, but also a significant decrease in week to week variance. The average dose was 23.5 mg/kg/hr, with a standard deviation of 2.1.
Table 2.
Number and percentage of CRRT treatments by effluent range before and after QI interventions.
| Dose Range | Before Interventions (N=837) | After Interventions (N=952) | p-value |
|---|---|---|---|
| Dose Range | <0.001 | ||
| <20 ml/kg/hr | 173 (20.7) | 112 (11.7) | |
| 20–25 mL/kg/hr | 279 (33.3) | 631 (66.3) | |
| 25.1–30 mL/kg/hr | 198 (23.7) | 172 (18.1) | |
| >30 mL/kg/h | 187 (22.3) | 37 (3.9) | |
| Mean Dose, mL/kg/hr (SD) | 26.1 (7.4) | 23.5 (2.1) | <0.001 |
Numbers given are figures and percents, unless otherwise specified.
Figure 3.
Control Chart before and after intervention implementation
Secondary Objective Outcomes –
Secondary outcomes are shown in the outcomes section of Table 1. There was a statistically significant decrease in mortality, with a mortality rate of 60% pre-intervention compared to 45% post-intervention. However, there were no differences in days of CRRT treatment, ICU length of stay, or hospital length of stay. As shown in Table 3, compliance rates for CRRT Day 1 treatments rose significantly from 42 to 53%, but this was less than the overall increase observed in all treatments from 33 to 66%.
Table 3.
Number and percentage of first-day only CRRT treatments by effluent range before and after QI interventions.
| CRRT Day 1 Values Only | Before Interventions (N=125) | After Interventions (N=122) | p-value |
|---|---|---|---|
| Dose Range | 0.002 | ||
| <20 ml/kg/hr | 27 (21.6) | 16 (13.1) | |
| 20–25 mL/kg/hr | 52 (41.6) | 65 (53.3) | |
| 25.1–30 mL/kg/hr | 20 (16.0) | 32 (26.2) | |
| >30 mL/kg/h | 26 (20.8) | 9 (7.4) | |
| Mean Dose, mL/kg/hr (SD) | 26.1 (10.7) | 23.9 (4.3) | 0.04 |
Process and Balancing Outcomes –
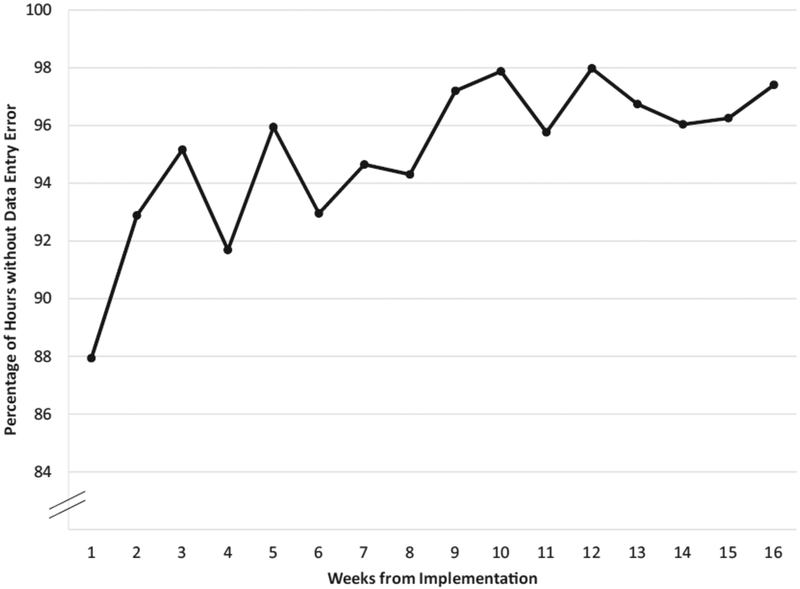
Data accuracy rates were 87.9% initially, and rose to 97.4% after 4 months (Figure 4). Whereas none of the procedure notes included dosing data initially, 100% did following modification of the procedure note template. Nurses surveyed did not feel that the changes to the CRRT program increased their workload.
Figure 4.
Weekly percentage of flowsheet hours without data entry errors
Discussion
In this quality improvement initiative, our group was able to dramatically increase the proportion of CRRT treatments at UCH in which an average dose of 20–25 mL/kg/hr was delivered. The interventions, which included modification of the EMR to reliably calculate and display delivered CRRT dose and changes to the CRRT procedure note, doubled the rate of compliance with national recommendations.
Two large randomized controlled trials have evaluated dose in critically ill patients requiring RRT. The Acute Renal Failure Trial Network (ATN) study randomized 1,124 patients to either more intense or less intense therapy, which in CRRT was an effluent flow rate of 20 mL/kg/hr vs 35 mL/kg/hr. Patients receiving hemodialysis and slow low-efficiency dialysis (SLED) were also studied, and with these modalities the comparison was 6 days of therapy a week vs 3 days. Results were published in 2008, and there was no observed benefit to higher doses of RRT10. The Intensity of Continuous Renal-Replacement Therapy in Critically Ill Patients (RENAL) study randomized 1508 patients to higher-intensity or lower-intensity CRRT therapy, defined as 40 mL/kg/hr vs 25 mL/kg/hr. Results were published in 2009, and also did not show a benefit to higher-intensity therapy17.
Although there are no randomized studies evaluating doses less than 20 mL/kg/hr, there is some reason to believe that survival may decrease as dose falls below this level. In a study evaluating the effect of increasing the frequency of intermittent hemodialysis, the 3x per week group on average received a kt/v of 0.94, less than the 1.2 recommended per session, and had higher mortality than the daily dialysis group18.
While randomized-controlled trials have not demonstrated increased mortality with high doses of CRRT, there are several notable complications with increased dosing. Hypophosphatemia is more likely to result with higher CRRT doses, and has been linked to prolonged ventilator times, rhabdomyolysis, cardiac dysfunction, and even impaired immune function17. There are significant amino acid losses during CRRT, and increased CRRT doses can more than double amino acid loss19. Finally, many antimicrobials are cleared by CRRT, and so increasing the dose without carefully monitoring drug levels can result in underdosing of these medications20. For these reasons, KDIGO guidelines recommend that patients treated with CRRT receive an effluent flow rate of 20–25 mL/kg/hr, and gave the recommendation a 1A rating.
CRRT dosing nationwide often does not comply with KDIGO guidelines. Even when the correct dose of CRRT is prescribed, actual delivered dose can be significantly different19 due to filter clotting, time off for surgery or imaging, or due to nursing models that require dialysis nurses to restart circuits16. Frequent monitoring of dose is necessary; however, nationwide survey results indicate that fewer than 50% of practitioners target a specific dose, and only 15% of patients have regular dose assessments15. At UCH prior to our intervention, only 33% of daily CRRT treatments delivered an average dose in the recommended range. Following interventions which included modification of the EHR to accurately report CRRT dosing, as well as changes to the CRRT procedure note and education of the renal fellows and ICU nurses, the percentage of treatments at goal doubled to 66%. We expect that these changes will be sustained over time, given the structural changes made to the CRRT flowsheet, CRRT order set, and CRRT procedure note, and will continue to monitor results at quarterly quality meetings.
There remain several important barriers to achieving 100% compliance with dosing recommendations. A major hurdle was the difficulty in predicting down time for a particular day. Patients often were temporarily taken off CRRT for OR visits, radiology, or due to circuit clotting. If the provider overestimated or underestimated time off, the dose often fell out of range. Circuit loss, especially, was difficult to anticipate. These difficulties were particularly pronounced in the first 24 hours of therapy. As shown in Table 2, a dose of 20–25 mL/kg/hr was achieved in 66% of patients overall following the intervention, but in only 53% in the first day of CRRT. These data suggest that additional QI interventions, such as to prolong circuit life, are necessary.
Notably, there was a statistically significant decrease in mortality in the post-intervention period of our quality initiative. While this is an encouraging finding, our study was not a randomized-controlled trial, and was not designed to determine the impact of changes in dosing on mortality. There are a myriad number of factors that contribute to mortality in the ICU, and the decreases in mortality are likely the result of a number of process changes in the ICU over an observation period of nearly 2 years. When CRRT dosing has been evaluated in large, controlled studies, no difference in mortality was demonstrated. It is possible that in real-world scenarios, standardized dosing of CRRT impacts mortality by decreasing variability in drug levels or by decreasing rates of hypophosphatemia, but we are unable to draw a conclusion based on this study.
This study has several strengths, including a large number of CRRT treatments evaluated, and a large observation time both before and after intervention implementation. Nevertheless, our work has limitations. Because this was a single-center study, our data may not be generalizable to other institutions, especially institutions with different nursing models for CRRT delivery. Also, the large variety of EMR systems nationwide may dictate the intervention be modified to the institution. In addition, this QI project did not aim to specifically evaluate dosing impacts on outcomes such mortality. Finally, data entered by ICU nurses did not attain 100% accuracy even after training. Future interventions may include direct transfer of data from CRRT machines to the medical record.
In conclusion, an important component of CRRT delivery is ensuring that patients on CRRT receive an average effluent dose of 20–25 mL/kg/hr. Using a series of interventions including changes to the EMR, changes to documentation templates, and specific education of renal fellows, we were able to double our institution’s rate of dosing compliance.
Supplementary Material
Support
The study was funded by a University of Colorado Clinical Effectiveness and Patient Safety Grant for Residents and Fellows (CEPS-RF) award - CEPSRF-2016001. BG is supported by a National Research Service Award (NRSA) Institutional Predoctoral Training Grant (T32), grant number T32 DK 007135. This study was supported in part by The University of Iowa Clinical and Translational Science Award granted with funds from the NIH (UL1TR002537).
None of these funding sources had a role in study design, data collection, analysis, reporting, or the decision to submit for publication.
Footnotes
Financial Disclosures
The authors declare that they have no financial conflicts of interest.
References
- 1.Uchino S, Bellomo R, Goldsmith D, Bates S, Ronco C. An assessment of the RIFLE criteria for acute renal failure in hospitalized patients. Crit Care Med. 2006;34(7):1913–1917. [DOI] [PubMed] [Google Scholar]
- 2.Star RA. Treatment of acute renal failure. Kidney Int. 1998;54(6):18171831. [DOI] [PubMed] [Google Scholar]
- 3.Metnitz PG, Krenn CG, Steltzer H, et al. Effect of acute renal failure requiring renal replacement therapy on outcome in critically ill patients. Crit Care Med. 2002;30(9):2051–2058. [DOI] [PubMed] [Google Scholar]
- 4.du Cheyron D, Bouchet B, Parienti JJ, Ramakers M, Charbonneau P. The attributable mortality of acute renal failure in critically ill patients with liver cirrhosis. Int Care Med. 2005;31(12):1693–1699. [DOI] [PubMed] [Google Scholar]
- 5.Bates DW, Su L, Yu DT, et al. Mortality and costs of acute renal failure associated with amphotericin B therapy. Clin Infect Dis. 2001;32(5):686–693. [DOI] [PubMed] [Google Scholar]
- 6.Coca SG, Peixoto AJ, Garg AX, Krumholz HM, Parikh CR. The prognostic importance of a small acute decrement in kidney function in hospitalized patients: a systematic review and meta-analysis. Am J Kidney Dis. 2007;50(5):712–720. [DOI] [PubMed] [Google Scholar]
- 7.Levy EM, Viscoli CM, Horwitz RI. The effect of acute renal failure on mortality. A cohort analysis. JAMA. 1996;275(19):1489–1494. [PubMed] [Google Scholar]
- 8.Xue JL, Daniels F, Star RA, et al. Incidence and mortality of acute renal failure in Medicare beneficiaries, 1992 to 2001. J Am Soc Nephrol. 2006;17(4):1135–1142. [DOI] [PubMed] [Google Scholar]
- 9.Uchino S, Bellomo R, Morimatsu H, et al. Continuous renal replacement therapy: a worldwide practice survey. The beginning and ending supportive therapy for the kidney (B.E.S.T. kidney) investigators. Int Care Med. 2007;33(9):1563–1570. [DOI] [PubMed] [Google Scholar]
- 10.Palevsky PM, Zhang JH, O’Connor TZ, et al. Intensity of renal support in critically ill patients with acute kidney injury. N Engl J Med. 2008;359(1):7–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Deepa C, Muralidhar K. Renal replacement therapy in ICU. J Anaesthesiol Clin Pharmacol. 2012;28(3):386–396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Claure-Del Granado R, Macedo E, Chertow GM, et al. Effluent volume in continuous renal replacement therapy overestimates the delivered dose of dialysis. Clin J Am Soc Nephrol. 2011;6(3):467–475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Stevens PE, Levin A. Evaluation and management of chronic kidney disease: synopsis of the kidney disease: improving global outcomes 2012 clinical practice guideline. Ann Intern Med. 2013;158(11):825–830. [DOI] [PubMed] [Google Scholar]
- 14.Palevsky PM, Liu KD, Brophy PD, et al. KDOQI US commentary on the 2012 KDIGO clinical practice guideline for acute kidney injury. Am J Kidney Dis. 2013;61:649–672. [DOI] [PubMed] [Google Scholar]
- 15.Koyner JL, Cerda J, Goldstein SL, et al. The daily burden of acute kidney injury: a survey of U.S. nephrologists on World Kidney Day. Am J Kidney Dis. 2014;64(3):394–401. [DOI] [PubMed] [Google Scholar]
- 16.Martin RK. Who should manage continuous renal replacement in the intensive care setting? A nursing viewpoint. EDTNA ERCA J. 2002;Suppl 2:43–45, 53. [DOI] [PubMed] [Google Scholar]
- 17.Bellomo R, Cass A, Cole L, et al. Intensity of continuous renal-replacement therapy in critically ill patients. N Engl J Med. 2009;361(17):1627–1638. [DOI] [PubMed] [Google Scholar]
- 18.Vijayan A, Palevsky PM. Dosing of renal replacement therapy in acute kidney injury. Am J Kidney Dis. 2012;59(4):569–576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Btaiche IF, Mohammad RA, Alaniz C, Mueller BA. Amino Acid requirements in critically ill patients with acute kidney injury treated with continuous renal replacement therapy. Pharmacotherapy. 2008(5);28:600–613. [DOI] [PubMed] [Google Scholar]
- 20.Venkataraman R, Kellum JA, Palevsky P. Dosing patterns for continuous renal replacement therapy at a large academic medical center in the United States. J Crit Care. 2002;17(4):246–250. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.