Abstract
Safety concerns on the toxic and carcinogenic effects of formalin exposure have drawn increasing attention to the search for alternative low risk fixatives for processing tissue specimens in laboratories worldwide. Alcohol-based fixatives are considered some of the most promising alternatives. We evaluated the performance of alcohol-fixed paraffin-embedded (AFPE) samples from Sprague-Dawley (SD) rats analyzing tissue morphology, protein and nucleic acid preservation after short and extremely long fixation times (up to 7 years), using formalin-fixed paraffin-embedded (FFPE) samples as a comparator fixative. Following short and long-term alcohol fixation, tissue morphology and cellular details in tissues, evaluated by scoring stained sections (Hematoxylin-Eosin and Mallory’s trichrome), were optimally preserved if compared to formalin fixation. Immunoreactivity of proteins (Ki67, CD3, PAX5, CD68), evaluated by immunohistochemistry, showed satisfactory results when the fixation period did not exceed 1 year. Finally, we confirm the superiority of alcohol fixation compared to formalin, in terms of quantity of nucleic acid extracted from paraffin blocks, even after an extremely long time of alcohol fixation.
Our results confirm that alcohol fixation is a suitable and safe alternative to formalin for pathological evaluations. There is a need for standardization of formalin-free methods and harmonization of diagnosis in pathology department worldwide.
Keywords: Fixative, Alcohol, Formaldehyde, Pathology, Immunohistochemistry, DNA, RNA
1. Introduction
Chemical fixation is an essential process to prevent tissue autolysis and degradation, while preserving morphology and cellular details for microscopic evaluations. For over a century, 10% solution of formalin (4% formaldehyde), diluted in water or in a buffered solution (Neutral Buffered Formalin, NBF), has been considered the fixative of choice in routine histopathology, thanks to its ability to quickly permeate cell walls and membranes, and to preserve tissue specimens for long periods of time at reasonable cost. Formalin creates covalent bonds between biological macromolecules, and ensures chemical activity and cellular antigenicity conservation in tissues (Warmington et al., 2000). Therefore, formalin-fixed paraffin-embedded (FFPE) tissues currently represent the most common material for clinical research and molecular diagnosis collected in pathology departments worldwide. But formalin does not always represent the “gold standard” fixative when considering other downstream biochemical and molecular analyses. In fact, the positive features of formalin as preservative for histology are counterbalanced by different disadvantages among which some of the most discussed are reduced immunohistochemical reactivity and rapid nucleic acids degradation (Cox et al., 2006; Gillespie et al., 2002; Moelans et al., 2011a, b). The crosslinking mechanism of formalin alters protein folding reducing the overall availability of epitopes that can be bound by antibody, although the degree of epitopes alteration varies with molecular targets (Bogen et al., 2009; Hayat, 2001; O’Leary et al., 2009; Otali et al., 2009; Paavilainen et al., 2010). To overcome this problem, several proteolytic or heat-induced antigen retrieval methods have been recently become available through commercial sources, with the purpose of restoring normal protein folding and to improve epitope accessibility on fixed tissues (Fowler et al., 2011; Paavilainen et al., 2010). To date, these antigen retrieval techniques are considered a standard procedure to achieve high quality staining, even if their mechanisms of action are not well understood. For this reason, antigen retrieval methods remain mainly empirical, requiring the optimization of several critical parameters in a systematic process (Miller et al., 2000).
In addition, the health risks stemming from formaldehyde exposure during tissue processing for preservation, have been widely discussed by many public health agencies for long time and there is increasing awareness of its toxic and carcinogenic properties (National Research Council, 2011). Already in 1987, the U.S. Environmental Protection Agency (EPA) classified formaldehyde as a probable human carcinogen following unusually high or prolonged exposure (US EPA, 1989), resulting in the lowering of acceptable exposure levels a few years later (OSHA, 1992). IARC reported a correlation between formaldehyde exposure and nasopharyngeal carcinoma in human (Cogliano et al., 2005), later supported by the scientific evidence of a link between formaldehyde exposure and leukemia (Baan et al., 2009). It is note-worthy that leukemogenic effects were also observed in our experiments (Soffritti et al., 1989, 2002). These announcements provided the basis for the last evaluation by IARC published in 2012 defining formaldehyde as “carcinogenic to humans” (Group 1) (IARC, 2012).
The recognition of the health risks related to formaldehyde use and the desire to improve diagnostics through molecular techniques, has encouraged the search for a substitute over the years (Buesa, 2008; Srinivasan et al., 2002). Among several alternative fixatives, alcohol-based ones are considered the most promising especially for molecular pathology, as they act by coagulation and do not mask antigenic sites (Stanta et al., 2006). Short fixation time, optimal preservation of DNA, RNA and proteins and a safer workplace environment, are some of the advantages of alcohol-fixation methods (Bostwick et al., 1994; Moelans et al., 2011a). Some disadvantages of alcohol-based fixatives have been reported, including tissue shrinkage and hardening, artifactual pigment deposition and lysis of erythrocytes (Bostwick et al., 1994; Moelans et al., 2011b). In order to overcome these problems, some additives for alcohol-based fixatives have been used over time, as these might improve the quality of histological and molecular analysis (Table 1), but none of them have met a widespread application (Boissière-Michot et al., 2013; Dotti et al., 2010; Lassalle et al., 2009; Masir et al., 2012; Moelans et al., 2011a, b; Stanta et al., 2006).
Table 1.
Summary of scientific studies on alcohol-based fixatives and their suitability on histopathology evaluation.
| References | Fixative | Type of tissue | Techniques | Main results and comments |
|---|---|---|---|---|
| Chung et al. J Histochem Cytochem. (2018); 66(2):121–135 | 10% NBF, 70% ethanol, buffered ethanol (BE70) | Mouse tissues | H&E, IHC, Western Blot, DNA and RNA extraction and quantification, Real Time RT-PCR | Ethanol-based fixatives show a broader time spectrum than NBF, preserving histomorphological features and quantity and quality of biomolecules from paraffin-embedded tissue. |
| Ghoddoosi and Masir J Med Surg Pathol. (2016); 1:2 | NBF, RCL2 diluted in 100% ethanol, RCL2 diluted in 95% ethanol | Benign fresh human surgical specimens | H&E, Perl’s stain, IHC, FISH, SISH, genomic DNA extraction and quantification | RCL2 is potentially a good substitute for formalin. |
| Perry et al. J Histochem Cytochem. (2016); 64(7):425–440 | 10% NBF, 70% ethanol, 70% ethanol + 0.5× phosphate-buffered saline, 70% ethanol + 1% glycerol + 0.5 × PBS, 70% ethanol + 0.5% glacial acetic acid + 0.5 × PBS, 70% ethanol + 1% glycerol + 0.5% glacial acetic acid + 0.5 × PBS, buffered ethanol (BE70) | Mouse tissues | H&E, IHC, Western Blot, DNA and RNA extraction and quantification, Real-Time RT-PCR, Multiplex RT-PCR | BE70 fixative may be a potential replacement for NBF in both research and clinical settings, with the benefit of better biomolecule preservation, without the trade-off of impaired histomorphology. |
| Stefanits H. et al. Clin Neuropathol. (2016); 35(1):3–12 | NBF, RCL2, KINFix | Neurosurgical biopsy specimens | Nuclear Magnetic Resonance (NMR) spectroscopy, H&E, Gomori-Trichrome, Alcian blue, Periodic acid Schiff, IHC, DNA and RNA extraction and quantification, PCR, HPLC-MS/ MS | RCL2 and KINFix offer comparable histomorphology and superior template for molecular analyses than formalin. |
| Belloni B. et al. J Clin Pathol. (2013); 66(2):124–135 | NBF, PAXgene | Human malignant melanoma biopsy specimens | H&E, IHC, DNA and extraction and quantification, qRT-PCR, Real-time RT-PCR, Sanger sequencing | In PAXPE samples, morphology is well preserved but immunohistochemistry requires re-evaluation of markers and staining procedures. PAXPE fixation offers some advantages concerning molecular analysis. |
| Chieco C. et al. Biotech Histochem. (2013); 88(2):109–119 | 1. FAA (formalin, acetic acid, 95% ethanol, distilled water, 10:5:50:35, v/v), FineFIX (70% ethanol concentration) 2. 2.5% glutaraldehyde in 0.1 M phosphate buffer; FineFIX (70% ethanol concentration) | Healthy leaves 1. T. cordata Mill. Tiliaceae, P. avium L. Rosaceae and P. persica (D.C.) C.A. Mey Hamamelidaceae 2. C. australis L. Ulmaceae, U. minor Mill Ulmaceae and G. biloba L. Ginkgoaceae | 1. Light microscopy: Histological staining (toluidine blue) 2. SEM | FineFIX is a viable alternative to formalin for both histological and SEM studies of vegetative plant tissues. |
| Groelz D. et al. Exp Mol Pathol. (2013); 94(1):188–194 | NBF, PAXgene | Rat tissues | H&E, RNA extraction, microcapillary electrophoresis, Real-time RT-PCR | PAXgene preserves histology similarly to formalin, and does not chemically modify RNA. |
| Gündisch S. et al. PLoS One. (2013); 8(3):e60638 | 3.5–3.7% NBF, PAXgene | Non-malignant and malignant human tissue specimens | Western Blot, Two-dimensional SDS-PAGE, ELISA | PAXgene preserves even after prolonged fixation or stabilization times, and is compatible with methods for protein analysis. |
| Milcheva et al. Acta Histochem. (2013); 115(3):279–289 | 10% NBF, ethanol + glacial acetic acid (EtAc), methanol + glacial acetic acid (MetAc) | Mouse tissues | H&E, IHC, RNA and extraction and quantification, quantitative real-time PCR analysis | Alcohol-based fixatives are an excellent tool for storage of tissue samples designed for IHC and mRNA expression studies. |
| Staff S. et al. J Clin Pathol. (2013); 66(9):807–810 | 10% NBF, zinc-based Z7 fixative, RCL2, PAXgene, Allprotect, RNAlater | Human tissue specimens | H&E, IHC, DNA and RNA extraction and quantification, qRT-PCR, Real-time RT-PCR, FISH, CISH | PAXgene provides the best alternative to both liquid nitrogen and formalin, enabling high quality molecular analyses, IHC and sufficient morphological examination. |
| References | Fixative | Type of tissue | Techniques | Main results and comments |
| Masir et al. Histopathology. (2012); 60(5):804–815 | 10% NBF, RCL2 diluted in 100% ethanol, RCL2 in 95% ethanol | Benign fresh surgical human specimens | H&E, Periodic acid-Schiff with diastase, Prussian blue and Masson trichrome, IHC, FISH, SISH, DNA extraction and quantification | RCL2 is a potential formalin substitute suitable as a fixative for use in routine histopathological examination. |
| Turashvili et al. Exp Mol Pathol. (2012); 92(1):33–43 | 10% NBF, Molecular Fixative (MF) | Non-cancerous human tissues | H&E, DNA and RNA extraction and quantification, PCR, RT-PCR | The Molecular Fixative were able to preserve large DNA and RNA fragments in paraffin blocks. |
| Zanini et al. Environ Health. (2012); 11:59 | NBF, Cell-Block, Neo-Fix, RCL2, ZBF, Z7, PAGA, PAGA-T, FineFixx, Carnoy’s fixative, B5 | Human surgical pathology specimens | H&E, Giemsa, trichromic stain, Alcian blue, PAS, IHC, RNA extraction and quantification | These fixatives are suitable for routine use for surgical pathology diagnostic work. |
| Arzt L. et al. Exp Mol Pathol. (2011); 91(2):490–495 | 10% NBF, FineFix, RCL-2, HOPE | Human lung cancer specimens | H&E, RNA extraction and quantification, miRNA amount, real-time RT-PCR analysis | Formalin-free fixatives are in general not superior for RNA studies. |
| References | Fixative | Type of tissue | Techniques | Main results and comments |
| Kap et al. PLoS One. (2011); 6(11):e27704 | 4% NBF, PAXgene | Human surgical pathology specimens | H&E, Periodic acid Schiff, resorcin fuchsin, sirius red, Gomori, IHC, CISH | Results obtained with PAXgene-fixed tissue are comparable to those of formalin-fixed tissue. |
| Kothmaier et al. Arch Pathol Lab Med. (2011); 135(6):744–752 | 10% NBF, FineFIX, RCL2, HOPE | Human lung cancer specimens | H&E, The Movat pentachrome stain, IHC, protein quantification, Western Blot | Formalin-free fixatives have the potential in routine pathology and research to replace formalin in histomorphology and protein preservation. |
| Moelans C.B. et al Am J Clin Pathol. (2011a),2011b; 136(4):548–56 | 4% NBF, F-solv, FineFIX, RCL2 | Fresh surgical human tissue specimens | H&E, periodic acid–Schiff (PAS) without and with diastase (PASD), alcian blue, azan, elastin van Gieson (EvG), and Gordon-Sweet (G&S) and Jones silver stains, IHC | None of the alcohol-based fixatives was comparable overall to NBF with regard to macroscopy, morphologic examination, and immunohistochemical studies. |
| Moelans C.B. et al Am J Clin Pathol. (2011a),2011b; 64(11):960–967 | 4% NBF, F-solv, FineFIX, RCL2 | Fresh surgical human tissue specimens | DNA and RNA extraction and quantification, epidermal growth factor receptor sequence analysis, microsatellite instability (MSI), qPCR, CISH, FISH | FineFIX and RCL2 performed better than F-solv and NBF with regard to DNA and RNA yield, quality and applicability in molecular diagnostics. |
| Dotti et al. Diagn Mol Pathol. (2010); 19(2):112–22 | NBF, methacarn, FineFIX | Cell line-based model | RNA extraction and quantification; rRNA and mRNA integrity, Northern Blot, Real-Time RT-PCR | Alcohol-based fixatives are a good solution for long-term fixation of both cytologic and tissue samples by virtue of their time-independent effects on mRNA preservation. |
| Ergin B. et al. J Proteome Res. (2010); 9(10):5188–5196 | NBF, PAXgene | Mouse tissues, non-malignant human specimens | One-dimensional SDS-PAGE, Western blot, reverse-phase protein microarrays (RPPA), MALDI Imaging MS, RNA extraction and quantification, PCR, electrophoresis | PAXgene has great potential to serve as a novel multimodal fixative for modern pathology, enabling extensive protein biomarker studies on clinical tissue samples. |
| Nykänen and Kuopio Exp Mol Pathol. (2010); 88(2):265–271 | NBF, LN-FIX, FineFIX | Breast cancer cell lines MCF7 (HTB-22) and T-47D (HTB-133) | H&E, IHC, RNA extraction and quantification, Real-Time PCR | Formalin or LN-FIX can be used as a fixative for molecular diagnostics preserving both morphology and nucleic acids; whereas FineFIX proved to be most unsuitable for gene expression analysis. |
| Paavilainen L. et al. J Histochem Cytochem. (2010); 58(3):237–246 | NBF, Glyo-fixx, Zink formalin, FineFIX, HOPE, NEO-FIX, and Zinc-based fixative | Fresh surgical human tissue specimens, cancer cell lines (RT-4, U-251, PC-3) | H&E, tissue microarray, IHC, protein concentration, SDS-PAGE, Western-Blot | Morphological resolution and immunoreactivity were superior in tissues fixed with aldehyde-based fixatives, whereas the use of non-aldehyde–based fixatives can be advantageous in obtaining high protein yield for Western blot analysis. |
| Preusser M. et al. Brain Pathol. (2010); 20(6):1010–1020. | 4.5% NBF, RCL2 | Human brain tumor specimens | H&E, IHC, DNA and RNA extraction, quantification and quality, multiplex PCR, electrophoresis, quantitative MGMT MSP | RCL2 fixation does not seem to significantly compromise histological tumor typing or IHC and preserves nucleic acids at a better quality than formalin fixation. |
| References | Fixative | Type of tissue | Techniques | Main results and comments |
| van Essen H.F. et al. J Clin Pathol. (2010); 63(12):1090–1094 | 4% NBF, RCL2, Boonfix | Fresh human tissue samples | IHC | Tissues fixed in non-crosslinking alcohol based fixatives like RCL2 and Boonfix can successfully be immunohistochemically stained for most antibodies following the usual NBF based protocols. |
| Balbi T. et al.Am J Forensic Med Pathol (2009); 30(3):242–245 | Ethanol-based fixation | Human tissue section | SEM | The details are clearer with respect to those obtainable with formalin fixatives. |
| Lassalle et al. Thyroid. (2009); 19(11):1239–1248 | 10% NBF, Glyo-Fixx, FineFIX, ExcellPlus, RCL2, liquid nitrogen | Human thyroid specimens | H&E, periodic acid Schiff, trichromic Masson, and Sweet-Gordon staining, IHC, DNA, miRNA and RNA extraction, quantification, integrity | All the formalin substitute fixatives tested provided good histomorphologic quality for the different stained thyroid tissues, but individually, some fixatives performed better for immunohistochemical and molecular biological procedures for different thyroid pathologies. |
| Nassiri et al.BMC Clin Pathol. (2008); 29;8:1 | 10% NBF, UMFIX | Human breast cancer specimen | H&E, IHC, FISH, CISH, RNA and DNA extraction, quantification and integrity, PCR | The formalin-free tissue fixation and processing system is a practical platform for evaluation of biomolecular markers in breast cancer and it allows reliable DNA and RNA and protein studies. |
| Lykidis D. et al. Nucleic Acids Res. (2007); 35(12):e85 | NBF, zinc-based Z2 fixative, HOPE, zinc-based Z7, Z8, Z16, Z17, Z18, Z19 fixatives; replacement of zinc solutions with manganese, magnesium, gallium or vanadium solutions; addition of chemicals to the standard zinc-based (Z2) fixation recipe (Z3, Z4, Z5, Z6, Z9, Z10, Z11, Z12, Z13, Z14, Z15) | Mouse tissues | H&E, IHC, DNA and RNA extraction and quantification, PCR, RT-PCR, Real-Time PCR, Real-Time RT-PCR, Two-dimensional (2-D) polyacrylamide gel electrophoresis | Z7 provides significantly improved preservation of DNA, RNA and proteins and allows improved PCR, Real-Time PCR and protein analysis, which may provide an excellent alternative to NBF for contemporary molecular pathobiology research. |
| References | Fixative | Type of tissue | Techniques | Main results and comments |
| Cox M.L. et al. Exp Mol Pathol. (2006); 80(2):183–191 | 10% NBF, modified Davidson’s solution II, 70% ethanol, UMFIX, modified Carnoy’s solution, modified methacarn, Bouin’s solution, PBS, 30% sucrose | Rat tissues | H&E, RNA extraction and quantification, laser capture microdissection, Taqman qRT-PCR | Modified methacarn provided the best results and can be considered a fixative of choice where tissue morphology and RNA integrity are being assessed in the same specimens |
| Delfour C. et al. J Mol Diagn. (2006); 8(2):157–169 | 4% NBF, FAAM, methacarn solution, RCL2 | MCF-7 cells, human breast carcinoma specimens | H&E, IHC, CISH, DNA and RNA extraction, laser capture microdissection, PCR, Real-Time RT-PCR | Methacarn and RCL2 have great potential for performing both morphological and molecular analyses on the same fixed tissue sample, even after laser-capture microdissection |
| Stanta et al. Diagn Mol Pathol. (2006); 15(2):115–123 | FineFIX | Human biopsy or surgery tissues | DNA and RNA extraction, PCR, RT-PCR, Western Blot, two dimensional electrophoresis | FineFIX fixed tissues preserved DNA and RNA better than formalin. Proteins obtained from FineFIX treated samples are amenable and comparable in quality with those obtained from fresh frozen tissues. |
| Nadji M. et al. Appl Immunohistochem Mol Morphol. (2005); 13(3):277–82 | NBF, UMFIX | Human neoplastic and non-neoplastic specimens | IHC | IHC staining results of tissues fixed in UMFIX and processed by the microwave-assisted system are comparable to those obtained on formalin-fixed, similarly processed specimens. |
| Titford and Horenstein Arch Pathol Lab Med. (2005); 129(4):502–506 | NBF, Glyo-Fixx, STF, Omnifix II, Histochoice, Histofix | Human surgical pathology specimens | H&E | Formalin fixation provided the highest histomorphologic quality for tissue stained with hematoxylin-eosin and examined for diagnostic surgical pathology. |
| Uhlig U. et al. Pathol Res Pract. (2004); 200(6):469–472 | NBF, HOPE | Human lung cancer | Western Blot | HOPE fixation maintains the antigenicity of proteins better than formalin fixation. |
| References | Fixative | Type of tissue | Techniques | Main results and comments |
| Soukup J. et al. Neoplasma. (2003); 50(4):300–304 | 10% NBF, 75% ethanol, formalin-ethanol fixation | Human B-cell lymphomas | DNA and RNA extraction, PCR, RT-PCR | The ethanol fixed samples retained a high quality of both DNA and RNA and provided reproducible PCR products similar to frozen samples and significantly better than those extracted from formalin fixed samples |
| Vincek V. et al. Lab Invest. (2003); 83(10):1427–35 | NBF, UMFIX | Mouse tissue, human tissues | H&E, DNA and RNA extraction, PCR, RT-PCR, Real-Time PCR, Protein extraction, Western Blot, histochemistry, IHC | The morphology of UMFIX-exposed tissue was comparable to that fixed in formalin. There were no significant differences between UMFIX-exposed and frozen tissues on PCR, RT-PCR, real-time PCR, and expression microarrays. |
| Gillespie et al. Am J Pathol. (2002);160(2):449–457 | 70% ethanol, 95% ethanol, 70% ethanol: 100% methanol (3:1), 95% ethanol: 100% methanol (3:1), SafeFix, Streck, 10% NBF, Omnifix | Human prostate and kidney specimens | H&E, IHC, One-Dimensional PAGE, immunoblot, Two-Dimensional PAGE, Layered Expression Scanning, DNA and RNA extraction, Agarose Gel Electrophoresis, RT-PCR | 70% ethanol fixation is a useful method for molecular profiling studies. |
| Vince D.G. et al. Anal Cell Pathol. (1997);15(2):119–129. | 10% NBF, Histochoice | Human tissue specimens | IHC | Histochoice produces staining intensity that is comparable, and in many cases superior, to formalin |
| Boon M.E. et al. Pathol Res Pract. (1992); 188(7):832–5 | NBF, Kryofix | Human tissues samples | IHC | All markers studied showed enhanced staining in the Kryofix blocks after 4 hours of fixation, whilst in some cases the immunostaining of the formalin blocks was even negative. |
As the use of specific fixatives remains a crucial point for the optimization of analysis method, fixation times also represent a critical parameter in terms of biomolecule preservation for optimal histomorphological analysis. This aspect has been evaluated by a few authors that have demonstrated that under- or over-fixation of tissues with NBF produces low quality of both immunohistochemical staining (Goldstein et al., 2007; Yaziji et al., 2008) and nucleic acid preservation (Chung et al., 2008). While other studies have reported uncertain results about over-fixation period (De Marzo et al., 2002; Shi et al., 2007; Wester et al., 2000), insufficient data have been collected about minimum tissue fixation times (Dapson, 2007; Kalkman et al., 2014). Chung et al. (2018), analyzed the effects of fixation time comparing crosslinking (NBF) and coagulative (BE70 and 70% ethanol) fixatives, reporting how the latter allows a good preservation of both antigens and nucleic acids, suggesting an optimal fixation period from 4 h to about 3 months. Instead, the authors suggested a fixation window from 12 h to 1 week for NBF, highlighting how the use of NBF, from a technical point of view, is more restricted. The optimization of the fixation time represents a primary feature in developing new high quality fixation protocol for clinical and basic research.
Since 1969, because of the concerns surrounding the safety of formaldehyde, the Ramazzini Institute (RI) is using alcohol-based fixatives to preserve tissue specimens. The decision to substitute formaldehyde and encourage and enforce formaldehyde-free laboratory procedures was, and still is, the only available possibility to protect lab personnel from exposure, although at that time formaldehyde was not yet classified as a carcinogen. In 1984, the RI started also a series of experiments on formaldehyde carcinogenicity. An increased incidence of total malignant tumours, haematopoietic tumours, and interstitial-cell adenomas of the testis were observed in male SD rats (Soffritti et al., 1989, 2002). Moreover, the studies showed an increased incidence of smooth-muscle tumours of the small intestine (leiomyosarcoma) in female SD rats (Soffritti et al., 1989). The results of these experiments, assessed by the IARC, have contributed to the classification of formaldehyde as a Group 1 carcinogen (“carcinogenic to humans”) (IARC, 2012).
To date, the RI has completed nearly 400 cancer bioassays on more than 200 compounds/agents for the identification of exogenous carcinogens, environmental and industrial above all, using about 148,000 SD rats monitored at least until 130 weeks or for the life span until their spontaneous death. The large tissue bank archived in over 40 years of activity, represents an ideal setting for exploring the suitability over time of alcohol-fixed paraffin-embedded (AFPE) samples for morphological and advanced molecular biology analysis. Particularly, in the present study, we evaluated the effects of fixation in alcohol after relatively short time (48 h) up to 6–7 years, on cellular morphology, protein and nucleic acid preservation. The performances of AFPE samples were also compared with the standard 48 h FFPE samples.
2. Materials and methods
2.1. Tissue samples
Spleen, liver and kidney from fourteen SD rats (seven males and seven females) of the colony of the Cesare Maltoni Cancer Research Center (CMCRC) were collected during necropsy (Soffritti et al., 2006). Tissues were obtained from untreated animals of experiments performed by the RI from 2003 to 2013. All these experiments were approved, at the time, by the local scientific ethical committee on animal experimentation (Organismo Preposto al Benessere degli Animali - OPBA) of the RI.
Tissue samples were fixed either in formalin for 48 h (10% NBF) or in 70% alcohol (mixture of ca. 40% ethyl alcohol and ca. 60% isopropyl alcohol; Solvanol, Vital Srl, Italy) diluted in distilled water, for 48 h, 1 week, 1 month, 1–2 years, 4 years, 6–7 years. Following fixation at room temperature, samples were processed according to the standard operating procedures of the CMCRC with 80% (2X), 95% (3X), 100% (3X) alcohol, K-clear (2X) (Kaltek srl, Padova, Italia) and subsequently infiltrated and embedded with paraffin wax. All blocks of fixed tissues were stored at room temperature in the dark until use.
Histochemical and immunohistochemical (IHC) staining Sections of FFPE and AFPE spleen (3 μm thick) were obtained using a rotating microtome (Leica Biosystem, Wetzlar, Germany) and collected on polylysine coated slides.
For histological analysis, oven-dried sections were deparaffinized with xylene and hydrated through a graded series of ethanol (100%, 95% and 80% ethanol) and distilled water. One section of each tissue was routinely stained with Hematoxylin and Eosin (H&E) or Mallory’s Trichrome for basic morphological evaluation. The staining was performed according to RI standard procedures and the sections were histologically verified in blinded fashion by two pathologists independently. Morphology assessment included both overall morphology and nuclear, cytoplasmic and membrane details in the tissues.
For IHC analysis, air dried sections were deparaffinized with xylene and hydrated through a graded series of ethanol (100%, 95% and 80% ethanol) and distilled water. On the basis of our previous work (Panzacchi et al., 2013), paraffin-embedded spleen sections were post-fixed with 10% NBF for 30 min at 4 °C before starting the staining. This process, developed by our group, allows the use of the same standard IHC protocols already in use for FFPE on AFPE samples. After this step, sections were transferred in 70% alcohol for 18–24 hours. Endogenous peroxidase activity was quenched with 3% hydrogen peroxide for 15 min. Slides were placed into a Tissue Tek® container with 1X Antigen Decloaker (pH 6) (Biocare Medical, Pacheco, CA, USA) inside the pan and decloaked for 5 min at 120 °C. Then, non-specific binding sites were blocked with serum obtained from the source species in which the secondary antibody is produced, for 15 min in a humidified chamber (Jackson Immunoresearch Laboratories, Inc., West Grove, PA, USA). Sections were incubated with primary antibody: monoclonal mouse anti-Ki67 (Dakocytomation Corporation, Carpinteria, CA, USA), polyclonal rabbit anti-CD3 (Abcam, Cambridge, MA, USA), polyclonal goat anti-PAX5 (Santa Cruz Biotechnology, Santa Cruz, CA, USA) or monoclonal mouse anti-CD68 (AbD Serotec, Raleigh, NC, USA). The selected target proteins include different cell populations: Ki67 a nuclear protein strictly associated with cell proliferation (Schluter et al., 1993); CD3, a constituent of the T-cell receptor complex (Rehg et al., 2012); PAX5 a transcription factor of the paired-box containing (PAX) family, expressed during early pro B-cell, pre B-cell and mature B-cell stages, but absent in plasma cells (Adams et al., 1992; Barberis et al., 1990; Rehg et al., 2012) and CD68, a glycoprotein associated with lysosomes highly expressed by cells of the monocyte/macrophage lineage (Rehg et al., 2012). Details about the protocols, including primary and secondary antibodies producers, dilutions and incubation time, are reported in Table 2. Positive and negative controls were included for each immunohistochemical analysis.
Table 2.
IHC analysis: technical specification of the primary antibodies used.
| Ab | Cell Marker | Manufacturer / Product N. | Lot # | Isotype | Clonality | Species | Dilution/Time | Secondary Ab | Manufacturer | Product Number | Lot # | Dilution/Time |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Ki67 | Growth fraction of normal and neoplastic cells | DakoCytomation / M7248 | 00070222 | IgG1 | Monoclonal (MIB-5) | Mouse | 1:100 60 min RT | Biotinylated Horse Anti-Mouse IgG (H + L) | Vector Lab | BA-2001 | W1225 | 1:100 30 min RT |
| CD3 | Early T cells | Abcam / ab5690 | GR95294–1 | IgG | Polyclonal | Rabbit | 1:100 60 min RT | Rabbit on Rodent HRP-Polymer Detection | Biocare Medical | RMR622 | 110612 | Ready to Use 30 min RT |
| PAX5 | Pro, pre and mature B cells | Santa Cruz Biotechnology / sc-1974 | 62909 | IgG | Polyclonal(C-20) | Goat | 1:500 60 min RT | Biotinylated Horse Anti-Goat IgG (H + L) | Vector Lab | BA-9500 | V0609 | 1:1000 30 min RT |
| CD68 | Myeloid cells | AbD Serotec / MCA341GA | 170210 | IgG1 | Monoclonal | Mouse | 1:500 15 min RT | Biotinylated Horse Anti-Mouse IgG (H + L) | Vector Lab | BA-2001 | W1225 | 1:1000 30 min RT |
A specific secondary antibody was used for each reaction: biotinylated horse anti-mouse IgG antibody (Vector Laboratories, Burlingame, CA, USA) for Ki67 or CD68 detection; biotinylated horse anti-goat IgG antibody (Vector Laboratories, Burlingame, CA, USA) for PAX5 detection and the rabbit on rodent HRP polymer reagent (Biocare Medical, Concord, CA, USA) for CD3 detection. An incubation with the SS Polymer-HRP Detection System (Biogenex Laboratories, San Ramon, CA, USA) was performed for Ki67, PAX5 and CD68 staining.
Finally, the entire antibody-enzyme complex was then made visible by the reaction with diaminobenzidine (DAB) until adequate color development was seen. Sections were rinsed in distilled water, counterstained with hematoxylin, dehydrated, and cleared in xylene. Mountant and cover slips were applied for optical microscopy analysis. Two pathologists performed the evaluation of the slides independently. Criteria for a sufficient staining were antibody binding specificity, tissue morphology and overall staining quality. Grading of the specific immunoreactivity was based on a four point scale with 0 being missing, 1 being weak, 2 being moderate and 3 being strong. Stars indicate light background (*), moderate background (**), or strong and diffuse background (***).
Extraction, quantification and quality assessment of DNA and RNA Sections (20 μm thick) of FFPE and AFPE kidney and liver tissues were prepared under RNase/DNase free conditions. Sections of each tissue (3 μm thick) were stained with H&E for morphological evaluation. Paraffin sections were deparaffinized employing a simple heating procedure, in the presence of a non-volatile Melting Buffer supplied with the PureLink Kit (Invitrogen, Carlsbad, CA, USA). Proteins were digested with Proteinase K buffer to free nucleic acids and then paraffin was separated by centrifugation. Nucleic Acids (RNA or DNA) in the tissue lysate were captured by selective binding to a silica–based membrane in the Spin Cartridge. Impurities were removed by thorough washing with a Wash Buffer. Total RNA and DNA was eluted in RNase- and DNase-free water. For RNA isolation, removal of any contaminating DNA was accomplished with an off-column digestion step using DNase I (RNase-free) for 10 min at room temperature (Quiagen, Hilden, Germany). For DNA isolation, RNA was digested by incubation with RNase I (Thermo Fisher Scientific, Waltham, Massachusetts, USA) for 30 min at 37 °C. Extracted nucleic acids were quantified by absorbance (NanoDrop ND-1000, Thermo Fisher Scientific, Waltham, Massachusetts, USA) and evaluated for impurities by A260/280 ratio for residual proteins.
3. Results
3.1. Tissue morphology (H&E and Mallory’s Trichrome)
We performed H&E and Mallory’s Trichrome staining on AFPE specimens and compared their histological features according to fixation time. Forty-eight hours FFPE samples served as reference. Morphology of tissues was evaluated by analyzing nuclear features, cytoplasmic and membrane details, tissue architecture and staining characteristics.
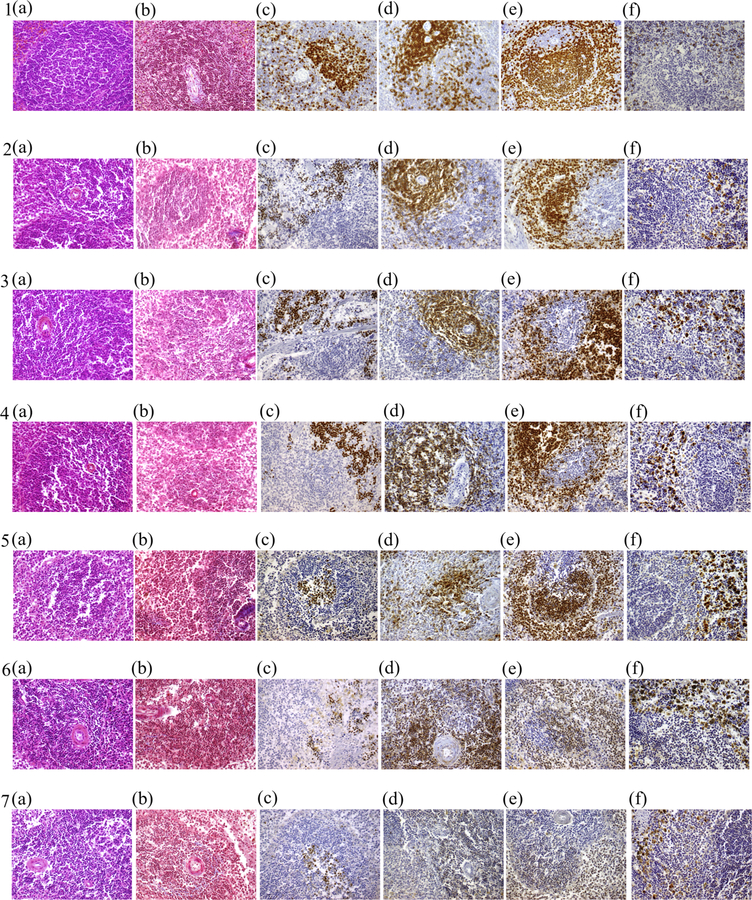
As can be seen in Fig. 1 (1 a,b and 2 a,b), both 48 h NBF and alcohol fixation gave generally comparable and satisfactory results regarding the structural status of tissues and the consequent identification of tissue components. Particularly, tissues were well preserved and all nuclear as well as cytoplasmic details were clearly visible. However, AFPE tissues showed some peculiarity such as shrinkage of tissue components, clarity of cytoplasmic elements, no evidence of eosinophils and red cell lysis. Finally, the overall quality of H&E and Mallory’s Trichrome staining were not altered by fixation time in AFPE samples (Fig. 1: 3 a,b, 4 a,b, 5 a,b, 6 a,b, 7 a,b).
Fig. 1.
Female Sprague-Dawley rat spleen tissues stained with: H&E, Mallory’s Trichrome, Ki67, CD3, PAX5, CD68 (10 X). Tissue samples were fixed either in 10% formalin (NBF) for 48 h (1a – 1f) or in 70% solution of Solvanol (ethyl alcohol 60%, isopropyl alcohol 40% and distilled water), for 48 h (2a – 2f), 1 week (3a – 3f), 1 month (4a – 4f), 1 year (5a – 5f), 4 years (6a – 6f), 7 years (7a – 7f).
3.2. Immunohistochemistry
We assessed the quality of IHC in spleen tissues fixed in alcohol from 48 h to 7 years in order to evaluate antigen preservation. Forty-eight hours FFPE tissues served as reference. IHC staining, performed to evaluate Ki67, CD3, PAX5, CD68 protein expression, has been optimized for each antibody through the choice of the appropriate antigen retrieval methods and the adjustment of the antibody concentration. No discrepancies in subcellular localization of protein expression were observed in the differently fixed samples. The immunostaining pattern observed for Ki67, CD3, PAX5 and CD68 was entirely retained up to 1 year alcohol fixation (Fig. 1: 2 c–f, 3 c–f, 4 c–f, 5 c–f), showing no remarkable differences if compared to the standard 48 h NBF fixation (Fig. 1: 1 c–f). From 4 to 7 years in alcohol fixation, the overall quality of the IHC analysis performed for the above mentioned proteins, gradually decreased, showing from weak to not specific staining (Fig. 1: 6 c–f, 7 c–f). Finally, increasing levels of non-specific background and artifactual pigmentation were observed following 4 year of alcohol fixation (Fig. 1: 6 c–f), with maximum effects following 7 years fixation (Fig. 1: 7 c–f). Grading of the immunoreactivity is summarized in Table 3 and the staining results on female rat tissues are shown in Fig. 1.
Table 3.
IHC analysis. Evaluation of specific immunostaining intensity obtained with rabbit polyclonal antibody against CD3, goat polyclonal antibody against PAX5, mouse monoclonal antibody against CD68 and mouse monoclonal antibody against Ki67 on Sprague-Dawley rat spleen tissues according to different time of fixation. Grading was 0 (missing), 1 (weak), 2 (moderate) and 3 (strong).
| Antibodies |
||||||
|---|---|---|---|---|---|---|
| Fixation time | Fixative | Sex | Ki67 | PAX5 | CD3 | CD68 |
| 48 hours | NBF | M | 3 | 3 | 3 | 3 |
| 48 hours | F | 3 | 3 | 3 | 3 | |
| 48 hours | Alcohol | M | 3 | 3 | 3 | 3 |
| 48 hours | F | 3 | 3 | 3 | 3 | |
| 1 week | Alcohol | M | 3 | 3 | 3 | 3 |
| 1 week | F | 3 | 3 | 3 | 3 | |
| 1 month | Alcohol | M | 3 | 3 | 3 | 3 |
| 1 month | F | 3 | 3 | 3 | 3 | |
| 2 years | Alcohol | M | 2* | 1** | 0*** | 3* |
| 1 year | F | 2* | 1* | 0*** | 2* | |
| 4 years | Alcohol | M | 1** | 0* | 0*** | 2** |
| 4 years | F | 1** | 0* | 0** | 2** | |
| 6 years | Alcohol | M | 0*** | 0* | 0*** | 2** |
| 7 years | F | 0*** | 0* | 0** | 2** | |
The stars indicate light background (*), moderate background (**), or strong and diffuse background (***).
3.3. DNA and RNA content and quality
We investigated the quantity and quality of nucleic acids extracted from AFPE tissues according to fixation times. DNA and RNA were successfully extracted from all fixed rat kidney and liver tissues. Results of the AFPE tissues were compared to the standard 48 h NBF fixation. Extracted nucleic acids were quantified by absorbance and evaluated for impurities by A260/280 ratios for residual proteins. Results are reported in Table 4.
Table 4.
Spectrophotometric measurement of extracted nucleic acids.
| RNA extraction |
DNA extraction |
||||||
|---|---|---|---|---|---|---|---|
| Tissue | Fixation Time | Sex | Fixative | Concentration (ng/μl) | A260/280 | Concentration (ng/μl) | A260/280 |
| Liver | 48 hours | M | NBF | 60,3 | 1,90 | 73,3 | 2,00 |
| 48 hours | F | NBF | 59,9 | 1,94 | 115,4 | 2,01 | |
| 48 hours | M | Alcohol | 123,6 | 2,01 | 250,3 | 2,00 | |
| 48 hours | F | Alcohol | 177,6 | 2,01 | 395,2 | 1,97 | |
| 1 week | M | Alcohol | 308,1 | 1,99 | 392,1 | 1,99 | |
| 1 week | F | Alcohol | 270,2 | 2,01 | 388,7 | 1,98 | |
| 1 month | M | Alcohol | 311,7 | 1,99 | 406,0 | 1,97 | |
| 1 month | F | Alcohol | 248,5 | 2,02 | 429,9 | 1,97 | |
| 2 years | M | Alcohol | 374,5 | 1,94 | 513,1 | 1,92 | |
| 1 year | F | Alcohol | 399,4 | 1,96 | 494,3 | 1,95 | |
| 4 years | M | Alcohol | 531,9 | 1,99 | 534,5 | 1,92 | |
| 4 years | F | Alcohol | 364,3 | 1,94 | 518,9 | 1,94 | |
| 6 years | M | Alcohol | 776,6 | 2,01 | 326,4 | 1,95 | |
| 7 years | F | Alcohol | 300,9 | 1,98 | 499,2 | 1,92 | |
| RNA extraction |
DNA extraction |
||||||
| Tissue | Fixation Time | Sex | Fixative | Concentration (ng/μl) | A260/280 | Concentration (ng/μl) | A260/280 |
| Kidneys | 48 hours | M | NBF | 59,60 | 1.92 | 357,6 | 2.01 |
| 48 hours | F | NBF | 135,3 | 2.00 | 221,8 | 2.00 | |
| 48 hours | M | Alcohol | 266,8 | 1.95 | 347,3 | 1.93 | |
| 48 hours | F | Alcohol | 141,4 | 1.93 | 201,1 | 1.95 | |
| 1 week | M | Alcohol | 312,6 | 1.91 | 322,3 | 1.93 | |
| 1 week | F | Alcohol | 338,4 | 1.81 | 315,2 | 1.88 | |
| 1 month | M | Alcohol | 328,6 | 1.92 | 216,2 | 1.94 | |
| 1 month | F | Alcohol | 210,2 | 1.91 | 251,6 | 1.93 | |
| 2 years | M | Alcohol | 342,6 | 1.87 | 384,4 | 1.91 | |
| 1 year | F | Alcohol | 384,5 | 1.86 | 153,0 | 1.93 | |
| 4 years | M | Alcohol | 327,3 | 1.87 | 366,6 | 1.90 | |
| 4 years | F | Alcohol | 355,6 | 1.88 | 115,6 | 1.93 | |
| 6 years | M | Alcohol | 394,2 | 1.85 | 220,4 | 1.92 | |
| 7 years | F | Alcohol | 628,3 | 1.91 | 216,1 | 1.91 | |
Absorbance of each extracted sample was measured by spectrophotometer (Nanodrop ND-1000) and nucleic acid sample purity assessed by 260 nm/280 nm (A260/ 280) ratio. Values of about 2.0 are considered as optimal for RNA. Values of about 1.8 or greater are considered as optimal for DNA.
DNA extraction yield was similar in all AFPE tissues regardless of fixation periods, and was higher than the standard 48 h NBF fixation. The A260/280 ratio of all AFPE tissues was similar to that of 48 h NBF. Likewise, RNA extraction yield from AFPE tissues did not undergo substantial changes from 48 h until 7 years of fixation. Particularly, the RNA recovery yield from AFPE tissues was higher than the standard 48 h FFPE, regardless of fixation period. The A260/280 ratio of all AFPE specimens was similar to that of 48 h NBF.
Results confirmed that alcohol fixation for up to 7 years was superior to 48 h NBF fixation in terms of quantity of DNA and RNA retrieved and comparable in terms of purity.
4. Discussion
Safety concerns regarding the health risks connected to formaldehyde exposure have motivated health care facilities (e.g. hospitals) and researchers to adopt alternative solutions in order to reduce staff exposure. In particular, pathology units are at high risk of exposure for the frequent use of NBF during several processes and activities (IARC, 2012). Diffusion of technical guidelines for the handling, storage, transportation and disposal of formaldehyde, together with the adoption of precautionary measures such as personal equipment, represents some of the tools used to protect personnel. Despite its widespread use, from a technical point of view, the choice of formalin for tissue fixation in pathology laboratories worldwide is not justified by its superior performances, but rather stems from the need to harmonize the diagnostic criteria (Zanini et al., 2012). Formalin, in fact, like all chemical fixatives, elicits protein modifications and hampers the extraction of intact nucleic acids (Bogen et al., 2009; Cox et al., 2006; Gillespie et al., 2002; Hayat, 2001; Moelans et al., 2011a; O’Leary et al., 2009; Otali et al., 2009). Therefore, the scientific community is focused on the search for alternative fixatives to substitute formalin, being alcohol-based fixatives some of the most promising candidates.
In this perspective, we evaluated the performance of AFPE samples from SD rats analyzing tissue morphology, protein and acid nucleic preservation after short and extremely long fixation times (up to 7 years), using FFPE samples as a comparator. Our study clearly shows that morphology of tissues following short and long-term alcohol-based fixation is optimally preserved, and tissues are suitable for most histological purposes. In fact, both H&E and Mallory’s Trichrome staining gave optimal overall results in terms of intensity and in cytoplasmic and nuclear detail definition in all the tested conditions. Indeed prolonged alcohol fixation, from 4 up to 7 years, is associated with a slightly lower score of the quality of tissue morphology, however this does not seem to interfere with H&E and Mallory’s Trichrome evaluation. Thus, from the point of view of morphological analysis, tissue can be stored in alcohol fixative for extremely long periods of time. This is not the case for the “gold standard” NBF, as emphasized in the work of Chung et al., (2018) in which scientists reported a progressive decrease of H&E staining intensity following 1 week to 6 months fixation. Finally, we observed some artifactual changes that have been previously described in alcohol-based fixed tissues, in particular shrinkage and hardening effects independently by fixation times (Perry et al., 2016). However, the degree of these alterations did not significantly influence the establishment of a proper diagnosis in our samples.
In carcinogenesis studies, the use of IHC markers are crucial to distinguish clonal expansions typical of malignant tumours and to determine specific tumour origin/type or progression of given neoplasm (Painter et al., 2010; Rehg et al., 2012). Thus, IHC represents an effective tool for protein patterns distribution analysis both in normal and pathological tissues and provide an excellent method to confirm diagnosis that might be subjected to inter- and intra-observer variability, particularly in border-line lesions. Scientific studies argue that alcohol based fixatives act by precipitation of proteins, which do not mask their antigenicity and make the antigen retrieval on slide unnecessary (Burns et al., 2009; Howat and Wilson, 2014; Kap et al., 2011; Nassiri et al., 2008; van Essen et al., 2010). This topic has been extensively evaluated in our previous work on the development of a standardized IHC method on AFPE tissue (Panzacchi et al., 2013). It is true that the immunoreactivity of membrane or cytoplasmic proteins, such as CD3 and CD68, unlike nuclear antigens, on alcohol-fixed tissues, could be evaluated according to standard IHC staining protocols. But in order to improve the immune reaction of AFPE tissues, in some cases a post-fixation in 30 min NBF before performing IHC staining on AF tissue might still be necessary (Kothmaier et al., 2011). The improvement of the antibody efficiency and of the overall IHC staining following NBF post-fixation step could be explained by the fact that commercially available antibodies have been developed and selected to be applied on formalin-fixed tissues. To date antibodies are not routinely tested by the manufacturers on alcohol-based fixed tissue and, for this reason, the producers do not guarantee a successful IHC staining on AFPE. Nevertheless, our results demonstrated that different antibodies are highly compatible with alcohol fixation and indeed the subcellular distribution of the corresponding proteins is preserved in the experimental conditions. The only limit of IHC staining following alcohol fixation might be represented by an extremely long fixation time. Tissues dwelled in alcohol for a maximum of 1 year reacts promptly with the antibodies; whereas, after 4 year fixation, a gradual decrease of staining intensity levels or absence of immune reactivity were observed. Moreover, as fixation time increase, non-specific background and artifactual pigmentation were more evident, complicating an accurate evaluation of protein expression by pathologists.
In the last few years, advanced molecular technologies, requiring high-quality nucleic acids, have been developed to support pathologists in diagnosis. As already reported in the scientific literature, the recovery of nucleic acids from FFPE tissues remains challenging, with low recovery and poor quality (Chung et al., 2018). Our study confirms the superiority of alcohol fixation compared to NBF, in terms of quantity and purity of nucleic acid extracted from paraffin blocks, even after an extremely long time of alcohol fixation (up to 7 years). A recent work of Chung et al. (2018) demonstrated that nucleic acid integrity is well-preserved from 1 to 6 months following 70% ethanol-fixation, while a rapid fragmentation is observed following NBF-fixation. Moreover, previous studies showed that nucleic acids extracted from AFPE are of high quality and slightly fragmented (Dotti et al., 2010; Giannella et al., 1997; Gillespie et al., 2002; Milcheva et al., 2013; Moelans et al., 2011a; Noguchi et al., 1997; Perry et al., 2016; Srinivasan et al., 2002). Because of the encouraging results on the quality and quantity of macromolecules preserved in AFPE tissues presented here, our next steps will be to explore deeply other parameters as DNA and RNA integrity, and to evaluate microRNA yield (Klopfleisch et al., 2011).
Our results reinforce the increasing available scientific evidences on low risk chemical compounds, such as alcohol, with optimal results in terms of tissue fixation. In light of the risks connected with the use of formaldehyde, no scientific reasons exist to justify the extensive use of formalin for processing tissue specimens in clinical and research laboratories. Standardization of formalin-free methods and harmonization of diagnosis in pathology department worldwide, should urgently aim to formalin substitution and the development of safer alternative protocols (Bostwick et al., 1994). Occupational health authorities throughout the world have introduced stricter limits and regulations to formalin use to protect workers and citizens. But the most effective form of prevention, that any pathology laboratory in the world can enforce, is the avoidance of any unnecessary use of formalin and its substitution with safer and cheap alternatives, such as alcohol-based fixatives.
Acknowledgements
Authors would like to thank the National Institute of Environmental Health Sciences (NIEHS) for the technical support and hospitality at the Research Triangle Park Laboratory of Dr. Simona Panzacchi in order to finalize this project. The Authors are grateful to Mrs. Luana De Angelis for her precious professional support.
Funding statement
The authors disclosed receipt of the following financial support for the research, authorship, and/or publication of this article. This study was supported by: the Ramazzini Institute, Bologna, Italy; Associazione Federide, Bologna, Italy; Lions Club Minerva Minerbio, Bologna, Italy.
Footnotes
Declaration of Competing Interest
The authors report no conflict of interest. The authors alone are responsible for the content and writing of the paper. The authors also declare that their funding sources had no direct role in the study design, data collection, analysis and interpretation of the data, in the writing of the manuscript, or in the decision to publish the work.
References
- Adams B, Dorfler P, Aguzzi A, Kozmik Z, Urbánek P, Maurer-Fogy I, Busslinger M, 1992. Pax-5 encodes the transcription factor BSAP and is expressed in B lymphocytes, the developing CNS, and adult testis. Genes Dev. 6, 1589–1607. 10.1101/gad.6.9.1589. [DOI] [PubMed] [Google Scholar]
- Arzt L, Kothmaier H, Quehenberger F, Halbwedl I, Wagner K, Maierhofer T, Popper HH, 2011. Evaluation of formalin-free tissue fixation for RNA and microRNA studies. Exp. Mol. Pathol 91 (2), 490–495. 10.1016/j.yexmp.2011.05.007. [DOI] [PubMed] [Google Scholar]
- Baan R, Grosse Y, Straif K, Secretan B, El Ghissassi F, Bouvard V, Benbrahim-Tallaa L, Guha N, Freeman C, Galichet L, Cogliano V, WHO International Agency for Research on Cancer Monograph Working Group, 2009. A review of human carcinogens-Part F: chemical agents and related occupations. Lancet Oncol. 10, 1143–1144. [DOI] [PubMed] [Google Scholar]
- Barberis A, Widenhorn K, Vitelli L, Busslinger M, 1990. A novel B-cell lineage-specific transcription factor present at early but not late stages of differentiation. Genes Dev. 4, 849–859. 10.1101/gad.4.5.849. [DOI] [PubMed] [Google Scholar]
- Balbi T, Cicognani A, Esposti PD, Pierini G, 2009. Microwave processing and ethanol-based fixation in forensic pathology: an addendum of further scanning electron microscope observations. Am. J. Forensic Med. Pathol 30 (3), 242–245. 10.1097/PAF.0b013e31819d222e. [DOI] [PubMed] [Google Scholar]
- Belloni B, Lambertini C, Nuciforo P, Phillips J, Bruening E, Wong S, Dummer R, 2013. Will PAXgene substitute formalin? A morphological and molecular comparative study using a new fixative system. J. Clin. Pathol 66 (2), 124–135. 10.1136/jclinpath-2012-200983. [DOI] [PubMed] [Google Scholar]
- Bogen SA, Vani K, Sompuram SR, 2009. Molecular mechanisms of antigen retrieval: antigen retrieval reverses steric interference caused by formalin-induced cross-links. Biotech. Histochem 84 (5), 207–215. 10.3109/10520290903039078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boissière-Michot F, Denouël A, Boulle N, Guillaume C, Orsetti B, Lopez-Crapez E, Chateau MC, Bibeau F, 2013. The non-crosslinking fixative RCL2®-CS100 is compatible with both pathology diagnosis and molecular analyses. Pathol. Oncol. Res 19 (1), 41–53. 10.1007/s12253-012-9556-2. [DOI] [PubMed] [Google Scholar]
- Bostwick DG, al Annouf N, Choi C, 1994. Establishment of the formalin-free surgical pathology laboratory. Utility of an alcohol-based fixative. Arch. Pathol. Lab. Med 118 (3), 298–302. [PubMed] [Google Scholar]
- Boon ME, Schmidt U, Cramer-Knijnenburg GI, van Krieken JH, 1992. Using Kryofix as alternative for formalin results in more optimal and standardized immunostaining of paraffin sections. Pathol. Res. Pract 188 (7), 832–835. 10.1016/S0344-0338(11)80240-2. [DOI] [PubMed] [Google Scholar]
- Buesa RJ, 2008. Histology without formalin? Ann. Diagn. Pathol 12, 387–396. 10.1016/j.anndiagpath.2008.07.004. [DOI] [PubMed] [Google Scholar]
- Burns JA, Li Y, Cheney CA, Ou Y, Franlin-Pfeifer LL, Kuklin N, Zhang ZQ, 2009. Choice of fixative is crucial to successful immunohistochemical detection of phosphoproteins in paraffin-embedded tumor tissues. J. Histochem. Cytochem 57 (3), 257–264. 10.1369/jhc.2008.952911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung JY, Song JS, Ylaya K, Sears JD, Choi L, Cho H, Rosenberg AZ, Hewitt SM, 2018. Histomorphological and molecular assessments of the fixation times comparing formalin and ethanol-based fixatives. J. Histochem. Cytochem 66 (2), 121–135. 10.1038/s41598-018-30582-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung JY, Braunschweig T, Williams R, Guerrero N, Hoffmann KM, Kwon M, Song YK, Libutti SK, Hewitt SM, 2008. Factors in tissue handling and processing that impact RNA obtained from formalin-fixed, paraffin-embedded tissue. J. Histochem. Cytochem 56 (11), 1033–1042. 10.1369/jhc.2008.951863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cogliano VJ, Grosse Y, Baan RA, Straif K, Secretan MB, El Ghissassi F, 2005. Meeting report: summary of IARC monographs on formaldehyde, 2-butoxyethanol, and 1-tert-butoxy-2-propanol. Environ. Health Perspect 113, 1205–1208 10.1289/ehp.7542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox ML, Schray CL, Luster CN, Stewart ZS, Korytko PJ, Khan KN, Paulauskis JD, Dunstan RW, 2006. Assessment of fixatives, fixation, and tissue processing on morphology and RNA integrity. Exp. Mol. Pathol 80 (2), 183–191. 10.1016/j.yexmp.2005.10.002. [DOI] [PubMed] [Google Scholar]
- Dapson RW, 2007. Macromolecular changes caused by formalin fixation and antigen retrieval. Biotech. Histochem 82 (3), 133–140. 10.1080/10520290701567916. [DOI] [PubMed] [Google Scholar]
- De Marzo AM, Fedor HH, Gage WR, Rubin MA, 2002. Inadequate formalin fixation decreases reliability of p27 immunohistochemical staining: probing optimal fixation time using high-density tissue microarrays. Hum. Pathol 33 (7), 756–760. 10.1053/hupa.2002.126187. [DOI] [PubMed] [Google Scholar]
- Delfour C, Roger P, Bret C, Berthe ML, Rochaix P, Kalfa N, Raynaud P, Bibeau F, Maudelonde T, Boulle N, 2006. RCL2, a new fixative, preserves morphology and nucleic acid integrity in paraffin-embedded breast carcinoma and microdissected breast tumor cells. J. Mol. Diagn 8 (2), 157–169. 10.2353/jmoldx.2006.050105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dotti I, Bonin S, Basili G, Nardon E, Balani A, Siracusano S, Zanconati F, Palmisano S, De Manzini N, Stanta G, 2010. Effects of formalin, methacarn, and fineFIX fixatives on RNA preservation. Diagn. Mol. Pathol 19 (2), 112–122. 10.1097/PDM.0b013e3181b520f8. [DOI] [PubMed] [Google Scholar]
- Ergin B, Meding S, Langer R, Kap M, Viertler C, Schott C, Ferch U, Riegman P, Zatloukal K, Walch A, Becker KF, 2010. Proteomic analysis of PAXgene-fixed tissues. J. Proteome Res 9 (10), 5188–5196. 10.1021/pr100664e. [DOI] [PubMed] [Google Scholar]
- Fowler CB, Evers DL, O’Leary TJ, Mason JT, 2011. Antigen retrieval causes protein unfolding evidence for a linear epitope model of recovered immunoreactivity. J. Histochem. Cytochem 59 (4), 366–381. 10.1369/0022155411400866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghoddoosi M, Masir N, 2016. RCL2: A potential formalin substitute for tissue fixation in routine pathological specimens. J. Med. Surg. Pathol 1, 2 10.4172/jmsp.1000112. [DOI] [PubMed] [Google Scholar]
- Giannella C, Zito FA, Colonna F, Paradiso A, Marzullo F, Alaibac M, Schittulli F, 1997. Comparison of formalin, ethanol, and Histochoice fixation on the PCR amplification from paraffin-embedded breast cancer tissue. Eur. J. Clin. Chem. Clin. Biochem 35, 633–635. [PubMed] [Google Scholar]
- Gillespie JW, Best CJ, Bichsel VE, Cole KA, Greenhut SF, Hewitt SM, Ahram M, Gathright YB, Merino MJ, Strausberg RL, Epstein JI, Hamilton SR, Gannot G, Baibakova GV, Calvert VS, Flaig MJ, Chuaqui RF, Herring JC, Pfeifer J, Petricoin EF, Linehan WM, Duray PH, Bova GS, Emmert-Buck MR, 2002. Evaluation of non-formalin tissue fixation for molecular profiling studies. Am. J. Pathol 160 (2), 449–457. 10.1016/S0002-9440(10)64864-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein NS, Hewitt SM, Taylor CR, Taylor CR, Yaziji H, Hicks DG, Members of Ad-Hoc Committee On Immunohistochemistry Standardization, 2007. Recommendations for improved standardization of immunohistochemistry. Appl. Immunohistochem. Mol. Morphol 15 (2), 124–133. 10.1097/PAI.0b013e31804c7283. [DOI] [PubMed] [Google Scholar]
- Groelz D, Sobin L, Branton P, Compton C, Wyrich R, Rainen L, 2013. Non-formalin fixative versus formalin-fixed tissue: a comparison of histology and RNA quality. Exp. Mol. Pathol 4 (1), 188–194. 10.1016/j.yexmp.2012.07.002. [DOI] [PubMed] [Google Scholar]
- Gündisch S, Schott C, Wolff C, Tran K, Beese C, Viertler C, Zatloukal K, Becker KF, 2013. The PAXgeneü Tissue System Preserves Phosphoproteins in Human Tissue Specimens and Enables Comprehensive Protein Biomarker Research. Plos One 8 (3), e60638 10.1371/journal.pone.0060638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayat MA, 2001. Microscopy, Immunohistochemistry, and Antigen Retrieval Methods: Light and Electron Microscopy. Kluwer Academic/Plenum Publishers, New York, Boston, Dordrecht, London, Moscow. [Google Scholar]
- Howat WJ, Wilson BA, 2014. Tissue fixation and the effect of molecular fixatives on downstream staining procedures. Methods. 70 (1), 12–19. 10.1016/j.ymeth.2014.01.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- IARC, 2012. Monographs on the evaluation of carcinogenic risks to humans volume 100F: a review of human carcinogens: chemical agents and related occupations. Michael Addit. Formaldehyde -dialkylhydrazones Β Γ-unsaturated Β-oxo Esters 2012
- Kalkman S, Barentsz MW, Witkamp AJ, van der Wall E, Verkooijen HM, van Diest PJ, 2014. Brief fixation does not affect assessment of hormone receptor expression in invasive breast carcinoma biopsies: paving the road for same-day tissue diagnostics. Am. J. Surg. Pathol. 38 (8), 1071–1078. 10.1097/PAS.0000000000000207. [DOI] [PubMed] [Google Scholar]
- Kap M, Smedts F, Oosterhuis W, Winther R, Christensen N, Reischauer B, Viertler C, Groelz D, Becker KF, Zatloukal K, Langer R, Slotta-Huspenina J, Bodo K, de Jong B, Oelmuller U, Riegman P, 2011. Histological assessment of PAXgene tissue fixation and stabilization reagents. PLoS One 6 (11), e27704 10.1371/journal.pone.0027704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klopfleisch R, Weiss AT, Gruber AD, 2011. Excavation of a buried treasure-DNA, mRNA, miRNA and protein analysis in formalin fixed, paraffin embedded tissues. Histol. Histopathol 26 (6), 797–810 https://doi:10.14670/HH-26.797. [DOI] [PubMed] [Google Scholar]
- Kothmaier H, Rohrer D, Stacher E, Quehenberger F, Becker KF, Popper HH, 2011. Comparison of formalin-free tissue fixatives: a proteomic study testing their application for routine pathology and research. Arch. Pathol. Lab. Med 135 (6), 744–752. 10.1043/2009-0676-OA.1. [DOI] [PubMed] [Google Scholar]
- Lassalle S, Hofman V, Marius I, Gavric-Tanga V, Brest P, Havet K, Butori C, Selva E, Santini J, Mograbi B, Hofman P, 2009. Assessment of morphology, antigenicity, and nucleic acid integrity for diagnostic thyroid pathology using formalin substitute fixatives. Thyroid. 19 (11), 1239–1248. 10.1089/thy.2009.0095. [DOI] [PubMed] [Google Scholar]
- Lykidis D, Van Noorden S, Armstrong A, Spencer-Dene B, Li J, Zhuang Z, Stamp GW, 2007. Novel zinc-based fixative for high quality DNA, RNA and protein analysis. Nucleic Acids Res. 35 (12), e85 10.1093/nar/gkm433 Epub 2007 Jun 18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masir N, Ghoddoosi M, Mansor S, Abdul-Rahman F, Florence CS, Mohamed-Ismail NA, Tamby MR, Md-Latar NH, 2012. RCL2, a potential formalin substitute for tissue fixation in routine pathological specimens. Histopathology. 60 (5), 804–815. 10.1111/j.1365-2559.2011.04127.x. [DOI] [PubMed] [Google Scholar]
- Milcheva R, Janega P, Celec P, Russev R, Babál P, 2013. Alcohol based fixatives provide excellent tissue morphology, protein immunoreactivity and RNA integrity in paraffin embedded tissue specimens. Acta Histochem. 115 (3), 279–289. 10.1016/j.acthis.2012.08.002. [DOI] [PubMed] [Google Scholar]
- Miller RT, Swanson PE, Wick MR, 2000. Fixation and epitope retrieval in diagnostic immunohistochemistry: a concise review with practical considerations. Appl. Immunohistochem. Mol. Morphol 8 (3), 228–235. [DOI] [PubMed] [Google Scholar]
- Moelans CB, Oostenrijk D, Moons MJ, van Diest PJ, 2011a. Formaldehyde substitute fixatives: effects on nucleic acid preservation. J. Clin. Pathol 64 (11), 960–967. 10.1136/jclinpath-2011-200152. [DOI] [PubMed] [Google Scholar]
- Moelans CB, ter Hoeve N, van Ginkel JW, ten Kate FJ, van Diest PJ, 2011b. Formaldehyde substitute fixatives. Analysis of macroscopy, morphologic analysis, and immunohistochemical analysis. Am. J. Clin. Pathol 136 (4), 548–556. 10.1309/AJCPHH1B0COCBGOM. [DOI] [PubMed] [Google Scholar]
- Nadji M, Nassiri M, Vincek V, Kanhoush R, Morales AR, 2005. Immunohistochemistry of tissue prepared by a molecular-friendly fixation and processing system. Appl. Immunohistochem. Mol. Morphol 13 (3), 277–282 [DOI] [PubMed] [Google Scholar]
- Nassiri M, Ramos S, Zohourian H, Vincek V, Morales AR, Nadji M, 2008. Preservation of biomolecules in breast cancer tissue by a formalin-free histology system. BMC Clin. Pathol 29 (8), 1 10.1186/1472-6890-8-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- National Research Council, 2011. Review of the Environmental Protection Agency’s Draft IRIS Assessment of Formaldehyde. The National Academies Press, Washington, DC: 10.17226/13142. [DOI] [Google Scholar]
- Noguchi M, Furuya S, Takeuchi T, Hirohashi S, 1997. Modified formalin and methanol fixation methods for molecular biological and morphological analyses. Pathol. Int 47, 685–691. 10.1111/j.1440-1827.1997.tb04442.x. [DOI] [PubMed] [Google Scholar]
- Nykänen M, Kuopio T, 2010. Protein and gene expression of estrogen receptor alpha and nuclear morphology of two breast cancer cell lines after different fixation methods. Exp Mol Pathol. 88 (2), 265–271. 10.1016/j.yexmp.2009.12.003. [DOI] [PubMed] [Google Scholar]
- O’Leary TJ, Fowler CB, Evers DL, Mason JT, 2009. Protein fixation and antigen retrieval: chemical studies. Biotech. Histochem 84 (5), 217–221. 10.3109/10520290903039086. [DOI] [PubMed] [Google Scholar]
- OSHA, 1992. Occupational exposure to Formaldehyde: response to Court remand; final rule. 29 CFR 1910. Fed. Regist 1992 (50), 22290–22328. [PubMed] [Google Scholar]
- Otali D, Stockard CR, Oelschlager DK, Wan W, Manne U, Watts SA, Grizzle WE, 2009. The combined effects of formalin fixation and individual steps in tissue processing on immuno-recognition. Biotech. Histochem 84 (5), 223–247. 10.3109/10520290903039094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paavilainen L, Edvinsson A, Asplund A, Hober S, Kampf C, Pontén F, Wester K, 2010. The impact of tissue fixatives on morphology and antibody-based protein profiling in tissues and cells. J. Histochem. Cytochem 58 (3), 237–246. 10.1369/jhc.2009.954321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Painter JT, Clayton NP, Herbert RA, 2010. Useful immunohistochemical markers of tumor differentiation. Toxicol. Pathol 38, 131–141 https://doi:10.1177/0192623309356449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panzacchi S, Boiani S, Mandrioli D, et al. , 2013. Applying immunohistochemistry to alcohol-fixed paraffin-embedded tissues: an innovative technique to reduce use of Formaldehyde. Eur. J. Oncol 18 (2), 75–83. [Google Scholar]
- Perry C, Chung JY, Ylaya K, Choi CH, Simpson A, Matsumoto KT, Smith WA, Hewitt SM, 2016. A buffered alcohol-based fixative for histomorphologic and molecular applications. J. Histochem. Cytochem 64 (7), 425–440 https://doi:10.1369/0022155416649579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rehg JE, Bush D, Ward JM, 2012. The utility of immunohistochemistry for the identification of hematopoietic and lymphoid cells in normal tissues and interpretation of proliferative and inflammatory lesions of mice and rats. Toxicol. Pathol 40 (2), 345–374 https://doi:10.1177/0192623311430695. [DOI] [PubMed] [Google Scholar]
- Schluter C, Duchrow M, Wohlenberg C, Becker MH, Key G, Flad HD, Gerdes J, 1993. The cell proliferation-associated antigen of antibody Ki-67: a very large, ubiquitous nuclear protein with numerous repeated elements, representing a new kind of cell cycle-maintaining proteins. J. Cell Biol 123 (3), 513–522. 10.1083/jcb.123.3.513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi SR, Liu C, Taylor CR, 2007. Standardization of immunohistochemistry for formalin-fixed, paraffin-embedded tissue sections based on the antigen-retrieval technique: from experiments to hypothesis. J. Histochem. Cytochem 55 (2), 105–109 https://doi:10.1369/jhc.6P7080.2006. [DOI] [PubMed] [Google Scholar]
- Soffritti M, Belpoggi F, Degli Esposti D, 2006. Cancer prevention: the lesson from the lab In: Biasco G, Tanneberger S (Eds.), Cancer Medicine at the Dawn of the 21st. 2006. Bonomia University, pp. 49–64 Century: the view from Bologna. [Google Scholar]
- Soffritti M, Belpoggi F, Lambertini L, Lauriola M, Padovani M, Maltoni C, 2002. Results of long-term experimental studies on the carcinogenicity of formaldehyde and acetaldehyde in rats. Ann. N. Y. Acad. Sci 982, 87–105 10.1111/j.1749-6632.2002.tb04926.x. [DOI] [PubMed] [Google Scholar]
- Soffritti M, Maltoni C, Maffei F, Biagi R, 1989. Formaldehyde: an experimental multipotential carcinogen. Toxicol. Ind. Health 5 (5), 699–730. 10.1177/074823378900500510. [DOI] [PubMed] [Google Scholar]
- Soukup J, Krskova L, Hilska I, Kodet R, 2003. Ethanol fixation of lymphoma samples as an alternative approach for preservation of the nucleic acids. Neoplasma 50 (4), 300–304 [PubMed] [Google Scholar]
- Srinivasan M, Sedmak D, Jewell S, 2002. Effect of fixatives and tissue processing on the content and integrity of nucleic acids. Am. J. Pathol 161 (6), 1961–1971. 10.1016/S0002-9440(10)64472-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanta G, Mucelli SP, Petrera F, Bonin S, Bussolati G, 2006. A novel fixative improves opportunities of nucleic acids and proteomic analysis in human archive’s tissues. Diagn. Mol. Pathol 15 (2), 115–123. [DOI] [PubMed] [Google Scholar]
- Stefanits H, Bieńkowski M, Galanski M, Mitulović G, Ströbel T, Gelpi E, Ribalta T, Broholm H, Hartmann C, Kros JM, Preusser M, Hainfellner JA, 2016. KINFix – A formalin-free non-commercial fixative optimized for histological, immunohistochemical and molecular analyses of neurosurgical tissue specimens. Clin. Neuropathol 35 (1), 3–12. 10.5414/NP300907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Titford ME, Horenstein MG, 2005. Histomorphologic assessment of formalin substitute fixatives for diagnostic surgical pathology, Arch Pathol, Lab. Med 129 (4), 502–506. . [DOI] [PubMed] [Google Scholar]
- Turashvili G, Yang W, McKinney S, Kalloger S, Gale N, Ng Y, Chow K, Bell L, Lorette J, Carrier M, Luk M, Aparicio S, Huntsman D, Yip S, 2012. Nucleic acid quantity and quality from paraffin blocks: defining optimal fixation, processing and DNA/RNA extraction techniques. Exp. Mol. Pathol 92 (1), 33–43. 10.1016/j.yexmp.2011.09.013. [DOI] [PubMed] [Google Scholar]
- Uhlig U, Uhlig S, Branscheid D, Zabel P, Vollmer E, Goldmann T, 2004. HOPE technique enables Western blot analysis from paraffin-embedded tissues. Pathol. Res. Pract 200 (6), 469–472. [DOI] [PubMed] [Google Scholar]
- U.S. Environmental Protection Agency, 1989. Office of Air and Radiation: Report to Congress on Indoor Air Quality, Volume II Chapter 4: Assessment and Control of Indoor Air Pollution, vol. 1989 pp. 12–27. [Google Scholar]
- van Essen HF, Verdaasdonk MA, Elshof SM, de Weger RA, van Diest PJ, 2010. Alcohol based tissue fixation as an alternative for formaldehyde: influence on immunohistochemistry. J. Clin. Pathol 63 (12), 1090–1094 https://doi:10.1136/jcp.2010.079905. [DOI] [PubMed] [Google Scholar]
- Vince DG, Tbakhi A, Gaddipati A, Cothren RM, Cornhill JF, Tubbs RR, 1997. 1997. Quantitative comparison of immunohistochemical staining intensity in tissues fixed in formalin and Histochoice, Anal. Cell Pathol 15 (2), 119–129 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vincek V, Nassiri M, Nadji M, Morales AR, 2003. A tissue fixative that protects macromolecules (DNA, RNA, and protein) and histomorphology in clinical samplesm. Lab. Invest 83 (10), 1427–1435 [DOI] [PubMed] [Google Scholar]
- Warmington AR, Wilkinson JM, Riley CB, 2000. Evaluation of ethanol-based fixatives as a substitute for formalin in diagnostic clinical laboratories. J. Histotechnol 23, 299–308. [Google Scholar]
- Wester K, Wahlund E, Sundström C, Ranefall P, Bengtsson E, Russell PJ, Ow KT, Malmström PU, Busch C, 2000. Paraffin section storage and immunohistochemistry. Effects of time, temperature, fixation, and retrieval protocol with emphasis on p53 protein and MIB1 antigen. Appl. Immunohistochem. Mol. Morphol 8 (1), 61–70. [PubMed] [Google Scholar]
- Yaziji H, Taylor CR, Goldstein NS, Dabbs DJ, Hammond EH, Hewlett B, Floyd AD, Barry TS, Martin AW, Badve S, Baehner F, Cartun RW, Eisen RN, Swanson PE, Hewitt SM, Vyberg M, Hicks DG, 2008. Members of the Standardization Ad-Hoc Consensus Committee, 2008. Consensus recommendations on estrogen receptor testing in breast cancer by immunohistochemistry. Appl. Immunohistochem. Mol. Morphol 16 (6), 513–520. 10.1097/PAI.0b013e31818a9d3a. [DOI] [PubMed] [Google Scholar]
- Zanini C, Gerbaudo E, Ercole E, Vendramin A, Forni M, 2012. Evaluation of two commercial and three home-made fixatives for the substitution of formalin: a formaldehyde-free laboratory is possible. Environ. Health 4 (11), 59 10.1186/1476-069X-11-59. [DOI] [PMC free article] [PubMed] [Google Scholar]