Abstract
Providing robust links between mitochondrial genotype and phenotype is of major importance given that mitochondrial DNA (mtDNA) variants can affect reproductive success. Because of the strict maternal inheritance (SMI) of mitochondria in animals, haplotypes that negatively affect male fertility can become fixed in populations. This phenomenon is known as ‘mother's curse’. Doubly uniparental inheritance (DUI) of mitochondria is a stable exception in bivalves, which entails two mtDNA lineages that evolve independently and are transmitted separately through oocytes and sperm. This makes the DUI mitochondrial lineages subject to different sex-specific selective sieves during mtDNA evolution, thus DUI is a unique model to evaluate how direct selection on sperm mitochondria could contribute to male reproductive fitness. In this study, we tested the impact of mtDNA variants on sperm performance and bioenergetics in DUI and SMI species. Analyses also involved measures of sperm performance following inhibition of main energy pathways and sperm response to oocyte presence. Compared to SMI, DUI sperm exhibited (i) low speed and linearity, (ii) a strict OXPHOS-dependent strategy of energy production, and (iii) a partial metabolic shift towards fermentation following egg detection. Discussion embraces the adaptive value of mtDNA variation and suggests a link between male-energetic adaptation, fertilization success and paternal mitochondria preservation.
This article is part of the theme issue ‘Linking the mitochondrial genotype to phenotype: a complex endeavour’.
Keywords: mitochondria, OXPHOS, glycolysis, sperm, bivalves, DUI
1. Introduction
As accumulating evidence undermines the assumption of selective neutrality of mitochondrial DNA (mtDNA) variability, inferring links between mitochondrial genotype and phenotype becomes a major issue in evolutionary biology [1,2]. Non-neutral mtDNA variations can influence mitochondrial functionality [3,4], longevity [5–8], susceptibility to diseases [9], adaptation to specific environments [10–12] and could even drive speciation [2,13,14]. An added layer of complexity in the relationship between mtDNA evolution and fitness is the strict maternal inheritance (SMI) of mitochondria in most animal species [15]. This sex-specific selective sieve in mtDNA evolution enables male-harming mutations with a bland repercussion on female fitness to persist and reach high frequencies in natural populations, a phenomenon known as ‘mother's curse’ [16–18]. Evidence of this phenomenon comes, for example, from studies linking specific mtDNA haplotypes with decreased sperm motility and male fertility, while being of low impact on female reproduction [19–21].
A potential but uncommon compensatory mechanism resides in the paternal inheritance of mitochondria, the only stable example in animals being the doubly uniparental inheritance (DUI) of mitochondria in bivalve molluscs [22–24]. The DUI system entails two sex-linked mtDNAs (the female or F-type and the male or M-type) transmitted separately through oocytes and sperm. These two mtDNA lineages evolve independently and remarkably exhibit from 8% to 40% of DNA sequence divergence [22]. Because the fidelity of gamete-specific transmission of the two mtDNAs is a basic requirement for explaining the evolutionary stability of DUI, this system does not represent a case of biparental inheritance of organelles [22–24]. The oocytes carry the female-derived mitotype, whereas sperm only bear the male-derived mitotype [25,26]. In a few cases, the maternal mt lineage has been found to invade the male route and take the place of the paternal lineage. This has only been documented in Mytilus spp, a rare phenomenon named ‘masculinization’ [24]. No evidence of masculinization events has been recorded in other DUI species [22–24,26]. As such, in all other DUI species, a strict sex-specific mtDNA segregation in the germ line is the stable rule, with sperm carrying exclusively the M-type mitochondria [25,26].
The opportunity for natural selection to act directly on sperm mitochondria makes the DUI system an attractive model to evaluate the phenotype resulting from a male-specific evolution of mitochondria and thus the adaptive value of paternally inherited mtDNA variants [27]. Furthermore, comparing the functions of male gametes carrying either male- or female-derived mitochondria (DUI versus SMI) brings an exceptional opportunity to test the effectiveness of the mother's curse hypothesis in bivalves. To date, DUI has been detected in more than 100 bivalve species and its distribution appears to be scattered [28]. Although a single origin of DUI near the origin of the modern class Bivalvia would represent the most parsimonious hypothesis, there is evidence for multiple independent origins of this peculiar system [24,28,29]. This is reflected at the phylogenetic level, where F- and M-mitotypes of different species sometimes join according to their gender linkage, as seen in freshwater mussels, or they cluster together according to species relatedness, as seen in several marine species [28–31]. In a recent paper, the presence of selective signatures in the mitochondrial genomes of DUI species was investigated and few DUI-specific mutations were identified that gave support to the hypothesis of multiple independent origins [29]. Interestingly, they documented episodes of acute directional selection associated with the origins of different DUI systems in six mt genes (i.e. atp6, cox1, cox2, cox3, nad4 L and nad6). As such, even in a scenario of multiple independent origins of the DUI system, a common increase in mutational events and selective pressure on specific mt genes appear to take place at the base of a DUI clade [29].
In accordance, a convergent phenotypic evolution has been suggested in the DUI marine clam, Arctica islandica, and marine mussel, Mytilus edulis, for which the mitochondrial phenotypes of the F- and M-type mitochondria have been recently characterized [3]. Compared to F-type mitochondria in eggs and gills, M-type mitochondria in sperm exhibit (i) low respiratory activity compared to their maximum capacity (coupled oxidative phosphorylation (OXPHOS) rate/ uncoupled rate) because of a limitation by the phosphorylation system, and (ii) low excess capacity of cytochrome c oxidase (complex IV or CIV), which could link to a tight flux control of CIV over the upstream complexes. This energetic remodelling, that appears specific of DUI sperm even across distantly related DUI species, has been proposed to be involved in the preservation of the paternal mitochondrial lineage across generations, linking male-energetic adaptation with selection and inheritance of cytoplasmic organelle genomes [3,32,33].
Little is known about the extent to which the retention of a male-specific mitotype (and the expression of a rearranged mitochondrial phenotype) could affect sperm performance. For example, selection acting directly on male mitochondria has been proposed to lead to the evolution of genomes specifically adapted for sperm functions, fostering male reproductive success in DUI species [22,34]. So far, studies on My. edulis did not find any evidence that M-type mitochondria are linked to higher sperm swimming speed [35,36], suggesting that the adaptive value of DUI could embrace other sperm fitness traits, such as endurance, longevity, or response to either competing sperm or egg-derived chemical attractants (chemoattractants) [22,36,37]. Concerning adenosine triphosphate (ATP) production, knowing the flexible energetic metabolism of bivalve species [38] and the putative downregulation of both the OXPHOS and the swimming speed in sperm bearing M-type mitochondria [3,35,36], the question arises whether DUI species would rely more on aerobic or glycolytic energy metabolism to sustain spermatic functions. Because DUI allows selection to act directly on male mt-encoded components, and keeping in mind the mother curse's effect in SMI systems, one prediction could be that the sperm of DUI species use OXPHOS while the sperm of SMI species might rely primarily on glycolysis. In other words, because mt genes are only or mainly involved in OXPHOS, the sperm of DUI species might rely more heavily on OXPHOS because selection can act more efficiently on their (mt) OXPHOS genes.
In animals, there is still controversy regarding the main energetic pathway of energy production in sperm, and the two processes are linked and non-mutually exclusive [39–43]. Species strongly differ in the proportion of use of these two pathways [21,39–47]. The balance between the aerobic and anaerobic capacity allows a flexible metabolic strategy to meet sperm energetic demand, which could vary depending on the surrounding environment and the presence of different substrates/chemicals [39,41,42]. For example, the sperm flagellar movement of the pacific oyster, Crassostrea gigas, passes from a phosphagen- and glycolytic-dependant metabolism to OXPHOS, when changing from the early to the long motility phase [47]. However, although the role played by OXPHOS has been confirmed in the sperm of various bivalve species [3,33,48], there is still a lack of knowledge about the importance of the anaerobic metabolism. Moreover, although the presence of chemoattractants has been found to exert changes in sperm swimming behaviour and physiology in bivalves [49–52], whether egg detection can influence sperm bioenergetics is still unknown. Beyond promoting gamete encounter, egg-derived chemoattractants also seem to mediate bivalves' mate choice, as gametes could exploit these molecules to select for genetically compatible partners. This suggests a link between sperm chemotaxis and gamete-level sexual selection, increasing the role of gamete chemical signals in sessile marine invertebrates [50–52]. A change in steady-state speed following egg detection has been proposed for Mytilus galloprovincialis sperm. Specifically, mussel sperm would conserve energy by swimming slowly and in tight circles if eggs are absent in the water environment, but faster and straighter towards the more genetically compatible oocytes once detecting them [53]. Whether the link between sperm chemotaxis and sexual selection at the gamete-level could be in some way related to DUI remains to be examined.
The goal of the present study was to test the impact of bearing paternal or maternal mitotypes upon bivalve sperm bioenergetics and performance. We aimed to infer: (i) if bivalve species rely more on oxidative or glycolytic energy metabolism to sustain spermatic functions, (ii) whether gamete chemoattraction may influence the metabolic pathways of spermatozoa, and (iii) whether a different energetic strategy may be the result of natural selection shaping the evolution of paternally inherited mitochondria, thus reflecting male-specific energetic adaptation in DUI species. Sperm motility parameters were evaluated in five bivalve species. We compared sperm of the DUI species My. edulis (Order Mytilida) and Ruditapes philippinarum (Order Venerida), bearing their male-specific mitochondria (i.e. the DUI M-type), with sperm of the SMI species Mercenaria mercenaria (Order Venerida), Nuttallia obscurata (Order: Cardiida) and Placopecten magellanicus (Order Pectinida), bearing their own species-specific and maternally derived mitochondria (i.e. the SMI maternally inherited type). To avoid potential taxon-driven bias in the results, the five bivalve species tested were selected to be distantly related. The strong evolutionary divergence between the mitochondrial lineages of these species is reflected in how their entire mt genomes cluster separately in a phylogenetic tree, with their last common ancestor being dated to the mid-Cambrian, ≈510 Ma [30]. Moreover, the DUI species used for this research probably represent two independent origins of DUI, as their sex-linked genomes (F- and M-type) cluster according to the species rather than by sex specificity [24,28–30]. The nucleotidic divergence between the F and M genomes is gene-specific and ranges between 10%and 22% in My. edulis [3,24,54,55] and between 16% and 32% in R. philippinarum [31,56].
The equilibrium between the aerobic and anaerobic metabolism to sustain sperm motility was assessed following the inhibition of the main pathways of energy production, and the potential change in this balance was assessed following the introduction of oocyte-derived chemoattractants. Our results are discussed in the light of the adaptive value of mtDNA variation, the paternal inheritance of mtDNA, male-energetic adaptation and its evolutionary implications.
2. Material and methods
(a). Animal collection
Adult bivalves were ordered from culture farms or bought in fish markets during their spawning period between June and August 2018, acclimated for four weeks in a 12°C recirculating seawater aquarium and fed with a mix of microalgae. We tested five different broadcast spawning bivalve species: the DUI species My. edulis (Linnaeus, 1758) from Kensington (Prince Edward Island, Canada) and R. philippinarum (Adams & Reeve, 1850) from Vancouver (British Columbia, Canada), as well as the SMI species Me. mercenaria (Linnaeus, 1758) from Barnstable (MA, USA), N. obscurata (Reeve, 1857) from Vancouver (British Columbia, Canada) and P. magellanicus (Gmelin, 1791) from Newport (Québec, Canada). Sex and maturity of individuals were assessed through microscopic examination of gonadal smears. The absence of masculinization in the My. edulis sperm sample was tested by amplifying part of the M-mtDNA (654 bp) using the male-haplotype specific primers: MyEd-M-for (TACTGTTGGCACATACGAGAG) and MyEd-M-rev (TACTGTTGGCACATACGAGAG), designed on the complete My. edulis M-mtDNA (accession numbers AY823623.1). The specific primers were already tested on this species [3]. Mytilus edulis oocytes (carrying the only F-mtDNA lineage) were tested to confirm the M-mtDNA specificity of the primers adopted. Results confirmed the presence of M-mtDNA in sperm and its absence in eggs.
(b). Gamete sample preparation
To test the effect of oocyte-derived chemoattractants on sperm motility, prior to experiments and for each species, one egg sample was collected, adjusted to 1 : 5 w/v with artificial seawater (ASW), homogenized (3 × 30 s at medium speed) using a PT 1200 homogenizer (Polytron, Kinematica), microfiltered and stored at −20°C until use. Male gonads were excised and placed in a Petri dish containing 5 ml of ASW. Gametes were stripped by performing incisions in the gonads and allowing the motile mature sperm to actively swim out for 5 min. Total sperm count was determined by using a Petroff-Hausser counting chamber and the final concentration was corrected to 5 × 106 sperm ml−1 by the addition of ASW. Sperm suspensions were divided into two aliquots (475 µl each), one supplemented with 25 µl of ASW (‘normal’ group) and the other with 25 µl of species-specific egg-derived chemoattractants (‘chemoattractants’ group, 1 : 100 w/v). To assess the effect on sperm performance of metabolic inhibitors together with (or without) chemoattractants, each group was further divided into five aliquots (100 µl each): (i) ASW (‘control’ group), and four treatments: (ii) 1 µM rotenone (Rot, inhibitor of mitochondrial respiratory complex I—NADH-dehydrogenase), (iii) 1 µM antimycin A (Ama, inhibitor of mitochondrial respiratory complex III—coenzyme Q: cytochrome c oxidoreductase), (iv) 5 µM oligomycin (Omy, inhibitor of mitochondrial ATP-synthase), and (v) 30 mM of sodium oxamate (Oxa, inhibitor of lactate dehydrogenase 4 (LDH4)). The effectiveness of these mitochondrial inhibitors to target specific mitochondrial complexes in bivalves and other animal models, as well as their optimal concentrations, have already been tested and verified through titration in previous studies [3,57–59]. After inhibitor addition, sperm aliquots were incubated at 15°C for 30 min prior to sperm motility parameters assessment [59]. All chemicals were purchased from Sigma-Aldrich (Oakville, Ontario, Canada).
(c). Sperm performance parameters
After incubation, 10 µl of each sperm suspension was placed in a 20 µm deep microscopy chamber. A minimum of 500 sperm per treatment were analysed using a CEROS microscope (Hamilton Thorne Inc, Beverly, USA) with a 20× negative phase contrast objective. Recorded videos were manually verified to exclude drifting particles and drifting immotile sperm from the analysis. The following sperm motility parameters were estimated through a computer-aided sperm analyser (CASA system): distance of average path (DAP, µm), straight-line distance (DSL, µm), curvilinear distance (DCL, µm), curvilinear velocity (VCL, µm s−1), straight-line velocity (VSL, µm s−1), average path velocity (VAP, µm s−1), linearity (LIN = VSL · VCL−1), straightness (STR = VSL · VAP−1), wobble coefficient (WOB = VAP · VCL−1), amplitude of lateral head displacement (ALH, µm) and beat-cross frequency (BCF, Hz). For each sample, the value of each parameter represents the mean of all its individual sperm values. All these parameters describe various motility traits of male gametes, such as speed and linearity of the trajectory, and are widely implied to infer the reproductive fitness of individuals [35–37,44–47,51,53,59–62].
(d). Data and statistical analysis
Sperm performances were measured for n = 11 My. edulis, n = 9 R. philippinarum, n = 9 Me. mercenaria, n = 5 N. obscurata and n = 11 P. magellanicus. As sperm kinetic parameters have already been shown to be highly correlated [59], all parameters were combined and resumed by performing a principal component analysis (PCA) (electronic supplementary material, figure S1 and table S1). The first principal component (PC1) accounted for 58% of the variability of the original parameters and reflects sperm velocity, as all the velocity parameters (VAP, VSL and VCL) heavily load on it. The second principal component (PC2) accounted for 21% of the variability and reflects the linearity of the path, owing to the heavy load that LIN, WOB and STR have on it (electronic supplementary material, figure S1 and tables S1). The assumptions of normality and homoscedasticity were verified using Shapiro and Levene's tests, respectively. Sperm motility parameters have been analysed in function of the factors: ‘species’ (five levels), ‘treatment’ (five levels) and the presence of egg-derived chemoattractants (factor ‘chemoattractants’, two levels). Statistical analyses were performed considering single or multiple factors, depending on the biological question of interest. Interspecific differences in basal sperm motility (effect of factor ‘species’) in both absence or presence of egg chemical cues have been tested by means of one-way ANOVAs followed by a post hoc Tukey's multi comparison test (figure 1; electronic supplementary material, figure S2). The fixed effect of metabolic inhibition (factor ‘treatment’), chemoattractants absence/presence (factor ‘chemoattractants’) and species (factor ‘species’) on sperm motility parameters were assessed either separately or combined through linear mixed effect models that controlled for by-subject variability and for the individual variability in the response to egg detection (figures 2–4; electronic supplementary material, figure S3). The significance of the fixed variables was determined by using a type III ANOVA, followed by a post hoc pairwise comparison with Holm correction for multiple testing. All the analyses and graphs have been made using R software [63]. Statistical significance was set at p ≤ 0.05. Results are presented as means ± standard error of the mean (s.e.m.).
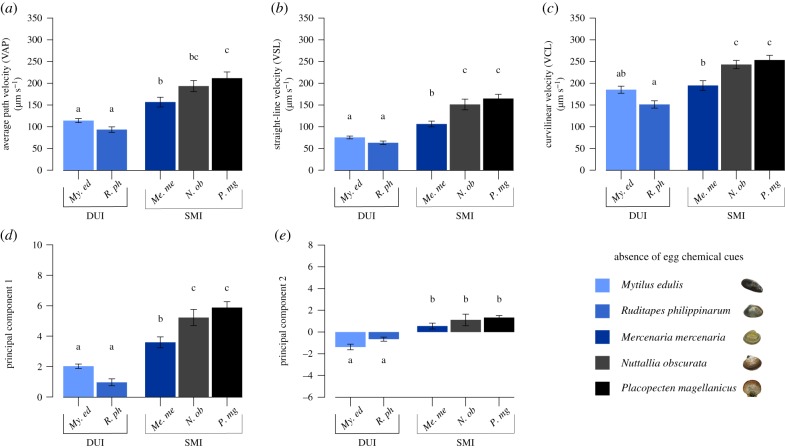
Figure 1.
Basal sperm motility parameters in five bivalve species, DUI and SMI, without chemoattractants. (a) Average path velocity (µm s−1). (b) Straight-line velocity (µm s−1). (c) Curvilinear velocity (µm s−1). (d) First principal component of the PCA combining sperm velocity parameters. (e) Second principal component of the PCA. Data are presented as means ± s.e.m. Differences (p ≤ 0.05) in a post hoc Tukey's test are indicated by different letters. DUI species: My. edulis (My. ed, n = 11), R. philippinarum (R. ph, n = 9). SMI species: Me. mercenaria (Me. me, n = 9), N. obscurata (N. ob, n = 5), P. magellanicus (P. mg, n = 11). A detailed summary is reported in the electronic supplementary material, tables S2 and S3. (Online version in colour.)
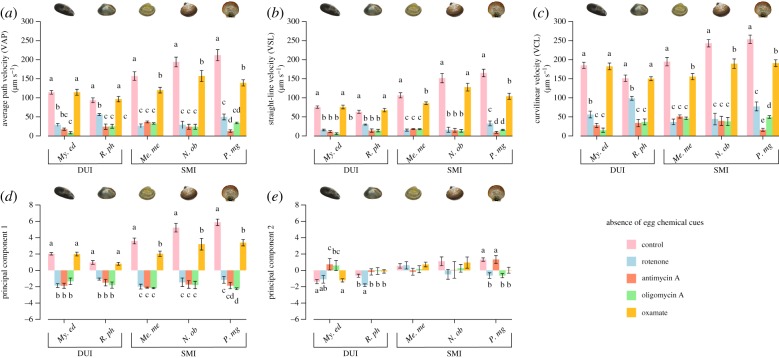
Figure 2.
Effect of metabolic inhibitors on sperm motility parameters in five bivalve species, DUI and SMI, without chemoattractants. (a) Average path velocity (µm s−1). (b) Straight-line velocity (µm s−1). (c) Curvilinear velocity (µm s−1). (d) First principal component of the PCA. (e) Second principal component of the PCA. Data are presented as means ± s.e.m. Statistical difference was set at p ≤ 0.05. Difference among treatments are indicated by letters determined through a post hoc comparison adjusted using Holm's correction for multiple testing. For abbreviations refer to figure 1. A detailed summary is reported in the electronic supplementary material, tables S2 and S5. (Online version in colour.)
Figure 4.
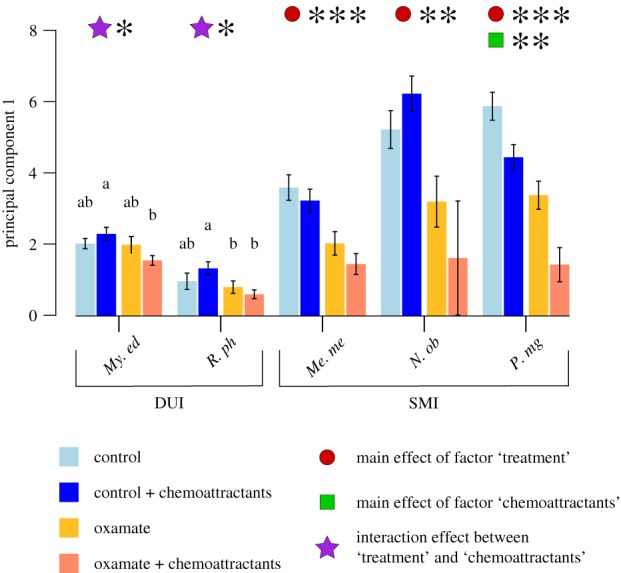
Interaction effect between glycolysis inhibition and addition of chemoattractants on the first principal component of the PCA, reflecting sperm velocity. Values are presented as means ± s.e.m. The main effect of the two fixed factors ‘treatment’ and ‘chemoattractants’ are indicated with a circle and square, respectively. Interaction effect is indicated with a star. *p ≤ 0.05, **p ≤ 0.01, ***p ≤ 0.001. Letters indicate differences following a post hoc pairwise comparison. DUI, doubly uniparental inheritance; SMI, strict maternal inheritance. Species: My. edulis (My. ed, n = 11); R. philippinarum (R. ph, n = 9); Me. mercenaria (Me. me, n = 9); N. obscurata (N. ob, n = 5); P. magellanicus (P. mg, n = 11). A detailed summary is reported in the electronic supplementary material, tables S2 and S7. (Online version in colour.)
3. Results and discussion
(a). Sperm carrying paternally inherited mitochondria exhibit low speed and accentuate curvilinear trajectory
The comparison of sperm motility parameters of DUI and SMI species is represented in figure 1 and the electronic supplementary material, figure S2, respectively, in the absence or presence of egg-derived chemoattractants. Significant differences across species were detected for all the motility traits, in the absence or presence of egg-derived chemoattractants. A detailed summary of the results is provided in the electronic supplementary material, tables S2 and S3. Among sperm velocity parameters, differences were reported for the VAP (figure 1a; electronic supplementary material, figure S2a), VSL (figure 1b; electronic supplementary material, figure S2b), VCL (figure 1c; electronic supplementary material, figure S2c) and are resumed in PC1 (figure 1d, F = 41.92, p = 8.45 × 10−14; electronic supplementary material, figure S2d, F = 32.18, p = 5.1 × 10−12), representing a proxy of the sperm velocity itself. Interspecific differences were also observed for all sperm trajectory parameters (LIN, WOB, STR, ALH, BCF, see the electronic supplementary material, table S3), as resumed in PC2 (figure 1e, F = 20.93, p = 2.25 × 10−9; electronic supplementary material, figure S2e, F = 14.44, p = 2.2 × 10−7), which expresses the linearity of the path. This finding is corroborated in the electronic supplementary material, figure S3, where a strong main effect of the factor ‘species’ is found widespread among all motility parameters (electronic supplementary material, table S4).
Interestingly, sperm of both DUI species (My. edulis and R. philippinarum) have a consistent lower speed (VAP, VSL, VCL and PC1) and a less linear path (LIN, WOB, STR and PC2) than sperm of the three SMI species (Me. mercenaria, N. obscurata and P. magellanicus), regardless of the absence/presence of egg chemoattractants (figure 1; electronic supplementary material, figures S2 and S3). Egg-derived chemoattractants have been shown to exert an effect on sperm motility behaviour, specifically swimming speed and direction [50–53]. Contrary to our expectations, we did not detect any significant impact of egg presence on sperm velocity parameters (only a trend of increasing speed), and differences in velocity were explained by the only fixed factor ‘species’ (electronic supplementary material, figure S3 and table S4). Specifically, interspecific differences were detected for VAP (electronic supplementary material, figure S3a), VSL (electronic supplementary material, figure S3b), VCL (electronic supplementary material, figure S3c) and are resumed in PC1 (electronic supplementary material, figure S3d, F = 53.22, p = 1.71 × 10−15). These results are consistent with a previous work on My. edulis in which no increase in sperm velocity parameters were observed under sperm competition and detection of oocytes [37]. Conversely, sperm trajectory was influenced by both factors ‘species’ and addition of ‘chemoattractants’ (electronic supplementary material, figure S3e and table S4). Specifically, DUI and SMI sperm cluster separately based on a less linear trajectory of the former, while the addition of chemoattractants produced a trend of decreased linearity in both groups.
In DUI species, the preservation of sex-linked mtDNAs in gametes has been proposed as a way to avoid sex-linked constraints of mitochondrial inheritance, and an opportunity for mitochondria to evolve adaptively for male and sperm fitness [22]. Our results on bivalve sperm carrying either a female or a male-derived mitotype suggest that selection on sperm function might be acting differently in these groups, possibly owing to the DUI versus SMI system of organelle inheritance, favouring both low sperm speed and linearity in DUI species. This is congruent with previous studies in the species My. edulis that found sperm bearing the paternally inherited mtDNA having an equal or even lower speed than ‘masculinized’ sperm carrying the maternally inherited mtDNA [35,36]. The present findings thus provide additional evidence that the adaptive value of paternal mitochondria preservation in DUI species might embrace different sperm phenotypic traits than higher velocity or straightness, although it is still unclear whether the traits seen in DUI sperm increase or decrease sperm fitness (or are neutral) [22,35,36,64].
Swimming speed is just one sperm fitness trait among many, and even a decreased velocity could represent an advantage depending on the fertilization strategy adopted. For instance, slower sperm with pronounced curved trajectories and a high angle change rate have already been associated with the highest fertilization rates in My. galloprovincialis [60,61]. As a trade-off between sperm rapidity and endurance has already been demonstrated [62], a slow sperm speed may reflect a strategy linked with energy preservation and/or swim endurance in the DUI species tested so far, shifting the selective pressure towards stamina rather than speed. Even in presence of eggs, selection may favour slow but constant-speed sperm that survive for a longer time and cover a larger distance due also to an increased oscillation around the average path, rather than faster sperm with a shorter lifespan and a straighter path. Based on the phylogenetic distance between the two DUI species pertaining to different Orders, i.e. Mytilida and Venerida, and probably representing two independent origins of DUI, the intriguing hypothesis that such sperm phenotype might reflect a shared DUI feature can be considered. We speculate that the fertilization success contributed to the evolution and preservation of the paternally inherited and highly divergent M-mtDNA lineage in DUI species. Also, the link between energy production limitation and reactive oxygen species production should be considered, as a lower metabolic rate could reduce the oxidative stress and in turn preserve the integrity of the paternal mtDNA to be passed through generations. These hypotheses, however, remain to be tested.
(b). Sperm carrying paternally inherited mitochondria show a flexible metabolic strategy depending on the presence of egg-derived chemoattractants
The importance of aerobic and anaerobic pathways of energy production has been investigated through the addition of specific metabolic inhibitors and the results are reported in figure 2; electronic supplementary material, tables S2 and S5. For all five species, the inhibition of the OXPHOS (i.e. through the separate addition of rotenone, antimycin A and oligomycin A, respectively, inhibiting complex I, complex III and ATP-synthase) strongly hampered all sperm velocity parameters analysed (VAP, VSL, VCL, PC1) (figure 2a–d; electronic supplementary material, table S5). By contrast, sperm trajectory parameters were only marginally affected by inhibitors and no congruent trend was detectable (figure 2e; electronic supplementary material, table S5). Our results thus suggest that, contrary to some other animal species including humans [40,42,43], the energy production through the OXPHOS is mandatory to sustain sperm velocity in these bivalve species. The importance of the anaerobic pathway of energy production, assessed through the addition of sodium oxamate, an inhibitor of LDH, revealed that lactic fermentation plays a different role in sperm bearing the paternally or the maternally inherited mitochondria. Indeed, contrary to the sperm of SMI species (carrying the maternal mt lineage), for which the inhibition of LDH impacted motility, sperm of DUI species (carrying the paternal mt lineage) remained unaffected (figure 2a–d; electronic supplementary material, table S5).
Marine bivalves exhibit a panoply of energy production strategies, including aerobic respiration, various cytosolic fermentation pathways (i.e. lactate and opine pathways) and even an oxygen-independent mitochondrial functioning through the malate-dismutation pathway [38,65–67]. A previous study on the pacific oyster C. gigas suggested that the ATP-dependent flagellar movement is sustained by both phosphagen and glycolytic metabolism during the early phase of movement, whereas OXPHOS would support sperm motility in the long motility phase [47]. Likewise, our results reveal that, in the absence of oocytes, both fermentation and aerobic metabolism are important to sustain sperm motility in SMI species, but not in the two DUI species. Although the aerobic metabolism appears mandatory in both SMI and DUI species, a strictly OXPHOS-dependent strategy, or at least not dependent on lactic fermentation, could represent a DUI-specific and evolutionary conserved sperm metabolic rearrangement. Our results are congruent with the previous finding that, compared to maternally transmitted mitochondria of either DUI or SMI species, male mitochondria in DUI species exhibit a reorganization of the OXPHOS system that may influence ATP production efficiency [3,64]. These variations entail differences in the catalytic capacity of various enzyme complexes [64] and the expression of a rearranged mitochondrial phenotype, characterized by a limitation of the aerobic metabolism by ATP-synthase and by a potential tight control of cytochrome c oxidase over the upstream respiratory enzymes [3], strongly suggesting an evolutionary link between the OXPHOS mechanism and the DUI system itself. Taken all together, these results are somewhat in line with the prediction of the mother's curse hypothesis, i.e. that sperm of DUI species use OXPHOS (as mt-encoded components can be selected for sperm function) while sperm of SMI species (for which selection might be less efficient) might compensate reduced (or compromised) OXPHOS function with glycolysis. However, more species will have to be tested to clearly confirm the trend observed in the present study.
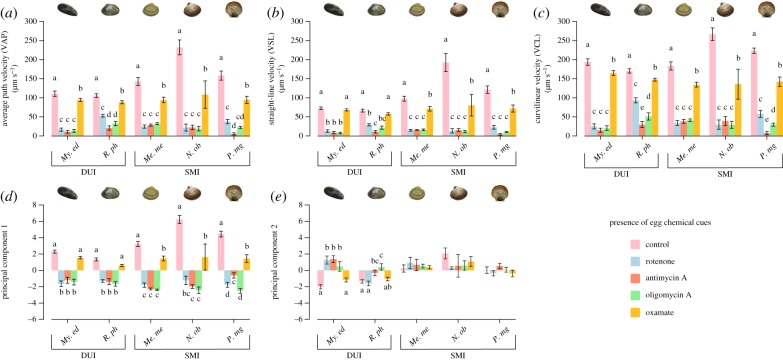
The equilibrium between the aerobic and anaerobic pathways was also investigated in the presence of egg chemical cues, and results are reported in figure 3 and the electronic supplementary material, table S6. In the three SMI species, addition of chemoattractants did not exert any change in the balance between the two pathways (i.e. both OXPHOS and lactic fermentation are required, with or without chemoattractants), whereas in DUI species, the presence of chemoattractants affected their proportion, i.e. both My. edulis and R. philippinarum sperm motility became sensitive to oxamate (for both average path and curvilinear velocities; figure 3a,c). No effect was detected for the VSL nor for the PC1 parameter (figure 3b,d), although for the latter a decreasing trend is detectable. For sperm trajectory no trend was detectable (figure 3e).
Figure 3.
Effect of metabolic inhibitors on sperm motility parameters in five bivalve species, DUI and SMI, with chemoattractant. (a) Average path velocity (µm s−1). (b) Straight-line velocity (µm s−1). (c) Curvilinear velocity (µm s−1). (d) First principal component of the PCA. (e) Second principal component of the PCA. Data are presented as means ± s.e.m. Statistical difference was set at p ≤ 0.05. Difference among treatments are indicated by letters determined through a post hoc comparison adjusted using Holm's correction for multiple testing. For abbreviations refer to figure 1. A detailed summary is reported in the electronic supplementary material, tables S2 and S6. (Online version in colour.)
Overall, the analysis of the energetic metabolism suggests that: (i) both SMI and DUI species strongly rely on OXPHOS to sustain sperm motility; (ii) for the SMI species analysed, both aerobic and anaerobic pathways of energy production appear to play a role in sustaining sperm motility, no matter the presence of female gamete compounds; and (iii) only the DUI species show a flexible metabolic strategy depending on the presence of egg-derived chemoattractants. Specifically, My. edulis and R. philippinarum sperm appear to exclusively rely on OXPHOS activity after spawning but switch to a combined metabolic strategy in the presence of egg-derived compounds. This can also be seen in figure 4, where the interaction effect between LDH-inhibition (factor ‘treatment’) and the presence of oocytes (factor ‘chemoattractants’) was investigated. For the three SMI species, no interaction effect is found for the velocity parameters, resumed in PC1 (figure 4). Sperm velocity was only affected by the addition of oxamate (i.e. Me. mercenaria and N. obscurata) or, separately, by both oxamate and addition of chemoattractants (P. magellanicus). Conversely, for both DUI species, an interaction effect of glycolysis inhibition and chemoattractants addition was observed. The post hoc simple main effect analysis confirmed that the effect of glycolysis inhibition is dependent on egg presence and that this outcome does not derive solely from an increased speed after addition of chemoattractants nor a higher sensibility to lactic fermentation inhibition, but mainly by a combined influence of both (figure 4; electronic supplementary material, table S7).
One possible explanation for the glycolytic switch relates to the ATP diffusion throughout sperm. While mitochondrial ATP diffusion from the mitochondrial midpiece would be slower and may not reach all areas, the colocalization of glycolytic enzymes close to the flagellum would make the switch to a more glycolytic-dependent energy production a good strategy to increase and sustain sperm swimming speed during sperm competition [42,43]. However, as our analyses did not reveal any significant increase in sperm velocity (figures 3 and 4; electronic supplementary material, figure S3), the question arises on the purpose of such a strategy in DUI species only in the presence of eggs.
Although it will be important to extend the analysis to other SMI and DUI species to confirm our finding, we propose that the detected metabolic shift in DUI sperm (passing from a completely OXPHOS-dependent energy production strategy towards a combined aerobic and anaerobic strategy) could reflect (i) the importance of the lactate shuttle mechanism and (ii) a potential programmed increase in Δψm of sperm mitochondria, just before the fertilization event (preliminary analyses on Δψm support this hypothesis, electronic supplementary material, figure S4). In turn, this could potentially allow for paternal mitochondria to escape the classic SMI and be inherited across generations [68]. Lactate is erroneously seen as a merely waste product of anaerobic glycolysis, and increasing evidence points towards the aerobic and anaerobic metabolism to be well linked, with lactate produced under fully aerobic conditions and readily oxidized in mitochondria (i.e. lactate shuttle mechanism) [69,70]. This mechanism has already proved to be important in sperm metabolism and is supported by a sperm-specific mitochondrial LDH isoform in mammals [40,42,43,69,71–73]. Lactate uptake and oxidation in the mitochondrial intermembrane space have been proposed to (i) favour the import of pyruvate into the matrix, where it participates in the tricarboxylic acid cycle, and (ii) actively contribute to the mitochondrial electrochemical gradient by releasing protons in the proximity of the inner mitochondrial membrane [69,70]. The mitochondrial membrane potential (Δψm) designates active mitochondria and its role in the preservation of the DUI paternal mitochondria has already been proposed [32]. Potential support comes from the direct observation of a high Δψm in sperm mitochondria of DUI species [33], and from a metabolic remodelling specific of DUI male mitochondria in line with the maintenance of a high electrochemical gradient [3]. Our results based on two distantly related DUI species support this hypothesis.
4. Conclusion
Linking the mitochondrial genotype to the phenotype is a complex endeavour. Given the deleterious effect that the uniparental inheritance of mitochondria could have for male fertility, the DUI system reflects an unprecedent opportunity for mitochondria to evolve adaptively for male functions. Our results highlighted a significant divergence in sperm performance and partially in energy metabolism strategy between DUI and SMI species. The paternal mtDNAs of both DUI species associate with sperm swimming slower and in a more curvilinear trajectory compared to sperm of SMI species, carrying maternally inherited mitotypes. In DUI species, this fitness trait could be under selection for male functions (e.g. potentially increasing the fertilization success owing to a higher endurance, longevity or distance covered by male gametes). The analysis of the energy metabolism revealed that, in absence of egg chemical cues, DUI sperm strictly rely on OXPHOS to sustain their motility, whereas sperm of SMI species combined both aerobic and anaerobic pathways of energy production, although still relying mostly on aerobic metabolism. Our results highlighted not only the importance of OXPHOS for bivalve sperm motility, but also revealed how its specific importance could vary between DUI and SMI species. These results are congruent with the previous finding of a rearranged mitochondrial metabolism characterizing the male mitotype in DUI species and with the prediction that a male-driven selection of mt-encoded components for sperm function could favour OXPHOS. Remarkably, the detection of egg-derived chemoattractants produced a partial metabolic shift in the DUI sperm we tested, implying a combined strategy of energy production, whereas it did not affect the energy pathway equilibrium in SMI sperm. However, even with an increased importance of lactic fermentation in the presence of eggs, the OXPHOS still remain mandatory to sustain sperm movement in these species and no increment in sperm swimming speed was detected. We thus propose a potential alternative role of this metabolic shift involving a programmed increase of the mitochondrial membrane potential in DUI species following egg detection, linking the lactic oxidation pathway of ATP production with paternal mitochondria preservation at fertilization.
As sperm mitochondria in DUI species are not an evolutionary dead-end, the overmentioned rearranged phenotype can reflect the selective forces driving the evolution of sperm mitochondria in the absence of SMI. The authors herein propose that a metabolic remodelling is indeed associated with the existence and adaptive value of paternal mitochondria inheritance and that these male-specific energetic adaptations in DUI species could reflect selection for both fertilization success and male mitotype preservation. Even though additional species need to be tested to confirm the trend found in the present study, these results based on five distantly related species of bivalves point in that direction, providing a clear reference for future experiments to confirm this trend. Further investigations are definitively necessary to test the intriguing hypothesis of a link between male-specific mtDNA variants, sperm energetic adaptation, paternal mitochondria preservation and inheritance.
Supplementary Material
Acknowledgements
We thank the guest editors for the invitation to contribute to the special issue and we are grateful to five anonymous referees who greatly contributed to improving this manuscript by providing thoughtful and constructive comments.
Data accessibility
The datasets supporting this article have been uploaded as part of the electronic supplementary material.
Authors' contributions
S.Be. carried out the laboratory work, data analysis, designed the experiment and drafted the manuscript; S.N. and A.D. participated in the laboratory work; L.M. and P.U.B. supervised the study; S.Br. conceived, coordinated and supervised the study. All authors gave final approval for publication.
Competing interests
We have no competing interests.
Funding
This work was supported by the Fonds de Recherche du Québec—nature et technologie (to S.Br.), the Natural Sciences and Engineering Research Council of Canada (to P.U.B.) and the Italian Ministry of Education, University and Research MIUR-SIR Programme (to L.M.).
References
- 1.Blier PU, Dufresne F, Burton RS. 2001. Natural selection and the evolution of mtDNA-encoded peptides: evidence for intergenomic co-adaptation. Trends Genet. 17, 400–406. ( 10.1016/S0168-9525(01)02338-1) [DOI] [PubMed] [Google Scholar]
- 2.Dowling DK, Friberg U, Lindell J. 2008. Evolutionary implications of non-neutral mitochondrial genetic variation. Trends Ecol. Evol. 23, 546–554. ( 10.1016/j.tree.2008.05.011) [DOI] [PubMed] [Google Scholar]
- 3.Bettinazzi S, Rodríguez E, Milani L, Blier PU, Breton S. 2019. Metabolic remodelling associated with mtDNA: insights into the adaptive value of doubly uniparental inheritance of mitochondria. Proc. R. Soc. B 286, 20182708 ( 10.1098/rspb.2018.2708) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pichaud N, Ballard JW, Tanguay RM, Blier PU. 2012. Naturally occurring mitochondrial DNA haplotypes exhibit metabolic differences: insight into functional properties of mitochondria. Evolution 66, 3189–3197. ( 10.1111/j.1558-5646.2012.01683.x) [DOI] [PubMed] [Google Scholar]
- 5.Niemi AK, Hervonen A, Hurme M, Karhunen PJ, Jylha M, Majamaa K. 2003. Mitochondrial DNA polymorphisms associated with longevity in a Finnish population. Hum. Genet. 112, 29–33. ( 10.1007/s00439-002-0843-y) [DOI] [PubMed] [Google Scholar]
- 6.Coskun PE, Ruiz-Pesini E, Wallace DC. 2003. Control region mtDNA variants: longevity, climatic adaptation, and a forensic conundrum. Proc. Natl Acad. Sci. USA 100, 2174–2176. ( 10.1073/pnas.0630589100) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dato S, Passarino G, Rose G, Altomare K, Bellizzi D, Mari V, Feraco E, Franceschi C, De Benedictis G. 2004. Association of the mitochondrial DNA haplogroup J with longevity is population specific. Eur. J. Hum. Genet. 12, 1080–1082. ( 10.1038/sj.ejhg.5201278) [DOI] [PubMed] [Google Scholar]
- 8.Zhang J, et al. 2003. Strikingly higher frequency in centenarians and twins of mtDNA mutation causing remodeling of replication origin in leukocytes. Proc. Natl Acad. Sci. USA 100, 1116–1121. ( 10.1073/pnas.242719399) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Taylor RW, Turnbull DM. 2005. Mitochondrial DNA mutations in human disease. Nat. Rev. Genet. 6, 389–402. ( 10.1038/nrg1606) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mishmar D, et al. 2003. Natural selection shaped regional mtDNA variation in humans. Proc. Natl Acad. Sci. USA 100, 171–176. ( 10.1073/pnas.0136972100) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ruiz-Pesini E, Mishmar D, Brandon M, Procaccio V, Wallace DC. 2004. Effects of purifying and adaptive selection on regional variation in human mtDNA. Science 303, 223–226. ( 10.1126/science.1088434) [DOI] [PubMed] [Google Scholar]
- 12.Lajbner Z, Pnini R, Camus MF, Miller J, Dowling DK. 2018. Experimental evidence that thermal selection shapes mitochondrial genome evolution. Sci. Rep. 8, 9500 ( 10.1038/s41598-018-27805-3) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gershoni M, Templeton AR, Mishmar D. 2009. Mitochondrial bioenergetics as a major motive force of speciation. Bioessays 31, 642–650. ( 10.1002/bies.200800139) [DOI] [PubMed] [Google Scholar]
- 14.Lane N. 2009. Biodiversity: on the origin of bar codes. Nature 462, 272–274. ( 10.1038/462272a) [DOI] [PubMed] [Google Scholar]
- 15.Birky CW. 1995. Uniparental inheritance of mitochondrial and chloroplast genes: mechanisms and evolution. Proc. Natl Acad. Sci. USA 92, 11 331–11 338. ( 10.1073/pnas.92.25.11331) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gemmell NJ, Metcalf VJ, Allendorf FW. 2004. Mother's curse: the effect of mtDNA on individual fitness and population viability. Trends Ecol. Evol. 19, 238–244. ( 10.1016/j.tree.2004.02.002) [DOI] [PubMed] [Google Scholar]
- 17.Frank SA, Hurst LD. 1996. Mitochondria and male disease. Nature 383, 224 ( 10.1038/383224a0) [DOI] [PubMed] [Google Scholar]
- 18.Innocenti P, Morrow EH, Dowling DK. 2011. Experimental evidence supports a sex-specific selective sieve in mitochondrial genome evolution. Science 332, 845–848. ( 10.1126/science.1201157) [DOI] [PubMed] [Google Scholar]
- 19.Ruiz-Pesini E, et al. 2000. Human mtDNA haplogroups associated with high or reduced spermatozoa motility. Am. J. Hum. Genet. 67, 682–696. ( 10.1086/303040) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Montiel-Sosa F, Ruiz-Pesini E, Enriquez JA, Marcuello A, Diez-Sanchez C, Montoya J, Wallace DC, Lopez-Perez MJ. 2006. Differences of sperm motility in mitochondrial DNA haplogroup U sublineages. Gene 368, 21–27. ( 10.1016/j.gene.2005.09.015) [DOI] [PubMed] [Google Scholar]
- 21.Nakada K, Sato A, Yoshida K, Morita T, Tanaka H, Inoue S, Yonekawa H, Hayashi J. 2006. Mitochondria-related male infertility. Proc. Natl Acad. Sci. USA 103, 15 148–15 153. ( 10.1073/pnas.0604641103) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Breton S, Beaupre HD, Stewart DT, Hoeh WR, Blier PU. 2007. The unusual system of doubly uniparental inheritance of mtDNA: isn't one enough? Trends Genet. 23, 465–474. ( 10.1016/j.tig.2007.05.011) [DOI] [PubMed] [Google Scholar]
- 23.Passamonti M, Ghiselli F. 2009. Doubly uniparental inheritance: two mitochondrial genomes, one precious model for organelle DNA inheritance and evolution. DNA Cell Biol. 28, 79–89. ( 10.1089/dna.2008.0807) [DOI] [PubMed] [Google Scholar]
- 24.Zouros E. 2012. Biparental inheritance through uniparental transmission: the doubly uniparental inheritance (DUI) of mitochondrial DNA. Evol. Biol. 40, 1–31. ( 10.1007/s11692-012-9195-2) [DOI] [Google Scholar]
- 25.Venetis TI, Zouros E, Rodakis GC. 2006. No evidence for presence of maternal mitochondrial DNA in the sperm of Mytilus galloprovincialis males. Proc. R. Soc. B 273, 2483–2489. ( 10.1098/rspb.2006.3607) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ghiselli F, Milani L, Passamonti M. 2010. Strict sex-specific mtDNA segregation in the germ line of the DUI species Venerupis philippinarum (Bivalvia: Veneridae). Mol. Biol. Evol. 28, 949–961. ( 10.1093/molbev/msq271) [DOI] [PubMed] [Google Scholar]
- 27.Milani L, Ghiselli F. 2019. Faraway, so close. The comparative method and the potential of non-model animals in mitochondrial research. Phil. Trans. R. Soc. B 375, 20190186 ( 10.1098/rstb.2019.0186) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gusman A, Lecomte S, Stewart DT, Passamonti M, Breton S. 2016. Pursuing the quest for better understanding the taxonomic distribution of the system of doubly uniparental inheritance of mtDNA. PeerJ 4, e2760 ( 10.7717/peerj.2760) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Plazzi F, Passamonti M. 2019. Footprints of unconventional mitochondrial inheritance in bivalve phylogeny: signatures of positive selection on clades with doubly uniparental inheritance. J. Zool. Syst. Evol. Res. 57, 258–271. ( 10.1111/jzs.12253) [DOI] [Google Scholar]
- 30.Plazzi F, Puccio G, Passamonti M. 2016. Comparative large-scale mitogenomics evidences clade-specific evolutionary trends in mitochondrial DNAs of Bivalvia. Genome Biol. Evol. 8, 2544–2564. ( 10.1093/gbe/evw187) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bettinazzi S, Plazzi F, Passamonti M. 2016. The complete female- and male-transmitted mitochondrial genome of Meretrix lamarckii. PLoS ONE 11, e0153631 ( 10.1371/journal.pone.0153631) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Milani L. 2015. Mitochondrial membrane potential: a trait involved in organelle inheritance? Biol. Lett. 11, 20150732 ( 10.1098/rsbl.2015.0732) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Milani L, Ghiselli F. 2015. Mitochondrial activity in gametes and transmission of viable mtDNA. Biol. Direct. 10, 22 ( 10.1186/s13062-015-0057-6) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Burt A, Trivers R. 2006. Selfish mitochondrial DNA. Genes in conflict: the biology of selfish genetic elements. Cambridge, MA: Belknap Press of Harvard University. [Google Scholar]
- 35.Jha M, Côté J, Hoeh WR, Blier PU, Stewart DT, Swalla B. 2008. Sperm motility in Mytilus edulis in relation to mitochondrial DNA polymorphisms: implications for the evolution of doubly uniparental inheritance in bivalves. Evolution 62, 99–106. [DOI] [PubMed] [Google Scholar]
- 36.Everett EM, Williams PJ, Gibson G, Stewart DT. 2004. Mitochondrial DNA polymorphisms and sperm motility in Mytilus edulis (Bivalvia: Mytilidae). J. Exp. Zool. A Comp Exp. Biol. 301, 906–910. ( 10.1002/jez.a.122) [DOI] [PubMed] [Google Scholar]
- 37.Stewart DT, Jha M, Breton S, Hoeh WR, Blier PU. 2012. No effect of sperm interactions or egg homogenate on sperm velocity in the blue mussel, Mytilus edulis (Bivalvia: Mytilidae). Can. J. Zool. 90, 1291–1296. ( 10.1139/z2012-099) [DOI] [Google Scholar]
- 38.Muller M, et al. 2012. Biochemistry and evolution of anaerobic energy metabolism in eukaryotes. Microbiol. Mol. Biol. Rev. 76, 444–495. ( 10.1128/MMBR.05024-11) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ruiz-Pesini E, Díez-Sánchez C, López-Pérez MJ, Enríquez JA. 2007. The role of the mitochondrion in sperm function: is there a place for oxidative phosphorylation or is this a purely glycolytic process? Curr. Top. Dev. Biol. 77, 3–19. ( 10.1016/S0070-2153(06)77001-6) [DOI] [PubMed] [Google Scholar]
- 40.Storey BT. 2008. Mammalian sperm metabolism: oxygen and sugar, friend and foe. Int. J. Dev. Biol. 52, 427–437. ( 10.1387/ijdb.072522bs) [DOI] [PubMed] [Google Scholar]
- 41.du Plessis SS, Agarwal A, Mohanty G, van der Linde M. 2015. Oxidative phosphorylation versus glycolysis: what fuel do spermatozoa use? Asian J. Androl. 17, 230–235. ( 10.4103/1008-682X.135123) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Moraes CR, Meyers S. 2018. The sperm mitochondrion: organelle of many functions. Anim. Reprod. Sci. 194, 71–80. ( 10.1016/j.anireprosci.2018.03.024) [DOI] [PubMed] [Google Scholar]
- 43.Ferramosca A, Zara V. 2014. Bioenergetics of mammalian sperm capacitation. BioMed Res. Int. 2014, 8 ( 10.1155/2014/902953) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Miki K, Qu W, Goulding EH, Willis WD, Bunch DO, Strader LF, Perreault SD, Eddy EM, O'Brien DA. 2004. Glyceraldehyde 3-phosphate dehydrogenase-S, a sperm-specific glycolytic enzyme, is required for sperm motility and male fertility. Proc. Natl Acad. Sci. USA 101, 16 501–16 506. ( 10.1073/pnas.0407708101) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Tourmente M, Villar-Moya P, Rial E, Roldan ER. 2015. Differences in ATP generation via glycolysis and oxidative phosphorylation and relationships with sperm motility in mouse species. J. Biol. Chem. 290, 20 613–20 626. ( 10.1074/jbc.M115.664813) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Davila MP, Munoz PM, Bolanos JM, Stout TA, Gadella BM, Tapia JA, da Silva CB, Ferrusola CO, Pena FJ. 2016. Mitochondrial ATP is required for the maintenance of membrane integrity in stallion spermatozoa, whereas motility requires both glycolysis and oxidative phosphorylation. Reproduction 152, 683–694. ( 10.1530/REP-16-0409) [DOI] [PubMed] [Google Scholar]
- 47.Boulais M, Soudant P, Le Goïc N, Quéré C, Boudry P, Suquet M. 2015. Involvement of mitochondrial activity and OXPHOS in ATP synthesis during the motility phase of spermatozoa in the Pacific oyster, Crassostrea gigas. Biol. Reprod. 93, 118, 1–7. ( 10.1095/biolreprod.115.128538) [DOI] [PubMed] [Google Scholar]
- 48.Ghiselli F, Breton S, Milani L. 2018. Mitochondrial activity in gametes and uniparental inheritance: a comment on ‘What can we infer about the origin of sex in early eukaryotes?’ Phil. Trans. R. Soc. B 373, 20170147 ( 10.1098/rstb.2017.0147) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Eisenbach M, Giojalas LC. 2006. Sperm guidance in mammals: an unpaved road to the egg. Nat. Rev. Mol. Cell Biol. 7, 276–285. ( 10.1038/nrm1893) [DOI] [PubMed] [Google Scholar]
- 50.Evans JP, Garcia-Gonzalez F, Almbro M, Robinson O, Fitzpatrick JL. 2012. Assessing the potential for egg chemoattractants to mediate sexual selection in a broadcast spawning marine invertebrate. Proc. R. Soc. B 279, 2855–2861. ( 10.1098/rspb.2012.0181) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Oliver M, Evans JP. 2014. Chemically moderated gamete preferences predict offspring fitness in a broadcast spawning invertebrate. Proc. R. Soc. B 281, 20140148 ( 10.1098/rspb.2014.0148) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Lymbery RA, Kennington WJ, Evans JP. 2017. Egg chemoattractants moderate intraspecific sperm competition. Evol. Lett. 1, 317–327. ( 10.1002/evl3.34) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Eads AR, Kennington WJ, Evans JP. 2016. Interactive effects of ocean warming and acidification on sperm motility and fertilization in the mussel Mytilus galloprovincialis. Mar. Ecol. Prog. Ser. 562, 101–111. ( 10.3354/meps11944) [DOI] [Google Scholar]
- 54.Stewart DT, Saavedra C, Stanwood RR, Ball AO, Zouros E. 1995. Male and female mitochondrial DNA lineages in the blue mussel (Mytilus edulis) species group. Mol. Biol. Evol. 12, 735–747. ( 10.1093/oxfordjournals.molbev.a040252) [DOI] [PubMed] [Google Scholar]
- 55.Breton S, Burger G, Stewart DT, Blier PU. 2006. Comparative analysis of gender-associated complete mitochondrial genomes in marine mussels (Mytilus spp.). Genetics 172, 1107–1119. ( 10.1534/genetics.105.047159) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Passamonti M, Boore JL, Scali V. 2003. Molecular evolution and recombination in gender-associated mitochondrial DNAs of the Manila clam Tapes philippinarum. Genetics 164, 603–611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Bettinazzi S, Gendron AD, Breton S. 2019. The effect of cryopreservation on mitochondrial function in freshwater mussel tissue samples (Bivalvia: Unionida). Cryobiology 88, 106–109. ( 10.1016/j.cryobiol.2019.04.006) [DOI] [PubMed] [Google Scholar]
- 58.Munro D, Pichaud N, Paquin F, Kemeid V, Blier PU. 2013. Low hydrogen peroxide production in mitochondria of the long-lived Arctica islandica: underlying mechanisms for slow aging. Aging Cell 12, 584–592. ( 10.1111/acel.12082) [DOI] [PubMed] [Google Scholar]
- 59.Tourmente M, Hirose M, Ibrahim S, Dowling DK, Tompkins DM, Roldan ERS, Gemmell NJ. 2017. mtDNA polymorphism and metabolic inhibition affect sperm performance in conplastic mice. Reproduction 154, 341–354. ( 10.1530/REP-17-0206) [DOI] [PubMed] [Google Scholar]
- 60.Fitzpatrick JL, Simmons LW, Evans JP. 2012. Complex patterns of multivariate selection on the ejaculate of a broadcast spawning marine invertebrate. Evolution 66, 2451–2460. ( 10.1111/j.1558-5646.2012.01627.x) [DOI] [PubMed] [Google Scholar]
- 61.Liu G, Innes D, Thompson RJ. 2011. Quantitative analysis of sperm plane circular movement in the blue mussels Mytilus edulis, M. trossulus and their hybrids. J. Exp. Zool. A Ecol. Genet. Physiol. 315A, 280–290. ( 10.1002/jez.674) [DOI] [PubMed] [Google Scholar]
- 62.Levitan DR. 2000. Sperm velocity and longevity trade off each other and influence fertilization in the sea urchin Lytechinus variegatus. Proc. R. Soc. Lond. B 267, 531–534. ( 10.1098/rspb.2000.1032) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.R Core Team. 2016. R: a language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing; See https://www.R-project.org/. [Google Scholar]
- 64.Breton S, Stewart DT, Blier PU. 2009. Role-reversal of gender-associated mitochondrial DNA affects mitochondrial function in Mytilus edulis (Bivalvia: Mytilidae). J. Exp. Zool. B 312, 108–117. ( 10.1002/jez.b.20251) [DOI] [PubMed] [Google Scholar]
- 65.de Zwaan A, Wijsman TCM. 1976. Anaerobic metabolism in bivalvia (Mollusca) characteristics of anaerobic metabolism. Comp. Biochem. Physiol. B Comp. Biochem. 54, 313–323. ( 10.1016/0305-0491(76)90247-9) [DOI] [PubMed] [Google Scholar]
- 66.Lee A-C, Lee K-T. 2011. The enzyme activities of opine and lactate dehydrogenases in the gills, mantle, foot, and adductor of the hard clam Meretrix lusoria. J. Mar. Sci. Technol. 19, 361–367. [Google Scholar]
- 67.Dando PR, Storey KB, Hochachka PW, Storey JM. 1981. Multiple dehydrogenases in marine molluscs: electrophoretic analysis of alanopine dehydrogenase, strombine dehydrogenase, octopine dehydrogenase and lactate dehydrogenase. Mar. Biol. Lett. 2, 249–257. ( 10.7717/peerj.1956/supp-1) [DOI] [Google Scholar]
- 68.Knorre DA. 2019. Intracellular quality control of mitochondrial DNA: evidence and limitations. Phil. Trans. R. Soc. B 375, 20190176 ( 10.1098/rstb.2019.0176) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Brooks GA, Dubouchaud H, Brown M, Sicurello JP, Butz CE. 1999. Role of mitochondrial lactate dehydrogenase and lactate oxidation in the intracellular lactate shuttle. Proc. Natl Acad. Sci. USA 96, 1129–1134. ( 10.1073/pnas.96.3.1129) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Kane DA. 2014. Lactate oxidation at the mitochondria: a lactate-malate-aspartate shuttle at work. Front. Neurosci. 8, 366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Gallina FG, Deburgos NMG, Burgos C, Coronel CE, Blanco A. 1994. The lactate/pyruvate shuttle in spermatozoa: operation in vitro. Arch. Biochem. Biophys. 308, 515–519. ( 10.1006/abbi.1994.1072) [DOI] [PubMed] [Google Scholar]
- 72.Storey BT, Kayne FJ. 1977. Energy metabolism of spermatozoa. VI. Direct intramitochondrial lactate oxidation by rabbit sperm mitochondria. Biol. Reprod. 16, 549–556. [PubMed] [Google Scholar]
- 73.Passarella S, de Bari L, Valenti D, Pizzuto R, Paventi G, Atlante A. 2008. Mitochondria and l-lactate metabolism. FEBS Lett. 582, 3569–3576. ( 10.1016/j.febslet.2008.09.042) [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets supporting this article have been uploaded as part of the electronic supplementary material.