Abstract
Mitochondria provide the vast majority of cellular energy available to eukaryotes. Therefore, adjustments in mitochondrial function through genetic changes in mitochondrial or nuclear-encoded genes might underlie environmental adaptation. Environmentally induced plasticity in mitochondrial function is also common, especially in response to thermal acclimation in aquatic systems. Here, we examined mitochondrial function in mayfly larvae (Baetis and Drunella spp.) from high and low elevation mountain streams during thermal acclimation to ecologically relevant temperatures. A multi-substrate titration protocol was used to evaluate different respiratory states in isolated mitochondria, along with cytochrome oxidase and citrate synthase activities. In general, maximal mitochondrial respiratory capacity and oxidative phosphorylation coupling efficiency decreased during acclimation to higher temperatures, suggesting montane insects may be especially vulnerable to rapid climate change. Consistent with predictions of the climate variability hypothesis, mitochondria from Baetis collected at a low elevation site with highly variable daily and seasonal temperatures exhibited greater thermal tolerance than Baetis from a high elevation site with comparatively stable temperatures. However, mitochondrial phenotypes were more resilient than whole-organism phenotypes in the face of thermal stress. These results highlight the complex relationships between mitochondrial and organismal genotypes, phenotypes and environmental adaptation.
This article is part of the theme issue ‘Linking the mitochondrial genotype to phenotype: a complex endeavour’.
Keywords: Oroboros Oxygraph 2k, flux control factor, mitochondrial respiration, G × E effects, climate variability hypothesis, thermal tolerance
1. Introduction
Mitochondria are a near-ubiquitous characteristic of eukaryotes and provide the vast majority of ATP used to maintain eukaryotic cellular functions [1]. The acquisition of the original mitochondrial endosymbiont has been hypothesized as the driver that allowed eukaryotes to achieve levels of organismal and genomic complexity not observed in prokaryotes [2–5]. A role for mitochondria has also been implicated in nearly all major aspects of eukaryotic evolution, from ageing to sexual reproduction [6–8]. It is, therefore, unsurprising that mitochondria have been suggested to play a role in environmental adaptation.
Mitochondria function in a variety of cellular processes, from calcium sequestration to anti-oxidant defence, but their central bioenergetic task is ATP production [9]. Because energy production is such a vital cellular process, changes in mitochondrial function can alter many organismal phenotypes. Mitochondrial functions and the organismal phenotypes they influence are dependent on multiple genomes. Most genetic products that influence mitochondrial functions are encoded by the nuclear genome. However, almost all mitochondria maintain an independent mitochondrial genome [10,11] and integration of mitochondrial and nuclear gene products dictates mitochondrial functionality [12]. Despite the observation that mutations in human mitochondrial genomes can cause disease [13], it was long assumed that standing mitochondrial genomic variation in natural populations was effectively neutral [14]. This paradigm has now been largely discarded with the recognition that selection can act on mitochondrial genomic variation, which can in turn influence organismal phenotype and fitness [15,16]. Meta-analysis confirms that when the influence of cytoplasmic genomes on organismal phenotypes are examined, the effects are generally significant [17].
Mitochondrial phenotypes are shaped not only by their underlying mitochondrial and nuclear genotypes but also by the environment in which they are expressed [18]. For example, the same mitonuclear genotype might show a different rate of mitochondrial respiration after acclimation to a new environment. Such mitochondrial plasticity has been investigated thoroughly in aquatic systems in response to changes in environmental temperature (table 1). Water temperatures are usually more stable than air temperatures due to the high heat capacity of water [39]. Moreover, aquatic species are usually ectotherms, meaning that their body temperatures conform to environmental temperatures. Both traits have made aquatic species exemplars for studying thermal plasticity.
Table 1.
Examples of systems with evidence for thermal acclimation of mitochondrial phenotypes.
| taxon | mitochondrial phenotype | references |
|---|---|---|
| fishes Oncorhynchus, Notothenia, Gadus, Perca, Myoxocephalus, Fundulus, Salvelinus, Zoarces, Pachycara, Pleuragramma |
respiration, enzyme activities, membrane potential, membrane composition | [19–30] |
| insects Eurosta, Epiblema |
enzyme activities | [31,32] |
| annelids Lumbricus, Arenicola |
respiration, enzyme activities, membrane composition | [33,34] |
| molluscs Crassostrea, Sepia, Mercenaria |
respiration, enzyme activities, membrane potential, membrane composition | [29,35–38] |
One outstanding question in thermal biology is the generality of the climate variability hypothesis (CVH), which states that thermal tolerance should be higher in species from environments that experience greater thermal variability [40]. Originally, the CVH was formulated to explain latitudinal patterns of biodiversity, with one prediction being that temperate species should show greater thermal tolerance compared to their tropical counterparts. This prediction has been supported in a wide range of taxa based on measures of thermal tolerance [41–49]. Predictions of the CVH should extend to acclimation ability as well—species from more variable environments should be able to acclimate to a wider range of temperatures [47], although this may depend on the scale of environmental variability (e.g. daily versus yearly) and its predictability. Thermal plasticity in mitochondrial function has also been compared between populations from different environments [19–21,33]. These studies usually focus on comparing species from different latitudes, although some work has shown or implied altered mitochondrial phenotypes across elevation gradients [50–52]. The CVH should apply among populations across elevations in the temperate latitudes, where low elevation environments exhibit much more thermal variation throughout the year than high elevation environments, especially with regard to mountain streams [45–47,53,54].
Larval aquatic insects are a powerful system to test thermal plasticity of mitochondrial phenotypes. Thermal variation in streams is vital to the life history of aquatic insects and influences dormancy duration, growth rates, and time of emergence [55]. In temperate mountains, aquatic insects carry out most of their development in streams, often at near-freezing temperatures (figure 1a) before emerging as winged adults. High elevation species generally show greater thermal sensitivity compared to low elevation species, especially at temperate latitudes [45,53], making them ideal for extending predictions of the CVH. Here, for the first time, we investigate thermal acclimation of mitochondrial respiration and enzyme activities in aquatic insects from temperate mountain streams. We predicted that mitochondrial phenotypes would show thermal plasticity, as documented in other aquatic species (table 1). Under the CVH, we also predicted mitochondrial phenotypes from low elevation insects would show greater thermal plasticity compared to those from high elevations.
Figure 1.
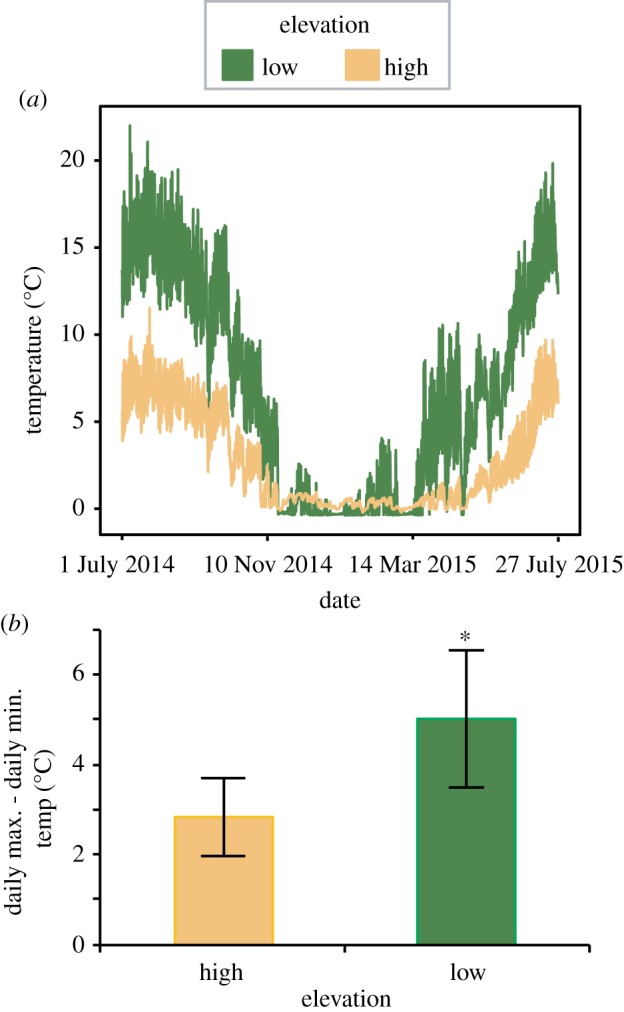
Thermal variation at high elevation (Killpecker) and low elevation (Elkhorn) streams in Colorado, USA. (a) Temperature measured every 30 min at each site over a year. (b) The difference between maximum and minimum temperatures recorded during a 24 h time period at each site averaged for July and August 2014. Error bars show standard deviation. Asterisk indicates a significant difference between high and low elevation (p < 0.001, Student's t-test). (Online version in colour.)
2. Methods
(a). Animal collection and acclimation
Mayfly larvae were collected during July and August 2017 from two streams in the Cache la Poudre river drainage in Colorado, USA: a low elevation site, Spring Creek, in Fort Collins (40°33'45.5″ N, 105°04'39.9″ W, elevation 1519 m) and a high elevation site, Killpecker Creek, in the Arapahoe and Roosevelt National Forests, near Red Feather Lakes (40°48'51.9″ N, 105°42'34.0″ W, elevation 2788 m). Thermal data from streams at similar elevations within the same river drainage confirm that high elevation sites show less seasonal variation (figure 1a) as well as less diel variation (figure 1b).
We collected mayflies between 09.00 and 12.00 with a D-frame kick net (mesh size: 500 µm). Insects were identified in the field to genus based on morphology. Species in the genus Baetis were the most common at both sites and were targeted for this study. Although B. tricaudatus and B. bicaudatus separate along an elevation gradient in many streams [56], a recent study has suggested these species hybridize at approximately 2700 m [57]. Therefore, we refer to these samples as being from the Baetis species complex for this study. Drunella coloradensis were also found in abundance at the high elevation site. Therefore, elevation effects associated with predictions of the CVH were investigated for Baetis samples, while general thermal plasticity was investigated for Drunella. Insects were transported to the laboratory in Fort Collins, within a few hours of collection in aerated, chilled water from the stream sites. In the laboratory, we randomly assigned insects to cold (6°C) or warm (16°C) acclimation treatments, which represented the average summer temperatures at high and low elevation streams, respectively (figure 1a). To mimic stream conditions, we also provided oxygenation and flow in the acclimation containers and maintained insects for 2–6 days before isolating mitochondria for experiments. This duration was chosen because it is sufficient to cause an acclimation response in the critical thermal maximum (CTMAX) for these insects [58].
Warm acclimation appeared to induce higher rates of mortality and emergence, especially in Drunella. Therefore, during the last collection of Drunella, we quantified how many individuals could not be tested due to either mortality or metamorphosis in each acclimation treatment (n = 50 per acclimation temperature).
(b). Mitochondrial isolation
After acclimation, we isolated mitochondria from insects following a procedure developed for Drosophila [59]. Briefly, insects were anaesthetized on ice, excess water was wicked away with KimWipes, and then animals were placed into a vial containing approximately 3 ml of ice-cold isolation buffer (0.32 M sucrose, 10 mM EDTA, 10 mM Tris/HCl, 2% BSA at pH 7). We pooled 1–7 Drunella (depending on size) to create a single biological sample, and 16–74 Baetis per sample. Each sample was approximately 25 mg wet weight. Samples were ground using Potter-Elvehjem tissue grinders (DWK Life Sciences, Millville, NJ) attached to a drill press at maximal speed (1000 r.p.m.) for 1–2 min on ice until homogenized. Mitochondrial homogenates were then filtered through two layers of cheesecloth and a layer of Miracloth and aliquoted into two 2 ml microcentrifuge tubes. Samples were centrifuged at 600g for 5 min, and the resulting supernatant was centrifuged at 2200g for 10 min at 4°C. The resulting pellet was washed and then resuspended with BSA-free isolation buffer and centrifuged again at 2200g for 10 min to obtain functionally robust organelles while minimizing contamination with damaged/fragmented mitochondria or other cellular components [59,60]. The pellets from each of the two tubes were resuspended in 100 µl BSA-free isolation buffer and then combined into a single tube to produce a final 200 µl mitochondrial isolate. Protein concentration was then quantified using a Qubit fluorometer (ThermoFisher Scientific) with the accompanying Qubit protein assay kit and final data were expressed per mg protein. Mitochondrial isolates were kept on ice until respiration experiments.
(c). Mitochondrial respiration
We quantified respiration in fresh mitochondrial isolates using an Oxygraph 2k (O2K) high-resolution respirometer (Oroboros Instruments GmbH, Innsbruck, Austria). A volume of isolate equivalent to 0.5–1.0 mg of total protein (depending on the size and number of animals in each sample) was added for Drunella, and 0.25 mg was used for Baetis. Respiration rates were expressed relative to the total protein added in each sample. Two machines, each with two respiration chambers, were used to enable four samples to be run simultaneously. Up to eight samples were run per day, requiring mitochondrial isolates to be on ice for either 1 or 4 h prior to experimentation. Before adding mitochondrial isolates, chambers were rinsed with 70% ethanol (3×) and milliQ H2O (6×), then filled with MiR05 respiration buffer containing (in mM) 0.5 EGTA, 3 MgCl2, 60 K-lactobionate, 20 taurine, 10 KH2PO4, 20 HEPES, 110 sucrose and 1 g l−1 fatty-acid free BSA, and air-calibrated to a starting oxygen content of 160 µM.
A multi-substrate titration protocol was used to investigate three distinct respiratory states in aquatic insect mitochondria (figure 2). The first was a low flux LEAK respiration state generated by adding a combination of substrates (1 mM malate, 10 mM pyruvate and 10 mM glutamate) in the absence of ADP. LEAK respiration supports NADH-linked electron flow facilitated by non-specific proton leak across the inner mitochondrial membrane. The next state was oxidative phosphorylation (OXPHOS)-linked (state 3) respiration generated by the addition of 3 mM ADP to the same chamber, which enables a higher rate of NADH-linked electron flow by dissipating the inner membrane proton gradient through the ATP synthase. Finally, a maximal OXPHOS-linked respiration rate was generated by the addition of 20 mM succinate to provide additional electron supply through succinate dehydrogenase (CII), thereby fully reconstituting the supply of reducing equivalents from the tricarboxylic acid cycle to the electron transfer system. During experimentation, oxygen concentration in the chambers was recorded every 2 s and oxygen flux was calculated based on the previous 10 s of data. The average of at least 2 min of stable oxygen flux recordings were used as the respiration rate for each state.
Figure 2.
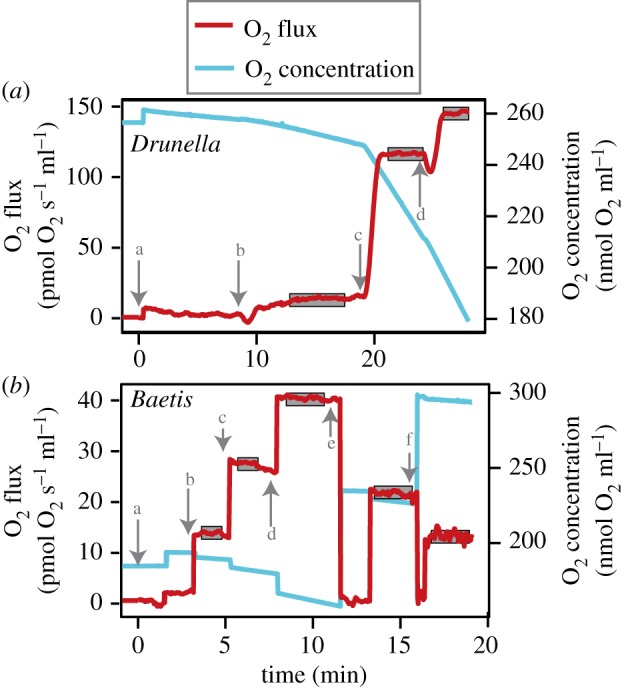
The protocol used here for quantifying mitochondrial respiration from aquatic insects. In both (a) Drunella and (b) Baetis, a representative set of data from a single experiment are shown. Lower case letters and arrows represent adding the following: (a) mitochondrial isolate, (b) malate, pyruvate and glutamate, (c) ADP and (d) succinate. For Baetis, (e) and (f) indicate when the temperature of the respiration chamber was decreased to 16°C and 6°C, respectively. Grey boxes represent stable stretches of data used to calculate oxygen consumption for each respiratory state. (Online version in colour.)
In addition to generating multiple respiration states from a single biological sample, substrate titration protocols enable calculation of flux control factors (FCFs), which reveal the extent of respiratory control exerted by a selected metabolite or pathway by expressing flux relative to an internal reference state from the same experiment [61]. We calculated two FCFs: OXPHOS coupling efficiency and CII flux control. OXPHOS coupling efficiency was calculated as (1 – (LEAK/OXPHOS)) to represent the extent of ADP control over CI-supported respiration (linearized form of the classic respiratory control ratio [61–63]), ranging from 0 (non-ADP controlled) to 1 (maximally coupled to ADP). CII flux control was calculated as (1 – (O2 flux prior to adding succinate divided by flux after adding succinate)), representing the proportion of maximal respiration contributed by CII, which can vary from 0 to 1.
For Drunella, samples were tested at either 6°C or 16°C to investigate effects of test temperature. For Baetis, limited sample sizes precluded the use of multiple test temperatures. However, we investigated how maximal respiration varied with test temperature by decreasing the temperature in the respiration chambers after maximal respiration rates were reached. The beginning test temperature (and the one used to calculate FCFs) was 28°C. After maximal respiration was reached, the temperature was dropped gradually to 16°C and a new steady state of maximal respiration was recorded (figure 2b). A final drop to 6°C yielded one last reading for maximal respiration (figure 2b). O2 concentration was recalibrated in the chambers at each test temperature before collecting data.
(d). Mitochondrial enzyme activities
To complement respiration measurements, mitochondrial isolates were frozen after respiration experiments and were later used to quantify cytochrome c oxidase (COX) and citrate synthase activities. For both enzymes, reactions were performed in 96 well plates and read using a Spectramax 2e spectrofluorometer plate reader. At least two technical replicates were averaged per sample. For COX, the reaction is based on COX in the sample oxidizing reduced cytochrome c, resulting in a linear decrease in absorbance at 550 nm [64]. For each reaction, 200 µl of sample buffer (120 mM Tris–KCl and 250 mM Tris–sucrose), 2–10 µg of mitochondrial isolate and 0.22 mM of reduced CytC were added and absorbance was recorded every 30 s for 10 min at 550 nm. COX activity was taken as the absolute slope of a line through these data and was normalized to protein content. For citrate synthase, we followed a similar procedure: 200 µl of sample buffer (20 mM HEPES, 1 mM EGTA, 220 mM sucrose, 40 mM KCl, 0.1 mM DNTB and 0.1 mM acetyl CoA), 5 µg of mitochondrial isolate and 0.05 mM oxaloacetate was added per reaction and absorbance at 412 nm was read every 30 s for 10 min. The reaction is based on the formation of 5-thio-2-nitrobenzoic acid (TNB), which results in an increase in absorbance at 412 nm [65]. All COX and citrate synthase reactions were performed at 25°C.
(e). Statistical analyses
R v.3.4.4 was used to test for effects of acclimation temperature, test temperature, elevation, and their interactions on the mitochondrial phenotypes measured based on general linear models (i.e. 2 × 2 ANOVAs) and the lm function. We included the number of acclimation days and the time since mitochondrial isolation as random factors in preliminary tests (using the lme function) but results were not qualitatively different as neither acclimation duration nor incubation time on ice significantly affected the parameters measured here.
3. Results
(a). Thermal acclimation of mitochondrial respiration
Mitochondrial respiration phenotypes were strongly influenced by thermal acclimation in Drunella (figure 3). LEAK respiration did not vary with acclimation temperature (figure 3a, p = 0.680), test temperature (p = 0.150), or their interaction (p = 0.533). However, maximal (i.e. CI + CII-supported) OXPHOS-linked respiration was higher in cold-acclimated animals at both test temperatures (figure 3b), although this was only statistically significant at 6°C (p = 0.179 main effect, ANOVA; p = 0.037 t-test at 6°C). Maximal respiration was highest at the warmer test temperature (p = 0.014), and there was no interaction between test and acclimation temperature (p = 0.504). Animals acclimated to the colder temperature showed 15% higher OXPHOS coupling efficiency compared to those acclimated to the warmer temperature (figure 3c, p = 0.022). Using the warmer test temperature also resulted in higher OXPHOS coupling efficiency (p = 0.035). While not statistically significant, the difference in OXPHOS coupling efficiency between acclimation treatments appeared to be more extreme at the lower test temperature (interaction p = 0.073). The proportion of maximum respiration attributable to CII (i.e. the CII FCF) was approximately 10% higher in insects acclimated to warm temperatures (figure 3d, p = 0.030), and was lower in insects tested at warm temperatures (p = 0.016). There was no suggestion of an interaction with test temperature (p = 0.916). Finally, in the last collection of Drunella, mortality/metamorphosis rates were 10% and 43% in the cold and warm acclimation treatment, respectively.
Figure 3.
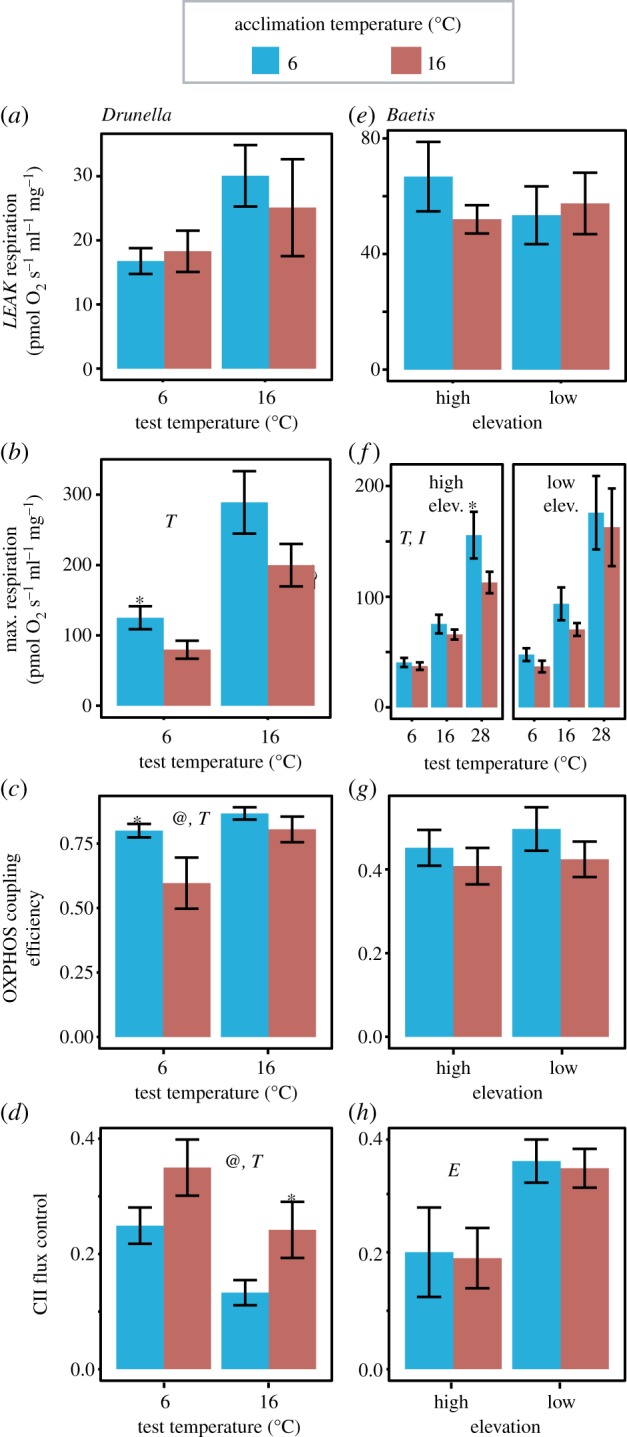
Mitochondrial respiration in Drunella (a–d) and Baetis (e–h) mayfly larvae acclimated to either cold (6°C) or warm (16°C) temperatures. LEAK (a,e) and maximal respiration (i.e. CI + CII) is presented (b,f) along with two flux control factors that were analysed: OXPHOS coupling efficiency (c,g) and CII flux control (d,h). Elevation and test temperature effects are presented for Baetis in (f). Error bars show ±s.e.m. @, T, E and I indicate significant main effects for acclimation temperature, test temperature, elevation or their interactions in the 2 × 2 ANOVAs, respectively. Asterisks indicate significant effects of acclimation temperature for individual comparisons based on Student's t-tests. For Drunella, n = 10 for 6°C acclimation, n = 9 for 16°C acclimation tested at 16°C and n = 6 for 16°C acclimation tested at 6°C. For Baetis, n = 10 for all treatments. (Online version in colour.)
Baetis showed no difference in LEAK respiration attributable to elevation, acclimation temperature, or their interaction (figure 3e, p > 0.290 for all main effects). Maximal respiration was quantified at three test temperatures for Baetis and, like Drunella, respiration increased with increasing test temperatures (figure 3f, average Q10 = 1.84, p < 0.001). Also similar to Drunella, Baetis acclimated to the lower temperature tended to have higher maximal respiration, although this was not statistically significant (p = 0.121 main acclimation effect, ANOVA). There was an especially large difference due to acclimation in animals from high elevation and tested at the high temperature, resulting in a weakly significant interaction between acclimation temperature and elevation (p = 0.049) and a significant interaction between acclimation temperature and test temperature (p < 0.001). As with Drunella, there was a trend for higher OXPHOS coupling efficiency in Baetis when acclimated to the colder temperature, although the difference was not statistically significant (figure 3g, p = 0.200, main acclimation effect). Elevation did not influence OXPHOS efficiency and there was no interaction between elevation and acclimation (p > 0.570 for both). The CII FCF was approximately 80% higher in Baetis from the low elevation site (figure 3h, p = 0.004), although acclimation temperature did not influence the CII FCF and there was no interaction between elevation and acclimation (p > 0.891 for both).
(b). Thermal acclimation of mitochondrial enzyme activities
For Drunella, acclimation temperature did not influence the activities of COX (figure 4a, p = 0.387) or citrate synthase (figure 4b, p = 0.943). For Baetis, elevation, acclimation temperature, or the interaction between the two did not influence COX or citrate synthase activity (figure 4c,d, p > 0.076).
Figure 4.
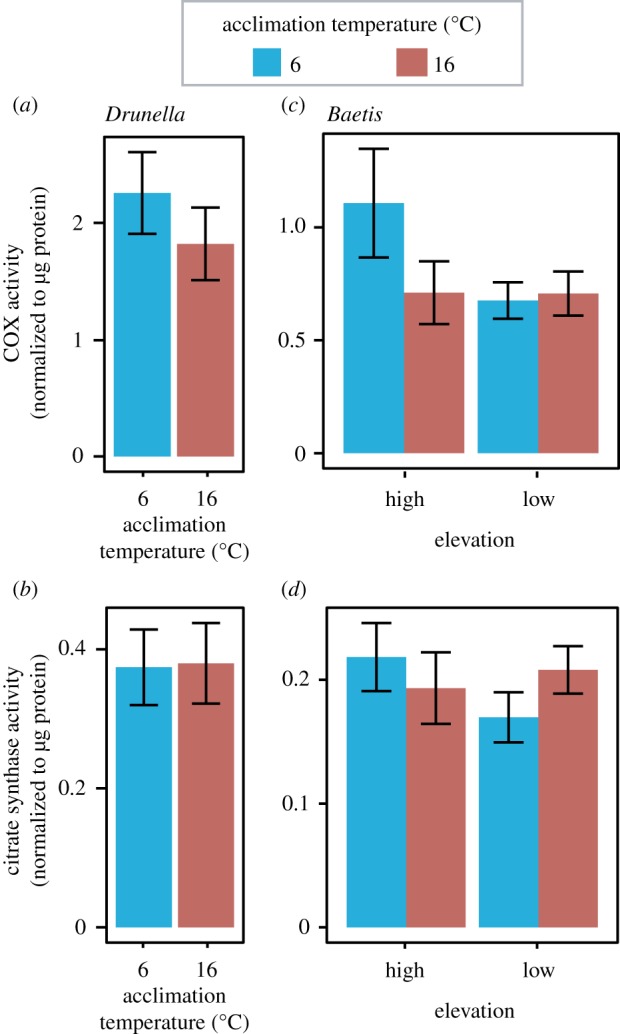
Cytochrome c oxidase (a,c) and citrate synthase (b,d) activities in Drunella (a,b) and Baetis (c,d) mayfly larvae acclimated to either cold (6°C) or warm (16°C) temperatures. Elevation effects are presented for Baetis (c,d). Error bars show ±s.e.m. For Drunella, n = 5–9. For Baetis, n = 9–11. No significant effects were found. (Online version in colour.)
4. Discussion
(a). Mitochondrial phenotypic plasticity in aquatic insect larvae
Here, we demonstrate thermal plasticity in mitochondrial phenotypes in mayfly larvae from mountain streams, as in many aquatic systems (table 1). In both genera examined, OXPHOS coupling efficiency and maximal mitochondrial respiration were higher in insects acclimated to low temperatures at each test temperature (figure 3). This suggests mitochondrial respiration is most closely coupled to ATP production and respiratory capacity is highest in insects acclimated to relatively low temperatures. This is in agreement with previous studies suggesting cold acclimation increases mitochondrial respiratory capacity, possibly as a way to maintain high activity levels in the cold [18,66–69]. In some cases, this is attributed to increased mitochondrial proliferation with cold acclimation, which is often approximated using citrate synthase assays [22,70]. Here, citrate synthase activities did not change in response to cold acclimation, suggesting mitochondrial populations remained fairly stable. The contribution of CII to mitochondrial respiration was also plastic (figure 3d). For Drunella, increased CII flux control at warm acclimation temperatures may reflect a reduced capacity for CI-supported respiration (figure 3c). For Baetis, the CII FCF was not affected by acclimation temperature, suggesting different mitochondrial responses to thermal acclimation in this genus compared to Drunella.
One consistent pattern in these data is that acclimation to ecologically relevant warm temperatures caused altered mitochondrial phenotypes. We observed this pattern in Baetis even at low elevation sites where the warm acclimation temperature (16°C) is commonly exceeded in the summer (figure 1a). Warm acclimation has been shown to cause decreased mitochondrial coupling in other ectotherms, possibly due to proton leak increasing more rapidly with temperature than OXPHOS [66,71]. However, in this study, proton leak did not increase with temperature (figure 3a,e).
We did observe elevated levels of mortality and emergence in the warm acclimation treatment compared to the cold acclimation, suggesting that altered mitochondrial functions might be indicative of stress. Even though warm acclimation temperatures are common, they may be offset by cooler night-time temperatures, and experiencing such elevated temperatures constantly for days, as in the experiment here, may therefore induce thermal stress. Future experiments may benefit from using fluctuating acclimation temperatures. The finding that even modestly elevated temperatures may cause stress in these systems is in line with previous studies suggesting montane insects may be especially vulnerable to rapid climate change [53]. However, it should be noted that when tested at their acclimation temperatures, both warm- and cold-acclimated groups showed similar OXPHOS coupling efficiency and maximum respiration, suggesting mitochondrial function may recover with prolonged warm acclimation.
Another possibility is that altered mitochondrial functions may be under compensation at higher organization levels. Some evidence from the mayfly system supports this hypothesis. Critical thermal maxima (CTMAX) are well above 16°C for all populations of the taxa examined here [45,53,58] and whole-organism oxygen consumption increases somewhat linearly with temperature up to 25°C (AA Shah et al. 2019, unpublished data). Therefore, while mitochondrial phenotypes may be compromised at high temperatures, organismal stress may be ameliorated by compensatory mechanisms.
The data presented here indicate that understanding the relationship between mitochondrial genotype and phenotype is complex. In this system, acclimation to a constant 16°C was enough to result in decreased OXPHOS coupling efficiency and declines in maximum respiration, although this temperature results in increased respiration at the whole-animal level and is well below the CTMAX [45,53] (AA Shah et al. 2019, unpublished data). This observed disconnect between whole-animal and mitochondrial physiology seems to be common. For example, in the killifish Fundulus heteroclitus mitochondrial oxygen consumption continues to increase with temperature up to 40°C, even though the CTMAX is below this temperature [72]. In another mayfly system, whole-animal oxygen consumption and aerobic scope likewise did not decrease even at temperatures above the CTMAX [73]. The result here that maximal mitochondrial respiration increased with test temperature (figure 3c,f), even up to 28°C (approaching CTMAX), echoes these previous findings. One reason for this disconnect may be that isolated mitochondria are more likely to follow Arrhenius-like processes compared to more complex systems such as whole organisms [72,74]. Examining the relationships between genotypes, mitochondrial phenotypes, whole-organism phenotypes, and ecologically relevant responses is, therefore, a worthwhile endeavour for future studies.
(b). Mitochondrial plasticity and the climate variability hypothesis
Although thermal plasticity in mitochondrial phenotypes has been investigated in many aquatic systems (table 1), few studies have compared mitochondrial plasticity among different populations [19–21,33]. In general, such studies have examined acclimation of mitochondrial function in the context of cold-adapted versus warm-adapted populations. For example, cod (Gadus morhua) from a cold-adapted population showed a larger increase in citrate synthase activity in white muscle when acclimated to 4°C compared to a more temperate population [22]. Lugworms from cold-adapted populations also showed increased mitochondrial populations and COX activities [33,75]. Schulte and colleagues have also described increased mitochondrial respiration, uncoupling responses, and enzyme activities during cold acclimation in northern killifish in comparison to southern populations [23,24,76–78]. Although warm-adapted populations also typically experience more climate variability, previous studies have rarely explicitly considered mitochondrial thermal plasticity in the context of stable versus variable thermal environments.
Key predictions of the CVH could be tested by studying Baetis mayflies collected from high and low elevation streams. Both sites routinely experience freezing temperatures in the winter and early spring, when newly hatched larvae enter a four-month growth phase prior to emerging by late summer [79]. However, the low elevation site experiences much warmer maximum temperatures and wider daily thermal fluctuations than the high elevation site during the summer months (figure 1), leading to the prediction that low elevation insects should exhibit greater thermal tolerance than high elevation insects. Consistent with this prediction, warm acclimation temperatures caused a decline in the OXPHOS-linked respiratory capacity of mitochondria from Baetis collected at the high elevation site, but not the low elevation site (figure 3f). This was only observed at the test temperature of 28°C, which is warmer than the maximum summer temperatures experienced even at the low elevation site (22°C, figure 1a). However, thermal ranges of mitochondrial phenotypes may be disconnected from those of whole organisms, as discussed above. Moreover, Baetis do occur at elevations lower than those examined here and small lowland streams can get much warmer, making high test temperatures ecologically relevant. Although streams at both elevations routinely freeze, one caveat to using this study system is that while different elevations show different degrees of thermal variability, populations across elevations could also be considered as cold- versus warm-adapted.
Although not statistically significant, COX activity in Baetis followed a similar trend as OXPHOS-linked respiration to decline following warm acclimation only in high elevation insects (figure 4c), which is consistent with results from other cold-adapted populations [20,22,33]. By contrast, FCFs and citrate synthase did not vary in accordance with the CVH, suggesting that thermal sensitivity of mitochondria is at the level of the membrane electron transfer complexes, and perhaps COX in particular. This emphasizes the benefit of the protocol employed here to disentangle multiple aspects of mitochondrial function that might be obscured by evaluating only single enzyme markers of mitochondrial content or matrix volume such as citrate synthase.
When examining CTMAX and CTMIN in this system, predictions of the CVH are generally supported, with CTMAX decreasing at higher elevation (dropping from 31 to 27°C) and CTMIN being low and consistent across elevation (approx. 1°C) [45,53]. As with mitochondrial plasticity, there seems to be some disconnect between mitochondrial phenotypes, organismal phenotypes, and ecology in relation to the CVH. While some aspects of mitochondrial function support predictions of the CVH, they show weaker patterns than those at the whole-organism level. One explanation in this system may be high levels of genetic connectivity among high and low elevation sites [53,57]. If genotypes are shared among sites, levels of plasticity in mitochondrial phenotypes (dictated by mitochondrial and nuclear genotypes) may be similar among sites. In comparable aquatic insect species in the tropics, genetic connectivity across elevation is low, and differences in acclimation responses among sites are much more extreme than in the temperate sites investigated here [53]. This is consistent with Janzen's classic hypothesis of why mountain passes are ‘higher’ in the tropics [54]. Clearly, a future goal should be to expand studies of mitochondrial plasticity to tropic versus temperate systems in order to more definitively test predictions of the CVH.
Acknowledgements
We are grateful to the Funk, Ghalambor and Sloan labs at CSU for generously providing equipment used in this study. Luke Link assisted in performing preliminary enzyme activity assays.
Data accessibility
All raw data generated in this study, including mitochondrial respiration values and enzyme activities, are provided at https://figshare.com/s/ab40bed830d2f5b4d7db.
Authors' contributions
All authors designed the experiments and wrote the paper. J.C.H. and A.A.S. performed the experiments.
Competing interests
We declare we have no competing interests.
Funding
This work was supported by NIH grant no. F32GM116361 to J.C.H. and funds from the University of Texas at Austin.
References
- 1.Karnkowska A, et al. 2016. A eukaryote without a mitochondrial organelle. Curr. Biol. 26, 1274–1284. ( 10.1016/j.cub.2016.03.053) [DOI] [PubMed] [Google Scholar]
- 2.Lane N. 2014. Bioenergetic constraints on the evolution of complex life. Cold Spring Harbor Perspect. Biol. 6, a015982 ( 10.1101/cshperspect.a015982) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lane N, Martin W. 2010. The energetics of genome complexity. Nature 467, 929–934. ( 10.1038/nature09486) [DOI] [PubMed] [Google Scholar]
- 4.Lane N, Martin WF. 2016. Mitochondria, complexity, and evolutionary deficit spending. Proc. Natl Acad. Sci. USA 113, E666 ( 10.1073/pnas.1522213113) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lynch M, Marinov GK. 2015. The bioenergetic costs of a gene. Proc. Natl Acad. Sci. USA 112, 15 690–15 695. ( 10.1073/pnas.1421641112) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lane N. 2011. Mitonuclear match: optimizing fitness and fertility over generations drives ageing within generations. Bioessays 33, 860–869. ( 10.1002/bies.201100051) [DOI] [PubMed] [Google Scholar]
- 7.Havird JC, Hall MD, Dowling DK. 2015. The evolution of sex: a new hypothesis based on mitochondrial mutational erosion. Bioessays 37, 951–958. ( 10.1002/bies.201500057) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hill GE. 2015. Mitonuclear ecology. Mol. Biol. Evol. 32, 1917–1927. ( 10.1093/molbev/msv104) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nicholls DG. 2002. Mitochondrial function and dysfunction in the cell: its relevance to aging and aging-related disease. Int. J. Biochem. Cell B 34, 1372–1381. ( 10.1016/S1357-2725(02)00077-8) [DOI] [PubMed] [Google Scholar]
- 10.Santos HJ, Makiuchi T, Nozaki T. 2018. Reinventing an organelle: the reduced mitochondrion in parasitic protists. Trends Parasitol. 34, 1038–1055. ( 10.1016/j.pt.2018.08.008) [DOI] [PubMed] [Google Scholar]
- 11.Roger AJ, Munoz-Gomez SA, Kamikawa R. 2017. The origin and diversification of mitochondria. Curr. Biol. 27, R1177–R1192. ( 10.1016/j.cub.2017.09.015) [DOI] [PubMed] [Google Scholar]
- 12.Sloan DB, Warren JM, Williams AM, Wu ZQ, Abdel-Ghany SE, Chicco AJ, Havird JC. 2018. Cytonuclear integration and co-evolution. Nat. Rev. Genet. 19, 635–648. ( 10.1038/s41576-018-0035-9) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wallace DC. 1992. Diseases of the mitochondrial DNA. Annu. Rev. Biochem. 61, 1175–1212. ( 10.1146/annurev.bi.61.070192.005523) [DOI] [PubMed] [Google Scholar]
- 14.Ballard JWO, Kreitman M. 1995. Is mitochondrial-DNA a strictly neutral marker. Trends Ecol. Evol. 10, 485–488. ( 10.1016/S0169-5347(00)89195-8) [DOI] [PubMed] [Google Scholar]
- 15.Ballard JWO, Whitlock MC. 2004. The incomplete natural history of mitochondria. Mol. Ecol. 13, 729–744. ( 10.1046/j.1365-294X.2003.02063.x) [DOI] [PubMed] [Google Scholar]
- 16.Bock DG, Andrew RL, Rieseberg LH. 2014. On the adaptive value of cytoplasmic genomes in plants. Mol. Ecol. 23, 4899–4911. ( 10.1111/mec.12920) [DOI] [PubMed] [Google Scholar]
- 17.Dobler R, Rogell B, Budar F, Dowling DK. 2014. A meta-analysis of the strength and nature of cytoplasmic genetic effects. J. Evol. Biol. 27, 2021–2034. ( 10.1111/jeb.12468) [DOI] [PubMed] [Google Scholar]
- 18.Seebacher F, Brand MD, Else PL, Guderley H, Hulbert AJ, Moyes CD. 2010. Plasticity of oxidative metabolism in variable climates: molecular mechanisms. Physiol. Biochem. Zool. 83, 721–732. ( 10.1086/649964) [DOI] [PubMed] [Google Scholar]
- 19.Baris TZ, Crawford DL, Oleksiak MF. 2016. Acclimation and acute temperature effects on population differences in oxidative phosphorylation. Am. J. Physiol. Regul. Integr. Comp. Physiol. 310, R185–R196. ( 10.1152/ajpregu.00421.2015) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lannig G, Eckerle LG, Serendero I, Sartoris FJ, Fischer T, Knust R, Johansen T, Portner HO. 2003. Temperature adaptation in eurythermal cod (Gadus morhua): a comparison of mitochondrial enzyme capacities in boreal and Arctic populations. Mar. Biol. 142, 589–599. ( 10.1007/s00227-002-0967-6) [DOI] [Google Scholar]
- 21.Guderley H, St-Pierre JS. 2002. Going with the flow or life in the fast lane: contrasting mitochondrial responses to thermal change. J. Exp. Biol. 205, 2237–2249. [DOI] [PubMed] [Google Scholar]
- 22.Lucassen M, Koschnick N, Eckerle LG, Portner HO. 2006. Mitochondrial mechanisms of cold adaptation in cod (Gadus morhua L.) populations from different climatic zones. J. Exp. Biol. 209, 2462–2471. ( 10.1242/jeb.02268) [DOI] [PubMed] [Google Scholar]
- 23.Chung DJ, Schulte PM. 2015. Mechanisms and costs of mitochondrial thermal acclimation in a eurythermal killifish (Fundulus heteroclitus). J. Exp. Biol. 218, 1621–1631. ( 10.1242/jeb.120444) [DOI] [PubMed] [Google Scholar]
- 24.Chung DJ, Sparagna GC, Chicco AJ, Schulte PM. 2018. Patterns of mitochondrial membrane remodeling parallel functional adaptations to thermal stress. J. Exp. Biol. 221, jeb174458 ( 10.1242/jeb.174458) [DOI] [PubMed] [Google Scholar]
- 25.Sappal R, Fast M, Stevens D, Kibenge F, Siah A, Kamunde C. 2015. Effects of copper, hypoxia and acute temperature shifts on mitochondrial oxidation in rainbow trout (Oncorhynchus mykiss) acclimated to warm temperature. Aquat. Toxicol. 169, 46–57. ( 10.1016/j.aquatox.2015.10.006) [DOI] [PubMed] [Google Scholar]
- 26.Sappal R, MacDougald M, Fast M, Stevens D, Kibenge F, Siah A, Kamunde C. 2015. Alterations in mitochondrial electron transport system activity in response to warm acclimation, hypoxia-reoxygenation and copper in rainbow trout, Oncorhynchus mykiss. Aquat. Toxicol. 165, 51–63. ( 10.1016/j.aquatox.2015.05.014) [DOI] [PubMed] [Google Scholar]
- 27.Strobel A, Leo E, Portner HO, Mark FC. 2013. Elevated temperature and PCO2 shift metabolic pathways in differentially oxidative tissues of Notothenia rossii. Comp. Biochem. Phys. B 166, 48–57. ( 10.1016/j.cbpb.2013.06.006) [DOI] [PubMed] [Google Scholar]
- 28.Ekstrom A, Sandblom E, Blier PU, Cyr BAD, Brijs J, Pichaud N. 2017. Thermal sensitivity and phenotypic plasticity of cardiac mitochondrial metabolism in European perch, Perca fluviatilis. J. Exp. Biol. 220, 386–396. ( 10.1242/jeb.150698) [DOI] [PubMed] [Google Scholar]
- 29.Cherkasov AS, Biswas PK, Ridings DM, Ringwood AH, Sokolova IM. 2006. Effects of acclimation temperature and cadmium exposure on cellular energy budgets in the marine mollusk Crassostrea virginica: linking cellular and mitochondrial responses. J. Exp. Biol. 209, 1274–1284. ( 10.1242/jeb.02093) [DOI] [PubMed] [Google Scholar]
- 30.Pichaud N, Ekstrom A, Hellgren K, Sandblom E. 2017. Dynamic changes in cardiac mitochondrial metabolism during warm acclimation in rainbow trout. J. Exp. Biol. 220, 1674–1683. ( 10.1242/jeb.152421) [DOI] [PubMed] [Google Scholar]
- 31.Joanisse DR, Storey KB. 1994. Mitochondrial-enzymes during overwintering in 2 species of cold-hardy gall insects. Insect. Biochem. Mol. Biol. 24, 145–150. ( 10.1016/0965-1748(94)90080-9) [DOI] [Google Scholar]
- 32.McMullen DC, Storey KB. 2008. Mitochondria of cold hardy insects: responses to cold and hypoxia assessed at enzymatic, mRNA and DNA levels. Insect Biochem. Mol. Biol. 38, 367–373. ( 10.1016/j.ibmb.2007.12.003) [DOI] [PubMed] [Google Scholar]
- 33.Sommer AM, Portner HO. 2002. Metabolic cold adaptation in the lugworm Arenicola marina: comparison of a North Sea and a White Sea population. Marine Ecol. Prog. Ser. 240, 171–182. ( 10.3354/meps240171) [DOI] [Google Scholar]
- 34.Crockett EL, Dougherty BE, McNamer AN. 2001. Effects of acclimation temperature on enzymatic capacities and mitochondrial membranes from the body wall of the earthworm Lumbricus terrestris. Comp. Biochem. Phys. B 130, 419–426. ( 10.1016/S1096-4959(01)00456-0) [DOI] [PubMed] [Google Scholar]
- 35.Ivanina AV, Kurochkin IO, Leamy L, Sokolova IM. 2012. Effects of temperature and cadmium exposure on the mitochondria of oysters (Crassostrea virginica) exposed to hypoxia and subsequent reoxygenation. J. Exp. Biol. 215, 3142–3154. ( 10.1242/jeb.071357) [DOI] [PubMed] [Google Scholar]
- 36.Oellermann M, Portner HO, Mark FC. 2012. Mitochondrial dynamics underlying thermal plasticity of cuttlefish (Sepia officinalis) hearts. J. Exp. Biol. 215, 2992–3000. ( 10.1242/jeb.068163) [DOI] [PubMed] [Google Scholar]
- 37.Sokolova IM. 2004. Cadmium effects on mitochondrial function are enhanced by elevated temperatures in a marine poikilotherm, Crassostrea virginica Gmelin (Bivalvia: Ostreidae). J. Exp. Biol. 207, 2639–2648. ( 10.1242/jeb.01054) [DOI] [PubMed] [Google Scholar]
- 38.Gillis TE, Ballantyne JS. 1999. Influences of subzero thermal acclimation on mitochondrial membrane composition of temperate zone marine bivalve mollusks. Lipids 34, 59–66. ( 10.1007/s11745-999-338-z) [DOI] [PubMed] [Google Scholar]
- 39.Mohseni O, Stefan HG. 1999. Stream temperature air temperature relationship: a physical interpretation. J. Hydrol. 218, 128–141. ( 10.1016/S0022-1694(99)00034-7) [DOI] [Google Scholar]
- 40.Stevens GC. 1989. The latitudinal gradient in geographical range—how so many species coexist in the tropics. Am. Nat. 133, 240–256. ( 10.1086/284913) [DOI] [Google Scholar]
- 41.Sheldon KS, Tewksbury JJ. 2014. The impact of seasonality in temperature on thermal tolerance and elevational range size. Ecology 95, 2134–2143. ( 10.1890/13-1703.1) [DOI] [PubMed] [Google Scholar]
- 42.Deutsch CA, Tewksbury JJ, Huey RB, Sheldon KS, Ghalambor CK, Haak DC, Martin PR. 2008. Impacts of climate warming on terrestrial ectotherms across latitude. Proc. Natl Acad. Sci. USA 105, 6668–6672. ( 10.1073/pnas.0709472105) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Compton TJ, Rijkenberg MJA, Drent J, Piersma T. 2007. Thermal tolerance ranges and climate variability: a comparison between bivalves from differing climates. J. Exp. Mar. Biol. Ecol. 352, 200–211. ( 10.1016/j.jembe.2007.07.010) [DOI] [Google Scholar]
- 44.Clusella-Trullas S, Blackburn TM, Chown SL. 2011. Climatic predictors of temperature performance curve parameters in ectotherms imply complex responses to climate change. Am. Nat. 177, 738–751. ( 10.1086/660021) [DOI] [PubMed] [Google Scholar]
- 45.Shah AA, et al. 2017. Climate variability predicts thermal limits of aquatic insects across elevation and latitude. Funct. Ecol. 31, 2118–2127. ( 10.1111/1365-2435.12906) [DOI] [Google Scholar]
- 46.Currie DJ. 2017. Mountain passes are higher not only in the tropics. Ecography 40, 459–460. ( 10.1111/ecog.02695) [DOI] [Google Scholar]
- 47.Ghalambor CK, Huey RB, Martin PR, Tewksbury JJ, Wang G. 2006. Are mountain passes higher in the tropics? Janzen's hypothesis revisited. Integr. Comp. Biol. 46, 5–17. ( 10.1093/icb/icj003) [DOI] [PubMed] [Google Scholar]
- 48.Sunday JM, Bates AE, Kearney MR, Colwell RK, Dulvy NK, Longino JT, Huey RB. 2014. Thermal-safety margins and the necessity of thermoregulatory behavior across latitude and elevation. Proc. Natl Acad. Sci. USA 111, 5610–5615. ( 10.1073/pnas.1316145111) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Araujo MB, Ferri-Yanez F, Bozinovic F, Marquet PA, Valladares F, Chown SL. 2013. Heat freezes niche evolution. Ecol. Lett. 16, 1206–1219. ( 10.1111/ele.12155) [DOI] [PubMed] [Google Scholar]
- 50.Dahlhoff EP, et al. 2019. Getting chased up the mountain: high elevation may limit performance and fitness characters in a montane insect. Funct. Ecol. 33, 809–818. ( 10.1111/1365-2435.13286) [DOI] [Google Scholar]
- 51.Scott GR, Guo KH, Dawson NJ. 2018. The mitochondrial basis for adaptive variation in aerobic performance in high-altitude deer mice. Integr. Comp. Biol. 58, 506–518. ( 10.1093/icb/icy056) [DOI] [PubMed] [Google Scholar]
- 52.Ma XH, Kang JL, Chen WT, Zhou CJ, He SP. 2015. Biogeographic history and high-elevation adaptations inferred from the mitochondrial genome of glyptosternoid fishes (Sisoridae, Siluriformes) from the southeastern Tibetan Plateau. BMC Evol. Biol. 15, ARTN 233 ( 10.1186/s12862-015-0516-9) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Polato NR, et al. 2018. Narrow thermal tolerance and low dispersal drive higher speciation in tropical mountains. Proc. Natl Acad. Sci. USA 115, 12 471–12 476. ( 10.1073/pnas.1809326115) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Janzen DH. 1967. Why mountain passes are higher in tropics. Am. Nat. 101, 233–249. ( 10.1086/282487). [DOI] [Google Scholar]
- 55.Ward JV, Stanford JA. 1982. Thermal responses in the evolutionary ecology of aquatic insects. Annu. Rev. Entomol. 27, 97–117. ( 10.1146/annurev.en.27.010182.000525) [DOI] [Google Scholar]
- 56.Gill BA, Harrington RA, Kondratieff BC, Zamudio KR, Poff NL, Funk WC. 2014. Morphological taxonomy, DNA barcoding, and species diversity in southern Rocky Mountain headwater streams. Freshw. Sci. 33, 288–301. ( 10.1086/674526) [DOI] [Google Scholar]
- 57.Polato NR, et al. 2017. Genetic diversity and gene flow decline with elevation in montane mayflies. Heredity 119, 107–116. ( 10.1038/hdy.2017.23) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Shah AA, Funk WC, Ghalambor CK. 2017. Thermal acclimation ability varies in temperate and tropical aquatic insects from different elevations. Integr. Comp. Biol. 57, 977–987. ( 10.1093/icb/icx101) [DOI] [PubMed] [Google Scholar]
- 59.Ferguson M, Mockett RJ, Shen Y, Orr WC, Sohal RS. 2005. Age-associated decline in mitochondrial respiration and electron transport in Drosophila melanogaster. Biochem. J. 390, 501–511. ( 10.1042/Bj20042130) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Venditti P, Costagliola IR, Di Meo S. 2002. H2O2 production and response to stress conditions by mitochondrial fractions from rat liver. J. Bioenerg. Biomembr. 34, 115–125. ( 10.1023/A:1015175925756) [DOI] [PubMed] [Google Scholar]
- 61.Pesta D, Gnaiger E. 2012. High-resolution respirometry: OXPHOS protocols for human cells and permeabilized fibers from small biopsies of human muscle. Methods Mol. Biol. 810, 25–58. ( 10.1007/978-1-61779-382-0_3) [DOI] [PubMed] [Google Scholar]
- 62.Chicco AJ, et al. 2014. High fatty acid oxidation capacity and phosphorylation control despite elevated leak and reduced respiratory capacity in northern elephant seal muscle mitochondria. J. Exp. Biol. 217, 2947–2955. ( 10.1242/jeb.105916) [DOI] [PubMed] [Google Scholar]
- 63.Chance B, Williams GR. 1955. Respiratory enzymes in oxidative phosphorylation. I. Kinetics of oxygen utilization. J. Biol. Chem. 217, 383–393. [PubMed] [Google Scholar]
- 64.Storrie B, Madden EA. 1990. Isolation of subcellular organelles. Methods Enzymol. 182, 203–225. ( 10.1016/0076-6879(90)82018-W) [DOI] [PubMed] [Google Scholar]
- 65.Medja F, et al. 2009. Development and implementation of standardized respiratory chain spectrophotometric assays for clinical diagnosis. Mitochondrion 9, 331–339. ( 10.1016/j.mito.2009.05.001) [DOI] [PubMed] [Google Scholar]
- 66.Blier PU, Lemieux H, Pichaud N. 2014. Holding our breath in our modern world: will mitochondria keep the pace with climate changes? Can. J. Zool. 92, 591–601. ( 10.1139/cjz-2013-0183) [DOI] [Google Scholar]
- 67.Glanville EJ, Seebacher F. 2006. Compensation for environmental change by complementary shifts of thermal sensitivity and thermoregulatory behaviour in an ectotherm. J. Exp. Biol. 209, 4869–4877. ( 10.1242/jeb.02585) [DOI] [PubMed] [Google Scholar]
- 68.White CR, Alton LA, Frappell PB. 2012. Metabolic cold adaptation in fishes occurs at the level of whole animal, mitochondria and enzyme. Proc. R. Soc. B 279, 1740–1747. ( 10.1098/rspb.2011.2060) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Portner HO, van Dijk PLM, Hardewig I, Sommer A. 2000. Levels of metabolic cold adaptation: tradeoffs in eurythermal and stenothermal ectotherms. In Antarctic ecosystems: models for a wider ecological understanding (eds Davison W, Howard-Williams C, Broady P), pp. 109–122. Christchruch, New Zealand: Caxton Press. [Google Scholar]
- 70.Lucassen M, Schmidt A, Eckerle LG, Portner HO. 2003. Mitochondrial proliferation in the permanent vs. temporary cold: enzyme activities and mRNA levels in Antarctic and temperate zoarcid fish. Am. J. Physiol. Regul. Integr. Comp. Physiol. 285, R1410–R1420. ( 10.1152/ajpregu.00111.2003) [DOI] [PubMed] [Google Scholar]
- 71.Chamberlin ME. 2004. Top-down control analysis of the effect of temperature on ectotherm oxidative phosphorylation. Am. J. Physiol. Regul. Integr. Comp. Physiol. 287, R794–R800. ( 10.1152/ajpregu.00240.2004) [DOI] [PubMed] [Google Scholar]
- 72.Schulte PM. 2015. The effects of temperature on aerobic metabolism: towards a mechanistic understanding of the responses of ectotherms to a changing environment. J. Exp. Biol. 218, 1856–1866. ( 10.1242/jeb.118851) [DOI] [PubMed] [Google Scholar]
- 73.Kim KS, Chou H, Funk DH, Jackson JK, Sweeney BW, Buchwalter DB. 2017. Physiological responses to short-term thermal stress in mayfly (Neocloeon triangulifer) larvae in relation to upper thermal limits. J. Exp. Biol. 220, 2598–2605. ( 10.1242/jeb.156919) [DOI] [PubMed] [Google Scholar]
- 74.Arrhenius S. 1915. Quantitative laws in biochemical chemistry. London, UK: Bell. [Google Scholar]
- 75.Sommer AM, Portner HO. 2004. Mitochondrial function in seasonal acclimatization versus latitudinal adaptation to cold in the lugworm Arenicola marina (L.). Physiol. Biochem. Zool. 77, 174–186. ( 10.1086/381468) [DOI] [PubMed] [Google Scholar]
- 76.Bryant HJ, Chung DJ, Schulte PM. 2018. Subspecies differences in thermal acclimation of mitochondria function and the role of uncoupling proteins in killifish. J. Exp. Biol. 221, 186320 ( 10.1242/jeb.186320) [DOI] [PubMed] [Google Scholar]
- 77.Chung DJ, Bryant HJ, Schulte PM. 2017. Thermal acclimation and subspecies-specific effects on heart and brain mitochondrial performance in a eurythermal teleost (Fundulus heteroclitus). J. Exp. Biol. 220, 1459–1471. ( 10.1242/jeb.151217) [DOI] [PubMed] [Google Scholar]
- 78.Fangue NA, Richards JG, Schulte PM. 2009. Do mitochondrial properties explain intraspecific variation in thermal tolerance? J. Exp. Biol. 212, 514–522. ( 10.1242/jeb.024034) [DOI] [PubMed] [Google Scholar]
- 79.Rader RB, Ward JV. 1987. Mayfly production in a Colorado mountain stream—an assessment of methods for synchronous and nonsynchronous species. Hydrobiologia 148, 145–150. ( 10.1007/Bf00008400) [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All raw data generated in this study, including mitochondrial respiration values and enzyme activities, are provided at https://figshare.com/s/ab40bed830d2f5b4d7db.


