Abstract
Although O2-(2,4-dinitrophenyl) derivatives of diazeniumdiolate-based nitric oxide (NO) prodrugs bearing a free carboxylic acid group were activated by glutathione to release NO, these compounds were poor sources of intracellular NO and showed diminished anti-proliferative activity against human leukemia HL-60 cells. The carboxylic acid esters of these prodrugs, however, were found to be superior sources of intracellular NO and potent inhibitors of HL-60 cell proliferation.
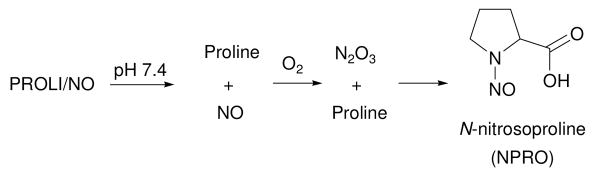
Nitric oxide (NO) donors of the diazeniumdiolate class are routinely used as sources of nitric oxide for chemical and biological applications.1 For example, the 1-[2-(carboxylato)pyrrolidin-1-yl]diazen-1-ium-1,2-diolate (PROLI/NO) anion dissociates in pH 7.4 phosphate buffer to form nitric oxide with a half-life of 2 s (Scheme 1).2
Scheme 1.
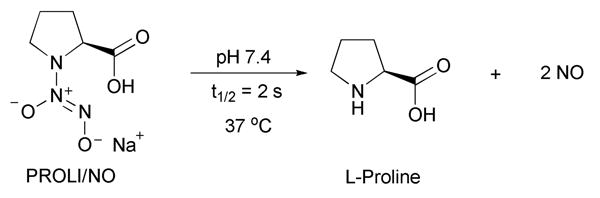
Dissociation of PROLI/NO to form NO.
Under aerobic conditions, one of the possible byproducts of PROLI/NO decomposition is N-nitrosoproline (NPRO, Scheme 2).2e
Scheme 2.
Proposed mechanism for the formation of NPRO from the decomposition of PROLI/NO under aerobic conditions.
In contrast to most other N-nitrosamines,3 which are potent carcinogens, NPRO showed no carcinogenic activity at a range of dosages in numerous animal models.2e,4 For example, N-nitrosodiethylamine was shown to induce tumors in a rat model in different organs and sites (Table 1, entry 2).3b
Table 1.
N-Nitrosamine-induced carcinogenesis in a rat modela
| entry | N-nitroso | % of treated animals with tumors (organ developing tumors) |
|---|---|---|
| 1 | proline (NPRO)b | 0 |
| 2 | diethylaminec | 95 (esophagus), 65 (liver) |
| 3 | pyrrolidined | 100 (liver) |
| 4 | piperidined | 100 (nasal), 67 (esophagus), 27 (liver) |
| 5 | pipecolic acidd | 0 |
| 6 | isonipecotic acidd | 0 |
administrated through drinking water; reference 3b.
Total dose was 100 mmol/animal.
Total dose was 1.0 mmol/animal.
Total dose was 3.9-4.6 mmol/animal.
However, even at 100-fold higher doses than those of N-nitrosodiethylamine, NPRO showed no evidence of tumor formation (Table 1, entry 1).3b Not just NPRO, but other N-nitrosamines with a carboxylic acid group were reported to show no carcinogenic activity in a rodent model (Table 1, entries 5, 6).3b Thus, the use of diazeniumdiolate anions with a carboxylic acid functionality (such as PROLI/NO) would be preferable in clinical settings due to the formation of relatively innocuous byproducts.
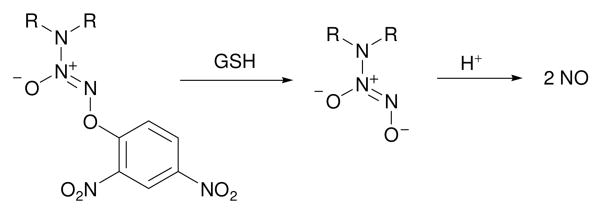
O2-Derivatization of diazeniumdiolate anions using a suitable protective group facilitates site-directed delivery of nitric oxide.5 For example, O2-(2,4-dinitrophenyl) diazeniumdiolates are reported to be activated by glutathione (GSH) to form NO (Scheme 3).6 Glutathione is an essential component of the biochemical machinery and its intracellular distribution ranges from 0.1-10 mM.7
Scheme 3.
Glutathione-activated nitric oxide prodrugs.
Earlier, we prepared the O2-(2,4-dinitrophenyl) derivative of PROLI/NO, 2, from diazeniumdiolate salt 1 in two steps (Scheme 4).8
Scheme 4.
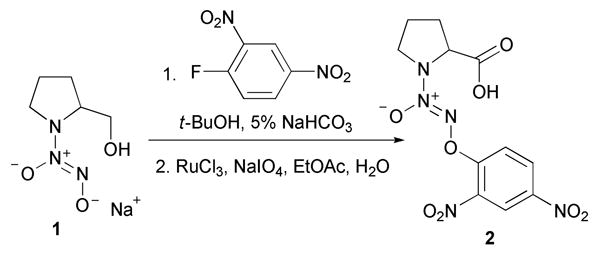
Synthesis of 2 from 1.
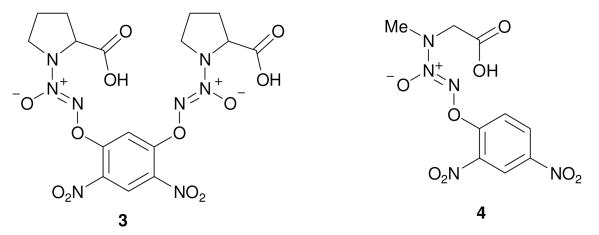
Using a similar procedure, carboxylic acids 39 and 4 were prepared (Figure 1).
Figure 1.
Compounds 3 and 4.
A chemiluminescence assay was used to study glutathione-activated nitric oxide formation in aqueous buffer (Table 2). Next, the intracellular NO release by these compounds was determined using the nitric oxidesensitive fluorophore, 4-amino-5-methylamino-2′,7′-difluorofluorescein diacetate (DAF-FM diacetate);10 briefly, human leukemia HL-60 cells were pre-loaded with DAF-FM diacetate, followed by treatment with DMSO solutions of 2, 3, and 4; fluorescence measurements after 40 min provided estimates of levels of intracellular NO (Table 2).
Table 2.
Nitric oxide release, fluorescence measurements, and in vitro anti-proliferative activity
| compd | nitric oxide yield (%)a | fluorescencec ± S. D.d (a.u.) | relative fluorescence (%) | IC50 (μM)e |
|---|---|---|---|---|
| DMSO | 0 | 7.7 ± 0.25 | 100 | - |
| 2 | 100 | 9.5 ± 0.13 | 124 | >20f |
| 3 | 87b | 7.9 ± 0.09 | 103 | 9.6g |
| 4 | 96 | 7.4 ± 0.18 | 96 | >20 |
| 5 | 98 | 17.0 ± 0.49 | 221 | 5.4 |
| 6 | 100b | 28.9 ± 1.26 | 375 | 2.7 |
| 7 | 100 | 17.0 ± 0.49 | 221 | 6.0 |
| 8 | 100 | 24.3 ± 0.85 | 315 | 3.4 |
| 9 | 100 | 26.7 ± 0.85 | 346 | 4.8 |
| 10 | 99 | 24.9 ± 0.70 | 324 | 1.4 |
Nitric oxide yields from the decomposition of the compound (50-100 μL of a 0.1 mM DMSO solution) in the presence of GSH (3.6 mM) in 0.1 M phosphate buffer (3.5 mL) containing 50 μM diethylenetriamine pentaacetic acid (DTPA) at pH 7.4 and 37 °C as measured by chemiluminescence.
Calculated based on 4 moles of NO per mole of compound.
Mean intracellular NO release measured using the NO-sensitive DAF-FM diacetate dye in HL-60 cells measured in arbitrary units (a.u.).
Standard deviations of fluorescence measurements (3 independent experiments).
The 50% inhibitory concentrations are reported for activity against proliferation of HL-60 cells.
While they were excellent sources of nitric oxide in the presence of glutathione in aqueous phosphate buffer, the carboxylic acids 2, 39 and 4 did not form significantly higher levels of intracellular NO than the DMSO control (Table 2). These intracellular NO release observations are consistent with their diminished ability to inhibit in vitro proliferation of human leukemia HL-60 cells (Table 2).9,11
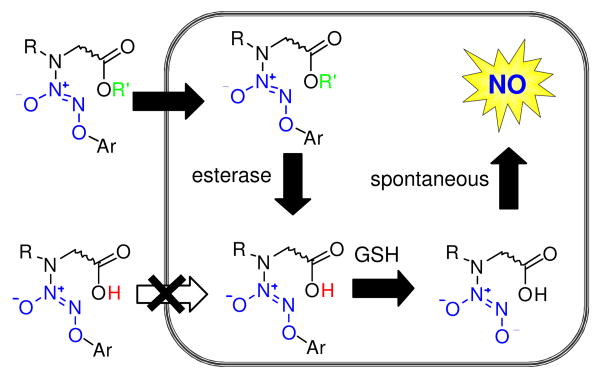
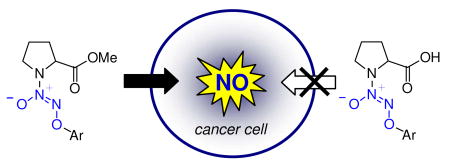
In order to improve cell permeability, it was envisaged that a free carboxylic acid group be masked as an ester; as a neutral, non-ionizable species, an ester should be able to cross the cell membrane.12 Subsequent intracellular ester hydrolysis and glutathione activation should generate the spontaneously nitric oxide-forming diazeniumdiolate anion, which upon decomposition would generate a secondary amine linked to a carboxylic acid such as L-proline (Figure 2).
Figure 2.
Design of cell-permeable nitric oxide prodrugs.
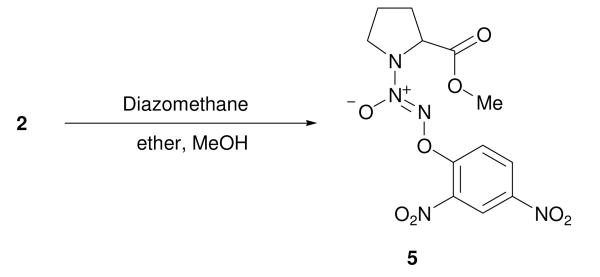
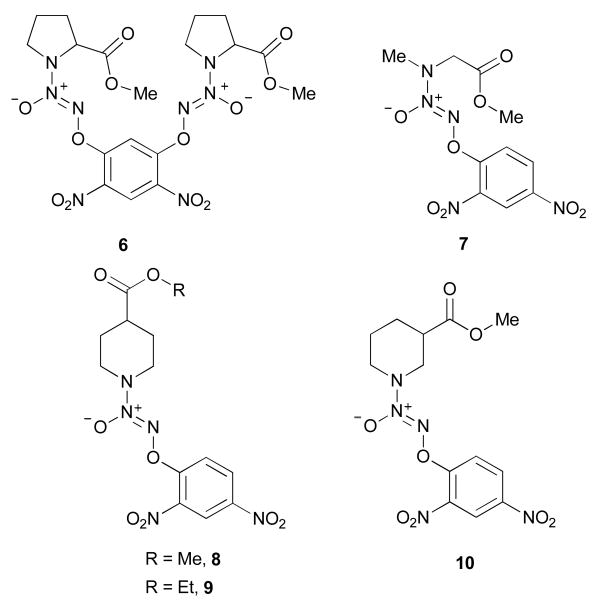
Accordingly, 5, the methyl ester of 2, was prepared by treating the carboxylic acid with diazomethane (Scheme 3). Using a similar procedure, compounds 6 and 7 were prepared from 3 and 4, respectively (Figure 3). Next, diazeniumdiolation of the requisite secondary amines, followed by arylation, produced esters of isonipecotic acid, 8 and 9, and nipecotic acid, 10 (Figure 3).
Scheme 3.
Synthesis of 5 by methylation of 2.
Figure 3.
Compounds 6, 7, 8, 9 and 10.
Glutathione-activated nitric oxide release for these esters was determined. Quantitative nitric oxide yields from a majority of the prodrug esters were observed (Table 2). Then, these esters were tested for their ability to deliver nitric oxide intracellularly using the DAF-FM diacetate assay. All the esters were found to release much higher levels of intracellular NO than their carboxylic acid counterparts (Table 2). Under these conditions, the prodrug 6, which released 4 moles of NO in the presence of GSH, produced the most intracellular nitric oxide. A plot of the relative levels of intracellular NO shows that esters 8, 9 and 10 formed nearly 3-fold higher levels of NO than 2 (Figure 4).
Figure 4.
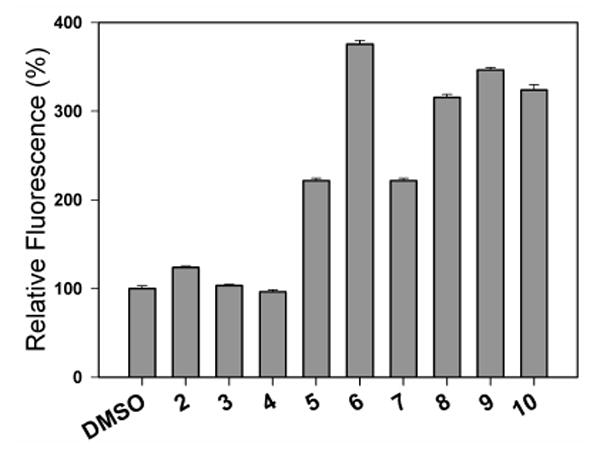
Relative levels of intracellular nitric oxide formation upon treatment of HL-60 cells with compounds 2-10 (5 μM DMSO solutions) and DMSO (control) as determined by DAF-FM diacetate fluorescence study.
Finally, the ability of these compounds to inhibit proliferation of human leukemia HL-60 cells was determined. Esterification of the carboxylic acids 2-4 significantly improved in vitro anti-proliferative activity of the resulting esters 5-7 against HL-60 human leukemia cells (Table 2). For example, the PROLI/NO ester analogues 5 and 6 were superior inhibitors of HL-60 cell proliferation relative to their carboxylic acid counterparts 2 and 3 (Table 2). All the other esters (7-10) showed excellent anti-proliferative activity that was consistent with elevated levels of intracellular nitric oxide. While other mechanisms might be operational, cell permeability of O2-(2,4-dinitrophenyl) diazeniumdiolates to release nitric oxide intracellularly appears to be a crucial determinant of inhibitory potential.
Supplementary Material
Preparative and cell culture procedures, analytical data and NMR spectra for new compounds. This information is available free of charge via the Internet at http://pubs.acs.org.
Acknowledgments
This research was supported in part by the Intramural Research Program of the NIH, National Cancer Institute, Center for Cancer Research, as well as by National Cancer Institute contract N01-CO-12400 to SAIC-Frederick.
References
- 1.(a) Scatena R, Bottoni P, Martorana GE, Giardina B. Expert Opin Investig Drugs. 2005;14:835–846. doi: 10.1517/13543784.14.7.835. [DOI] [PubMed] [Google Scholar]; (b) Keefer LK. Curr Top Med Chem. 2005;5:625–636. doi: 10.2174/1568026054679380. [DOI] [PubMed] [Google Scholar]; (c) King SB. Free Rad Biol Med. 2004;37:735–736. doi: 10.1016/j.freeradbiomed.2004.01.013. [DOI] [PubMed] [Google Scholar]; (d) Keefer LK. Annu Rev Pharmacol Toxicol. 2003;43:585–607. doi: 10.1146/annurev.pharmtox.43.100901.135831. [DOI] [PubMed] [Google Scholar]; (e) Hrabie JA, Keefer LK. Chem Rev. 2002;102:1135–1154. doi: 10.1021/cr000028t. [DOI] [PubMed] [Google Scholar]; (f) Keefer LK, Nims RW, Davies KM, Wink DA. Methods Enzymol. 1996;268:281–293. doi: 10.1016/s0076-6879(96)68030-6. [DOI] [PubMed] [Google Scholar]
- 2.(a) Saavedra JE, Southan GJ, Davies KM, Lundell A, Markou C, Hanson SR, Adrie C, Hurford WE, Zapol WM, Keefer LK. J Med Chem. 1996;39:4361–4365. doi: 10.1021/jm960616s. [DOI] [PubMed] [Google Scholar]; (b) Chen C, Hanson SR, Keefer LK, Saavedra JE, Davies KM, Hutsell TC, Hughes JD, Ku DN, Lumsden AB. J Surg Res. 1997;67:26–32. doi: 10.1006/jsre.1996.4915. [DOI] [PubMed] [Google Scholar]; (c) Champion HC, Bivalacqua TJ, Wang R, Kadowitz PJ, Keefer LK, Saavedra JE, Hrabie JA, Doherty PC, Hellstrom WJG. J Urol. 1999;161:2013–2019. [PubMed] [Google Scholar]; (d) Bivalacqua TJ, Champion HC, De Witt BJ, Saavedra JE, Hrabie JA, Keefer LK, Kadowitz PJ. J Cardiovasc Pharmacol. 2001;38:120–129. doi: 10.1097/00005344-200107000-00013. [DOI] [PubMed] [Google Scholar]; (e) Waterhouse DJ, Saavedra JE, Davies KM, Citro ML, Xu X, Powell DA, Grimes GJ, Potti GK, Keefer LK. J Pharm Sci. 2006;95:108–115. doi: 10.1002/jps.20486. [DOI] [PubMed] [Google Scholar]
- 3.(a) Lijinsky W. Chemistry and Biology of N-Nitroso Compounds. Cambridge University Press; Cambridge: 1992. [Google Scholar]; (b) Lijinsky W. Cancer Metastasis Rev. 1987;6:301–356. doi: 10.1007/BF00144269. [DOI] [PubMed] [Google Scholar]; (c) Lijinsky W. In: Chemical Carcinogenesis. Feo F, Pani P, Columbano A, Garcea R, editors. Plenum Publishing Corp.; New York: 1988. pp. 639–647. [Google Scholar]; (d) Lijinsky W. In: Genotoxicology of N-Nitroso Compounds. Rao TK, Lijinsky W, Epler JL, editors. Plenum Publishing Corp.; New York: 1984. pp. 189–231. [Google Scholar]; (e) Pool BL, Eisenbrand G, Preussmann R, Schlehofer JR, Schmezer P, Weber H, Wiessler M. Food Chem Toxic. 1986;24:685–691. doi: 10.1016/0278-6915(86)90158-4. [DOI] [PubMed] [Google Scholar]; (f) Druckrey H, Preussmann R, Ivankovic R, Schmal D. Z Krebsforsch. 1967;69:103–121. [PubMed] [Google Scholar]; (g) Lijinsky W, Reuber MD, Riggs CW. Cancer Res. 1981;41:4997–5003. [PubMed] [Google Scholar]
- 4.(a) Brunnemann KD, Enzmann HG, Perrone CE, Iatropoulos MJ, Williams GM. Arch Toxicol. 2002;76:606–612. doi: 10.1007/s00204-002-0380-4. [DOI] [PubMed] [Google Scholar]; (b) Negishi T, Shiotani T, Fujikawa K, Hayatsu H. Mutat Res. 1991;252:119–128. doi: 10.1016/0165-1161(91)90012-w. [DOI] [PubMed] [Google Scholar]; (c) Mirvish SS, Bulay O, Runge RG, Patil K. J Natl Cancer Inst. 1980;64:1435–1442. doi: 10.1093/jnci/64.6.1435. [DOI] [PubMed] [Google Scholar]; (d) Nixon JE, Wales JH, Scanlan RA, Bills DD, Sinnhuber RO. Food Cosmetics Toxicol. 1976;14:133–135. doi: 10.1016/s0015-6264(76)80257-x. [DOI] [PubMed] [Google Scholar]
- 5.(a) Wu X, Tang X, Xian M, Wang PG. Tetrahedron Lett. 2001;42:3779–3782. [Google Scholar]; (b) Showalter BM, Reynolds MM, Valdez CA, Saavedra JE, Davies KM, Klose JR, Chmurny GN, Citro ML, Barchi JJ, Jr, Merz SI, Meyerhoff ME, Keefer LK. J Am Chem Soc. 2005;127:14188–14189. doi: 10.1021/ja054510a. [DOI] [PubMed] [Google Scholar]; (c) Saavedra JE, Billiar TR, Williams DL, Kim YM, Watkins SC, Keefer LK. J Med Chem. 1997;40:1947–1954. doi: 10.1021/jm9701031. [DOI] [PubMed] [Google Scholar]; (d) Saavedra JE, Shami PJ, Wang LY, Davies KM, Booth MN, Citro ML, Keefer LK. J Med Chem. 2000;43:261–269. doi: 10.1021/jm9903850. [DOI] [PubMed] [Google Scholar]; (e) Valdez CA, Saavedra JE, Showalter BM, Davies KM, Wilde TC, Citro ML, Barchi JJ, Jr, Deschamps JR, Parrish D, El-Gayar S, Schleicher U, Bogdan C, Keefer LK. J Med Chem. 2008;51:3961–3970. doi: 10.1021/jm8000482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.(a) Shami PJ, Saavedra JE, Bonifant CL, Chu J, Udupi V, Malaviya S, Carr BI, Kar S, Wang M, Jia L, Ji X, Keefer LK. J Med Chem. 2006;49:4356–4366. doi: 10.1021/jm060022h. [DOI] [PubMed] [Google Scholar]; (b) Chakrapani H, Wilde TC, Citro ML, Goodblatt MM, Keefer LK, Saavedra JE. Bioorg Med Chem. 2008;16:2657–2664. doi: 10.1016/j.bmc.2007.11.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Meister A. J Biol Chem. 1988;263:17205–17208. [PubMed] [Google Scholar]
- 8.Chakrapani H, Showalter BM, Kong L, Keefer LK, Saavedra JE. Org Lett. 2007;9:3409–3412. doi: 10.1021/ol701419a. [DOI] [PubMed] [Google Scholar]
- 9.Andrei D, Maciag AE, Chakrapani H, Citro ML, Keefer LK, Saavedra JE. Journal of Medicinal Chemistry. 2008 doi: 10.1021/jm800831y. submitted to. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wardman F. Free Rad Biol Med. 2007;43:995–1022. doi: 10.1016/j.freeradbiomed.2007.06.026. [DOI] [PubMed] [Google Scholar]
- 11.Chakrapani H, Goodblatt MM, Udupi V, Malaviya S, Shami PJ, Keefer LK, Saavedra JE. Bioorg Med Chem Lett. 2008;18:950–953. doi: 10.1016/j.bmcl.2007.12.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.(a) Rautio J, Kumpulainen H, Heimbach T, Oliyai R, Oh D, Jarvinen T, Savolainen J. Nature Rev Drug Disc. 2008;7:255–270. doi: 10.1038/nrd2468. [DOI] [PubMed] [Google Scholar]; (b) Liderer BM, Borchardt RT. J Pharm Sci. 2006;95:1177–1195. doi: 10.1002/jps.20542. [DOI] [PubMed] [Google Scholar]; (c) Potter PM, Wadkins RM. Curr Med Chem. 2006;13:1045–1054. doi: 10.2174/092986706776360969. [DOI] [PubMed] [Google Scholar]; (d) Beaumont K, Webster R, Gardner I, Dack I. Curr Drug Metabol. 2003;4:461–485. doi: 10.2174/1389200033489253. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Preparative and cell culture procedures, analytical data and NMR spectra for new compounds. This information is available free of charge via the Internet at http://pubs.acs.org.