Abstract
Parkinson disease (PD) is a neurodegenerative disorder characterized by motor and non-motor symptoms which relentlessly and progressively lead to substantial disability and economic burden. Pathologically, these symptoms follow the loss of dopaminergic neurons in the substantia nigra pars compacta (SNpc) associated with abnormal α-synuclein (α-Syn) deposition as cytoplasmic inclusions called Lewy bodies in pigmented brainstem nuclei, and in dystrophic neurons in striatal and cortical regions (Lewy neurites). Pharmacotherapy for PD focuses on improving quality of life and primarily targets dopaminergic pathways. Dopamine acts through two families of receptors, dopamine D1-like and dopamine D2-like; dopamine D3 receptors (D3R) belong to dopamine D2 receptor (D2R) family. Although D3R’s precise role in the pathophysiology and treatment of PD has not been determined, we present evidence suggesting an important role for D3R in the early development and occurrence of PD. Agonist activation of D3R increases dopamine concentration, decreases α-Syn accumulation, enhances secretion of brain derived neurotrophic factors (BDNF), ameliorates neuroinflammation, alleviates oxidative stress, promotes neurogenesis in the nigrostriatal pathway, interacts with D1R to reduce PD associated motor symptoms and ameliorates side effects of levodopa (L-DOPA) treatment. Furthermore, D3R mutations can predict PD age of onset and prognosis of PD treatment. The role of D3R in PD merits further research. This review elucidates the potential role of D3R in PD pathogenesis and therapy.
Keywords: Dopamine D3 receptor, Parkinson disease, α-synuclein, BDNF, neuroinflammation
1. Introduction
Parkinson disease (PD), the second most common progressive neurodegenerative disorder, affects ~1% population over 60 years old (Macdonald et al., 2018), and is characterized by resting tremor, bradykinesia, rigidity and postural instability (Kalia and Lang, 2015). PD is typically not diagnosed until motor symptoms develop, although non-motor manifestations including depression, sleep problems and loss of smell, typically begin years earlier (Srivanitchapoom et al., 2018). Current treatment focuses on relief of motor symptoms and no approved treatment slows disease progression. Ideally, disease modifying interventions should be applied as early as possible. While most pharmacotherapies have focused on dopamine D1 receptor (D1R) and dopamine D2 receptor (D2R) (Lewis et al., 2006), the stimulation in D3R plays an important role in PD pathogenesis. Thus, D3R may both provide a biomarker for early-stage pathological changes and also represent a target for development of novel therapeutics. In this communication we review the characteristics of D3R, its role in the pathogenesis of PD and as a potential target for therapeutics.
Dopamine (3-hydroxytyramine, DA) plays a critical part in movement, cognition, emotion, memory, reward, and drug addiction, and involves in a number of distinct neurodegenerative diseases, in which PD is the most widely recognized. Early motor manifestations of PD result from degeneration of nigral dopaminergic cell bodies in the substantia nigra pars compacta (SNpc) and the subsequent dysfunction of the terminal fields of those neurons producing a reduction in striatal DA (Girault and Greengard, 2004). DA binds to five closely related yet functionally distinct G protein-coupled receptors (GPCRs) named dopamine receptors (DRs). These DRs are divided into two major groups: the D1-like receptors (D1, D5), and the D2-like receptors (D2, D3, D4). It is commonly believed that D1Rs couple to Gαs/olf if and induce production of cyclic adenosine monophosphate (cAMP) and the activation of protein kinase A (PKA), while D2Rs activate Gαi/o and inhibit cAMP production (Beaulieu and Gainetdinov, 2011). These receptors differ in their distribution, expression, affinity and functional properties (see Table 1).
Table 1.
Structural, distributional and pharmacological properties of DR subtypes
Caudate-Putamen (CPu), Prefrontal Cortex (PFC), Nucleus Accumbens (NAc), Olfactory Bulb (OB), Amygdala (AM), Frontal Cortex (FrC), Hippocampus (Hip), Cerebellum (CB), Prefrontal Cortex (PFC), Premotor Cortex (PMC), Cingulated Cortex (Cg), Entorhinal Cortex (Ent), Hypothalamus (Hypo), Dentate Gyrus (DG), Striatum (Str), Olfactory Tubercle (OTB), Ventral Tegmental Area (VTA), Septum (Sept), Islands of Calleja (IC), Globus Pallidus (GP), Substantia Nigra pars reticulate (SNr), Thalamus (Thal), Pre-synaptic location (Pre), Post-synaptic location (Post), Restless Legs Syndrome (RLS), Schizophrenia(SCZ), Substance Addiction (SA), Depression (Depr), Attention Deficit Hyperactivity Disorder(ADHD)
DA can not cross the blood brain barrier, so the prodrug levodopa (L-DOPA) was administered to patients with PD. This therapeutic approach provided dramatic clinical benefit but motor complications like “on-off’ fluctuations soon limited its clinical application (Wijeyekoon and Barker, 2009). Subsequently, DR agonists were developed but these agonists were non-selective or selective only for D1Rs or D2Rs families (Hori and Kunugi, 2013; Moore et al., 2014). DR agonist therapy is more prone to produce non-motor side effects that include neuropsychiatric problems such as hallucinations, delusions, impulse control disorders (ICD), cognitive impairment and non-neuropsychiatric problems such as gastrointestinal symptoms, orthostatic hypotension and valvular heart diseases (Brusa et al., 2003; Fenelon et al., 2000; Hori and Kunugi, 2013; Lang, 2001; Poewe, 2003; Voon et al., 2011). Some of these side effects are related to the lack of subtype specificity. Unfortunately, high amino acid sequence homogeneity across DR subtypes creates challenges in developing subtype selective agonists.
Although many studies have focused on D1R and D2R specific drugs for PD, D3R may be an appropriate alternative target. Evidence supporting this strategy comes from: 1.) Although the density of D1R and D2R are 2~3 times higher than D3R density in the striatum of aged human brain (Sun et al., 2012), DA binds to D3R with more than 100-fold higher affinity than it binds to D2R or D1R. Furthermore, therapeutic activation of D3R may be highly relevant since the basal concentrations of DA in extracellular (5-10 nM) and synaptic (50 nM) spaces (Prieto, 2017) are much lower than the Ki of DA at D1R or D2R, but higher than its Ki at D3R (see Table 1); 2.) Among all classes of pharmacological drugs that have been evaluated, D2R/D3R agonists are considered to be the most effective treatment for motor deficits in PD (Magnard et al., 2016). However, due to the lack of clearly defined subtype-selective therapeutics, it is hard to clarify which DR subtype is responsible for beneficial effects when treating patients with PD (Magnard et al., 2016). Pramipexole, apomorphine, ropinirole and rotigotine are currently the most commonly used DA agonists. Pramipexole, which was thought to be a D1R&D2R receptor agonist in 1990 (Kaneko et al., 1990), was later found to be a potent D3R agonist (Chen et al., 2014) and preferentially stimulates D3R compared with D2R (D2R/D3R selectivity of 24-800) (Cortes et al., 2016) with little affinity (>1000 nM) to D1R (Wood et al., 2015). Apomorphine, ropinirole and rotigotine, first reported as D2R agonists (Eden et al., 1990; Umegaki et al., 1997; Van der Weide et al., 1988), were found to bind to D3R with 2~4, 19~94 and 19~20 fold higher affinity than D2R (reviewed in (Cortes et al., 2016)). Thus D3R action of these drugs may be more important than initially expected, and early studies should be reevaluated.
D3R was firstly discovered in 1990 (Sokoloff et al., 1990), but initial researches into its action were hampered due to lack of potent and selective D3R ligands. D3R belongs to the D2R family and possesses seven membrane spanning-helices, an intercellular carboxyl terminus, and an extracellular amino terminus linked with intra and extra-cellular protein loops (Maramai et al., 2016). D3R is both a presynaptic autoreceptor and a postsynaptic receptor, distinguishing it from most of other DR types except D2R (see Table 1). Studies indicate that D3R plays an important role in in the CNS by modulating motor activity, emotions, memory and reward mechanisms (Beaulieu and Gainetdinov, 2011), thus D3R therapies initially focused on schizophrenia and drug addiction. Recent studies have addressed the potential involvement of D3R in PD. Here we review the role of D3Rin the pathogenesis, treatment and prognosis of PD and potential mechanisms underlying its role in treatment of PD.
2. The D3R regulation in the nigrostriatal regions of PD
2.1. The importance of biomarkers in PD pathogenesis
PD is pathologically defined post-mortem by abnormal α-synuclein (α-Syn) deposition (Lewy bodies/neurites) in the SNpc, striatal, and cortical regions, as well as a marked loss of dopamine projection from SN to striatum (Dauer and Przedborski, 2003; Zhang et al., 2018). The development of therapies for PD is limited by lacking in objective markers that show the pathogenesis and severity of PD. Pathologically, α-Syn is closely related to PD and considerable research efforts were made to find a suitable biomarker for α-Syn. However, no in vivo imaging biomarkers of α-Syn deposition are currently available. Thus, detection of α-Syn concentration in bodily fluid is used to evaluate if they are related to the progression of PD(Fayyad et al., 2019). Blood is the most accessible bodily fluid but the detection α-Syn in blood has yielded inconsistent results. Blood tests have given both increased (Miller et al., 2004) and decreased α-Syn levels (Li et al., 2007) in PD patients when compared with controls. Most of the results using cerebrospinal fluid (CSF) have shown that total α-Syn is down-regulated in PD (Fayyad et al., 2019), except one reported no differences between PD and control cohorts (Ohrfelt et al., 2009). However, this is a difficult method to apply clinically, because cerebrospinal fluid is obtained by lumbar puncture which is especially hard for elderly PD patients. As a result, researchers have turned their attention toward tracers, which can detect the release (via DAT and VMAT) and activation (DRs) of dopamine. The measures of SN uptake of [11C]DTBZ, a marker for vesicular monoamine transporter type 2 (VMAT2), and [11C]CFT, a marker for dopamine active transporter (DAT), do correlate with nigral cell counts throughout the full range of nigral cell loss (Tabbal et al., 2012). These findings are consistent with a postmortem study of PD that demonstrated a flooring effect of presynaptic dopaminergic measures in patients with moderate disease (Kordower et al., 2013) and in a longitudinal PET study in people with PD (Kuramoto et al., 2013). However, as we mentioned earlier, no tracers can accurately detect D2R or D3R because of the high structural similarity between die two receptors.
2.2. Quantitative autoradiography assay for measuring D2R and D3R densities
We developed a quantitative autoradiography based mathematical model for calculating the absolute densities of D2R and D3R using in vitro binding data obtained from D3R preferring antagonist [3H]WC-10 (Xu et al., 2009) and D2R preferring antagonist [3H]raclopride (Xu et al., 2010). [3H]WC-10 and [3H]raclopride bind to D2R and D3R with different labeling proportions. The specific bound amount of receptors of single concentration of [3H]WC-10 or [3H]raclopride binding can be expressed by the formulas:
Where a1 and b1 are the fractional occupancies of [3H]WC-10 to D2R and D3R; B1 is the apparent receptor binding density (D2+D3) directly measured from autoradiography studies of [3H]WC-10; a2, b2, and B2 are the same parameters for [3H]raclopride; D2, D3 are the absolute densities of D2R and D3R. The absolute densities of D2R and D3R were calculated by solving the simultaneous equations:
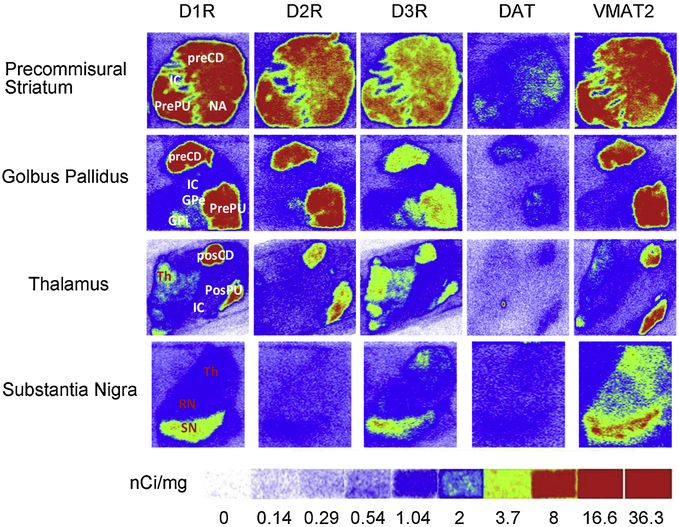
This novel assay has been used for evaluating DR subtypes alterations in mouse models of sleep deprivation and attention deficiency (Brown et al., 2011; Lim et al., 2011), and genetic PD models including DJ-1 and Pink-1 knockout (KO) rats (Sun et al., 2013b). The first comprehensive analysis of presynaptic (VMAT2 and DAT) and postsynaptic (DR) biomarkers in the striatal and extrastriatal regions was conducted in aged human brains (Sun et al., 2012). The differential distribution and reported density of D1R, D2R, D3R, DAT and VMAT2 are shown in Figure 1 (Sun et al., 2012). D3R was upregulated, whereas D1R and D2R were not, in striatum and downregulated in SN in PD dementia brains. The observed increase in striatal D3R may reflect a compensatory change upon dopaminergic denervation, while the reduction of nigral D3R density reflects neuronal loss in the nigrostriatal system in PD with dementia (Sun et al., 2013a).
Fig 1. Autoradiograms show the differential distribution of D1R, D2R, D3R, DAT and VMAT2 in the striatal and extrastriatal regions of human brain sections.
[3H]Microscale standards (ranging from 0 to 36.3 nCi/mg) were also counted for the calibration of radioactivity). The following CNS anatomical regions have been denoted: Precommissural putamen (PrePu); Precommissural caudate (PreCd); Nucleus accumbens (NAc); Internal capsule (IC). Globus pallidus external part (GPe); Globus pallidus internal part (GPi); Postcommissural putamen (PostPu); Postcommissural caudate (PosCd); Thalamus (Th); Substantia nigra (SN); Red nucleus (RN). D3R density is higher than D2R in Th, RN, SN and GPi. dopamine active transporter (DAT); vesicular monoamine transporter 2 (VMAT2).
3. The change of D3R expression in preclinical or prodromal stage of PD
Due to limitations in ligand selectivity, although several early studies used nonselective D2R/D3R ligands which indicated a potential role of D2R in PD, the potential influence of D3R in PD pathogenesis cannot be excluded. For example, studies using raclopride and fallypride demonstrated that D2R density increase in the striatum of early-stage PD patients (Fisher et al., 2013; Rinne et al., 1995). Another study, using raclopride, showed that there was increased binding of D2R/D3R in the early symptomatic stage of MPTP-intoxicated monkey model (Ballanger et al., 2016). However, both raclopride and fallypride do not differentiate between D2R and D3R (Le Foll et al., 2014b; Mukherjee et al., 2015). One research also proved that it is the D3R, rather than D2R mediated modulation was enhanced to induce Ca2+ current and synaptic GABA release in striatal neurons after DA depletion which mimics the early pathological feature of PD in an in vitro study (Prieto et al., 2011).
Why D3R is increased in the early stage of PD? Increased D3R promotes T-cell activation and induces neuroinflammation, which may underlie the role D3R activation plays in PD pathogenesis. The activation of D3R on the surface of T-cells reduces cAMP levels and extracellular regulated protein kinases 2 (ERK2)-phosphorylation, increases proliferation and adhesion of T-cells, enhances CD4+ T-cell activation(Franz et al., 2015), further attenuates T-helper 2 (Th2) differentiation, and promotes Th1 responses by regulating cytokine signaling 5, a negative regulator of Th2 development (Pacheco, 2017). The activation of D3R produces changes in T cells that eventually leads to their ability to cross the BBB, changes in their cytokine profile, increases in inflammatory cells, and aggravates inflammatory response (Ilani et al., 2004). This phenomenon may play a critical role in PD pathogenesis as the CD4+ T-cells obtained from PD patients have higher Th1-biased differentiation than those obtained from healthy control, which lead to 2 fold higher frequency of Th1 cells in PD individuals than in healthy control (Elgueta et al., 2019). It has also been observed that the T-cells with higher densities of D3R infiltrate through the BBB and into the SN and induce the activation of microglia (Gonzalez et al., 2013), resulting in the production of more inflammatory factors when compared with D3R−/− counterparts (Xue et al., 2016). Activated microglia further induce neuroinflammation and subsequent neurodegeneration of DAergic neurons in the SN - one of the main pathological characteristics of PD (Elgueta et al., 2019). One study of note, directly correlate, the expression of D3R in peripheral blood correlated with neuroinflammation in the CNS, degeneration of DAergic neurons in SN, and the occurrence of PD. In this instance, RAG1 KO mice - which lack T-cells - were resistant to MPTP-induced neurodegeneration. However, after these mice were reconstituted with wild-type CD4+ T-cells, they were susceptible to MPTP-induced DAergic neuronal damage. In contrast, RAG1 mice that were administrated D3R−/− CD4+ T-cells were completely refractory to neuroinflammation and consequent neurodegeneration (Gonzalez et al., 2013).
Therefore, it was proposed that high expression of D3R mRNA in the blood be considered as the disease specific biomarker during the preclinical stage of PD. A research observed the change of D3R mRNA blood cells of MPTP-treated mice at the presymptomatic stage. The results showed that D3R mRNA was enhanced in the mice at the presymptomatic PD stage, however decreased at the symptomatic PD stage (Kim et al., 2018). All mice in the study eventually showed significant decrease of DA content in the striatum at the symptomatic stage (Kim et al., 2018). Thus, Inhibition of D3R prior to the onset of clinical conditions may hinder the pathogenesis of PD. One research showed that before the administration of MPTP, which implies the pathogenesis of PD, injection with D3R selective antagonist PG01037 or using D3R−/− mice could completely reverse neuroinflammation and consequent neurodegeneration, thus eliminate parkinsonian symptoms (Elgueta et al., 2017).
However, the evidence for a protective role of D3R inhibition in mitigating PD pathogenesis is not completely consistent. There is a common notion that chronic tobacco use is associated with a lower risk for PD. Researchers observed that nicotine induced D3R activation to help signaling structural plasticity in mesencephalic DAergic neurons (Collo et al., 2013). Moreover, another study proposed that this stimulation of D3R by nicotine induced D3R-nAChR heteromer activation that attenuated α-syn aggregation (Bono et al., 2019). In addition, others reported a significant decrease of D3R mRNA expression in peripheral blood lymphocytes (PBLs) of PD patients compared with healthy controls (Gui et al., 2011). These studies suggests conflicting conclusions; either a decreased density of D3R or the inactivation of D3R is an indication of the early stage of PD.
There are many factors that may be influencing the inconsistent results in this line of research. First, there is a possibility that a high density of D3Rs may reflect a compensatory mechanism, common for many receptors, due to the dopamine depletions or inactivation of D3R. Thus, the increased D3R expression in early PD and the treatment of diagnosed PD with D3R agonists are not contradictive. Second, it has been suggested that although D3R densities are observed to change, it is actually the ratio of D3R to related neuroreceptors that is associated with the onset of PD. Animal model studies have shown that the continuous increase in the D1R/D3R ratio may be a physiological basis of PD among middle-aged and elderly populations (Keeler et al., 2016). Third, in recent years there has been a focus on the relationship between D3R polymorphism and PD pathogenesis. A study evaluating the association between the polymorphisms of D2R and D3R genes (D2R: rs1800497, rs1079597, rs6278, rs6279, rs273482, rs1799732 and rs1076563; D3R: rs6280 and rs2134655) and their influence in PD pathogenesis was conducted among 4279 PD cases and 5661 controls. The results showed a significant association between PD occurrence and D3Rrs2134655 polymorphism (Dai et al., 2014). Another study discussing the relationship between D3R polymorphisms and the age of PD occurrence showed that D3R polymorphism (rs6280), rather than D2R (Taq 1A, rs1800497), correlated with younger PD onset with a 4.4-year decrease in mean age of onset (Hassan et al., 2016). These observations lead us to believe that in order to determine whether D3R density changes contribute to the development of PD, we need to take different genotypes of D3R into consideration.
In other words, there is no clear indication that D3R expression can be used as a diagnostic marker for early onset of PD. Although most studies mentioned above suggested the possibility, further studies are needed to clarify the role D3R plays in PD prediction. Further studies should clarify whether changes in D3R appear because of the pathological changes of PD or D3R changes induce PD pathogenesis. Furthermore, the degree to which one D3R mutation or various genotypes influence PD pathogenesis is of importance for further research. It should be noted, there is debate about evidence of changes in D3R being used as signs of preclinical or prodromal PD. However, we hope that in-depth research can help people find an early stage marker of PD as soon as possible.
4. Involvement of D3R in PD treatment
4.1. Change of D3R in PD development
Similar to the role of D3R in predicting PD occurrence, the changes of D3R in the SN and striatum during PD development are conflicting. Some studies have shown reduced D3R expression within the striatum in parkinsonism rats (Favier et al., 2017), monkeys (Morissette et al., 1998), and drug-naïve PD patients (Boileau et al., 2009). However, other studies indicated that D3R or mRNA expression was up-regulated in Parkinsonism rats (Sun et al., 2013b) and cats (Wade et al., 2001).
The controversies involving D3R expression in PD models and patients may be related to the time course of PD. One study indicated that D3R mRNA increased six-fold by 2 weeks and then decreased in baboons after MPTP administration (Todd et al., 1996). Medication may also alter the expression of D3R protein. Researchers believe that decreased expression of D3R may be reversed by long term L-DOPA treatment (Bezard et al., 2003), or other D1R agonist therapies (Morissette et al., 1998). Furthermore, although D3R may lead to the decrease of DA in the synaptic cleft due to its role as autoreceptor in the presynaptic membrane; it should be mentioned that the formation of D1R-D3R heteromers which appears mostly postsynaptically(Marcellino et al., 2008) leads to postsynaptic signaling similar to D1R activation. A function significantly different than D3R’s role as autoreceptor(Schmauss, 2000). D3R stimulation potentiated significant D1R mediated locomotor activation in reserpinized mice PD models (Marcellino et al., 2008), likely due to the fact that there is only weak coupling between the D3R promoter and Gi/o in D1R-D3R heteromer. Instead, D3R enhances D1R signaling over the Gs/olf complex, through allosteric receptor-receptor interactions (Fuxe et al., 2015). Therefore, this does not exclude the possibility that increased D3R could be a diagnostic criterion for early onset of PD as we mentioned earlier in this review.
Despite inconsistencies that exist, we believe the activities of D3R are reduced in all these studies. Only D3R agonists are routinely used in preclinical and clinical interventions of PD; PD research studies seldom mention D3R antagonists.
4.2. D3R agonists in alleviating PD symptoms
Therefore, D3R agonists attract more and more attention in the treatment of PD in in vivo and in vitro models.
In animal studies, D3R agonists alleviated DA depletion in the striatum, attenuated DAergic neuron cell death in the SN, ameliorated microglial activation, and improved behavioral performance in Parkinsonism mice. These effects appear to be D3R dependent as they did not appear in D3R KO mice or in mice that received D3R antagonist pretreatment (Lao et al., 2013; Li et al., 2010a).
In clinical research trials, the efficacy of D3R preferential agonist, rotigotine, has been demonstrated in a series of phase III clinical studies with good tolerability and safety. Studies included patients with early and advanced stages of PD and established a dose-response relationship between escalating doses of rotigotine and improvements in PD symptoms (Benitez et al., 2014; Elmer et al., 2012; LeWitt et al., 2007; Pham and Nogid, 2008). Pramipexole, a D3R preferring agonist with a higher receptor selectivity, is effective as a monotherapy in early stage PD and able to delay the occurrence of dyskinesia in participants that fail to respond well to L-DOPA treatment(Antonini et al., 2010). Pramipexole can also be used as an adjunctive therapy in advanced PD (Antonini et al., 2010; Schapira et al., 2011) and should be established as a treatment option for all stages of PD patients (Frampton, 2014).
However, the principal for D3R agonists in PD treatment is still obscure, and we will discuss the potential mechanisms in detail in the following part.
5. Possible mechanism involving in D3R activation
5.1. D3R activation inhibits DA reuptake and breakdown in synaptic terminals
D3Rs are located both postsynaptically and presynaptically on DA target cells on DAergic neurons, respectively. Thus D3R works as an autoreceptor and generally provides an important negative feedback mechanism for neurotransmitter release that works in response to changes in extracellular neurotransmitter levels (Beaulieu and Gainetdinov, 2011). Therefore, it is believed that increased sensitivity or activation of D3R results in decreased DA content (Bustos et al., 2004). However, in certain circumstances, the activation of D3R might inhibit two main processes of DA metabolism including DA reuptake and breakdown, which may underlie the possible mechanisms by which D3R agonists alleviate PD symptoms.
a. D3R stimulation inhibits DA reuptake
The released DA in the synaptic space can be removed within milliseconds via DA reuptake through DAT. DAT pumps DA back into the cell to avoid persistent DA-induced biological effects. The transported DA can be encapsulated in vesicles for recycle by VMAT2. If effect of VMAT2 is hindered, there is a blockade of DA reuse and causes PD.
D3R activation reduces the affinity of DA for DAT and hinders DAT activity to induce persistence of DA biological effects. Castro-Hernandez et al found that a physical interaction between D3R and DAT reduced DA affinity for DAT in the absence of any changes in DAT density and subcellular distribution, and led to a decrease in DA uptake. This modulatory effect was D3R but not D2R dependent as the protective effect of pramipexole was absent in D3R−/− mice, but present in D2R−/− mice (Castro-Hernandez et al., 2015). Another demonstrated that D3R mediated long-term deficits in DAT function, and D3R inactivation attenuated methamphetamine-induced decreases in striatal DAT (Baladi et al., 2014). The direct modulation of DAT expression by D3R agonists was also examined. Pramipexole was able to reduced DAT immunoreactivity and lowered the Vmax for DA uptake into striatal synaptosomes in WT type mice, while this effect was not observed in D3R KO mice (Joyce et al., 2004). It is an interesting observation that the blocking of DAT may further activate D3R (Swant and Wagner, 2006), thus forming a positive feedback loop to block DAT.
D3R has also shown a close relationship with VMAT2. The D3R preferring agonist, quinpirole, can increase vesicular DA uptake in purified rat striatal vesicles; this effect was associated with redistribution of VMAT2 within nerve terminals (Truong et al., 2004). Pramipexole was also proved to be able to rapidly increase vesicular DA uptake in synaptic vesicles prepared from striatum of treated rats and this effect is associated with a redistribution of VMAT2 immunoreactivity within nerve terminals (Truong et al., 2003).
b. D3R stimulation inhibits DA breakdown
Although we cannot provide a direct example of interactions between D3R and DA metabolic enzymes, apomorphine, a D3R preferring agonist (D3R affinity 2~4 fold greater than for D2R) acts as a reversible competitive inhibitor of monoamine oxidase (MAO) A and B with IC50 values of 93 and 214 μM, respectively (Youdim et al., 1999).
While there is considerable evidence that D3R activation may regulate its transport and inhibit its metabolism (Figure 2), some studies do not support this conclusion. For example, some believe D3R antagonists promote the release of DA from mesolimbic DAergic neurons (Rivet et al., 1994), some believe that the protective effect of D3R agonists is independent of changes in DA concentration (Svensson et al., 1994). These views have some merits; in both PD patients and in animal models of PD, damage to the nigrostriatal pathway might result in a majority of the indicators below normal levels, making it easy to observe changes in protein expression and DA levels in these cases. Additionally, those changes in protein expression and dopamine level may be due to the overall therapeutic effect of D3R agonists.
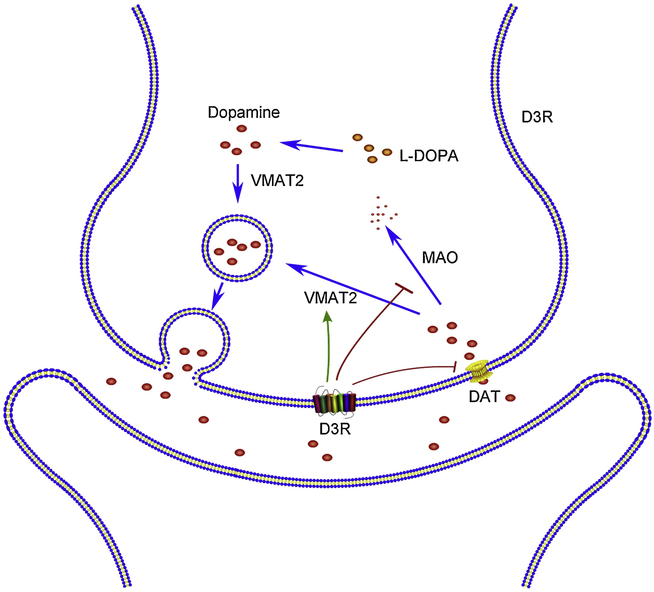
Fig 2. Effects of presynaptic D3R activation in modulating D3R content.
D3R promotes the function of VMAT2 to boost the release of DA, hinders DAT to restrain the reuptake of DA, and inhibits MAO to reduce the breakdown of DA, eventually augments the content of DA in the synaptic cleft. dopamine active transporter (DAT); vesicular monoamine transporter 2 (VMAT2); monoamine oxidase (MAO).
These conflicting observations provide novel insights into the long-term antiparkinsonian actions of D3R agonists. From all these results, we believe that D3R agonists can be regarded as regulators of DA fluctuation, and might thus contribute to the treatment of PD.
5.2. D3R activation decreases α-Syn accumulation
The protein α-Syn is detrimental to DAergic neurons in the SN, and may result in the formation of Lewy bodies and subsequent cell death. Many studies suggested that D3R agonists may exert neuroprotective effect through modulating abnormal aggregates of α-Syn (Figure 3).
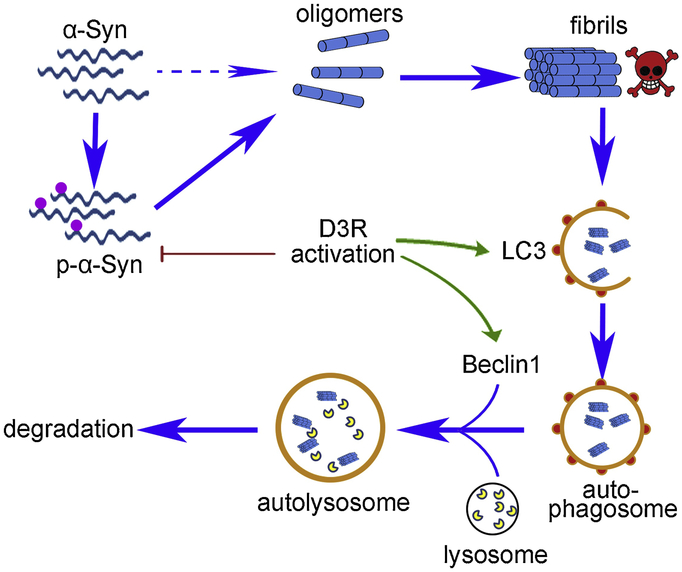
Fig 3. D3R activation inhibits abnormal aggregation and enhances clearance of α-Syn.
D3R activation hinders the phosphorylation of α-Syn to inhibit the abnormal formation of fibrils. Besides, D3R activation also enhances autophagy-dependent degradation of toxic fibrils by modulating autophagy related protein LC3 and Beclin1. α-Synuclein (α-Syn); microtubule-associated protein 1 light chain 3 (LC3).
a. D3R activation decreases α-Syn expression
D3R activation is believed to inhibit α-Syn expression in animal models and PD patients. Pramipexole was proved to be able to decrease α-Syn expression in a mouse PD model (Zhang et al., 2017) and in PD patients (Luo et al., 2016), measured by western blot or serum exosomes analysis.
b. D3R activation inhibits α-Syn aggregation
D3R activation also inhibits the aggregation of α-Syn. When co-incubated with an α-Syn solution, ropinirole inhibited α-Syn oligomer formation in an in vitro study (Ono et al., 2013). A study showed that α-Syn aggregate inclusions distributed both in mesencephalic DAergic neuron cell bodies and dendrites were dramatically reduced by quinpirole. This effect was significantly antagonized by the D2R/D3R antagonist sulpiride (Bono et al., 2018). A recent study published by the same group showed that nicotine could inhibit the accumulation of α-Syn though a D3R-nAChR heteromer. This effect was disrupted by D3R-nAChR heteromer specific interfering TAT-peptides (Bono et al., 2019). Another study proved that the co-incubation of D-520, a newly developed D3R selective agonist with Ki(D2R)/Ki(D3R)=119. inhibited α-Syn aggregation, reduced the toxicity of α-Syn when compared to control group (Modi et al., 2014). It is worth mentioning that some researchers believe that phosphorylated α-Syn accounts for 90% of α-Syn aggregates in Lewy bodies(Karampetsou et al., 2017), while pramipexole inhibited α-Syn phosphorylation to alleviate the formation of this neurotoxic aggregation (Chau et al., 2013).
c. D3R activation promotes α-Syn clearance through autophagy
The scavenging effect of D3R agonists on α-Syn may depend on autophagy (Figure 3). Autophagy helps remove abnormally aggregated proteins via lysosomal degradation, and its dysfunction is involved in the occurrence of PD.
One in vitro study demonstrated that co-incubation of rotenone treated PC12 cells with pramipexole led to a promoted clearance of α-Syn secondary to the up-regulation of Beclin1 (BECN1), an autophagy-associated protein. This effect was DR dependent because the R(+) enantiomer of pramipexole, which is devoid of agonist activity, showed no similar effect on autophagic markers or autophagosome formation (Wang et al., 2015a). And we believe that D3R may play an more important role than D2R as that D3R blockade seemed to be more effective than D2R blockade in suppressing the expression of autophagy related proteins, such as microtubule-associated protein 1 light chain 3(LC3)-II and BECN1 (Wang et al., 2015a). Similar results were also observed in an animal study which showed a massive aggregation of autophagosomes/autophagic vacuoles when using pramipexole (Li et al., 2010b).
5.3. D3R activation enhances secretion of BDNF
Brain derived neurotrophic factor (BDNF) plays an important role in processing chemical communication, facilitating the survival and maturation of DAergic neurons and enhancing functional re-innervation in the adult brain striatum. Degeneration of DAergic neurons is found in the absence of BDNF while enhanced levels of BDNF may improve symptoms in PD patients (Jiang et al., 2019).
In an in vitro experiment, researchers found that pramipexole rescued TH+ and Microtubule-Associated Protein(MAP)+ neurons injury, and this protective effect was dependent on the release of BDNF (Imamura et al., 2008). Another study demonstrated that when primary mesencephalic cultures were co-incubated with conditioned medium from pramipexole or ropinirole treated primary cultures, the number of DAergic neurons was significantly increased. This protective effect could be blocked or diminished by BDNF neutralizing antibodies or by D3R antagonists, but not by D2R antagonists (Du et al., 2005). Other groups have reported similar findings. The protective effect of D3R activation disappeared when neurons were antagonized by selective D3R antagonists, or by immuno-neutralization of BDNF. Besides, the protective effect is D3R and BDNF dependent, but not D1R and D2R dependent (Collo et al., 2018; Presgraves et al., 2004).
Moreover, not only does D3R modulate expression of BNDF, but also BDNF influences the normal expression of D3R in neurons (Guillin et al., 2001). BDNF increases striatal level of D3R via tyrosine kinase receptor B (TrkB), thereby activate the relevant pathways (Jeanblanc et al., 2006). Saylor et al found that young BDNF+/− mice expressed lower levels of D3R mRNA than wild type mice, while a single microinjection of BDNF restored the expression of D3R. This suggests that both D3R and BDNF play a synergistic role in protecting striatal neurons (Saylor and McGinty, 2010).
5.4. D3R activation ameliorates neuroinflammation
Neuroinflammation is a double-edged sword. Inflammation plays an important defensive role in maintaining CNS homeostasis. However, uncontrolled neuroinflammation can disrupt blood-brain barrier (BBB) patency and may continuously trigger neuronal cell death. Obvious symptoms of enhanced neuroinflammation have been observed in post mortem brains of PD cases while non-steroidal anti-inflammatory drugs (NSAIDs) have been proposed to play a protective role in PD patients (Vivekanantham et al., 2015; Wahner et al., 2007).
It was observed that pramipexole treatment significantly alleviated microglial activation, attenuated DAergic neuron loss in SN and improved rotarod performance in an animal study (Li et al., 2010b). Other studies demonstrated that pramipexole could block the secretion of inflammatory factors, alleviate nerve inflammation and inhibit astrocytes activation (Kang et al., 2016; Lieberknecht et al., 2017).
The anti-inflammatory effect observed above is D3R dependent. A novel D3R preferring agonist D264 was shown to exert a protective effect in two mouse models of PD. Pretreatment with D264 seven days before MPTP or lactacystin administration dramatically improved behavioral performance, attenuated neuron loss through ameliorating microglial activation and increasing BDNF levels; these protective effects could be blocked by administering a D3R antagonist U99194 (Li et al., 2010a).
Contradictory evidence has also been presented. Although continuous pramipexole administration produced significant preservation of TH+ cells degeneration and alleviated TH+ terminals loss in parkinsonism rats who received stereotaxic injection of lipopolysaccharide (LPS) into SN, there was no effect on the expression of inflammatory markers; the authors believed that the protective effect of pramipexole could be due to enhanced tolerance to inflammation (Iravani et al., 2008).
We have proposed that D3R activation might enhance inflammation in the early stage of PD pathogenesis. So what leads to conflicting evidence? We think that is partly due to the complicated role of D3R in modulating inflammation. A study has shown that when Chinese hamster ovary cell (CHO) cells which overexpress D3R were activated by low concentration of DA, the release of arachidonic acid (AA) was inhibited, while high concentration of DA increased the release of AA. This suggested that excessive activation of D3R might be associated with inflammation, while moderate activation might play an anti-inflammatory role (Nilsson et al., 1999).
5.5. D3R activation alleviates oxidative stress injury
Oxidative stress is believed to be involved in PD. Increased lipid peroxidation and impaired mitochondrial function are found in the SN of PD patients. In numerous experimental models, exogenous toxins such as MPTP produce free radicals which induce PD (Zou et al., 2000). Rotigotine induced neuroprotective effect against glutamate toxicity in primary dopaminergic cultures through significantly decreasing the production of free radicals via dopamine receptor stimulation (presumably via the D3R) (Oster et al., 2014) . Thus, it is possible that D3R activation may influence PD progression through its antioxidant effect.
D3R activation was shown to inhibit reactive oxygen species (ARO) generation in neurons or animals. Pramipexole inhibited the production of mitochondrial ROS and prevented cell death, when compared with SH-SY5Y cells treating with H2O2 (Ferrari-Toninelli et al., 2010). Rotigotine significantly alleviated rotenone-induced primary mesencephalic neuronal death due to the significant attenuation of ROS production (Radad et al., 2014). An in vivo study showed that treating mice with MPTP increased lipid peroxidation products and loss of TH+ DA neurons in SN, while pramipexole treatment inhibited lipid peroxidation and protected SN injury (Zou et al., 2000).
The reason may be that D3R activation increases the level of enzymes which reduce ROS. Pramipexole was shown to normalize the level of Glutathione (GSH) and GSH peroxidase (Lieberknecht et al., 2017) in animal models of PD. Ropinirole is believed to increase the activities of GSH, catalase, and Superoxide dismutase which scavenge ROS, while pretreatment with D2R/D3R antagonist blockers abolished this protective effect (Iida et al., 1999).
Although most authors believed that D3R activation is necessary for the demonstrated reduction in ROS injury (Li et al., 2010a), some have shown that this can be independent of D3R activation (Le et al., 2000). Although more research is needed to clarify this issue, both the antioxidant and D3R activating engagements of D3R agonists play critical roles in its neuroprotective actions in PD treatment (Ling et al., 1999).
5.6. D3R activation promotes neurogenesis
PD demonstrates obvious impaired neurogenesis. A hallmark of PD is the loss of TH+ neurons in the SNpc and the reduction of DAergic projections to the striatum, therefore increasing neurogenesis helps alleviate the symptoms of PD (Salvi et al., 2016). Although it was once believed that adult neurogenesis occurs only in the hippocampal dentate gyms (DG) and subventricular zone (SVZ) regions, recent studies have shown that newly generated neurons may also occur in areas previously considered as non-neurogenic, e.g., the striatum (Salvi et al., 2016).
There is close relationship between D3R and neurogenesis. The expression of D3R in the CNS occurs in the very early stages of development, and mainly exists in the site of neurogenesis (Van Kampen and Robertson, 2005). It was observed that in hiPSC-derived DAergic neurons, treatment with D3R preferring agonists such as quinpirole and ropinirole promoted the reproduction of neuronal progenitor cells (Bono et al., 2018). In one study, the results showed that there was significantly increased proliferation of neurons in the DG and doublecortin (DCX)+ neuroblasts in the striatum of pramipexole treated mice (Salvi et al., 2016). Other studies showed that the maximal dendrite length, number of primary dendrites, number of DAergic neurons and [3H]dopamine uptake of TH+ neurons were significantly enhanced when treated with quinpirole and D3R agonist 7-OH-DPAT, while the neurotrophic effects were all eliminated when D3R was blocked (Collo et al., 2008; Van Kampen and Eckman, 2006). Another study in lesioned zebra finches showed that 7-OH-DPAT led to significant reduction of lesion size and increased numbers of migrating neuroblasts and newborn cells in the striatal vocal nucleus area X, while the same effect was not seen in D3R antagonist U99194 treated cohorts (Lukacova et al., 2016).
The studies above suggested that the increased neuronal production effect was conducted via a direct effect on D3R. In addition, rat experiments showed that D3R activation could significantly increase neurogenesis in SVZ and SNpc (Van Kampen and Robertson, 2005), which are also the most vulnerable regions in PD. Pramipexole significantly promoted neurogenesis in the SVZ area of adult mice or rats, manifested by enhanced neuron number, neuronal differentiation, S-phase population at cell cycle and incorporation of BrdU, which could all be blocked by D3R antagonist U99194A. Pramipexole also increased the number of GFAP-positive cells and enhanced the expression of neuronal markers DCX and MAP2, and resulted in enhanced motor activity (Merlo et al., 2011; Winner et al., 2009).
5.7. D3R synergistically promote biological effect of D1R stimulation
Most DR agonists are for the D1R and D2R families, or the D2R family. The use of D1-selective/preferring agonists in PD therapeutics is an ongoing area of research. However, among all of these DRs, D1R is the most abundantly expressed receptor in the striatum (Perachon et al., 1999) and is believed to play a critical role in PD pathogenesis and treatment. Clinically, dopamine agonists with the highest D1R affinity have an efficacy most similar to L-DOPA, although not as effective. The full activation of D1R is the key to directly alleviate PD related symptoms (Lewis et al., 2006). Increased D1R expression is considered one of the critical elements for establishing a given dopamine agonists’ clinical sensitivity to reduce PD symptoms (Hisahara and Shimohama, 2011). Deficiencies in D1R expression have led to Parkinsonian motor symptoms in animal studies (Centonze et al., 2003). It could be a logical conclusion that the synergistic effect between D3R and D1R may also participate in the alleviating effect of D3R agonists in PD.
The interaction between GPCRs may interfere with the GPCR signaling in both quantitative (ligand affinity) and qualitative (downstream pathway disruption) ways. It was proved that the interaction of D3R on D1R increases the affinity of D1R with its ligand, but has little effect on the affinity of D3R with its ligand (Marcellino et al., 2008). For example, selective D3R agonist PD128907 reduced the ED50 for the D1R agonist SKF 38393 from 56 to 4 nM in certain circumstances (Avalos-Fuentes et al., 2013). Once the affinity of DA to D1R is increased, G-protein signaling and cAMP accumulation is enhanced (Leggio et al., 2016). Deleting or pharmacologically blocking D3R attenuated important molecular markers in the D1R-neurons such as FBJ murine osteosarcoma viral oncogene homolog B(FosB), ERK, and histone-3 (H3)-activation (Solis et al., 2017), while activating D3R in the SN enhanced D1Rs to induce γ-aminobutyric acid (GABA) release in SN (Avalos-Fuentes et al., 2013) and striatum (Cruz-Trujillo et al., 2013). All these directly showed how D3R might enhance the physiological function of D1R neurons by activating D1R.
In one study, using risepinized mice in which DA was exhausted, locomotor activity was enhanced by the administration of D1R agonist. The effect of D1R agonist was potentiated through co-administration with the D3R agonist PD128907 dose-dependently. Furthermore, in light of the contested D3R selection properties of PD128907, D3R KO mice were also used. It was shown that although D1R agonist significantly enhanced the motor activation in both WT and D3R KO mice by itself, PD128907 significantly potentiated the locomotor activity induced by D1R stimulation in WT mice. However, similar results were not obtained with D3R KO mice (Marcellino et al., 2008). These results suggest that it is the activation of D3R which plays a critical synergistic role in promoting the D1R mediated behavioral effect.
5.8. Different voices
Although we have mentioned much evidence asserting the critical role that D3R may play in alleviating PD symptoms via differing mechanisms, studies have shown conclusions which are at odds with ours. Some studies conclude that D3R stimulation may be the source of debilitating PD symptoms. One study found that when S33084 - a selective D3R antagonist - was administered alone, Parkinsonian disability was alleviated in 6-OHDA injured rats (Mela et al., 2010) and a low dose of S33084 potentiated the anti-Parkinsonian effect of both L-DOPA and ropinirole (Silverdale et al., 2004). Nafadotride, another D3R antagonist, was reported to enhance locomotor activity at low doses in rats (Sautel et al., 1995). It was also reported that BP897, a D3R agonist, weakened the anti-Parkinson effect of L-DOPA (Hsu et al., 2004).
When looked at on the whole, the conflicting nature of these reports lead us to believe that the role of D3R in the treatment of PD needs to be further discussed. We believe that in-depth discussion of these contradictions can help the research community better understand the role of D3R in PD. We can think of several reasons for the conflicting evidence present across the various studies. One, the therapeutic effects of D3R antagonists on PD may vary with the dosage. Although the mechanisms of D3R antagonists are still unknown. Some reports indicate that low doses of preferential D3R antagonist, S33138, was shown to potentiate the antiparkinson action of ropinirole. However, the benefits of this drug diminished at a high dose (Millan et al., 2008). Another D3R antagonist, nafadotride, similarly enhanced locomotor activity at a low dose, while this locomotor activation was not observed at a high dose or in some instance even decreased (Sautel et al., 1995). Still another study has shown that high doses of D3R antagonists produce an increase in disability, while low doses do not give such effects (Hadj Tahar et al., 1999). Two, the difference in D3R expression and binding state with its ligand should also be taken into consideration. Contrary results may be produced relative to differing states of stimulation or inhibition of D3R. Discrepancies may arise from changes relative to PD stage, treatment, or between species. For example, in one report that utilized MPTP-lesioned baboons, expression of D3R increased for two weeks to a peak of six fold before starting to decrease (Todd et al., 1996). The decline in caudate D3R expression after nigrostriatal degeneration was reversed after L-DOPA treatment (Quik et al., 2000). Changes were not limited to the expression of D3R but also the binding to exogenous ligands was shown to have been enhanced in the striatum of rats following repeated administration of L-DOPA (Bordet et al., 1997). Furthermore, the differences of D3R among species should also be taken into consideration. There is more widely distributed D3R in the striatum in humans and primates than in rats (Silverdale et al., 2004), a difference that may yield contradictory results when sorting out the effects of D3R agonists/antagonists. Three, the hypothesis that the inhibition of D3R alleviates PD symptoms has also been challenged due to the poor selectivity of some of the aforementioned D3R antagonists. Nafadotride, for example, is believed to be a D3R blocker but also binds with the 5-HT receptor (Audinot et al., 1998) and only shows 6.2 fold selectivity for D3R over D2R (Audinot et al., 1998). Another study used homozygous (D3−/−), heterozygous (D3+/−) mutant and wild-type (D3+/+) mice to analyze the effect of D3R agonists and antagonist in locomotor activity. Results of this study concluded that all D3R agonists decreased locomotion to a similar extent in all three genotypes while D3 receptor antagonist increased locomotor activity. However, there were no apparent difference between the D3−/−, D3+/−, and D3+/+ genotypes (Boulay et al., 1999). This research illustrates the possibility that there might be other alternative adaptive mechanisms involving still other receptors. Four, there is basis for reinterpreting some of the opposing results. In the instance where authors believe that D3R agonist weaken the anti-Parkinson effects of L-DOPA, it was concluded that D3R stimulation aggravated parkinsonian symptoms. However, it is reasonable to conclude that the partial D3R antagonist, BP897, could antagonize the anti-Parkinson effect of L-DOPA, whose metabolites are considered a complete agonist. After the dose of L-DOPA increased, the inhibitory effect of BP897 on the therapeutic effect of L-DOPA disappeared(Hsu et al., 2004), this supports our deduction and is a common phenomenon when discussing the effect of partial agonists. In addition, the above results also confirm that the dosage of L-DOPA may affect our judgment relative to the role of D3R in PD. Lastly, other authors believed that among various methods used to evaluate motor ability in different species of animals may lead to differences in the evaluation of the PD improvement effect in question (Hsu et al., 2004).
6. the role of D3R in PD associated non-motor symptoms
It is believed that D3R distributes abundantly in mesolimbic areas: nucleus accumbens, olfactory tubercle, amygdala, islands of Calleja, and in the striatum (Gurevich and Joyce, 1999; Jaber et al., 1996). Thus, activation or inhibition of D3R may be associated with the emotional, behavioral, motivational, and memory fluctuations which are commonly seen in PD patients. Common symptoms of these states include depression, anxiety, psychosis and cognitive defects.
6.1. Sensory and mood deficits
a. Depression
A reduction of the mesolimbic pathway precipitates the loss of motivation and anhedonia - symptoms of depression (Leggio et al., 2013). There is evidence that the down-regulation of expression and function of D3R at the mesencephalon exists in major depression (Leggio et al., 2013). The deficiency of D3R has also be posited to contribute to increased incidence of depressive symptoms (Moraga-Amaro et al., 2014), while D3R agonists are reported to exhibit antidepressant effects in a rat model(Rogoz et al., 2004).
Studies have reported that commonly used antidepressants could increase the expression, binding, or responsiveness of D3R in relevant brain areas such as the islands of Calleja and the nucleus accumbens - major projection areas of the mensolimbic dopaminergic system (Lammers et al., 2000; Maj et al., 1998). Therapeutic effects may depend on D3R as the preferential D3R agonist, cariprazine, exerted antidepressant effects in WT mice. However, this effect did not exist in D3R KO mice (Duric et al., 2017). Thus suggesting that activation of D3R be considered a potential method for the treatment of depression in PD rats model (Carnicella et al., 2014). Common clinically used D3R agonists, ropinirole and pramipexole, are reported to alleviate depressive symptoms in rats and PD patients further lending support to this hypothesis (Antonini et al., 2010; Mavrikaki et al., 2014).
b. Anxiety
D3R also plays an important role in anxiety. Results indicate that D3R KO mice show anxiety-like symptoms. Evidenced by thigmotaxis in openfield tests, a significant lower time spent in the lit compartment in the light-dark box test (Moraga-Amaro et al., 2014). 7-OH-DPAT and cariprazine, both are preferential D3R agonists, were found to exert anxiolytic-like effect in rats and mice models (Duric et al., 2017; Rogoz et al., 2004). Cariprazine was also believe to alleviate anxiety/depression vs placebo in clinical application (Marder et al., 2019). One clinically used anti-PD compound, ropinirole was proved to produce anxiolytic-like effects in the mouse, rat, and marmoset models (Rogers et al., 2000). Another in vivo study suggested that activation of D3R be considered a potential method of treatment for anxiety and depression in PD rat models when compared with D1R and D2R activation (Carnicella et al., 2014).
c. psychosis
Psychosis is a common neuropsychiatric symptom of PD (Bleickardt et al., 2012). Treatment with dopaminergic medication can also induce psychosis. DR agonists are more likely to induce psychoses than L-DOPA(Bleickardt et al., 2012) - making DR agonist therapy a critical risk factor for PD psychosis (Han et al., 2018). The effect of dopamine agonists on psychosis may be closely related to prepulse inhibition (PPI). PPI is a neurological phenomenon defined by the reduction in startle magnitude following a weak prepulse (or prestimulus) and is used for the measurement of sensorimotor gating (Weber et al., 2009). PPI reduction (or deficit) is detected in schizophrenia patients and is among the most consistent and robust markers for this disorder (Swerdlow et al., 2006).
Many studies have shown that PPI is related to 5-HT and NMDA receptors, while D3R has been given more attention recently. Apomorphine (Yang et al., 2017), ropinirole(Giakoumaki et al., 2007), quinpirole (Nespor and Tizabi, 2008), and pramipexole (Chang et al., 2010) were all believed to induce a significant PPI reduction or deficit in animal models or human studies. It is thought that this effect of PPI reduction may be mediated by D3R agonism. Selective D3R antagonist, Y-QA31, was shown to reverse PPI perturbation in methamphetamine induce schizophrenia models (Sun et al., 2016). A D3R active drug, (S)-pramipexole, decreased PPI when infused into the NAc and islands of Calleja, in a dose-dependent manner. While (R)-pramipexole, a D3R-inactive molecule, had no effect. Moreover, the PPI disruptive effect of pramipexole were prevented by D3R-selective antagonist (Chang et al., 2012). Buspirone was also found to counteract the disruption of PPI induced by MK801 in WT mice, subsequently exerting an antipsychotic-like effect. This effect may be mainly driven by antagonist activity at D3Rs as the counteracting effect disappeared in D3R−/− mice (Torrisi et al., 2017).
These observations indicate that the PPI disruption seen with DR agonists may be mediated by D3R, however the role of other DRs cannot be ruled out(Varty and Higgins, 1998). Alternative explanations posit that D1R (Ralph-Williams et al., 2003) or D2R (Ralph et al., 1999) are essential for the disruption of PPI but not D3R (Ralph et al., 1999). This may be due to the various mechanisms in which DR agonists induce psychosis. For example, it was reported D2R antagonists inhibited apomorphine but not pramipexole induced PPI deficit, while D3R antagonists inhibited pramipexole but not apomorphine induced PPI deficit (Weber et al., 2008; Weber et al., 2009; Zhang et al., 2007). Furthermore, D3R Ser9Gly polymorphism may also influence their effect on PPI, demonstrated in one human study by the fact that Gly/Gly individuals had the lowest PPI while Ser/Ser individuals had the highest PPI (Roussos et al., 2008). Despite the controversies surrounding the mechanisms underlying psychosis-like symptoms, much consideration to this side effect is needed when using DR agonists. Best clinical practices, such as the reduction of doses or introducing antipsychotic medications if necessary, should be followed.
6.2. Dementia
The striatum is not only critically important in motor functions, but also plays important role in cognitive functions. D3R receptors are proved to specifically participate in cognitive aspects of striatal functions (Morissette et al., 1998).
D3R expression was shown to be severely reduced in AD patients (Kumar and Patel, 2007). Having PD with an additional diagnosis of dementia correlated with lower D3 receptor density. Correspondingly, non-demented PD cases have elevated levels of D3R binding (Joyce et al., 2001).
When pramipexole was used to treat mild-moderate AD individuals, 13/16 patients showed improved cognition (Bennett et al., 2016). Another report in bipolar disorder also confirmed the therapeutic effect of pramipexole in cognitive impairment (Aprahamian et al., 2013). However, the reasons are still obscure.
Some rat experiments showed that D3R activation could significantly increase neurogenesis in SVZ and striatum (Van Kampen and Robertson, 2005), the vulnerable regions in PD. That may partially explain why treating with pramipexole could restore the synaptic transmission and Long-Term Potentiation (Castro-Hernandez et al., 2017).
However, there are also studies which do not support the view that D3R activation promotes cognitive function (Chang et al., 2018; Sun et al., 2013a). Whether activation of D3R may alleviate dementia in PD needs validation through large-scale clinical trials.
6.3. Impulse control disorders
ICDs (such as, but not limited to, gambling disorders, hypersexuality, and compulsive eating and shopping) have been reported in PD. ICD incidences are especially pronounced in PD patient populations who have received DA replacement therapies (Voon and Fox, 2007), and as such DR agonists are considered a major risk factor for ICD (Marvanova, 2016). ICDs were more frequent in patients taking agonists than a L-DOPA monotherapy (Fasano and Petrovic, 2010), while the D3-acting effect was statistically associated with ICD development (Fasano and Petrovic, 2010; Moore et al., 2014). Incidences of ICD are also associated with the use of L-DOPA, and the combination of L-DOPA and dopamine receptor agonists showed higher incidence of ICD than dopamine receptor agonists alone(Weintraub et al., 2010). The reason for these outcomes may lie in enhancement of D3R expression and binding activity as a result of chronic L-DOPA treatments (Cote and Kuzhikandathil, 2015), however there are many issue that still need to be elucidated further regarding this hypothesis. For one, D3R binding in ventral striatum has been shown to be lower in PD patients with ICD compared with those without, and also correlated with the severity of ICD (Payer et al., 2015).
Controversy between these studies also may be due to the differing genotypes of D3R. Researchers testing the association between ICD and D3R allelic variants, showed that D3R Ser9Gly (rs6280) genotype was significantly associated with ICD. In patients with this genotype, it is likely that increased D3R-ligand affinity, due to the gain-of-function conferred by the glycine residues, could impair reward-risk assessment and ultimately lead to ICD (Krishnamoorthy et al., 2016). However, another study discussed the association between the polymorphisms of 13 candidate genes and suggest that the independent contribution of a single D3R polymorphism in ICD is too low to be detected (Kraemmer et al., 2016). Some researchers believe that the selectivity for D3R over D2R should be taken into account and suggest that the incidence of ICD may be related to the affinity of D3R/D2R. This theory concludes that for a medicine - the higher the affinity of D3R/D2R, the higher the proportion of ICD induced by the medicine - for a given patient population (Seeman, 2015). Other possible reasons for variable incidences of ICD may also include: routes of administration (oral D3R agonists have higher risk of ICD development than transdermal(Garcia-Ruiz et al., 2014)), differing dosage (high D3R agonists dosage often means high frequency of ICD(Hassan et al., 2011)), or age of subjects (younger people may be better able to act on their impulses than seniors (Hassan et al., 2011)).
7. Role of D3R in treating L-DOPA induced dyskinesia
L-DOPA therapy remains the gold standard for PD treatment, however, long term L-DOPA exposure is closed related with many side effects, among which the commonest one is L-DOPA-induced dyskinesia (LID), or L-DOPA-induced abnormal involuntary movements (AIMs). The incidence of LID in PD patients receiving L-DOPA treatment for over 5 years is 50% (Dragasevic-Miskovic et al., 2019). Investigators are therefore committed to developing new PD drugs that will not cause LID, or drugs that can be combined with L-DOPA to reduce the onset of LID. D3R might be a target.
Some researchers believe that D3R expression is uppregulated in the LID patients receiving long-term treatment with L-DOPA (Aristieta et al., 2012; Bezard et al., 2003; Payer et al., 2016). Not only the expression of D3R, but also the binding of D3R was higher in LID monkeys or patients when compared to non-dyskinetic PD or normal counterparts (Guigoni et al., 2005; Payer et al., 2016), which hinted the role of D3R in LID. The overexpression of D3R induces behavioral sensitization in parkinsonian subjects, which subsequently leads to the occurrence of LID when treated with L-DOPA (Guillin et al., 2001). Down-regulating the expression of D3R in the striatum (Wang et al., 2015b), or blocking D3R (Cote et al., 2014), reversed motor fluctuations in rat PD models in which D3R expression was elevated.
D3R selective agonists can be used for the treatment of LID (Simms et al., 2016; Utsumi et al., 2013). However, what puzzled us is why D3R agonists hinder the occurrence of LID when D3R elevation or activation is believed to contribute to LID. Here is a brief explanation. Actually, it is D1R rather than the D3R activation contribute to LID. Long term L-DOPA administration may induced hypersensitivity of D1R, thus enhance GABA release and cAMP accumulation, which may play a role in LID occurrence (Rangel-Barajas et al., 2011). But D1R hypersensitivity can be modulated by D3R. L-DOPA-induced D3R overexpression principally occurs in D1R-containing neurons but shows relatively lower co-expression in the D2R-containing neurons (Solis et al., 2017). That may because D3R combines with D1R to form a heteromeric complex which plays an important part in LID (Fiorentini et al., 2008; Solis et al., 2017). There is a close relationship between the expression of D1R-D3R heterodimer and the occurrence of LID (Solis and Moratalla, 2018). An interaction between D3R and D1R could increase the binding affinity between D1R and DA, and enhance the density of D1R due to the lack of internalization of D1R (Fanni et al., 2018). In addition, the expression of D3R induced by long term L-DOPA treatment is instrumental in activating the downstream signal pathway of D1R, which aggravates the oversensitivity of D1R (Azkona et al., 2014).
This proposed explanation leads to an interesting conclusion, that is, balancing and normalizing the activation of D3R may relieve the fluctuation of D1R sensitivity, thus avoiding the occurrence of LID; overactivation of D3R may induce LID, while blocking D3R activation may lead to PD symptoms. Some studies confirmed our deduction. When D3R full agonists, such as PD128907 (Ki was 0.4 nM (Scheideler et al., 1997)), were co-administered with D1R agonists (such as SKF38393) in rat experiments, there were severe exacerbation of LID (Lanza et al., 2018). When a partial agonist BP897 (Ki was 0.92 nM (Garcia-Ladona and Cox, 2003)) was administrated to animals in combination with L-DOPA, not only LID was attenuated, there was also no influence on the therapeutic effect of L-DOPA (Bezard et al., 2003). However, when a specific D3 receptor antagonist was co-administrated with L-DOPA, LID severity was alleviated but PD-like symptoms reappeared (Bezard et al., 2003). The suggestion that both D3R selective antagonists and partial agonists are pharmacotherapeutic candidates for the treatment of L-DOPA-associated AIMs in patients with PD (Mela et al., 2010; Riddle et al., 2011) can also be supported by our deduction.
Normalizing the activation of D3 receptor might attenuate LID without affecting the therapeutic effect produced by L-DOPA. Overactivation of D3R may induce LID because it enhances the density of D1R or augments D1R activity. Inhibiting D3R totally may cause damaged alleviation in PD symptoms due to the protective roles D3R played in PD therapy. The best approach is to mildly activate D3R (see Figure 4).
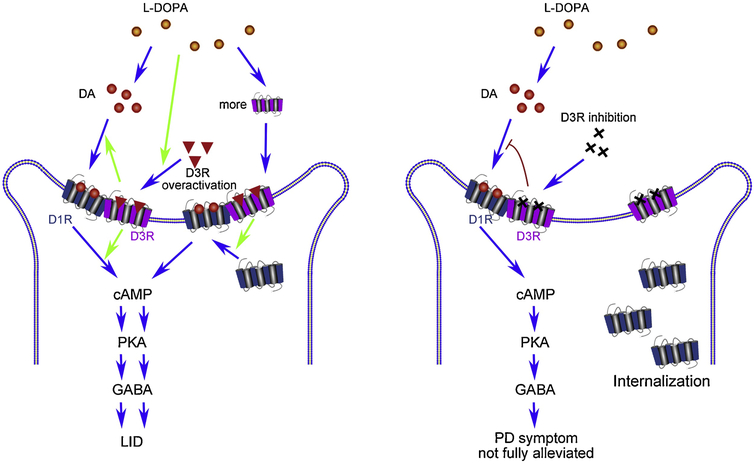
Fig 4. The relationship between D3R with LID.
Left penal: D3R exacerbates D1R hypersensitivity and leads to LID. D3R enhances the expression of D1R in the surface of neurons, increases the affinity of DA to D1R, and activates the downstream signal pathway of D1R, finally induces the oversensitivity of D1R, which leads to LID. Right panel: D3R inhibition relieves LID, but shows no effect on alleviating PD symptoms. D3R inhibition lead to the lack of D1R in the surface of neurons and the decreased affinity of DA on D1R, which induces damaged alleviation of DA agonists in PD symptoms. Dopamine (DA); cyclic adenosine monophosphate (cAMP); protein kinase A (PKA); γ -aminobutyric acid (GABA); L-DOPA induced dyskinesias (LID).
This view is in accordance with animal tests and clinical practice. SK609 (a D3R selective agonist, Ki=103 nM (Xu et al., 2017b)) notably improved the performance of the Parkinson like symptoms but did not induce AIMs and even reduced AIMs when co-administrated with L-DOPA (Simms et al., 2016). Pramipexole (Ki=10~12.6 nM (Millan et al., 2000; Millan et al., 2002)) was shown to be more effective than ergotamine D1R/D2R agonists in alleviating LID when co administrated with L-DOPA. It has been reported that when treated with a partial D3R agonist, the improvement in LID is associated with a worsening of Parkinsonism. In other words, the reduction in LID occurs at the expense of the anti-parkinsonian effects of L-DOPA (Hsu et al., 2004). This conclusion is in good agreement with our results.
Although we have shown many results that demonstrate the reliability of our conclusion, there are still some reports which give inconsistencies. Some results show no differences in striatal D3R expression between normal and PD patients (Hurley et al., 1996). Others studies - where chronic L-DOPA treatments produced AIMs - reported that co-treatment with D3R antagonist, S33084, and L-DOPA shows no alleviating effect either in terms of AIM frequency or amplitude in rats (Mela et al., 2010). Similar results were also obtained in MPTP treated marmosets (Silverdale et al., 2004). Other influencing factors for these results could be to blame. One, doses of D3R agonists/antagonists may exert influence on LID presentation. One Study has shown that high doses of D3R antagonists show a reduction of LID, while low doses do not show such an effect (Hadj Tahar et al., 1999). Two, doses of L-DOPA may influence the D3R antagonists’ effect on LID. According to the receptor competition theory, increased dopamine induced by enhanced doses of L-DOPA can competitively inhibit the binding of antagonist to D3R. Therefore, the dosage of L-DOPA may affect the therapeutic benefits of D3R antagonist on LID. Three, there is evidence that the pathogenesis of PD may also change with respect to the progress of LID. There is a hypothesis that the development of LID reflects D3R insensitivity in an early age and sensitivity in the later age (Mela et al., 2010).Four, as mentioned previously, the discrepancy of D3R expression and binding state of its exogenous ligand between different PD stages, treatments, or species may also lead to deviations in experimental results. Lastly, we also acknowledge that in some studies, the activation of partial D3R agonists on other receptors may also help to relieve LID. For example, partial D3R agonist, BP897, was shown to attenuate LID and is also believed to be a potent ligand at 5-HT receptor (Heidbreder et al., 2005). Furthermore, activation of 5-HT also participates in alleviating LID (Bibbiani et al., 2001).
Therefore, we must mention that the hypothesis of the important role that D3R may play in the pathogenesis of LID is only a conjecture based on the review of the relevant literature, which we believe may too simplistic for discussing such a common and complicated side effect of L-DOPA. We cannot explain that why LID occurs after long term exposure to L-DOPA rather than immediately after several doses of L-DOPA or why a single dose of selective D3R antagonist shows no effect upon established LID. Others posit that the chronic aggravation induced via pathological damage, such as a loss of homeostatic synaptic plasticity in striatum or SN is also considered as a possible cause of LID (Borgkvist et al., 2018).
8. D3R changes for predicting prognosis of PD treatment
The change in D3R expression or function helps predict prognosis of PD treatment. For example, it was suggested that if D3R is elevated, response to DAergic drugs will be maintained. Correspondingly, loss of D3R density correlated with loss of response to DAergic drugs (Joyce et al., 2002). In an animal study, DR agonists showed better improvement in alleviating PD symptoms in animals with striatal over-expression of both D2R and D3R, when compared to those with over-expression of only D2R (Matsukawa et al., 2007).
However, most studies focus on D3R genotype in determining treatment plans. Changes in a single residue in D3R may cause fluctuation in agonist-dependent tolerance property (Gil-Mast et al., 2013), and affect the therapeutic effect of related drugs. In D3R, the most commonly researched polymorphic site is Ser9Gly. A study showed that D3R ser/Gly mutant is the main factor determining dose of DR agonists, and patients who carry Gly/Gly need higher doses of DR agonist to achieve identical treatment (Xu et al., 2017a).
Determining the precise D3R genotype may help identify potential side effects of PD therapy. A study has shown that the D3R Ser/Ser genotype is a predictor of gastrointestinal symptoms after L-DOPA treatment (Rieck et al., 2018). Other results showed that D3R Ser/Gly may lead to essential tremor in PD (Paus et al., 2010).
9. Conclusions
For a long time, D1R and D2R have long been considered as critical participants in PD occurrence and progression. However, with in-depth understanding of the structure and function of D3R, people are paying more and more attention in the role of D3R in PD. But it is difficult to develop D3R-specific tracers and agonists as D2R and D3R show high similarity. We developed a quantitative autoradiography procedure for measuring the densities and mapping the regional distributions of D2R and D3R in the brain, and found that the striatal D3R is closely related with pathophysiological symptoms and prognosis results in PD patients. Therefore, we review the role of D3R in the pathogenesis, treatment and prognosis of PD, and try to provide insights for novel PD pharmaceutics via targeting D3R.
By summarizing literatures, we find that alterations of D3R involve in the early stage of PD and may be considered as an early stage biomarker for predicting PD occurrence. D3R agonists are also thought to be efficient in the treatment of PD, with their effect in increasing DA content, decreasing α-Syn accumulation, enhancing BDNF secretion, ameliorating oxidative stress and neuroinflammation, promoting neurogenesis and interacting with other DRs. Besides, Targeting D3R also helps in relieving some PD associated non motor symptoms and side effects of L-DOPA. Furthermore, D3R is also considered as an indicator for predicting prognosis of PD treatment. All of these suggested that D3R may be of great importance in the diagnosis, treatment and prognosis of PD. However, many questions need further clarification.
Whether the development of highly selective tracers or drugs for D3R will yield better diagnostic or therapeutic effects without unwanted side effects. We should avoid similar phenomena as valdecoxib, a highly selective inhibitor of COX2 with strong anti-inflammatory effects, which was unfortunately withdraw from the market due to severe cardiovascular side effects (Atukorala and Hunter, 2013).
Should the interactions between D3R and other GPCRs or other related proteins be taken into account in the diagnosis and treatment of PD? Our literature review indicates that interactions between D3R and other GPCRs or other related proteins (such as VMAT2) may be an important factor in the occurrence and development of PD, rather than D3R itself.
As mentioned earlier, D3R activation not only participates in the treatment of PD, but also help in alleviating the occurrence of LID. Both effects are achieved partly by regulating D1R activity. The critical questions are what’s the ideal affinity and selectivity of D3R agonists and what’s the optimized individual dosage in clinical practice?
Although much scientific research is needed to further reveal the roles of D3R in PD, we believe that the development of D3R specific tracers and drugs is important for the diagnosis and treatment of PD. While we do not expect D3R-related drugs will solve all the problems, each step of understanding D3R will bring us closer to an improved cure for PD.
Highlights.
A novel procedure for quantitatively calculating the densities of D2R and D3R is recommended.
The role of D3R in the pathogenesis, treatment and prognosis of PD is systematically summarized.
Possible role of D3R in alleviating L-DOPA-induced dyskinesia and possible D3R agonists are introduced.
The potential mechanisms of D3R activation in treating PD are extensively expounded.
Acknowledgments
We thank Mr. William Knight and Ms. Lynne Jones for editorial assistance. Funding: This work was supported by the National Institutes of Health [grant number R01 NS092865, R01 AG052550, P01 AG026276, P01 AG03991 and P50 AG05681].
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of interest The authors have no conflicts of interest.
Reference
- Antonini A, Barone P, Ceravolo R, Fabbrini G, Tinazzi M, Abbruzzese G, 2010. Role of pramipexole in the management of Parkinson's disease. CNS drugs 24, 829–841. [DOI] [PubMed] [Google Scholar]
- Aprahamian I, Nunes PV, Forlenza OV, 2013. Cognitive impairment and dementia in late-life bipolar disorder. Current opinion in psychiatry 26, 120–123. [DOI] [PubMed] [Google Scholar]
- Ariano MA, Aronin N, Difiglia M, Tagle DA, Sibley DR, Leavitt BR, Hayden MR, Levine MS, 2002. Striatal neurochemical changes in transgenic models of Huntington's disease. Journal of neuroscience research 68, 716–729. [DOI] [PubMed] [Google Scholar]
- Aristieta A, Azkona G, Sagarduy A, Miguelez C, Ruiz-Ortega JA, Sanchez-Pernaute R, Ugedo L, 2012. The role of the subthalamic nucleus in L-DOPA induced dyskinesia in 6-hydroxydopamine lesioned rats. PloS one 7, e42652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atukorala I, Hunter DJ, 2013. Valdecoxib : the rise and fall of a COX-2 inhibitor. Expert opinion on pharmacotherapy 14, 1077–1086. [DOI] [PubMed] [Google Scholar]
- Audinot V, Newman-Tancredi A, Gobert A, Rivet JM, Brocco M, Lejeune F, Gluck L, Desposte I, Bervoets K, Dekeyne A, Millan MJ, 1998. A comparative in vitro and in vivo pharmacological characterization of the novel dopamine D3 receptor antagonists (+)-S 14297, nafadotride, GR 103,691 and U 99194. The Journal of pharmacology and experimental therapeutics 287, 187–197. [PubMed] [Google Scholar]
- Augood SJ, Faull RL, Emson PC, 1997. Dopamine D1 and D2 receptor gene expression in the striatum in Huntington's disease. Annals of neurology 42, 215–221. [DOI] [PubMed] [Google Scholar]
- Avalos-Fuentes A, Loya-Lopez S, Flores-Perez A, Recillas-Morales S, Cortes H, Paz-Bermudez F, Aceves J, Erlij D, Floran B, 2013. Presynaptic CaMKIIalpha modulates dopamine D3 receptor activation in striatonigral terminals of the rat brain in a Ca(2)(+) dependent manner. Neuropharmacology 71, 273–281. [DOI] [PubMed] [Google Scholar]
- Azkona G, Sagarduy A, Aristieta A, Vazquez N, Zubillaga V, Ruiz-Ortega JA, Perez-Navarro E, Ugedo L, Sanchez-Pernaute R, 2014. Buspirone anti-dyskinetic effect is correlated with temporal normalization of dysregulated striatal DRD1 signalling in L-DOPA-treated rats. Neuropharmacology 79, 726–737. [DOI] [PubMed] [Google Scholar]
- Baladi MG, Newman AH, Nielsen SM, Hanson GR, Fleckenstein AE, 2014. Dopamine D(3) receptors contribute to methamphetamine-induced alterations in dopaminergic neuronal function: role of hyperthermia. European journal of pharmacology 732, 105–110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ballanger B, Beaudoin-Gobert M, Neumane S, Epinat J, Metereau E, Duperrier S, Broussolle E, Thobois S, Bonnefoi F, Tourvielle C, Lavenne F, Costes N, Lebars D, Zimmer L, Sgambato-Faure V, Tremblay L, 2016. Imaging Dopamine and Serotonin Systems on MPTP Monkeys: A Longitudinal PET Investigation of Compensatory Mechanisms. The Journal of neuroscience : the official journal of the Society for Neuroscience 36, 1577–1589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beaulieu JM, Gainetdinov RR, 2011. The physiology, signaling, and pharmacology of dopamine receptors. Pharmacological reviews 63, 182–217. [DOI] [PubMed] [Google Scholar]
- Benitez A, Edens H, Fishman J, Moran K, Asgharnejad M, 2014. Rotigotine transdermal system: developing continuous dopaminergic delivery to treat Parkinson's disease and restless legs syndrome. Annals of the New York Academy of Sciences 1329, 45–66. [DOI] [PubMed] [Google Scholar]
- Bennett J, Burns J, Welch P, Bothwell R, 2016. Safety and Tolerability of R(+) Pramipexole in Mild-to-Moderate Alzheimer's Disease. Journal of Alzheimer's disease : JAD 49, 1179–1187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bezard E, Ferry S, Mach U, Stark H, Leriche L, Boraud T, Gross C, Sokoloff P, 2003. Attenuation of levodopa-induced dyskinesia by normalizing dopamine D3 receptor function. Nature medicine 9, 762–767. [DOI] [PubMed] [Google Scholar]
- Bibb JA, Yan Z, Svenningsson P, Snyder GL, Pieribone VA, Horiuchi A, Nairn AC, Messer A, Greengard P, 2000. Severe deficiencies in dopamine signaling in presymptomatic Huntington's disease mice. Proceedings of the National Academy of Sciences of the United States of America 97, 6809–6814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bibbiani F, Oh JD, Chase TN, 2001. Serotonin 5-HT1A agonist improves motor complications in rodent and primate parkinsonian models. Neurology 57, 1829–1834. [DOI] [PubMed] [Google Scholar]
- Bleickardt CJ, Lashomb AL, Merkel CE, Hodgson RA, 2012. Adenosine A(2A) Receptor Antagonists Do Not Disrupt Rodent Prepulse Inhibition: An Improved Side Effect Profile in the Treatment of Parkinson's Disease. Parkinsons Dis 2012, 591094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boeckler F, Gmeiner P, 2007. Dopamine D3 receptor ligands: recent advances in the control of subtype selectivity and intrinsic activity. Biochimica et biophysica acta 1768, 871–887. [DOI] [PubMed] [Google Scholar]
- Boileau I, Guttman M, Rusjan P, Adams JR, Houle S, Tong J, Hornykiewicz O, Furukawa Y, Wilson AA, Kapur S, Kish SJ, 2009. Decreased binding of the D3 dopamine receptor-preferring ligand [11C]-(+)-PHNO in drug-naive Parkinson's disease. Brain : a journal of neurology 132, 1366–1375. [DOI] [PubMed] [Google Scholar]
- Bono F, Mutti V, Savoia P, Barbon A, Bellucci A, Missale C, Fiorentini C, 2019. Nicotin prevents alpha-synuclein accumulation in mouse and human iPSC-derived dopaminergic neurons through activation of the dopamine D3- acetylcholine nicotinic receptor heteromer. Neurobiology of disease 129, 1–12. [DOI] [PubMed] [Google Scholar]
- Bono F, Savoia P, Guglielmi A, Gennarelli M, Piovani G, Sigala S, Leo D, Espinoza S, Gainetdinov RR, Devoto P, Spano P, Missale C, Fiorentini C, 2018. Role of Dopamine D2/D3 Receptors in Development, Plasticity, and Neuroprotection in Human iPSC-Derived Midbrain Dopaminergic Neurons. Molecular neurobiology 55, 1054–1067. [DOI] [PubMed] [Google Scholar]
- Bordet R, Ridray S, Carboni S, Diaz J, Sokoloff P, Schwartz JC, 1997. Induction of dopamine D3 receptor expression as a mechanism of behavioral sensitization to levodopa. Proceedings of the National Academy of Sciences of the United States of America 94, 3363–3367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borgkvist A, Lieberman OJ, Sulzer D, 2018. Synaptic plasticity may underlie l-DOPA induced dyskinesia. Curr Opin Neurobiol 48, 71–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boulay D, Depoortere R, Rostene W, Perrault G, Sanger DJ, 1999. Dopamine D3 receptor agonists produce similar decreases in body temperature and locomotor activity in D3 knock-out and wild-type mice. Neuropharmacology 38, 555–565. [DOI] [PubMed] [Google Scholar]
- Brown JA, Xu J, Diggs-Andrews KA, Wozniak DF, Mach RH, Gutmann DH, 2011. PET imaging for attention deficit preclinical drug testing in neurofibromatosis-1 mice. Experimental neurology 232, 333–338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brusa L, Bassi A, Stefani A, Pierantozzi M, Peppe A, Caramia MD, Boffa L, Ruggieri S, Stanzione P, 2003. Pramipexole in comparison to l-dopa: a neuropsychological study. Journal of neural transmission (Vienna, Austria : 1996) 110, 373–380. [DOI] [PubMed] [Google Scholar]
- Bustos G, Abarca J, Campusano J, Bustos V, Noriega V, Aliaga E, 2004. Functional interactions between somatodendritic dopamine release, glutamate receptors and brain-derived neurotrophic factor expression in mesencephalic structures of the brain. Brain research. Brain research reviews 47, 126–144. [DOI] [PubMed] [Google Scholar]
- Cao JL, Covington HE 3rd, Friedman AK, Wilkinson MB, Walsh JJ, Cooper DC, Nestler EJ, Han MH, 2010. Mesolimbic dopamine neurons in the brain reward circuit mediate susceptibility to social defeat and antidepressant action. The Journal of neuroscience : the official journal of the Society for Neuroscience 30, 16453–16458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carnicella S, Drui G, Boulet S, Carcenac C, Favier M, Duran T, Savasta M, 2014. Implication of dopamine D3 receptor activation in the reversion of Parkinson's disease-related motivational deficits. Translational psychiatry 4, e401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castro-Hernandez J, Adlard PA, Finkelstein DI, 2017. Pramipexole restores depressed transmission in the ventral hippocampus following MPTP-lesion. Scientific reports 7, 44426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castro-Hernandez J, Afonso-Oramas D, Cruz-Muros I, Salas-Hernandez J, Barroso-Chinea P, Moratalla R, Millan MJ, Gonzalez-Hernandez T, 2015. Prolonged treatment with pramipexole promotes physical interaction of striatal dopamine D3 autoreceptors with dopamine transporters to reduce dopamine uptake. Neurobiology of disease 74, 325–335. [DOI] [PubMed] [Google Scholar]
- Centonze D, Grande C, Saulle E, Martin AB, Gubellini P, Pavon N, Pisani A, Bernardi G, Moratalla R, Calabresi P, 2003. Distinct roles of D1 and D5 dopamine receptors in motor activity and striatal synaptic plasticity. The Journal of neuroscience : the official journal of the Society for Neuroscience 23, 8506–8512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang PK, Yu L, Chen JC, 2018. Dopamine D3 receptor blockade rescues hyper-dopamine activity-induced deficit in novel object recognition memory. Neuropharmacology 133, 216–223. [DOI] [PubMed] [Google Scholar]
- Chang WL, Swerdlow NR, Breier MR, Thangaraj N, Weber M, 2010. Parametric approaches towards understanding the effects of the preferential D3 receptor agonist pramipexole on prepulse inhibition in rats. Pharmacol Biochem Behav 95, 473–478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang WL, Weber M, Breier MR, Saint Marie RL, Hines SR, Swerdlow NR, 2012. Stereochemical and neuroanatomical selectivity of pramipexole effects on sensorimotor gating in rats. Brain research 1437, 69–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chau KY, Cooper JM, Schapira AH, 2013. Pramipexole reduces phosphorylation of alpha-synuclein at serine-129. Journal of molecular neuroscience : MN 51, 573–580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen J, Jiang C, Levant B, Li X, Zhao T, Wen B, Luo R, Sun D, Wang S, 2014. Pramipexole derivatives as potent and selective dopamine D(3) receptor agonists with improved human microsomal stability. ChemMedChem 9, 2653–2660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coghill D, Banaschewski T, 2009. The genetics of attention-deficit/hyperactivity disorder. Expert review of neurotherapeutics 9, 1547–1565. [DOI] [PubMed] [Google Scholar]
- Collins GT, Butler P, Wayman C, Ratcliffe S, Gupta P, Oberhofer G, Caine SB, 2012. Lack of abuse potential in a highly selective dopamine D3 agonist, PF-592,379, in drug self-administration and drug discrimination in rats. Behavioural pharmacology 23, 280–291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collo G, Bono F, Cavalleri L, Plebani L, Mitola S, Merlo Pich E, Millan MJ, Zoli M, Maskos U, Spano P, Missale C, 2013. Nicotine-induced structural plasticity in mesencephalic dopaminergic neurons is mediated by dopamine D3 receptors and Akt-mTORC1 signaling. Molecular pharmacology 83, 1176–1189. [DOI] [PubMed] [Google Scholar]
- Collo G, Cavalleri L, Bono F, Mora C, Fedele S, Invernizzi RW, Gennarelli M, Piovani G, Kunath T, 2018. Ropinirole and Pramipexole Promote Structural Plasticity in Human iPSC-Derived Dopaminergic Neurons via BDNF and mTOR Signaling. 2018, 4196961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collo G, Zanetti S, Missale C, Spano P, 2008. Dopamine D3 receptor-preferring agonists increase dendrite arborization of mesencephalic dopaminergic neurons via extracellular signal-regulated kinase phosphorylation. The European journal of neuroscience 28, 1231–1240. [DOI] [PubMed] [Google Scholar]
- Cooper AJ, Stanford IM, 2001. Dopamine D2 receptor mediated presynaptic inhibition of striatopallidal GABA(A) IPSCs in vitro. Neuropharmacology 41, 62–71. [DOI] [PubMed] [Google Scholar]
- Cortes A, Moreno E, Rodriguez-Ruiz M, Canela EI, Casado V, 2016. Targeting the dopamine D3 receptor: an overview of drug design strategies. Expert opinion on drug discovery 11, 641–664. [DOI] [PubMed] [Google Scholar]
- Cote SR, Chitravanshi VC, Bleickardt C, Sapru HN, Kuzhikandathil EV, 2014. Overexpression of the dopamine D3 receptor in the rat dorsal striatum induces dyskinetic behaviors. Behavioural brain research 263, 46–50. [DOI] [PubMed] [Google Scholar]
- Cote SR, Kuzhikandathil EV, 2015. Chronic levodopa treatment alters expression and function of dopamine D3 receptor in the MPTP/p mouse model of Parkinson's disease. Neuroscience letters 585, 33–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cruz-Trujillo R, Avalos-Fuentes A, Rangel-Barajas C, Paz-Bermudez F, Sierra A, Escartin-Perez E, Aceves J, Erlij D, Floran B, 2013. D3 dopamine receptors interact with dopamine D1 but not D4 receptors in the GABAergic terminals of the SNr of the rat. Neuropharmacology 67, 370–378. [DOI] [PubMed] [Google Scholar]
- Dai D, Wang Y, Wang L, Li J, Ma Q, Tao J, Zhou X, Zhou H, Jiang Y, Pan G, Xu L, Ru P, Lin D, Pan J, Xu L, Ye M, Duan S, 2014. Polymorphisms of DRD2 and DRD3 genes and Parkinson's disease: A meta-analysis. Biomedical reports 2, 275–281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dal Toso R, Sommer B, Ewert M, Herb A, Pritchett DB, Bach A, Shivers BD, Seeburg PH, 1989. The dopamine D2 receptor: two molecular forms generated by alternative splicing. The EMBO journal 8, 4025–4034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dauer W, Przedborski S, 2003. Parkinson's disease: mechanisms and models. Neuron 39, 889–909. [DOI] [PubMed] [Google Scholar]
- Del Bello F, Bonifazi A, Giorgioni G, Cifani C, Micioni Di Bonaventura MV, Petrelli R, Piergentili A, 2018. 1-[3-(4-Butylpiperidin-1-yl)propyl]-1,2,3,4-tetrahydroquinolin-2-one (77-LH-28-1) as a Model for the Rational Design of a Novel Class of Brain Penetrant Ligands with High Affinity and Selectivity for Dopamine D4 Receptor. 61, 3712–3725. [DOI] [PubMed] [Google Scholar]
- Diaz J, Pilon C, Le Foll B, Gros C, Triller A, Schwartz JC, Sokoloff P, 2000. Dopamine D3 receptors expressed by all mesencephalic dopamine neurons. The Journal of neuroscience : the official journal of the Society for Neuroscience 20, 8677–8684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dragasevic-Miskovic N, Petrovic I, Stankovic I, Kostic VS, 2019. Chemical management of levodopa-induced dyskinesia in Parkinson's disease patients. Expert Opin Pharmacother 20, 219–230. [DOI] [PubMed] [Google Scholar]
- Du F, Li R, Huang Y, Li X, Le W, 2005. Dopamine D3 receptor-preferring agonists induce neurotrophic effects on mesencephalic dopamine neurons. The European journal of neuroscience 22, 2422–2430. [DOI] [PubMed] [Google Scholar]
- Duric V, Banasr M, Franklin T, Lepack A, Adham N, Kiss B, Gyertyan I, Duman RS, 2017. Cariprazine Exhibits Anxiolytic and Dopamin D3 Receptor-Dependent Antidepressant Effects in the Chronic Stress Model. The international journal of neuropsychopharmacology 20, 788–796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eden RJ, Wallduck MS, Patel B, Owen DA, 1990. Autonomic and haemodynamic responses to SK & F 101468 (ropinirole), a DA2 agonist, in anaesthetised cats. European journal of pharmacology 175, 333–340. [DOI] [PubMed] [Google Scholar]
- Elgueta D, Aymerich MS, Contreras F, Montoya A, Celorrio M, Rojo-Bustamante E, Riquelme E, Gonzalez H, Vasquez M, Franco R, Pacheco R, 2017. Pharmacologic antagonism of dopamine receptor D3 attenuates neurodegeneration and motor impairment in a mouse model of Parkinson's disease. Neuropharmacology 113, 110–123. [DOI] [PubMed] [Google Scholar]
- Elgueta D, Contreras F, Prado C, Montoya A, Ugalde V, Chovar O, Villagra R, Henriquez C, Abellanas MA, Aymerich MS, Franco R, Pacheco R, 2019. Dopamine Receptor D3 Expression Is Altered in CD4(+) T-Cells From Parkinson's Disease Patients and Its Pharmacologic Inhibition Attenuates the Motor Impairment in a Mouse Model. Front Immunol 10, 981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elmer LW, Surmann E, Boroojerdi B, Jankovic J, 2012. Long-term safety and tolerability of rotigotine transdermal system in patients with early-stage idiopathic Parkinson's disease: a prospective, open-label extension study. Parkinsonism & related disorders 18, 488–493. [DOI] [PubMed] [Google Scholar]
- Fanni S, Scheggi S, Rossi F, Tronci E, Traccis F, Stancampiano R, De Montis MG, Devoto P, Gambarana C, Bortolato M, Frau R, Carta M, 2018. 5alpha-reductase inhibitors dampen L-DOPA-induced dyskinesia via normalization of dopamine D1-receptor signaling pathway and D1-D3 receptor interaction. Neurobiology of disease 121, 120–130. [DOI] [PubMed] [Google Scholar]
- Fasano A, Petrovic I, 2010. Insights into pathophysiology of punding reveal possible treatment strategies. Mol Psychiatry 15, 560–573. [DOI] [PubMed] [Google Scholar]
- Favier M, Carcenac C, Drui G, Vachez Y, Boulet S, Savasta M, Carnicella S, 2017. Implication of dorsostriatal D3 receptors in motivational processes: a potential target for neuropsychiatric symptoms in Parkinson's disease. Scientific reports 7, 41589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fayyad M, Salim S, Majbour N, Erskine D, Stoops E, Mollenhauer B, El-Agnaf OMA, 2019. Parkinson's disease biomarkers based on alpha-synuclein. Journal of neurochemistry 150, 626–636. [DOI] [PubMed] [Google Scholar]
- Fenelon G, Mahieux F, Huon R, Ziegler M, 2000. Hallucinations in Parkinson's disease: prevalence, phenomenology and risk factors. Brain : a journal of neurology 123 ( Pt 4), 733–745. [DOI] [PubMed] [Google Scholar]
- Ferrari-Toninelli G, Maccarinelli G, Uberti D, Buerger E, Memo M, 2010. Mitochondria-targeted antioxidant effects of S(−) and R(+) pramipexole. BMC pharmacology 10, 2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fiorentini C, Busi C, Spano P, Missale C, 2008. Role of receptor heterodimers in the development of L-dopa-induced dyskinesias in the 6-hydroxydopamine rat model of Parkinson's disease. Parkinsonism & related disorders 14 Suppl 2, S159–164. [DOI] [PubMed] [Google Scholar]
- Fisher BE, Li Q, Nacca A, Salem GJ, Song J, Yip J, Hui JS, Jakowec MW, Petzinger GM, 2013. Treadmill exercise elevates striatal dopamine D2 receptor binding potential in patients with early Parkinson's disease. Neuroreport 24, 509–514. [DOI] [PubMed] [Google Scholar]
- Frampton JE, 2014. Pramipexole extended-release: a review of its use in patients with Parkinson's disease. Drugs 74, 2175–2190. [DOI] [PubMed] [Google Scholar]
- Franz D, Contreras F, Gonzalez H, Prado C, Elgueta D, Figueroa C, Pacheco R, 2015. Dopamine receptors D3 and D5 regulate CD4(+)T-cell activation and differentiation by modulating ERK activation and cAMP production. Journal of neuroimmunology, 284, 18–29. [DOI] [PubMed] [Google Scholar]
- Fuxe K, Guidolin D, Agnati LF, Borroto-Escuela DO, 2015. Dopamine heteroreceptor complexes as therapeutic targets in Parkinson's disease. Expert opinion on therapeutic targets 19, 377–398. [DOI] [PubMed] [Google Scholar]
- Garcia-Ladona FJ, Cox BF, 2003. BP 897, a selective dopamine D3 receptor ligand with therapeutic potential for the treatment of cocaine-addiction. CNS drug reviews 9, 141–158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia-Ruiz PJ, Martinez Castrillo JC, Alonso-Canovas A, Herranz Barcenas A, Vela L, Sanchez Alonso P, Mata M, Olmedilla Gonzalez N, Mahillo Fernandez I, 2014. Impulse control disorder in patients with Parkinson's disease under dopamine agonist therapy: a multicentre study. Journal of neurology, neurosurgery, and psychiatry 85, 840–844. [DOI] [PubMed] [Google Scholar]
- Giakoumaki SG, Roussos P, Frangou S, Bitsios P, 2007. Disruption of prepulse inhibition of the startle reflex by the preferential D(3) agonist ropinirole in healthy males. Psychopharmacology 194, 289–295. [DOI] [PubMed] [Google Scholar]
- Gil-Mast S, Kortagere S, Kota K, Kuzhikandathil EV, 2013. An amino acid residue in the second extracellular loop determines the agonist-dependent tolerance property of the human D3 dopamine receptor. ACS chemical neuroscience 4, 940–951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giorelli M, Livrea P, Trojano M, 2005. Dopamine fails to regulate activation of peripheral blood lymphocytes from multiple sclerosis patients: effects of IFN-beta. Journal of interferon & cytokine research : the official journal of the International Society for Interferon and Cytokine Research 25, 395–406. [DOI] [PubMed] [Google Scholar]
- Girault JA, Greengard P, 2004. The neurobiology of dopamine signaling. Archives of neurology 61, 641–644. [DOI] [PubMed] [Google Scholar]
- Gizer IR, Ficks C, Waldman ID, 2009. Candidate gene studies of ADHD: a meta-analytic review. Human genetics 126, 51–90. [DOI] [PubMed] [Google Scholar]
- Gonzalez H, Contreras F, Prado C, Elgueta D, Franz D, Bernales S, Pacheco R, 2013. Dopamine receptor D3 expressed on CD4+ T cells favors neurodegeneration of dopaminergic neurons during Parkinson's disease. Journal of immunology (Baltimore, Md. : 1950) 190, 5048–5056. [DOI] [PubMed] [Google Scholar]
- Grandy DK, Zhang YA, Bouvier C, Zhou QY, Johnson RA, Allen L, Buck K, Bunzow JR, Salon J, Civelli O, 1991. Multiple human D5 dopamine receptor genes: a functional receptor and two pseudogenes. Proceedings of the National Academy of Sciences of the United States of America 88, 9175–9179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gross G, Wicke K, Drescher KU, 2013. Dopamine D(3) receptor antagonism--still a therapeutic option for the treatment of schizophrenia. Naunyn-Schmiedeberg's archives of pharmacology 386, 155–166. [DOI] [PubMed] [Google Scholar]
- Gui YX, Wan Y, Xiao Q, Wang Y, Wang G, Chen SD, 2011. Verification of expressions of Kir2 as potential peripheral biomarkers in lymphocytes from patients with Parkinson's disease. Neuroscience letters 505, 104–108. [DOI] [PubMed] [Google Scholar]
- Guigoni C, Aubert I, Li Q, Gurevich VV, Benovic JL, Ferry S, Mach U, Stark H, Leriche L, Hakansson K, Bioulac BH, Gross CE, Sokoloff P, Fisone G, Gurevich EV, Bloch B, Bezard E, 2005. Pathogenesis of levodopa-induced dyskinesia: focus on D1 and D3 dopamine receptors. Parkinsonism & related disorders 11 Suppl 1, S25–29. [DOI] [PubMed] [Google Scholar]
- Guillin O, Diaz J, Carroll P, Griffon N, Schwartz JC, Sokoloff P, 2001. BDNF controls dopamine D3 receptor expression and triggers behavioural sensitization. Nature 411, 86–89. [DOI] [PubMed] [Google Scholar]
- Gurevich EV, Joyce JN, 1999. Distribution of dopamine D3 receptor expressing neurons in the human forebrain: comparison with D2 receptor expressing neurons. Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacology 20, 60–80. [DOI] [PubMed] [Google Scholar]
- Hadj Tahar A, Grondin R, Doad VD, 1999. Behavioural effects of selective dopamine D3 receptor agonists/antagonist in primate model of Parkinson's disease. Abstr.-Soc. Neurosci 25, 1598. [Google Scholar]
- Han JW, Ahn YD, Kim WS, Shin CM, Jeong SJ, Song YS, Bae YJ, Kim JM, 2018. Psychiatric Manifestation in Patients with Parkinson's Disease. J Korean Med Sci 33, e300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hassan A, Bower JH, Kumar N, Matsumoto JY, Fealey RD, Josephs KA, Ahlskog JE, 2011. Dopamine agonist-triggered pathological behaviors: surveillance in the PD clinic reveals high frequencies. Parkinsonism & related disorders 17, 260–264. [DOI] [PubMed] [Google Scholar]
- Hassan A, Heckman MG, Ahlskog JE, Wszolek ZK, Serie DJ, Uitti RJ, van Gerpen JA, Okun MS, Rayaprolu S, Ross OA, 2016. Association of Parkinson disease age of onset with DRD2, DRD3 and GRIN2B polymorphisms. Parkinsonism & related disorders 22, 102–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heidbreder CA, Gardner EL, Xi ZX, Thanos PK, Mugnaini M, Hagan JJ, Ashby CR Jr., 2005. The role of central dopamine D3 receptors in drug addiction: a review of pharmacological evidence. Brain research. Brain research reviews 49, 77–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hisahara S, Shimohama S, 2011. Dopamine receptors and Parkinson's disease. International journal of medicinal chemistry 2011, 403039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hori H, Kunugi H, 2013. Dopamine agonist-responsive depression. Psychogeriatrics : the official journal of the Japanese Psychogeriatric Society 13, 189–195. [DOI] [PubMed] [Google Scholar]
- Hsu A, Togasaki DM, Bezard E, Sokoloff P, Langston JW, Di Monte DA, Quik M, 2004. Effect of the D3 dopamine receptor partial agonist BP897 [N-[4-(4-(2-methoxyphenyl)piperazinyl)butyl]-2-naphthamide] on L-3,4-dihydroxyphenylalanine-induced dyskinesias and parkinsonism in squirrel monkeys. The Journal of pharmacology and experimental therapeutics 311, 770–777. [DOI] [PubMed] [Google Scholar]
- Hurley MJ, Stubbs CM, Jenner P, Marsden CD, 1996. D3 receptor expression within the basal ganglia is not affected by Parkinson's disease. Neuroscience letters 214, 75–78. [DOI] [PubMed] [Google Scholar]
- Iida M, Miyazaki I, Tanaka K, Kabuto H, Iwata-Ichikawa E, Ogawa N, 1999. Dopamine D2 receptor-mediated antioxidant and neuroprotective effects of ropinirole, a dopamine agonist. Brain research 838, 51–59. [DOI] [PubMed] [Google Scholar]
- Ilani T, Strous RD, Fuchs S, 2004. Dopaminergic regulation of immune cells via D3 dopamine receptor: a pathway mediated by activated T cells. FASEB journal : official publication of the Federation of American Societies for Experimental Biology 18, 1600–1602. [DOI] [PubMed] [Google Scholar]
- Imamura K, Takeshima T, Nakaso K, Ito S, Nakashima K, 2008. Pramipexole has astrocyte-mediated neuroprotective effects against lactacystin toxicity. Neuroscience letters 440, 97–102. [DOI] [PubMed] [Google Scholar]
- Iravani MM, Sadeghian M, Leung CC, Tel BC, Rose S, Schapira AH, Jenner P, 2008. Continuous subcutaneous infusion of pramipexole protects against lipopolysaccharide-induced dopaminergic cell death without affecting the inflammatory response. Experimental neurology 212, 522–531. [DOI] [PubMed] [Google Scholar]
- Jaber M, Robinson SW, Missale C, Caron MG, 1996. Dopamine receptors and brain function. Neuropharmacology 35, 1503–1519. [DOI] [PubMed] [Google Scholar]
- Jeanblanc J, He DY, McGough NN, Logrip ML, Phamluong K, Janak PH, Ron D, 2006. The dopamine D3 receptor is part of a homeostatic pathway regulating ethanol consumption. The Journal of neuroscience: the official journal of the Society for Neuroscience 26, 1457–1464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang L, Zhang H, Wang C, Ming F, Shi X, Yang M, 2019. Serum level of brain-derived neurotrophic factor in Parkinson's disease: a meta-analysis. Progress in neuro-psychopharmacology & biological psychiatry 88, 168–174. [DOI] [PubMed] [Google Scholar]
- Joyce JN, Ryoo H, Gurevich EV, Adler C, Beach T, 2001. Ventral striatal D(3) receptors and Parkinson's Disease. Parkinsonism & related disorders 7, 225–230. [DOI] [PubMed] [Google Scholar]
- Joyce JN, Ryoo HL, Beach TB, Caviness JN, Stacy M, Gurevich EV, Reiser M, Adler CH, 2002. Loss of response to levodopa in Parkinson's disease and co-occurrence with dementia: role of D3 and not D2 receptors. Brain research 955, 138–152. [DOI] [PubMed] [Google Scholar]
- Joyce JN, Woolsey C, Ryoo H, Borwege S, Hagner D, 2004. Low dose pramipexole is neuroprotective in the MPTP mouse model of Parkinson's disease, and downregulates the dopamine transporter via the D3 receptor. BMC biology 2, 22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalia LV, Kalia SK, 2015. alpha-Synuclein and Lewy pathology in Parkinson's disease. Current opinion in neurology 28, 375–381. [DOI] [PubMed] [Google Scholar]
- Kalia LV, Lang AE, 2015. Parkinson's disease. Lancet (London, England) 386, 896–912. [DOI] [PubMed] [Google Scholar]
- Kaneko S, Eisner GM, Jose PA, 1990. Effect of pramipexole, a dopamine-1/dopamine-2 receptor agonist, on sodium excretion and blood pressure in spontaneously hypertensive rats. Journal of autonomic pharmacology 10 Suppl 1, s53–60. [DOI] [PubMed] [Google Scholar]
- Kang R, Zeng L, Xie Y, Yan Z, Zhou B, Cao L, Klionsky DJ, Tracey KJ, Li J, Wang H, Billiar TR, Jiang J, Tang D, 2016. A novel PINK1- and PARK2-dependent protective neuroimmune pathway in lethal sepsis. Autophagy 12, 2374–2385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karampetsou M, Ardah MT, Semitekolou M, Polissidis A, Samiotaki M, Kalomoiri M, Majbour N, Xanthou G, El-Agnaf OMA, Vekrellis K, 2017. Phosphorylated exogenous alpha-synuclein fibrils exacerbate pathology and induce neuronal dysfunction in mice. Scientific reports 7, 16533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kebabian JW, Britton DR, DeNinno MP, Perner R, Smith L, Jenner P, Schoenleber R, Williams M, 1992. A-77636: a potent and selective dopamine D1 receptor agonist with antiparkinsonian activity in marmosets. European journal of pharmacology 229, 203–209. [DOI] [PubMed] [Google Scholar]
- Keck TM, Banala AK, Slack RD, Burzynski C, Bonifazi A, Okunola-Bakare OM, Moore M, Deschamps JR, Rais R, Slusher BS, Newman AH, 2015. Using click chemistry toward novel 1,2,3-triazole-linked dopamine D3 receptor ligands. Bioorganic & medicinal chemistry 23, 4000–4012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keeler BE, Lallemand P, Patel MM, de Castro Bras LE, Clemens S, 2016. Opposing aging-related shift of excitatory dopamine D1 and inhibitory D3 receptor protein expression in striatum and spinal cord. Journal of neurophysiology 115, 363–369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim A, Nigmatullina R, Zalyalova Z, Soshnikova N, Krasnov A, Vorobyeva N, Georgieva S, Kudrin V, Narkevich V, Ugrumov M, 2018. Upgraded Methodology for the Development of Early Diagnosis of Parkinson's Disease Based on Searching Blood Markers in Patients and Experimental Models. [DOI] [PubMed] [Google Scholar]
- Kordower JH, Olanow CW, Dodiya HB, Chu Y, Beach TG, Adler CH, Halliday GM, Bartus RT, 2013. Disease duration and the integrity of the nigrostriatal system in Parkinson's disease. Brain : a journal of neurology 136, 2419–2431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kraemmer J, Smith K, Weintraub D, Guillemot V, Nalls MA, Cormier-Dequaire F, Moszer I, Brice A, Singleton AB, Corvol JC, 2016. Clinical-genetic model predicts incident impulse control disorders in Parkinson's disease. Journal of neurology, neurosurgery, and psychiatry 87, 1106–1111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krishnamoorthy S, Rajan R, Banerjee M, Kumar H, Sarma G, Krishnan S, Sarma S, Kishore A, 2016. Dopamine D3 receptor Ser9Gly variant is associated with impulse control disorders in Parkinson's disease patients. Parkinsonism & related disorders 30, 13–17. [DOI] [PubMed] [Google Scholar]
- Kumar U, Patel SC, 2007. Immunohistochemical localization of dopamine receptor subtypes (D1R-D5R) in Alzheimer's disease brain. Brain research 1131, 187–196. [DOI] [PubMed] [Google Scholar]
- Kuramoto L, Cragg J, Nandhagopal R, Mak E, Sossi V, de la Fuente-Fernandez R, Stoessl AJ, Schulzer M, 2013. The nature of progression in Parkinson's disease: an application of non-linear, multivariate, longitudinal random effects modelling. PloS one 8, e76595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lammers CH, Diaz J, Schwartz JC, Sokoloff P, 2000. Selective increase of dopamine D3 receptor gene expression as a common effect of chronic antidepressant treatments. Molecular psychiatry 5, 378–388. [DOI] [PubMed] [Google Scholar]
- Lang AE, 2001. Acute orthostatic hypotension when starting dopamine agonist therapy in parkinson disease: the role of domperidone therapy. Archives of neurology 58, 835. [DOI] [PubMed] [Google Scholar]
- Lanza K, Meadows SM, Chambers NE, Nuss E, Deak MM, Ferre S, Bishop C, 2018. Behavioral and cellular dopamine D1 and D3 receptor-mediated synergy: Implications for L-DOPA-induced dyskinesia. Neuropharmacology 138, 304–314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lao CL, Kuo YH, Hsieh YT, Chen JC, 2013. Intranasal and subcutaneous administration of dopamine D3 receptor agonists functionally restores nigrostriatal dopamine in MPTP-treated mice. Neurotoxicity research 24, 523–531. [DOI] [PubMed] [Google Scholar]
- Le Foll B, Collo G, Rabiner EA, Boileau I, Merlo Pich E, Sokoloff P, 2014a. Dopamine D3 receptor ligands for drug addiction treatment: update on recent findings. Progress in brain research 211, 255–275. [DOI] [PubMed] [Google Scholar]
- Le Foll B, Gallo A, Le Strat Y, Lu L, Gorwood P, 2009. Genetics of dopamine receptors and drug addiction: a comprehensive review. Behavioural pharmacology 20, 1–17. [DOI] [PubMed] [Google Scholar]
- Le Foll B, Wilson AA, Graff A, Boileau I, Di Ciano P, 2014b. Recent methods for measuring dopamine D3 receptor occupancy in vivo: importance for drug development. Frontiers in pharmacology 5, 161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le WD, Jankovic J, Xie W, Appel SH, 2000. Antioxidant property of pramipexole independent of dopamine receptor activation in neuroprotection. Journal of neural transmission (Vienna, Austria : 1996) 107, 1165–1173. [DOI] [PubMed] [Google Scholar]
- Leggio GM, Bucolo C, Platania CB, Salomone S, Drago F, 2016. Current drug treatments targeting dopamine D3 receptor. Pharmacology & therapeutics 165, 164–177. [DOI] [PubMed] [Google Scholar]
- Leggio GM, Salomone S, Bucolo C, Platania C, Micale V, Caraci F, Drago F, 2013. Dopamine D(3) receptor as a new pharmacological target for the treatment of depression. European journal of pharmacology 719, 25–33. [DOI] [PubMed] [Google Scholar]
- Lewis MM, Huang X, Nichols DE, Mailman RB, 2006. D1 and functionally selective dopamine agonists as neuroprotective agents in Parkinson's disease. CNS & neurological disorders drug targets 5, 345–353. [DOI] [PubMed] [Google Scholar]
- LeWitt PA, Lyons KE, Pahwa R, 2007. Advanced Parkinson disease treated with rotigotine transdermal system: PREFER Study. Neurology 68, 1262–1267. [DOI] [PubMed] [Google Scholar]
- Li C, Biswas S, Li X, Dutta AK, Le W, 2010a. Novel D3 dopamine receptor-preferring agonist D-264: Evidence of neuroprotective property in Parkinson's disease animal models induced by 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine and lactacystin. Journal of neuroscience research 88, 2513–2523. [DOI] [PubMed] [Google Scholar]
- Li C, Guo Y, Xie W, Li X, Janokovic J, Le W, 2010b. Neuroprotection of pramipexole in UPS impairment induced animal model of Parkinson's disease. Neurochemical research 35, 1546–1556. [DOI] [PubMed] [Google Scholar]
- Li QX, Mok SS, Laughton KM, McLean CA, Cappai R, Masters CL, Culvenor JG, Horne MK, 2007. Plasma alpha-synuclein is decreased in subjects with Parkinson's disease. Experimental neurology 204, 583–588. [DOI] [PubMed] [Google Scholar]
- Lieberknecht V, Junqueira SC, Cunha MP, Barbosa TA, de Souza LF, Coelho IS, Santos AR, Rodrigues AL, Dafre AL, Dutra RC, 2017. Pramipexole, a Dopamine D2/D3 Receptor-Preferring Agonist, Prevents Experimental Autoimmune Encephalomyelitis Development in Mice. Molecular neurobiology 54, 1033–1045. [DOI] [PubMed] [Google Scholar]
- Lim MM, Xu J, Holtzman DM, Mach RH, 2011. Sleep deprivation differentially affects dopamine receptor subtypes in mouse striatum. Neuroreport 22, 489–493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ling ZD, Robie HC, Tong CW, Carvey PM, 1999. Both the antioxidant and D3 agonist actions of pramipexole mediate its neuroprotective actions in mesencephalic cultures. The Journal of pharmacology and experimental therapeutics 289, 202–210. [PubMed] [Google Scholar]
- Lukacova K, Pavukova E, Kostal L, Bilcik B, Kubikova L, 2016. Dopamine D3 receptors modulate the rate of neuronal recovery, cell recruitment in Area X, and song tempo after neurotoxic damage in songbirds. Neuroscience 331, 158–168. [DOI] [PubMed] [Google Scholar]
- Luo D, Sharma H, Yedlapudi D, Antonio T, Reith MEA, Dutta AK, 2016. Novel multifunctional dopamine D2/D3 receptors agonists with potential neuroprotection and anti-alpha synuclein protein aggregation properties. Bioorganic & medicinal chemistry 24, 5088–5102. [DOI] [PubMed] [Google Scholar]
- Macdonald R, Barnes K, Hastings C, Mortiboys H, 2018. Mitochondrial abnormalities in Parkinson's disease and Alzheimer's disease: can mitochondria be targeted therapeutically? Biochemical Society transactions. [DOI] [PubMed] [Google Scholar]
- Magnard R, Vachez Y, Carcenac C, Krack P, David O, Savasta M, Boulet S, Carnicella S, 2016. What can rodent models tell us about apathy and associated neuropsychiatric symptoms in Parkinson's disease? Translational psychiatry 6, e753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maj J, Dziedzicka-Wasylewska M, Rogoz R, Rogoz Z, 1998. Effect of antidepressant drugs administered repeatedly on the dopamine D3 receptors in the rat brain. European journal of pharmacology 351, 31–37. [DOI] [PubMed] [Google Scholar]
- Maramai S, Gemma S, Brogi S, Campiani G, Butini S, Stark H, Brindisi M, 2016. Dopamine D3 Receptor Antagonists as Potential Therapeutics for the Treatment of Neurological Diseases. Frontiers in neuroscience 10, 451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marcellino D, Ferre S, Casado V, Cortes A, Le Foll B, Mazzola C, Drago F, Saur O, Stark H, Soriano A, Barnes C, Goldberg SR, Lluis C, Fuxe K, Franco R, 2008. Identification of dopamine D1-D3 receptor heteromers. Indications for a role of synergistic D1-D3 receptor interactions in the striatum. The Journal of biological chemistry 283, 26016–26025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marder S, Fleischhacker WW, Earley W, Lu K, Zhong Y, Nemeth G, Laszlovszky I, Szalai E, Durgam S, 2019. Efficacy of cariprazine across symptom domains in patients with acute exacerbation of schizophrenia: Pooled analyses from 3 phase I/III studies. European neuropsychopharmacology : the journal of the European College of Neuropsychopharmacology 29, 127–136. [DOI] [PubMed] [Google Scholar]
- Marvanova M, 2016. Introduction to Parkinson disease (PD) and its complications. The mental health clinician 6, 229–235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsukawa N, Maki M, Yasuhara T, Hara K, Yu G, Xu L, Kim KM, Morgan JC, Sethi KD, Borlongan CV, 2007. Overexpression of D2/D3 receptors increases efficacy of ropinirole in chronically 6-OHDA-lesioned Parkinsonian rats. Brain research 1160, 113–123. [DOI] [PubMed] [Google Scholar]
- Mavrikaki M, Schintu N, Nomikos GG, Panagis G, Svenningsson P, 2014. Ropinirole regulates emotionality and neuronal activity markers in the limbic forebrain. The international journal of neuropsychopharmacology 17, 1981–1993. [DOI] [PubMed] [Google Scholar]
- Mela F, Millan MJ, Brocco M, Morari M, 2010. The selective D(3) receptor antagonist, S33084, improves parkinsonian-like motor dysfunction but does not affect L-DOPA-induced dyskinesia in 6-hydroxydopamine hemi-lesioned rats. Neuropharmacology 58, 528–536. [DOI] [PubMed] [Google Scholar]
- Merlo S, Canonico PL, Sortino MA, 2011. Distinct effects of pramipexole on the proliferation of adult mouse sub-ventricular zone-derived cells and the appearance of a neuronal phenotype. Neuropharmacology 60, 892–900. [DOI] [PubMed] [Google Scholar]
- Millan MJ, Girardon S, Monneyron S, Dekeyne A, 2000. Discriminative stimulus properties of the dopamine D3 receptor agonists, PD128,907 and 7-OH-DPAT: a comparative characterization with novel ligands at D3 versus D2 receptors. Neuropharmacology 39, 586–598. [DOI] [PubMed] [Google Scholar]
- Millan MJ, Maiofiss L, Cussac D, Audinot V, Boutin JA, Newman-Tancredi A, 2002. Differential actions of antiparkinson agents at multiple classes of monoaminergic receptor. I. A multivariate analysis of the binding profiles of 14 drugs at 21 native and cloned human receptor subtypes. The Journal of pharmacology and experimental therapeutics 303, 791–804. [DOI] [PubMed] [Google Scholar]
- Millan MJ, Svenningsson P, Ashby CR Jr., Hill M, Egeland M, Dekeyne A, Brocco M, Di Cara B, Lejeune F, Thomasson N, Munoz C, Mocaer E, Crossman A, Cistarelli L, Girardon S, Iob L, Veiga S, Gobert A, 2008. S33138 [N-[4-[2-[(3aS,9bR)-8-cyano-1,3a,4,9b-tetrahydro[1]-benzopyrano[3,4-c]pyrrol-2(3H )-yl)-ethyl]phenylacetamide], a preferential dopamine D3 versus D2 receptor antagonist and potential antipsychotic agent. II. A neurochemical, electrophysiological and behavioral characterization in vivo. The Journal of pharmacology and experimental therapeutics 324, 600–611. [DOI] [PubMed] [Google Scholar]
- Miller DW, Hague SM, Clarimon J, Baptista M, Gwinn-Hardy K, Cookson MR, Singleton AB, 2004. Alpha-synuclein in blood and brain from familial Parkinson disease with SNCA locus triplication. Neurology 62, 1835–1838. [DOI] [PubMed] [Google Scholar]
- Mishra A, Singh S, Shukla S, 2018. Physiological and Functional Basis of Dopamine Receptors and Their Role in Neurogenesis: Possible Implication for Parkinson's disease. Journal of experimental neuroscience 12, 1179069518779829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Missale C, Nash SR, Robinson SW, Jaber M, Caron MG, 1998. Dopamine receptors: from structure to function. Physiological reviews 78, 189–225. [DOI] [PubMed] [Google Scholar]
- Modi G, Voshavar C, Gogoi S, Shah M, Antonio T, Reith ME, Dutta AK, 2014. Multifunctional D2/D3 agonist D-520 with high in vivo efficacy: modulator of toxicity of alpha-synuclein aggregates. ACS chemical neuroscience 5, 700–717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monti JM, Jantos H, 2018. The effects of local microinjection of selective dopamine D1 and D2 receptor agonists and antagonists into the dorsal raphe nucleus on sleep and wakefulness in the rat. Behavioural brain research 339, 11–18. [DOI] [PubMed] [Google Scholar]
- Moore TJ, Glenmullen J, Mattison DR, 2014. Reports of pathological gambling, hypersexuality, and compulsive shopping associated with dopamine receptor agonist drugs. JAMA internal medicine 174, 1930–1933. [DOI] [PubMed] [Google Scholar]
- Moraga-Amaro R, Gonzalez H, Pacheco R, Stehberg J, 2014. Dopamine receptor D3 deficiency results in chronic depression and anxiety. Behavioural brain research 274, 186–193. [DOI] [PubMed] [Google Scholar]
- Morissette M, Goulet M, Grondin R, Blanchet P, Bedard PJ, Di Paolo T, Levesque D, 1998. Associative and limbic regions of monkey striatum express high levels of dopamine D3 receptors: effects of MPTP and dopamine agonist replacement therapies. The European journal of neuroscience 10, 2565–2573. [DOI] [PubMed] [Google Scholar]
- Mukherjee J, Constantinescu CC, Hoang AT, Jerjian T, Majji D, Pan ML, 2015. Dopamine D3 receptor binding of (18)F-fallypride: Evaluation using in vitro and in vivo PET imaging studies. Synapse (New York, N.Y.) 69, 577–591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muly EC, Maddox M, Khan ZU, 2010. Distribution of D1 and D5 dopamine receptors in the primate nucleus accumbens. Neuroscience 169, 1557–1566. [DOI] [PubMed] [Google Scholar]
- Muly EC, Senyuz M, Khan ZU, Guo JD, Hazra R, Rainnie DG, 2009. Distribution of D1 and D5 dopamine receptors in the primate and rat basolateral amygdala. Brain structure & function 213, 375–393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakajima S, Gerretsen P, Takeuchi H, Caravaggio F, Chow T, Le Foll B, Mulsant B, Pollock B, Graff-Guerrero A, 2013. The potential role of dopamine D(3) receptor neurotransmission in cognition. European neuropsychopharmacology : the journal of the European College of Neuropsychopharmacology 23, 799–813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nespor AA, Tizabi Y, 2008. Effects of nicotine on quinpirole- and dizocilpine (MK-801)-induced sensorimotor gating impairments in rats. Psychopharmacology 200, 403–411. [DOI] [PubMed] [Google Scholar]
- Nilsson CL, Hellstrand M, Ekman A, Eriksson E, 1999. Both dopamine and the putative dopamine D3 receptor antagonist PNU-99194A induce a biphasic inhibition of phorbol ester-stimulated arachidonic acid release from CHO cells transfected with the dopamine D3 receptor. Life sciences 64, 939–951. [DOI] [PubMed] [Google Scholar]
- Ohrfelt A, Grognet P, Andreasen N, Wallin A, Vanmechelen E, Blennow K, Zetterberg H, 2009. Cerebrospinal fluid alpha-synuclein in neurodegenerative disorders-a marker of synapse loss? Neuroscience letters 450, 332–335. [DOI] [PubMed] [Google Scholar]
- Ono K, Takasaki J, Takahashi R, Ikeda T, Yamada M, 2013. Effects of antiparkinsonian agents on beta-amyloid and alpha-synuclein oligomer formation in vitro. Journal of neuroscience research 91, 1371–1381. [DOI] [PubMed] [Google Scholar]
- Oster S, Radad K, Scheller D, Hesse M, Balanzew W, Reichmann H, Gille G, 2014. Rotigotine protects against glutamate toxicity in primary dopaminergic cell culture. European journal of pharmacology 724, 31–42. [DOI] [PubMed] [Google Scholar]
- Pacheco R, 2017. Targeting dopamine receptor D3 signalling in inflammation. Oncotarget 8, 7224–7225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paus S, Gadow F, Kaut O, Knapp M, Klein C, Klockgether T, Wullner U, 2010. Tremor in Parkinson's disease is not associated with the DRD3 Ser9Gly polymorphism. Parkinsonism & related disorders 16, 381–383. [DOI] [PubMed] [Google Scholar]
- Payer DE, Guttman M, Kish SJ, Tong J, Adams JR, Rusjan P, Houle S, Furukawa Y, Wilson AA, Boileau I, 2016. D3 dopamine receptor-preferring [11C]PHNO PET imaging in Parkinson patients with dyskinesia. Neurology 86, 224–230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Payer DE, Guttman M, Kish SJ, Tong J, Strafella A, Zack M, Adams JR, Rusjan P, Houle S, Furukawa Y, Wilson AA, Boileau I, 2015. [(1)(1)C]-(+)-PHNO PET imaging of dopamine D(2/3) receptors in Parkinson's disease with impulse control disorders. Movement disorders : official journal of the Movement Disorder Society 30, 160–166. [DOI] [PubMed] [Google Scholar]
- Perachon S, Schwartz JC, Sokoloff P, 1999. Functional potencies of new antiparkinsonian drugs at recombinant human dopamine D1, D2 and D3 receptors. European journal of pharmacology 366, 293–300. [DOI] [PubMed] [Google Scholar]
- Pham DQ, Nogid A, 2008. Rotigotine transdermal system for the treatment of Parkinson's disease. Clinical therapeutics 30, 813–824. [DOI] [PubMed] [Google Scholar]
- Poewe W, 2003. Psychosis in Parkinson's disease. Movement disorders : official journal of the Movement Disorder Society 18 Suppl 6, S80–87. [DOI] [PubMed] [Google Scholar]
- Presgraves SP, Borwege S, Millan MJ, Joyce JN, 2004. Involvement of dopamine D(2)/D(3) receptors and BDNF in the neuroprotective effects of S32504 and pramipexole against 1-methyl-4-phenylpyridinium in terminally differentiated SH-SY5Y cells. Experimental neurology 190, 157–170. [DOI] [PubMed] [Google Scholar]
- Prieto GA, 2017. Abnormalities of Dopamine D3 Receptor Signaling in the Diseased Brain. Journal of central nervous system disease 9, 1179573517726335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prieto GA, Perez-Burgos A, Palomero-Rivero M, Galarraga E, Drucker-Colin R, Bargas J, 2011. Upregulation of D2-class signaling in dopamine-denervated striatum is in part mediated by D3 receptors acting on Ca V 2.1 channels via PIP2 depletion. Journal of neurophysiology 105, 2260–2274. [DOI] [PubMed] [Google Scholar]
- Quik M, Police S, He L, Di Monte DA, Langston JW, 2000. Expression of D(3) receptor messenger RNA and binding sites in monkey striatum and substantia nigra after nigrostriatal degeneration: effect of levodopa treatment. Neuroscience 98, 263–273. [DOI] [PubMed] [Google Scholar]
- Radad K, Scheller D, Rausch WD, Reichmann H, Gille G, 2014. Neuroprotective effect of rotigotine against complex I inhibitors, MPP(+) and rotenone, in primary mesencephalic cell culture. Folia neuropathologica 52, 179–186. [DOI] [PubMed] [Google Scholar]
- Ralph-Williams RJ, Lehmann-Masten V, Geyer MA, 2003. Dopamine D1 rather than D2 receptor agonists disrupt prepulse inhibition of startle in mice. Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacology 28, 108–118. [DOI] [PubMed] [Google Scholar]
- Ralph RJ, Varty GB, Kelly MA, Wang YM, Caron MG, Rubinstein M, Grandy DK, Low MJ, Geyer MA, 1999. The dopamine D2, but not D3 or D4, receptor subtype is essential for the disruption of prepulse inhibition produced by amphetamine in mice. The Journal of neuroscience : the official journal of the Society for Neuroscience 19, 4627–4633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rangel-Barajas C, Silva I, Lopez-Santiago LM, Aceves J, Erlij D, Floran B, 2011. L-DOPA-induced dyskinesia in hemiparkinsonian rats is associated with up-regulation of adenylyl cyclase type V/VI and increased GABA release in the substantia nigra reticulata. Neurobiology of disease 41, 51–61. [DOI] [PubMed] [Google Scholar]
- Riddle LR, Kumar R, Griffin SA, Grundt P, Newman AH, Luedtke RR, 2011. Evaluation of the D3 dopamine receptor selective agonist/partial agonist PG01042 on L-dopa dependent animal involuntary movements in rats. Neuropharmacology 60, 284–294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rieck M, Schumacher-Schuh AF, Altmann V, Callegari-Jacques SM, Rieder CRM, Hutz MH, 2018. Association between DRD2 and DRD3 gene polymorphisms and gastrointestinal symptoms induced by levodopa therapy in Parkinson's disease. The pharmacogenomics journal 18, 196–200. [DOI] [PubMed] [Google Scholar]
- Rinne JO, Laihinen A, Ruottinen H, Ruotsalainen U, Nagren K, Lehikoinen P, Oikonen V, Rinne UK, 1995. Increased density of dopamine D2 receptors in the putamen, but not in the caudate nucleus in early Parkinson's disease: a PET study with [11C]raclopride. Journal of the neurological sciences 132, 156–161. [DOI] [PubMed] [Google Scholar]
- Rivet JM, Audinot V, Gobert A, Peglion JL, Millan MJ, 1994. Modulation of mesolimbic dopamine release by the selective dopamine D3 receptor antagonist, (+)-S 14297. European journal of pharmacology 265, 175–177. [DOI] [PubMed] [Google Scholar]
- Rogers DC, Costall B, Domeney AM, Gerrard PA, Greener M, Kelly ME, Hagan JJ, Hunter AJ, 2000. Anxiolytic profile of ropinirole in the rat, mouse and common marmoset. Psychopharmacology 151, 91–97. [DOI] [PubMed] [Google Scholar]
- Rogoz Z, Skuza G, Kllodzinska A, 2004. Anxiolytic- and antidepressant-like effects of 7-OH-DPAT, preferential dopamine D3 receptor agonist, in rats. Pol J Pharmacol 56, 519–526. [PubMed] [Google Scholar]
- Rondou P, Haegeman G, Van Craenenbroeck K, 2010. The dopamine D4 receptor: biochemical and signalling properties. Cellular and molecular life sciences : CMLS 67, 1971–1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roussos P, Giakoumaki SG, Bitsios P, 2008. The dopamine D(3) receptor Ser9Gly polymorphism modulates prepulse inhibition of the acoustic startle reflex. Biol Psychiatry 64, 235–240. [DOI] [PubMed] [Google Scholar]
- Salvi R, Steigleder T, Schlachetzki JC, Waldmann E, Schwab S, Winner B, Winkler J, Kohl Z, 2016. Distinct Effects of Chronic Dopaminergic Stimulation on Hippocampal Neurogenesis and Striatal Doublecortin Expression in Adult Mice. Frontiers in neuroscience 10, 77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sampson D, Zhu XY, Eyunni SV, Etukala JR, Ofori E, Bricker B, Lamango NS, Setola V, Roth BL, Ablordeppey SY, 2014. Identification of a new selective dopamine D4 receptor ligand. Bioorganic & medicinal chemistry 22, 3105–3114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanchez-Soto M, Bonifazi A, Cai NS, Ellenberger MP, Newman AH, Ferre S, Yano H, 2016. Evidence for Noncanonical Neurotransmitter Activation: Norepinephrine as a Dopamine D2-Like Receptor Agonist. Molecular pharmacology 89, 457–466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sautel F, Griffon N, Sokoloff P, Schwartz JC, Launay C, Simon P, Costentin J, Schoenfelder A, Garrido F, Mann A, et al. , 1995. Nafadotride, a potent preferential dopamine D3 receptor antagonist, activates locomotion in rodents. The Journal of pharmacology and experimental therapeutics 275, 1239–1246. [PubMed] [Google Scholar]
- Saylor AJ, McGinty JF, 2010. An intrastriatal brain-derived neurotrophic factor infusion restores striatal gene expression in Bdnf heterozygous mice. Brain structure & function 215, 97–104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schapira AH, Barone P, Hauser RA, Mizuno Y, Rascol O, Busse M, Salin L, Juhel N, Poewe W, 2011. Extended-release pramipexole in advanced Parkinson disease: a randomized controlled trial. Neurology 77, 767–774. [DOI] [PubMed] [Google Scholar]
- Scheideler MA, Martin J, Hohlweg R, Rasmussen JS, Naerum L, Ludvigsen TS, Larsen PJ, Korsgaard N, Crider AM, Ghosh D, Cruse SF, Fink-Jensen A, 1997. The preferential dopamine D3 receptor agonist cis-8-OH-PBZI induces limbic Fos expression in rat brain. European journal of pharmacology 339, 261–270. [DOI] [PubMed] [Google Scholar]
- Schmauss C, 2000. A single dose of methamphetamine leads to a long term reversal of the blunted dopamine D1 receptor-mediated neocortical c-fos responses in mice deficient for D2 and D3 receptors. The Journal of biological chemistry 275, 38944–38948. [DOI] [PubMed] [Google Scholar]
- Seeman P, 2015. Parkinskin’s disease treatment may cause impulse-control disorder via dopamine D3 receptors. Synapse (New York, N.Y.) 69, 183–189. [DOI] [PubMed] [Google Scholar]
- Seeman P, Weinshenker D, Quirion R, Srivastava LK, Bhardwaj SK, Grandy DK, Premont RT, Sotnikova TD, Boksa P, El-Ghundi M, O'Dowd B F, George SR, Perreault ML, Mannisto PT, Robinson S, Palmiter RD, Tallerico T, 2005. Dopamine supersensitivity correlates with D2High states, implying many paths to psychosis. Proceedings of the National Academy of Sciences of the United States of America 102, 3513–3518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silverdale MA, Nicholson SL, Ravenscroft P, Crossman AR, Millan MJ, Brotchie JM, 2004. Selective blockade of D(3) dopamine receptors enhances the anti-parkinsonian properties of ropinirole and levodopa in the MPTP-lesioned primate. Experimental neurology 188, 128–138. [DOI] [PubMed] [Google Scholar]
- Simms SL, Huettner DP, Kortagere S, 2016. In vivo characterization of a novel dopamine D3 receptor agonist to treat motor symptoms of Parkinson's disease. Neuropharmacology 100, 106–115. [DOI] [PubMed] [Google Scholar]
- Sokoloff P, Giros B, Martres MP, Bouthenet ML, Schwartz JC, 1990. Molecular cloning and characterization of a novel dopamine receptor (D3) as a target for neuroleptics. Nature 347, 146–151. [DOI] [PubMed] [Google Scholar]
- Solis O, Garcia-Montes JR, Gonzalez-Granillo A, Xu M, Moratalla R, 2017. Dopamine D3 Receptor Modulates l-DOPA-Induced Dyskinesia by Targeting D1 Receptor-Mediated Striatal Signaling. Cerebral cortex (New York, N.Y. : 1991) 27, 435–446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solis O, Moratalla R, 2018. Dopamine receptors: homomeric and heteromeric complexes in L-DOPA-induced dyskinesia. 125, 1187–1194. [DOI] [PubMed] [Google Scholar]
- Srivanitchapoom P, Pitakpatapee Y, Suengtaworn A, 2018. Parkinsonian syndromes: A review. Neurology India 66, S15–s25. [DOI] [PubMed] [Google Scholar]
- Stanwood GD, Artymyshyn RP, Kung MP, Kung HF, Lucki I, McGonigle P, 2000. Quantitative autoradiographic mapping of rat brain dopamine D3 binding with [(125)I]7-OH-PIPAT: evidence for the presence of D3 receptors on dopaminergic and nondopaminergic cell bodies and terminals. The Journal of pharmacology and experimental therapeutics 295, 1223–1231. [PubMed] [Google Scholar]
- Sun J, Cairns NJ, Perlmutter JS, Mach RH, Xu J, 2013a. Regulation of dopamine D(3) receptor in the striatal regions and substantia nigra in diffuse Lewy body disease. Neuroscience 248, 112–126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun J, Kouranova E, Cui X, Mach RH, Xu J, 2013b. Regulation of dopamine presynaptic markers and receptors in the striatum of DJ-1 and Pink1 knockout rats. Neuroscience letters 557 Pt B, 123–128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun J, Xu J, Cairns NJ, Perlmutter JS, Mach RH, 2012. Dopamine D1, D2, D3 receptors, vesicular monoamine transporter type-2 (VMAT2) and dopamine transporter (DAT) densities in aged human brain. PloS one 7, e49483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun X, Gou HY, Li F, Lu GY, Song R, Yang RF, Wu N, Su RB, Cong B, Li J, 2016. Y-QA31, a novel dopamine D3 receptor antagonist, exhibits antipsychotic-like properties in preclinical animal models of schizophrenia. Acta pharmacologica Sinica 37, 322–333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sunahara RK, Guan HC, O'Dowd BF, Seeman P, Laurier LG, Ng G, George SR, Torchia J, Van Tol HH, Niznik HB, 1991. Cloning of the gene for a human dopamine D5 receptor with higher affinity for dopamine than D1. Nature 350, 614–619. [DOI] [PubMed] [Google Scholar]
- Svensson K, Carlsson A, Waters N, 1994. Locomotor inhibition by the D3 ligand R-(+)-7-OH-DPAT is independent of changes in dopamine release. Journal of neural transmission. General section 95, 71–74. [DOI] [PubMed] [Google Scholar]
- Swant J, Wagner JJ, 2006. Dopamine transporter blockade increases LTP in the CA1 region of the rat hippocampus via activation of the D3 dopamine receptor. Learning & memory (Cold Spring Harbor, N.Y.) 13, 161–167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swerdlow NR, Light GA, Cadenhead KS, Sprock J, Hsieh MH, Braff DL, 2006. Startle gating deficits in a large cohort of patients with schizophrenia: relationship to medications, symptoms, neurocognition, and level of function. Arch Gen Psychiatry 63, 1325–1335. [DOI] [PubMed] [Google Scholar]
- Tabbal SD, Tian L, Karimi M, Brown CA, Loftin SK, Perlmutter JS, 2012. Low nigrostriatal reserve for motor parkinsonism in nonhuman primates. Experimental neurology 237, 355–362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Todd RD, Carl J, Harmon S, O'Malley KL, Perlmutter JS, 1996. Dynamic changes in striatal dopamine D2 and D3 receptor protein and mRNA in response to 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP) denervation in baboons. The Journal of neuroscience : the official journal of the Society for Neuroscience 16, 7776–7782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torrisi SA, Salomone S, Geraci F, Caraci F, Bucolo C, Drago F, Leggio GM, 2017. Buspirone Counteracts MK-801-Induced Schizophrenia-Like Phenotypes through Dopamine D3 Receptor Blockade. Frontiers in pharmacology 8, 710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Truong JG, Newman AH, Hanson GR, Fleckenstein AE, 2004. Dopamine D2 receptor activation increases vesicular dopamine uptake and redistributes vesicular monoamine transporter-2 protein. European journal of pharmacology 504, 27–32. [DOI] [PubMed] [Google Scholar]
- Truong JG, Rau KS, Hanson GR, Fleckenstein AE, 2003. Pramipexole increases vesicular dopamine uptake: implications for treatment of Parkinson's neurodegeneration. European journal of pharmacology 474, 223–226. [DOI] [PubMed] [Google Scholar]
- Umegaki H, Chernak JM, Ikari H, Roth GS, Ingram DK, 1997. Rotational behavior produced by adenovirus-mediated gene transfer of dopamine D2 receptor into rat striatum. Neuroreport 8, 3553–3558. [DOI] [PubMed] [Google Scholar]
- Utsumi H, Okuma Y, Kano O, Suzuki Y, Iijima M, Tomimitsu H, Hashida H, Kubo S, Suzuki M, Nanri K, Matsumura M, Murakami H, Hattori N, 2013. Evaluation of the efficacy of pramipexole for treating levodopa-induced dyskinesia in patients with Parkinson's disease. Internal medicine (Tokyo, Japan) 52, 325–332. [DOI] [PubMed] [Google Scholar]
- Van der Weide J, De Vries JB, Tepper PG, Krause DN, Dubocovich ML, Horn AS, 1988. N-0437: a selective D-2 dopamine receptor agonist in in vitro and in vivo models. European journal of pharmacology 147, 249–258. [DOI] [PubMed] [Google Scholar]
- Van Kampen JM, Eckman CB, 2006. Dopamine D3 receptor agonist delivery to a model of Parkinson's disease restores the nigrostriatal pathway and improves locomotor behavior. The Journal of neuroscience : the official journal of the Society for Neuroscience 26, 7272–7280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Kampen JM, Robertson HA, 2005. A possible role for dopamine D3 receptor stimulation in the induction of neurogenesis in the adult rat substantia nigra. Neuroscience 136, 381–386. [DOI] [PubMed] [Google Scholar]
- Varty GB, Higgins GA, 1998. Dopamine agonist-induced hypothermia and disruption of prepulse inhibition: evidence for a role of D3 receptors? Behavioural pharmacology 9, 445–455. [DOI] [PubMed] [Google Scholar]
- Vivekanantham S, Shah S, Dewji R, Dewji A, Khatri C, Ologunde R, 2015. Neuroinflammation in Parkinson's disease: role in neurodegeneration and tissue repair. The International journal of neuroscience 125, 717–725. [DOI] [PubMed] [Google Scholar]
- Voon V, Fox SH, 2007. Medication-related impulse control and repetitive behaviors in Parkinson disease. Archives of neurology 64, 1089–1096. [DOI] [PubMed] [Google Scholar]
- Voon V, Sohr M, Lang AE, Potenza MN, Siderowf AD, Whetteckey J, Weintraub D, Wunderlich GR, Stacy M, 2011. Impulse control disorders in Parkinson disease: a multicenter case--control study. Annals of neurology 69, 986–996. [DOI] [PubMed] [Google Scholar]
- Wade TV, Rothblat DS, Schneider JS, 2001. Changes in striatal dopamine D3 receptor regulation during expression of and recovery from MPTP-induced parkinsonism. Brain research 905, 111–119. [DOI] [PubMed] [Google Scholar]
- Wahner AD, Bronstein JM, Bordelon YM, Ritz B, 2007. Nonsteroidal anti-inflammatory drugs may protect against Parkinson disease. Neurology 69, 1836–1842. [DOI] [PubMed] [Google Scholar]
- Wang C, Liu Y, Wang H, Wu H, Gong S, He D, 2014. Molecular characterization, expression profile, and polymorphism of goose dopamine D1 receptor gene. Molecular biology reports 41, 2929–2936. [DOI] [PubMed] [Google Scholar]
- Wang JD, Cao YL, Li Q, Yang YP, Jin M, Chen D, Wang F, Wang GH, Qin ZH, Hu LF, Liu CF, 2015a. A pivotal role of FOS-mediated BECN1/Beclin 1 upregulation in dopamine D2 and D3 receptor agonist-induced autophagy activation. Autophagy 11, 2057–2073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang T, Duan SJ, Wang SY, Lu Y, Zhu Q, Wang LJ, Han B, 2015b. Coadministration of hydroxysafflor yellow A with levodopa attenuates the dyskinesia. Physiology & behavior 147, 193–197. [DOI] [PubMed] [Google Scholar]
- Weber M, Chang WL, Breier M, Ko D, Swerdlow NR, 2008. Heritable strain differences in sensitivity to the startle gating-disruptive effects of D2 but not D3 receptor stimulation. Behavioural pharmacology 19, 786–795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber M, Chang WL, Durbin JP, Park PE, Luedtke RR, Mach RH, Swerdlow NR, 2009. Using prepulse inhibition to detect functional D3 receptor antagonism: effects of WC10 and WC44. Pharmacol Biochem Behav 93, 141–147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weintraub D, Koester J, Potenza MN, Siderowf AD, Stacy M, Voon V, Whetteckey J, Wunderlich GR, Lang AE, 2010. Impulse control disorders in Parkinson disease: a cross-sectional study of 3090 patients. Archives of neurology 67, 589–595. [DOI] [PubMed] [Google Scholar]
- Wijeyekoon R, Barker RA, 2009. Cell replacement therapy for Parkinson's disease. Biochimica et biophysica acta 1792, 688–702. [DOI] [PubMed] [Google Scholar]
- Willner P, 1997. The mesolimbic dopamine system as a target for rapid antidepressant action. International clinical psychopharmacology 12 Suppl 3, S7–14. [DOI] [PubMed] [Google Scholar]
- Winner B, Desplats P, Hagl C, Klucken J, Aigner R, Ploetz S, Laemke J, Karl A, Aigner L, Masliah E, Buerger E, Winkler J, 2009. Dopamine receptor activation promotes adult neurogenesis in an acute Parkinson model. Experimental neurology 219, 543–552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wood M, Dubois V, Scheller D, Gillard M, 2015. Rotigotine is a potent agonist at dopamine D1 receptors as well as at dopamine D2 and D3 receptors. British journal of pharmacology 172, 1124–1135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu J, Chu W, Tu Z, Jones LA, Luedtke RR, Perlmutter JS, Mintun MA, Mach RH, 2009. [(3)H]4-(Dimethylamino)-N-[4-(4-(2-methoxyphenyl)piperazin- 1-yl)butyl]benzamide, a selective radioligand for dopamine D(3) receptors. I. In vitro characterization. Synapse 63, 717–728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu J, Hassanzadeh B, Chu W, Tu Z, Jones LA, Luedtke RR, Perlmutter JS, Mintun MA, Mach RH, 2010. [3H]4-(dimethylamino)-N-(4-(4-(2-methoxyphenyl)piperazin-1-yl) butyl)benzamide: a selective radioligand for dopamine D(3) receptors. II. Quantitative analysis of dopamine D(3) and D(2) receptor density ratio in the caudate-putamen. Synapse (New York, N.Y.) 64, 449–459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu S, Liu J, Yang X, Qian Y, Xiao Q, 2017a. Association of the DRD2 CAn-STR and DRD3 Ser9Gly polymorphisms with Parkinson's disease and response to dopamine agonists. Journal of the neurological sciences 372, 433–438. [DOI] [PubMed] [Google Scholar]
- Xu W, Wang X, Tocker AM, Huang P, Reith ME, Liu-Chen LY, Smith AB 3rd, Kortagere S, 2017b. Functional Characterization of a Novel Series of Biased Signaling Dopamine D3 Receptor Agonists. ACS chemical neuroscience 8, 486–500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xue L, Geng Y, Li M, Jin YF, Ren HX, Li X, Wu F, Wang B, Cheng WY, Chen T, Chen YJ, 2016. The effects of D3R on TLR4 signaling involved in the regulation of METH-mediated mast cells activation. International immunopharmacology 36, 187–198. [DOI] [PubMed] [Google Scholar]
- Yang T, Xiao T, Sun Q, Wang K, 2017. The current agonists and positive allosteric modulators of alpha7 nAChR for CNS indications in clinical trials. Acta Pharm Sin B 7, 611–622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Youdim MB Grunblatt E, Mandel S, 1999. The pivotal role of iron in NF-kappa B activation and nigrostriatal dopaminergic neurodegeneration. Prospects for neuroprotection in Parkinson's disease with iron chelators. Annals of the New York Academy of Sciences 890, 7–25. [DOI] [PubMed] [Google Scholar]
- Zhang M, Ballard ME, Unger LV, Haupt A, Gross G, Decker MW, Drescher KU, Rueter LE, 2007. Effects of antipsychotics and selective D3 antagonists on PPI deficits induced by PD128907 and apomorphine. Behavioural brain research 182, 1–11. [DOI] [PubMed] [Google Scholar]
- Zhang QS, Heng Y, Mou Z, Huang JY, Yuan YH, Chen NH, 2017. Reassessment of subacute MPTP-treated mice as animal model of Parkinson's disease. Acta pharmacologica Sinica 38, 1317–1328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Z, Chu SF, Wang SS, Jiang YN, Gao Y, Yang PF, Ai QD, Chen NH, 2018. RTP801 is a critical factor in the neurodegeneration process of A53T alpha-synuclein in a mouse model of Parkinson's disease under chronic restraint stress. 175, 590–605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zou L, Xu J, Jankovic J, He Y, Appel SH, Le W, 2000. Pramipexole inhibits lipid peroxidation and reduces injury in the substantia nigra induced by the dopaminergic neurotoxin 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine in C57BL/6 mice. Neuroscience letters 281, 167–170. [DOI] [PubMed] [Google Scholar]