Abstract
The CellMax (CMx®) platform was developed to enrich for epithelial circulating tumor cells (CTCs) in the whole blood. This report provides assay performance data, including accuracy, linearity, limit of blank, limit of detection (LOD), specificity, and precision of enumeration of cancer cell line cells (CLCs) spiked in cell culture medium or healthy donor blood samples. Additionally, assay specificity was demonstrated in 32 young healthy donors and clinical feasibility was demonstrated in a cohort of 47 subjects consisting of healthy donors and patients who were colonoscopy verified to have colorectal cancer, adenomas, or a negative result. The CMx platform demonstrated high accuracy, linearity, and sensitivity for the enumeration of all CLC concentrations tested, including the extremely low range of 1 to 10 cells in 2 mL of blood, which is most relevant for early cancer detection. Theoretically, the assay LOD is 0.71 CTCs in 2 mL of blood. The analytical specificity was 100% demonstrated using 32 young healthy donor samples. We also demonstrated precision across multiple days and multiple operators, with good reproducibility of recovery efficiency. In a clinical feasibility study, the CMx platform identified 8 of 10 diseased subjects as positive (80% clinical sensitivity) and 4 of 5 controls as negative (80% clinical specificity). We also compared processing time and transportation effects for similar blood samples from two different sites and assessed an artificial intelligence-based counting method. Finally, unlike other platforms for which captured CTCs are retained on ferromagnetic beads or tethered to the slide surface, the CMx platform’s unique airfoam-enabled release of CTCs allows captured cells to be transferred from a microfluidic chip to an Eppendorf tube, enabling a seamless transition to downstream applications such as genetic analyses and live cell manipulations.
Keywords: Circulating tumor cell (CTC), liquid biopsy, biomarker, colorectal cancer, metastasis, CellMax CTC platform, microfluidics, analytical validation
Introduction
In the United States, the risk of developing cancer in one’s lifetime is 40%,1 with most cancers detected too late for treatments to have any significant impact on survival. The World Health Organization defined the current dilemma the best, “…we cannot treat our way out of the cancer problem.” There is a need for “more commitment to the prevention and early detection” of cancer.2 Colorectal cancer (CRC) in particular is a disease, afflicting countries with a high human development index, and CRC incidence is positively correlated with increasing prosperity. Early detection of CRC disease and recurrence has been shown to significantly improve overall survival, inclusive of colorectal polyp or adenoma detection.3,4 There exists an unmet medical need for a novel test that can detect precancerous lesions or early stage diseases.
Circulating epithelial cells, commonly referred to as circulating tumor cells (CTCs), provide rich and varied information in the context of detecting and staging cancer. This information can be mined for early detection, characterization of metastasis, and treatment monitoring.5,6 CTCs were first discovered in the late 19th century.7 They present an opportunity for diagnosis of cancer via liquid biopsy performed on less than 10 mL of blood from a single blood draw, superseding solid tissue biopsy and related invasive procedures.8 This is of great benefit to patients weakened by the disease and possibly by chemotherapy. Secondly, the cost of testing can be lowered since radiographic image guidance is not required and less time needs to be invested by skilled health-care personnel. Thirdly, peripheral venous phlebotomy is a more suitable technique for the repeat sampling required to monitor disease progression or recurrence. Monitoring cancer by the detection of CTCs has several key advantages over solid tissue biopsies.9,10
CTCs in the peripheral blood are involved in tumor metastasis, with greater numbers detected in metastatic patients compared to patients with localized and benign disease.11,12 CTCs increase in the hematologic phase of tumor metastasis. A tumor that grows larger than 2 mm13 may undergo angiogenesis and shed tumor cells that enter the vascular system and migrate to distant locations. Clinical validation has supported the prognostic value of CTC enumeration to predict progression-free survival and overall survival in metastatic breast cancer,14,15 prostate cancer,16,17 and colorectal carcinoma.12 Beyond enumeration, the molecular characterization of CTCs has the potential to predict response to therapy.12,16 The CMx assay is distinguished by its ability to harvest live cells for analysis via gentle airfoam release, without damaging these cells.18 This facilitates downstream analyses of CTCs comprising enumeration, gene expression, methylation, and mutations.
Although CTCs have been extensively studied for more than two decades, there are few reliable methods of detecting and isolating CTCs in the early stages of cancer.19–21 Experimental in vivo studies have suggested that CTCs are present early in the natural history of solid tumor growth, before the development of metastasis.22 More recently, CTCs have been detected in the blood at early stages and at recurrence in women with breast cancer,14,23 and in premalignant stages of prostate cancer tumor progression.24 The problem of isolating rare CTCs is technologically challenging and complex.25 CTCs exist in frequencies in the range of one in one billion blood cells.11,26 Numerous research and commercial efforts have failed to isolate CTCs in early stage cancer, utilizing techniques ranging from gradient centrifugation,27 affinity separation24,28 to filtration.29–33 Currently, there are at least three clinical trials registered with the US National Institutes of Health that use CTCs for cancer screening. They include lung cancer34 (https://clinicaltrials.gov/ct2/show/NCT02500693), breast cancer35 (https://clinicaltrials.gov/ct2/show/NCT01322750), and CRC trials (https://clinicaltrials.gov/ct2/show/NCT02005913). In studies utilizing the CellSearch® FDA-cleared CTC test with an EpCAM cell enrichment strategy, authors concluded that multiple sample processing steps resulted in the loss of CTCs36 and suggested that gentler methods could reduce this loss and enable the detection of CTCs at earlier stages of disease.21
While isolating CTCs in patients with precancerous lesions or early stage CRC has been a formidable challenge, a recent prospective study correlating CTCs to outcomes in nonmetastatic CRC concluded that preoperative CTC detection is a powerful prognostic marker in nonmetastatic CRC.37,38 Technologies that increase sensitivity have the potential to enable early cancer detection.21 Peripheral blood CTCs can be detected in patients with precancerous colorectal polyps as well as CRC.39 A significant difference in peripheral CTC counts has been observed between benign and malignant disease; a CTC count >3/3 mL has been correlated with the presence of a primary tumor. CTC counts also vary with respect to anatomical location and degree of tissue differentiation of the primary tumor.39
The CellMax (CMx®) platform is uniquely suited for CTC detection in early stage disease due to technological aspects that include (i) a biomimetic surface coating on a microfluidic chip that reduces non-specific binding and enables capture of CTCs with threefold greater sensitivity and sixfold greater purity (less contamination than white blood cells) than conventional coating, (ii) proprietary high-affinity antibodies with sixfold greater affinity for cancer cells than conventional antibodies, and (iii) a gentle airfoam release mechanism that enables the capture and collection of low EpCAM expressing cancer cells that can be further used for several downstream applications.18,37,40 This study validated the accuracy, linearity, limits of blank and detection, and the reproducibility of the CMx platform. It also explored the possibility of a CMx test for the early detection of CRC with the inclusion of a clinical feasibility study.
Methods
CMx CTC assay
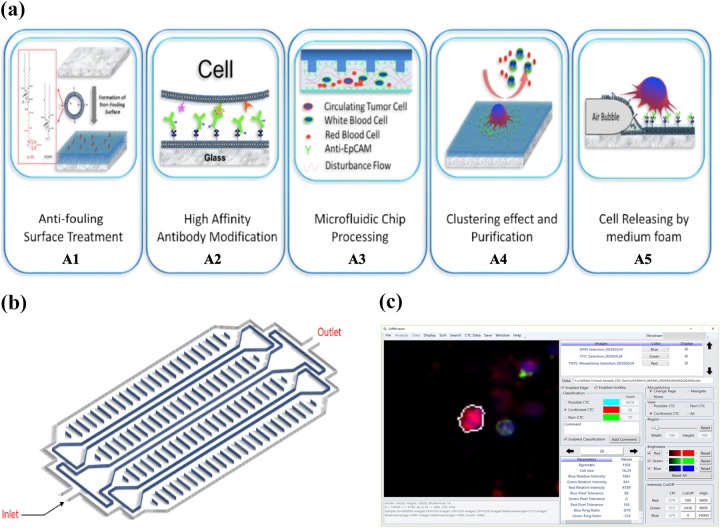
The CMx CTC assay (Figure 1(a)) utilizes a microfluidic chip (Figure 1(b)) consisting of a top layer of poly(methyl methacrylate) with a proprietary etched pattern and a glass bottom coverslip sandwiched together with double-sided 3M tape. The inner surface of the glass layer is coated with a supported lipid bilayer (SLB). This SLB mimics the cell membrane and provides an antifouling surface property that reduces nonspecific binding to the chip surface (Figure 1(a), A1). The epithelial cell adhesion molecule (EpCAM) is a glycosylated membrane protein that is common to epithelial cells and often overexpressed on CTCs derived from solid tumors. The CMx platform uses a high-affinity, monoclonal, anti-EpCAM antibody conjugated to the SLB to capture CTCs from the peripheral blood (Figure 1(a), A2). This conjugation is based on the NeutrAvidin-Biotin chemistry. Whole blood and reagents enter the chip at the inlet port and exit the chip at the outlet port (Figure 1(b)). The etched pattern on the chip produces an optimal flow disturbance that results in maximal contact41 between CTCs and the capture antibody (Figure 1(a), A3). The fluidic nature of the SLB enhances CTC binding to the EpCAM antibody molecules by allowing additional proximal antibody molecules to migrate toward the cell. This “clustering effect” further strengthens the binding force and increases capture efficiency (Figure 1(a), A4).18 Residual white blood cells (WBCs) and red blood cells are efficiently removed from the adhered CTCs with a phosphate-buffered saline (PBS) buffer wash.18 Then the unique airfoam generation and release mechanism recovers captured cells from the chip at the inlet port. The airfoam is generated by mixing cell culture medium and air from two syringes and applied to the chip with a syringe pump. The hydrophilic outside and hydrophobic core property of the airfoam facilitates the interaction with the hydrophobic side of the SLB layer. This allows for the gentle release of the CTC from the SLB surface (Figure 1(a), A5).41 This unique release method causes minimum stress to the CTC and improves recovery while keeping the cells intact. These released cells are collected in an Eppendorf tube for further analysis. For CTC enumeration, the cell solution is transferred to a slide equipped with Millipore filter membrane for immunofluorescent staining.18
Figure 1.
CellMax Life’s CMx CTC assay platform. The CellMax Life CTC assay is performed on the CMx platform (a) using a microfluidic chip (b) that includes several distinctive features to isolate rare circulating tumor cells from peripheral blood and employs specific antibodies to identify the origin of these cells. The CMx technology (a) includes chip surface design and treatment (A1), antibody modification (A2), microfluidic chip processing (A3), purification (A4), and target cell release by air foam (A5). A diagram of the chip is represented in (b). The final step in the assay is the software-assisted enumeration of CTCs using AI-based software and in-house developed imaging software—CellReviewer (c). CTC: circulating tumor cell.
The final steps in the assay involve CTC counting using proprietary artificial intelligence (AI)-based software to screen and identify CTCs on images and quantify stain parameters and cellular features using CellMax developed proprietary software—CellReviewer (Figure 1(c)).
CLC preparation, sample collection, processing, and CTC detection
Peripheral blood (4–8 mL) is drawn from the median decubitus vein by a trained phlebotomist and collected in a BD vacutainer tube containing K2EDTA as the anticoagulant. Streck cell preservative (Streck Inc., Omaha, Nebraska, USA) is added to the tube, typically within 2–4 h at a ratio of 4:1 (blood:preservative), then gently inverted 5 to 10 times to mix, and stored at room temperature until delivery. The sample tubes are delivered in ambient conditions to the CellMax Laboratories in Sunnyvale (CLIA#: 05D2119031, CAP #: 9478056) or Taiwan (CAP #: 9258554) for processing.
The standard operating procedures for microfluidic chip fabrication, micropattern etching, chip surface modification (lipid and EpCAM coating), and airfoam generation and application originate from published research studies at Academia Sinica.18,40 Whole blood (2 mL mixed with 0.5 mL preservative) is loaded by syringe into the inlet port and pulled through a microfluidic channel by a syringe pump connected to the outlet port at a flow rate of 1.5 mL/h. The unbound cells are washed out of the chip with three washes of PBS buffer (0.2 mL at 3 mL/h). The bound cells are fixed on the chip with 4% paraformaldehyde and are recovered using the airfoam mechanism connected to the outlet port. Released cells are collected in an Eppendorf tube via the inlet port. A small volume of ethanol is added to de-bubble the foam–cell mixture in the tube. These cells are then transferred to the membrane chip for counting.
Contrived samples for analytical validation included both donor blood and cell culture medium (Dulbecco’s modified Eagle’s medium, Thermo Scientific 11965084 with 10% fetal bovine serum, Thermo Scientific 26140079) spiked with prestained cancer cell line cells (CLCs) HT29 (HTB-38, ATCC). Prestaining was performed by adding CellTracker™ Green CMFDA or Deep Red Dye (ThermoFisher C2925 or C34565) to the cell culture prior to harvesting, following the manufacturer’s recommendation. The stained cells were fixed with 4% paraformaldehyde and stored in the refrigerator for use. The prestained cell concentration was first estimated with a Scepter 2.0 automated cell counter (EMD Millipore PHCC20060) and then diluted to appropriate concentrations. Aliquots of prestained cells for the final working concentrations were mounted on membranes, imaged with the microscope, and counted by two separate operators to determine precise counts for spiking. Post-capture and release, the cell solution was carefully placed on a membrane, and liquid was wicked away with a blotting pad. The sample was then mounted for imaging and counting.
For samples collected for the clinical feasibility analysis, the released cells were incubated with goat serum (10%) for 60 min at room temperature. Primary antibodies were incubated overnight at 4°C in a refrigerator. The cells were washed with PBS and incubated with fluorescently tagged secondary antibodies. After final washes, the cells were mounted with mounting medium containing 4′,6-diamidino-2-phenylindole (DAPI) antifade (Thermo Fisher P36931) onto a glass slide for image capture.
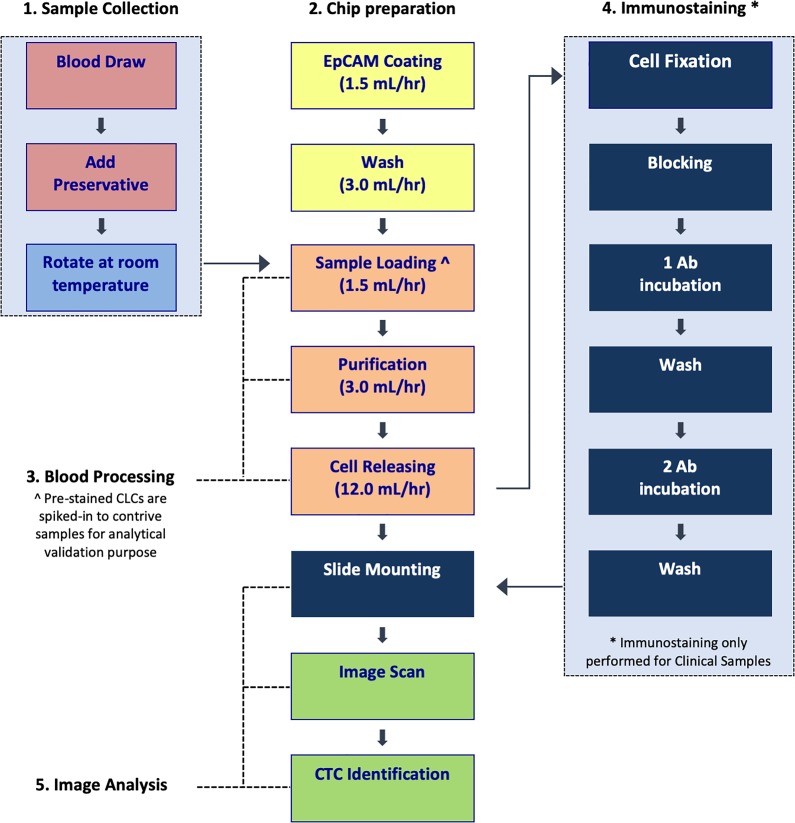
Figure 2 provides a schematic depiction of the CMx assay workflow. The samples are accessioned on day 1 and blood processing commences on day 2. For contrived samples, captured cell release, slide mounting and image capture are performed on day 2, followed by CLC counting. For clinical samples, immunostaining, slide mounting, and CTC enumeration are performed on day 3.
Figure 2.
The CMx CTC assay workflow. The entire CTC process from sample collection to image analysis is summarized in this diagram. Step 1 is performed on day 1, while steps 2, 3 and 5 are performed on day 2 for contrived CLC samples. For clinical samples, steps 2, 3 and 4 up to primary antibody (1 Ab) staining are performed on day 2, while secondary antibody (2 Ab) staining to step 5 are performed on day 3. CTC: circulating tumor cell; CLC: cancer cell line cell.
CLC enumeration
A CLC is defined as a cell that is round to oval in shape and that demonstrates both a nucleus stain (DAPI, blue color) and a prestain (green color). Cells with a single stain were not counted. Counting of prestained HT29 cells was performed with the commercial software MetaMorph (Version 7.8, Molecular Device). The LAS-X stitched images (from Step I described below) were loaded into MetaMorph, assigned colors (DAPI-blue, prestain-green), and merged into a single image. This image was then magnified and reviewed by an operator using a raster scanning mechanism with the aid of location marks on the monitor.
CTC enumeration
Step I: Image capture
For each CTC sample (cells on 10-mm diameter membrane), 100 (10 × 10) frames of images are captured in each of three channels: red, green, and blue, corresponding to TRITC for cytokeratin 20 (CK20), FITC for lymphocyte common antigen (CD45), and DAPI for nucleus counterstain. The Leica autofocus mapping mechanism is applied to ensure images are in focus. Using the built-in stitching function of Leica Las-X software, these 100 image frames are stitched together as one image volume for each of the three channels.
Step II: Identification of candidate cells
We use custom software based on an AI algorithm developed specifically to search the stitched images for regions that have the characteristics of a cell. The coordinates of each target of interest on the image are recorded. The AI algorithm is repeatedly trained with confirmed CTCs and WBCs to improve its sensitivity and specificity. Each of the candidate cells is assigned a confidence index, which allows identification for further morphology-based review and confirmation.
Step III: Reviewing candidates to enumerate CTCs
For CTC confirmation and enumeration, CellMax Life has developed stringent criteria to avoid false positivity using a custom software—CellReviewer. The CTC candidate cell’s morphology is reviewed by a trained technician. CTCs must be round-to-oval shaped, have a cell size between 8 µm and 40 µm and be CK20+, CD45−, and DAPI+ (have a genuine DAPI-stained nucleus). Additionally, WBCs identified by the characteristic multi-segmented (multi-lobed) nucleus are further excluded. CTCs are further reviewed by a technical expert or an experienced pathologist to confirm the absence of WBCs as needed. Final CTC counts are recorded.
Patient cohort
In the clinical feasibility study, we enrolled nine patients with CRC and one patient with colorectal polyp. Among the patients diagnosed with CRC, two were at stage 1, one was at stage 2, three were at stage 3, and three were at stage 4 cancer based on tumor–node–metastasis staging. The patient with colorectal polyp had a tubular adenoma 2 mm in size.
Results
Analytical validation design
We validated the following CTC assay characteristics: accuracy, linearity, limit of blank (LOB), limit of detection (LOD), specificity, and precision (reproducibility). To more closely mimic the cells expected from patients, HT29—a CRC cell line was chosen as a CTC surrogate for this assay validation. HT29 is an epithelial cancer cell line derived from human colorectal adenocarcinomas and has stable expression of both EpCAM and CK20, the two markers used to identify a colorectal CTC. To measure assay performance characteristics, the HT29 CLCs were prestained (see “Methods” section) and each dilution is carefully counted under a microscope prior to spiking into healthy donor blood or culture medium. Sample processing and CLC enumeration of spiked samples followed the protocol described in the “Methods” section. Table 1 lists the analytical performance characteristics validated in this study.
Table 1.
Performance characteristics measured in the analytical validation of CMx CTC assay.
| Performance characteristics | Definition | Sample composition | Measured parameter | |
|---|---|---|---|---|
| 1 | Accuracy (recovery efficiency) | Percent (recovered cells/spiked-in cells) | 33 Spiked samples in 4 concentrations in medium and blood | Percent recovery efficiency |
| 2 | Linearity | Linearity plot of percent (recovered cells/spiked-in cells) | 33 Spiked samples of 2 to 189 cells in medium and blood | Regression curve in linear plot for recovered versus spiked cells |
| 3 | LOD | Limit of detection | 52 Spiked blood samples containing 2 to 281 cells | Lowest measurable and quantifiable CLC count per 2 mL blood |
| 4 | LOB and specificity | Limit of blank and rate of false-positive detection | 8 Nonspiked medium and 32 healthy control blood samples | CTC detection in nonspiked medium and blood |
| 5 | Precision (reproducibility) | Intra-assay variability (3 tests) | Spiked blood samples in 3 concentrations: high (96–181 cells), medium (12–21 cells), and low (2–11 cells) | Percent coefficient of variation of recovery efficiency for 3 concentrations (high, medium, low) in 3 test conditions |
| Inter-assay variability (3 days) | ||||
| Inter-operator variability (3 operators) |
CTC: circulating tumor cell; CLC: cancer cell line cell.
Assay accuracy
The assay accuracy is defined as percentage recovery of spiked HT29 cells across a range that covers meaningful concentrations of CTCs. For this test, 33 dilutions of cells in four concentration groups: high (101–200 cells), medium (51–100 cells), low (11–50 cells), and rare (1–10 cells) were counted under the microscope prior to spiking into 2 mL medium or healthy donor blood. The overall efficiency was defined as the cell numbers (recovered cells from the process) observed under a microscope as a proportion of the spiked cell numbers. The average overall efficiency for the spiked medium was 78.1%, with relatively low standard deviation (6%) (Figure 3 and Table 2). The average overall efficiency was 64.2% ± 11% in blood across the entire spiked cell range (Figure 3 and Table 3). Inherently, donor blood can interact with spiked cells and increase variability in the overall efficiency. For this reason, we also quantified the overall recovery efficiency by spiking HT29 into cell culture medium to factor out the variability of blood from the system performance. Note that at the “rare” concentration level, numbers as low as 2 and 3 cells were counted prior to spiking into 2 mL (assay volume) medium and blood. Instead of estimating cell counts in serial dilutions, we selected this precise counting technique to correctly contrive samples and thus determine the assay accuracy at a very low spiked concentration, thus simulating rare CTC events in early stage disease.
Figure 3.
Overall recovery efficiency of CLCs in medium and blood. The bar plot displays the overall efficiency, defined as the cell count (recovered from the process) observed under a microscope as a proportion of the spiked cell count, in both medium (blue) and blood (gray). The error bars represent the standard deviations. CLC: cancer cell line cell.
Table 2.
Overall recovery efficiency in medium.
| CLC dilution range | Mean number of cells spiked | Mean number of cells recovered | Average recovery (%) | Standard deviation (%) | Coefficient of variation (%) |
|---|---|---|---|---|---|
| High (101–200) | 146.7 | 109.6 | 74.7 | 9.76 | 13.1 |
| Medium (51–100) | 66.4 | 53.4 | 80.4 | 4.73 | 5.9 |
| Low (11–50) | 19.9 | 16.1 | 80.8 | 9.53 | 11.8 |
| Rare (1–10) | 7.4 | 5.7 | 76.5 | 24.1 | 31.5 |
CLC: cancer cell line cell
Table 3.
Overall recovery efficiency in blood.
| CLC dilution range | Mean number of cells spiked | Mean number of cells recovered | Average recovery (%) | Standard deviation (%) | Coefficient of variation (%) |
|---|---|---|---|---|---|
| High (101–200) | 160.4 | 96.8 | 60.3 | 15.37 | 25.5 |
| Medium (51–100) | 88.8 | 60.6 | 68.2 | 7.56 | 11.1 |
| Low (11–50) | 18.9 | 13.2 | 69.6 | 10.94 | 15.7 |
| Rare (1–10) | 6.3 | 3.7 | 58.7 | 26.43 | 45.0 |
Assay linearity
The assay linearity was measured by plotting observed cell counts as a function of spiked in cell counts across various concentrations. Thirty three dilutions of cells with concentrations ranging from 2 to 189 cells were spiked into 2 mL medium or healthy donor blood. The assay was shown to be linear across all spiked concentrations from 2 to 189 cells with a slope of 0.7521 and R 2 = 0.9765 in medium (Figure 4(a)), and with a slope of 0.7166 and R 2 = 0.9822 in blood (Figure 4(b)).
Figure 4.
CMx assay linearity in medium (a) and blood (b). Linearity was characterized by plotting the observed cells (Y-axis) versus the spiked in number of cells (X-axis) for 33 cell dilutions (ranging from 2–189 cells) and calculating the linear regression.
LOB and LOD
The LOB is defined as the highest CTC count expected to be found when replicates of a blank sample containing no CTCs are tested.42 In the current study, we tested replicates of eight non-spiked medium samples and found no CTCs. Therefore, the LOB is estimated to be zero CTCs. There are no acceptance criteria for the LOB.
The LOD is defined as the CTC count for which the probability of falsely claiming the absence of a CTC is 5%, given a 5% (or lower) probability of falsely claiming the presence of a CTC.42 Figure 5 displays the (natural logarithm of) observed CTC counts (plus 1) versus the (natural logarithm of) known CTC counts (plus 1) shown as the blue line along with two-sided 90% confidence intervals for 52 donor blood samples spiked with 2 to 281 HT29 cells. Note that the known CTC count is the number of CLCs counted and spiked into each blood sample. The intersection of the lower one-sided 95% confidence interval for the least squares fitted mean with the LOB (i.e. 0) was 0.71 CTCs. Therefore, the estimated theoretical LOD of the CMx assay is 0.71 CTCs. There are no acceptance criteria for the LOD.
Figure 5.
CMx assay limit of detection. The plot displays the natural logarithm of observed CTC counts (plus 1) versus the natural logarithm of known CTC counts (plus 1) shown as the blue line along with two-sided 90% confidence intervals for 52 donor blood samples spiked with 2 to 281 HT29 cells. The intersection of the lower one-sided 95% confidence interval for the least squares fitted mean with the LOB (i.e. 0) was 0.71 CTCs. The red line represents 100% recovery efficiency. CTC: circulating tumor cell; LOB: limit of blank.
Assay specificity
Assay specificity was demonstrated via analysis of donor samples from 32 young healthy adults (<35 years of age); it has been observed previously that young adults (aged 20–40 years) have a very low (1–2%) prevalence of adenomas.43 The CMx CTC assay was performed on 4 mL blood from each donor and run in duplicate (two chips with 2 mL for each donor). CTCs were counted for each chip, and average counts are reported in Table 4. Zero CTCs were found in 18 of 32 samples, 9 of 32 samples had 1 CTC, and 5 of 32 samples had 1.5 CTCs. Using a cutoff of 2 CTCs/2 mL (as described in “Clinical feasibility” section below), all of the samples were negative, resulting in 100% analytical specificity (Table 4).
Table 4.
Summary of CTC counts in young healthy (<35 years old) donor blood samples to demonstrate specificity.a
| Patient number | Sample number | Patient age | Average CTC counts |
|---|---|---|---|
| Patient 1 | CRC232 | 26 | 0 |
| Patient 2 | CRC243 | 33 | 0.5 |
| Patient 3 | CRC244 | 33 | 0 |
| Patient 4 | CRC245 | 33 | 0 |
| Patient 5 | CRC246 | 24 | 0 |
| Patient 6 | CRC247 | 29 | 0 |
| Patient 7 | CRC231 | 24 | 1.5 |
| Patient 8 | CRC235 | 20 | 0 |
| Patient 9 | CRC236 | 29 | 1 |
| Patient 10 | CRC248 | 26 | 0 |
| Patient 11 | CRC255 | 34 | 2 |
| Patient 12 | CRC256 | 33 | 2 |
| Patient 13 | CRC233 | 30 | 0 |
| Patient 14 | CRC234 | 19 | 0.5 |
| Patient 15 | CRC257 | 22 | 0 |
| Patient 16 | CRC258 | 22 | 1.5 |
| Patient 17 | CRC259 | 34 | 0.5 |
| Patient 18 | CRC267 | 20 | 0 |
| Patient 19 | CRC268 | 17 | 1 |
| Patient 20 | CRC283 | 19 | 0 |
| Patient 21 | CRC284 | 20 | 1 |
| Patient 22 | CRC269 | 28 | 0 |
| Patient 23 | CRC270 | 29 | 0.5 |
| Patient 24 | CRC271 | 31 | 0 |
| Patient 25 | CRC272 | 24 | 0 |
| Patient 26 | CRC280 | 29 | 0 |
| Patient 27 | CRC291 | 31 | 0 |
| Patient 28 | CRC292 | 28 | 1.5 |
| Patient 29 | CRC293 | 29 | 1 |
| Patient 30 | CRC294 | 31 | 0 |
| Patient 31 | CRC295 | 30 | 0.5 |
| Patient 32 | CRC296 | 24 | 0 |
CTC: circulating tumor cell; CRC: colorectal cancer.
a Eighteen of 32 samples had zero CTCs detected, 9 of 32 had one CTC detected, and only 5 had more than one (but fewer than 2) CTCs detected.
Precision (reproducibility)
The precision of the CMx platform was assessed by evaluating variation in overall recovery efficiency for triplicate samples of HT29 cells spiked in blood. To better characterize assay precision, recovery efficiency was further evaluated across multiple days and among multiple operators.
Intra-assay, inter-assay, and inter-perator reproducibility
Triplicate (3) contrived samples of spiked HT29 cells in blood for three concentrations (high, medium, and low) with known CTC counts were processed, and recovered cells were recounted to determine the overall recovery efficiency (Table 5). Intra-assay variability was measured for one operator who ran triplicate samples in three concentrations for a total of nine samples (n = 9), whereas inter-assay variability was measured using triplicate samples across three concentrations for three different days for a total of 27 samples (n = 27). Inter-operator repeatability was measured for three operators; each operator ran triplicate samples across three concentrations for a total of 27 samples (n = 27). Results for the precision analyses are listed in Table 5. For intra-assay precision, coefficient of variation (CV) for overall efficiency is reported for the triplicate samples in three concentrations. For inter-assay precision, CV for the overall efficiency is reported for 3 days, with triplicate samples run in three concentrations on each day. For inter-operator precision, CV for overall efficiency is reported for three operators, with each operator processing triplicate samples in three concentrations.
Table 5.
Precision analyses of CMx assay showed the percentage CV of overall recovery efficiencies for triplicate blood samples spiked with HT29 cells.
| Precision parameter | CV, overall efficiency | ||
|---|---|---|---|
| High concentration (96–181 cells) (%) | Medium concentration (12–21 cells) (%) | Low concentration (2–11 cells) (%) | |
| Intra-assay (n = 9) | 8.8 | 21.9 | 37.0 |
| Inter-assay (3 days, n = 27) | 9.9 | 15.8 | 36.6 |
| Inter-operator (3 operators, n = 27) | 13.7 | 11.0 | 35.3 |
CV: coefficient of variation
It is worth noting that precision studies at very low spike concentrations are challenging, with high inherent variability likely at these cell concentrations due to difficulty in controlling spiked cell counts. However, we were able to visualize and count spiked cells at concentrations as low as 2–11 cells per 2 mL of blood and thus demonstrate the reproducibility of rare cell recovery.
Clinical feasibility
To establish clinical feasibility for the CMx test, we enrolled 47 study subjects, consisting of 15 subjects with known colonoscopy results (nine CRC patients, one adenoma, five negative) and 32 self-declared young healthy subjects under 35 years of age.
The 15 colonoscopy verified samples were collected in Taiwan and processed both in Taiwan and the United States. The samples from young healthy subjects were collected in the United States and processed only in the United States. CTC counting for all samples was conducted with CellMax Life’s proprietary AI-based software and CellReviewer. The clinical feasibility study had two goals: (1) to compare CTC counts for the same samples processed at two different sites, Taiwan versus the United States and (2) to compare CTC counts in colonoscopy-negative subjects and young self-declared healthy subjects. The cohort, mean subject age, and mean CTC counts for samples processed at two sites are listed in Table 6.
Table 6.
CTC counts in healthy and diseased subpopulations.
| Subject category (total = 47 patients) | Mean CTC counts | ||||
|---|---|---|---|---|---|
| Type | Number of subjects | Mean age | Processed in the United States | Processed in Taiwan | Average |
| Cancer | 9 | 51 | 6.6 | 15.7 | 11.1 |
| Adenoma | 1 | 66 | 3.0 | 9.0 | 6.0 |
| Colonoscopy negative | 5 | 59 | 1.2 | 3.0 | 2.1 |
| Young healthy | 32 | 26 | 0.5 | N/A | N/A |
CTC: circulating tumor cell.
The colonoscopy-verified subjects’ samples were processed in CellMax’s CAP accredited laboratories in Taipei, Taiwan, and Sunnyvale, California, USA. The young healthy adults’ samples were processed only in the United States. CTC counts for the same samples processed in United States were generally lower than those processed in Taiwan, likely due to transportation to the United States. Although preservative was added to each sample collected in the hospital in Taiwan upon blood draw, transition time and transportation impact may have contributed to the decrease in CTC counts.
The average across sites of the mean CTC counts for Taiwan and the United States were 11.1 for cancer, 6.0 for polyp (adenoma), and 2.1 for colonoscopy-negative subjects. For the 32 young self-declared healthy donors processed in the United States only, the mean CTC count was 0.5.
More clinical data need to be collected for this comparison, but the CMx test with its AI-based image pattern recognition shows promise for CTC identification. Since a CTC count of greater than three CTCs/3 mL has been correlated with the presence of a primary tumor,39 we chose a threshold of 2 CTCs/2 mL (three or more CTCs as positive). Using this cutoff, for the samples processed in the United States, the CMx test identified 8 of 10 diseased (cancer and adenoma) samples as positive (80% clinical sensitivity) and 4 of 5 controls as negative (80% clinical specificity).
Discussion
This study addresses the analytical validation of the CMx CTC assay platform by evaluating its overall recovery accuracy, assay linearity, LOB and LOD, specificity, and precision. Performance characteristics were evaluated with precise counting of spiked CLCs at concentrations as low as two cells in 2 mL of blood or medium, with the aim of quantifying the performance for detecting true rare events. Spiking error, including dilution, pipetting, and aliquoting can contribute significantly to the variation at low cell concentrations. The purpose of the assay accuracy analysis was to determine the true overall recovery of the CMx process, independent of this error. Hence, we chose to prestain CLCs and perform precise cell counting under a fluorescent microscope prior to spiking, unlike previous validation studies in which cells were diluted without further counting prior to spiking and recovered cell counts sometimes exceeded 100% of spiked in cells.44,45 At 64.2% recovery when spiked in blood, the assay achieved 11% standard deviation across all spiked concentrations, and without the interference of blood components mean recovery was 78.1% with a relatively low standard deviation (6%) across all dilutions (Tables 2 and 3; Figure 3). Assay specificity was determined to be 100%, derived from 32 young healthy donor blood samples (Table 4). Moreover, using 8 unspiked medium samples (blank), we demonstrated LOB of zero CTCs.
The CMx platform also demonstrated excellent assay linearity (R 2 = 0.98) (Figure 4) across a wide range of cell dilutions from 2 cells to 189 cells per 2 mL of blood. At low, medium, and high concentrations (11–50, 51–100, and 101–200) of spiked cells, the assay is reproducible with low variation in overall recovery efficiency (11.1–25.5% CV). At an extremely rare concentration (1–10 cells/ 2 mL blood), the CMx assay still demonstrated a variability of only 45% CV. Assuming a Poisson distribution for the detected CTCs/sample, the theoretical CV for a set of measurements carried out on the same samples is 44.7% at a comparable concentration of 5 CTCs/7.5 mL.46 Without the interference of donor blood, variability was further reduced to 31.5% when HT29 cells were spiked in medium at this rare concentration. We were able to quantify the system accuracy at such low concentrations with a combination of prestaining and precise counting. Dilutions and/or counting errors likely led to previous attempts resulting in 120–200% recovery of spiked cells when concentrations were below 10 cells per sample.45 From the analysis of 52 donor blood samples spiked with 2 to 281 HT29 cells, we were able to establish a theoretical LOD of 0.71 cells per 2 mL of blood (Figure 5).
With the ability to precisely control for spiking and counting, we further proved reproducibility by studying intra-assay, inter-assay, and inter-operator variability. At high spike concentrations (96–181 cells/2 mL), wherein mixing and aliquoting are less likely to contribute to variation, the CVs for triplicate samples remained at a low level (<15%). At medium concentrations (12–21 cells/2 mL), the CV for triplicate samples was in the range of 11.1–21.9% and at very low concentrations (2–11 cells/2 mL), the CVs were between 35% and 37%. The higher variability at the lowest concentration is expected as error for quantification in each step is greater at these concentrations. The ability to maintain a similar, narrow range of precision across multiple days and among multiple operators is important for a reproducible test, and we were able to demonstrate it with spiked cells across relevant concentrations as low as 2–11 cells/sample (Table 5).
A recent study of another CTC detection platform44 demonstrated 88% accuracy in CTC recovery, but at dilution rates of 6 to 300 CLCs/slide, rather than this study’s more realistic measured range of 2 to 189 CLCs/2 mL blood. Linearity and specificity were comparable, while the precision achieved by the CMx assay was superior for the same number of CLCs/slide. With regard to the CellSearch® system, Cummings et al. state that the exact determination of low CTC counts (<10 cells/7.5 mL of blood) is crucial for clinical significance and assigned patients to groups with a relatively favorable (<5 CTCs/7.5 mL of blood) or unfavorable prognosis (≥ 5 CTCs/7.5 mL of blood) based on CTC count.46 However, major limitations have been reported with regard to the heterogeneity of CTC populations.19 Results from controls and spiked blood confirmed a three- to fourfold higher degree of imprecision at low cell counts, and individual analysts introduced a highly specific error into the interpretation of CTC images, correlated to their level of training and experience.46 Although our data demonstrated the ability to precisely count and characterize CMx assay performance at clinically relevant CTC count of <5 CTCs, we only provide limited clinical data as part of a clinical feasibility study. We hope to address some of these challenges by performing clinical validation in future studies.
The core strength of our assay is that the CMx platform enables the harvesting of live cells for analysis via the gentle air foam release of the underlying lipid layer from the chip, without breaking antibody–antigen bonds. Unlike other CTC platforms in which cells cannot be separated from substrates such as ferromagnetic beads without disrupting or negatively influencing the cells for further analysis,47 the CMx platform was designed to allow gentle release of cells using airfoam. This allows for downstream analyses of CTCs, including (but not limited to) enumeration, gene expression, methylation, mutations, and so on, with these cells conveniently contained in an Eppendorf tube. We have developed the assay keeping in mind that several epithelial-derived cancer types (e.g. renal cell carcinoma) do not express EpCAM; however, some EpCAM expressing cancers (e.g. breast cancer) can lose EpCAM expression when they are treated and will display cancer stem cell phenotype. The CMx assay can be easily adapted to other capture markers, including an antibody cocktail, as the microfluidic chip coating and manufacture process is based on the well-established NeutrAvidin-Biotin chemistry. However, the assay does have some limitations. The reproducibility of the current manual process is highly dependent on the skills of the trained technical staff. We anticipate that the automation of the cell capture and release will greatly reduce variability among operators. Therefore, a prototype CTC instrument is being employed for this purpose. Another limitation is the result of immunostaining on the Millipore filter membrane; this membrane retains captured cells while allowing liquid to pass through. The membrane also reduces wash efficiency due to the small amount of buffer that can pass through. A revision to this process is currently being implemented by staining cells while they are retained in-chip and then applying airfoam to release stained cells.
Finally, the clinical feasibility study results demonstrate sensitivity and specificity of the CMx assay for CRC and adenoma detection, despite being derived from a limited data set. Based on the samples processed in the United States, the mean CTC counts were 6.6 for cancer, 3.0 for adenomas, and 1.2 for colonoscopy-negative subjects (Table 6). Since colonoscopy can fail to detect polyps at a rate of 6–27%,48 the odds are that some of the CTCs detected in “colonoscopy-negative” subjects may not be truly “false positive.” In a necropsy analysis, young adults (20–40 years old) were found to have only a 1–2% adenoma prevalence rate.43 We thereby enrolled young adults aged 18–35 years to test the CTC detection specificity by the CMx process. On average, only 0.5 CTCs were found in 32 young self-declared healthy young adults (Table 4), thus providing stronger evidence for the specificity of the CMx assay. There are possibilities that the CTCs detected in young healthy donors and colonoscopy-negative patients could be tumor cells (of colorectal or other origin) or non-neoplastic cells (EpCAM and CK20 positive epithelial cells in circulation from benign diseases) or simply false positive for other reasons. Hence, further studies of a larger cohort of asymptomatic subjects are necessary and already underway to validate the clinical performance of the CMx assay.
Conclusions
This validation study indicates the potential of the CMx assay for diagnosing individuals at risk for CRC. Identifying, isolating, and enumerating CTCs in peripheral blood could be an important new tool for cancer detection and management, while liquid biopsy is an effective test modality that drives compliance for routine testing. Further validation of the CMx CTC assay with a multi-analyte approach is underway, and studies focused on its clinical utility will identify the range of indications for which it may be suitable.
Acknowledgements
The authors would like to acknowledge Dr Wen-Sy Tsai of Chang Gung Memorial Hospital who provided clinical samples for this study. We would also like to thank Sumedha Sinha and Rebecca Suttmann for writing assistance.
Footnotes
Declaration of Conflicting Interests: The author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: All authors are employees of CellMax Life, which provided funding for this study.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: CellMax Life provided funding for this study.
ORCID iD: Rui Mei  https://orcid.org/0000-0001-7903-2846
https://orcid.org/0000-0001-7903-2846
Research ethics and patient consent: Analytical control cell lines were acquired from a commercial vendor. Institutional review board approval and informed consent were obtained from Chang Gung Memorial Hospital for 15 colonoscopy patients for clinical feasibility study. Informed consent was obtained from all 47 study patients including 32 healthy donors, whose samples were processed by CellMax Life’s CAP-accredited lab in Taiwan and CLIA certified and CAP accredited US laboratory. The study was conducted in accordance with the Declaration of Helsinki. All patient information presented as been de-identified and anonymized.
References
- 1. Noone AM, Cronin KA, Altekruse SF, et al. Cancer incidence and survival trends by subtype using data from the surveillance epidemiology and end results program, 1992-2013. Cancer Epidemiol Biomarkers Prev 2017; 26(4): 632–641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. McGuire S. World Cancer Report 2014. Geneva, Switzerland: World Health Organization, International Agency for Research on Cancer, WHO Press, 2015; Adv Nutr 2016; 7(2): 418–419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Corley DA, Jensen D, Marks AR, et al. Adenoma detection rate and risk of colorectal cancer and death. N Engl J Med 2014; 370(14): 1298–1306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Bonnington SN, Rutter MD. Surveillance of colonic polyps: Are we getting it right? World J Gastroenterol 2016; 22(6): 1925–1934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Joosse SA, Gorges TM, Pantel K. Biology, detection, and clinical implications of circulating tumor cells. EMBO Mol Med 2015; 7(1): 1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Millner LM, Linder MW, Valdes R., Jr Circulating tumor cells: a review of present methods and the need to identify heterogeneous phenotypes. Ann Clin Lab Sci 2013; 43(3): 295–304. [PMC free article] [PubMed] [Google Scholar]
- 7. Ashworth TR. A case of cancer in which cells similar to those in the tumours were seen in the blood after death. Med J Aust 1869; 14: 146–147. [Google Scholar]
- 8. Jeong KY, Kim EK, Park MH, et al. Perspective on cancer therapeutics utilizing analysis of circulating tumor cells. Diagnostics (Basel) 2018; 8(2): 23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Nesteruk D, Rutkowski A, Fabisiewicz S, et al. Evaluation of prognostic significance of circulating tumor cells detection in rectal cancer patients treated with preoperative radiotherapy: prospectively collected material data. Biomed Res Int 2014; 2014: 71 2827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Pantel K, Alix-Panabieres C. Real-time liquid biopsy in cancer patients: fact or fiction? Cancer Res 2013; 73(21): 6384–6388. [DOI] [PubMed] [Google Scholar]
- 11. Miller MC, Doyle GV, Terstappen LW. Significance of circulating tumor cells detected by the cellsearch system in patients with metastatic breast colorectal and prostate cancer. J Oncol 2010; 2010: 617421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Cohen SJ, Punt CJ, Iannotti N, et al. Relationship of circulating tumor cells to tumor response, progression-free survival, and overall survival in patients with metastatic colorectal cancer. J Clin Oncol 2008; 26(19): 3213–3221. [DOI] [PubMed] [Google Scholar]
- 13. Siegel RL, Miller KD, Jemal A. Cancer statistics, 2016. CA Cancer J Clin 2016; 66(1): 7–30. [DOI] [PubMed] [Google Scholar]
- 14. Budd GT, Cristofanilli M, Ellis MJ, et al. Circulating tumor cells versus imaging--predicting overall survival in metastatic breast cancer. Clin Cancer Res 2006; 12(21): 6403–6409. [DOI] [PubMed] [Google Scholar]
- 15. Hayes DF, Cristofanilli M, Budd GT, et al. Circulating tumor cells at each follow-up time point during therapy of metastatic breast cancer patients predict progression-free and overall survival. Clin Cancer Res 2006; 12(14 Pt 1): 4218–4224. [DOI] [PubMed] [Google Scholar]
- 16. Gorin MA, Verdone JE, van der Toom E, et al. Circulating tumour cells as biomarkers of prostate, bladder, and kidney cancer. Nat Rev Urol 2017; 14(2): 90–97. [DOI] [PubMed] [Google Scholar]
- 17. de Bono JS, Scher HI, Montgomery RB, et al. Circulating tumor cells predict survival benefit from treatment in metastatic castration-resistant prostate cancer. Clin Cancer Res 2008; 14(19): 6302–6309. [DOI] [PubMed] [Google Scholar]
- 18. Wu JC, Tseng PY, Tsai WS, et al. Antibody conjugated supported lipid bilayer for capturing and purification of viable tumor cells in blood for subsequent cell culture. Biomaterials 2013; 34(21): 5191–5199. [DOI] [PubMed] [Google Scholar]
- 19. Andree KC, van Dalum G, Terstappen LW. Challenges in circulating tumor cell detection by the CellSearch system. Mol Oncol 2016; 10(3): 395–407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Ren C, Chongxu H, Daxin W, et al. Detection of circulating tumor cells: Clinical relevance of a novel metastatic tumor marker. Exp Ther Med 2011; 2(3): 385–391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Parkinson DR, Dracopoli N, Petty BG, et al. Considerations in the development of circulating tumor cell technology for clinical use. J Transl Med 2012; 10: 138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Glaves D. Correlation between circulating cancer cells and incidence of metastases. Br J Cancer 1983; 48(5): 665–673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Rack B, Schindlbeck C, Jückstock J, et al. Circulating tumor cells predict survival in early average-to-high risk breast cancer patients. J Natl Cancer Inst 2014; 106(5): dju066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Nagrath S, Sequist LV, Maheswaran S, et al. Isolation of rare circulating tumour cells in cancer patients by microchip technology. Nature 2007; 450(7173): 1235–1239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Li P, Stratton ZS, Dao M, et al. Probing circulating tumor cells in microfluidics. Lab Chip 2013; 13(4): 602–609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Huang MY, Tsai HL, Huang JJ, et al. Clinical implications and future perspectives of circulating tumor cells and biomarkers in clinical outcomes of colorectal cancer. Transl Oncol 2016; 9(4): 340–347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Gertler R, Rosenberg R, Fuehrer K, et al. Detection of circulating tumor cells in blood using an optimized density gradient centrifugation. Recent Results Cancer Res 2003; 162: 149–155. [DOI] [PubMed] [Google Scholar]
- 28. Stott SL, Hsu CH, Tsukrov DI, et al. Isolation of circulating tumor cells using a microvortex-generating herringbone-chip. Proc Natl Acad Sci U S A 2010; 107(43): 18392–18397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Gupta V, Jafferji I, Garza M, et al. ApoStream(), a new dielectrophoretic device for antibody independent isolation and recovery of viable cancer cells from blood. Biomicrofluidics 2012; 6(2): 24133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Hur SC, Henderson-MacLennan NK, McCabe ER, et al. Deformability-based cell classification and enrichment using inertial microfluidics. Lab Chip 2011; 11(5): 912–920. [DOI] [PubMed] [Google Scholar]
- 31. Hur SC, Mach AJ, Di Carlo D. High-throughput size-based rare cell enrichment using microscale vortices. Biomicrofluidics 2011; 5(2): 22206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Zheng S, Lin HK, Lu B, et al. 3D microfilter device for viable circulating tumor cell (CTC) enrichment from blood. Biomed Microdevices 2011; 13(1): 203–213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Tan SJ, Yobas L, Lee GY, et al. Microdevice for the isolation and enumeration of cancer cells from blood. Biomed Microdevices 2009; 11(4): 883–892. [DOI] [PubMed] [Google Scholar]
- 34. Ilie M, Hofman V, Long-Mira E, et al. “Sentinel” circulating tumor cells allow early diagnosis of lung cancer in patients with chronic obstructive pulmonary disease. PLoS One 2014; 9(10): e111597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Murray NP, Miranda R, Ruiz A, et al. Diagnostic yield of primary circulating tumor cells in women suspected of breast cancer: the BEST (Breast Early Screening Test) study. Asian Pac J Cancer Prev 2015; 16(5): 1929–1934. [DOI] [PubMed] [Google Scholar]
- 36. Flores LM, Kindelberger DW, Ligon AH, et al. Improving the yield of circulating tumour cells facilitates molecular characterisation and recognition of discordant HER2 amplification in breast cancer. Br J Cancer 2010; 102(10): 1495–1502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Tsai WS, Chen JS, Shao HJ, et al. Circulating tumor cell count correlates with colorectal neoplasm progression and is a prognostic marker for distant metastasis in non-metastatic patients. Sci Rep 2016; 6: 24517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Bork U, Rahbari NN, Schölch S, et al. Circulating tumour cells and outcome in non-metastatic colorectal cancer: a prospective study. Br J Cancer 2015; 112(8): 1306–1313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Yang C, Zhuang W, Hu Y, et al. Clinical significance of peripheral circulating tumor cell counts in colorectal polyps and non-metastatic colorectal cancer. World J Surg Oncol 2018; 16(1): 13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Lai JM, Shao HJ, Wu JC, et al. Efficient elusion of viable adhesive cells from a microfluidic system by air foam. Biomicrofluidics 2014; 8(5): 052001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Chen JY, Tsai WS, Shao HJ, et al. Sensitive and specific biomimetic lipid coated microfluidics to isolate viable circulating tumor cells and microemboli for cancer detection. PLoS ONE 2016; 11(3): e0149633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Armbruster DA, Pry T. Limit of blank, limit of detection and limit of quantitation. Clin Biochem Rev 2008; 29(Suppl 1): S49–52. [PMC free article] [PubMed] [Google Scholar]
- 43. Pendergrass CJ, Edelstein DL, Hylind LM, et al. Occurrence of colorectal adenomas in younger adults: an epidemiologic necropsy study. Clin Gastroenterol Hepatol 2008; 6(9): 1011–1015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Werner SL, Graf RP, Landers M, et al. Analytical validation and capabilities of the epic CTC platform: enrichment-free circulating tumour cell detection and characterization. J Circ Biomark 2015; 4:3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Veridex_LLC_510 K, CellSearch 510 K Summary k071729. 2003.
- 46. Cummings J, Morris K, Zhou C, et al. Method validation of circulating tumour cell enumeration at low cell counts. BMC Cancer 2013; 13: 415–415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Lv SW, Wang J, Xie M, et al. Photoresponsive immunomagnetic nanocarrier for capture and release of rare circulating tumor cells. Chem Sci 2015; 6(11): 6432–6438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Ahn SB, Han DS, Bae JH, et al. The miss rate for colorectal adenoma determined by quality-adjusted, back-to-back colonoscopies. Gut Liver 2012; 6(1): 64–70. [DOI] [PMC free article] [PubMed] [Google Scholar]