Short abstract
Background
When deciding treatment options for patients with colon cancer, accurate staging is required. In Sweden, the main preoperative evaluation modality to determine tumor and nodal stage is computed tomography (CT).
Purpose
The aim of this study was to investigate how well the preoperative (CT-determined) clinical tumor and nodal stage (cTN) correlated with the postoperative histopathological stage (pTN). Another aim was to validate the tumor and nodal stage data in the Swedish Colorectal Cancer Registry (SCRCR).
Material and Methods
The SCRCR was used to identify patients with colon cancer, treated at a Swedish high-volume center during 2013–2016 (n = 974). Data were gathered from medical records regarding cTN and pTN stage, and predefined patient and tumor variables. The agreement between cTN and pTN was analyzed using kappa statistics.
Results
After excluding patients with either pre- or postoperative TN stage missing, 383 patients remained for further analyses. The analyses showed an agreement between cT and pT of κ: 0.27 and between cN and pN of κ: 0.21 (fair agreement). When comparing tumors with low (T1–3; N0) versus high risk (T4; N1–2), the kappa value was 0.19 (slight agreement). When comparing the SCRCR to medical records, 78% of completely staged tumors had been correctly reported.
Conclusion
The agreement between cTN and pTN was low in this study population, indicating a need for enhanced precision of the preoperative staging process. A high frequency of erroneous preoperative staging data in the SCRCR shows the need for further efforts of ensuring correct data transfers into the registry.
Keywords: Colon cancer, stage, computed tomography
Introduction
The most important prognostic marker for colon cancer is tumor stage (1). The most widely used and accepted staging classification is the tumor/node/metastasis (TNM) Classification of Malignant Tumors (2). In Sweden, preoperative staging is recommended in national guidelines. The modality most often used is computed tomography (CT) (3). Usage of CT in the staging of distant metastases (M) is well established (3) and some studies have indicated that CT is a valid method for determining tumor and nodal stage (4,5). These results, however, have been contradicted by a recent study from Sweden by Sjövall et al. (6), which showed low values of agreement when comparing preoperative (CT determined) clinical tumor and nodal (cTN) stage and postoperative tumor and nodal stage based on histopathology (pTN). The study by Sjövall et al. was based solely on information retrieved from the Swedish Colorectal Cancer Registry (SCRCR), a national quality registry, covering >98% of all adenocarcinomas of the colon in Sweden, containing information such as tumor localization, patient characteristics, and pre- and postoperative stage (7).
The aim of the current study was to investigate the agreement between the cTN stage, determined by CT, and the pTN stage, based on histopathology analysis, at a single high-volume colorectal center with data retrieved from medical journals. A secondary aim was to validate the data available in the SCRCR.
Material and Methods
Study population
Patients diagnosed with adenocarcinoma of the colon treated at a Swedish high-volume center during the time period January 2013 to December 2016 were identified through the SCRCR and considered eligible for the study. Exclusion criteria were synchronous tumors, non-resectional surgery, neoadjuvant treatment, and/or missing explicit stage according to the TNM classification.
Data retrieval
Information was retrieved from the patients’ medical journals and SCRCR, including sex, age at surgery, body mass index (BMI) registered within one month of surgery, and tumor location categorized into right colon (appendix, caecum, ascending colon, and hepatic flexure), transverse colon, or left colon (splenic flexure, descending colon, and sigmoid colon). In addition, information whether the surgical procedure was elective (scheduled procedure) or acute, cTN stage reported in the initial CT result or at a multidisciplinary team conference, and finally the pTN stage according to the histopathology report was also retrieved. The CT scans were generally performed by use of CT scanners SOMATOM® Definition Flash (Siemens, Munich, Germany) or Aquilion™ ONE (Canon, Zoetermer, the Netherlands), with a slice thickness of 1, 2, or 5 mm. If the preoperative stage assessed by the initial CT result and the multidisciplinary team conference differed, the results of the latter were used. In cases when a range of T stage or N stage was stated in the report, the most severe stage, i.e. the highest number, was used. No second readings of CT scans with reassessment of cTN were performed for this study.
Statistical analysis
Agreement between pre- and postoperative T stage and N stage was calculated by use of kappa statistics. To determine the degree of agreement, the range between 0 and 1 was divided into five equal groups in accordance with the often-used categorization of Landis and Koch (8). For matrices larger than 2 × 2 quadratic, weighting was applied unless otherwise specified. The analyses were performed on consecutive groups merged into larger groups. The merging performed was T1–2, T1–3, and N1–2. T and N staging were also combined into low- and high-risk tumors. Low-risk was defined as T1–3 and N0. High-risk was defined as T4 or N1–2. To determine whether the agreement was related to other patient and tumor characteristics, further analyses of kappa values were performed after stratification of the chosen potential confounding variables.
Accuracy of the preoperative staging was calculated as the ratio of correct observations, using the pTN as reference, divided by the total number of observations and multiplied by 100. Sensitivity and specificity were calculated with the higher stages, i.e. T4, N2, and high-risk, respectively, considered as the positive outcomes.
95% confidence intervals were calculated using bootstrapping for the kappa values and binomial proportion confidence intervals for accuracy, sensitivity, and specificity.
The SCRCR data of cTN was validated by dividing the cases with TNM stages corresponding to the medical records with all cases, including those with non-correctly reported TNM values. This produced a percentage of correctly staged tumors in the registry. A sample of 20 patients was randomly selected of those with a non-correctly reported stage to more closely examine the medical journals to determine a potential cause for them to have been not correctly reported. All statistical analyses were performed using SPSS version 24 and Matlab version R2016a.
The Ethics Committee of Lund University approved the study.
Results
Study cohort and clinical characteristics
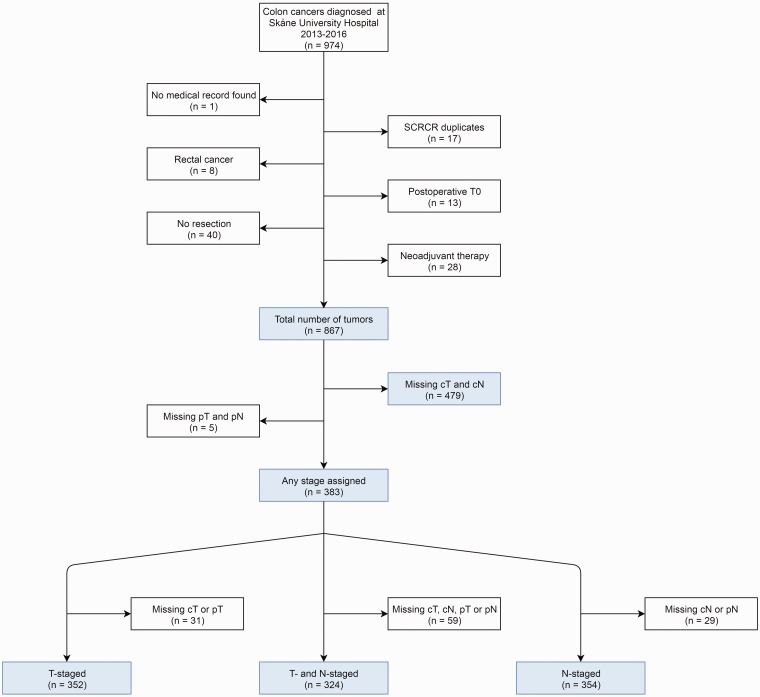
In all, 974 patients were identified through the SCRCR as eligible for the present study. After the exclusion of 107 patients not fulfilling the criteria of having a unique adenocarcinoma of the colon that was resected without prior neoadjuvant therapy and 484 patients missing both cT and cN stage or both pT and pN stage, 383 patients comprised the final study population (Fig. 1). In Table 1, the clinical characteristics of the study cohort are presented along with characteristics of the patients who were excluded. The percentage of adequately staged tumors increased each year, reaching 37% in 2016.
Fig. 1.
Study flow chart.
Table 1.
Patient characteristics.
| T-staged | cT- and cN-staged | N-staged | cT and cN missing | |
|---|---|---|---|---|
| n = 352 (%) | n = 324 (%) | n = 354 (%) | n = 479 (%) | |
| Sex | ||||
| Male | 175 (49.7) | 162 (50.0) | 175 (49.4) | 229 (47.8) |
| Female | 177 (50.3) | 162 (50.0) | 179 (50.6) | 250 (52.2) |
| Median age, years (range) | 74 (33–95) | 74 (33–95) | 75 (33–95) | 73 (33–102) |
| Tumor location | ||||
| Right colon | 185 (52.6) | 169 (52.2) | 183 (51.7) | 205 (42.8) |
| Colon transversum | 24 (6.8) | 23 (7.1) | 31 (8.8) | 47 (9.8) |
| Left colon | 143 (40.6) | 132 (40.7) | 140 (39.5) | 227 (47.4) |
| Type of surgery | ||||
| Elective surgery | 345 (98.0) | 318 (98.1) | 341 (96.3) | 313 (65.3) |
| Acute surgery | 7 (2.0) | 6 (1.9) | 13 (3.7) | 166 (34.7) |
| Median BMI, kg/m2 (range) | 25 (17–43) | 25 (17–43) | 25 (17–43) | 25 (14–43) |
| Source of staging | ||||
| CT | 42 (11.9) | 40 (12.3) | 58 (16.4) | 279 (58.2) |
| MDT conference | 310 (88.1) | 284 (87.7) | 296 (83.6) | 124 (25.9) |
| Missing | 0 (0.0) | 0 (0.0) | 0 (0.0) | 76 (2.1) |
| Validated against SCRCR | ||||
| Pre- and postop correct | 264 (75.0) | 252 (77.8) | 268 (75.7) | 212 (44.3) |
| Pre- or postop incorrect | 86 (24.4) | 71 (21.9) | 85 (24.0) | 257 (53.7) |
| Pre- or postop missing | 2 (0.6) | 1 (0.3) | 1 (0.3) | 10 (2.1) |
| Year | ||||
| 2013 | 26 (7.4) | 19 (5.9) | 19 (5.4) | 163 (34.0) |
| 2014 | 75 (21.3) | 65 (20.1) | 66 (18.6) | 146 (30.5) |
| 2015 | 114 (32.4) | 109 (33.6) | 134 (37.9) | 78 (16.3) |
| 2016 | 137 (38.9) | 131 (40.4) | 135 (38.1) | 92 (19.2) |
BMI, body mass index; CT, computed tomography; MDT, multidisciplinary team; SCRCR, Swedish Colorectal Cancer Registry.
Agreement and accuracy
The agreement between the pre- and postoperative T stage for the 352 patients for whom the tumors were adequately T-staged is shown in Table 2. For the merged T1–2 group, the weighted accuracy was 81% and a weighted kappa value of 0.28, indicating fair agreement. When merging T1–3, the analysis still indicated a fair agreement (κ = 0.27), although with a wider confidence interval.
Table 2.
Agreement of preoperative (cT) versus postoperative (pT) tumor stage.
|
pT |
|||
|---|---|---|---|
| cT | T1–2 | T3 | T4 |
| T1–2 | 33 | 81 | 28 |
| T3 | 11 | 104 | 48 |
| T4 | 1 | 16 | 30 |
| Weighted accuracy | 81% (95% CI 76–85) | ||
| Weighted kappa | 0.27 (95% CI 0.21–0.33) | ||
|
cT |
T1–3 |
T4 |
|
| T1–3 | 229 | 76 | |
| T4 | 17 | 30 | |
| Accuracy (%) | 74 (95% CI 69–78) | ||
| Kappa | 0.25 (95% CI 0.15–0.36) | ||
| Sensitivity (%) | 28 (95% CI 20–38) | ||
| Specificity (%) | 93 (95% CI 89–96) | ||
CI, confidence interval.
In Table 3, the agreement between the pre- and postoperative N stage is demonstrated for the 354 patients with complete N stage reported. Involvement of lymph nodes could be correctly judged in 61% with a weighted kappa coefficient of 0.21, suggesting a fair agreement. Merging N1 and N2 did not show a statistically significant difference in agreement between the pre- and postoperative N stage (data not shown).
Table 3.
Agreement of preoperative (cN) versus postoperative (pN) nodal stage.
| pN | ||
|---|---|---|
| cN | N0 | N1–2 |
| N0 | 128 | 71 |
| N1–2 | 67 | 88 |
| Accuracy (%) | 61 (95% CI 56–66) | |
| Kappa | 0.21 (95% CI 0.11–0.31) | |
| Sensitivity (%) | 55 (95% CI 47–63) | |
| Specificity (%) | 66 (95% CI 59–72) | |
CI, confidence interval.
The analysis of high-risk tumors compared against low-risk tumors showed an accuracy of 60% and a kappa coefficient of 0.19, denoting a slight agreement between pre- and postoperative staging.
There was no difference in agreement between cTN and pTN in relation to sex, age, BMI, type of surgery, tumor location, or whether it was entered correctly or not in the SCRCR (data not shown).
Non-validated data
When including non-validated data available from the SCRCR of all patients (n = 867), the agreement, calculated with weighted kappa, between pre- and postoperative stage was fair both for tumor (0.32, Suppl. Table 1) and nodal stage (0.26, Suppl. Table 2).
Validation of the SCRCR
Only 57% of the tumors included in the study (n = 867) were correctly reported to the SCRCR, i.e. the stage reported to the SCRCR was identical to the medical records. However, when excluding tumors not completely staged (n = 324), the number increased to 78%. In the sample of 20 randomly selected tumors with incomplete preoperative staging, it was observed that the findings in the CT scan report were often described in a way that a radiologist/colon cancer specialist may interpret the TNM classification of the tumor, i.e. description of the growth pattern, size, and morphology, etc., but not necessarily in a clear enough way that all personnel who transfer the information into the SCRCR could understand.
Discussion
This study shows that the agreement between CT-assessed cTN stage of colon cancer and pTN stage verified by histopathology was limited at a high-volume colorectal center. Kappa values corresponding to fair agreements between cT/pT and cN/pN were detected in this study population. Subgrouping into low- and high-risk tumors did not improve the agreement. Neither patient nor tumor characteristics were found to have any effect on the results.
M stage was not analyzed, as the main focus of this study was the radiological assessment of the primary tumor and the locoregional nodal involvement. The potential for using CT to produce a valid cTN stage has been discussed in several other studies. Some previous studies have shown acceptable agreement between cTN and pTN (5, 9), while others have, as in the present study, shown poor levels of agreement (10,11). Although some studies have presented moderately superior results compared to the current study, they have all struggled to identify high-risk tumors (4,6,9–11).
When comparing the results of the current study to those of a similar Swedish study by Sjövall et al. (6), the levels of agreement were for cT/pT 0.27 versus 0.44 and for cN/pN 0.21 versus 0.28. That the levels of agreement were inferior in the current study is somewhat surprising, taking into account that it explored a later time period and contained data validated against medical records. The difference between the studies could potentially be a result of discrepancies between patient medical records and the SCRCR rather than being an actual difference in the CT staging, as it was also shown in the current study that a mere 57% of data was correctly reported to the registry.
In Sweden, efforts have been made in recent years to increase the quality of radiological reports with standardized protocols in order to decrease missing data and to ensure the presence of preoperative staging. No formal educational efforts regarding CT staging of colon cancer had yet been undertaken at the hospital before or during the study period. However, in the fall of 2017, a structured protocol for CT staging in colon cancer was introduced at the Radiology Department to improve completeness and accuracy of cTN.
Currently, with the CT technology available today, perfect agreement between cTN and pTN cannot be expected. T-staging is dependent on invasion into different layers of the colon wall, not clearly visible on a CT scan. In the same way, N-staging is based on enlarged and irregular lymph nodes, not necessarily corresponding to cancer spread, whereas normal-sized lymph nodes may contain cancer cells (4). In addition, inter- and intra-reader variability between radiologists can account for different readings of the same images (11). In a study from 2017, Rollvén et al. (12) presented criteria for detection of metastases on preoperative CT. They could as best show moderate sensitivity (85%) and specificity (75%) when the criteria internal heterogeneity and/or irregular outer borders were combined.
Other modalities could be used to assess cTN stage. Magnetic resonance imaging (MRI) may be a more accurate and radiation-free alternative to the traditional CT, which does not have the same resolution, especially with regard to soft tissue separation (13), but is costly and time-consuming. CT colonography (CTC) is also a potential modality to use for assessment of the colon and some studies have shown superior results concerning agreement between preoperative radiological staging and histological stage compared to the current study. Kim et al. (14) showed accuracies of T stage of 86% and N stage of 70% using CTC. A more recent study by Maupoey Ibanez et al. (15) showed an 87% accuracy of differentiating T1/T2 from T3/T4 stage (κ = 0.7) and an N-stage accuracy of 69% (κ = 0.37). The current main use of CTC is as an alternative to conventional colonoscopy in cases of obstructive colorectal cancer (3).
The present study underlines the importance of validating registry data since erroneous entries may be higher than expected. It was shown that 57% of TNM stages reported were identical to those in the medical records. When excluding patients with not completely staged tumors, 78% were correct.
The erroneous data most likely derived from the medical records missing explicit staging according to the TNM classification. Accordingly, unstaged tumors were more often incorrectly entered in the SCRCR. The analysis of a smaller sample of 20 tumors showed that the misinformation seemed to arise in the transfer from medical records to the registry. This indicates a need for proper guidelines regarding documentation of TNM stage in the patient’s medical record in order for these to be correctly transformed to the SCRCR. The validity of the SCRCR data has been previously examined with a better result than in the current study (16,17).
The main strength of this study was that all SCRCR data were validated using the medical records. Furthermore, it was performed at a single center, limiting the number of radiologists and thereby potentially improving the consistency between CT scan assessments.
However, surprisingly, fewer than half of the patients were completely staged, limiting the number of tumors included and producing a risk of selection bias. After excluding unstaged tumors, 383 remained for statistical analysis. There is a risk that patients with correctly staged tumors could have either easier or more difficult tumors to assess by CT, making it difficult to determine if the levels of accuracy in the study sample match those of the population intended to study. However, in the analyses in which tumors with TN stage reported in the SCRCR but not in the medical records were included, the levels of accuracy were similar.
When comparing staged and unstaged tumors, it could be noted that the proportion of staged tumors increased each year. This was possibly caused by an increased awareness among radiologists on how to properly report TNM staging. A comparison of baseline characteristics showed that the patients with available cTN were somewhat older and their tumors were more often located in the right colon. The age difference is to be considered minor. We currently have no data indicating that different tumor locations in the colon affect cTN.
In the group with unstaged tumors, there was a higher frequency of emergency procedures and the staging was more often based on CT scan assessment rather than after a multidisciplinary team conferences. The cause of this could be the necessity to prioritize treatment to solve the acute situation over an optimal staging procedure and team conference.
These differences between staged and unstaged tumors should be taken into account when drawing conclusions from the results. The kappa values of agreement between cTN and pTN seen in this study may not correspond to a study population with a higher frequency of patients undergoing emergency procedures. However, the results are still interesting and some conclusions may be drawn.
In conclusion, only a fair agreement was shown in this study between the CT-assessed cTN stage and the pTN stage based on histopathological analysis. The CT-assessed staging gained no benefit from a merged as opposed to a precise TNM classification and the accuracy of preoperative cTN stage did not increase when dividing tumors into low- and high-risk groups. Educational efforts could potentially increase the level of accuracy. As correctly identifying high-risk versus low-risk tumors is of higher clinical importance than determining precise stages, efforts should be focused on how to detect high-risk tumors. As a surprisingly high level of erroneous data were detected in the SCRCR in this study, continuous efforts of ensuring valid data in the registry are necessary.
Supplemental Material
Supplemental material, ARR888713 Supplemental Material for Tumor and nodal staging of colon cancer: accuracy of preoperative computed tomography at a Swedish high-volume center by Kevin Korsbakke, Cecilia Dahlbäck, Niklas Karlsson, Sophia Zackrisson and Pamela Buchwald in Acta Radiologica Open
Declaration of conflicting interests
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding
The author(s) received no financial support for the research, authorship, and/or publication of this article.
ORCID iD
Cecilia Dahlbäck https://orcid.org/0000-0002-6663-7843
References
- 1.Ponz de Leon M, Sant M, Micheli A, et al. Clinical and pathologic prognostic indicators in colorectal cancer. A population-based study. Cancer 1992; 69:626–635. [DOI] [PubMed] [Google Scholar]
- 2.James D., Brierley MKG, Christian Wittekind. TNM Classification of Malignant Tumours. 8th edn West Sussex, UK: Wiley-Blackwell, 2017. [Google Scholar]
- 3.Colon and rectal cancer. National guidelines 2016. (Swedish) [Tjock- och ändtarmscancer. Nationellt vårdprogram 2016] Regional Cancer Centres in Collaboration, 2016. Available at: https://www.cancercentrum.se/globalassets/cancerdiagnoser/tjock-och-andtarmanal/vardprogram/nvpkolorektalcancer_2016-03-15.pdf (last accessed 16 September 2019).
- 4.Dighe S, Purkayastha S, Swift I, et al. Diagnostic precision of CT in local staging of colon cancers: a meta-analysis. Clin Radiol 2010; 65:708–719. [DOI] [PubMed] [Google Scholar]
- 5.Wiegering A, Kunz M, Hussein M, et al. Diagnostic value of preoperative CT scan to stratify colon cancer for neoadjuvant therapy. Int J Colorectal Dis 2015; 30:1067–1073. [DOI] [PubMed] [Google Scholar]
- 6.Sjövall A, Blomqvist L, Egenvall M, et al. Accuracy of preoperative T and N staging in colon cancer–a national population-based study. Colorectal Dis 2016; 18:73–79. [DOI] [PubMed] [Google Scholar]
- 7.Colon cancer. National quality report 2018 from the Swedish Colorectal Cancer Registry (Swedish) [Koloncancer. Nationell kvalitetsrapport för år 2018 från Svenska Kolorektalcancerregistret] Regional Cancer Centre North 2019. Available at: https://www.cancercentrum.se/globalassets/cancerdiagnoser/tjock-och-andtarm-anal/kvalitetsregister/tjock-och-andtarm-fr.-2018/kolonrapport2018.pdf (last accessed 16 September 2019).
- 8.Landis JR, Koch GG. The measurement of observer agreement for categorical data. Biometrics 1977; 33:159–174. [PubMed] [Google Scholar]
- 9.Dighe S, Blake H, Koh MD, et al. Accuracy of multidetector computed tomography in identifying poor prognostic factors in colonic cancer. Br J Surg 2010; 97:1407–1415. [DOI] [PubMed] [Google Scholar]
- 10.Lao IH, Chao H, Wang YJ, et al. Computed tomography has low sensitivity for the diagnosis of early colon cancer. Colorectal Dis 2013; 15:807–811. [DOI] [PubMed] [Google Scholar]
- 11.de Vries FE, da Costa DW, van der Mooren K, et al. The value of pre-operative computed tomography scanning for the assessment of lymph node status in patients with colon cancer. Eur J Surg Oncol 2014; 40:1777–1781. [DOI] [PubMed] [Google Scholar]
- 12.Rollvén E, Abraham-Nordling M, Holm T, et al. Assessment and diagnostic accuracy of lymph node status to predict stage III colon cancer using computed tomography. Cancer Imaging 2017; 17:3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nerad E, Lambregts DM, Kersten EL, et al. MRI for local staging of colon cancer: can MRI become the optimal staging modality for patients with colon cancer? Dis Colon Rectum 2017; 60:385–392. [DOI] [PubMed] [Google Scholar]
- 14.Kim JH, Kim WH, Kim TI, et al. Incomplete colonoscopy in patients with occlusive colorectal cancer: usefulness of CT colonography according to tumor location. Yonsei Med J 2007; 48:934–941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Maupoey Ibanez J, Pamies Guilabert J, Frasson M, et al. Accuracy of CT colonography in the preoperative staging of colon cancer: a prospective study of 217 patients. Colorectal Dis 2019; 21: 1151–1163. [DOI] [PubMed] [Google Scholar]
- 16.Jörgren F, Johansson R, Damber L, et al. Validity of the Swedish Rectal Cancer Registry for patients treated with major abdominal surgery between 1995 and 1997. Acta Oncol 2013; 52:1707–1714. [DOI] [PubMed] [Google Scholar]
- 17.Boström P, Rutegård J, Haapamaki M, et al. Arterial ligation in anterior resection for rectal cancer: A validation study of the Swedish Colorectal Cancer Registry. Acta Oncol 2014; 53:892–897. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, ARR888713 Supplemental Material for Tumor and nodal staging of colon cancer: accuracy of preoperative computed tomography at a Swedish high-volume center by Kevin Korsbakke, Cecilia Dahlbäck, Niklas Karlsson, Sophia Zackrisson and Pamela Buchwald in Acta Radiologica Open