Abstract
Background
Activation of the intracrine renin angiotensin systems (RAS) is increasingly recognized as contributing to human pathologies, yet non-canonical renin-independent mechanisms for angiotensin II (Ang II) biosynthesis remain controversial. Direct Ang II generation from angiotensin-(1-12) [Ang-(1-12)] by chymase is an essential intracrine source for regulation of cardiac function. Using a transgenic rat model that overexpresses the human angiotensinogen gene [TGR(hAGT)L1623] and displays increased cardiac Ang II levels, this study aimed to provide evidence for intracrine activation of L-type calcium currents (ICa-L) mediated by the Ang-(1–12)/chymase axis.
Methods and Results
On patch clamp, ICa-L density was significantly higher in TGR(hAGT)L1623 (−6.4 ± 0.3 pA/pF) compared to Sprague Dawley (SD) cardiomyocytes (−4.8, ±0.5 pA/pF). Intracellular administration of Ang II and Ang-(1–12) elicited an ICa-L increase in both SD and TGR(hAGT)L1623 cardiomyocytes, albeit blunted in transgenic cells. ICa-L activation by intracellular Ang II and Ang-(1–12) was abolished by the specific Ang II type 1 receptor blocker E-3174. Co-administration of a chymase inhibitor prevented activation of ICa-L by Ang-(1–12). Confocal micrographs revealed abundant chymase (mast cell protease 5) immunoreactive protein in SD and TGR(hAGT)L1623 cardiomyocytes.
Conclusions
Our data demonstrate the existence in cardiomyocytes of a calcium channel modulatory activity responsive to Ang II generated by the Ang-(1–12)/chymase axis that signals via intracellular receptors. Chronically elevated Ang II in TGR(hAGT)L1623 hearts leading to increased intracellular calcium through ICa-L suggests that activation of this Ang-(1–12)/chymase-governed cardiac intracrine RAS may contribute to the phenotypes observed in the humanized model of chronic hypertension and cardiac hypertrophy.
Keywords: angiotensin II type 1 receptor, hypertension, hypertrophy, Ang-(1–12), Ang II, chymase
INTRODUCTION
The physiological role of the circulating renin-angiotensin system (RAS) in governing arterial blood pressure and electrolyte homeostasis is well established. Moreover, defective regulation of the RAS has been extensively demonstrated to contribute to hypertension and hypertension-induced myocardial damage. As such, pharmacological targeting of the RAS is a predominant therapeutic strategy in cardiovascular medicine that continues to inspire significant research efforts aimed at developing new RAS targets and drugs.1 In the course of these investigations, alternative mechanisms for the production, delivery and/or action of effector angiotensin peptides have been discovered with the promise of improving patient outcomes.2 Indeed, non-canonical intracrine angiotensin II (Ang II) forming mechanisms are emerging as critical determinants of the evolution of myocardial remodeling and may provide the basis for novel and clinically relevant treatments.2
Non-canonical pathways for the conversion of angiotensinogen (AGT) into shorter biologically-active peptides exist both in the circulation and in defined tissues.2,3 In the heart, a robust yet underappreciated literature shows that chymase rather than ACE is the enzyme responsible for Ang II production.4 The more recent discovery of Ang II generation directly from the dodecapeptide angiotensin-(1–12) [Ang-(1–12)] in rodent tissues5,6 and the human heart7,8 has put forward the possibility that this non-renin dependent alternate Ang II-forming substrate may constitute a primary mechanism of intracrine contractile and trophic Ang II actions.8,9 Characterization of functional Ang-(1–12) actions in brainstem pathways involved in blood pressure regulation and cardiac lusitropic mechanisms10,11 builds on robust evidence implicating a critical role of cardiac chymase in the generation of cardiac dysfunction.12–14
Transgenic rats expressing the human sequence of AGT (hAGT) [TGR(hAGT)L1623] have emerged as a valuable tool to investigate the existence of a tissue-delimited renin-independent and chymase-mediated biosynthetic mechanism in which Ang-(1–12) serves as the substrate for paracrine/intracrine Ang II formation. An earlier detailed study by Ferrario et al.15 demonstrated that TGR(hAGT)L1623 rats are hypertensive and display cardiac hypertrophy and systolic dysfunction associated with a 4-fold increase in the cardiac, but not circulating, Ang II content.15 As rat renin is unable to cleave the AGT protein encoded by the hAGT transgene due to species-specific differences in the amino-acid sequence (specifically, the 11th through 12th amino acids are changed from Leu10-Leu11-Tyr12 in rats to Leu10-Val11-Ile12 in humans),16 the high levels of human Ang-(1–12) in cardiac tissue of TGR(hAGT)L1623 rats, together with the high cardiac tissue expression of Ang II, provided evidence for a non-renin dependent enzymatic mechanism cleaving hAng-(1–12) into Ang II.4–8 This result gave us the opportunity to, by the combined use of patch clamp electrophysiology and immunofluorescence, test the hypothesis that Ang-(1–12) and Ang-(1–12)-derived Ang II in freshly isolated cardiac myocytes directly impact cellular contractile and electrical functions. Our study findings further support the existence of a non-canonical intracrine RAS in cardiomyocytes governed by an Ang-(1–12)/chymase axis and functionally influencing cellular ion homeostasis.
METHODS
Animals
Male Sprague Dawley (SD; n=8) and TGR(hAGT)L1623 (n=16) rats aged 15-24–weeks old and weighing 360-600g were obtained from the Hypertension and Vascular Research Center animal colony at Wake Forest School of Medicine. Rats were housed in an American Association of Laboratory Animal Care-approved facility in a temperature-controlled room (22 ± 2°C) with a 12:12 -h light-dark cycle (lights on 6:00 AM to 6:00 PM), allowed free access to food and water, and handled following National Institutes of Health guidelines. Experimental protocols were approved by the Wake Forest School of Medicine Institutional Animal Care and Use Committee.
Cardiomyocyte isolation
Calcium-tolerant left ventricular cardiomyocytes were isolated by enzymatic dissociation of hearts from isoflurane (3% induction, 1.5% maintenance) anesthetized TGR(hAGT)L1623 and wildtype SD rats as previously described with slight modifications.17 Briefly, excised hearts were retrogradely perfused in a gravity-based Langendorff system with oxygenated Tyrode solution containing, in mmol/L: 136.5 NaCl, 5.4 KCl, 5.5 glucose, 1.0 CaCl2, 1 MgCl2, 5.5 HEPES, for 5 min at 37°C. Hearts were then perfused with a low-Ca2+ medium containing, in mmol/L: 100 NaCl, 10 KCl, 1.2 KH2PO4, 5 MgSO4; 20 glucose, 50 taurine, 10 HEPES, supplemented with 2.5 mM EGTA, until cardiac arrest. EGTA was replaced with type II collagenase (0.05%, w/v; 320 U/mg, Worthington, USA), 1 mg/mL bovine serum albumin (fraction V), and 35 μmol/L CaCl2. When the heart appeared opaque and flaccid (after approximately 20 min), the left ventricle was cut into small fragments, transferred to a 50 mL tube with type II collagenase-containing solution (i.e., enzyme solution) and shaken vigorously for 10 s. Tissue chunks were allowed to decant, and the supernatant was transferred to a clean tube containing wash solution (enzyme solution without collagenase) through a 500 μm cell strainer. Single cells were allowed to precipitate for at least 20 min, washed twice and stored in wash solution. Calcium recovery for patch clamp recordings was achieved by changing the [CaCl2] in the wash solution every 10 min to 100, 400, 800 and 1200 μmol/L, and finally resuspending cells in the bath solution used for experiments containing 1800 μmol/L CaCl2. Only rod-shaped myocytes with clear edges on morphological examination were used for experiments.
Patch clamp electrophysiology
L-type calcium channel currents (ICa-L) in isolated cardiomyocytes were measured using the whole-cell mode of the patch clamp technique, with patch pipettes (3–5 MΩ) backfilled with, in mmol/L: 130 CsCl, 5 MgSO4, 10 HEPES-KOH, pH 7.25. This pipette solution was used to dilute angiotensin peptides and drugs for intracellular delivery. The bath solution contained, in mmol/L: 80 NaCl, 1.8 CaCl2, 5 MgSO4, 1.2 KH2PO4, 10 CsCl, 20 tetraethyl ammonium chloride (TEA-Cl), 20 glucose, 5 ATP 10 HEPES-CsOH, pH 7.25; with Cs and TEA ions used to diminish K+ currents. ICa-L was elicited by depolarizing 200 ms-long, 50 mV rectangular pulses from a holding potential of −50 mV at a pacing rate of 0.03–0.04 Hz. Membrane capacitance, measured using pCLAMP 8 software (Axon Instruments, San Jose, CA), was used to calculate current density (in pA/pF). Peak ICa-L density was measured at steady state at least 5 minutes after obtaining whole cell patches.
Immunostaining and confocal microscopy
Isolated cardiomyocytes suspended in wash solution (see above) were plated onto 1 mM laminin-coated chamber slides before calcium recovery, and allowed to adhere to the surface for at least 3 h. Cells were fixed with fresh 4% paraformaldehyde for 10 min, washed 3x with PBS and stained with Wheat Germ Agglutinin (1:200, Invitrogen, Carlsbad, CA) for 10 min at room temperature (RT). Cells were then permeabilized for 30 min with 0.15% Triton X-100 and incubated with a primary antibody against the rat α-chymase (CloudClone PAG515Ra01; 1:100) overnight at 4°C. Chamber slides were then incubated with goat anti-rabbit Alexa Fluor 594 secondary antibody (Thermo Fisher, Waltham, MA ; 1:1000) for 1 h at RT and mounted with Vectashield hard set antifade mounting media with DAPI (Vector Laboratories, Burlingame, CA). Images were taken on an Olympus FV1200 confocal microscope and analyzed using Olympus FluoView 4.2 software.
Statistical analysis
Data are presented as mean ± SEM. ANOVA with post hoc Student’s t-test or Student’s t-test alone were used as appropriate to determine statistical significance at pre-established p < 0.05 using Prism 7 (GraphPad Software, San Diego, CA) or Excel (Microsoft, Redmond, WA).
RESULTS
L-type calcium channel current density is increased in TGR(hAGT)L1623 rats at baseline
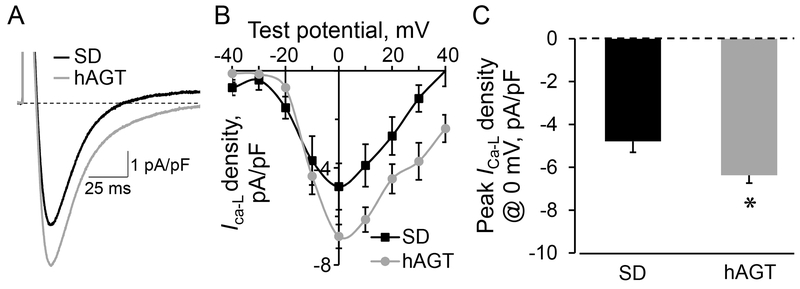
The TGR(hAGT)L1623 rat model is characterized by left ventricular hypertrophy and systolic dysfunction, yet the characteristics of isolated left ventricular cardiomyocytes have not been explored. As an indicator of cellular (electro)physiological status, we set to establish the baseline properties of L-type calcium channel currents in freshly isolated cardiomyocytes obtained from the left ventricle of TGR(hAGT)L1623 and SD rats using the whole cell patch clamp technique. Under control conditions, where no exogenous angiotensin peptides or drugs were administered, cardiomyocytes from TGR(hAGT)L1623 rat hearts exhibited significantly increased peak ICa-L density compared to SD control counterparts as elicited by single rectangular pulse stimulation to 0 mV from a −50 mV holding potential (−6.4 ± 0.3 pA/pF, n=28 vs. −4.8 ± 0.5 pA/pF, n=13, respectively; p = 0.0056; Figure 1A and 1C). The increased peak ICa-L density in TGR(hAGT)L1623 cardiomyocytes is in line with activation of L-type calcium channels resulting from augmented intracellular Ang II, as independently demonstrated by our previous finding of a 4-fold increase in left ventricular Ang II content in the hypertrophied heart of TGR(hAGT)L1623 rats.15
Figure 1.
A, Representative examples of peak ICa-L tracings recorded in cardiomyocytes isolated from SD and TGR(hAGT)L1623 (hAGT) rat hearts under control conditions. Dotted horizontal line indicates zero current level. B, L-type Ca2+ channel voltage-current (V-I) relationship obtained from SD and TGR(hAGT)L1623 (hAGT) cardiomyocytes. C, Mean and SEM values of peak ICa-L for SD (n=13) and hAGT (n=28) cardiomyocytes. * denotes p < 0.01 compared to SD.
Activation of ICa-L by intracellular administration of Ang II and Ang-(1–12) is impaired in TGR(hAGT)L1623 cardiomyocytes
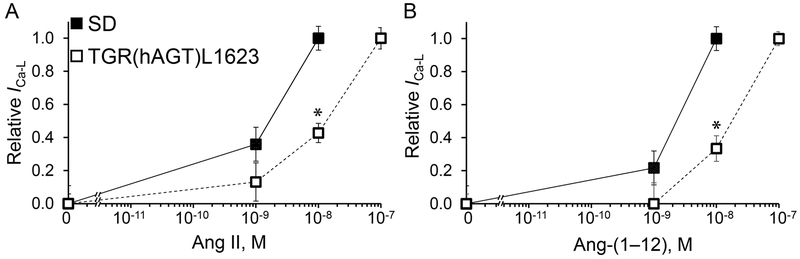
To further test the status of the cardiac intracrine RAS in TGR(hAGT)L1623 cardiomyocytes, exogenous Ang II was delivered to the cardiomyocyte cytosol via patch pipettes upon creation of the whole cell configuration. These experiments showed a rightward shift in the response curve to intracellular Ang II in transgenic cardiac myocytes compared to those obtained from SD controls. As illustrated in Figure 2A, SD cardiomyocytes displayed a progressive activation of ICa-L peaking at 10−8 mol/L in response to changing concentrations of Ang II. On the other hand, increases in ICa-L peaked at 10−7 mol/L in TGR(hAGT)L1623 cardiomyocytes (Figure 2A).
Figure 2.
Peak ICa-L response to intracellular administration of increasing concentrations of angiotensin peptides to SD and TGR(hAGT)L1623 cardiomyocytes. A, ICa-L density relative to maximum activation in response to increasing concentrations of intracellular Ang II; n=7-11 for each dose and group. B, ICa-L density relative to maximum activation in response to different concentrations of intracellular Ang-(1–12) ; n=6–10 for each dose and group. * denotes p < 0.05 compared to the same dose in SD control cardiomyocytes.
A similar rightward shift in the response to intracellular Ang-(1–12) administration was demonstrated in TGR(hAGT)L1623 compared to SD cardiomyocytes (Figure 2B). While ICa-L peaked with administration of Ang-(1–12) at 10−8 mol/L in SD (Figure 2B), 10−7 mol/L of Ang-(1–12) was required to reach a maximum response in TGR(hAGT)L1623 cardiomyocytes (Figure 2B).
Ang-(1–12)-induced increase in cardiomyocyte ICa-L is mediated by its processing to Ang II by chymase
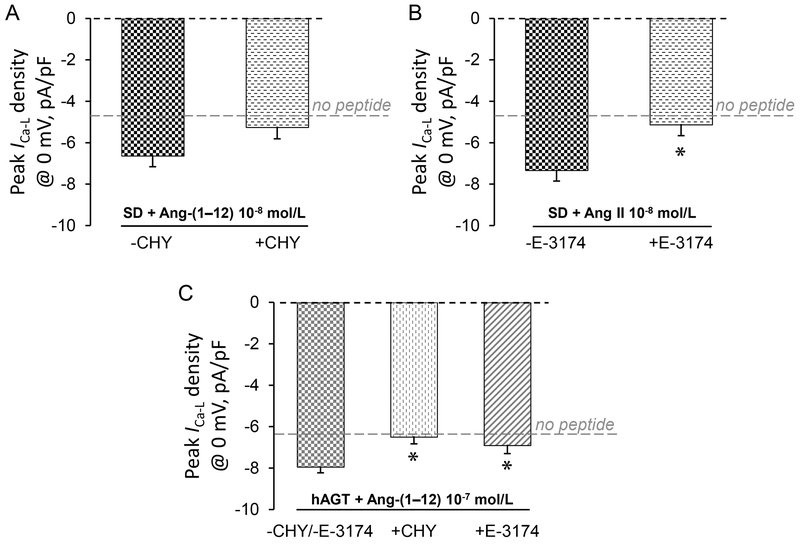
We reported previously that intracellular Ang-(1–12) decreases total potassium current in cardiomyocytes isolated from Wistar-Kyoto (WKY) rat hearts,10 yet whether this change was a direct effect of Ang-(1–12) or a result of the conversion of Ang-(1–12) to Ang II remained inconclusive. As illustrated in Figure 3, co-administration via patch pipettes of the chymase inhibitor chymostatin (50·10−6 mol/L) had different effects in SD and TGR(hAGT)L1623 cardiomyocytes. While the maximum ICa-L response to intracellular administration of Ang-(1–12) was only partially inhibited by the concomitant administration of chymostatin in SD cells (−6.6 ± 0.5 pA/pF, n=10 in the absence vs. −5.3 ± 0.5 pA/pF, n=4 in the presence of chymostatin; p = 0.1722; Figure 3A), chymase inhibition abolished the ICa-L -activating effect of Ang-(1–12) in TGR(hAGT)L1623 cardiomyocytes (−7.9 ± 0.3 pA/pF, n=13 in the absence vs. −6.5 ± 0.4 pA/pF, n=14 in the presence of chymostatin; p = 0.0107; Figure 3C).
Figure 3.
Effect of the chymase inhibitor chymostatin (CHY) and the AT1R-specific blocker E-3174 on the maximally-stimulated ICa-L by Ang II or Ang-(1–12) in SD or TGR(hAGT)L1623 (hAGT) cardiomyocytes. A, Effect of chymase on the maximally-activated ICa-L by Ang-(1–12) 10−8 M in SD cardiomyocytes. B, Effect of E-3174 on the maximally-activated ICa-L by Ang II 10−8 M in SD cardiomyocytes. C, Effect of chymase and E-3174 on the maximally-activated ICa-L by Ang-(1–12) 10−7 M in TGR(hAGT)L1623 (hAGT) cardiomyocytes. ANOVA p = 0.0163. * denotes p < 0.05 (post hoc t-test) compared to no-drug control.
The conversion of Ang-(1–12) to Ang II by chymase in the cardiomyocyte cytoplasm was further probed by co-administering the specific irreversible blocker of Ang II type 1 receptors (AT1R) (losartan metabolite E-3174)18 intracellularly with angiotensin peptides via patch pipettes. Co-administration of E-3174 10−6 mol/L with Ang II or Ang-(1–12) into the cytoplasm of SD or TGR(hAGT)L1623 cardiomyocytes via the patch pipette prevented the maximal ICa-L response to angiotensin peptides via blockade of intracellular AT1R signaling (Figure 3). Indeed, intracellular E-3174 effectively inhibited the maximal activation of L-type Ca2+ channel currents by Ang II 10−8 mol/L in SD cardiomyocytes (−7.3 ± 0.5 pA/pF, n=14 in the absence vs. −5.1 ± 0.4 pA/pF, n=7 in the presence of E-3174; p = 0.0125; Figure 3B) and by Ang-(1–12) 10−7 mol/L in TGR(hAGT)L1623 cells (−7.9 ± 0.3 pA/pF, n=13 in the absence vs. −6.9 ± 0.2 pA/pF, n=7 in the presence of E-3174; p = 0.0445; Figure 3C).
Chymase protein is present in the sarcolemma of isolated cardiomyocytes
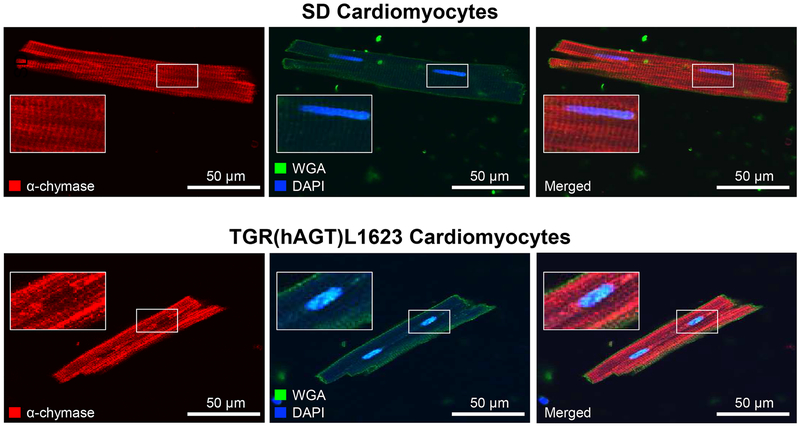
To build on our pharmacological studies and to confirm the presence of chymase immunoreactive protein in cardiomyocytes, we performed immunofluorescence staining with an antibody against rat mast cell protease 5 (MCP-5), the alpha form and most abundant subtype of rat chymase.19 Confocal imaging revealed the presence of α-chymase in isolated cardiomyocytes from both SD and TGR(hAGT)L1623 rats (red signal), in an expression pattern that did not align with wheat germ agglutinin (green signal, Figure 4).
Figure 4.
α-chymase (MCP-5) is expressed in SD and TGR(hAGT)L1623 cardiomyocytes. Confocal micrographs of freshly isolated cardiomyocytes from SD (top) and TGR(hAGT)L1623 left ventricles (bottom) taken at 100x magnification. Wheat Germ Agglutinin (WGA) is used to visualize the plasma membrane. DAPI stains cell nuclei.
DISCUSSION
In this study, we document activation of cardiomyocyte function by intracellular Ang II generation from Ang-(1–12) via a chymase mediated pathway. A transgenic rat model expressing the human AGT gene sources Ang II by a non-renin dependent mechanism, as the bond at which rat renin cleaves Ang I from the AGT substrate differs in the human substrate at positions 10 and 11 (H2N-Asp1-Arg2-Val3-Tyr4-Ile5-His6-Pro7-Phe8-His9-Leu10-Val11-Ile12-His13-COOH in humans versus H2N-Asp1-Arg2-Val3-Tyr4-Ile5-His6-Pro7-Phe8-His9-Leu10-Leu11-Tyr12-Tyr13-COOH) in rats.15,16 Extending previous studies, we now demonstrate that: i) increased cardiac Ang II in TGR(hAGT)L1623 rats15 contributed to or directly caused increased cardiomyocyte L-type Ca2+ channel currents at baseline; ii) locally-produced Ang II from Ang-(1–12) by chymase in cardiomyocytes elicits an effect on ICa-L; and iii) these effects are mediated through AT1Rs. Taken together, these data directly demonstrated cellular effects of Ang-(1–12) through an Ang II biosynthetic system governed by chymase in the cardiomyocytes of a clinically-relevant transgenic model of hypertension in which cardiac hypertrophy is accompanied by a 4-fold increased left ventricular Ang II content. This study highlights a critical role for a cardiomyocyte-specific intracrine RAS governed by the Ang-(1–12)/chymase axis in contributing to cardiac disease.20
Activation of the proposed cardiac intracrine RAS alters the electrophysiological properties of cardiomyocytes.10,21–23 In our most recent study, we reported that Ang-(1–12) in cardiomyocytes isolated from WKY rat caused depolarization of the surface cell membrane and an increase the action potential duration followed by the generation of early afterdepolarizations due to a reduction of potassium currents secondary to the activation of protein kinase C.10 Extending these observations to transgenic rats with increased cardiac Ang II content, we now use ICa-L as a readout to test whether expression of the human AGT gene in TGR(hAGT)L1623 cardiomyocytes will constitutively activate a cardiac intracrine RAS that is independent from the extracellular space or other cardiac cell types. Results showing a higher ICa-L at baseline in TGR(hAGT)L1623 cells, and a right shift in the response to intracellular administration of Ang II or Ang-(1–12), support our hypothesis. Furthermore, our results are in line with recently published data demonstrating that superfused Ang-(1–12) induces an increase in intracellular Ca2+ load and stimulates cardiomyocyte contractility via a chymase-mediated mechanism in normal rats.11 Whether both Ang-(1–12) intracellular uptake or formation account for its contractile augmenting properties remains to be determined; our data suggest that both pathways are at play.
Importantly, Ang-(1–12) inotropic activity was significantly blunted in isolated myocytes from isoproterenol-induced heart failure.11 In that study, altered Ang-(1–12) and Ang II inotropic responses were linked to cAMP-dependent mechanisms that are coupled to both stimulatory G and inhibitory PTX-sensitive G proteins.11 Here, the response to intracellular Ang-(1–12) in TGR(hAGT)L1623 cardiomyocytes was also blunted compared to control, suggesting that a pathological remodeling process of intracellular signaling pathways may be activated in transgenic cells exposed to a high local Ang II environment. Indeed, increased Ca2+ influx through L-type calcium channels is known to activate a calcium-dependent remodeling gene program in cardiomyocytes,24,25 which can be abolished by L-type calcium channel blockade. Chronically elevated mechanical, energetic and calcium load can underlie the left ventricular hypertrophy and systolic dysfunction observed in TGR(hAGT)L1623 hearts,15 providing a clinically relevant biological function to a cardiac-limited RAS. Furthermore, together with our previous report of impaired potassium currents,10 the effects of Ang-(1–12) on ICa-L presented here could be the underlying basis of the progressive diastolic dysfunction seen in rats with elevated recruitment of the cardiac intracrine RAS as measured by increasing levels of chymase mRNA.17
Based on our recent first characterization of chymase gene expressions in WKY and SHR rats,17 we selected an antibody against the alpha form of rat chymase (mast cell protease 5; MCP-5), as this is the isoform with Ang II forming activity and one of the two mast cell proteases with highest expression in the rat heart.17 Although the MCP-1 transcript is also highly expressed in TGR(hAGT)L1623 cardiomyocytes (our unpublished observations), this isoform enzyme does not cleave Ang II from Ang-(1–12).17
Besides indicating that chymase-dependent Ang II production does in fact occur in isolated cardiomyocytes, independent of the circulatory RAS, our data suggest that this catalytic reaction occurs intracellularly. Chymase immunoreactive protein, as shown here by confocal microscopy, did not co-localize with the membrane marker wheat germ agglutinin, yet displayed a cytoplasmic expression pattern that was more prominent underneath t-tubules, suggesting its probable localization to the sarcomeric z-disc.26 The presence of chymase in the cardiomyocyte along with the effects on ICa-L of intracellular administration of the Ang-(1–12) precursor, adds a layer of evidence for an intracrine RAS in the heart with functional relevance to myocyte performance. While there is now substantial evidence that chymase transcript is expressed directly in cardiomyocytes,17 the source of the Ang-(1–12) precursor warrants further investigation to complete the evolving picture of the cardiac intracrine RAS. Our findings suggest that the effector of the Ang-(1–12)/chymase axis in cardiomyocytes could be intracellular AT1 receptors. Indeed, E-3174, the biologically active losartan metabolite with non-competitive receptor blocking action, inhibited the effects of Ang II and Ang-(1–12) when co-administered intracellularly. Elucidating the organization of this heart-delimited Ang II production system and its effectors will provide necessary information for the development of newer therapies to improve the current paradigms of RAS blockade in heart disease.
Intracellular administration of the angiotensin peptides was achieved through the patch pipette, meaning the intracellular delivery occurred by passive diffusion immediately following creation of the whole cell configuration. Care was taken to minimize the time between pipette immersion in the batch and seal formation to prevent peptide leakage into the bath solution. Furthermore, a constant flow of peptide-free bath solution through the chamber was set to continuously wash the cardiomyocyte outer membrane and avoid activation of surface receptors. These precautions ensure that the changes recorded here represent direct actions of the peptides within the intact cell environment.
Progress for a more complete understanding of the molecular and physiological complexity of the enzymatic pathways from which Ang II is generated have been handicapped by the acceptance that renin and ACE are the unquestionable nodes participating in Ang II formation. Alternate mechanisms for Ang II formation through enzymes such as chymase and from other substrates such as Ang-(1–12) have been neglected based on equivocal interpretation of the published literature or results from imperfect studies.27,28 Yet, the increased impact of meta-analysis regarding the clinical effectiveness of ACE inhibitors, ARBs, and even direct renin inhibitors document their limited ability to reduce cardiovascular events beyond 30% of the treated population.2,3,29–32 The disconnect among the theoretical considerations, experimental findings and clinical outcomes needs to be resolved by pursuing the hypothesis that intracellular Ang II production may be stimulated by reduced AT1-R occupancy or interstitial Ang II levels following conventional RAS blockade. In this scenario, targeting chymase in addition to ACE may be the critical intervention leading to better inhibition of cardiac tissue Ang II production directly from Ang-(1–12).9 As reviewed elsewhere, 33 blockade of these non-canonical Ang II-forming mechanisms may yield better protection against end-organ damage, in line with the emerging RAS model in which a specific combination of angiotensin peptides results from the action of a specific combination of enzymes locally expressed in tissues.34
In summary, these results provide evidence that chymase is expressed and functionally active in rat adult cardiomyocytes where it mediates the conversion of Ang-(1–12) to Ang II. Downstream activation of intracellular AT1R through Ang-(1–12) dependent conversion into Ang II supports a role for the cardiomyocyte-specific intracrine RAS in contributing to cardiac disease. By focusing on a humanized model of hypertension due to overexpression of the human sequence of the AGT gene, the data gathered in these studies has direct relevance to the mechanisms by which Ang II contributes to human cardiovascular disease.35
HIGHLIGHTS.
Ang-(1–12)/chymase axis is active in cardiomyocytes of Ang II-mediated heart disease model.
Intracellular Ang II and Ang-(1–12) control L-type calcium currents.
Chymase inhibition prevents Ang-(1–12) L-type calcium channel activation.
Ang II formed by Ang-(1–12)/chymase axis acts via AT1 receptors.
Targeting Ang-(1–12)/chymase axis in cardiac tissue may add benefit to current care.
Acknowledgments
Grant Support
This work was funded by P01-HL-051952 Program Project Grant from the National Heart, Lung and Blood Institute (NHLBI) of NIH to CM Ferrario, by R01-AG-049770 Grant from the National Institute on Aging (NIA) of NIH to CP Cheng, and by the P01-HL051952-23S1 Diversity Supplement Award to CM Ferrario and S Reyes.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest
None
REFERENCES
- [1].Düsing R Mega clinical trials which have shaped the RAS intervention clinical practice. Ther Adv Cardiovasc Dis. 2016; 10:133–150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Reyes S, Varagic J, Ahmad S, et al. Novel cardiac intracrine mechanisms based on Ang-(1–12)/chymase axis require a revision of therapeutic approaches in human heart disease. Curr Hypertens Rep. 2017; 19:16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Ferrario CM, Ahmad S, Varagic J, et al. Intracrine Ang II functions originate from noncanonical pathways in the human heart. Am J Physiol Heart Circ Physiol. 2016; 311:H404–414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Urata H, Kinoshita A, Misono KS, Bumpus FM, Husain A. Identification of a highly specific chymase as the major angiotensin II-forming enzyme in the human heart. J Biol Chem. 1990; 265:2234–2237. [PubMed] [Google Scholar]
- [5].Nagata S, Kato J, Sasaki K, Minamino N, Eto T, Kitamura K. Isolation and identification of proangiotensin-12, a possible component of the renin–angiotensin system. Biochem Biophys Res Commun. 2006; 350:1026–1031. [DOI] [PubMed] [Google Scholar]
- [6].Trask AJ, Jessup JA, Chappell MC, Ferrario CM. Angiotensin-(1–12) is an alternate substrate for angiotensin peptide production in the heart. Am J Physiol Heart Circ Physiol. 2008; 294:H2242–2247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Ahmad S, Simmons T, Varagic J, Moniwa N, Chappell MC, Ferrario CM. Chymase-dependent generation of angiotensin II from angiotensin-(1–12) in human atrial tissue. PLoS One. 2011; 6:e28501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Ahmad S, Wei CC, Tallaj J, et al. Chymase mediates angiotensin-(1–12) metabolism in normal human hearts. J Am Soc Hypertens. 2013; 7:128–136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Ahmad S, Varagic J, VonCannon JL, et al. Primacy of cardiac chymase over angiotensin converting enzyme as an angiotensin-(1–12) metabolizing enzyme. Biochem Biophys Res Commun. 2016;478:559–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].De Mello WC, Dell’Itallia LJ, Varagic J, Ferrario CM. Intracellular angiotensin-(1–12) changes the electrical properties of intact cardiac muscle. Mol Cell Biochem. 2016; 422:31–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Li T, Zhang X, Cheng HJ, et al. Critical role of the chymase/angiotensin-(1–12) axis in modulating cardiomyocyte contractility. Int J Cardiol. 2018; 1;264:137–144 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Tsai CT, Lai LP, Hwang JJ, et al. Renin-angiotensin system component expression in the HL-1 atrial cell line and in a pig model of atrial fibrillation. J Hypertens. 2008; 26:570–582. [DOI] [PubMed] [Google Scholar]
- [13].Tojo H, Urata H. Chymase inhibition and cardiovascular protection. Cardiovasc Drugs Ther. 2013; 27:139–143. [DOI] [PubMed] [Google Scholar]
- [14].Yahiro E, Miura S, Imaizumi S, Uehara Y, Saku K. Chymase inhibitors. Curr Pharm Des. 2013; 19:3065–171. [DOI] [PubMed] [Google Scholar]
- [15].Ferrario CM, VonCannon J, Jiao Y, et al. Cardiac angiotensin-(1-12) expression and systemic hypertension in rats expressing the human angiotensinogen gene. Am J Physiol Heart Circ Physiol. 2016; 310:H995–1002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Ganten D, Wagner J, Zeh K, et al. Species specificity of renin kinetics in transgenic rats harboring the human renin and angiotensinogen genes. Proc Natl Acad Sci USA. 1992; 89:7806–7810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Zhang X, Cheng HJ, Zhou P, et al. Cellular basis of angiotensin-(1–7)-induced augmentation of left ventricular functional performance in heart failure. Int J Cardiol. 2017; 236:405–412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Suzuki J, Ohta H, Hanada K, Kawai N, Ikeda T, Nakao M, Ikemoto F, Nishikibe M. Acute effects of E-3174, a human active metabolite of losartan, on the cardiovascular system in tachycardia-induced canine heart failure. Hypertens Res. 2001; 24:65–74. [DOI] [PubMed] [Google Scholar]
- [19].Wang H, Sun X, Ahmad S, Su J, Ferrario CM, Groban L. Estrogen modulates the differential expression of cardiac myocyte chymase isoforms and diastolic function. Mol Cell Biochem. 2019; 456:85–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Dell’Italia LJ, Collawn JF, Ferrario CM. Multifunctional role of chymase in acute and chronic tissue injury and remodeling. Circ Res. 2018; 122:319–336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].De Mello WC. Intracellular angiotensin II regulates the inward calcium current in cardiac myocytes. Hypertension. 1998; 32:976–982. [DOI] [PubMed] [Google Scholar]
- [22].De Mello WC, Monterrubio J. Intracellular and extracellular angiotensin II enhance the L-type calcium current in the failing heart. Hypertension. 2004; 44:360–364. [DOI] [PubMed] [Google Scholar]
- [23].De Mello WC. Intracrine action of angiotensin II in the intact ventricle of the failing heart: angiotensin II changes cardiac excitability from within. Mol Cell Biochem. 2011; 358:309–315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Kane GC, Behfar A, Dyer RB, et al. KCNJ11 gene knockout of the Kir6.2 KATP channel causes maladaptive remodeling and heart failure in hypertension. Hum Mol Genet. 2006; 15:2285–2297. [DOI] [PubMed] [Google Scholar]
- [25].Yamada S, Kane GC, Behfar A, et al. Protection conferred by myocardial ATP-sensitive K+ channels in pressure overload-induced congestive heart failure revealed in Kcnj11 Kir6.2-null mutant. J Physiol. 2006; 577:1053–1065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Powell PC, Wei CC, Fu L, et al. Chymase Uptake by cardiomyocytes results in myosin degradation in cardiac volume overload. Heliyon. 2019; 5:e01397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Campbell DJ. Angiotensin II generation in vivo: does it involve enzymes other than renin and angiotensin-converting enzyme? J Renin Angiotensin Aldosterone Syst. 2012; 13:314–316. [DOI] [PubMed] [Google Scholar]
- [28].Chai W, Danser AH. Is angiotensin II made inside or outside of the cell? Curr Hypertens Rep. 2005; 7:124–127. [DOI] [PubMed] [Google Scholar]
- [29].Roig E, Perez-Villa F, Morales M, et al. Clinical implications of increased plasma angiotensin II despite ACE inhibitor therapy in patients with congestive heart failure. Eur Heart J. 2000; 21:53–57. [DOI] [PubMed] [Google Scholar]
- [30].Turnbull F, Neal B, Ninomiya T, et al. Effects of different regimens to lower blood pressure on major cardiovascular events in older and younger adults: meta-analysis of randomised trials. BMJ. 2008; 336:1121–1123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Zanchetti A, Thomopoulos C, Parati G. Randomized controlled trials of blood pressure lowering in hypertension: a critical reappraisal. Circ Res. 2015; 116:1058–1073. [DOI] [PubMed] [Google Scholar]
- [32].Ferrario CM, Mullick AE. Renin angiotensin aldosterone inhibition in the treatment of cardiovascular disease. Pharmacol Res. 2017; 125(Pt A):57–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Nehme A, Zouein FA, Deris Zayeri Z, Zibara K. An update on the tissue renin angiotensin system and its role in physiology and pathology. J Cardiovasc Dev Dis. 2019; 6: E14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Nehme A, Zibara K. Efficiency and specificity of RAAS inhibitors in cardiovascular diseases: how to achieve better end-organ protection? Hypertens Res. 2017; 40: 903–909. [DOI] [PubMed] [Google Scholar]
- [35].Akasu M, Urata H, Kinoshita A, Sasaguri M, Ideishi M, Arakawa K. Differences in tissue angiotensin II-forming pathways by species and organs in vitro. Hypertension. 1998; 32:514–520. [DOI] [PubMed] [Google Scholar]