Abstract
We previously reported increased current density through P-type voltage-gated Ca2+ channels in inferior colliculus (IC) neurons during alcohol withdrawal. However, the molecular correlate of this increased P-type channel current is currently unknown. Here, we probe changes in mRNA and protein expression of the pore-forming CaV2.1-α1 (P/Q-type) subunits in IC neurons during the course of alcohol withdrawal-induced seizures (AWSs). Rats received three daily doses of ethanol or the vehicle every 8 h for 4 consecutive days. The IC was dissected at various time intervals following alcohol withdrawal, and the mRNA and protein levels of the CaV2.1-α1 subunits were measured. In separate experiments, rats were tested for acoustically evoked seizure susceptibility 3, 24, and 48 h after alcohol withdrawal. AWSs were observed 24 h after withdrawal; no seizures were observed at 3 or 48 h or in the control-treated rats. Compared to control-treated rats, the mRNA levels of the CaV2.1α1 subunit were increased 1.9-fold and 2.1-fold at 3 and 24 h, respectively; change in mRNA expression was nonsignificant at 48 h following alcohol withdrawal. Western blot analyses revealed that protein levels of the CaV2.1-α1 subunits were not altered in IC neurons following alcohol withdrawal. We conclude that expression of the Cacna1a mRNA increased in before the onset of AWS susceptibility, suggesting that altered CaV2.1 channel expression may play a role in AWS pathogenesis.
Keywords: Cacna1a, P/Q-type calcium channel, audiogenic seizures
Introduction
Generalized tonic-clonic seizures (GTCSs) are one of the most severe withdrawal symptoms following the cessation of chronic alcohol intake (Alldredge & Lowenstein, 1993; Hillbom, Pieninkeroinen, & Leone, 2003; Pilke, Partinen, & Kovanen, 1984). A robust rat model of the human alcohol withdrawal-induced generalized tonic-clonic seizures has been developed to identify their underlying mechanisms. In this model, the inferior colliculus (IC) is critically involved in the initiation of acoustically evoked reflex seizures (i.e., audiogenic seizures) during alcohol withdrawal (i.e., alcohol withdrawal-induced seizures or AWSs) ( Chakravarty & Faingold, 1998; Eckardt et al., 1986; Faingold & Riaz, 1995; Frye, McCown, & Breese, 1983; McCown & Breese, 1990, 1993). Electrophysiology studies revealed elevated excitability of IC neurons following alcohol withdrawal (Evans, Li, & Faingold, 2000; Faingold, Li, & Evans, 2000; N’Gouemo, Caspary, & Faingold, 1996). Interestingly, increased firing of IC neurons was observed both prior to and during AWS (Chakravarty & Faingold, 1998; Faingold & Riaz, 1995). These findings support the importance of IC neurons in the networks that underlie AWSs (Faingold, N’Gouemo, & Riaz, 1998). The mechanism underlying alcohol withdrawal-induced IC neuronal hyperexcitability are not fully understood.
Altered voltage-gated Ca2+ (CaV) channels play an important role in the mechanisms that underlie AWSs (Whittington, Lambert, & Little, 1993). We previously reported that the increase in current density through CaV channels observed 3 h (i.e., when no AWS susceptibility is observed) and 24 h after alcohol withdrawal (i.e., when AWS susceptibility peaks) in IC neurons returns to control levels 48 h later, when the animal is no longer susceptible to AWSs (N’Gouemo, 2015). Interestingly, changes in CaV current density follow a parallel time course with the occurrence of AWS susceptibility (Faingold, 2008; N’Gouemo & Morad, 2003; N’Gouemo, 2015). Because alcohol withdrawal preferentially affects P-type of CaV2.1 channels (N’Gouemo & Morad, 2003), we examined the extent to which mRNA and protein expression of pore-forming CaV2.1-α1 (P/Q-type) subunit is altered in IC neurons following alcohol withdrawal and whether these changes are correlated with the development of AWS susceptibility.
Material and methods
Animals.
Male Sprague-Dawley rats (250–300 g; Taconic, Germantown, MD) were used for these experiments. The animals were maintained in a temperature- and humidity-controlled room on a 12-h/12-h light/dark cycle with food and water available ad libitum. All efforts were made to minimize the number of animals used in these experiments. All experimental procedures were approved by the Georgetown University Animal Care and Use Committee and were performed in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals (National Research Council (U.S.), 2011).
Ethanol administration.
Ethanol (prepared from a 95% stock solution) was administered by oral gavage as a 30% (v/v) solution in ISOMIL Infant Formula Concentrate (the ISOMIL formula was diluted 1:1 with water). Ethanol was administered three times per day (at 8-h intervals) for 4 days, and the level of intoxication was evaluated using a standard behavior-rating scale (Faingold, 2008). The first dose of ethanol was 5 g/kg body weight, and subsequent doses were adjusted for each animal in order to achieve a moderate (i.e., an accentuated staggering gait and considerable elevation of the pelvis) but not severe (i.e., lethargy without pelvic or abdominal elevation) degree of intoxication (Faingold, 2008); the total amount of ethanol administered was 2–12 g/kg/day. On the fourth day, ethanol treatment was terminated after the second dose. The behavioral signs of ethanol intoxication and subsequent doses of ethanol were determined based on a well-described intoxication scale (Faingold, 2008; Majchrowicz, 1975): 0, Neutrality or absence of signs of intoxication or withdrawal (5 g/kg); 1, Sedation (4 g/kg); 2, Ataxia 1 characterized by the lowest degree of gait impairment (4 g/kg); 3, Ataxia 2 corresponding to an intermediate degree of gait impairment (3 g/kg); 4, Ataxia 3 characterized by a marked level of intoxication or recovery of the righting reflex (2 g/kg); 5, Loss of righting reflex (1 g/kg); and 6, Coma or absence of movements, eye closure, and absence of the eye blink reflex (0 g/kg). The behavioral signs of ethanol withdrawal include hyperactivity, tremors, tail spasticity, spontaneous seizures (myoclonus and forelimb clonic seizures), and acoustically evoked startle responses and seizures (Faingold, 2008; N’Gouemo, Yasuda, & Morad, 2006). The control-treated animals were maintained under similar conditions but received three times daily the ISOMIL formula alone (without ethanol).
Seizure testing.
To determine the animals’ susceptibility to develop acoustically evoked AWSs, each control-treated (n=10) and ethanol-treated (n=10) rat was selected at random and tested repetitively for seizures during the course of ethanol withdrawal: 2-3 h (the 3-h group), 23-24 h (the 24-h group), and 47-48 h (the 48-h group) after the last dose of ethanol. The acoustic stimulus consisted of 100-110-decibel sound pressure level pure tones (Med Associates, St. Albans, VT) that were presented until wild running seizure (WRS) was elicited or for 60 sec if no seizure activity was observed. Convulsive seizure behavior was classified into four stages based on the scale for acoustically evoked seizures (Jobe, Mishra, & Dailey, 1992): stage 0, no seizures in response to acoustic stimuli; stage 1, one episode of WRS; stage 2, two episodes of WRS; stage 3, one episode of WRS and/or jumping followed by bouncing GTCSs (clonus) involving forelimbs and hindlimbs; stage 4, two episodes of WRS followed by clonus; stage 5, one episode of WRS and clonus followed by tonic forelimb extension (FLE) and clonus of the hindlimbs. Animals that did not exhibit a seizure during the first trial were tested again 2 h later using mixed sounds delivered via an electrical bell, based on the fact that seizure threshold varies between among animals. The animals used in the molecular studies were not subjected to acoustically evoked seizure testing, as evoked seizures can induce a long-lasting increase in extracellular GABA levels (Ueda & Tsuru, 1995). Such GABA release and various degrees of seizure severity and duration may alter of CaV2.1 channels expression.
Blood ethanol concentrations.
In a separate set of experiments, blood ethanol concentration (BEC) was measured in the control group (n=4) and in the ethanol-treated group 3 h (n=4), 24 h (n=4), and 48 (n=4) h during ethanol withdrawal. The rats were anesthetized (50 mg/kg Nembutal; intraperitoneally [i.p.]), and blood was extracted by intracardiac sampling using a 21-gauge needle. Serum ethanol concentration was measured using an Analox model GM7 analyzer (Analox Instruments, London, UK).
Western blot analysis.
At 3, 24, and 48 h during ethanol withdrawal, the animals were deeply anesthetized with pentobarbital (100 mg/kg; i.p.), and the colliculi were immediately dissected and stored at –80°C until use. Tissue homogenates from each animal were lysed in 50 mM Tris-HCl (pH 7.4), 300 mM NaCl, 1% IGEPAL (Sigma-Aldrich, St. Louis, MO), 10% glycerol, 1 mM EDTA, and 1 mM Na3VO4. The homogenates were cleared by centrifugation (13,800xg, 4°C, and 30 min). The supernatants were transferred to sterile microtubes and stored at ‒80°C until use. Protein concentration in the supernatants was measured using the bicinchoninic acid (BCA) assay and a Bio-Rad Model 680 spectrophotometer (Bio-Rad, Hercules, CA). For each sample, 60 μg of total protein was separated by electrophoresis in a 7.5% sodium dodecyl sulfate-polyacrylamide gel and electro-transferred to a nitrocellulose membrane (Bio-Rad). The membranes were blocked in Odyssey blocking buffer (LI-COR Biosciences, Lincoln, NE) for 1 h and then probed overnight at 4°C with primary rabbit antibodies against either the α1A subunit (CaV2.1-α1) (1:200; Alomone Lab, Jerusalem, Israel). The membranes were also incubated with anti-glyceraldehyde 3-phosphate dehydrogenase (anti-GAPDH) antibody (1:10,000; Thermo Fisher Scientific, Waltham, MA) overnight at 4°C as an internal control. The membranes were probed with goat anti-mouse IRDye800 (1:10,000; LI-COR Biosciences) and goat anti-rabbit IR-Dye680 (1:10,000; LI-COR Biosciences) for 1 h at room temperature, then scanned using an Odyssey Fc imager (LI-COR Biosciences).
RNA isolation and quantitative real-time PCR.
To quantify the mRNA levels of the CaV2.1α1 subunits in the IC, another set of rats (n=8 per group) were subjected to the 4-day ethanol intoxication paradigm (or control treatment), then anesthetized 3, 24, and 48 h after ethanol withdrawal. The brains were removed, and the IC was immediately dissected and stored at ‒80°C until use. Total RNA was extracted from the IC using the RNeasy mini kit in accordance with the manufacturer’s instructions (Qiagen, Valencia, CA). RNA quality and concentration was measured using a Bioanalyzer (Agilent Technologies, Palo Alto, CA) and tissues were stored at ‒20°C. Based on RNA quality, only n=5 IC samples per group were used for qPCR. Complementary DNA was synthesized using 5x iScript reaction mix (Bio-Rad, Hercules, CA) RNA by reverse transcription using the Quantitect reverse transcription kit (Qiagen) and stored at ‒20°C. The specific CaV2.1-α1 subunit primers (rat Cacna1a; Cat. #: Rn.PT.58.18040441, GenBank accession no. NM_012918, Integrated DNA Technologies, Coralville, IA) were pre-validated and tested for PCR efficiency by the manufacturer; the assay will perform with PCR efficiencies between 90–110% and R2 >0.99. Using the SsoAdvanced Universal SYBR green supermix (Bio-Rad), qPCR was performed on CFX96 real-time PCR detection system (Bio-Rad) using the following temperature settings: 95°C (denaturation) for 5 min followed by 40 cycles of 95°C for 15 sec, and 58°C (annealing/extension) for 1 min. After amplification, a crossing threshold (CT) value (representing the number of cycles required to reach a specific level of fluorescence) was determined for each sample using the BioRad CFX manager software.
Data analysis.
The protein levels of the CaV2.1-α1 subunit were measured using densitometry of the corresponding bands (Odyssey Image Studio v3.2 software, LI-COR Biosciences). The integrated intensity of each band was normalized to the intensity of GAPDH. The mean of the control-treated values was calculated, and the normalized intensity value of each control-treated and alcohol-treated sample was expressed as a ratio relative to the mean control-treated value. We used the comparative CT (ΔΔCT) method to calculate changes in the mRNA expression of CaV2.1-α1 subunit in ethanol-treated samples relative to control-treated samples and β-actin was used for data normalization (Livak & Schmittgen, 2001). The prevalence of each type of seizure was analyzed using the chi-square (χ2) test and the seizure severity was analyzed using the Kruskall Wallis test followed by the Student-Newman-Keuls or Dunn post hoc correction. To evaluate differences in the protein and mRNA levels, one-way analyses of variance (ANOVA; F) followed by a Bonferroni post-hoc correction were performed. Differences were considered significant at P<0.05, and the summary data are presented as the mean ± S.E.M. or as median ± median average of the mean (M.A.D)
Results
Ethanol intoxication-related behaviors and amount of ethanol administered to maintain intoxication
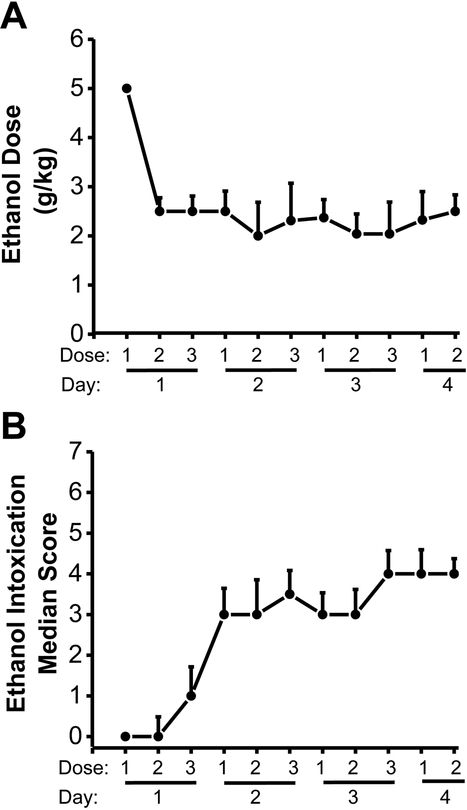
Fig. 1 summarizes the amount of ethanol administered (Fig. 1A) and the median scores of ethanol intoxication-related behaviors (Fig. 1B) obtained from 24 rats subjected to ethanol intoxication/withdrawal used in western blot experiments (n=8 per group). The amount of ethanol administered decreased steadily over the first 4 doses and stabilized at approximately 2.5 g/kg (from an initial 5 g/kg; Fig. 1A). The median score of ethanol intoxication-related behavior increased steadily to the level of ataxia (Fig. 1B).
Figure 1: Ethanol doses administered and ethanol intoxication scores.
Data are shown as the mean±SEM dosages of ethanol administered (A) and the median±MAD ethanol intoxication scores measured (B) at 8-h intervals over 4 days (n=24 ethanol-treated rats from Western blot experiments).
Ethanol withdrawal seizure susceptibility and blood ethanol levels
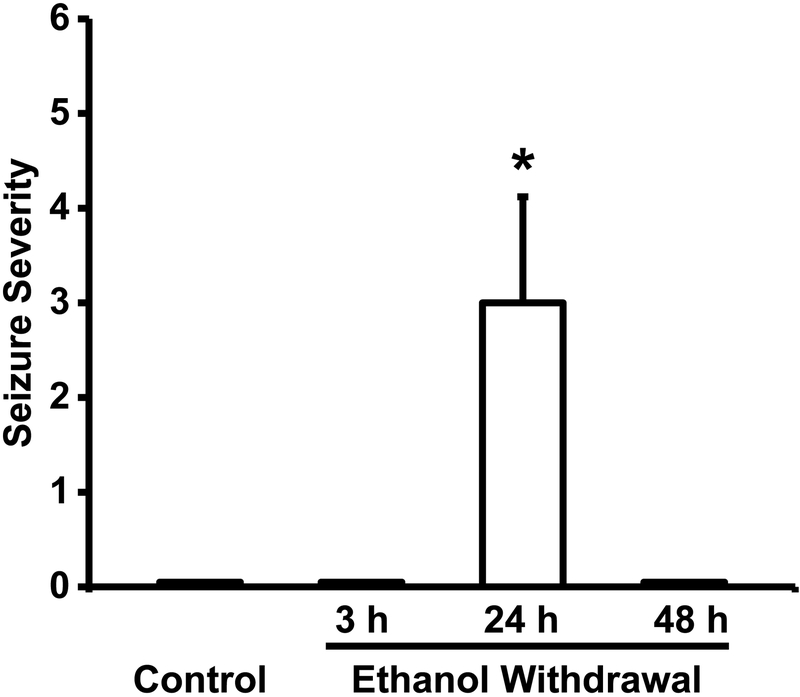
Figure 2 summarizes the severity of acoustically evoked AWS following control treatment or 3 h, 24 h, and 48 h during ethanol withdrawal. Acoustically evoked AWSs consisted of WRSs, which evolved into GTCSs followed occasionally by tonic forelimb extension. Twenty-four hours after ethanol withdrawal, these seizure types were observed in 100% (χ2 = 196, p < 0.001 vs. control), 60% (χ2 = 83, p < 0.001 vs. control) and 10% of ethanol-treated rats, respectively. This susceptibility to acoustically evoked AWS was transient, as these seizures were observed only in the 24-h group, but not in the 3-h or 48-h group or the control group (Fig. 2). Thus, ethanol withdrawal significantly (Kruskal-Wallis H = 38.14, df = 3, p = 0.0001) increased the seizure severity median score to 3.0 ± 1.12 (n = 10) 24 h following ethanol withdrawal from 0 ± 0 (n = 10) in the control-treated group (t = 10.69, p < 0.05) as well as in the 3-h (t = 5.41, p < 0.05) and 48-h (t = 7.18, p < 0.05) ethanol-treated group. Quantification also revealed that BEC group means were significantly different (F(3,12) = 40.25, p = 0.001). The BECs were significantly (t = 9, p < 0.05) elevated in the 3-h group (0.260 ± 0.040 g/dL, n = 4) compared with those of the 24-h (0.007 ± 0.001 g/dL, n = 4) and 48-h (0.006 ± 0.001 g/dL, n = 4) ethanol-treated group, and the control-treated group (0.005 ± 0.001 g/dL, n = 4). It is noteworthy to mention that BEC values in the control-treated group as well as in the 24-h and 48-h ethanol-treated groups were below the threshold for detection.
Figure 2.
Ethanol withdrawal increases seizure susceptibility. Data shown are median±MAD seizure severity score measured repetitively in the control-treated group and the ethanol-treated group at 3-h, 24-h, and 48-h time point following ethanol withdrawal (n=10 rats per group). *P<0.05 vs control, 3-h and 48-h time point, Kruskal-Wallis test and Student-Newman-Keuls or Dunn post hoc correction).
Ethanol withdrawal upregulates CaV2.1-α1 subunit at the mRNA level
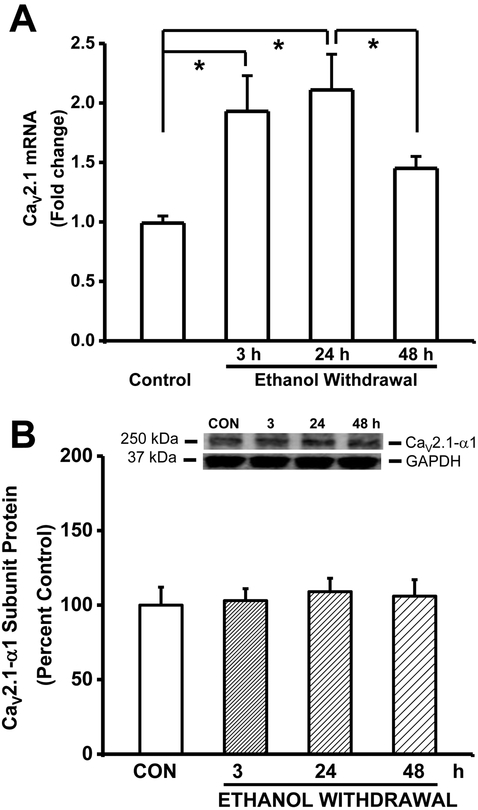
Figure 3A summarizes the mRNA levels of the CaV2.1-α1 subunits (Cacna1a) in IC tissues obtained from the control and ethanol-treated groups. The expression of Cacna1a (F3,16=10.92, P=0.0003) mRNA was increased significantly in the IC following ethanol withdrawal. Interestingly, the expression (presented as fold change±SEM) of Cacna1a mRNA was significantly increased at both the 3-h (2−∆∆CT=1.93±0.14, n=5, t=4.34) and the 24-h (2−∆∆CT=2.11±0.15, n=5, t=5.21, P<0.05) time point, but not at the 48-h (2−∆∆CT=1.45±0.22, n=5) time point during ethanol withdrawal, in comparison to the control group (1.0±0.06, n=6; Fig. 3A). The levels of Cacna1a mRNA was also significantly increased 24 h following ethanol withdrawal compared to the 48-h (t=3.06, P<0.05) group.
Figure 3.
Ethanol withdrawal upregulates Cacna1a mRNA but not CaV2.1-α1 subunit protein expression in the IC. A. The levels of Cacna1a mRNA were measured in the IC; the data are expressed as the fold change relative to the control group. The mRNA levels of Cacna1a was significantly elevated 3 h and 24 h after alcohol withdrawal compared to the control group. The summary data are shown as mean ± S.E.M. (n=5 rats per group). B. Shown in insets are representative immunoblots of the and CaV2.1 subunits measured from control IC samples and IC samples obtained at the indicated times after ethanol withdrawal. The bar graphs summarize the relative protein levels of CaV2.1-α1 subunits in the IC, expressed as a percentage of the control group. The density of the 250-kDa immunoreactive band (i.e., CaV2.1-α1 subunit) did not change significantly following ethanol withdrawal. The summary data are shown as the mean ± S.E.M. (8 rats per group). *P<0.05 versus control (ANOVA followed by a Bonferroni correction).
Ethanol withdrawal does not alter CaV1 channels at the protein level
Figure 3B summarizes the protein levels of CaV2.1-α1 subunits in IC neurons during the course of alcohol withdrawal. In contrast to the mRNA expression, no significant changes in the protein expression levels of the CaV2.1-α1 subunits were found at 3-h (103±16%, n=8), 24-h (109±19%, n=8) and 48-h (106±22%, n=8) during ethanol withdrawal. Ethanol withdrawal also did not significantly alter GAPDH expression in IC neurons (3-h group: 114±32%, n=8; 24-h group: 118±18%, n=8; 48-h group: 109±18%, n=8) compared to the control-treated group (100±23%, n=8).
Discussion
We previously reported increased currents through CaV2.1 P-type channels in IC neurons during alcohol withdrawal at the time point at which susceptibility to seizures peaks (N’Gouemo & Morad, 2003). Here, we report that the mRNA but not protein levels of the CaV2.1-α1 subunits increase in the IC during AWS susceptibility. In particular, expression of the CaV2.1-α1 subunit was significantly increased 3 h (before the onset of AWS susceptibility) and 24 h (when AWS susceptibility peaked) after alcohol withdrawal compared to the control-treated group. A significant increase in CaV2.1-α1 subunit mRNA was also observed 24 h following alcohol withdrawal compared to the 48-h group. Thus, increase in the expression of Cacna1a mRNA occurred before the onset of AWS susceptibility suggestion that these changes may be an antecedent but not a consequence of seizure activity. A lack of positive correlation between the expression of Cacna1a mRNA and the levels of CaV2.1-α1 subunit proteins in the IC could be due to different mRNA and protein degradation.
At the protein level, western blot analyses revealed no change in the protein expression of CaV2.1-α1 subunit in the IC. Such lack of CaV2.1-α1 subunit proteins was also found in the cortex of mice subjected to chronic alcohol intoxication (Katsura et al., 2006). Thus, a lack of positive correlation between the expression of Cacna1a mRNA and the levels of CaV2.1-α1 subunit proteins in the IC could be due to different mRNA and protein degradation. The lack of change in the protein expression of CaV2.1-α1 subunits suggests that metabolic factors such phosphorylation may contribute to increase CaV2.1 P-type currents in IC neurons following alcohol withdrawal. Consistent with this hypothesis, we recently reported elevated protein kinase A (PKA) expression and function in IC neurons during the development of AWS susceptibility (Akifiresoye, Miranda, Lovinger, & N’Gouemo, 2016). Whether increased PKA phosphorylation affects CaV2.1-α1 subunits in IC neurons remains unknown. The increased CaV2.1 P-type currents could also be due to upregulation of CaV channel regulatory subunits such as the α2/δ1 subunits, which increased CaV channel current amplitude and enhanced the open probability of CaV channels (Felix, Gurnett, DeWaard, Campbell, 1997; Klugbauer, Lacinova, Marais, Hobom, & Hofmann, 1999; Shistik, Ivanina, Puri, Hosey, & Dascal, 1995). Interestingly, increased protein expression of α2/δ1 subunit proteins, was increased in the cerebral cortex following alcohol intoxication (Katsura et al., 2006). Such upregulation of α2/δ1 subunits may also occur in IC neurons following alcohol withdrawal. However, in previous study, we found no change in the protein expression of CaV channel regulatory β subunit proteins in IC neurons 24 h following alcohol withdrawal (N’Gouemo et al., 2006).
In the brain, CaV2.1 channels are concentrated in presynaptic terminals, dendrites and cell bodies (Westenbroek et al., 1995), providing evidence for a key role of these channels in transmitter release. The IC plays an important role in initiating AWSs (Chakravarty & Faingold, 1998; Faingold & Riaz, 1995; Frey et al., 1983; Riaz & Faingold, 1994). Blockade of P-type CaV2.1 channels in the IC suppresses AWS (N’Gouemo, 2015). It is tempting to speculate that P-type CaV2.1 channels may contribute to the release of excitatory neurotransmitters in the IC during alcohol withdrawal, which in turn may contribute to neuronal hyperexcitability leading to AWSs.
Alcohol withdrawal-induced GTCSs originate primarily in the brainstem; these resemble seizures observed in the genetically epilepsy-prone (GEPR-3) rat (Faingold, 1999; Faingold et al., 1998). In GEPR-3s, the protein expression of CaV2.1-α1 subunits was increased in IC neurons of seizure-experienced animals but not in seizure-naïve animals when compared to controls (N’Gouemo, Yasuda, & Faingold, 2010). These findings suggest that CaV2.1 channels in IC neurons may not play an important role in neuronal hyperexcitability that leads to generalized tonic-clonic seizures, but a seizure episode can alter the expression of these channels. In the Mongolian gerbil model of inherited GTCSs, CaV2.1-α1 subunits were significantly increased the hippocampus and dentate gyrus during the pre-seizure state, as well as post-ictally in seizure-sensitive gerbils; although a transient decrease was found in the hippocampus (Kang et al., 2004). Similarly, CaV2.1-α1 subunits were increased in cerebellar Purkinje cells of seizure-sensitive gerbils (Park, Hwang, An, Wong, & Kang, 2003). Whether these changes also occur in the brainstem of the gerbil model is currently unknown. The increase in CaV2.1 channels in the hippocampus may represent the molecular correlates of increased Ca2+-dependent excitability in this model of GTCSs.
Altered mRNA levels of CaV1 channels have also been reported in various models of seizures and epilepsy. Notably, significant increases in the mRNA levels of CaV2.1-α1 subunit were found in the dorsal part of the lateral geniculate nucleus (dLGN) of the thalamus in epileptic Wistar Albino Glaxo from Rijswijk (WAG/Rij) rats, a model of inherited absence epilepsy; however, no changes were found in CaV2.1-α1 subunit proteins (Kanyshkova et al., 2014). In a kindling model of epileptogenesis, the mRNA levels of CaV2.1-α1 subunit channels were increased in the hippocampus during the non-convulsive limbic phase (Hendriksen, Kamphuis, & Lopes da Silva, 1997). In contrast, no change in the expression of CaV2.1-α1 subunit mRNA was found in the hippocampus of animals with GTCSs (Hendriksen et al., 1997). These findings suggest that altered CaV2.1-α1 channels may play a role in kindling epileptogenesis but not in the expression of kindled seizures. In kainate-induced status epilepticus, another model of limbic seizures, an upregulation of mRNA expression of CaV2.1-α1 subunit that lasts up to seven days was reported in the hippocampus (Vignes et al., 1999). However, no changes in the mRNA expression of CaV2.1-α1 subunit was reported in hippocampus two weeks after the occurrence of kainate-induce status epilepticus (Westenbroek et al., 1998). Thus, these findings suggest that upregulation of CaV2.1-α1 subunit mRNA in the brain is associated primarily with both non-convulsive seizures and generalized tonic-clonic seizures.
In summary, AWS susceptibility increased the expression of CaV2.1-α1 subunit mRNA in the rat IC neurons; this increase occurred before the onset of AWSs, suggesting that these changes may play a role in the pathogenesis of AWSs.
Acknowledgements
This work was supported by NIH Public Health Service Grants (AA020073 to P.N.) and the NIAAA Division of Intramural Clinical and Biological Research (to D.M.L.).
Footnotes
Statement of Interest
The authors have no conflicts of interest.
References
- Akinfiresoye LR, Miranda C, Lovinger DM, & N’Gouemo P (2016). Alcohol withdrawal increases protein kinase A activity in the rat inferior colliculus. Alcoholism: Clinical and Experimental Research, 40, 2359–2367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alldredge BK, & Lowenstein DH (1993). Status epilepticus related to alcohol abuse. Epilepsia, 34, 1033–1037. [DOI] [PubMed] [Google Scholar]
- Chakravarty DN, & Faingold CL (1998) Comparison of neuronal response patterns in the external and central nuclei of inferior colliculus during ethanol administration and ethanol withdrawal. Brain Research, 783, 102–108. [DOI] [PubMed] [Google Scholar]
- Eckardt MJ, Campbell GA, Marietta CA, Majchrowicz E, Wixon HN, & Weight FF (1986) Cerebral 2-deoxyglucose uptake in rats during ethanol withdrawal and post-withdrawal. Brain Research, 366, 1–9. [DOI] [PubMed] [Google Scholar]
- Evans MS, Li Y, & Faingold CL (2000) Inferior colliculus intracellular response abnormalities in vitro associated with susceptibility to ethanol withdrawal seizures. Alcoholism: Clinical and Experimental Research, 24, 1180–1186. [PubMed] [Google Scholar]
- Faingold CL (1999). Neuronal networks in the genetically epilepsy-prone rat. Advances in Neurology, 79, 311–321. [PubMed] [Google Scholar]
- Faingold CL (2008) The Majchrowicz binge alcohol protocol: an intubation technique to study alcohol dependence in rats. Current Protocols in Neuroscience.Jul; Chapter 9: Unit 9.28. [DOI] [PubMed] [Google Scholar]
- Faingold CL, & Riaz A (1995) Ethanol withdrawal induces increased firing in the inferior colliculus neurons associated with audiogenic seizure susceptibility. Experimental Neurology, 132, 91–98. [DOI] [PubMed] [Google Scholar]
- Faingold CL, N’Gouemo P, & Riaz A (1998) Ethanol and neurotransmitter interaction-from molecular to integration effects. Progress in Neurobiology, 55, 509–535. [DOI] [PubMed] [Google Scholar]
- Faingold CL, Li Y, Evans MS (2000) Decreased GABA and increase glutamate-mediated activity on inferior colliculus neurons in vitro are associated with susceptibility to ethanol withdrawal seizures. Brain Research, 868, 287–295. [DOI] [PubMed] [Google Scholar]
- Felix R, Gurnett CA, De Waard M, & Campbell KP (1997). Dissection of functional domains of the voltage-dependent Ca2+ channel α2δ subunit. Journal of Neuroscience, 17, 6884–6891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frye GD, McCown TJ, Breese GR (1983) Characterization of susceptibility to audiogenic seizures in ethanol-dependent rats after microinjection of gamma-aminobutyric acid (GABA) agonists into the inferior colliculus, substantia nigra or medial septum. Journal of Pharmacology and Experimental Therapeutics, 227, 663–670. [PMC free article] [PubMed] [Google Scholar]
- Hendriksen H, Kamphuis W, & Lopes da Silva FH (1997). Changes in voltage-dependent calcium channel alpha1-subunit mRNA levels in the kindling model of epileptogenesis. Brain Research. Molecular Brain Research, 50, 257–266. [DOI] [PubMed] [Google Scholar]
- Hillborm M, Pieninkeroinen I, & Leone M (2003). Seizures in alcohol-dependent patients: epidemiology, pathophysiology and management. CNS Drugs, 17, 1013–1030. [DOI] [PubMed] [Google Scholar]
- Jobe PC, Mishra PK, & Dailey JW (1992). Genetically epilepsy-prone rats—Actions of antiepileptic drugs and monoaminergic neurotransmitters In Faingold CL & Fromm GH (Eds.), Drugs for control of epilepsy— Actions on neuronal networks involved in seizure disorders (pp. 253–276). New York: CRC Press. [Google Scholar]
- Kang TC, Kim DS, Yoo KY, Hwang IK, Kwak SE, Kim JE, Jung JY, Won MH, Suh JG, & Oh YS (2004) Elevated voltage-gated Ca2+ channel immunoreactivities in the hippocampus of seizure-prone gerbil. Brain Research, 1029, 168–178. [DOI] [PubMed] [Google Scholar]
- Kanyshkova T, Ehling P, Cerina M, Meuth P, Zobeiri M, Meuth SG, Pape HC, & Budde T (2014). Regionally specific expression of high-voltage-activated calcium channels in thalamic nuclei of epileptic and non-epileptic rats. Molecular and Cellular Neurosciences, 61, 110–122. [DOI] [PubMed] [Google Scholar]
- Katsura M, Shibasaki M, Hayashida S, Torigoe F, Tsujimura A, & Ohkuma S (2006). Increase in expression of alpha1 and alpha2/delta1 subunits of L-type high voltage-gated calcium channels after sustained ethanol exposure in cerebral cortical neurons. Journal of Pharmacological Sciences, 102, 221–230. [DOI] [PubMed] [Google Scholar]
- Klugbauer N, Lacinová L, Marais E, Hobom M, & Hofmann F (1999). Molecular diversity of the calcium channel α2δ subunit. Journal of Neuroscience, 19, 684–691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Livak KJ, & Schmittgen TD (2001). Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) method. Methods, 25, 402–408. [DOI] [PubMed] [Google Scholar]
- Majchrowicz E (1975). Induction of physical dependence upon ethanol and the associated behavioral changes in rats. Psychopharmacologia, 43, 245–254. [DOI] [PubMed] [Google Scholar]
- McCown TJ, & Breese GR (1990). Multiple withdrawals from chronic ethanol “kindles” inferior collicular seizure activity: evidence for kindling seizures associated with alcoholism. Alcoholism: Clinical and Experimental Research, 14, 394–399. [DOI] [PubMed] [Google Scholar]
- McCown TJ, & Breese GR (1993). A potential contribution to ethanol withdrawal kindling: reduced GABA function in inferior collicular cortex. Alcoholism: Clinical and Experimental Research, 17, 1290–1294. [DOI] [PubMed] [Google Scholar]
- National Research Council (U.S.). (2011) Guide for the care and use of laboratory animals Institute for Laboratory Animal Research 8th ed.). Washington, D.C: National Academies Press. [Google Scholar]
- N’Gouemo P (2015). Altered voltage-gated calcium channels in the rat inferior colliculus neurons contribute to alcohol withdrawal seizures. European Journal of Neuropsychopharmacology, 25, 1342–1352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- N’Gouemo P, Caspary DM, & Faingold CL (1996). Decreased GABA effectiveness in the inferior colliculus neurons during ethanol withdrawal in rat susceptible to audiogenic seizures. Brain Research, 724, 200–204. [DOI] [PubMed] [Google Scholar]
- N’Gouemo P, & Morad M (2003) Ethanol withdrawal seizure susceptibility is associated with upregulation of L- and P-type Ca2+ channels currents in rat inferior colliculus neurons. Neuropharmacology, 45, 429–437. [DOI] [PubMed] [Google Scholar]
- N’Gouemo P, Yasuda RP, & Morad M (2006). Ethanol withdrawal is accompanied by downregulation of calcium channel alpha 1B subunit in rat inferior colliculus neurons. Brain Research, 1108, 216–220. [DOI] [PubMed] [Google Scholar]
- N’Gouemo P, Yasuda R, & Faingold CL (2010). Seizure susceptibility is associated with altered protein expression of voltage-gated calcium channel subunits in inferior colliculus neurons of the genetically epilepsy-prone rat. Brain Research, 1308, 153–157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- N’Gouemo P, & Morad M (2014). Alcohol withdrawal is associated with a downregulation of large-conductance Ca2+-activated K+ channels in rat inferior colliculus neurons. Psychopharmacology, 231, 2009–2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park SK, Hwang IK, An SJ, Won MH, & Kang TC (2003). Elevated P/Q-type (a1A) and L2 type (a1D) Purkinje cell voltage-gated calcium channels in the cerebella of seizure prone gerbils. Molecules and Cells, 16, 297–301. [PubMed] [Google Scholar]
- Pilke A, Partinen M, & Kovanen J (1984). Status epilepsticus and alcohol abuse: an analysis of 82 status epilepsticus admission. Acta Neurological Scandinavica, 70, 443–450. [DOI] [PubMed] [Google Scholar]
- Riaz A, & Faingold CL (1994). Seizures during ethanol withdrawal are blocked by focal microinjection of excitant amino acid antagonists in the inferior colliculus and pontine reticular formation. Alcoholism: Clinical and Experimental Research 18, 1456–1462. [DOI] [PubMed] [Google Scholar]
- Shistik E, Ivanina T, Puri T, Hosey M, Dascal N (1995). Ca2+ current enhancement by α2/δ and β subunits in Xenopus oocytes: contribution of changes in channel gating and α1 protein level. Journal of Physiology, 489, 55–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ueda Y, Tusru N (1995) Simultaneous monitoring of the seizure related change in extracellular glutamate and gamma-aminobutyric acid concentration in bilateral hippocampi following development of amygdaloid kindling. Epilepsy Research, 20, 213–219. [DOI] [PubMed] [Google Scholar]
- Vignes S, Gastaldi M, Chabret C, Massacrier A, Cau P, & Valmier J (1999). Regulation of calcium channel α1A subunit splice variant mRNA in kainate-induced temporal lobe epilepsy. Neurobiology of Disease, 6, 288–301. [DOI] [PubMed] [Google Scholar]
- Westenbroek RE, Sakurai T, Elliott EM, Hell JW, Starr TV, Snutch TP, & Catterall WA (1995). Immunochemical identification and subcellular distribution of the alpha 1A subunits of brain calcium channels. Journal of Neuroscience, 15, 6403–6418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westenbroek RE, Bausch SB, Lin RC, Franck JE, Noebels JL, & Catterall WA (1998). Upregulation of L-type Ca2+ channels in reactive astrocytes after brain injury, hypomyelination, and ischemia. Journal of Neuroscience, 18, 2321–2334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whittington MA, Lambert JD, Little HJ (1993). Increases in synaptic activation of calcium current as a mechanism for generation of alcohol withdrawal seizures. Alcohol Alcohol Supplement, 2, 391–394. [PubMed] [Google Scholar]