Abstract
Background
Epiaortic ultrasound detects and localizes ascending aortic atherosclerosis. In this analysis we investigated the association between epiaortic ultrasound-based atheroma grade during surgical aortic valve replacement (SAVR) and perioperative adverse outcomes.
Methods
SAVR patients in a randomized trial of two embolic protection devices underwent a protocol-defined 5-view epiaortic ultrasound read at a core-laboratory. Aortic atherosclerosis was quantified with Katz atheroma grade and patients were categorized into mild (grade I-II) versus moderate/severe (grade III-V). Multivariable logistic regression was used to estimate associations between atheroma grade and adverse outcomes including death, clinically apparent stroke, cerebral infarction on diffusion-weighted magnetic resonance imaging (DW-MRI), delirium, and acute kidney injury (AKI) by 7 and 30 days.
Results
Of the 383 randomized patients, 326 (85.1%) had pre-cannulation epiaortic ultrasound data available. Of these, 106 (32.5%) had moderate/severe Katz atheroma grade at any segment of the ascending aorta. While there were no significant differences in the composite of death, stroke or cerebral infarction on DW-MRI by 7 days, moderate/severe atheroma grade was associated with a greater risk of AKI by 7 days (adjusted odds ratio [OR]: 2.63; 95% confidence interval [CI]: 1.24–5.58; p=0.01). At 30 days, patients with moderate/severe atheroma grade had a greater risk of death, stroke or AKI (adjusted OR: 1.97; 95%CI: 1.04–3.71; p=0.04).
Conclusions
Moderate/severe aortic atherosclerosis was associated with an increased risk of adverse events following SAVR. Epiaortic ultrasound may serve as a useful adjunct for identifying patients who may benefit from strategies to reduce atheroembolic complications during SAVR.
Although there have been significant improvements in outcomes after surgical aortic valve replacement (SAVR), stroke and other end-organ damage from atheroemboli remain a relevant and often morbid perioperative complication(1–5). Proximal aortic atherosclerosis is the most important risk factor for stroke following cardiac surgery(6–8). Transesophageal echocardiography (TEE), digital palpation of the ascending aorta, and epiaortic ultrasound have been utilized to detect ascending aortic atherosclerosis(9–11). However, no pre or intraoperative imaging technique has been shown to consistently predict subsequent embolic complications. Epiaortic ultrasound can detect and localize ascending aortic atherosclerosis, but even with more than 15 years of clinical use and research, its predictive utility remains uncertain(12–13).
Palpation alone underestimates the degree of aortic atherosclerosis as compared to TEE and epiaortic ultrasound (10,12,14). Palpation may underestimate noncalcified intimal thickening which is associated with intracranial lesions detected on MRI(15–16). Epiaortic ultrasound identifies more ascending aortic atherosclerosis than palpation or TEE alone(12). Transesophageal echo has a “blind spot” in the distal ascending aorta and proximal arch because of drop out from the right mainstem bronchus(10). Reported associations between epiaortic ultrasound-defined ascending aortic atherosclerosis and postoperative stroke exist, but these are largely retrospective analyses with no independent review of epiaortic ultrasound data(9,17–20). Although epiaortic ultrasound is the optimal imaging modality, few large-scale, prospective studies have demonstrated better outcomes with its routine use(13). Epiaortic ultrasound is currently recommended for patients undergoing cardiac surgical procedures felt to be at increased risk for embolic stroke(21).
This analysis of data from a previously reported randomized trial, comparing two different embolic protection devices to standard aortic cannula in SAVR patients, investigated the association between epiaortic ultrasound-based atheroma grade during SAVR and perioperative adverse outcomes(2).
PATIENTS AND METHODS
Data source
The patients for this secondary analysis were enrollees in a randomized trial of neuroprotection in SAVR recipients(2). From March 2015 to July 2016, 383 patients were randomized 1:1:1 to receive a standard aortic cannula, a cannula with intra-aortic filtration, or a cannula with suction-based extraction. The trial was conducted by the Cardiothoracic Surgical Trials Network (CTSN) in 18 North American Centers with a data coordinating center, an independent adjudication committee, and a data and safety monitoring board that oversaw trial progress. The institutional review board at the participating centers approved the protocol, and all patients signed a written informed consent. The inclusion and exclusion criteria for the trial have been published previously(2).
Epiaortic ultrasound
Patients underwent epiaortic ultrasound examination prior to aortic cannulation and after decannulation. A protocol-defined 5-view evaluation of the ascending aorta from the sinotubular junction to the origin of the innominate artery and the aortic arch were required. First, a long axis (LAX) view of the ascending aorta including visualization of the proximal, mid, and distal segments was acquired. Next, a short axis (SAX) view of ascending aorta was obtained by positioning the ultrasound probe on the ascending aorta as proximally as possible, with the orientation marker directed toward the patient’s left shoulder. Imaging plane was perpendicular to the aorta with the aorta centered within the imaging plane. In this view, the three views collected were proximal-level, mid-level, and distal-level. Lastly, a LAX view of proximal aortic arch was performed including a view of the origin of the innominate artery and proximal aortic arch. With digital archiving, at least 3 – 5 cardiac cycles video loops were required for each view.
Measurement of atheroma burden
All epiaortic ultrasound data were transmitted to an echocardiographic core laboratory for review. Assessment of atheroma for each of the three ascending aortic SAX segments and the aortic arch was performed utilizing the Katz’s atheroma grade as described by the American Society of Echocardiography (ASE): Katz I: normal to mild intimal thickening; Katz II: severe intimal thickening without protruding atheroma; Katz III: atheroma protruding < 5 mm into the aorta lumen; Katz IV: atheroma protruding ≥ 5 mm into the aorta lumen; Katz V: any thickness with mobile component(21). In this analysis, the maximum atheroma grade across the segments of the aorta was categorized into mild (grade I-II) versus moderate/severe (grade III-V).
Study endpoints
The primary aim of this study was to assess the association between epiaortic-based Katz atheroma grade and the risk of adverse perioperative outcomes after SAVR. Specific adverse events assessed at 7 days included any clinically apparent stroke, delirium, any acute kidney injury (AKI), and a composite outcome that included death, clinically apparent stroke, or cerebral infarct on diffusion-weighted magnetic resonance imaging (DW-MRI). At 30 days, a composite of death, clinically apparent stroke, or AKI, and any AKI were evaluated. Clinically apparent stroke was ascertained through serial National Institutes of Health Stroke Scale (NIHSS) assessments at days 1, 3 and 7 or through adverse event reporting; AKI was defined according to the AKI Network criteria; and delirium defined according to the Confusion Assessment Method (CAM) [for intubated (CAM-ICU) and non-intubated patients (3D-CAM)]. Delirium was assessed up to 7 days post-operatively (22).
Statistical analysis
Baseline and operative characteristics were compared between Katz atheroma groups using T-tests or Wilcoxon tests for continuous measures and χ2 or Fisher exact tests for categorical measures as appropriate. Univariable and multivariable logistic regression analyses were used to assess the association of aortic atherosclerosis grade with perioperative adverse events. Variables entered in the model were prespecified and perceived as having clinical relevance, including age, sex, history of peripheral vascular disease, diabetes, history of atrial fibrillation, cardiopulmonary bypass time, concomitant coronary artery bypass grafting, use of an embolic protection device, and log ratio of white matter to intracranial volume. Overall model fit was assessed using Pearson’s Chi-square and Hosmer-Lemeshow goodness-of-fit tests. Each outcome model demonstrated no evidence of lack of fit. Results were reported as odds ratios (ORs) with 95% confidence intervals (CI). A value of P < 0.05 was considered statistically significant. All analyses were performed using SAS version 9.4 (SAS Institute Inc, Cary, NC).
RESULTS
Patient characteristics
Of the 383 patients randomized in the neuroprotection trial, 326 (85.1%) are included in the current analysis. One patient was excluded due to thrombus and 56 others were excluded because of missing pre-cannulation epiaortic ultrasound data (data on missing patients are included in Appendix I and II). For scans with image quality issues as reported by the core laboratory, there was no pattern of unreadability by site. Baseline patient characteristics as well as operative procedural data are shown in Table 1. In the study cohort, 67.5% (n=220) of patients had mild Katz atheroma grade whereas 32.5% (n=106) had moderate/severe grades. Between Katz atheroma groups, patient demographics were similar, however, patients with moderate/severe grade were significantly older than patients with mild KAG (75.1±6.2 versus 73.4±6.7, p=0.03) and had a significantly higher frequency of peripheral vascular disease (24.5% versus 8.2%; p<0.001). Patients with moderate/severe grade had a significantly longer mean operative time among (336.3±100.9 versus 311.8±85.4 minutes, p=0.03). There was no significant difference in the frequency of carotid stenosis or the distribution of embolic protection device between groups. Further, among those who were randomized to an embolic protection device, the presence and volume of debris did not differ between Katz groups.
Table 1:
Baseline Patient and Operative Characteristics
| Katz Atheroma Grade | |||
|---|---|---|---|
| Mild (I or II) (N=220) | Moderate or Severe (III-V) (N=106) | p-value | |
| Age | 73.4±6.7 | 75.1±6.2 | 0.03 |
| BMI | 29.5±5.9 | 30.2±6.1 | 0.28 |
| Male | 135(61.4) | 67(63.2) | 0.75 |
| White Race | 207(94.1) | 96(90.6) | 0.35a |
| Medical History | |||
| Severe Carotid Artery Disease(>80% stenosis) | 7(3.2) | 2(1.9) | 0.72 |
| Peripheral Vascular Disease | 18(8.2) | 26(24.5) | <0.001 |
| Hypertension | 179(81.4) | 89(84.0) | 0.57 |
| Diabetes | 62(28.2) | 39(36.8) | 0.12 |
| History of AF | 22(10.0) | 16(15.1) | 0.18 |
| History of Stroke or TIA | 19(8.6) | 12(11.3) | 0.44 |
| Chronic Renal Insufficiency | 26 (11.8) | 16 (15.1) | 0.41 |
| Creatinine (mg/dl) | 1.0 (0.8,1.1) | 1.0 (0.8,1.2) | 0.87 |
| White Matter Lesion Volume(mm3) | 5433 (2679,8870) | 4782 (2258,10242) | 0.66 |
| White Matter Lesion Volume/Intracranial Volume | 0.005 (0.002,0.008) | 0.004 (0.002,0.009) | 0.67 |
| Operative Characteristics | |||
| Use of Embolic Protection Filter Deviceb | 138(62.7) | 73(68.9) | 0.28 |
| Largest particle size(mm)c | 0.7 (0.3,1.2) | 0.8 (0.4,1.1) | 0.76 |
| Presence of debrisc | 116(87.9) | 63(87.5) | 0.94 |
| Concomitant CABG | 85(38.6) | 47(44.3) | 0.33 |
| Operation Time(min) | 311.8±85.4 | 336.3±100.9 | 0.03 |
| CPB Time(min) | 104.2±41 | 108.5±42.6 | 0.39 |
Results are reported as n(%), mean±SD, or median (IQR).
P-value for race is computed excluding those who are unknown
In this patient sample, 4 patients who were randomized to a device received the standard cannula. The numbers provided are based on the device used rather than randomization assignment.
Among patients who received an embolic protection filter device.
AF: atrial fibrillation; BMI: body mass index; CABG: coronary artery bypass graft; CPB: cardiopulmonary bypass; TIA: transient ischemic attack
Distribution of aortic atherosclerosis
The distribution of Katz atheroma grade by segments of the aorta is presented in Table 2. Less than 2% of patients were noted by epiaortic ultrasound at either extreme of the Katz atheroma grade (I or V). Within all segments of the aorta that were analyzed, the majority of patients were either grade II or III.
Table 2:
Katz Atheroma Grade by Segment of Aorta
| Katz Atheroma Grade | All | |||||
|---|---|---|---|---|---|---|
| I | II | III | IV | V | ||
| Aortic Arch | 5(1.7) | 233(79) | 47(15.9) | 10(3.4) | 0 | 295 |
| Ascending Aorta Proximal-Level | 8(2.5) | 273(85) | 33(10.3) | 5(1.6) | 2(0.6) | 321 |
| Ascending Aorta Mid-Level | 7(2.2) | 288(88.9) | 24(7.4) | 5(1.5) | 0 | 324 |
| Ascending Aorta Distal-Level | 6(1.9) | 266(83.4) | 37(11.6) | 9(2.8) | 1(0.3) | 319 |
| Maximum Gradea | 3(0.9) | 217(66.6) | 83(25.5) | 20(6.1) | 3(0.9) | 326 |
Results are reported as n(%) and not all segments were readable by the core laboratory. Maximum grade is defined as an individual’s highest KAG across the segments of the aorta.
Clinical outcomes and adverse events
Clinical outcomes by maximum pre-cannulation Katz atheroma grade are shown in Table 3. Overall, in unadjusted analyses, there was no significant difference between groups in the frequency of death within 7 days (p=0.54) as well as the frequency of clinically apparent stroke (p=0.38) or radiographic infarcts on DW-MRI (p=0.17). There was also no significant difference between groups in the frequency of delirium within 7 days (p=0.52). However, there was a significantly higher risk of AKI by 7 days among patients with moderate/severe atheroma grade (26.7% versus 11.9%,p=0.001). At 30 days, patients with moderate/severe grade also had a higher frequency of the composite endpoint of clinically apparent stroke, death, or AKI (38.1% versus 20.2%,p=0.001) as well as an overall higher risk of AKI (31.4% versus 14.7%,p<0.001).
Table 3:
Clinical Outcomes by Maximum Pre-cannulation Katz Atheroma Grade
| Katz Atheroma Grade | |||
|---|---|---|---|
| Mild (I or II) (N=220) | Moderate or Severe (III-V) (N=106) | p-value | |
| Composite Primary Endpoint in First 7 Days | 140/195 (71.8) | 63/95 (66.3) | 0.34 |
| Death < 7 daysa | 1/219 (0.5) | 1/105 (1.0) | 0.54 |
| Clinically apparent stroke < 7 daysa’b | 13/219 (5.9) | 9/105 (8.6) | 0.38 |
| Radiographic Infarcts on DW-MRI | 138/193 (71.5) | 59/93 (63.4) | 0.17 |
| Count and Volume of Lesions on DW-MRI | |||
| T otal Count | 1 (0,3) | 1 (0,2) | 0.14 |
| Total Volume(mm3) | 55.7 (0,253.9) | 42 (0,129.7) | 0.09 |
| Largest Lesion Volume(mm3) | 35 (0,141.8) | 31.5 (0,76.3) | 0.14 |
| Delirium | |||
| Day 1 | 36/208 (17.3) | 20/90 (22.2) | 0.32 |
| Day 3 | 28/204 (13.7) | 15/95 (15.8) | 0.64 |
| Day 7 | 21/208 (10.1) | 9/97 (9.3) | 0.82 |
| Within 7 daysc | 59/213 (27.7) | 30/96 (31.3) | 0.52 |
| AKI Adverse Events by Day 7 | |||
| Stage 1 | 21/219 (9.6) | 20/105 (19) | 0.02 |
| Stage 2 | 2/219 (0.9) | 3/105 (2.9) | 0.33 |
| Stage 3 | 3/219 (1.4) | 5/105 (4.8) | 0.12 |
| Overall | 26/219 (11.9) | 28/105 (26.7) | 0.001 |
| Composite Endpoint in 30 Daysa | |||
| Composite | 44/218 (20.2) | 40/105 (38.1) | 0.001 |
| Clinically Apparent Stroke | 13/218 (6) | 9/105 (8.6) | 0.38 |
| Death | 6/218 (2.8) | 2/105 (1.9) | >0.99 |
| AKI (Stage 1–3, Serious & Non-serious) | 32/218 (14.7) | 33/105 (31.4) | <0.001 |
Results are reported as no. of patients/no. observed(%) or median(IQR).
2 patients withdrew from the trial prior to 7 days and are not included in the denominators; 1 additional patient withdrew prior to 30 days
Clinically apparent stroke was ascertained through the reporting of an adverse event by 7 days or through adjudication by the neurological EAC of an NIHSS >2 on day 1, 3 or 7.
Evidence of delirium during hospitalization is defined as those who had any indication of delirium at visits on day 1, 3, or 7. Patients who scored positive for delirium at any visit (1, 3, or 7) are counted as having evidence of delirium. In order to be counted as having no evidence of delirium, a patient had to have at least 2 non-missing CAM assessment that indicated no delirium.
AKI: acute kidney index; CAM: Confusion Assessment Method; DW-MRI: diffusion weighted magnetic resonance imaging; EAC: event adjudication committee; NIHSS: National Institutes of Health Stroke Scale.
Association of Katz atheroma grade and perioperative adverse events
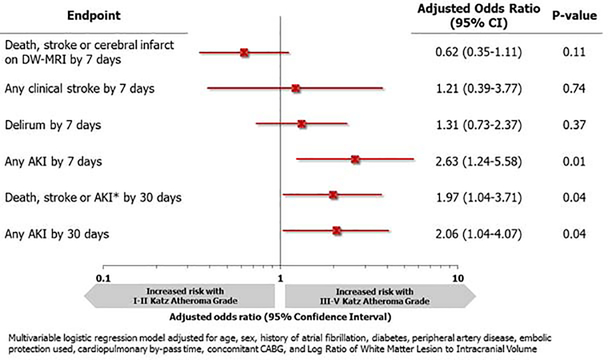
Figure 1 depicts the association between atheroma grade and perioperative outcomes. Patients with moderate/severe Katz atheroma grade had higher risk of any AKI by 7 days (OR:2.63, 95% CI: 1.24–5.58,p=0.01), AKI by 30 days (OR:2.06, 95% CI:1.04–4.07,p=0.04), and the composite of death, stroke, or AKI at 30 days (OR:1.97, 95% CI:1.04–3.71,p=0.04). Additional adjustments with creatinine and renal insufficiency were explored and did not alter our results for the impact of atheroma grade on AKI by 7 days or by 30 days (Appendix III). The odds of a patient with clinically apparent stroke at 7 days was 1.21 (95% CI 0.39–3.77) times higher for those with moderate/severe atheroma grade compared to mild; however, this was not statistically significant (p=0.74).
Figure 1.
Adjusted odds ratio for adverse events by 7 and 30 days after surgical aortic valve replacement.
COMMENT
In this analysis we observed that the degree of ascending aortic atherosclerotic burden as measured through Katz atheroma grade was associated with an increased risk of AKI as well as the composite of death, stroke, or AKI by 30 days. Our analysis focused on a secondary endpoint of a randomized trial of embolic protection after SAVR, which found no significant difference between 2 embolic protection devices and standard cannula in radiographic and clinical brain infarcts at 7 days, although there was a significant difference in delirium(2) with one of the embolic protection devices. All patients underwent intra-operative epiaortic ultrasound in five pre-defined views that were recorded and transmitted to an independent core laboratory. The majority of patients in this sub-study had mild atherosclerotic disease as measured by a Katz atheroma score of II throughout all segments of the ascending aorta and arch. Overall, 66.6% of patients were grade II and 25.5% were grade III.
The distribution of atheroma grade observed in our analysis is consistent with a larger series by Butler and colleagues who examined 22,304 consecutive epiaortic ultrasounds from cardiac operations and found 85% of patients to have mild atherosclerotic disease and 15% of patients to have moderate/severe disease (22). In our study, we observed a higher frequency of moderate/severe atherosclerotic disease of 32.5%, which is likely driven by our study population of only SAVR patients. Given that nearly one third of patients presenting for surgical AVR had moderate to severe atherosclerotic disease highlights the necessity of further quantifying how atheroma grade correlates with perioperative outcomes.
Currently, ASE recommends epiaortic imaging primarily for patients with an increased risk for embolic stroke(21). Such high-risk patients are defined as those with a history of cerebrovascular or peripheral vascular disease and patients who have evidence of aortic atherosclerosis or calcification by other imaging modalities. In our analysis, patients with peripheral vascular disease had a higher risk of moderate/severe atherosclerotic disease onepiaortic ultrasound, and there was a trend toward a higher burden of atherosclerotic disease among patients with diabetes and a history of atrial fibrillation.
While no prospective, randomized trials have been carried out to assess the relationship between routine epiaortic ultrasound and perioperative adverse events, several authors have retrospectively examined the association between aortic atherosclerosis and adverse events. Van der Linden and colleagues studied data from epiaortic images on 921 consecutive patients undergoing cardiac surgery(9). The overall incidence of atherosclerotic disease in the cohort was 26.2% and the incidence of stroke among patients with atherosclerosis of the ascending aorta was 8.7%. Specifically, the authors found that patients with atherosclerotic disease occupying more than 50% of the ascending aorta had a 33.3% incidence of postoperative stroke. Goto and colleagues also examined the association between aortic atherosclerosis, as assessed by epiaortic ultrasound, and neurological dysfunction(17). The authors found that patients with moderate atherosclerosis had 7% risk of neuropsychological dysfunction and 1.8% risk routine epiaortic ultrasound and perioperative adverse events, several authors have retrospectively examined the association between aortic atherosclerosis and adverse events. Van der Linden and colleagues studied data from epiaortic images on 921 consecutive patients undergoing cardiac surgery(9). The overall incidence of atherosclerotic disease in the cohort was 26.2% and the incidence of stroke among patients with atherosclerosis of the ascending aorta was 8.7%. Specifically, the authors found that patients with atherosclerotic disease occupying more than 50% of the ascending aorta had a 33.3% incidence of postoperative stroke. Goto and colleagues also examined the association between aortic atherosclerosis, as assessed by epiaortic ultrasound, and neurological dysfunction(17). The authors found that patients with moderate atherosclerosis had 7% risk of neuropsychological dysfunction and 1.8% risk of intraoperative stroke, whereas those with severe atherosclerosis had a 26% risk of neuropsychological dysfunction and a 10.5% risk of stroke. In our analysis, the rate of clinically apparent stroke within 7 days was 6.8%. Among patients with mild Katz atheroma grade, the risk of stroke was 5.9% whereas those with moderate/severe had a risk of 8.6%, nearly 50% higher. This difference was not significantly different as we were underpowered to detect a difference in stroke rates due to low number of events. Also, although not statistically significant, in adjusted analyses there was a 31% increase in risk of delirium among patients with moderate/severe Katz atheroma grade.
At 30 days, we did observe that among patients with moderate/severe aortic atherosclerosis, there was almost 2 times higher odds of the composite endpoint of death, stroke, or AKI. This finding highlights the potential long-term consequences of significant atheroma grade in the proximal aorta. As there was no significant difference in mortality between groups at 30 days, the composite endpoint was driven largely by a 15% higher risk of AKI among those with moderate/severe atheroma grade. The increased risk of AKI among patients with significant atherosclerosis was also observed within 7 days, and limited primarily to stage I AKI. Dávila-Román and colleagues examined the influence of aortic atherosclerosis assessed by epiaortic ultrasound on renal dysfunction among 978 consecutive cardiac surgery patients(23). The authors concluded that in multivariable analysis, ascending aortic atherosclerosis was a significant, independent predictor of postoperative renal dysfunction from preoperative to postoperative day 1 (OR:3.06, 95%CI 1.06–8.85) and from preoperative to postoperative day 6 (OR:2.13, 95%CI 1.40–3.26). The proposed mechanism of renal dysfunction is likely related to athero-embolism to the kidneys during cardiopulmonary bypass, and because proximal aortic atherosclerosis may be a marker of more distal, visceral atherosclerotic disease.
Study Limitations
There are several limitations to this analysis. First, 15% of patients from the original neuroprotection trial had missing pre-cannulation epiaortic ultrasound data. Second, data was not collected on whether findings from epiaortic ultrasound altered the operative cannulation strategy if, upon epiaortic ultrasound examination, the aorta appeared hostile to traditional cannulation at the time of surgery. Third, two neuroprotection devices were used in the trial in addition to standard cannulas raising the possibility for differences in outcomes based on cannula use. Importantly, however, the frequency of neuroprotection cannula devices was similar between mild and moderate/severe Katz atheroma groups. Fourth, while we include serum creatinine and diabetes in our models, AKI is multifactorial and the relationship between atheroma burden and AKI requires further investigation. Lastly, variability may exist in the acquisition of the standardized 5-view of the ascending aorta, however, all images were transferred and independently reviewed at a core laboratory in this trial.
Conclusions
Moderate to severe aortic atherosclerotic burden, as measured through epiaortic ultrasound, was associated with an increased risk of adverse events following SAVR. While there was no significant association with clinically apparent stroke at 7 days, there was a significant association with AKI and the composite endpoint of death, stroke, and AKI. Intraoperative epiaotic ultrasound may serve as a useful adjunct for identifying patients who may benefit from strategies to reduce atheroembolic complications during SAVR.
Supplementary Material
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Thiagarajan K, Jeevanantham V, Van Ham R, et al. Perioperative Stroke and Mortality After Surgical Aortic Valve Replacement: A Meta-Analysis. Neurologist. 2017;22(6):227–233. [DOI] [PubMed] [Google Scholar]
- 2.Mack MJ, Acker MA, Gelijns AC, et al. Effect of Cerebral Embolic Protection Devices on CNS Infarction in Surgical Aortic Valve Replacement: A Randomized Clinical Trial. JAMA. 2017;318(6):536–547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Udesh R, Mehta A, Gleason T, Thirumala PD. Carotid artery disease and perioperative stroke risk after surgical aortic valve replacement: A nationwide inpatient sample analysis. J Clin Neurosci. 2017;42:91–96. [DOI] [PubMed] [Google Scholar]
- 4.Agarwal S, Garg A, Parashar A, et al. In-hospital mortality and stroke after surgical aortic valve replacement: A nationwide perspective. J Thorac Cardiovasc Surg. 2015;150(3):571–8.e8. [DOI] [PubMed] [Google Scholar]
- 5.Idrees JJ, Schiltz NK, Johnston DR, et al. Trends, Predictors, and Outcomes of Stroke After Surgical Aortic Valve Replacement in the United States. Ann Thorac Surg. 2016;101(3):927–35. [DOI] [PubMed] [Google Scholar]
- 6.Roach GW, Kanchuger M, Mangano CM, et al. Adverse cerebral outcomes after coronary bypass surgery. Multicenter Study of Perioperative Ischemia Research Group and the Ischemia Research and Education Foundation Investigators. N Engl J Med. 1996;335(25):1857–63. [DOI] [PubMed] [Google Scholar]
- 7.van der Linden J, Bergman P, Hadjinikolaou L. The topography of aortic atherosclerosis enhances its precision as a predictor of stroke. Ann Thorac Surg. 2007;83(6):2087–92. [DOI] [PubMed] [Google Scholar]
- 8.Bar-Yosef S, Anders M, Mackensen GB, et al. Aortic atheroma burden and cognitive dysfunction after coronary artery bypass graft surgery. Ann Thorac Surg. 2004;78(5):1556–62. [DOI] [PubMed] [Google Scholar]
- 9.van der Linden J, Hadjinikolaou L, Bergman P, Lindblom D. Postoperative stroke in cardiac surgery is related to the location and extent of atherosclerotic disease in the ascending aorta. J Am Coll Cardiol. 2001;38(1):131–5. [DOI] [PubMed] [Google Scholar]
- 10.Royse AG, Royse CF. Epiaortic ultrasound assessment of the aorta in cardiac surgery. Best Pract Res Clin Anaesthesiol. 2009;23(3):335–41. [DOI] [PubMed] [Google Scholar]
- 11.Yamaguchi A, Adachi H, Tanaka M, Ino T. Efficacy of intraoperative epiaortic ultrasound scanning for preventing stroke after coronary artery bypass surgery. Ann Thorac Cardiovasc Surg. 2009;15(2):98–104. [PubMed] [Google Scholar]
- 12.Das S, Dunning J. Can epiaortic ultrasound reduce the incidence of intraoperative stroke during cardiac surgery? Interact Cardiovasc Thorac Surg. 2004;3(1):71–5. [DOI] [PubMed] [Google Scholar]
- 13.Ostrowski JW, Kanchuger MS. Con: epiaortic scanning is not routinely necessary for cardiac surgery. J Cardiothorac Vasc Anesth. 2000;14(1):91–4. [DOI] [PubMed] [Google Scholar]
- 14.Royse C, Royse A, Blake D, Grigg L. Screening the thoracic aorta for atheroma: a comparison of manual palpation, transesophageal and epiaortic ultrasonography. Ann Thorac Cardiovasc Surg. 1998;4(6):347–50. [PubMed] [Google Scholar]
- 15.Djaiani G, Fedorko L, Borger M, et al. Mild to moderate atheromatous disease of the thoracic aorta and new ischemic brain lesions after conventional coronary artery bypass graft surgery. Stroke. 2004;35(9):e356–8. [DOI] [PubMed] [Google Scholar]
- 16.Dávila-Román VG, Phillips KJ, Daily BB, Dávila RM, Kouchoukos NT, Barzilai B. Intraoperative transesophageal echocardiography and epiaortic ultrasound for assessment of atherosclerosis of the thoracic aorta. J Am Coll Cardiol. 1996;28(4):942–7. [DOI] [PubMed] [Google Scholar]
- 17.Goto T, Baba T, Matsuyama K, Honma K, Ura M, Koshiji T. Aortic atherosclerosis and postoperative neurological dysfunction in elderly coronary surgical patients. Ann Thorac Surg. 2003;75(6):1912–8. [DOI] [PubMed] [Google Scholar]
- 18.Rosenberger P, Shernan SK, Löffler M, et al. The influence of epiaortic ultrasonography on intraoperative surgical management in 6051 cardiac surgical patients. Ann Thorac Surg. 2008;85(2):548–53. [DOI] [PubMed] [Google Scholar]
- 19.Ikram A, Mohiuddin H, Zia A, et al. Does epiaortic ultrasound screening reduce perioperative stroke in patients undergoing coronary surgery? A topical review. J Clin Neurosci. 2018;50:30–34. [DOI] [PubMed] [Google Scholar]
- 20.Zingone B, Rauber E, Gatti G, et al. The impact of epiaortic ultrasonographic scanning on the risk of perioperative stroke. Eur J Cardiothorac Surg. 2006;29(5):720–8. [DOI] [PubMed] [Google Scholar]
- 21.Glas KE, Swaminathan M, Reeves ST, et al. Guidelines for the performance of a comprehensive intraoperative epiaortic ultrasonographic examination: recommendations of the American Society of Echocardiography and the Society of Cardiovascular Anesthesiologists; endorsed by the Society of Thoracic Surgeons. J Am Soc Echocardiogr. 2007;20(11):1227–35. [DOI] [PubMed] [Google Scholar]
- 22.Butler CG, Ho Luxford JM, Huang CC, et al. Aortic Atheroma Increases the Risk of Long-Term Mortality in 20,000 Patients. Ann Thorac Surg. 2017;104(4):1325–1331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dávila-Román VG, Kouchoukos NT, Schechtman KB, Barzilai B. Atherosclerosis of the ascending aorta is a predictor of renal dysfunction after cardiac operations. J Thorac Cardiovasc Surg. 1999;117(1):111–6. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.