Alisertib inhibits aurora A kinase (AAK), a serine/threonine kinases required for mitotic entry, chromosome alignment, centrosome maturation and separation, and spindle assembly checkpoint.1 As a single agent, alisertib can produce an overall response rate (ORR) of 27% in patients with relapsed refractory aggressive B-cell and T-cell non-Hodgkin lymphomas (NHL), ranging between 33% and 50% in patients with peripheral T-cell lymphoma (PTCL).2,3 However, the majority of responses are partial and short-lasting, and when compared in a randomized phase 3 trial to investigate the choice of pralatrexate, romidepsin, or gemcitabine, no superiority was observed.4
Romidepsin is a class I histone deacetylase (HDAC) inhibitor, which was approved by the Food and Drug Administration (FDA) in 2011 for the treatment of relapsed/refractory PTCL.5 In patients with relapsed/refractory PTCL, single agent romidepsin was associated with an ORR of 25-38%, a complete remission (CR) rate of 15-18%, and a median response duration of 9-28 months.6–8
While AAK regulates mitosis through its action at the G2-M transition point, HDAC induce G1-S transition, supporting the combination of inhibitors affecting both targets. In addition, HDAC inhibitors can degrade AAK and aurora B kinase (ABK) and can modify kinetochore assembly through hyperacetylation of pericentromeric histones, providing further pre-clinical rational for their combination with AAK inhibitors.9,10 In preclinical models of Hodgkin lymphomas (HL) and B-cell NHL, HDAC inhibitors have shown to induce transcriptional and post-transcriptional changes, including repression of C-Myc and C-Myc-responsive micro RNAs, that create a pro-apoptotic environment sensitizing cells to AAK inhibitors. In addition, in pre-clinical models of T-cell NHL, these two classes have shown synergistic activity secondary to cytokinesis failure.11,12
Here we report a phase 1 clinical trial combining romidepsin and alisertib in patients with relapsed/refractory aggressive B-cell and T-cell lymphoma.
This open-label single institute phase I study was conducted at the MD Anderson Cancer Center in Houston, in accordance with the principles of the Declaration of Helsinki, and approved by the institutional review board. The primary objective of the study was to assess the safety profile of romidepsin and alisertib combination therapy and to determine the maximum tolerated dose (MTD). Secondary objectives included ORR and CR rate. This phase 1 trial is registered at clinicaltrials gov. identifier: 0189701.
Patients with histologically confirmed HL, Burkitt’s lymphoma, diffuse large-B cell lymphoma or peripheral T-cell lymphoma, relapsed or refractory after at least one line of systemic treatment, were eligible for this study. Patients with low-grade B-cell lymphoma were not included in the study. Patients should have at least one measurable disease site ≥ 1.5 cm, Eastern Cooperative Oncology Group (ECOG) performance status ≤2, age ≥ 18 years, and adequate hematological, renal and hepatic function. Patients were excluded from the study if they had central nervous involvement.
Romidepsin and alisertib were given at eight different dose levels, the schedule is outlined in the Online Supplementary Table.
Toxicity was graded using the Common Terminology Criteria for Adverse Events, version 4.0. Dose limiting toxicity (DLT) was assessed during cycle 1: non-hematologic DLT was defined as any grade 3 or 4 toxicity attributed to study drugs that could not be controlled or prevented by supportive care; DLT for hematologic toxicity was defined as grade 4 neutropenia or thrombocytopenia lasting longer than 14 days. A standard “3+3” design was used to determine the MTD. The patients were assessed for response after every even numbered cycle using disease-specific response criteria.
Progression-free survival (PFS) was calculated from the date of study entry to the date of progression, death of any cause or the last follow-up. Overall survival (OS) was calculated from the date of study entry to the date of death of any cause or the last follow-up.
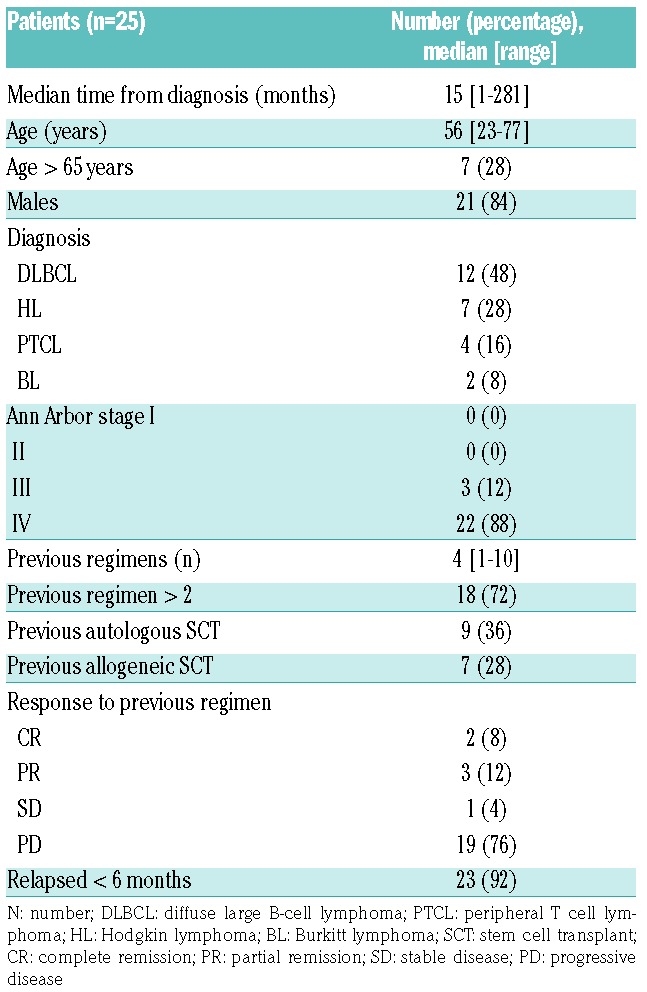
Twenty-five patients were included in the study. The baseline characteristics are shown in Table 1.
Table 1.
Patient baseline characteristics.
Three patients were enrolled at each of the eight dose levels. One additional patient was enrolled at dose level 4 because one patient decided to discontinue treatment before the DLT assessment window was completed. Of interest, all patients with classic HL were enrolled at dose level > 4. The median time on study was two months (range, 1-8 months); the median number of delivered cycles was two (range, 1-8) and the median interval between cycles was four weeks (range, 3-9 weeks).
Twenty-four (96%) patients have discontinued treatment; the reasons for the study discontinuation were: progression (N=19; 79%), completion of treatment (N=2; 9%), indication for stem cell transplantation (SCT) (N=1; 4%), patient’s choice (N=1; 4%), and toxicity (atrial fibrillation; N=1; 4%).
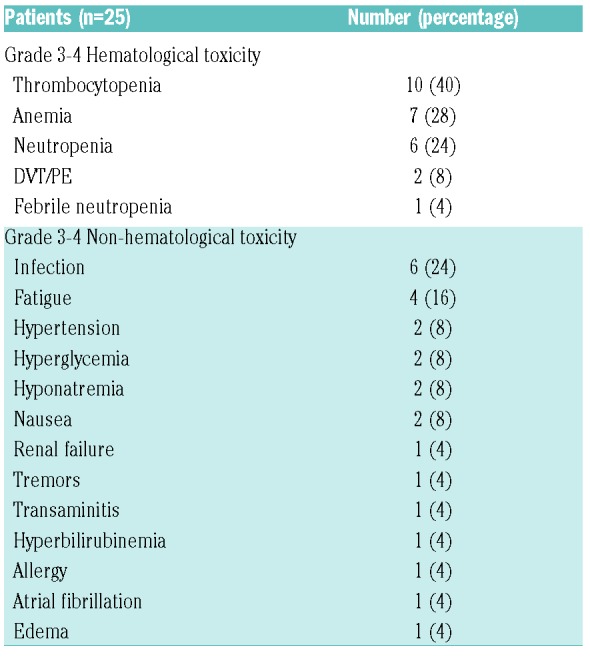
No DLTs have been observed. Grade 3 or higher toxicities are shown in Table 2.
Table 2.
Grade 3-4 toxicity.
Twenty-four patients were evaluable for response and one patient opted to withdraw from the study after four weeks. The ORR in the entire cohort was 28% (7 of 25) based on intent-to-treat analysis, up to 71% (5 out of 7) when limited to patients with cHL. Three patients achieved CR (12%)(two with cHL and one with mycosis fungoides), four (16%) partial remission (PR) (one with a double hit diffuse large B-cell lymphoma (DLBCL) and three with cHL), and five (20%) stable disease (SD).
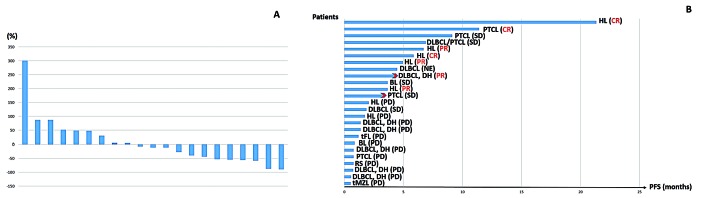
Twenty-one patients were evaluable for response by imaging studies, while three patients had clinical progression before imaging. Four patients stopped treatment only after one cycle because of clinical progression (N=3) and patient’s consent withdrawal (N=1). Twelve patients had a decrease in tumor burden by imaging studies (Figure 1A). Only one (5%) patient (with mycosis fungoides, who had achieved SD) proceeded to allogeneic SCT after 3 months of treatment.
Figure 1.
Response and survival. (A) Waterfall plot showing the best response in tumor size from baseline. (B) Swimmer plot showing progression-free survival. DLBCL: diffuse large B-cell lymphoma; PTCL: peripheral T cell lymphoma; HL: Hodgkin lymphoma; BL: Burkitt lymphoma; DH: double hit; tFL: transformed follicular lymphoma; tMZL: transformed marginal zone lymphoma; RS: Richter Syndrome; CR: complete remission; PR: partial remission; SD: stable disease; PD: progressive disease; PFS: progression-free survival
After a median follow-up of 5 months (range, 1-46 months), 23 (92%) patients have progressed, one after allogeneic SCT. The longest PFS (> 6 months) was observed in two patients with heavily pretreated cHL (21 months and 7 months), two patients with PTCL (one with angioimmunoblastic T-cell lymphoma and one with mycosis fungoides; 11 months and 9 months) and one patient with Epstein-Barr virus-related DLBCL (of interest, with a component of PTCL NOS; 7 months)(Figure 1B).
At the most recent follow-up (data cut-off 02/01/2018), 11 (44%) patients had died, all of disease progression, and the median OS was 12 months (range, 1-46 months).
Alisertib has been investigated as a single agent for the treatment of patients with relapsed/refractory B-cell and T-cell NHL in two separate phase 2 studies, at the dose of 50 mg twice daily for seven days in 21-day cycles; grade (G)3-4 myelosuppression was observed in 24-63% of cases, and G3-4 infections in 13-14% of cases.2,3 Romidespin has been investigated as a single agent for the treatment of patients with relapsed T-cell lymphoma in two separate phase 2 studies, administered as a 4-hour infusion on days 1, 8, and 15 of a 28-day cycle with a starting dose of 14 mg/m2; G3-4 myelosuppression was reported in 6-32% of patients, and G3-4 infectious complications in 2-19% of cases.6,8 In this study, alisertib was investigated at a dose ranging between 20 and 40 mg twice daily, with various schedules, while romidepsin was investigated at a dose ranging between 8 and 12 mg/m2, on a day 1, 8 or day 2, 9, 16 schedule. Despite the study population including heavily-pretreated patients, G3-4 myelosuppression was observed in 24-40% of cases and G3-4 infections in 24% of cases. No DLT was observed, the median time between cycles was 4 weeks, and only one patient interrupted treatment as a consequence of toxicity. Future phase 1/2 studies investigating a full dose of romidepsin (14 mg/m2) and alisertib (50 mg BID) in patients with HL may help to further identify the recommended phase 2 dose for this regimen.
In two separate phase 2 studies conducted in patients with relapsed/refractory B-cell and T-cell NHL, alisertib was associated with an ORR of 27-30% and a CR rate of 7-10%, almost exclusively reported in PTCL.2,3 Similarly, in two separate phase 2 studies conducted in patients with relapsed/refractory PTCL, single agent romidepsin produced an ORR of 25-38% and a CR rate of 15-18%.6,8 In our study, the combination of alisertib and romidepsin resulted in an ORR of 28% and a CR rate of 12%, not comparing favorably to previous experiences with the single agents. In fact, 56% of patients included in this study had a diagnosis of relapsed/refractory B-cell NHL, and two separate phase 2 trials (the results of one of these had not yet been published at the time of the study design) have reported significant myelosuppression and limited ORR (< 20%) for these patients, not supporting further clinical evaluation of this agent in patients with relapsed/refractory B-cell NHL.2,13 Of interest, pre-clinical models suggesting synergy of alisertib and romidepsin on cytokinesis failure was not observed in B cells, but only in T cells.12
The achievement of CR or PR in five out of seven patients with heavily pre-treated cHL (all having failed chemotherapy, brentuximab vedotin, PD-1 inhibitors and/or SCT) observed with this combination is promising. In pre-clinical models, HDAC inhibitors have shown to enhance HL cell killing mediated by AAK inhibitors.11 In addition, clinical activity has been observed for HDAC inhibitors in patients with relapsed/refractory cHL, while the clinical activity of AAK inhibitors in cHL has not been previously described.14,15
In conclusion, the combination of alisertib and romidepsin is a safe regimen for patients with relapsed/refractory lymphomas. Further exploration in a phase 2 study including patients with relapsed/refractory cHL is warranted.
Footnotes
Funding: NCI-CTEP U01 Grant Program at MDACC, Celgene, Millennium/Takeda, and National Stem Cell Foundation.
Information on authorship, contributions, and financial & other disclosures was provided by the authors and is available with the online version of this article at www.haematologica.org.
References
- 1.Goldenson B, Crispino JD. The aurora kinases in cell cycle and leukemia. Oncogene. 2015;34(5):537–545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Friedberg JW, Mahadevan D, Cebula E, et al. Phase II study of alisertib, a selective Aurora A kinase inhibitor, in relapsed and refractory aggressive B- and T-cell non-Hodgkin lymphomas. J Clin Oncol. 2014;32(1):44–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Barr PM, Li H, Spier C, et al. Phase II Intergroup Trial of Alisertib in Relapsed and Refractory Peripheral T-Cell Lymphoma and Transformed Mycosis Fungoides: SWOG 1108. J Clin Oncol. 2015;33(21):2399–2404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.O’Connor OA, Ozcan M, Jacobsen ED, et al. First Multicenter, Randomized Phase 3 Study in Patients (Pts) with Relapsed/Refractory (R/R) Peripheral T-Cell Lymphoma (PTCL): Alisertib (MLN8237) Versus Investigator’s Choice (Lumiere trial; NCT01482962). Blood. 2015;126(23):341. [Google Scholar]
- 5.West AC, Johnstone RW. New and emerging HDAC inhibitors for cancer treatment. J Clin Invest. 2014;124(1):30–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Coiffier B, Pro B, Prince HM, et al. Results from a pivotal, open-label, phase II study of romidepsin in relapsed or refractory peripheral T-cell lymphoma after prior systemic therapy. J Clin Oncol. 2012;30(6):631–636. [DOI] [PubMed] [Google Scholar]
- 7.Coiffier B, Pro B, Prince HM, et al. Romidepsin for the treatment of relapsed/refractory peripheral T-cell lymphoma: pivotal study update demonstrates durable responses. J Hematol Oncol. 2014;7:11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Piekarz RL, Frye R, Prince HM, et al. Phase 2 trial of romidepsin in patients with peripheral T-cell lymphoma. Blood. 2011;117(22):5827–5834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cha TL, Chuang MJ, Wu ST, et al. Dual degradation of aurora A and B kinases by the histone deacetylase inhibitor LBH589 induces G2-M arrest and apoptosis of renal cancer cells. Clin Cancer Res. 2009;15(3):840–850. [DOI] [PubMed] [Google Scholar]
- 10.Park JH, Jong HS, Kim SG, et al. Inhibitors of histone deacetylases induce tumor-selective cytotoxicity through modulating Aurora-A kinase. J Mol Med (Berl). 2008;86(1):117–128. [DOI] [PubMed] [Google Scholar]
- 11.Kretzner L, Scuto A, Dino PM, et al. Combining histone deacetylase inhibitor vorinostat with aurora kinase inhibitors enhances lymphoma cell killing with repression of c-Myc, hTERT, and microRNA levels. Cancer Res. 2011;71(11):3912–3920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zullo KM, Guo Y, Cooke L, et al. Aurora A Kinase Inhibition Selectively Synergizes with Histone Deacetylase Inhibitor through Cytokinesis Failure in T-cell Lymphoma. Clin Cancer Res. 2015;21(18):4097–4109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cohen JB, Maddocks KJ, Huang Y, et al. A phase 2 trial of alisertib in patients with relapsed or refractory B-cellnon-Hodgkin lymphoma. Leuk Lymphoma. 2017;58(9):1–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kirschbaum MH, Goldman BH, Zain JM, et al. A phase 2 study of vorinostat for treatment of relapsed or refractory Hodgkin lymphoma: Southwest Oncology Group Study S0517. Leuk Lymphoma. 2012;53(2):259–262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Garrido-Laguna I, Velez-Bravo V, Falchook GS, et al. Significant Activity Of The mTOR Inhibitor Sirolimus and HDAC Inhibitor Vorinostat In Heavily Pretreated Refractory Hodgkin Lymphoma Patients. Blood. 2013;122(21):3048–3048. [Google Scholar]