Abstract
Here, we report the outcome of 226 myeloma patients presenting with extramedullary plasmacytoma or paraosseous involvement in a retrospective study conducted in 19 centers from 11 countries. Extramedullary disease was detected at diagnosis or relapse between January 2010 and November 2017. Extramedullary plasmacytoma and paraosseous involvement were observed in 130 patients at diagnosis (92 of 38) and in 96 at relapse (84 of 12). The median time from multiple myeloma diagnosis to the development of extramedullary disease was 25.1 months (range 3.1-106.3 months) in the relapse group (median follow up: 15 months). Imaging approach for extramedullary disease was computed tomography (n=133), positron emission tomography combined with computed tomography (n=50), or magnetic resonance imaging (n=35). The entire group received a median two lines of treatment and autologous stem cell transplantation (44%) following the diagnosis of extramedullary disease. Complete response was higher for paraosseous involvement versus extramedullary plasmacytoma at diagnosis (34.2% vs. 19.3%; P=NS.) and relapse (54.5% vs. 9%; P=0.001). Also paraosseous involvement patients had a better progression-free survival (PFS) when recognized at initial diagnosis of myeloma than at relapse (51.7 vs. 38.9 months). In addition, overall survival was better for paraosseous involvement compared to extramedullary plasmacytoma at diagnosis (not reached vs. 46.5 months). Extramedullary plasmacytoma at relapse had the worst prognosis with a PFS of 13.6 months and overall survival of 11.4 months. In the multivariate analysis, paraosseous involvement, extramedullary disease at diagnosis, International Staging System (ISS-I), and undergoing autologous stem cell transplantation improved overall survival independently. This cohort demonstrated that extramedullary disease benefits from front-line autologous stem cell transplantation and extramedullary plasmacytoma differs from paraosseous involvement in terms of rate and duration of response, with even worse outcomes when detected at relapse, constituting an unmet clinical need.
Introduction
Multiple myeloma (MM) originates from the proliferation of clonal malignant plasma cells (PC) with a strong interaction with the bone marrow microenvironment. Although the disease is considered generally incurable, overall survival (OS) has improved substantially in the past 15 years and more than 25% of patients can now expect to live for more than ten years.1 However, there is still a group of patients presenting with very poor prognostic features whose outcome has not improved; presentation with disease at extramedullary sites is included among these.
Once the plasma cells acquire independence from the cellular microenvironment, plasma cell leukemia or metastasis to soft tissues in the form of plasmacytomas may occur creating an unmet clinical need, even in the era of novel agents.2,3 Such an escape is driven by pathophysiological alterations including decreased expression of adhesion molecules, low expression of cytokine receptors or increased angiogenesis.2 Two types of soft tissue involvement in myeloma have been defined: extramedullary plasmacytomas (EMP) resulting from hematogenous spread and involving only soft tissues, and paraskeletal or paraosseous (PO) plasmacytomas, consisting of tumor masses adjacent to bones and arising from focal skeletal lesions.3,4 The incidence of extra-medullary involvement and paraskeletal plasmacytomas at diagnosis ranges from 1.7% to 3.5% and from 6% to 34.4%, respectively; at relapse, the presence of extramedullary disease (EMD) increases up to 10%.3–6 There is no clear evidence that the incidence of EMD is higher at relapse after allogeneic transplantation or after exposure to novel anti-myeloma agents.7,8 At present, there are limited data regarding the basic features of EMD, such as incidence, prevalence, clinical characteristics, laboratory features, and response to novel drugs.6–11 Two previous publications reported the incidence of EMD at diagnosis and relapse to be 15% and 20%, respectively.12,13 In the largest study to date, Varettoni et al. report the results of 1,003 consecutive MM patients who presented to the University of Pavia in Italy between 1971 and 2007 with an incidence of 13% (7% EMD at diagnosis and 6% at relapse). Of note, cytogenetic data were not available for all patients and were not included in the analysis.6
Extramedullary disease both clinically and morphologically resembles lymphoma transformation in terms of laboratory features, such as frequent association with high serum levels of lactate dehydrogenase.14 In addition, the majority of patients presenting with EMD have highly complex cytogenetic abnormalities and, as found most recently, high-risk features on gene expression profiling.15 In a classic monoclonal immunoglobulin-secreting tumor, EMD may present as light chain secretory, hypo-secretory, or non-secretory disease as a sign of disease de-differentiation and transformation.16
Moreover, an increase in the incidence is probably due to the availability of highly sensitive imaging techniques and the prolongation of survival. Modern imaging techniques, especially 18F-fluorodeoxyglucose (FDG) PET, have become extremely helpful in documenting suspected EMP.8
Except for solitary plasmacytoma, there is no standard approach for EMD management.17 Neither response to EMD within the clinical trials nor case reports have been extensively analyzed and, therefore, no evidence-based consensus has been reached. Therefore, the objectives of this study were to determine the demographic and clinical characteristics of EMD (EMP or PO) among myeloma patients at initial diagnosis or relapse to evaluate its impact on treatment outcomes. The response, progression-free survival (PFS) and overall survival (OS) of this real-world data based on 226 patients will serve as a reference for future studies addressing EMD.
Methods
This is a retrospective, multi-institutional study conducted in 19 centers from 11 countries in Europe. Patients were identified through a database search at each of the participating institutions. Adult (≥18 years) patients with MM who had a pathological and/or radiological diagnosis of extramedullary involvement at any time of follow up between January 2010 and November 2017 were included. Ethical committee approvals and consents were collected from each patient on admission depending on the local regulations of each country. The diagnosis of EMD was made in accordance with the International Myeloma Working Group Guidelines.18 Eligibility criteria included EMD at any time following the initial diagnosis of MM excluding plasma cell leukemia or solitary plasmacytoma. Those patients with pathological or radiological evidence of neoplastic plasma cells in the soft tissues adjacent to axial skeleton were considered to have PO involvement of locally advanced myeloma, but not EMP. On the basis of type of extramedullary involvement, we defined two groups of myeloma patients: PO group and extramedullary organ/tissue involvement (EMP). Cases with both PO and EMP were included in the EMP group. Disease stage at diagnosis was determined according to the International Staging System (ISS; I-III). Remission, progression, and relapse were defined according to standard International Myeloma Working Group (IMWG) criteria. Progression was calculated from the date of diagnosis of EMD until the date of progression of myeloma or isolated EMD, whichever occurred first.
Clinical data included age at the time of MM diagnosis and at the time of EMD, ISS stage, cytogenetic abnormalities, radiological findings (PET-CT/MRI/CT), number and types of therapies including chemo/radiotherapy, autologous stem cell transplantation (ASCT) for EMD, response, PFS and OS.
Categorical variables were compared with the use of the Fisher’s exact test or the χ2 test. Continuous variables were analyzed using the Kruskal-Wallis test for independent samples. Survival probabilities were estimated by the Kaplan-Meier method,19 and the Log-Rank test was used for univariate comparison. Outcomes were determined as response to treatment, PFS and OS. We also compared the PFS and OS between the time of EMD diagnosis and PO/EMP cohorts. To assess the multivariate factors for each end point, we used Cox proportional hazard model to estimate hazard ratios (HR) with 95% confidence intervals (CI). All tests were two-sided, with the type 1 error rate fixed at α=0.05. All analyses and graphs were obtained using the statistical software SPSS Statistics 21 (SPSS; IBM Corp., Armonk, NY, USA).
Results
Clinical characteristics
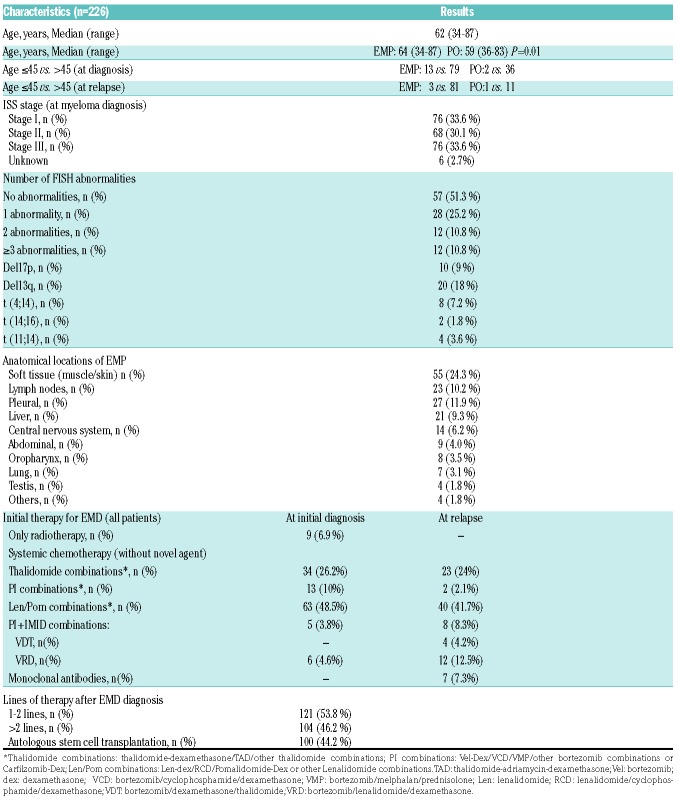
A total of 226 patients met the predetermined criteria for inclusion in this study. Baseline clinical characteristics are summarized in Table 1. Median age at diagnosis of EMD was 62 years (range 34-87 years). EMP/PO were observed in 130 patients at the time of initial diagnose (92 of 38) and in 96 during disease relapse (84 of 12). The median time from MM diagnosis to the development of EMD in the relapsed group was 25.1 months (range 3.1-106.3 months) with relatively faster progression among the EMP patients (PO: median 9.8 months; EMP: median 5.7 months; P=NS). Since Jurczyszyn et al.20 have demonstrated a survival advantage among younger patients, an age cut-off of 45 years was adopted (Table 1) revealing younger age for PO (59 years) versus EMP (64 years) (P=0.01). Median ages of patients presenting with PO (58.5 years) or EMP (62 years) at diagnosis were not significantly different. The imaging modalities used for the diagnosis were CT (n=133), PET-CT (n=50) and MRI (n=35). The anatomical distribution of EMD is depicted in Table 1. Most patients with EMP (65%) presented with one involved site, 16% had two sites, and 11% had three sites, while involvement in four and five sites was present in 7% of patients, respectively.
Table 1.
Baseline characteristics of patients.
Cytogenetic analysis of clonal plasma cells in the bone marrow at the time of MM diagnosis was available for 111 of 226 (49.1%) of the patients with EMD (Table 1).
Therapeutic interventions and response
Treatments of patients are summarized in Table 1. The most commonly used treatment was combination chemotherapy with or without radiotherapy followed by cyclophosphamide/bortezomib/dexamethasone (45.6%). A total of 100 patients received ASCT, of which 67 (51.5%) with EMD at diagnosis. Median interval from EMD diagnosis to ASCT was 11.3 months (2-91 months). Transplant was a more frequent treatment approach among patients presenting with PO (31 of 38) compared to those with EMP (36 of 92) In addition, 29 patients (12.8%) had already been transplanted prior to the diagnosis of EMD, which developed after a median of 30.8 months post ASCT. Only four EMD patients diagnosed at relapse underwent ASCT. The entire group received a median of two lines of treatment following the diagnosis of EMD. Seventy-five (57.7%) myeloma patients with EMD at diagnosis went on to receive second line of therapy and 48 (37.2%) received more than two lines of therapy. Among 96 patients with EMD at relapse, 56 (58.3%) of them received more than two lines therapy.
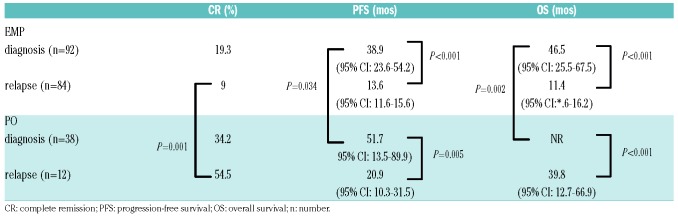
As can be seen in Table 2 there were significant differences in outcomes when EMP was compared with PO. A statistically significant difference in complete response rate (CR) (PO: 38.8% vs. EMP: 14.8%; P=0.001) was observed following first line of treatment (not shown in Table 2). Of the 88 newly diagnosed EMP patients, with response to induction available, 35 had received radiotherapy without (n=6) or with (n=29) systemic treatment. These patients achieved a CR rate (11.4 %) that was considerably less than the CR (24.5%) achieved with chemotherapy alone. Among those who received ASCT, there was an improved CR rate of 29% versus 19% (at diagnosis) 41.7% versus 9.5% (at relapse). However, regardless of treatment, 51.4% of even those who achieved CR progressed within median 18.1 months versus 12.1 months in PO and EMP groups, respectively (P=NS). Among the newly diagnosed patients who underwent ASCT (n=67), the median PFS from diagnosis was 49 months (95%CI: 22.7-75.3) (PO: 51.7 months (95%CI: 18.3-85.1) and EMP: 46.5 months (95%CI: 32.8-60.2); P=NS). Among those who did not receive ASCT the median PFS was 28.1 months (95%CI: 20.3-35.9) (P<0.001). Post-ASCT depth of response (>VGPR vs. <VGPR) did not affect PFS. Type of therapy did not significantly impact PFS: immunomodulatory drug (IMID)-based (median 18.4 months, 95%CI: 6-32) or proteasome inhibitors (PI)-based (median 24.3 months, 95%CI: 20-29).
Table 2.
Comparison of response, survival outcomes of extramedullary plasmacytomas (EMP) or paraosseous (PO) patients either at diagnosis or at relapse.
Survival analyses and prognostic factors
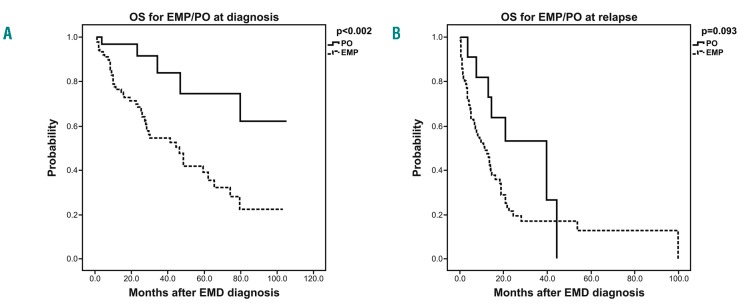
We did not find any association between EMP, ISS or age (Table 3). At the time of this report, 118 patients (52.2%) have died and the median follow up after EMD diagnosis is 15 months (range: 2-105 months). The estimated median PFS and OS from initial diagnosis of myeloma for the EMP and PO groups with a median follow up of 24.4 months are summarized (Table 2 and Figure 1). At initial MM diagnosis, PFS and OS were 38.9 months and 46.5 months for EMP, whereas 51.7 months (P=0.034) and not reached (P=0.002) for PO, respectively. However, if diagnosed at relapse, PFS and OS were 13.6 months and 11.4 months for EMP compared to 20.9 months (P=0.249) and 39.8 months (P=0.093) for PO, respectively (Table 2 and Figure 1).
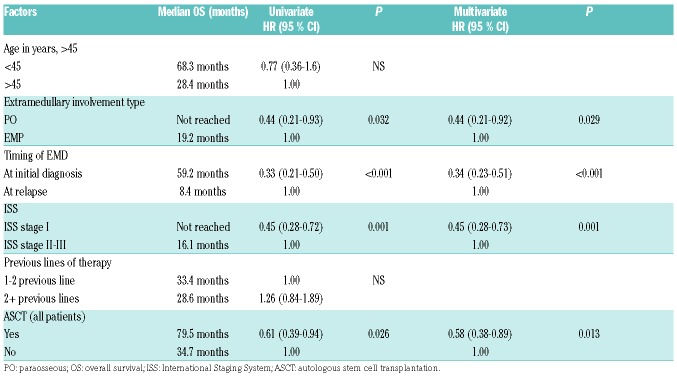
Table 3.
Univariate and multivariate analysis for overall survival in myeloma patients with extramedullary disease (EMD).
Figure 1.
Overall survival (OS) estimates comparing patients with extramedullary plasmacytomas (EMP) to those with paraosseous (PO) lesions (A) at diagnosis and (B) at relapse. EMD: extramedullary disease.
In the group of patients with EMD at initial diagnosis, the OS was 46.5 months (95%CI: 10.3-31.5) with 2- and 5-year OS rates of 74.1±0.4 % and 47.1±0.6 %, respectively (P<0.001). For the PO group, median OS after ASCT was not reached versus 43.5 months in the EMP group (P=0.018).
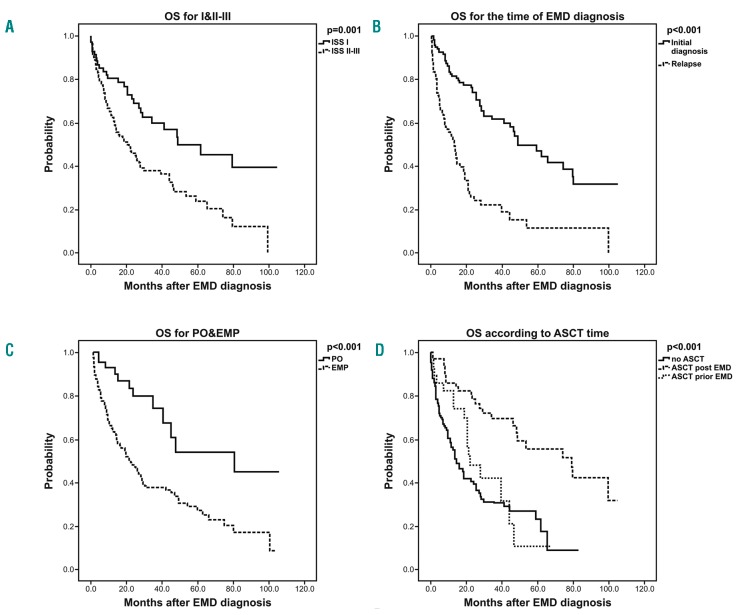
In the univariate analysis, ISS staging (II/III vs. I) at the time of initial MM diagnosis (Figure 2A), time of EMD diagnosis (relapse vs. initial diagnosis) (Figure 2B), type of extra-medullary involvement (EMP vs. PO) (Figure 2C), and not undergoing ASCT (all patients) (Figure 2D) were associated with a worse OS. In the multivariate analysis, ISS stage I versus II-III, EMD at diagnosis versus relapse, PO versus EMP and yes versus no for ASCT were independently associated with better OS. The univariate and multivariate models are shown in Table 3.
Figure 2.
Overall survival (OS) estimates comparing the risk factors in (A) extramedullary disease (EMD) patients at diagnosis according to International Staging System (ISS) stage, (B) EMD patients according to disease stage, (C) all patients according to paraosseous (PO) versus extramedullary plasmacytomas (EMP) and (D) all patients according to autologous stem cell transplantation (ASCT) treatment at diagnosis versus at relapse versus no ASCT.
Discussion
Extramedullary disease (EMD) is generally considered to be a poor prognostic factor. This multi-institutional real-world retrospective analysis on 226 patients has shown PFS/OS similar to the general myeloma population for those presenting with PO but not EMP.9 However, EMD at diagnosis when treated with ASCT was able to reach a median PFS of 79.5 months (95%CI: 42.4-116.6) versus 30.1 months (95%CI: 11.2-48.9) depending on the depth of response (≥VGPR). Thus, although deep responses are reachable they are not sustainable for EMP even with ASCT.
In a report from the Spanish PETHEMA group, an upfront comparison was made of patients treated with three induction regimens: (i) thalidomide/dexamethasone; (ii) bortezomib/thalidomide/dexamethasone; and (iii) vin-cristine/carmustine/melphalan/cyclophosphamide plus prednisone/vincristine/carmustine/adriamycin/bortezomib with the lowest rate of progressive disease being observed in the bortezomib/thalidomide/ dexamethasone arm. EMD was reported in 18% of patients across this study and the response among EMD patients were not specified.9 There are limited data concerning the efficacy of novel agents in myeloma patients with EMD. Different groups reported successful use of bortezomib.21,22,23 The efficacy of other proteasome inhibitor (PI) (carfilzomib and ixazomib) is still unknown. The efficacy of IMID is also limited. Rosinòl et al. reported the data on the lack of efficacy of thalidomide in myeloma patients with EMD in different series.24,25 In addition, Anagnostopoulos et al. recently demonstrated that relapses may occur under thalidomide maintenance with an increase in bone marrow plasma cells and no increase in the M-protein size.26 The efficacy of lenalidomide on plasmacytomas has not yet been reported. Concerning pomalidomide and dexamethasone, different groups have reported conflicting results.27
In a retrospective study, a subset of 101 EMD patients (66 at diagnosis and 35 at relapse), were compared to patients without any EMD but enrolled in Total Therapy (TT) or non-TT protocols.28 Regardless of therapy, EMD was associated with shorter PFS and OS: EMP at diagnosis was associated with poor PFS (TT: 27%, non-TT: 12% after 5 years) and OS (TT: 35%, non-TT: 34% after 5 years) regardless of whether or not the patients were treated on TT protocols.14 The PFS and OS in our study is comparable to the survival durations reported by the Arkansas group. Usmani et al., but not Pour et al., found fluorescence in situ hybridization (FISH) detectable abnormalities to be associated with EMD and poor outcome.21,24 In our study, FISH analysis was available in half of the patients and was similar to the experience of the Czech group revealing results comparable to the general myeloma population. In our experience, only 13q del (18%), was observed less frequently than expected.
In a recent paper, Kumar et al. have analyzed data of 44 (16.2%) EMD out of 271 consecutive ASCT recipients. Although they did not discriminate EMP from PO, both OS and PFS was shorter for patients with EMD; median OS was 19.2 months (95%CI: 10.6-27.8) with a median PFS of 19 months (95%CI: 12.6-25.4). Achievement of CR post transplant was found to be the most important predictor for OS and PFS in this study.29 In our cohort, 67 myeloma patients with EMD at diagnosis underwent ASCT within a median of 10.7 months and 39 patients (66.1%) achieved ≥very good partial response (VGPR) following ASCT. We were also able to demonstrate the impact of transplant on OS in our newly diagnosed EMD ASCT cohort in univariate and multivariate analysis. Although PFS was comparable to the standard myeloma population, we were not able to see the impact of response ≥VGPR, which may be attributable to the differences among the imaging tools used.
The European Group of Blood and Marrow Transplantation (EBMT) recently reported on 682 EMD subjects (EMP/PO: 139/543) who have received ASCT. In this report, PO (14.5%) involvement was found to be more frequent compared to EMP (3.7%). They noted a gradual increase in frequency of EMD from 2005 to 2014.30 Similar to our results, they also report ISS to have a poor prognostic effect on PFS and OS. Organ distributions are similar between the EBMT report and ours. We have to consider selection bias in their report, as elderly patients not transplanted are not included. Our study did not aim to analyze the frequency of EMD, but rather the response and survival outcomes. Our results for patients detected at diagnosis differ from the EBMT report as their median PFS (PO vs. EMP) values were 36 versus 24 months, compared to our results, which were 51.7 versus 38.9 months, respectively. In our study, ASCT was performed among 44% of our patients. Among the EMD following ASCT cases, a shorter 3-year PFS: 28.4±1.6% was observed compared to the PFS of those who were transplanted with EMD at diagnosis (55.8±6.7%; P<0.001). Usmani et al. have also concluded that even with the Total Therapy approach EMD is not controllable. In their study, non-EMD patients were able to improve their PFS from four to six years, but PFS of patients with EMD were approximately one year regardless of being included in the TT programs with a 5-year PFS of 50% versus 21% in no EMD versus EMD prior to ASCT groups, respectively (P=0.08).28 Pour et al. reported on 55 cases in an extramedullary relapse setting and the most important finding in their study was the significant difference in prognosis for PO and EMP. If the extramedullary myeloma infiltration was not bone-related, the OS was extremely short and not longer than four months. They were not able to observe any association between EMD relapse and novel agents (thalidomide or bortezomib).31 This multi-national study includes widely heterogeneous drug approvals and access to novel agents. In our relapsed EMD cohort, 24% (23 of 96) of patients received initial therapy without any novel agents. Although there was a trend in favor of PI, we were not able to observe an effect of novel agents on PFS. In addition, our cohort of relapsed EMP also had the worst prognosis with an OS of 11.4 months.
Our study results highlight a lack of an association between EMD and younger age at diagnosis. In our study, the age cut-off of 45 years was selected arbitrarily to distinguish younger patients from the general myeloma population (65+/− 20 years). In general, the outcome of younger patients is better than that of elderly myeloma patients because of their better performance status and treatment tolerability. Median ages of EMP and PO of our patients were similar to the median value of 59 years reported in the EBMT study (EMP/PO: 64/59 years).30
A standard approach for EMD has still not been established. Neither response to EMD within the clinical trials nor case reports have been extensively analyzed in order to arrive at an evidence-based consensus. The most standardized modality is to give radiotherapy and treat patients with multiple agents as if treating lymphoma. Given the dismal outcome of EMD reported by others and us, there is an unmet need to improve PFS and OS. Prospective clinical trials focusing on EMD are needed. Despite the limitations of a retrospective approach, the response kinetics reported in our real-world study may provide guidance in designing future EMD clinical trials. Since PO versus EMP, EMP at initial diagnosis versus relapse, ISS I versus II and III, ASCT yes/no are found to improve OS, these parameters need to be balanced in future studies comparing novel treatment approaches.
Acknowledgments
Authors are grateful to additional members of Balkan Myeloma Study Group who also participated but could not qualify for authorship: Guenova M, Markovic O, Djurdjevic P, Kinda SB, Karanfilsky O, Dapcevic M, Zver S.
Footnotes
Check the online version for the most updated information on this article, online supplements, and information on authorship & disclosures: www.haematologica.org/content/105/1/201
References
- 1.Kumar SK, Rajkumar SV, Dispenzieri A, et al. Improved survival in multiple myeloma and the impact of novel therapies. Blood. 2008;111(5):2516–2520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Palumbo A, Anderson K. Multiple myeloma. N Eng J Med. 2011;364(11):1046–1060. [DOI] [PubMed] [Google Scholar]
- 3.Blade J, Larrea CF, Rosinol L, et al. Soft-tissue plasmacytomas in multiple myeloma: incidence, mechanisms of extramedullary spread, ad treatment approach. J Clin Oncol. 2011;29(28):3805–3812. [DOI] [PubMed] [Google Scholar]
- 4.Touzeau C, Moreau P. How I treat extramedullary myeloma. Blood. 2016; 127(8):971–976. [DOI] [PubMed] [Google Scholar]
- 5.Oriol A. Multiple myeloma with extramedullary disease. Adv Ther. 2011; 28(Suppl 7):1–6. [DOI] [PubMed] [Google Scholar]
- 6.Varettoni M, Corso A, Pica G, Mangiacavalli S, Pascutto C, Lazzarino M. Incidence, presenting features and outcome of extramedullary disease in multiple myeloma: a longitudinal study on 1003 consecutive patients. Ann Oncol. 2010; 21(2):325–330. [DOI] [PubMed] [Google Scholar]
- 7.Varga C, Xie W, Laubach J, et al. Development of extramedullary myeloma in the era of novel agents: no evidence of increased risk with lenalidomide-bortezomib combinations. Br J Haematol. 2015;169(6):843–850. [DOI] [PubMed] [Google Scholar]
- 8.Weinstock M, Aljwai Y, Morgan EA, et al. Incidence and clinical features of extramedullarty multiple myeloma in patents who underwent stem cell transplantation. Br Hematol. 2015;169(6):851–858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rosiñol L, Oriol A, Teruel AI, et al. Superiority of bortezomib, thalidomide, and dexamethasone (VTD) as induction pre- transplantation therapy in multiple myeloma: a randomized phase 3 PETHE-MA/GEM study. Blood. 2012; 12:120(8): 1589–1596. [DOI] [PubMed] [Google Scholar]
- 10.Usmani SZ, Rodriguez-Otero P, Bhutani M, Mateos MV, Miguel JS. Defining and treating high-risk myeloma. Leukemia. 2015; 29(11):2119–2125. [DOI] [PubMed] [Google Scholar]
- 11.Sonneveld P, Avet-Loiseau H, Lonial S, et al. Treatment of multiple myeloma with high risk cytogenetics: a consensus of the International Myeloma Working Group. Blood. 2016;127(24):2955–2962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bladé J, Lust J, Kyle RA. Immunoglobulin D multiple myeloma: presenting features response to therapy, and survival in a series of 53 cases. J Clin Oncol. 1994;12(11):2398–2404. [DOI] [PubMed] [Google Scholar]
- 13.Bladé J, Kyle RA, Greipp PR. Presenting features and prognosis in 72 patients with multiple myeloma who were younger than 40 years. Br J Haematol. 1996;93:345–351. [DOI] [PubMed] [Google Scholar]
- 14.Barlogie B, Smallwood L, Smith T, et al. High serum levels of lactic dehydrogenase identify a high-grade lymphoma-like myeloma. Ann Intern Med. 1989; 110(7):521–525. [DOI] [PubMed] [Google Scholar]
- 15.Rasche L, Bernard C, Topp M, et al. Features of extramedullary myeloma relapse: high proliferation, minimal marrow nvolvement, adverse cytogenetics: a retrospective single centre study of 24 cases. Ann Hematol. 2012;91(7):1031–1037. [DOI] [PubMed] [Google Scholar]
- 16.Weinstock M, Ghobrial IM. Extramedullary multiple myeloma. Leuk Lymphoma. 2013; 54(6):1135–1141. [DOI] [PubMed] [Google Scholar]
- 17.Caers J, Paiva B, Zamagni E, et al. Diagnosis, treatment, and response assessment in solitary plasmacytoma: updated recommendations from a European Expert Panel. J Hematol Oncol. 2018;11(1):10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rajkumar SV, Dimopoulos MA, Palumbo A, et al. International Myeloma Working Group updated criteria fort he diagnosis of multiple myeloma. Lancet Oncol. 2014;15(12):PE538–E5448. [DOI] [PubMed] [Google Scholar]
- 19.Kaplan E, Meier P. Nonparametric estimation from incomplete observations. J Am Stat Assoc. 1958;53(282):457–481. [Google Scholar]
- 20.Jurczyszyn A, Nahi H, Avivi I, et al. Characteristics and outcomes of patients with multiple myeloma aged 21-40 years versus 41-60 years: a multi-institutional case-control study. Br J Haematol. 2016;175(5):884–891. [DOI] [PubMed] [Google Scholar]
- 21.Patriarca F, Prosdocimo S, Tomadini V, et al. Efficacy of bortezomib therapy for extramedullary relapse of myeloma after autologous and non-myeloablative allogeneic transplantation. Haematologica. 2005;90(2):278–279. [PubMed] [Google Scholar]
- 22.Paubelle E, Coppo P, Garderet L, et al. Complete remission with bortezomib on plasmacytomas in an end-stage patient with refractory multiple myeloma who failed all other therapies including haematopoietic stem cell transplantation: possible enhancement of graft-vs-tumor effect. Leukemia. 2005;19(9):1702–1704. [DOI] [PubMed] [Google Scholar]
- 23.Rosiñol L, Cibeira MT, Uriburu C, et al. Bortezomib: an effective agent in extramedullary disease in multiple myeloma. Eur J Haematol. 2006;76(5):405–408. [DOI] [PubMed] [Google Scholar]
- 24.Rosiñol L, Cibeira MT, Bladé J, et al. Extramedullary multiple myeloma escapes the effect of thalidomide. Haematologica. 2004;89(7):832–836. [PubMed] [Google Scholar]
- 25.Bladé J, Perales M, Rosiñol L, et al. Thalidomide in multiple myeloma: lack of response of soft-tissue plasmacytomas. Br J Haematol. 2001;113(2):422–425. [DOI] [PubMed] [Google Scholar]
- 26.Anagnostopoulos A, Gika D, Hamilos G, et al. Treatment of relapsed refractory multiple myeloma with thalidomide-based regimens: identification of prognostic factors. Leuk Lymphoma. 2004;45(11):2275–2279. [DOI] [PubMed] [Google Scholar]
- 27.Jiménez-Segura R, Granell M, Gironella M, et al. Pomalidomida/dexametasona en el tratamiento de la enfermedad extramedular en pacientes con myeloma multiple en recaída/refractario: análisis de 16 casos. Haematologica. 2016;101(s4):103. [Google Scholar]
- 28.Usmani SZ, Heuck C, Mitchell A, et al. Extramedullary disease portends poor prognosis in multiple myeloma and is overrepresented in high-risk disease even in the era of novel agents. Hematologica. 2012;97(11):1761–1767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kumar L, Gogi R, Patel AK, et al. Multiple myeloma with extramedullary disease: impact of autologous stem cell transplantation on outcome. Bone Marrow Transplant. 2017;52(10):1473–1475. [DOI] [PubMed] [Google Scholar]
- 30.Gagelmann N, Eikerna DJ, Iacobelli S, et al. Impact of extramedullary disease in patients with newly diagnosed multiple myeloma undergoing autologous stem cell transplantation: a study from the Chronic Malignancies Working Party of the EBMT. Haematologica. 2018;103(5):890–897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pour L, Sevcikova S, Greslikova H, et al. Soft-tissue extramedullary multiple myeloma prognosis is significantly worse in comparison to bone-related extramedullary relapse. Hematologica. 2014;99(2):360–364. [DOI] [PMC free article] [PubMed] [Google Scholar]