Abstract
Immunosuppressive therapy (IST) is one therapy option for treatment of patients with lower-risk myelodysplastic syndromes (MDS). However, the use of several different immunosuppressive regimens, the lack of high-quality studies, and the absence of validated predictive biomarkers pose important challenges. We conducted a systematic review and meta-analysis according to the Meta-Analysis of Observational Studies in Epidemiology (MOOSE) guidelines and searched MEDLINE via PubMed, Ovid EMBASE, COCHRANE registry of clinical trials (CENTRAL), and the Web of Science without language restriction from inception through September 2018, as well as relevant conference proceedings and abstracts, for prospective cohort studies or clinical trials investigating IST in MDS. Fixed and Random-effects models were used to pool response rates. We identified nine prospective cohort studies and 13 clinical trials with a total of 570 patients. Overall response rate was 42.5% [95% confidence interval (CI): 36.1-49.2%] including a complete remission rate of 12.5% (95%CI: 9.3-16.6%) and red blood cell transfusion independence rate of 33.4% (95% CI: 25.1–42.9%). The most commonly used forms of IST were anti-thymocyte globulin alone or in combination with cyclosporin A with a trend towards higher response rates with combination therapy. Progression rate to acute myeloid leukemia was 8.6% per patient year (95%CI: 3.3-13.9%). Overall survival and adverse events were only inconsistently reported. We were unable to validate any biomarkers predictive of a therapeutic response to IST. IST for treatment of lower-risk MDS patients can be successful to alleviate transfusion burden and associated sequelae.
Introduction
Myelodysplastic syndromes (MDS) comprise a spectrum of clonal hematopoietic stem cell disorders that are characterized by peripheral blood cytopenias and dysplastic changes due to ineffective hematopoiesis, recurrent cytogenetic abnormalities, and an increased risk of progression to acute myeloid leukemia (AML).1,2 As a heterogenous group of diseases, treatment regimens for MDS patients need to be individualized and mainly based on the extent of MDS-associated symptoms and the risk of progression to AML, as assessed by various risk stratification tools such as the International Prognostic Scoring System (IPSS) and its revised version (IPSS-R).3–5 For patients with lower-risk MDS (which is usually defined as patients with very low, low or intermediate-1 risk based on IPSS and IPSS-R) several treatment options including lenalidomide, erythropoiesis-stimulating agents, immunosuppressive therapy (IST), and hypomethylating agents are available.3,5–7 The rationale for the use of IST in MDS is based on studies showing that up to 48% of patients with MDS had evidence of autoimmune disease, but the impact of this finding on prognosis is controversial.8,9 Additionally, dysregulation of T-cell function has been linked to impaired hematopoiesis in patients with both aplastic anemia and lower- risk MDS and can potentially be restored by IST.9–11 Several forms of IST have been tested in MDS treatment with varying degrees of success. Previous studies have reported durable objective responses and transfusion independence ranging up to 55% and 27%, respectively.12,13 Consensus guidelines recommend consideration of IST in patients with low or intermediate-1 risk, non-del(5q-) MDS patients.3,6,14 The most commonly used of these are anti-thymocyte globulin (ATG), cyclosporine A (CsA), and monoclonal antibodies (etanercept, alemtuzumab) which can be used either as monotherapy or in combination.13,15–20 Although IST has been used for over two decades in MDS treatment, response rates are highly heterogeneous between various patient subpopulations and studies. While several predictive response markers such as age, HLA-DR15 positivity, bone marrow cellularity, and disease duration have been identified in some studies, these findings could not be reproduced in others.12,16,21–23
Given this large heterogeneity among published studies, we performed a systematic literature review and meta-analysis on several forms of IST in MDS to objectively assess overall response rates (ORR), rates of achieving a complete remission (CR), erythroid hematologic improvement (HI-E), and red blood cell transfusion independence (TI) as well as the rate of AML progression per patient-year for patients receiving IST.
Methods
Date sources and search strategy
This systematic review and meta-analysis was conducted according to the Preferred Reporting Items for Systematic Reviews and Meta-Analysis (PRISMA) and Meta-Analysis of Observational Studies in Epidemiology (MOOSE) guidelines.24 MEDLINE via PubMed, Ovid EMBASE, the COHRANE registry of clinical trials (CENTRAL), and the Web of Science electronic databases were searched without language restriction from inception through September 2018, using the following combination of free-text terms linked by Boolean operators: (“MDS” OR “myelodysplasia” OR “myelodysplastic syndrome”) AND (“IST” OR “immunosuppressive therapy” OR “immunosuppression” OR “ATG” OR “anti-thymocyte globulin” OR “tacrolimus” OR “cyclosporine” OR “sirolimus” OR “prednisone” OR “prednisolone” OR “steroids” OR “etanercept” OR “alemtuzumbab”).
We performed a gray literature search through: 1) manual search of bibliographies of all identified studies; and 2) conference proceedings and abstracts of the following annual meetings: American Society of Hematology, American Society of Clinical Oncology, European Hematology Association, and European Society of Medical Oncology.
Study selection and endpoints
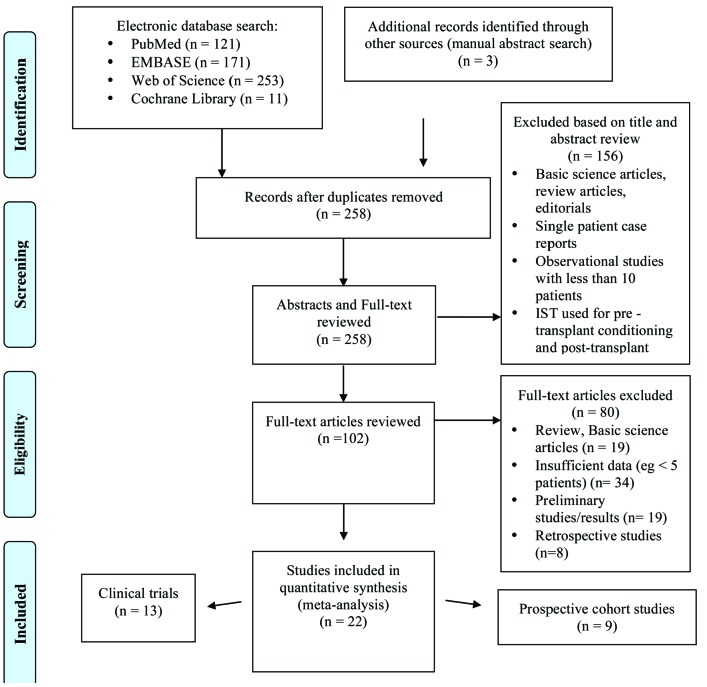
Two reviewers (MS and JPB) independently screened the titles and abstracts of all retrieved studies for eligibility and removed duplicates. Subsequently, full texts of the potentially eligible studies were reviewed for eligibility. We excluded studies that: 1) lack information on either ORR or CR rate; 2) review articles, editorials, and correspondence letters that did not report independent data; 3) case series and studies reporting outcomes on fewer than five patients; and 4) retrospective studies. There was no disagreement among the two reviewers regarding the inclusion of any study. The study selection process is illustrated in a flow diagram (Figure 1).
Figure 1.
Flow chart showing study selection as per the MOOSE guidelines. The search strategy and stepwise process of study selection used in this meta-analysis. MEDLINE via PubMed, Ovid EMBASE, the COHRANE registry of clinical trials (CENTRAL), and the Web of Science electronic databases were searched with no language restriction from inception through September 2018, using the following combination of free-text terms linked by Boolean operators: (“MDS” OR “myelodysplasia” OR “myelodysplastic syndrome”) AND (“IST” OR “immunosuppressive therapy” OR “immunosuppression” OR “ATG” OR “anti-thymocyte globulin” OR “tacrolimus” OR “cyclosporine” OR “sirolimus” OR “prednisone” OR “prednisolone” OR “steroids” OR “etanercept” OR “alemtuzumbab”). Two authors (MS and JPB) independently screened the titles and abstracts of all retrieved studies for eligibility and removed any duplicate records. In a second step, full texts of the potentially eligible studies were reviewed for the final eligibility. Review, basic science articles and articles with insufficient patient number (< 5 patients) as well as preliminary studies and retrospective studies were excluded.
Prospective cohort studies or clinical trials investigating the use of IST for the treatment of MDS were included. IST was defined as receipt of one or a combination of the following drugs: rabbit and horse ATG, CsA, sirolimus, mycophenolate mofetil and monoclonal antibodies (etanercept, alemtuzumab).
The primary outcomes were ORR and CR rate. Secondary outcomes included rates of HI-E, TI, and AML progression. ORR was defined based on the 2006 modified International Working Group (IWG) response criteria for MDS.25
Data extraction
Two investigators (MS and JPB) extracted data using a standardized data-extraction form, and a third investigator (SG) performed a cross-check for data accuracy. A more detailed description of the extracted information is provided in the Online Supplementary Methods.
Quality assessment
The quality of each study was assessed by two authors (MS and JPB) using the modified Down and Black checklist.26 Quality assessments for individual studies are provided in Online Supplementary Table S1.
Statistical analysis
Random-effects models were used to pool ORR, rates of CR, HI-E, TI, and progression to AML. All effect sizes underwent logarithmic transformation prior to pooling using an inverse variance weighting approach. Heterogeneity of studies was determined using Cochran Q and I2 indices and significant heterogeneity (defined as I2 > 60%) was further explored with sensitivity analyses.27 Subgroup analyses were planned based on the type of IST used. All analyses were performed with Comprehensive Meta-Analysis (CMA 2.2, Biostat).
Results
Description of included studies
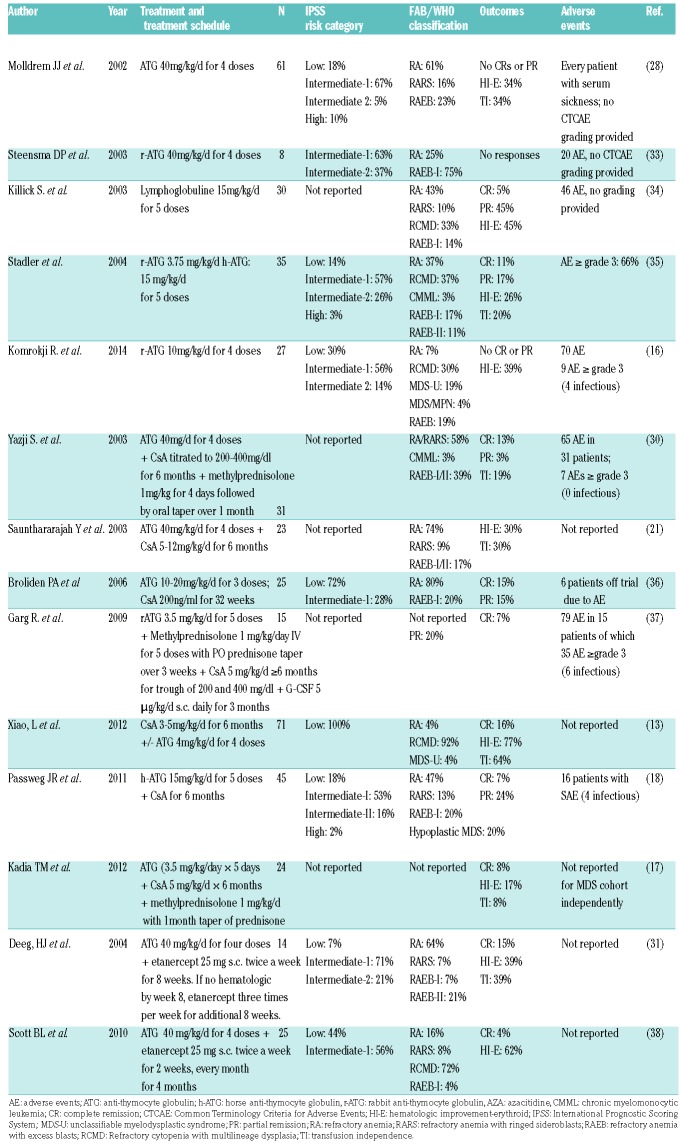
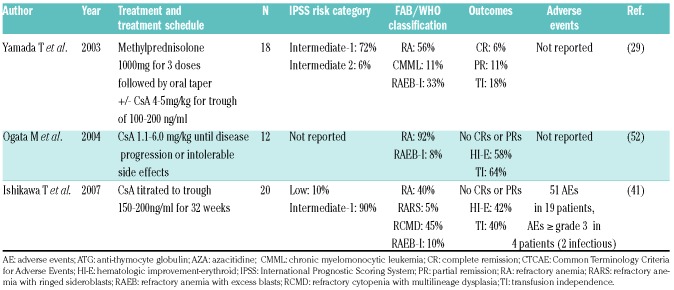
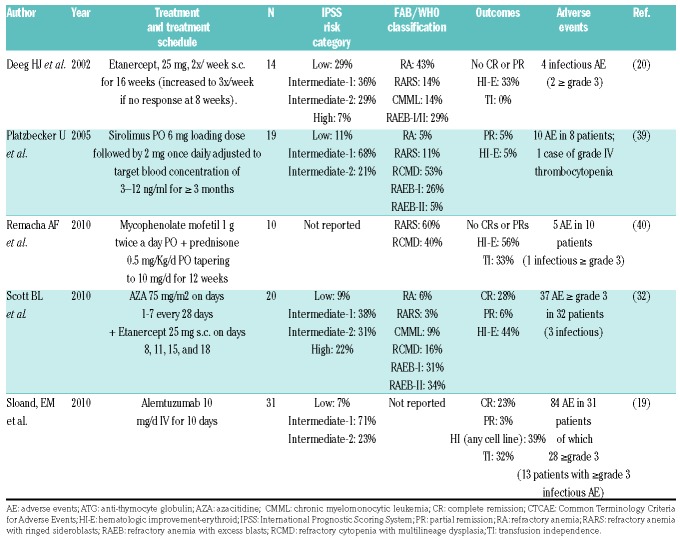
The flow diagram of study selection based on the MOOSE guidelines is shown in Figure 1. An electronic search of PubMed, EMBASE, the Cochrane Library, and the Web of Science plus a manual search retrieved a total of 258 publications after removal of duplicates. Of the 258 articles reviewed, 156 articles were excluded on the basis of the title and abstract review if the article was clearly labeled as a review article, editorial, correspondence letter, case series or retrospective study in the title or abstract. A total of 102 articles were reviewed in full text. Of these, 80 articles were excluded because they were reviews, basic science articles, presented insufficient data (<5 patients), only showed preliminary results, or were retrospective in nature. Of the 22 studies included, there were 9 prospective cohort studies13,21,28–32 and 13 clinical trials.16–20,33–40 Patients were treated with ATG, ATG + CsA, ATG + Etanercept (Table 1), CsA (Table 2), and other IST regimens (Table 3).
Table 1.
ATG.
Table 2.
Cyclosporine A.
Table 3.
Other immunosuppressive therapy regimens.
There was a total of 570 patients in the 22 included studies. The average median age was 62.0 years (range 12-87 years). Among the studies that reported IPSS scores, 360 (80.9%) patients had IPSS scores of low- or intermediate-1, while 71 patients (16.0%) had intermediate-2 and high IPSS scores. The median duration of follow up of individual studies, where reported, was 16.4 months (range 0-60 months).
Assessment of study quality
Except for three studies,18,29,35 all studies included in this meta-analysis used a single-arm design. Study quality was assessed using the Downs and Black checklist. Assessments for individual studies are provided in Online Supplementary Table S1.
Rates of overall response and complete remission
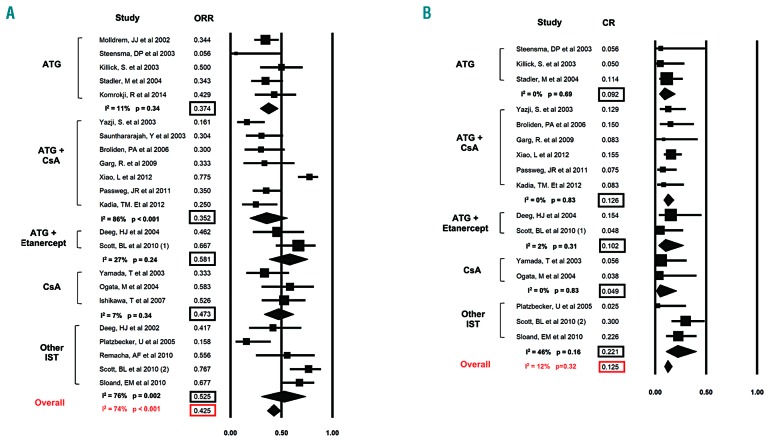
The ORR was reported by all 22 studies (Figure 2A). Overall, the ORR was 42.5% (95%CI: 36.1-49.2%). There was a significant heterogeneity among the various studies, with a Cochran’s Q statistic of 80% (P<0.001) and an I2 statistic of 74%.
Figure 2.
Overall and complete response rates to various forms of immunosuppressive therapy (IST). Forest plots of odds ratios (squares, proportional to study weights used in meta-analysis, 95% confidence intervals) for various forms of IST with the summary measures (center line of diamond) and associated confidence intervals (lateral tips of diamond) for overall response rate (ORR) and complete response (CR) rate are shown in panel (A) and (B), respectively.
A pre-specified subgroup analysis showed that the ORR was highest with ATG + Etanercept at 58.1% (95%CI: 37.8-75.9%; I2=27%), followed by other IST at 52.5% (95%CI: 30.4-73.7%; I2=76%), CsA at 47.3% (95%CI: 33-62%; I2=7%), ATG at 37.4% (95%CI: 29.1-46.6%; I2=11%), and ATG + CsA at 35.2% (95%CI: 18.9-55.9%; I2=86%), respectively (Figure 2A).
Complete remission rates were reported by 16 studies (Figure 2B). Overall, the CR rate was 12.5% (95%CI: 9.3-16.6%). Heterogeneity among the various studies was low, with a Cochran’s Q statistic of 17 (P=0.32) and an I2 statistic of 12%.
A pre-specified subgroup analysis for patients, who received ATG, ATG + CsA, ATG + Etanercept, CsA and other IST, showed that the CR was 9.2% (95%CI: 4.0-19.6%; I2=0%), 12.6% (95%CI: 8.6-18.1%; I2=0%), 10.2% (95%CI: 3.3-27.8%; I2=2%), 4.9% (95%CI: 1.0-21.1%; I2=0) and 22.1% (95%CI: 10.6-40.4%; I2=46%), respectively (Figure 2B).
Hematologic improvement and transfusion independence
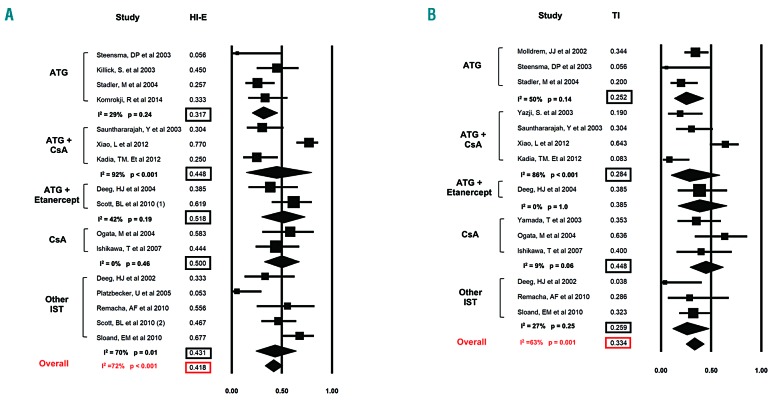
Erythroid hematologic improvement rates were reported by 16 studies (Figure 3A). Overall, the HI-E rate was 41.8% (95%CI: 33.3-50.8%). Heterogeneity among the various studies was high, with a Cochran’s Q statistic of 53.1 (P<0.001) and an I2 statistic of 72%.
Figure 3.
Rate of hematologic improvement in the erythroid lineage (HI-E) and red blood cell transfusion independence. Forest plots of odds ratios (squares, proportional to study weights used in meta-analysis, 95% confidence intervals) for various forms of immunosuppressive therapy (IST), with the summary measure (center line of diamond) and associated confidence intervals (lateral tips of diamond) for hematologic improvement in the erythroid lineage (HI-E) and achievement of red blood cell transfusion independence (TI) are shown in panel (A) and (B), respectively.
A pre-specified subgroup analysis showed that the HI-E rate was highest with ATG + Etanercept at 51.8% (95%CI: 29.8-73.1%; I2=42%), followed by CsA at 50% (95%CI: 32.7-67.3%; I2=0), ATG + CsA at 44.8% (95%CI: 14.3-79.8%; I2=92%), other IST agents at 43.1% (95%CI: 24.0-64.4%; I2=70%), and ATG at 31.7% (95%CI: 20.3-45.8%; I2=29%), respectively (Figure 3A).
The rates of TI were reported by 14 studies (Figure 3B). Overall, the TI was 33.4% (95%CI: 25.1-42.9%). There was a significant heterogeneity among the various studies, with a Cochran’s Q statistic of 35.1 (P=0.001) and an I2 statistic of 63%.
A pre-specified subgroup analysis showed that the TI rate was highest with CsA at 44.8% (95%CI: 28.8-61.9%; I2=9%) followed by ATG + Etanercept at 38.5% (95%CI: 17-65.6%; I2=0%), ATG at 25.2% (95%CI: 13.3-42.5%; I2=50%), ATG + CsA at 28.4% (95%CI: 10.0-58.6%; I2=86%), and other IST at 25.9% (95%CI: 11.7-48.0; I2=27%), respectively (Figure 3B).
Acute myeloid leukemia progression rate and adverse events
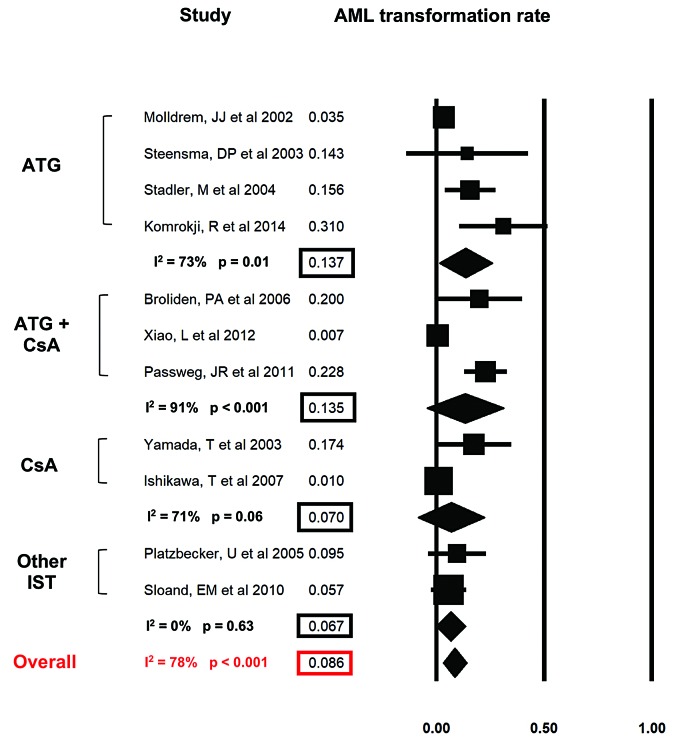
The rates of progression to AML were reported by 11 studies (Figure 4). Overall, the AML progression rate per person year of follow up was low (8.6%; 95%CI: 3.3-13.9%). There was a significant heterogeneity among the various studies, with a Cochran’s Q statistic of 45.2 (P<0.001) and an I2 statistic of 78%. Pre-specified subgroup analysis showed an AML transformation rate per patient year of 13.7% (95%CI: 1.4-25.9%; I2=73%), 13.5% (95%CI: 0-31.3; I2=91%), 7.0% (95%CI: 0-22.4%; I2=71%), and 6.7% (95%CI: 0-13.5%; I2=0%) for patients who received ATG, ATG + CsA, CsA and other IST, respectively.
Figure 4.
Rate of acute myeloid leukemia (AML) progression during study duration. Forest plots of odds ratios (squares, proportional to study weights used in meta-analysis, 95% confidence intervals) for various forms of immunosuppressive therapy (IST), with the summary measure (center line of diamond) and associated confidence intervals (lateral tips of diamond) for rate of transformation to AML during the study period.
Only 10 of the 22 studies reported grade 3/4 side effects.16,18,19,30,32,35,37,39–41 The data included in these papers were insufficient to conduct any further meta-analysis on the safety of IST in LR-MDS.
Sensitivity analysis
Separate sensitivity analyses for ORR, HI, TI and AML progression rate showed that exclusion of any one study did not change the overall effect direction but did change the effect size in subgroup analysis, and led to a reduction in heterogeneity.
For ORR, HI-E and TI, the study with the largest influence on the heterogeneity of these outcomes was the study by Xiao et al. examining the use of ATG + CsA.13 Removal of this study changed the ORR by 5.1% (from 42.5% to 37.4%) in the overall analysis and by 6.5% (from 35.2% to 28.7%) in the subgroup analysis of studies examining ATG + CsA. In addition, removal of this study led to a loss of heterogeneity in the overall analysis (I2=62%, Cochran’s Q statistic = 52.1, P=0.001) and in the subgroup analysis of studies examining ATG + CsA (I2= 0%, Cochran’s Q statistic = 3.4, P=0.64). Removal of the study by Xiao et al. also changed the HI-E by 3.9% (from 41.8% to 37.9%) in the overall analysis and 17.1% (from 44.8% to 27.7) in the subgroup analysis of studies examining ATG + CsA. This led to a loss of heterogeneity in the overall analysis (I2 = 55%, Cochran’s Q statistic = 31.1, P=0.005) and in the subgroup analysis of studies examining ATG + CsA (I2 = 0%, Cochran’s Q statistic = 0.17, P=0.68). For TI, removal of this study changed the outcome by 2.8% (from 33.4% to 30.6%) in the overall analysis and by 8.8% (from 28.4% to 19.6%) in the subgroup analysis of studies examining ATG + CsA. This also led to a loss of heterogeneity in the overall analysis (I2=35%, Cochran’s Q statistic = 18.6, P=0.1) and in the subgroup analysis of studies examining ATG + CsA (I2=40.6, Cochran’s Q statistic = 3.4, P=0.19).
The study with the largest influence on the AML progression rate was that reported by Passweg et al.,18 the removal of which changed the AML progression rate by 0.5% (from 8.6% to 8.1%). In addition, removal of this study led to a loss of heterogeneity (I2 = 67%, Cochran’s Q statistic = 27.6, P=0.001).
Discussion
To our knowledge, this is the first systematic review and meta-analysis on IST in MDS, and included a total of nine prospective cohort studies and 13 clinical trials. The meta-analysis of these studies demonstrated an ORR of 42.5% (with a CR rate of 12.5%) and achievement of red blood cell transfusion independence in one-third of the patients.
Previous retrospective studies reported similar ORR and TI rates among MDS patients with IST. A recent uncontrolled large international retrospective study in MDS patients treated with various different IST regimens demonstrated results very similar to our meta-analysis, with an ORR and TI rate of 48% (with 11.2% achieving a CR) and 30% of patients, respectively. In other large retrospective studies, the ORR and TI rates ranged from 30% to 42% and from 31% to 41%, respectively.22,23,42 In our meta-analysis, ATG +/− CsA was the most commonly used IST regimen, similar to the finding in a recent large retrospective study.12 Importantly, while the National Comprehensive Cancer Network (NCCN; Version 2.2019) guidelines suggest the use of ATG +/− CsA as a treatment option for certain types of MDS,14 they do not suggest other IST regimens. However, in our meta-analysis, multiple different IST regimens other than ATG +/− CsA were included, among them ATG + etanercept, CsA and several “other IST” regimens including etanercept +/− azacitidine, sirolimus, mycophenolate mofetil and alemtuzumab. It is important to point out that these other IST regimens do not have completely overlapping mechanisms of action, tolerability, and expected response rates compared to ATG and CsA. Acknowledging these differences, we included these IST regimens in our analysis as they all provide an element of immunosuppression regardless of their specific mechanism of action.
While the identification of clinical and molecular markers to predict response to IST would be of clinical importance to optimize treatment of individual patients, data for several of these co-variates that had been proposed, such as younger age, shorter disease duration, hypocellular bone marrow, or the presence of HLA DR15 and PNH clones, are controversial.12,21–23,42,43 Given the heterogeneity of the studies included in this meta-analysis, we were unable to address the utility of predictive biomarkers as they were only reported by a minority of studies. However, several studies, including the largest study to date by Stahl et al., were unable to validate any predictive biomarkers except of bone marrow hypocellularity.42–44
Based on available prospective data, the current National Comprehensive Cancer Network guidelines suggest IST with ATG +/− CsA as a treatment option for symptomatic anemia in low-risk, non-del(5q) MDS especially for patients younger than 60 years of age, ≤5% blasts in the bone marrow, or with a hypocellular bone marrow, PNH clone positivity, or STAT-3 mutant cytotoxic T-cell clones.14 However, further prospective studies are warranted to verify these predictive markers. It also remains to be shown how the wider availability of genetic testing, for example, by next generation sequencing, will impact individualized treatment decisions for MDS patients. Supporting a potential role in guiding management decisions, two recent phase II clinical trials on the transforming growth factor (TGF)-β pathway inhibitors luspatercept and sotatercept have shown that the presence of ≥15% ringed sideroblasts or of a SF3B1 mutation was predictive of a higher rate of treatment response.45–47
Given the small sample sizes of most studies, the different treatment regimens used, and the various diagnostic techniques employed, there was a high degree of heterogeneity among included studies. However, sensitivity analyses accounting for the type of IST as well as a ‘one-study removed’ analysis, found no significant impact of this heterogeneity on the overall results of the meta-analysis. The study by Xiao et al.13 demonstrated a significantly higher ORR and rate of HI-E and TI compared to other studies studying the application of ATG + CsA. This can be explained by the fact that in the study by Xiao et al.13 patients were strictly selected to have a high chance of responding to IST. Patients were required to have low risk MDS (IPSS score equal or less 1.0) and either expression of the HLA-DR15 allele or a BM cellularity of less than 30%, or an abnormal immune index of BM T lymphocytes. Furthermore, patients were excluded if they had >5% marrow myeloblasts or a poor risk karyotype or a diagnosis of concurrent non-hematologic malignancy. By excluding the study by Xiao et al.,13 from the analysis, heterogeneity in the subgroup analysis of studies examining ATG + CsA decreased significantly. This demonstrates that by strict selection of MDS patients, who are predicted to benefit from IST, the response to IST can be significantly increased.
While a randomized, placebo-controlled, double-blinded design is the gold standard of clinical studies, the het-erogenous patient population in terms of demographic, clinical (e.g. prior treatments), and molecular co-factors makes such a trial design challenging. As expected, this systematic review and meta-analysis confirmed the lack of published prospective, randomized controlled trials of IST in MDS. In this meta-analysis, 20 out of 22 included studies were single arm clinical trials or prospective cohort studies. As the Downs and Black checklist had been originally developed for the evaluation of multi-arm studies, several of its quality criteria such as randomization, equal distribution of confounding variables among study groups, and blinding were not applicable to the majority of the studies in our meta-analysis. However, when eliminating items from the modified Downs and Black check list that are only applicable to multi-arm studies, 12 out of 22 studies scored at least 15 out of the remaining 20 points. A notable exception in terms of methodological quality was the phase III trial by Passweg et al. comparing ATG + CsA to best supportive care.18 Importantly, treatment with ATG + CsA resulted in a hematologic response in 31% of patients in this trial which is comparable to the 33.7% for ATG + CsA in our meta-analysis. Although the limitations in terms of study quality must be kept in mind, the overall comparable results among different studies suggest that our meta-analysis provides robust evidence on the effect of IST in the treatment of MDS.
Previous studies have suggested that IST may contribute to the risk of progression to AML because of a suppression of immune surveillance. However, this seems to be more relevant in high-risk MDS subtypes rather than the lower risk MDS forms that constitute the patients primarily treated with IST.48,49 In our meta-analysis, we found a rate of progression to AML of 8.6% per patient year (95%CI: 3.3-13.9%). The risk of progression to AML varies substantially based on the IPSS risk category as well as the presence of certain cytogenetic abnormalities.50 The majority of patients in our meta-analysis had either low (32.8%) or intermediate-1 (49.2%) risk disease by IPSS (Tables 1–3). In previous studies, the time of progression to AML in the absence of treatment for 25% of patients with IPSS-low and intermediate-1 MDS patients was reported at 10.8 and 3.2 years, respectively.51 Although comparison of our data with these historical results is limited, our meta-analysis does not show a significantly higher AML transformation rate than expected for IPSS lower and intermediate-1 risk MDS patients in general.
Our study has several limitations. In many of these studies, the patients included were selected and judged by the investigators to potentially benefit from IST. Therefore, the efficacy results might be inflated and not necessarily apply to all lower-risk MDS patients. In addition to the heterogeneity of studies and the overall low methodological quality, there were insufficient data to assess adverse events in our meta-analysis. While at least a minimum amount of information on treatment-associated adverse events was provided in 17 out of 22 studies, only 5 studies provided CTCAE grading of adverse events and reported those on a patient level.18,35,39–41 Given the heterogeneity of adverse event reporting, we were unable to conduct a meta-analysis on the side effect profile of IST in MDS. As IST is most commonly used for lower-risk MDS patients to alleviate symptom burden resulting from red blood cell transfusion dependence and to limit the detrimental sequelae of resulting iron overload rather than modifying AML transformation risk, information on treatment-associated adverse events is essential for physicians to appropriately counsel patients. We were not able to analyze the effect of IST on platelet transfusion independence as it was reported in only the minority of studies. Lastly, publication bias could not be assessed in this meta-analysis because of the lack of a comparative treatment arm in the majority of the studies.
Given that the median overall survival rate among untreated patients with lower-risk MDS is 5.3 years, a long duration of follow up would be required to detect any survival benefit from IST and data on overall survival were provided in 5 out of 23 studies only. Therefore, we were unable to assess whether IST provides an actual survival benefit in MDS.
Conclusions
In summary, our data showed an ORR of 42.5% and a TI rate of 33.4% for IST in MDS, with ATG-based treatment regimens being the most commonly used option. Response rates tended to be higher for combination therapy of ATG in conjunction with mostly CsA compared to ATG monotherapy. While we were unable to assess the utility of various biomarkers in predicting response to IST, current guidelines recommend considering IST for symptomatic treatment of lower-risk MDS patients to alleviate transfusion burden and associated sequelae. However, given the lack of prospective, randomized, controlled studies, it is difficult to definitively determine the impact of IST on response and survival in patients with MDS, and randomized trials of IST are warranted to confirm our results.
Footnotes
Check the online version for the most updated information on this article, online supplements, and information on authorship & disclosures: www.haematologica.org/content/105/1/102
Funding
Amer Zeidan is a Leukemia and Lymphoma Society Scholar in Clinical Research and is also supported by a National Cancer Institute (NCI) Cancer Clinical Investigator Team Leadership Award (CCITLA). Research reported in this publication was supported by the NCI of the National Institutes of Health under Award Number P30 CA016359. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
References
- 1.Arber DA, Orazi A, Hasserjian R, et al. The 2016 revision to the World Health Organization classification of myeloid neoplasms and acute leukemia. Blood. 2016;127(20):2391–2405. [DOI] [PubMed] [Google Scholar]
- 2.Swerdlow SH, Harris NL, Jaffe ES, et al. WHO Classification of Tumours of Haematopoietic and Lymphoid Tissues. WHO Classification of Tumours, Revised 4th Edition. 2017; Volume 2. [Google Scholar]
- 3.Steensma DP. Myelodysplastic syndromes current treatment algorithm 2018. Blood Cancer J. 2018;8(5):47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Montalban-Bravo G, Takahashi K, Patel K, et al. Impact of the number of mutations in survival and response outcomes to hypomethylating agents in patients with myelodysplastic syndromes or myelodysplastic/myeloproliferative neoplasms. Oncotarget. 2018;9(11):9714–9727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Greenberg PL, Stone RM, Al-Kali A, et al. Myelodysplastic Syndromes, Version 2.2017, NCCN Clinical Practice Guidelines in Oncology. J Natl Compr Canc Netw. 2017;15(1):60–87. [DOI] [PubMed] [Google Scholar]
- 6.Fenaux P, Ades L. How we treat lower-risk myelodysplastic syndromes. Blood. 2013; 121(21):4280–4286. [DOI] [PubMed] [Google Scholar]
- 7.Stahl M, Zeidan AM. Lenalidomide use in myelodysplastic syndromes: Insights into the biologic mechanisms and clinical applications. Cancer. 2017;123(10):1703–1713. [DOI] [PubMed] [Google Scholar]
- 8.Montoro J, Gallur L, Merchan B, et al. Autoimmune disorders are common in myelodysplastic syndrome patients and confer an adverse impact on outcomes. Ann Hematol. 2018;97(8):1349–1356. [DOI] [PubMed] [Google Scholar]
- 9.Komrokji RS, Kulasekararaj A, Al Ali NH, et al. Autoimmune diseases and myelodysplastic syndromes. Am J Hematol. 2016; 91(5):E280–E283. [DOI] [PubMed] [Google Scholar]
- 10.Young NS. Aplastic Anemia. N Engl J Med. 2018;379(17):1643–1656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Olnes MJ, Sloand EM. Targeting immune dysregulation in myelodysplastic syn dromes. JAMA. 2011;305(8):814–819. [DOI] [PubMed] [Google Scholar]
- 12.Stahl M, DeVeaux M, de Witte T, Neukirchen J, et al. The use of immunosuppressive therapy in MDS: clinical outcomes and their predictors in a large international patient cohort. Blood Adv. 2018;2(14):1765–1772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Xiao L, Qi Z, Qiusheng C, et al. The use of selective immunosuppressive therapy on myelodysplastic syndromes in targeted populations results in good response rates and avoids treatment-related disease progression. Am J Hematol. 2012;87(1):26–31. [DOI] [PubMed] [Google Scholar]
- 14.National Comprehensive Cancer Network. NCCN Guidelines Version 2.2019: Myelodysplastic syndromes 2019. [cited 2018 20.12.2018]; Available from: https://www.nccn.org/store/login/login.aspx?ReturnURL=https%3a%2f%2fwww.nccn.org%2fprofessionals%2fphysician_gls%2fPDF%2fmds.pdf
- 15.Molldrem JJ, Caples M, Mavroudis D, et al. Antithymocyte globulin for patients with myelodysplastic syndrome. Br J Haematol. 1997;99(3):699–705. [DOI] [PubMed] [Google Scholar]
- 16.Komrokji RS, Mailloux AW, Chen DT, et al. A phase II multicenter rabbit anti-thymocyte globulin trial in patients with myelodysplastic syndromes identifying a novel model for response prediction. Haematologica. 2014;99(7):1176–1183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kadia TM, Borthakur G, Garcia-Manero G, et al. Final results of the phase II study of rabbit anti-thymocyte globulin, ciclosporin, methylprednisone, and granulocyte colony-stimulating factor in patients with aplastic anaemia and myelodysplastic syndrome. Br J Haematol. 2012;157(3):312–320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Passweg JR, Giagounidis AA, Simcock M, et al. Immunosuppressive therapy for patients with myelodysplastic syndrome: a prospective randomized multicenter phase III trial comparing antithymocyte globulin plus cyclosporine with best supportive care--SAKK 33/99. J Clin Oncol. 2011;29(3):303–309. [DOI] [PubMed] [Google Scholar]
- 19.Sloand EM, Olnes MJ, Shenoy A, et al. Alemtuzumab treatment of intermediate-1 myelodysplasia patients is associated with sustained improvement in blood counts and cytogenetic remissions. J Clin Oncol. 2010;28(35):5166–5173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Deeg HJ, Gotlib J, Beckham C, et al. Soluble TNF receptor fusion protein (etanercept) for the treatment of myelodysplastic syndrome: a pilot study. Leukemia. 2002;16(2):162–164. [DOI] [PubMed] [Google Scholar]
- 21.Saunthararajah Y, Nakamura R, Wesley R, et al. A simple method to predict response to immunosuppressive therapy in patients with myelodysplastic syndrome. Blood. 2003;102(8):3025–3027. [DOI] [PubMed] [Google Scholar]
- 22.Lim ZY, Killick S, Germing U, et al. Low IPSS score and bone marrow hypocellularity in MDS patients predict hematological responses to antithymocyte globulin. Leukemia. 2007;21(7):1436–1441. [DOI] [PubMed] [Google Scholar]
- 23.Sloand EM, Wu CO, Greenberg P, et al. Factors affecting response and survival in patients with myelodysplasia treated with immunosuppressive therapy. J Clin Oncol. 2008;26(15):2505–2511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Stroup DF, Berlin JA, Morton SC, et al. Meta-analysis of observational studies in epidemiology: a proposal for reporting. Meta-analysis Of Observational Studies in Epidemiology (MOOSE) group. JAMA. 2000;283(15):2008–2012. [DOI] [PubMed] [Google Scholar]
- 25.Cheson BD, Greenberg PL, Bennett JM, et al. Clinical application and proposal for modification of the International Working Group (IWG) response criteria in myelodysplasia. Blood. 2006;108(2):419–425. [DOI] [PubMed] [Google Scholar]
- 26.Downs SH, Black N. The feasibility of creating a checklist for the assessment of the methodological quality both of randomised and non-randomised studies of health care interventions. J Epidemiol Community Health. 1998;52(6):377–384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Collaboration TC. The Cochrane Collaboration. Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0.2011. 2011. [Google Scholar]
- 28.Molldrem JJ, Leifer E, Bahceci E, et al. Antithymocyte globulin for treatment of the bone marrow failure associated with myelodysplastic syndromes. Ann Intern Med. 2002;137(3):156–163. [DOI] [PubMed] [Google Scholar]
- 29.Yamada T, Tsurumi H, Kasahara S, et al. Immunosuppressive therapy for myelodysplastic syndrome: efficacy of methylprednisolone pulse therapy with or without cyclosporin A. J Cancer Res Clin Oncol. 2003;129(8):485–491. [DOI] [PubMed] [Google Scholar]
- 30.Yazji S, Giles FJ, Tsimberidou AM, et al. Antithymocyte globulin (ATG)-based therapy in patients with myelodysplastic syndromes. Leukemia. 2003;17(11):2101–2106. [DOI] [PubMed] [Google Scholar]
- 31.Deeg HJ, Jiang PY, Holmberg LA, et al. Hematologic responses of patients with MDS to antithymocyte globulin plus etanercept correlate with improved flow scores of marrow cells. Leuk Res. 2004;28(11):1177–1180. [DOI] [PubMed] [Google Scholar]
- 32.Scott BL, Ramakrishnan A, Storer B, et al. Prolonged responses in patients with MDS and CMML treated with azacitidine and etanercept. Br J Haematol. 2010;148(6):944–947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Steensma DP, Dispenzieri A, Moore SB, et al. Antithymocyte globulin has limited efficacy and substantial toxicity in unselected anemic patients with myelodysplastic syndrome. Blood. 2003;101(6):2156–2158. [DOI] [PubMed] [Google Scholar]
- 34.Killick SB, Mufti G, Cavenagh JD, et al. A pilot study of antithymocyte globulin (ATG) in the treatment of patients with ‘low-risk’ myelodysplasia. Br J Haematol. 2003;120(4):679–684. [DOI] [PubMed] [Google Scholar]
- 35.Stadler M, Germing U, Kliche KO, et al. A prospective, randomised, phase II study of horse antithymocyte globulin vs rabbit antithymocyte globulin as immune-modulating therapy in patients with low-risk myelodysplastic syndromes. Leukemia. 2004;18(3):460–465. [DOI] [PubMed] [Google Scholar]
- 36.Broliden PA, Dahl IM, Hast R, et al. Antithymocyte globulin and cyclosporine A as combination therapy for low-risk non-sideroblastic myelodysplastic syndromes. Haematologica. 2006;91(5):667–670. [PubMed] [Google Scholar]
- 37.Garg R, Faderl S, Garcia-Manero G, et al. Phase II study of rabbit anti-thymocyte globulin, cyclosporine and granulocyte colony-stimulating factor in patients with aplastic anemia and myelodysplastic syndrome. Leukemia. 2009;23(7):1297–1302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Scott BL, Ramakrishnan A, Fosdal M, et al. Anti-thymocyte globulin plus etanercept as therapy for myelodysplastic syndromes (MDS): a phase II study. Br J Haematol. 2010;149(5):706–710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Platzbecker U, Haase M, Herbst R, et al. Activity of sirolimus in patients with myelodysplastic syndrome--results of a pilot study. Br J Haematol. 2005;128(5):625–630. [DOI] [PubMed] [Google Scholar]
- 40.Remacha AF, Arrizabalaga B, Bueno J, et al. Treatment with mycophenolate mofetil followed by recombinant human erythropoi-etin in patients with low-risk myelodysplastic syndromes resistant to erythropoietin treatment. Haematologica. 2010; 95(2):339–340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ishikawa T, Tohyama K, Nakao S, et al. A prospective study of cyclosporine A treatment of patients with low-risk myelodys-plastic syndrome: presence of CD55(-)CD59(-) blood cells predicts platelet response. Int J Hematol. 2007;86(2):150–157. [DOI] [PubMed] [Google Scholar]
- 42.Haider M, Al Ali N, Padron E, et al. Immunosuppressive Therapy: Exploring an Underutilized Treatment Option for Myelodysplastic Syndrome. Clin Lymphoma Myeloma Leuk. 2016;16 Suppl:S44–S48. [DOI] [PubMed] [Google Scholar]
- 43.Komrokji RS, Haider M, Al Ali NH, et al. Somatic Gene Mutations Serve As Molecular Biomarkers Predictive for Response to Immunosuppressive Therapy (IST) in Myelodysplastic Syndromes (MDS). Blood. 2015;126(23):1664. [Google Scholar]
- 44.Shallis RM, Chokr N, Stahl M, et al. Immunosuppressive therapy in myelodysplastic syndromes: a borrowed therapy in search of the right place. Expert Rev Hematol. 2018;11(9):715–726. [DOI] [PubMed] [Google Scholar]
- 45.Fenaux P, Platzbecker U, Mufti GJ, et al. The Medalist Trial: Results of a Phase 3, Randomized, Double-Blind, Placebo-Controlled Study of Luspatercept to Treat Anemia in Patients with Very Low-, Low-, or Intermediate-Risk Myelodysplastic Syndromes (MDS) with Ring Sideroblasts (RS) Who Require Red Blood Cell (RBC) Transfusions. Blood. 2018;132(Suppl 1):1.29976776 [Google Scholar]
- 46.Platzbecker U, Germing U, Gotze KS, et al. Luspatercept for the treatment of anaemia in patients with lower-risk myelodysplastic syndromes (PACE-MDS): a multicentre, open-label phase 2 dose-finding study with long-term extension study. Lancet Oncol. 2017;18(10):1338–1347. [DOI] [PubMed] [Google Scholar]
- 47.Komrokji R, Garcia-Manero G, Ades L, et al. Sotatercept with long-term extension for the treatment of anaemia in patients with lower-risk myelodysplastic syndromes: a phase 2, dose-ranging trial. Lancet Haematol. 2018;5(2):e63–e72. [DOI] [PubMed] [Google Scholar]
- 48.Wang C, Yang Y, Gao S, et al. Immune dysregulation in myelodysplastic syndrome: Clinical features, pathogenesis and therapeutic strategies. Crit Rev Oncol Hematol. 2018;122:123–132. [DOI] [PubMed] [Google Scholar]
- 49.Kojima S, Ohara A, Tsuchida M, et al. Risk factors for evolution of acquired aplastic anemia into myelodysplastic syndrome and acute myeloid leukemia after immunosuppressive therapy in children. Blood. 2002;100(3):786–790. [DOI] [PubMed] [Google Scholar]
- 50.Santini V. Treatment of low-risk myelodysplastic syndromes. Hematology Am Soc Hematol Educ Program. 2016;2016(1):462–469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Greenberg PL, Tuechler H, Schanz J, et al. Revised international prognostic scoring system for myelodysplastic syndromes. Blood. 2012;120(12):2454–2465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ogata M, Ohtsuka E, Imamura T, et al. Response to cyclosporine therapy in patients with myelodysplastic syndrome: a clinical study of 12 cases and literature review. Int J Hematol. 2004;80(1):35–42. [DOI] [PubMed] [Google Scholar]