Abstract
Hematopoietic stem cell (HSC) aging was originally thought to be essentially an HSC-autonomous process, which is the focus of another review in the same issue of Haematologica. However, studies on the microenvironment that maintains and regulates HSC (HSC niche) over the past 20 years have suggested that microenvironmental aging contributes to declined HSC function over time. The HSC niches comprise a complex and dynamic molecular network of interactions across multiple cell types, including endothelial cells, mesenchymal stromal cells, osteoblasts, adipocytes, neuroglial cells and mature hematopoietic cells. Upon aging, functional changes in the HSC niches, such as microenvironmental senescence, imbalanced bone marrow mesenchymal stromal cell differentiation, vascular remodeling, changes in adrenergic signaling and inflammation, coordinately and dynamically influence the fate of HSC and their downstream progeny. The end result is lymphoid deficiency and myeloid skewing. During this process, aged HSC and their derivatives remodel the niche to favor myeloid expansion. Therefore, the crosstalk between HSC and the microenvironment is indispensable for the aging of the hematopoietic system and might represent a therapeutic target in age-related pathological disorders.
Introduction to hematopoietic stem cell aging
Adult hematopoiesis takes place in the bone marrow (BM), where hematopoietic stem cells (HSC) can self-renew, proliferate and differentiate to replenish the blood and immune systems. Given that most HSC are quiescent under homeostasis, mature blood and immune cell production is believed to derive mainly under steady state from progenitor cells (rather than HSC), which differentiate to produce mature blood cells. Cumulative studies have demonstrated that HSC are heterogeneous and contain subsets with distinct myeloid, platelet or lymphoid-biased potentials, although the existence of lymphoid-biased HSC has long been debated and remains controversial.1–5 Additionally, recent studies have shown that HSC can bypass the intermediate steps to generate mature progenies under certain conditions, such as chronic inflammation and aging.
Upon aging, HSC increase in number but their functions are impaired, characterized by reduced regenerative and homing capacity, loss of cell polarity, and myeloid-biased differentiation at the expense of lymphopoiesis.6–9 These changes were initially thought to cause only cell-intrinsic dysregulation,10 such as epigenetic deregulation,11 replication stress,12 deficient DNA repair,13 and transition from canonical to non-canonical Wnt signaling.14 Old HSC also suffer metabolic changes,15,16 impaired autophagy17 and altered protein homeostasis,18 which contribute to the decline of their regenerative potential. However, current studies are revealing that the BM microenvironment may contribute to HSC aging. This hypothesis is supported by an elegant study in which old HSC transplanted into young recipients exhibited reduced myeloid output as compared those transplanted into old recipients, suggesting that the old BM microenvironment contributes to myeloid skewing.19 This review will cover microenvironmental contributions to HSC aging, provide hypotheses for BM niche remodeling based on current knowledge, and discuss the potential implications for age-related myeloid malignancies. HSC-intrinsic aging mechanisms are the focus of a separate complementary review in this issue of Haematologica and will not be discussed here.
Evolving views on hematopoietic stem cell niches
HSC are surrounded by numerous cell types and the associated extracellular matrix in the BM, which form a unique microenvironment known as the “HSC niche’’. Osteoblasts were the first niche cells found to be involved in hematopoiesis. Early studies indicated that osteoblasts differentiate from BM osteoprogenitor cells, secrete hematopoietic cytokines and can maintain HSC in culture.20 In 2003, two studies described for the first time that transplanted HSC localize to the bone surface of BM and their numbers are regulated by osteoblastic cells. Long-term HSC were found to adhere to spindle-shaped N-cadherin+CD45− osteoblastic (SNO) cells, which control HSC size by BMP signaling.21 A recent study has shown that N-cadherin+ cells maintain a population of highly quiescent reserve HSC,22 suggesting the possibility that different BM niches might regulate steady-state vs. stress hematopoiesis. Another study showed that osteoblasts activated with parathyroid hormone/parathyroid hormone-related protein receptor produce high levels of Notch ligand Jagged 1 and increase HSC numbers.23 Later studies further identified Tie2/angiopoietin-1 signaling and thrombopoietin/MPL signaling as important regula tors of HSC quiescence through interactions with osteoblasts.24,25 A high calcium concentration in the endosteum also plays an indispensable role, maintaining HSC in the endosteal niche, since calcium-sensing receptor knockout HSC fail to migrate to the endosteal BM surface after transplantation.26 In addition, the endosteal BM area is enriched in CXCL1227 and stem cell factor,28 two of the most important molecules supporting hematopoiesis, strengthening the hypothesis that the endosteum is a major reservoir for HSC. However, the osteoblastic niche was thereafter challenged in studies in which osteoblastic-specific deletion of Cxcl12 or Scf only affected the maintenance of early lymphoid progenitors but had little impact on HSC.29 Furthermore, N-cadherin expression in osteolineage cells seems to be dispensable for HSC maintenance under homeostasis.30 Whereas N-cadherin might not be essential for HSC, N-cadherin+ cells appear necessary to maintain a reservoir population of quiescent HSC.22 Studies on these aspects raise the possibility that different niches might exist for activated/quiescent HSC, and/or for HSC contributing to steady-state/emergency hematopoiesis. The BM is highly vascularized, and the close developmental relationship between hematopoietic and endothelial lineages together suggest that HSC are housed and regulated in perivascular regions. To date, at least two functionally distinct perivascular niches that highly express Cxcl12 and Scf to dictate HSC cell fate have been identified in mice: (i) the arteriolar niches, composed mainly of arterioles (found throughout the BM) or endosteal transition-zone vessels, both of which are associated with sympathetic nerve fibers, Nestin-GFPbright and/or NG2+ cells; and (ii) the sinusoidal niches, where sinusoid-associated Cxcl12-abundant reticular cells, Nestin-GFPdim and LepR+ cells are located.31 Recent studies also reveal that megakaryocytes, which are mostly adjacent to sinusoids, regulate HSC quiescence through transforming growth factor-β, thrombopoietin and platelet factor-4 secretion.32–34 Currently, it remains controversial which specialized niches predominantly regulate HSC quiescence. It is possible that HSC quiescence is differently regulated between steady-state and emergency and/or malignant hematopoiesis. However, lineage commitment appears to be influenced by the location of HSC and their derivatives in the BM. Accumulating evidence suggests that lymphopoiesis preferentially occurs near the endosteum, while myelopoiesis/erythropoiesis/megakaryopoiesis mostly takes place in non-endosteal BM regions. Supporting this concept, a recent study using Vwf-eGFP to label different HSC populations demonstrated that Vwf+ platelet/myeloid-biased HSC are associated with megakaryocytes, whereas Vwf− lymphoid/unbiased HSC are located close to arterioles.35 Therefore, alterations in specialized niches might directly affect myeloid/lymphoid output, and the imbalanced production of mature hematopoietic cells at specific niches might in turn remodel the local microenvironment for these cells.
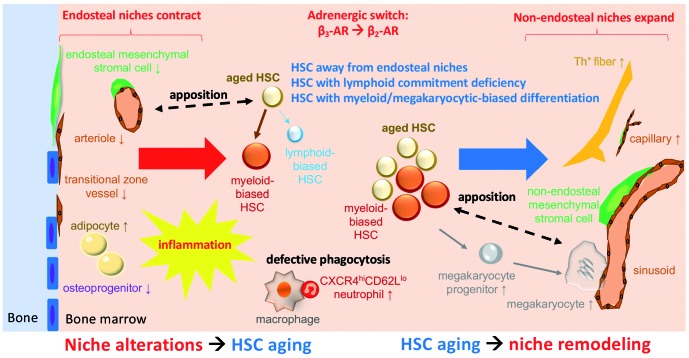
Figure 1.
Schematic model of the interplay between hematopoietic stem cells and the microenvironment during aging. Loss of β3-adrenergic receptor (β3-AR) activity reduces endosteal niches, pushes hematopoietic stem cells (HSC) away from the endosteum and favors myeloid bias at the expense of lymphopoiesis. Accumulation of aged HSC in the central bone marrow and increased β2-AR activity causes expansion of central capillaries, myeloid cells and megakaryocytes, which locate farther from HSC.
Hematopoietic stem cells change location as niches are remodeled during aging
A growing body of evidence has indicated that HSC redistribute within the BM upon aging. For instance, aged HSC locate away from the bone surface (endosteum), compared with young HSC, upon BM transplantation.36 This abnormal homing behavior correlates with increased BM HSC numbers and enhanced HSC egress into the circulation.37 Recent studies using whole-mount immunofluorescence staining of murine long bones further revealed that aged HSC are more distant from the endosteum, arterioles, Nestin-GFPhigh cells and megakaryocytes, but HSC distance from sinusoids and Nestin-GFPlow cells appears unchanged, compared with that of young HSC.38–40 These results strongly suggest that the BM microenvironment is altered with age, favoring HSC lodging near non-endosteal (central) niches, over endosteal niches. The following sections will discuss current studies on age-related BM niche remodeling, the key microenvironmental players and the associated mechanisms by which HSC localization and function are regulated.
Dysfunction of bone marrow mesenchymal stromal cells
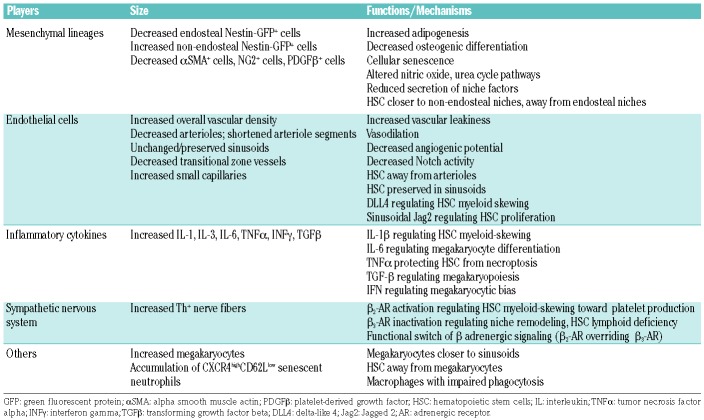
Studies regarding the absolute number of BM mesenchymal stromal cells (MSC) during aging have yielded controversial results, with some suggesting an overall increase,41,42 while others suggest unchanged43,44 or reduced numbers.45 It is noteworthy that BM MSC are heterogeneous, and the heterogeneity in the markers used to define BM MSC immunophenotypically might explain some of these controversies. Using Nestin-gfp to label murine BM MSC, different studies have reported reduced endosteal Nestin-GFP+ cells in the aged BM,39,40 consistent with reduced numbers of arteriolar αSMA+, PDGFRβ+ and NG2+ cells.38 The age-related contraction of endosteal BM might initiate lymphoid deficiency, since lymphoid niches have been previously described near bone.29,46–48 However, this notion has been refined more recently after elucidating dynamic interactions between B-cell progenitors and perivascular BM MSC, which provide key signals for B lymphopoiesis (such as Cxcl12 and Il7), both in endosteal and central sinusoidal BM niches.49–52 Functionally, old BM MSC exhibit reduced colony-forming unit-fibroblast (CFU-F) capacity in vitro and reduced expression of HSC niche factors.38 In this regard, revitalizing BM MSC to restore HSC niche factors has been proposed as a strategy to prevent DNA damage in cultured HSC.53
BM MSC exhibit reduced osteogenesis with age, which is associated with lower osteopontin secretion to the extracellular matrix.54 Osteopontin negatively regulates HSC proliferation,55–57 and its decline might accelerate HSC divisions during aging. Supporting this idea, treatment with thrombin-cleaved osteopontin partially reverses the age-associated phenotype of HSC.54
CC-chemokine ligand 5 (CCL5), a pro-inflammatory cytokine involved in bone remodeling,58 is reportedly increased with age. Researchers also reported a direct contribution to myeloid-biased differentiation at the cost of T cells by CCL5,19 suggesting that CCL5 is important for aging of the hematopoietic system and the microenvironment. In contrast, old BM MSC show adipocyte skewing.59 Adipocytes are a BM niche component that promotes HSC regeneration after irradiation, although their roles in hematopoiesis under homeostasis seem to be dispensable.60 However, altered functions of adipose tissue, including ectopic lipid deposition, insulin resistance and increased inflammation, have been described during aging.61 Accumulation of BM adipocytes upon aging not only reduces hematopoietic reconstitution, but also disrupts bone fracture repair.62 The latter likely contributes to the increased risk of osteoporosis and bone fracture in the elderly population.63,64
BM aging is also associated with senescence of BM MSC, evidenced by increased p53/p21-mediated DNA damage, upregulation of p16(INK4a) and elevated levels of reactive oxygen species.65–67 An age-dependent shortening of telomeres was found in telomerase-deficient (Terc−/−) BM MSC; consequently, lethally-irradiated Terc−/− mice carrying wildtype BM cells display accelerated myelopoiesis.68 More recently, proteome analyses of human BM have unraveled nitric oxide synthesis and the urea cycle pathways as potential mediators for the crosstalk between old BM MSC and HSC.69 Murine BM MSC show comparatively higher mRNA expression of neuronal nitric oxide synthase (encoded by the Nos1 gene), as compared with other nitric oxygen synthase isoforms, and Nos1−/− mice develop certain features of premature aging, such as remodeled BM vasculature and myeloid skewing.39 Given the importance of nitric oxide in vascular biology and balanced inflammatory responses, it is likely that nitric oxide pathways participate in the aged vascular remodeling and myeloid expansion partly by modulating inflammation.
Remodeling of bone marrow vasculature and endothelial cell functions
During aging, remodeling of the BM endothelial vasculature is notable. Studies using whole-mount confocal imaging, two-photon intravital microscopy and flow cytometry analysis demonstrated overall increased vascular density in aged mice.38,70 Yet distinct vascular beds show different, or even opposite alterations with age. Arterioles appear to be decreased, while sinusoids seem unchanged upon aging.39 Consistent with these observations, arteriole segments covered by Nestin-GFPbright cells appear shortened.38 Transitional zone vessels containing type-H endomucin (EMNC)-high endothelial cells (which are enriched in the murine trabecular BM, where they support developmental bone growth71) are reduced in old mice.39,70 In contrast, small capillaries (CD31highEMCN− cells <6 mm in diameter) are notoriously expanded in the central marrow.39
Table 1.
Microenvironmental players contributing to hematopoietic stem cell aging.
The functionality of vascular endothelium declines with age, as manifested by increased vascular leakiness, increased levels of reactive oxygen species and decreased angiogenic potential.72 Poulos et al. previously reported that HSC purified from young mice and co-cultured with endothelial cells from old mice lack long-term hematopoietic multilineage reconstitution, while old HSC co-cultured with young endothelial cells maintain their self-renewal ability.72 Infusion of young endothelial cells into aged, conditioned mice revives the old hematopoietic system. Kusumbe et al. identified high Notch activity in type-H endothelial cells and their associated subendothelial/perivascular cells,70 suggesting that contraction of endosteal vessels upon aging concomitantly occurs with impaired Notch signaling. Overexpression of the Notch ligand Dll4 in vascular endothelial cells can prevent myeloid skewing of hematopoietic progenitors73 but cannot completely rescue HSC aging,70 perhaps consistent with the finding of another study in which Dll4 was unchanged in the aged murine BM.40 A common finding is reduced endosteal activity of Notch ligand, since the latter study reported reduced expression of Jagged2 (Jag2) ligand in aged Nestin-GFPhigh cells. In contrast, Jag2 levels seem increased in the sinusoids, or their associated Nestin-GFPlow cells. Moreover, Jag2 blockade induces proliferation and clustering of aged HSC near the sinusoids. Therefore, whereas the specific role of Dll4 during aging is not clear, alterations of Notch signaling do seem to be important for hematopoietic aging. Together, these results strongly suggest that altered Notch signaling critically contributes to HSC aging in different ways depending on the niche: in the endosteal vessels, Notch signaling appears to regulate HSC lineage commitment, whereas it is required in the sinusoids to preserve old HSC (since HSC accumulate in sinusoidal niches as a function of age).40
Inflammation
Aging of the BM microenvironment is associated with increased pro-inflammatory cytokines, both in mice and humans.74 Several lines of evidence have indicated that these inflammatory cytokines drive myeloid/megakaryocytic differentiation. In aged-related myeloid malignancies, such as myeloid proliferative neoplasms and chronic myelogenous leukemia, serum interleukine (IL)-1β and IL-6 levels are elevated.75,76 Pietras et al. reported that chronic IL-1 exposure induces HSC myeloid skewing at the expense of self-renewal.77 IL-1α/β regulates thrombopoiesis in vitro,78,79 possibly explaining high platelet counts in aged mice.80 Defective phagocytosis of macrophages during aging induces expansion of platelet-biased HSC through Il-1β signaling.81 Il-6 promotes thrombopoiesis either through a direct effect on BM megakaryocyte differentiation39 or indirectly upregulating hepatic thrombopoietin levels.82 Yamashita et al. demonstrated that transient stimulation of tumor necrosis factor-α prevents HSC from necroptosis, and proposed that constitutive activation might lead to hyperproliferation of HSC and exacerbated myelopoiesis in aging and myeloproliferative disorders.83 Megakaryocytes express transforming growth factor-β to regulate HSC quiescence, while megakaryocyte-derived transforming growth factor-β also stimulates thrombopoietin synthesis by BM stromal cells to enhance megakaryopoiesis.84 An elegant study by Haas et al. found that acute inflammation induces proliferation of the stem cell-like megakaryocyte progenitor to quickly replenish platelet loss, and the process is in part mediated through the interferon family.85
Despite the well-known lymphocyte deficiency associated with aging, only the frequency and function (but not the absolute number of lymphoid-biased/balanced HSC) appear to decline with age.86 In fact, during aging both platelet/myeloid-biased and lymphoid-biased HSC expand, but exhibit altered gene expression programs and myeloid and platelet-skewing.80 These findings suggest a cell fate change of HSC upon aging, and the net outcome is an increase of the myeloid/platelet compartment at the expense of the lymphoid compartment. Two possible non-mutually exclusive explanations are: (i) different HSC suffer the same intrinsic abnormalities upon aging and (ii) microenvironmental alterations specifically influence HSC and direct their cell fate. In support of the second possibility, distinct HSC subpopulations respond differently to inflammatory challenges during aging.87 Moreover, old lymphoid-biased/balanced HSC seem to retain normal lymphoid commitment potential when removed from an old microenvironment.5 Exogenous addition of IL-1 can block lymphocyte differentiation from old lymphoid-biased HSC, confirming the indispensable role of IL-1 in HSC fate decisions. Consistently with this notion, IL-1 blockade seems sufficient to revert the age-dependent increase of megakaryocytic-biased HSC in vitro.81 IL-1 blockade can also attenuate myeloid expansion and inflammatory arthritis associated with the elderly.88
However, whether the inflamed BM niche is the cause or the consequence of HSC aging remains debated. It is notable that mature myeloid/megakaryocytic cells are a major source of inflammatory cytokines.89 Therefore, exacerbated myelopoiesis during aging might induce myeloid/megakaryocytic HSC skewing through inflammatory remodeling of the BM microenvironment. Many different cytokines directly affecting HSC (e.g., IL-6, tumor necrosis factor and interferon) increase during aging. A positive feedback loop between the myeloid cells and their derived inflammatory cytokines might increase both myelopoiesis and the cytokine storm. Future studies are needed to clarify the roles of other inflammatory cytokines, such as IL-6, in the regulation of HSC during aging.
Neuronal regulation by sympathetic adrenergic signaling
It has been reported that BM sympathetic stimulation of β2 or β3 adrenergic receptors (AR) regulates the egress and granulocyte – colony-stimulating factor -induced mobilization of HSC.90–92 A recent publication has suggested that noradrenergic nerve fibers are reduced in old murine BM.38 The study also indicated that surgical denervation of young BM induces premature aging of the hematopoietic system, although the inflammation caused by the surgical denervation might have had a certain influence. A similar reduction of nerve fibers was found in a mouse model of an age-related blood disorder, myeloproliferative neoplasm,75 suggesting that BM neuropathy might predispose to myeloid malignancies with age. However, a recent study did not find such a reduction of BM noradrenergic fibers in aged mice.93 Moreover, whole-mount imaging and three-dimensional reconstruction of different bones has revealed doubled BM area covered by noradrenergic nerve fibers in aged mice.39 This result is consistent with the well-known increase in sympathetic activity in the elderly, manifested for instance by an increased concentration of noradrenaline in the human plasma with age.94–97 Increased sympathetic activity causes myeloid skewing of old HSC through activating β2-AR, since exacerbated thrombopoiesis is present in old wildtype mice but absent in old Adrb2−/− mice or Adrb2−/−Adrb3−/− mice.39 A previous study showed that α-AR directly regulated megakaryocyte migration, adhesion and proplatelet formation under stress, but α-AR did not affect the earlier commitment of progenitor cells to the megakaryocyte lineage.98 Therefore, the age-dependent increase in sympathetic innervation might activate different AR as hematopoietic progenitor cells differentiate along the megakaryocyte lineage. Additionally, the sympathetic nervous system is known to control inflammation in a context-dependent manner.99 The concentration of catecholamines and the levels of expression of different AR influence the inflammatory state of innate immune cells. Increased sympathetic activity during aging might contribute to the cytokine storm by activating inflammatory cells, and subsequently affect lineage-bias of HSC.
Interestingly, β3-AR exhibits opposite effects on lympho-myeloid skewing, compared with β2-AR. Adult Adrb3−/−mice show a decreased frequency of endosteal lymphoid-biased HSC and/or lymphoid multipotent progenitors.38,39 Age-related remodeling of vasculature, such as reduced transitional zone vessels and expanded small capillaries, possibly explains the lymphoid deficiency in these mice.39 Altogether, these results suggest that lack of β3-AR in the microenvironment might accelerate aging of the hematopoietic system. One study suggested that administration of a β3-AR agonist to old mice rejuvenates most features of HSC aging, but overall changes of hematopoiesis in peripheral blood were not reported in this study.38 The same β3-AR agonist reduced myeloid expansion in a mouse model of myeloproliferative neoplasm75 and a murine model of Hutchinson-Gilford progeria syndrome (HGPS).39 However, hematopoietic rejuvenation was not detected in the peripheral blood of mice treated with this β3-AR agonist over 40 weeks in another study.75 Likewise, elderly individuals with myeloproliferative neoplasms who received a β3-AR agonist over 24 weeks did not show rejuvenation in the peripheral blood counts in a human study.100 Five-month old mice lacking β3-AR reportedly showed normal peripheral blood counts39 or premature, lymphoid deficiency and myeloid skewing.38 The discrepancies between these studies suggest that modulating a single neuronal pathway might not be sufficient to rejuvenate overall hematopoiesis. This may be due to the multiple compensatory/adjustment mechanisms of the autonomic nervous system revealed, for instance, in the cardiovascular system.101 It is worth mentioning that myeloid and lymphoid cells are also a source of catecholamines.102 Adrenal gland-derived adrenaline and immune cell-derived (nor)adrenaline might contribute to increased levels of adrenaline and noradrenaline in the circulation of aged individuals94–97 which, together with the increased BM noradrenergic innervation, might activate BM AR. We propose that sympathetic regulation of lympho-myeloid skewing pivots on activation or inactivation of different AR. A functional switch of neurotransmission (β2-AR overriding β3-AR) with age, rather than a general decline of BM noradrenergic innervation,38 might initiate BM niche remodeling and subsequently promote HSC myeloid skewing toward platelet production. Further investigation of other adrenergic and/or cholinergic signaling pathways possibly influencing aging is warranted.
Other players in the bone marrow microenvironment
Emerging data suggest that the progeny of HSC can feed back to regulate their activity under homeostasis, raising the possibility that mature hematopoietic cells (or their interactions with others) also contribute to HSC aging. For instance, clearance of senescent CD62LlowCXCR4high neutrophils by macrophages has been reported to modulate HSC niches.103 Frisch et al. discovered that aged macrophages are unable to engulf senescent neutrophils, leading to expansion of megakaryocytic-biased HSC through IL-1β signaling.81 Another key player is the megakaryocyte, reportedly expressing CXCL4, thrombopoietin and transforming growth factor-β to control HSC proliferation/quiescence.32–34 In murine BM, around 20% of HSC are spatially associated with megakaryocytes,32 and depletion of megakaryocytes expands platelet-biased HSC.35 During aging, megakaryocytes are found in increased numbers, lodging closer to sinusoids, and abundantly forming pseudopodial extensions (likely to be pro-platelets).39 Of note, the distance between HSC and megakaryocytes increases substantially,38,39 suggesting a remodeling of megakaryocyte niches with age. It is possible that megakaryocytes with these morphological changes fail to anchor HSC, inducing HSC hyperproliferation and lineage bias. However, whether and how age-related alterations of megakaryocytes regulate HSC aging is still unknown and requires further investigation.
Premature aging in Hutchinson-Gilford progeria syndrome
In HGPS, aberrant splicing of the LMNA gene (encoding lamin A and C) leads to nuclear assembly of the truncated protein, prelamin A (progerin).104,105 Certain hallmarks of murine hematopoietic aging, such as increased platelet counts, have been observed in HGPS.106 Given that cells aging naturally also express increased levels of progerin,107 it is possible that normal physiological conditions and progeria might share some aging mechanisms. Grigoryan et al. recently reported that HSC deficient of LMNA display a premature aging-like phenotype,108 suggesting a prominent role of lamin A/C in hematopoiesis at the level of HSC. The strong impact of progeria on growth and sexual maturation might be paralleled by altered endocrine regulation of HSC, since growth hormones and sex hormones regulate HSC survival, proliferation and lineage commitment.109–112 However, it remains unknown whether premature hematopoietic aging in HGPS is a consequence of progerin accumulation in HSC, other hematopoietic cells and/or the microenvironment. Using the LmnaG609G/G609G mouse model,113 we found that primary LmnaG609G/G609G mice exhibit features of premature hematopoietic aging; however, premature hematopoietic aging is not observed in wildtype recipients carrying LmnaG609G/G609G hematopoietic cells.39 Microenvironmental alterations are observed in LmnaG609G/G609G mice, some of which are shared between normally aged mice, such as elevated levels of pro-inflammatory cytokines (IL-3, IL-6, IL-1, interferon-γ), increased megakaryocytes with pro-platelet-like structure and megakaryocyte apposition to BM sinusoids. Of note, β3-AR agonism improves exacerbated myeloid expansion and restores the apposition of HSC to megakaryocytes. These results suggest that premature hematopoietic aging in HGPS is not HSC-autonomous, and certain aging features can be rejuvenated by targeting the microenvironment.39
Aged hematopoietic stem cells/microenvironment: chicken or egg?
One remaining key question relates to whether microenvironmental alterations initiate HSC aging and/or whether old HSC cause niche remodeling. It is noteworthy that HSC aging is not characterized by a single cellular feature, and different aging features might emerge individually at different developmental stages. For instance, in murine aging, defective lymphopoiesis starts early, at the age of 8 months old,114 while increased platelet counts do not seem to be pronounced until 18 months.39 BM nor -adrenergic nerve fibers appear to be decreased in adult, 8-month old mice75 (Supplementary Figure 5B in the report of that study), but these fibers appear increased in aged (20-month old) mice, when β3-AR signaling is already strongly reduced.39 A deficiency in β3-AR accelerates the loss of endosteal lymphoid-biased HSC in 4-month old mice, but it does not aggravate HSC aging in old mice.39 In contrast, a deficiency in β2-AR impairs megakaryopoiesis in young and old mice, and double knockout mice for β2-AR and β3-AR recapitulate the hematopoietic phenotype of single β2-AR-deficient mice. These results suggest that β2-AR overrides β3-AR during aging, and that this adrenergic remodeling contributes to imbalanced lymphoid/myeloid output. We propose that a lack of β3-AR activity near endosteum initiates the contraction of endosteal niches, which attenuates lymphopoiesis, favors myeloid bias and pushes HSC away from the endosteum. An adrenergic switch from β3-AR to β2-AR could feed back to worsen the reduction of endosteal niches, since activation of β2-AR on osteoblasts is known to restrain bone formation.115 However, the cell types expressing β2-AR and/or β3-AR involved in aged hematopoiesis are still elusive. Chances are that cells highly expressing β2-AR replace those with high expression of β3-AR over time, or BM niche cells ubiquitously increase β2-AR while decreasing β3-AR upon aging. These and other hypotheses need to be validated in future studies116 since the experiments were performed using global knockouts. Tissue-specific deletion of β2-AR and/or β3-AR will provide further insights into the mechanisms of β-adrenergic switching during aging. Importantly, β2-AR and β3-AR have different affinities for noradrenaline and adrenaline; whereas β3-AR shows higher affinity for noradrenaline over adrenaline, the opposite is true for β2-AR.117 Another possibility might be that BM concentrations of both neurotransmitters differ in aging, leading to imbalanced stimulation of the two receptors. The observation that old HSC home in the BM away from endosteal regions suggests that HSC-driven niche remodeling mainly occurs in non-endosteal domains. For instance, skewed myelopoiesis leading to increased numbers of neutrophils and defective phagocytosis of marrow macrophages might modulate the microenvironment favoring myeloid bias during aging.81 Increased myeloid cells might provide an additional source of catecholamines in a feed-forward loop promoting megakaryocyte differentiation by activating β2-AR. We propose that accumulation of old HSC results in microenvironmental remodeling by reinforcing adrenergic activity, expansion of non-endosteal niches and enhanced myeloid/megakaryocyte differentiation. As a secondary outcome, alteration of megakaryocyte niches (increased megakaryocyte numbers, proplatelet formation and apposition to sinusoids) might release HSC from their state of quiescence, further promoting HSC proliferation with age.
Niche alterations might predispose to hematologic neoplasms
The risk of developing myeloid malignancies increases significantly in individuals harboring clonal hematopoiesis-related somatic mutations. In fact some of these mutations are oncogenic drivers of myeloid malignancies.118 However, the factors limiting clonal expansion or, instead, allowing the mutant clones to become dominant and, in some cases, cause disease, remain unclear. Interestingly, similar niche alterations are shared between aging and myeloid disorders associated with age, which is an important selection pressure to expand aberrant HSC clones. For instance, a damaged neuro-MSC circuit promotes the development of a cytokine storm created by the mutant HSC, aggravating the progression of myeloproliferative neoplasms.75 In myelodysplastic syndromes, abnormal production of cytokines from the microenvironment, dysfunction of MSC and osteolineage cells, and vascular remodeling have been associated with disease initiation and progression.119,120 Targeting the abnormal microenvironment could be a promising complementary therapeutic approach to treat hematologic cancers in the future, especially when early diagnosis becomes available.
Conclusive remarks
Aging of the hematopoietic system might result from both HSC-intrinsic and microenvironmental alterations with changes in the location, function and regulation of HSC and their progeny. Future studies will determine the relative contribution of the aged microenviroment to altered hematopoiesis and increased incidence of age-related disorders originating in the BM.
Acknowledgments
Y-HH received fellowships from Alborada Scholarship (University of Cambridge), Trinity-Henry Barlow Scholarship (University of Cambridge) and R.O.C. Government Scholarship to Study Abroad (GSSA). This work was supported by core support grants from the Wellcome Trust and the MRC to the Cambridge Stem Cell Institute, National Health Service Blood and Transplant (United Kingdom), European Union’s Horizon 2020 research (ERC-2014-CoG-64765) and a Programme Foundation Award from Cancer Research UK to SM-F The authors regret that some relevant literature could not be discussed because of space constrictions.
Footnotes
Check the online version for the most updated information on this article, online supplements, and information on authorship & disclosures: www.haematologica.org/content/105/1/38
References
- 1.Carrelha J, Meng Y, Kettyle LM, et al. Hierarchically related lineage-restricted fates of multipotent haematopoietic stem cells. Nature. 2018;554(7690):106–111. [DOI] [PubMed] [Google Scholar]
- 2.Weksberg DC, Chambers SM, Boles NC, Goodell MA. CD150- side population cells represent a functionally distinct population of long-term hematopoietic stem cells. Blood. 2008;111(4):2444–2451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kiel MJ, Yilmaz OH, Morrison SJ. CD150-cells are transiently reconstituting multipotent progenitors with little or no stem cell activity. Blood. 2008;111(8):4413–4414; author reply 4414-4415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kent DG, Copley MR, Benz C, et al. Prospective isolation and molecular characterization of hematopoietic stem cells with durable self-renewal potential. Blood. 2009;113(25):6342–6350. [DOI] [PubMed] [Google Scholar]
- 5.Montecino-Rodriguez E, Kong Y, Casero D, et al. Lymphoid-biased hematopoietic stem cells are maintained with age and efficiently generate lymphoid progeny. Stem Cell Reports. 2019;12(3):584–596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Liang Y, Van Zant G, Szilvassy SJ. Effects of aging on the homing and engraftment of murine hematopoietic stem and progenitor cells. Blood. 2005;106(4):1479–1487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mohrin M, Shin J, Liu Y, et al. Stem cell aging. A mitochondrial UPR-mediated metabolic checkpoint regulates hematopoietic stem cell aging. Science. 2015;347(6228): 1374–1377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rossi DJ, Bryder D, Zahn JM, et al. Cell intrinsic alterations underlie hematopoietic stem cell aging. Proc Natl Acad Sci U S A. 2005;102(26):9194–9199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sudo K, Ema H, Morita Y, Nakauchi H. Age-associated characteristics of murine hematopoietic stem cells. J Exp Med. 2000;192(9):1273–1280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dykstra B, Olthof S, Schreuder J, Ritsema M, de Haan G. Clonal analysis reveals multiple functional defects of aged murine hematopoietic stem cells. J Exp Med. 2011;208(13):2691–2703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chambers SM, Shaw CA, Gatza C, et al. Aging hematopoietic stem cells decline in function and exhibit epigenetic dysregulation. PLoS Biol. 2007;5(8):e201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Flach J, Bakker ST, Mohrin M, et al. Replication stress is a potent driver of functional decline in ageing haematopoietic stem cells. Nature. 2014;512(7513):198–202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rossi DJ, Bryder D, Seita J, et al. Deficiencies in DNA damage repair limit the function of haematopoietic stem cells with age. Nature. 2007;447(7145):725–729. [DOI] [PubMed] [Google Scholar]
- 14.Florian MC, Nattamai KJ, Dorr K, et al. A canonical to non-canonical Wnt signalling switch in haematopoietic stem-cell ageing. Nature. 2013;503(7476):392–396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ito K, Suda T. Metabolic requirements for the maintenance of self-renewing stem cells. Nat Rev Mol Cell Biol. 2014;15(4):243–256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chandel NS, Jasper H, Ho TT, Passegue E. Metabolic regulation of stem cell function in tissue homeostasis and organismal ageing. Nat Cell Biol. 2016;18(8):823–832. [DOI] [PubMed] [Google Scholar]
- 17.Ho TT, Warr MR, Adelman ER, et al. Autophagy maintains the metabolism and function of young and old stem cells. Nature. 2017;543(7644):205–210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Vilchez D, Simic MS, Dillin A. Proteostasis and aging of stem cells. Trends Cell Biol. 2014;24(3):161–170. [DOI] [PubMed] [Google Scholar]
- 19.Ergen AV, Boles NC, Goodell MA. Rantes/Ccl5 influences hematopoietic stem cell subtypes and causes myeloid skewing. Blood. 2012;119(11):2500–2509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Taichman RS, Emerson SG. Human osteoblasts support hematopoiesis through the production of granulocyte colony-stimulating factor. J Exp Med. 1994;179(5):1677–1682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhang J, Niu C, Ye L, et al. Identification of the haematopoietic stem cell niche and control of the niche size. Nature. 2003;425(6960):836–841. [DOI] [PubMed] [Google Scholar]
- 22.Zhao M, Tao F, Venkatraman A, et al. N-cadherin-expressing bone and marrow stromal progenitor cells maintain reserve hematopoietic stem cells. Cell Rep. 2019;26(3):652–669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Calvi LM, Adams GB, Weibrecht KW, et al. Osteoblastic cells regulate the haematopoietic stem cell niche. Nature. 2003;425(6960):841–846. [DOI] [PubMed] [Google Scholar]
- 24.Arai F, Hirao A, Ohmura M, et al. Tie2/angiopoietin-1 signaling regulates hematopoietic stem cell quiescence in the bone marrow niche. Cell. 2004;118(2):149–161. [DOI] [PubMed] [Google Scholar]
- 25.Yoshihara H, Arai F, Hosokawa K, et al. Thrombopoietin/MPL signaling regulates hematopoietic stem cell quiescence and interaction with the osteoblastic niche. Cell Stem Cell. 2007;1(6):685–697. [DOI] [PubMed] [Google Scholar]
- 26.Adams GB, Chabner KT, Alley IR, et al. Stem cell engraftment at the endosteal niche is specified by the calcium-sensing receptor. Nature. 2006;439(7076):599–603. [DOI] [PubMed] [Google Scholar]
- 27.Sugiyama T, Kohara H, Noda M, Nagasawa T. Maintenance of the hematopoietic stem cell pool by CXCL12-CXCR4 chemokine signaling in bone marrow stromal cell niches. Immunity. 2006;25(6):977–988. [DOI] [PubMed] [Google Scholar]
- 28.Kinashi T, Springer TA. Steel factor and c-kit regulate cell-matrix adhesion. Blood. 1994;83(4):1033–1038. [PubMed] [Google Scholar]
- 29.Ding L, Morrison SJ. Haematopoietic stem cells and early lymphoid progenitors occupy distinct bone marrow niches. Nature. 2013;495(7440):231–235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Greenbaum AM, Revollo LD, Woloszynek JR, Civitelli R, Link DC. N-cadherin in osteolineage cells is not required for maintenance of hematopoietic stem cells. Blood. 2012;120(2):295–302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Boulais PE, Frenette PS. Making sense of hematopoietic stem cell niches. Blood. 2015;125(17):2621–2629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bruns I, Lucas D, Pinho S, et al. Megakaryocytes regulate hematopoietic stem cell quiescence through CXCL4 secretion. Nat Med. 2014;20(11):1315–1320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zhao M, Perry JM, Marshall H, et al. Megakaryocytes maintain homeostatic quiescence and promote post-injury regeneration of hematopoietic stem cells. Nat Med. 2014;20(11):1321–1326. [DOI] [PubMed] [Google Scholar]
- 34.Nakamura-Ishizu A, Takubo K, Fujioka M, Suda T. Megakaryocytes are essential for HSC quiescence through the production of thrombopoietin. Biochem Biophys Res Commun. 2014;454(2):353–357. [DOI] [PubMed] [Google Scholar]
- 35.Pinho S, Marchand T, Yang E, et al. Lineage-biased hematopoietic stem cells are regulated by distinct niches. Dev Cell. 2018;44(5): 634–641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Florian MC, Dorr K, Niebel A, et al. Cdc42 activity regulates hematopoietic stem cell aging and rejuvenation. Cell Stem Cell. 2012;10(5):520–530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Xing Z, Ryan MA, Daria D, et al. Increased hematopoietic stem cell mobilization in aged mice. Blood. 2006;108(7):2190–2197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Maryanovich M, Zahalka AH, Pierce H, et al. Adrenergic nerve degeneration in bone marrow drives aging of the hematopoietic stem cell niche. Nat Med. 2018;24(6):782–791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ho YH, Del Toro R, Rivera-Torres J, et al. Remodeling of bone marrow hematopoietic stem cell niches promotes myeloid cell expansion during premature or physiological aging. Cell Stem Cell. 2019;25(3):407–418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sacma M, Pospiech J, Bogeska R, et al. Haematopoietic stem cells in perisinusoidal niches are protected from ageing. Nat Cell Biol. 2019;21(11):1309–1320. [DOI] [PubMed] [Google Scholar]
- 41.Stolzing A, Jones E, McGonagle D, Scutt A. Age-related changes in human bone marrow-derived mesenchymal stem cells: consequences for cell therapies. Mech Ageing Dev. 2008;129(3):163–173. [DOI] [PubMed] [Google Scholar]
- 42.Garcia-Prat L, Sousa-Victor P, Munoz-Canoves P. Functional dysregulation of stem cells during aging: a focus on skeletal muscle stem cells. FEBS J. 2013;280(17):4051–4062. [DOI] [PubMed] [Google Scholar]
- 43.Siegel G, Kluba T, Hermanutz-Klein U, et al. Phenotype, donor age and gender affect function of human bone marrow-derived mesenchymal stromal cells. BMC Med. 2013;11:146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wagner W, Bork S, Horn P, et al. Aging and replicative senescence have related effects on human stem and progenitor cells. PloS One. 2009;4(6):e5846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ganguly P, El-Jawhari JJ, Burska AN, et al. The analysis of in vivo aging in human bone marrow mesenchymal stromal cells using colony-forming unit-fibroblast assay and the CD45(low)CD271(+) Phenotype. Stem Cells Int. 2019;2019: 5197983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Visnjic D, Kalajzic Z, Rowe DW, et al. Hematopoiesis is severely altered in mice with an induced osteoblast deficiency. Blood. 2004;103(9):3258–3264. [DOI] [PubMed] [Google Scholar]
- 47.Zhu J, Garrett R, Jung Y, et al. Osteoblasts support B-lymphocyte commitment and differentiation from hematopoietic stem cells. Blood. 2007;109(9):3706–3712. [DOI] [PubMed] [Google Scholar]
- 48.Wu JY, Purton LE, Rodda SJ, et al. Osteoblastic regulation of B lymphopoiesis is mediated by Gs{alpha}-dependent signaling pathways. Proc Natl Acad Sci U S A. 2008;105(44):16976–16981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Zehentmeier S, Pereira JP. Cell circuits and niches controlling B cell development. Immunol Rev. 2019;289(1):142–157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Fistonich C, Zehentmeier S, Bednarski JJ, et al. Cell circuits between B cell progenitors and IL-7(+) mesenchymal progenitor cells control B cell development. J Exp Med. 2018;215(10):2586–2599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Cordeiro Gomes A, Hara T, Lim VY, et al. Hematopoietic stem cell niches produce lineage-instructive signals to control multipotent progenitor differentiation. Immunity. 2016;45(6):1219–1231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Balzano M, De Grandis M, Vu Manh TP, et al. Nidogen-1 contributes to the interaction network involved in pro-B cell retention in the peri-sinusoidal hematopoietic stem cell niche. Cell Rep. 2019;26(12):3257–3271. [DOI] [PubMed] [Google Scholar]
- 53.Nakahara F, Borger DK, Wei Q, et al. Engineering a haematopoietic stem cell niche by revitalizing mesenchymal stromal cells. Nat Cell Biol. 2019;21(5):560–567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Guidi N, Sacma M, Standker L, et al. Osteopontin attenuates aging-associated phenotypes of hematopoietic stem cells. EMBO J. 2017;36(7):840–853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Nilsson SK, Johnston HM, Whitty GA, et al. Osteopontin, a key component of the hematopoietic stem cell niche and regulator of primitive hematopoietic progenitor cells. Blood. 2005;106(4):1232–1239. [DOI] [PubMed] [Google Scholar]
- 56.Stier S, Ko Y, Forkert R, et al. Osteopontin is a hematopoietic stem cell niche component that negatively regulates stem cell pool size. J Exp Med. 2005;201(11):1781–1791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Haylock DN, Nilsson SK. Osteopontin: a bridge between bone and blood. Br J Haematol. 2006;134(5):467–474. [DOI] [PubMed] [Google Scholar]
- 58.Wintges K, Beil FT, Albers J, et al. Impaired bone formation and increased osteoclastogenesis in mice lacking chemokine (C-C motif) ligand 5 (Ccl5). J Bone Miner Res. 2013;28(10):2070–2080. [DOI] [PubMed] [Google Scholar]
- 59.Kim M, Kim C, Choi YS, et al. Age-related alterations in mesenchymal stem cells related to shift in differentiation from osteogenic to adipogenic potential: implication to age-associated bone diseases and defects. Mech Ageing Dev. 2012;133(5):215–225. [DOI] [PubMed] [Google Scholar]
- 60.Zhou BO, Yu H, Yue R, et al. Bone marrow adipocytes promote the regeneration of stem cells and haematopoiesis by secreting SCF. Nat Cell Biol. 2017;19(8):891–903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Mancuso P, Bouchard B. The impact of aging on adipose function and adipokine synthesis. Front Endocrinol (Lausanne). 2019; 10:137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Ambrosi TH, Scialdone A, Graja A, et al. Adipocyte accumulation in the bone marrow during obesity and aging impairs stem cell-based hematopoietic and bone regeneration. Cell Stem Cell. 2017;20(6): 771–784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Fazeli PK, Horowitz MC, MacDougald OA, et al. Marrow fat and bone–new perspectives. J Clin Endocrinol Metab. 2013;98(3):935–945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Schwartz AV. Marrow fat and bone: review of clinical findings. Front Endocrinol (Lausanne). 2015;6:40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Zhang DY, Wang HJ, Tan YZ. Wnt/beta-catenin signaling induces the aging of mesenchymal stem cells through the DNA damage response and the p53/p21 pathway. PloS One. 2011;6(6):e21397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Zheng Y, He L, Wan Y, Song J. H3K9me-enhanced DNA hypermethylation of the p16INK4a gene: an epigenetic signature for spontaneous transformation of rat mesenchymal stem cells. Stem Cells Dev. 2013;22(2):256–267. [DOI] [PubMed] [Google Scholar]
- 67.Kornicka K, Marycz K, Tomaszewski KA, Maredziak M, Smieszek A. The effect of age on osteogenic and adipogenic differentiation potential of human adipose derived stromal stem cells (hASCs) and the impact of stress fctors in the course of the differentiation process. Oxid Med Cell Longev. 2015;2015: 309169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Ju Z, Jiang H, Jaworski M, et al. Telomere dysfunction induces environmental alterations limiting hematopoietic stem cell function and engraftment. Nat Med. 2007;13(6):742–747. [DOI] [PubMed] [Google Scholar]
- 69.Hennrich ML, Romanov N, Horn P, et al. Cell-specific proteome analyses of human bone marrow reveal molecular features of age-dependent functional decline. Nat Commun. 2018;9(1):4004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Kusumbe AP, Ramasamy SK, Itkin T, et al. Age-dependent modulation of vascular niches for haematopoietic stem cells. Nature. 2016;532(7599):380–384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Kusumbe AP, Ramasamy SK, Adams RH. Coupling of angiogenesis and osteogenesis by a specific vessel subtype in bone. Nature. 2014;507(7492):323–328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Poulos MG, Ramalingam P, Gutkin MC, et al. Endothelial transplantation rejuvenates aged hematopoietic stem cell function. J Clin Invest. 2017;127(11):4163–4178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Tikhonova AN, Dolgalev I, Hu H, et al. The bone marrow microenvironment at single-cell resolution. Nature. 2019;569(7755):222–228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Kovtonyuk LV, Fritsch K, Feng X, Manz MG, Takizawa H. Inflamm-aging of hematopoiesis, hematopoietic stem cells, and the bone marrow microenvironment. Front Immunol. 2016;7:502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Arranz L, Sanchez-Aguilera A, Martin-Perez D, et al. Neuropathy of haematopoietic stem cell niche is essential for myeloproliferative neoplasms. Nature. 2014;512(7512):78–81. [DOI] [PubMed] [Google Scholar]
- 76.Reynaud D, Pietras E, Barry-Holson K, et al. IL-6 controls leukemic multipotent progenitor cell fate and contributes to chronic myelogenous leukemia development. Cancer Cell. 2011;20(5):661–673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Pietras EM, Mirantes-Barbeito C, Fong S, et al. Chronic interleukin-1 exposure drives haematopoietic stem cells towards precocious myeloid differentiation at the expense of self-renewal. Nat Cell Biol. 2016;18(6): 607–618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Beaulieu LM, Lin E, Mick E, et al. Interleukin 1 receptor 1 and interleukin 1beta regulate megakaryocyte maturation, platelet activation, and transcript profile during inflammation in mice and humans. Arterioscler Thromb Vasc Biol. 2014;34(3):552–564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Nishimura S, Nagasaki M, Kunishima S, et al. IL-1alpha induces thrombopoiesis through megakaryocyte rupture in response to acute platelet needs. J Cell Biol. 2015;209(3):453–466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Grover A, Sanjuan-Pla A, Thongjuea S, et al. Single-cell RNA sequencing reveals molecular and functional platelet bias of aged haematopoietic stem cells. Nat Commun. 2016;7:11075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Frisch BJ, Hoffman CM, Latchney SE, et al. Aged marrow macrophages expand platelet-biased hematopoietic stem cells via interleukin1B. JCI Insight. 2019;5pii:124213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Kaser A, Brandacher G, Steurer W, et al. Interleukin-6 stimulates thrombopoiesis through thrombopoietin: role in inflammatory thrombocytosis. Blood. 2001;98(9): 2720–2725. [DOI] [PubMed] [Google Scholar]
- 83.Yamashita M, Passegue E. TNF-alpha Coordinates hematopoietic stem cell survival and myeloid regeneration. Cell Stem Cell. 2019;25(3):357–372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Sakamaki S, Hirayama Y, Matsunaga T, et al. Transforming growth factor-beta1 (TGF-beta1) induces thrombopoietin from bone marrow stromal cells, which stimulates the expression of TGF-beta receptor on megakaryocytes and, in turn, renders them susceptible to suppression by TGF-beta itself with high specificity. Blood. 1999;94(6):1961–1970. [PubMed] [Google Scholar]
- 85.Haas S, Hansson J, Klimmeck D, et al. Inflammation-induced emergency megakaryopoiesis driven by hematopoietic stem cell-like megakaryocyte progenitors. Cell Stem Cell. 2015;17(4):422–434. [DOI] [PubMed] [Google Scholar]
- 86.Beerman I, Bhattacharya D, Zandi S, et al. Functionally distinct hematopoietic stem cells modulate hematopoietic lineage potential during aging by a mechanism of clonal expansion. Proc Natl Acad Sci U S A. 2010;107(12):5465–5470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Mann M, Mehta A, de Boer CG, et al. Heterogeneous responses of hematopoietic stem cells to inflammatory stimuli are altered with age. Cell Rep. 2018;25(11): 2992–3005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Hernandez G, Mills TS, Rabe JL, et al. Pro-inflammatory cytokine blockade attenuates myeloid expansion in a murine model of rheumatoid arthritis. Haematologica. 2019. May 17 [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Pietras EM. Inflammation: a key regulator of hematopoietic stem cell fate in health and disease. Blood. 2017;130(15):1693–1698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Mendez-Ferrer S, Battista M, Frenette PS. Cooperation of beta(2)- and beta(3)-adrenergic receptors in hematopoietic progenitor cell mobilization. Ann N Y Acad Sci. 2010;1192:139–144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Katayama Y, Battista M, Kao WM, et al. Signals from the sympathetic nervous system regulate hematopoietic stem cell egress from bone marrow. Cell. 2006;124(2):407–421. [DOI] [PubMed] [Google Scholar]
- 92.Mendez-Ferrer S, Lucas D, Battista M, Frenette PS. Haematopoietic stem cell release is regulated by circadian oscillations. Nature. 2008;452(7186):442–447. [DOI] [PubMed] [Google Scholar]
- 93.Chartier SR, Mitchell SAT, Majuta LA, Mantyh PW. The changing sensory and sympathetic innervation of the young, adult and aging mouse femur. Neuroscience. 2018;387:178–190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Hart EC, Charkoudian N. Sympathetic neural regulation of blood pressure: influences of sex and aging. Physiology (Bethesda). 2014;29(1):8–15. [DOI] [PubMed] [Google Scholar]
- 95.Ng AV, Callister R, Johnson DG, Seals DR. Age and gender influence muscle sympathetic nerve activity at rest in healthy humans. Hypertension. 1993;21(4):498–503. [DOI] [PubMed] [Google Scholar]
- 96.Veith RC, Featherstone JA, Linares OA, Halter JB. Age differences in plasma norepinephrine kinetics in humans. J Gerontol. 1986;41(3):319–324. [DOI] [PubMed] [Google Scholar]
- 97.Ziegler MG, Lake CR, Kopin IJ. Plasma noradrenaline increases with age. Nature. 1976;261(5558):333–335. [DOI] [PubMed] [Google Scholar]
- 98.Chen S, Du C, Shen M, et al. Sympathetic stimulation facilitates thrombopoiesis by promoting megakaryocyte adhesion, migration, and proplatelet formation. Blood. 2016;127(8):1024–1035. [DOI] [PubMed] [Google Scholar]
- 99.Pongratz G, Straub RH. The sympathetic nervous response in inflammation. Arthritis Res Ther. 2014;16(6):504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Drexler B, Passweg JR, Tzankov A, et al. The sympathomimetic agonist mirabegron did not lower JAK2-V617F allele burden, but restored nestin-positive cells and reduced reticulin fibrosis in patients with myeloproliferative neoplasms: results of phase II study SAKK 33/14. Haematologica. 2019;104(4):710–716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Triposkiadis F, Karayannis G, Giamouzis G, et al. The sympathetic nervous system in heart failure physiology, pathophysiology, and clinical implications. J Am Coll Cardiol. 2009;54(19):1747–1762. [DOI] [PubMed] [Google Scholar]
- 102.Cosentino M, Marino F, Maestroni GJ. Sympathoadrenergic modulation of hematopoiesis: a review of available evidence and of therapeutic perspectives. Front Cell Neurosci. 2015;9:302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Casanova-Acebes M, Pitaval C, Weiss LA, et al. Rhythmic modulation of the hematopoietic niche through neutrophil clearance. Cell. 2013;153(5):1025–1035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.De Sandre-Giovannoli A, Bernard R, Cau P, et al. Lamin a truncation in Hutchinson-Gilford progeria. Science. 2003;300(5628): 2055. [DOI] [PubMed] [Google Scholar]
- 105.Eriksson M, Brown WT, Gordon LB, et al. Recurrent de novo point mutations in lamin A cause Hutchinson-Gilford progeria syndrome. Nature. 2003;423(6937):293–298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Merideth MA, Gordon LB, Clauss S, et al. Phenotype and course of Hutchinson-Gilford progeria syndrome. N Engl J Med. 2008;358(6):592–604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Scaffidi P, Misteli T. Lamin A-dependent nuclear defects in human aging. Science. 2006;312(5776):1059–1063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Grigoryan A, Guidi N, Senger K, et al. LaminA/C regulates epigenetic and chromatin architecture changes upon aging of hematopoietic stem cells. Genome Biol. 2018;19(1):189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Heo HR, Chen L, An B, et al. Hormonal regulation of hematopoietic stem cells and their niche: a focus on estrogen. Int J Stem Cells. 2015;8(1):18–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Stewart MH, Gutierrez-Martinez P, Beerman I, et al. Growth hormone receptor signaling is dispensable for HSC function and aging. Blood. 2014;124(20):3076–3080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Sanchez-Aguilera A, Arranz L, Martin-Perez D, et al. Estrogen signaling selectively induces apoptosis of hematopoietic progenitors and myeloid neoplasms without harming steady-state hematopoiesis. Cell Stem Cell. 2014;15(6):791–804. [DOI] [PubMed] [Google Scholar]
- 112.Nakada D, Oguro H, Levi BP, et al. Oestrogen increases haematopoietic stem-cell self-renewal in females and during pregnancy. Nature. 2014;505(7484):555–558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Osorio FG, Navarro CL, Cadinanos J, et al. Splicing-directed therapy in a new mouse model of human accelerated aging. Sci Transl Med. 2011;3(106):106ra107. [DOI] [PubMed] [Google Scholar]
- 114.Young K, Borikar S, Bell R, et al. Progressive alterations in multipotent hematopoietic progenitors underlie lymphoid cell loss in aging. J Exp Med. 2016;213(11):2259–2267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Kajimura D, Hinoi E, Ferron M, et al. Genetic determination of the cellular basis of the sympathetic regulation of bone mass accrual. J Exp Med. 2011;208(4):841–851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Raaijmakers M. Aging of the hematopoietic stem cell niche: an unnerving matter. Cell Stem Cell. 2019;25(3):301–303. [DOI] [PubMed] [Google Scholar]
- 117.Hoffmann C, Leitz MR, Oberdorf-Maass S, Lohse MJ, Klotz KN. Comparative pharmacology of human beta-adrenergic receptor subtypes–characterization of stably transfected receptors in CHO cells. Naunyn Schmiedebergs Arch Pharmacol. 2004;369(2):151–159. [DOI] [PubMed] [Google Scholar]
- 118.Deininger MWN, Tyner JW, Solary E. Turning the tide in myelodysplastic/myeloproliferative neoplasms. Nat Rev Cancer. 2017;17(7):425–440. [DOI] [PubMed] [Google Scholar]
- 119.Li AJ, Calvi LM. The microenvironment in myelodysplastic syndromes: niche-mediated disease initiation and progression. Exp Hematol. 2017;55:3–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Wang C, Yang Y, Gao S, et al. Immune dysregulation in myelodysplastic syndrome: clinical features, pathogenesis and therapeutic strategies. Crit Rev Oncol Hematol. 2018;122:123–132. [DOI] [PubMed] [Google Scholar]