Abstract
Extramedullary disease is relatively frequent in multiple myeloma, but our knowledge on the subject is limited and mainly relies on small case series or single center experiences. Little is known regarding the role of new drugs in this setting. We performed a meta-analysis of eight trials focused on the description of extramedullary disease characteristics, clinical outcome, and response to new drugs. A total of 2,332 newly diagnosed myeloma patients have been included; 267 (11.4%) had extramedullary disease, defined as paraosseous in 243 (10.4%), extramedullary plasmocytoma in 12 (0.5%), and not classified in 12 (0.5%) patients. Median progression-free survival was 25.3 months and 25.2 in extramedullary disease and non-extramedullary disease patients, respectively. In multivariate analysis the presence of extramedullary disease did not impact on progression-free survival (hazard ratio 1.15, P=0.06), while other known prognostic factors retained their significance. Patients treated with immunomodulatory drugs, mainly lenalidomide, or proteasome inhibitors had similar progression-free survival and progression-free survival-2 regardless of extramedullary disease presence. Median overall survival was 63.5 months and 79.9 months (P=0.01) in extramedullary and non-extramedullary disease patients, respectively, and in multivariate analysis the presence of extramedullary disease was associated with a reduced overall survival (hazard ratio 1.41, P<0.001), in line with other prognostic factors. With the limits of the use of low sensitivity imaging techniques, that lead to an underestimation of extramedullary disease, we conclude that in patients treated with new drugs the detrimental effect of extramedullary disease at diagnosis is limited, that lenalidomide is effective as are proteasome inhibitors, and that these patients tend to acquire a more aggressive disease in later stages. (EUDRACT2005-004714-32, NCT01063179. NCT00551928, NCT01091831, NCT01093196, NCT01190787, NCT01346787, NCT01857115).
Introduction
Multiple myeloma (MM) is a plasma cell neoplasia characterized by a diffuse tumor infiltration of the bone marrow, resulting, among others, in anemia, bone damage with hypercalcemia, and bone lesions. Occasionally, neoplastic plasma cells acquire a different growth pattern generating tumor masses, that are referred to as extra-medullary disease (EMD).1 EMD can arise from skeletal focal lesions, which disrupt the cortical bone and grow as extra-bone masses, and is referred to as paraosseous plasmocytoma (PO), or derive from hematogenous spread as manifestation in soft tissues, and is called extramedullary plasmocytomas (EMP). Incidence of EMD at diagnosis ranges between 6% and 10%,2–4 while later in the course of the disease this increases to 13%-26%,2,4 with a 32-35% peak in case of relapse after allogeneic stem cell transplantation.5,6 In the final stage of the disease, an extraskeletal involvement is observed in approximately 70% of cases studied with autopsy,7 with a peculiar involvement of visceral sites.8 As expected, patients with EMD at diagnosis tend to maintain the same pattern at relapse.2
The biological mechanisms behind the acquisition of the EMD-forming phenotype have not yet been fully elucidated. Increased expression of CXCR4 and CXCL12 plays a major role in promoting a bone marrow-independent behavior, favoring dissemination, and homing to distant and unusual sites.9,10 Other mechanisms are represented by reduced expression of several adhesion molecules, in particular VLA-4, CD44, and CD56, and chemokine receptors, such as CCR1, and CCR2. Diversely, the cyclin D1 pathway seems to favor the bone marrow homing, protecting from extramedullary localizations, as t(11;14) is not observed in MM patients with EMD.11
Despite its frequency and clinical relevance, EMD has often been neglected by the medical literature. In fact, almost all the available data derive from retrospective series and single center experiences, mainly reported in the pre-new drug era, with the limitations of this type of studies. In order to fill this gap and clarify the role of new drugs in MM with EMD, we conducted the largest meta-analysis so far reported, based on eight prospective trials by the same sponsors (Fonesa Onlus and Hovon Foundation).
Methods
Study design
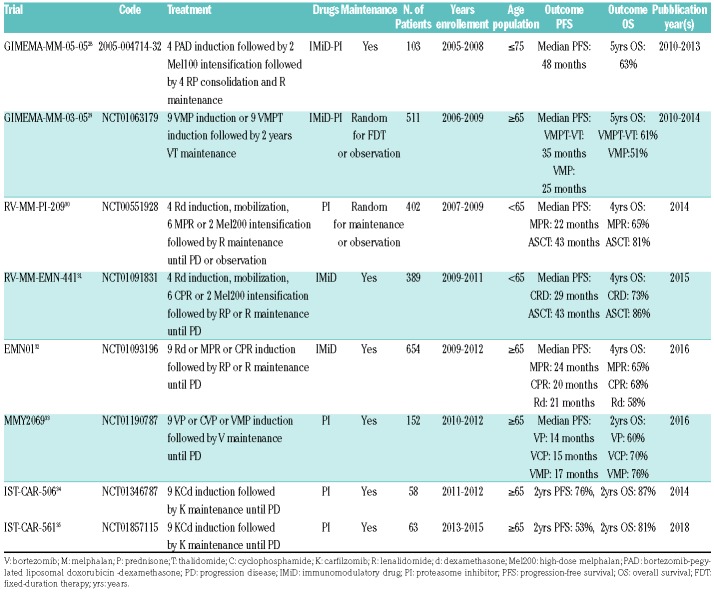
Patients with newly diagnosed MM enrolled in eight clinical trials were retrospectively analyzed. Details on trials and treatment regimens are summarized in Table 1. Briefly, three trials enrolled transplant eligible and five trials transplant ineligible patients. Three trials included an immunomodulatory (IMiD) drug in the treatment, lenalidomide in almost all cases, three trials a proteasome inhibitor (PI), and four trials both. Six out of eight trials included maintenance. Trials were approved by the Independent Ethics Committees/Institutional Review Boards at all participating centers. Patients provided written informed consent before entering the study, prepared in accordance with the Declaration of Helsinki. For the purpose of this meta-analysis, we considered the subgroup of patients with EMD, and compared them with patients without EMD.
Table 1.
Source studies.
Extramedullary disease definition and assessment
Extramedullary disease was classified as PO disease, consisting of tumor masses arising directly from bones, or EMP, consisting of masses not contiguous to the bones and derived from hematogenous spread. EMD was identified at study enrollment with the diagnostic procedure required by the patient’s study protocol, such as X-ray skeletal survey, magnetic resonance imaging (MRI), computed tomography (CT), and physical examination.
Statistical analysis
Differences in patients’ and disease characteristics for EMD patients versus non-EMD patients were investigated using Kruskal Wallis test for continuous variables and Fisher’s exact test for categorical variables. Data of trials were pooled together and analyzed. Time-to-event data were analyzed using the Kaplan-Meier method; EMD and non-EMD patients were compared with the log-rank test. The Cox proportional hazards models were used to estimate adjusted hazard ratios (HRs) and the 95% confidence intervals (CI) for the main comparisons, EMD patients versus non-EMD patients. To account for potential confounders, the Cox models were adjusted for the age, sex, International Staging System (ISS) stage (I vs. II; I vs. III), cytogenetic risk defined by fluorescence in situ hybridization (FISH) analysis [high, i.e. presence of del(17p), t(4;14), t(14;16), vs. standard risk; missing vs. standard risk], and autologous stem cell transplantation (ASCT) (ASCT vs. non-ASCT; not applicable, i.e. patients not candidate to ASCT, vs. non-ASCT). Subgroup analyses were performed to determine the consistency of the overall effect in different subgroups using interaction terms for the comparison between EMD versus non-EMD and each of the co-variates included in the Cox model plus Revised ISS stage (RISS) and type of therapies (IMID and PI). All Hazard Ratios (HR) were estimated with their 95%CI and two sided P-values. In order to evaluate the impact of different size and types of EMD, further subgroup analyses were performed: EMD size ≤ 3 > 3 cm; EMD size ≤ 5 vs. > 5 cm; PO or EMP. Data were analyzed as of December 2018 using and R (Version 3.1.1).
Results
Patients
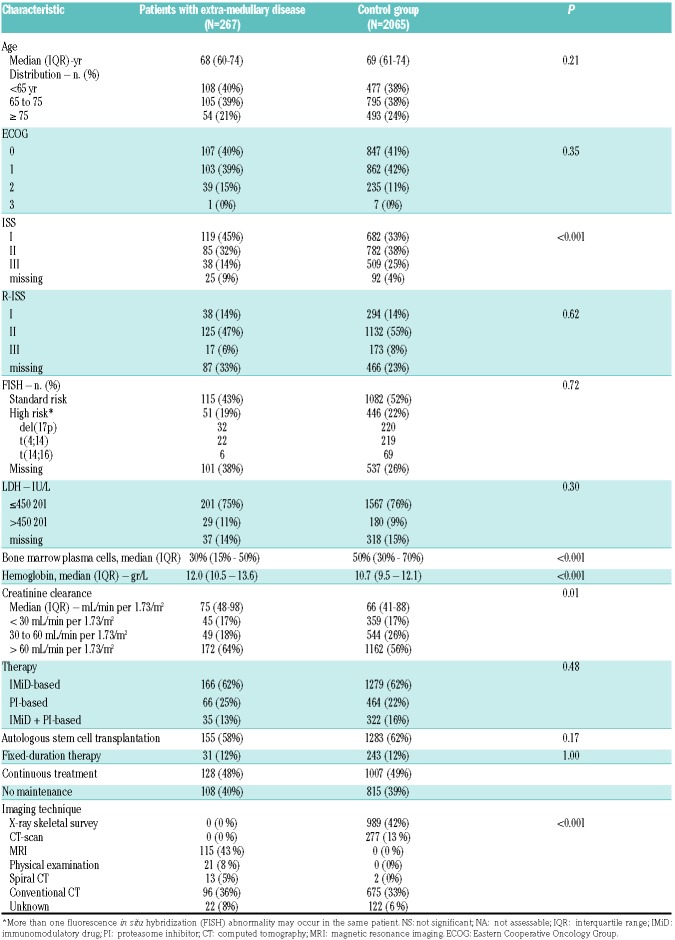
A total of 2,332 patients were included in this analysis: 267 (11%) had EMD, while 2,065 (89%) had no EMD. Median age of EMD patients was 68 years (IQ range 60-74), and 69 years (IQ range 61-74) in patients without EMD. International Staging System was I in 119 (45%) and 682 (33%), II in 85 (32%) and 782 (38%), and III in 38 (14%) and 509 (25%) patients with or without EMD, respectively. Clinical trials were based on IMiD in 166 (62%) and 1,279 (62%) patients, on a PI in 66 (25%) and 464 (22%) patients, or both in 35 (13%) and 322 (16%) patients with or without EMD, respectively. Patients’ characteristics are summarized in Table 2. Patients with EMD had PO in 243 (91%), and an EMP in 12 (4%) cases, while the information was not available for the other 12 (4%) patients. EMD localizations were single in 195 (73%), and multiple in 60 (22%) patients. Median EMD size was 4.2 cm (IQ range 3-7). EMD characteristics are summarized in Table 3. No differences were observed in patients with EMD ≤ or > 3 cm. EMD patients had a lower systemic tumor burden with respect to patients without EMD, as shown by: plasma cell bone marrow infiltration 30% (IQ range 15-50%) versus 50% (IQ range 30-70%), hemoglobin 12.0 gr/L (IQ range 10.5-13.6) versus 10.7 gr/L (IQ range 9.5-12.1), median creatinine clearance 75 mL/min per 1.73 m2 (IQ range 48-98) versus 66 (IQ range 41-88), respectively. EMD patients had ISS I stage in 45% of cases, compared to 33% in non-EMD patients (P<0.001).
Table 2.
Patients’ demographics and clinical characteristics.
Table 3.
Extramedullary disease characteristics.

Efficacy
Progression-free survival
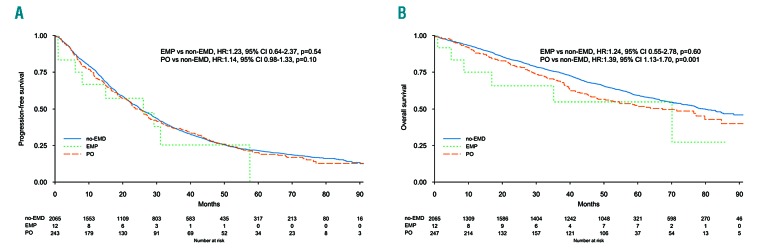
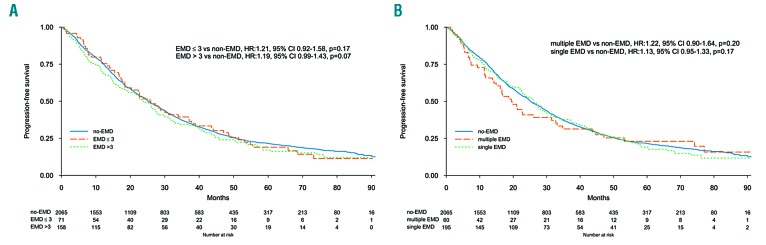
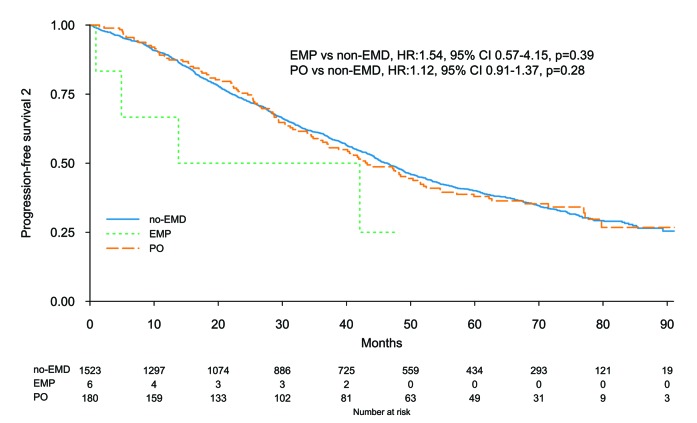
The median follow up was 62 months (IQ range 34-75) in EMD, and 65 months (IQ range 40-77) in non-EMD patients. Median PFS was 25.3 months (95%CI: 21.7-28.7) and 25.2 months (95%CI: 24.2-27.0) in EMD and non-EMD patients, respectively. Five-year PFS was 19% (95%CI: 15-25%) and 22% (95%CI: 20-24%) (P=0.46) in EMD and non-EMD patients, respectively (Online Supplementary Figure S1), and there were no differences between EMP, PO, and non-EMD (Figure 1A). In multivariate analysis the presence of EMD did not impact on PFS (HR 1.15, 95%CI: 0.99-1.33; P=0.06), while other known prognostic factors retained their significance: high risk versus standard cytogenetic (HR 1.35, 95%CI: 1.20-1.52; P<0.001), and ISS III versus I (HR 1.74, 95%CI: 1.53-1.98; P<0.001) (Online Supplementary Figure S2). Type of therapy had no impact on PFS: IMiD-based therapy (HR 1.14, 95%CI: 0.96-1.35) and no IMiD (HR 1.18, 95%CI: 0.87-1.59) (interaction P=0.86), PI-based therapy (HR 1.33, 95%CI: 1.04-1.71) and no PI, (HR 1.04, 95%CI: 0.87-1.25) (interaction P=0.12), and ASCT in eligible patients (HR 1.10, 95%CI: 0.81-1.50) and non-ASCT (HR 1.04, 95%CI: 0.73-1.47) (interaction P=0.72). A landmark analysis from maintenance start showed a median PFS of 23.4 months (95%CI: 19.1-30.1) and 23.5 months (95%CI: 21.8-25.7) (P=0.30) in EMD and non-EMD patients, respectively. EMD size was not correlated with median PFS: patients with EMD ≤3 cm 26.0 months (95%CI: 18.5-37.1), patients with EMD >3 cm 23.7 months (95%CI: 18.8-28.2), and patients without EMD 25.2 months (95%CI: 24.2-27.0) (Figure 2). The same results were observed with the EMD size threshold at 5 cm (Online Supplementary Figure S3). Median PFS according to EMD number was as follows: single EMD localization 26.1 months (95%CI: 22.5-30.1), multiple EMD localizations 19.4 months (95%CI: 14.9-33.1), and no EMD 25.2 months (95%CI: 24.2-27.0). Median PFS was not correlated with EMD site: PO 24.3 months (95%CI: 21.2-28.2), EMP 26.1 months (95%CI: 8.0-NR), and no EMD 25.2 months (95%CI: 24.2-27.0), PO versus no EMD (HR 1.14, 95%CI: 0.98-1.33; P=0.10), and EMP versus no EMD (HR 1.23, 95%CI: 0.64-2.37; P=0.54) (Figure 1A). Median PFS2 and 5-year PFS2 were 43.2 months (95%CI: 37.0-52.4) and 38% (95%CI: 31-47%) in PO, 27.9 months (95%CI: 4.9-NR) and NR in EMP, and 46.4 months (95%CI: 44.1-48.9) and 40% (95%CI: 37-43%) in non-EMD patients (Figure 3).
Figure 1.
(A) Progression-free survival (PFS) and (B) overall survival (OS) according to extramedullary disease presence and type. EMD: extramedullary disease; EMP: extramedullary plasmocytoma; PO: paraosseous plasmocytoma.
Figure 2.
Progression-free survival (PFS) according to extramedullary disease features. (A) PFS according to extramedullary disease (EMD) presence and size. (B) PFS according to single or multiple EMD localizations.
Figure 3.
Progression-free survival (PFS2). EMD: extramedullary disease; EMP: extramedullary plasmocytoma; PO: paraosseous plasmocytoma.
Overall survival
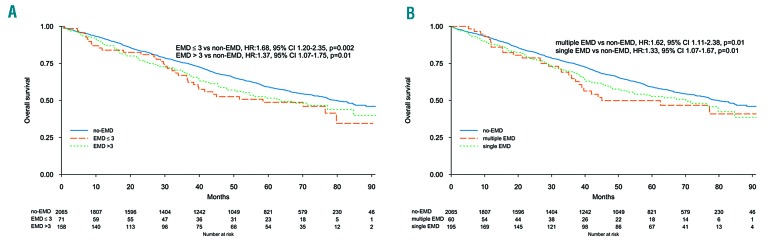
Median OS was 63.5 months (95%CI: 48.2-84.7) and 79.9 months (95%CI: 75.8-88.3; P=0.01) in EMD and non-EMD patients, respectively. Five-year OS was 51% (95%CI: 45-58%) and 59% (95%CI: 57-61%) (P=0.01) in EMD and non-EMD patients, respectively (Online Supplementary Figure S4), and there was a significant difference between PO and non-EMD (HR 1.39, 95%CI: 1.13-1.70; P=0.001) (Figure 1B). In multivariate analysis the presence of EMD was associated with a reduced OS (HR 1.41, 95%CI: 1.16-1.71; P<0.001), in line with other known prognostic factors: high risk versus standard cytogenetic (HR 1.68, 95%CI: 1.44-1.96; P<0.001), ISS III versus I (HR 2.36, 95%CI: 1.98-2.82; P<0.001) (Online Supplementary Figure S5). Type of therapy did not impact on OS: IMiD-based therapy (HR 1.38, 95%CI: 1.10-1.73) and no IMiD (HR 1.47, 95%CI: 1.01-2.13) (interaction P=0.78), PI-based therapy (HR 1.43, 95%CI: 1.04-1.97) and no PI, (HR 1.39, 95%CI: 1.09-1.76) (interaction P=0.87), and ASCT in eligible patients (HR 1.45, 95%CI: 0.95-2.20) and non-ASCT (HR 1.40, 95%CI: 0.88-2.25) (interaction P=0.99). A landmark analysis by maintenance start showed a median OS of 69.1 months (95%CI: 64.6-NR) and 87.8 months (95%CI: 87.8-NR) (P=0.22) in EMD and non-EMD patients, respectively. EMD size was not correlated with median OS: patients with EMD ≤3 cm 58.5 months (95%CI: 38.4-NR), patients with EMD >3 cm 63.7 months (95%CI: 48.2-NR), and patients without EMD 79.9 months (95%CI: 75.8-88.3) (Figure 4). The same analysis was done with the EMD size threshold at 5 cm (Online Supplementary Figure S6). Median OS according to EMD number was as follows: single EMD localization 70.1 months (95%CI: 50.4-NR), multiple EMD localizations 45 months (95%CI: 38.2-NR), and no EMD 79.9 months (95%CI: 75.8-88.3), single EMD versus no EMD (HR 1.33, 95%CI: 1.07-1.67; P=0.01), and multiple EMD localizations versus no EMD (HR 1.62, 95%CI: 1.11-2.38; P=0.01). Median OS was not correlated with EMD site: PO 67.3 months (95%CI: 50.4-NR), EMP 70.1 months (95%CI: 16.9-NR), and no EMD 79.9 months (95%CI: 75.8-88.3), PO versus no EMD (HR 1.39, 95%CI: 1.13-1.70; P=0.001), and EMP versus no EMD (HR 1.24, 95%CI: 0.55-2.78; P=0.60) (Figure 1B).
Figure 4.
Overall survival (OS) according to extramedullary disease (EMD) features. (A) OS according to EMD presence and size. (B) OS according to single or multiple EMD.
Discussion
To the best of our knowledge, this is the first meta-analysis of MM clinical trials focusing on patients with EMD so far reported. We included eight Fonesa Onlus and Hovon Foundation clinical trials that enrolled 2,332 newly diagnosed patients. In this population, we observed 267 (11%) patients with one or more EMD localizations, including 243 PO, 12 EMP, and 12 cases that were not classified. Since none of the clinical trials considered in this study had as primary end point the study of EMD, and a proportion of them were started around ten years ago, the most common imaging procedure performed at enrollment as screening was X-ray skeletal survey, and, only in case of a suspect of EMD, MRI or CT scan. X-ray skeletal survey is clearly suboptimal in detecting extramedullary asymptomatic disease. Nevertheless, the EMD incidence we observed is in line with other case series (in the range of 7-18%),1 suggesting that our patient population is quite representative of the daily clinical practice. In any case, it is expected that a wider use of more sensitive imaging techniques, such as positron emission tomography (PET), whole-body CT, and MRI will increase EMD detection.12,13 Interestingly, we observed that EMD patients had less disease burden, as shown by a more favorable ISS, lower bone marrow plasma cell infiltrate, higher hemoglobin levels, and a better renal function. This finding has been observed also by others in the first line setting,2,14 and may reflect a specific clinical picture, characterized by symptoms attributable to the EMD, rather than to larger disease burden. The presence of EMD at diagnosis did not impair the first line PFS, since EMD patients had a median PFS of 25.3 months, similar to the 25.2 months observed in patients without EMD. This finding is quite remarkable, since presence of EMD has long been recognized as an unfavorable prognostic factor, both in case of PO and EMP.4 Varettoni et al. described 76 EMD patients out of 1,003 MM patients at diagnosis, and with a treatment based on conventional chemotherapy the PFS of EMD was 18 versus the 30 months of patients without EMD (P=0.03).2 Only EMD patients who received an ASCT had a PFS similar to that of patients without EMD. Likewise, Wu et al. compared 75 EMD patients at diagnosis with 384 cases without EMD, and observed that EMD patients had an inferior PFS compared to that of patients without EMD, but this difference was overcome when EMD patients received ASCT.14 Hence, the presence of EMD at diagnosis has been incorporated as an adverse component of the Durie and Salmon PLUS prognostic score.15 Since we did not observe any significant difference in PFS between EMD and non-EMD patients, it is reasonable to speculate that the incorporation of new drugs in all the regimens tested in the studies included in this meta-analysis was able to overcome the unfavorable prognostic significance of EMD. In this perspective, several case reports, as well as a few trials, have shown that new drugs are effective in MM patients with EMD. In particular, Landau et al. have evaluated, in 42 high-risk MM at diagnosis including 14 patients with EMD, an induction with three cycles of bortezomib, liposomal doxorubicin and dexamethasone, followed by ASCT, with an acceptable median time-to-progression of 39 months.16 In our meta-analysis, 166 EMD patients were treated with IMiD-based therapies (lenalidomide in almost all cases) and have been compared with 1,279 non-EMD patients who received the same treatment. Quite surprisingly, also in this subset there was no difference in PFS between the two groups, suggesting that lenalidomide can be active also in this setting, as suggested by very few case reports.17 This is in contrast with the observation derived from studies involving thalidomide, the first-in-class IMiD, which resulted in having no effect on EMD,18 and this may be accounted for by the higher direct cytotoxic effect of lenalidomide respect to thalidomide.19 Interestingly, in our study EMD patients treated with IMiDs had the same PFS and OS as patients treated with PI (Online Supplementary Figure S7).
Previous studies showed that increasing the therapy intensity, i.e. intensifying the treatment with ASCT, overcame the negative prognostic significance of EMD presence.20 This has been confirmed in a large European Bone Marrow Transplantation registry study that considered 3,744 MM patients, including 353 with EMD, who received ASCT at diagnosis. This study has shown how patients with a single EMD had a similar PFS to patients without EMD.21 Since intensification seems to be the key to EMD control, it is possible to speculate that new drugs may offer a higher level of treatment intensity than conventional drugs. In the pre-new drug era, this goal was obtained only with ASCT. In order to evaluate whether the high efficacy of new drugs results in a more aggressive relapse, we analyzed PFS2, and we observed that EMD patients benefited from a similar disease control when compared to patients without EMD (42.3 vs. 46.4 months, respectively). This suggests that patients retain the benefit beyond the first line. Interestingly, also maintenance seems to have a similar efficacy in EMD and non-EMD patients. Median OS of EMD patients was inferior when compared with the control group (63.5 vs. 79.9 months, respectively), and this is irrespective of the type of therapy. Since PFS2 is similar between the two groups, it is safe to suggest that MM with EMD may acquire a more aggressive behavior in later stages of the disease.
Doubtless, the most sensitive technique for plasmacytoma identification is PET, which is able to upgrade myeloma-related lesion identification in more than half of patients when compared with X-ray skeletal survey.22 Unfortunately, in our study, PET was not used, since, at the time the trials were performed, this was not a standard technique. The recent IMAJEM trial, by the Intergroupe Francophone du Myelome (IFM), has shown that spine and pelvis MRI and PET are positive in 95% and 91% of patients at diagnosis, respectively, and that PET has a strong prognostic significance in terms of PFS and OS when evaluated both after the induction phase, represented by three cycles of lenalidomide plus bortezomib plus dexamethasone, and before maintenance start.23 Moreover, the IFM trial has shown that patients with EMD, evaluated with PET at diagnosis, have an increased risk of EMP relapse, progression or death (HR 3.4, 95%CI: 2.1-5.6; P<0.01). These data reinforce the concept that EMP has a strong detrimental effect on survival, but a specific analysis on the clinical significance of PO disease was not provided.
Surprisingly, we did not find any significant correlation between outcome and EMD size. A similar finding has been reported in the setting of solitary EMD. Eighty-four patients have been evaluated and no differences in terms of outcome have been seen between patients with EMD ≤5 cm, >5 and ≤10 cm, and >10 cm.24 Probably, the presence of a EMD is detrimental for the relevant biological features that are inherent in this variant of plasma cell neoplasm, rather than EMD size.25 Also the presence of single or multiple EMD localizations was not prognostically significant. Unfortunately, in our study, EMD was mainly represented by PO disease, since many EMP were probably missed due to the imaging techniques used at the time of trial design. Our observations are in contrast with the study by Rasche et al.,26 who evaluated with diffusion-weighted MRI 404 transplant-eligible patients and showed that the presence of three or more large focal lesions, defined as lesions with a product of the perpendicular diameters >5 cm2, were strong independent adverse prognostic factors. A possible explanation for this inconsistency can be attributed to the fact that Rasche et al. considered all types of focal lesions, including intraosseous focal lesions, while in our study we only analyzed EMD. Finally, we did not observe any significant correlation between EMP and outcome, but this is probably due to the limited number of cases observed in this study.
In conclusion, the main limitation of out study is an underestimation of EMD and, in particular, EMP incidence, caused by the low resolution of the imaging techniques employed at screening. Thus, our findings can be mainly referable to PO localizations, which are known to be less aggressive than EMP;27 this limits the value of our results. On the other hand, we performed the largest analysis of EMD patients at diagnosis, with the strength of using solid data derived from prospective trials. We confirmed that PI are effective towards EMD, and, for the first time, we provide evidence that also lenalidomide is effective in this difficult setting. We hope that our and other similar studies will draw attention to this unmet clinical need with trials specifically designed for MM patients with EMD.
Acknowledgments
The authors thank Anna De Filippo for assistance in formatting the paper.
Footnotes
Check the online version for the most updated information on this article, online supplements, and information on authorship & disclosures: www.haematologica.org/content/105/1/193
References
- 1.Blade J, Fernandez de Larrea C, Rosinol L, Cibeira MT, Jimenez R, Powles R. Soft-tissue plasmacytomas in multiple myeloma: incidence, mechanisms of extramedullary spread, and treatment approach. J Clin Oncol. 2011;29(28):3805–3812. [DOI] [PubMed] [Google Scholar]
- 2.Varettoni M, Corso A, Pica G, Mangiacavalli S, Pascutto C, Lazzarino M. Incidence, presenting features and outcome of extramedullary disease in multiple myeloma: a longitudinal study on 1003 consecutive patients. Ann Oncol. 2010; 21(2):325–330. [DOI] [PubMed] [Google Scholar]
- 3.Zamagni E, Patriarca F, Nanni C, et al. Prognostic relevance of 18-F FDG PET/CT in newly diagnosed multiple myeloma patients treated with up-front autologous transplantation. Blood. 2011;118(23):5989–5995. [DOI] [PubMed] [Google Scholar]
- 4.Pour L, Sevcikova S, Greslikova H, et al. Soft-tissue extramedullary multiple myeloma prognosis is significantly worse in comparison to bone-related extramedullary relapse. Haematologica. 2014;99(2):360–364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Minnema MC, van de Donk NW, Zweegman S, et al. Extramedullary relapses after allogeneic non-myeloablative stem cell transplantation in multiple myeloma patients do not negatively affect treatment outcome. Bone Marrow Transplant. 2008; 41(9):779–784. [DOI] [PubMed] [Google Scholar]
- 6.Perez-Simon JA, Sureda A, Fernandez-Aviles F, et al. Reduced-intensity conditioning allogeneic transplantation is associated with a high incidence of extramedullary relapses in multiple myeloma patients. Leukemia. 2006;20(3):542–545. [DOI] [PubMed] [Google Scholar]
- 7.Pasmantier MW, Azar HA. Extraskeletal spread in multiple plasma cell myeloma. A review of 57 autopsied cases. Cancer. 1969;23(1):167–174. [DOI] [PubMed] [Google Scholar]
- 8.Thomas FB, Clausen KP, Greenberger NJ. Liver disease in multiple myeloma. Arch Intern Med. 1973;132(2):195–202. [PubMed] [Google Scholar]
- 9.Roccaro AM, Mishima Y, Sacco A, et al. CXCR4 Regulates Extra-Medullary Myeloma through Epithelial-Mesenchymal-Transition-like Transcriptional Activation. Cell Rep. 2015; 12(4):622–635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Besse L, Sedlarikova L, Greslikova H, et al. Cytogenetics in multiple myeloma patients progressing into extramedullary disease. Eur J Haematol. 2016;97(1):93–100. [DOI] [PubMed] [Google Scholar]
- 11.Bink K, Haralambieva E, Kremer M, et al. Primary extramedullary plasmacytoma: similarities with and differences from multiple myeloma revealed by interphase cytogenetics. Haematologica. 2008;93(4):623–626. [DOI] [PubMed] [Google Scholar]
- 12.Hillengass J, Moulopoulos LA, Delorme S, et al. Whole-body computed tomography versus conventional skeletal survey in patients with multiple myeloma: a study of the International Myeloma Working Group. Blood Cancer J. 2017;7(8):e599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cavo M, Terpos E, Nanni C, et al. Role of (18)F-FDG PET/CT in the diagnosis and management of multiple myeloma and other plasma cell disorders: a consensus statement by the International Myeloma Working Group. Lancet Oncol. 2017; 18(4):e206–e217. [DOI] [PubMed] [Google Scholar]
- 14.Wu P, Davies FE, Boyd K, et al. The impact of extramedullary disease at presentation on the outcome of myeloma. Leuk Lymphoma. 2009;50(2):230–235. [DOI] [PubMed] [Google Scholar]
- 15.Durie BG. The role of anatomic and functional staging in myeloma: description of Durie/Salmon plus staging system. Eur J Cancer. 2006;42(11):1539–1543. [DOI] [PubMed] [Google Scholar]
- 16.Landau H, Pandit-Taskar N, Hassoun H, et al. Bortezomib, liposomal doxorubicin and dexamethasone followed by thalidomide and dexamethasone is an effective treatment for patients with newly diagnosed multiple myeloma with Internatinal Staging System stage II or III, or extramedullary disease. Leuk Lymphoma. 2012;53(2):275–281. [DOI] [PubMed] [Google Scholar]
- 17.Masárová K, Stefanikova Z, Mistrik M, Batorova A. Effectiveness of lenalidomide and dexamethasone for the treatment of extramedullary plasmacytoma in patients with multiple myeloma: Report of two cases. Blood. 2014;124(21):5760. [Google Scholar]
- 18.Rosinol L, Cibeira MT, Blade J, et al. Extramedullary multiple myeloma escapes the effect of thalidomide. Haematologica. 2004;89(7):832–836. [PubMed] [Google Scholar]
- 19.Kotla V, Goel S, Nischal S, et al. Mechanism of action of lenalidomide in hematological malignancies. J Hematol Oncol. 2009;2:36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Touzeau C, Moreau P. How I treat extramedullary myeloma. Blood. 2016; 127(8):971–976. [DOI] [PubMed] [Google Scholar]
- 21.Gagelmann N, Eikema DJ, Iacobelli S, et al. Impact of extramedullary disease in patients with newly diagnosed multiple myeloma undergoing autologous stem cell transplantation: a study from the Chronic Malignancies Working Party of the EBMT. Haematologica. 2018;103(5):890–897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zamagni E, Nanni C, Patriarca F, et al. A prospective comparison of 18F-fluorodeoxyglucose positron emission tomography-computed tomography, magnetic resonance imaging and whole-body planar radiographs in the assessment of bone disease in newly diagnosed multiple myeloma. Haematologica. 2007;92(1):50–55. [DOI] [PubMed] [Google Scholar]
- 23.Moreau P, Attal M, Caillot D, et al. Prospective evaluation of magnetic resonance imaging and [18F]fluorodeoxyglucose positron emission tomography-computed tomography at diagnosis and before maintenance therapy in symptomatic patients with multiple myeloma included in the IFM/DFCI 2009 Trial: results of the IMA-JEM Study. J Clin Oncol. 2017; 35(25):2911–2918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Reed V, Shah J, Medeiros LJ, et al. Solitary plasmacytomas: outcome and prognostic factors after definitive radiation therapy. Cancer. 2011;117(19):4468–4474. [DOI] [PubMed] [Google Scholar]
- 25.Bianchi G, Munshi NC. Pathogenesis beyond the cancer clone(s) in multiple myeloma. Blood. 2015;125(20):3049–3058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rasche L, Angtuaco EJ, Alpe TL, et al. The presence of large focal lesions is a strong independent prognostic factor in multiple myeloma. Blood. 2018;132(1):59–66 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jiménez R, Rosinol L, Cibeira MT, et al. Incidence and outcome of soft-tissue plasmacytomas in patients with multiple myeloma before and after the introduction of novel drugs. Blood. 2017;130(Suppl 1):3140. [Google Scholar]
- 28.Gay F, Magarotto V, Crippa C, et al. Bortezomib induction, reduced-intensity transplantation, and lenalidomide consolidation-maintenance for myeloma: updated results. Blood. 2013;122(8):1376–1383. [DOI] [PubMed] [Google Scholar]
- 29.Palumbo A, Bringhen S, Larocca A, et al. Bortezomib-melphalan-prednisone-thalidomide followed by maintenance with bortezomib-thalidomide compared with bortezomib-melphalan-prednisone for initial treatment of multiple myeloma: updated follow-up and improved survival. J Clin Oncol. 2014;32(7):634–640. [DOI] [PubMed] [Google Scholar]
- 30.Palumbo A, Cavallo F, Gay F, et al. Autologous transplantation and maintenance therapy in multiple myeloma. N Engl J Med. 2014;371(10):895–905. [DOI] [PubMed] [Google Scholar]
- 31.Gay F, Oliva S, Petrucci MT, et al. Autologous transplant vs oral chemotherapy and lenalidomide in newly diagnosed young myeloma patients: a pooled analysis. Leukemia. 2017;31(8):1727–1734. [DOI] [PubMed] [Google Scholar]
- 32.Magarotto V, Bringhen S, Offidani M, et al. Triplet vs doublet lenalidomide-containing regimens for the treatment of elderly patients with newly diagnosed multiple myeloma. Blood. 2016;127(9):1102–1108. [DOI] [PubMed] [Google Scholar]
- 33.Larocca A, Bringhen S, Petrucci MT, et al. A phase 2 study of three low-dose intensity subcutaneous bortezomib regimens in elderly frail patients with untreated multiple myeloma. Leukemia. 2016;30(6):1320–1326. [DOI] [PubMed] [Google Scholar]
- 34.Bringhen S, Petrucci MT, Larocca A, et al. Carfilzomib, cyclophosphamide, and dexamethasone in patients with newly diagnosed multiple myeloma: a multicenter, phase 2 study. Blood. 2014;124(1):63–69. [DOI] [PubMed] [Google Scholar]
- 35.Bringhen S, D’Agostino M, De Paoli L, et al. Phase 1/2 study of weekly carfilzomib, cyclophosphamide, dexamethasone in newly diagnosed transplant-ineligible myeloma. Leukemia. 2018;32(4):979–985. [DOI] [PubMed] [Google Scholar]