Current treatments for chronic lymphocytic leukemia (CLL) include targeted therapies like ibrutinib and monoclonal antibodies (rituximab and obinutuzumab). Although obinutuzumab shows better antitumor activity than rituximab, it induces more frequent and more severe infusion-related reactions (IRR).1 During this clinical trial (clinicaltrials.gov identifier: 02315768) we found that the combination of ibrutinib with obinutuzumab reduces the number and severity of IRR in previously untreated patients with CLL (Lujan et al., 2019, unpublished data). Additionally, we found that patients with higher baseline (pre-obinutuzumab-infusion) levels of CCL3, IFN-γ and IL-6 cytokines in peripheral blood, developed IRR, suggesting a potential predictive role for these cytokines. Moreover, after obinutuzumab infusion, we observed a significant increase in the levels of all analyzed cytokines (IFN-γ, IL-10, IL-6, IL-8, CCL3/MIP1-α, CCL4/MIP1-β and TNF-α), even in patients that did not develop IRR. However, CCL3, IFN-γ, and TNF-α reached statistically higher levels in patients who developed clinical IRR symptoms, indicating a possible role for these cytokines in the clinical manifestations observed.
Obinutuzumab is a glycoengineered type II anti-CD20 monoclonal antibody, that binds to CD20 (expressed on the surface of pre-B and mature B lymphocytes), and induces tumor clearance through direct cell death, antibody-dependent cell-mediated cytotoxicity, and, to a lesser extent, through complement-dependent phagocy-tosis.2 When compared with rituximab, obinutuzumab has shown better outcomes in patients with CLL and low-grade lymphoma.1,3,4 However, one limitation to obinutuzumab use is the presence of significantly more frequent and more severe IRR.1,3
Infusion-related reaction symptoms may affect any system and include, among others, fever, malaise, rigors, hypotension, dyspnea, pulmonary edema, and capillary leak syndrome; however, these are rarely lethal.6 Most commonly, IRR occurs during the first infusion and can be prevented by administrating pre-medications such as acetaminophen, diphenhydramine, and corticosteroids; IRR can be managed by decreasing the infusion rate or temporarily discontinuing the infusion.1,5,6
The incidence of any grade rituximab-induced IRR varies from 14% to 77%.7 The symptoms frequently appear during the infusion of the antibody but may also be delayed for 24 hours.7 Overall, the discontinuation rate of rituximab due to IRR is <1%.1 Instead, in the case of obinutuzumab, the overall rate of any grade IRR is 66-92%, and of grade 3-5 is 20-26%, with a permanent discontinuation rate due to IRR of 7-8%.2,5,7
The management of IRR leads not only to potentially serious medical consequences to patients, but also to an increased burden on patients, caregivers, and providers. The increase in mean costs for care of patients who experience IRR can range from $1,725 to $9,308, depending on whether they are managed as outpatients or are hospitalized, respectively. IRR also increases healthcare costs because it requires 31-80% more staff time compared to treating patients who do not experience IRR.8–11
We recently completed enrollment on a phase Ib/II clinical trial that combines obinutuzumab with the tyrosine kinase inhibitor ibrutinib in previously untreated CLL patients. Patients received ibrutinib before pre-medications, and at least one hour before infusion with obinutuzumab. We observed a significant reduction in obinutuzumab-induced IRR compared to previously reported data.2,6 Only 6 of 32 patients treated developed IRR symptoms (all grades: 19%, grade 1-2, 16% and grade 3, 3%). This rate of IRR is much lower than that in historical controls under monotherapy (all grades: 70-90%, grade ≥3, 2.5-20%),12,13 or as part of combination therapy (all grades: 65%, grade ≥3: 20%).2,6 Moreover, none of 32 patients treated in our study have required permanent discontinuation of obinutuzumab due to IRR.
To understand the biology of the beneficial effect that ibrutinib has over the reduced rate of obinutuzumab-associated IRR, we performed serial cytokine measurements on plasma samples from the first 23 patients enrolled in this study. Samples were taken at different time points during the first week of combined treatment: Cycle 1 prior to the first and post obinutuzumab infusion (at 2 and 4 hours, approximately); on day 1, day 2 and day 8.
Plasma and mononuclear blood cells were isolated from peripheral blood. After extraction and separation the samples were stored at −20°C and in liquid nitrogen, respectively, until further use.
A multiplex assay (Luminex) to measure seven different cytokines previously reported to be involved in ritux-imab and obinutuzumab IRR (IFN-γ, IL-10, IL-6, IL-8, CCL3/MIP1-α, CCL4/MIP1-β TNF-α) was designed.8,12 Standards were set up in duplicate yielding curves from 3.2 pg/mL to 10.000 pg/mL. Assays were performed according to the manufacturer’s instructions, with undiluted samples and overnight agitated incubation at 4°C. After measurement, we identified the maximum peak (at approx. 2 hours) of cytokine levels after obinutuzumab infusion, and compared those values with the baseline cytokine profile obtained before the first obinutuzumab infusion on Cycle 1 day 1.
Statistical analyses were performed with GraphPad Prism v7.04. Patients’ demographics and clinical characteristics were summarized using frequencies and corresponding percentages. Categorical variables were analyzed with Fisher’s exact test to determine if the incidence of IRR was related to age, gender, Rai stage, and lymph node size. Continuous variables were summarized using either mean with standard deviation (SD), or median with interquartile range (IQR), according to data distribution. For comparisons, we used unpaired t-test or Mann-Whitney U-test where appropriate. All P-values are two-sided; P<0.05 was considered significant.
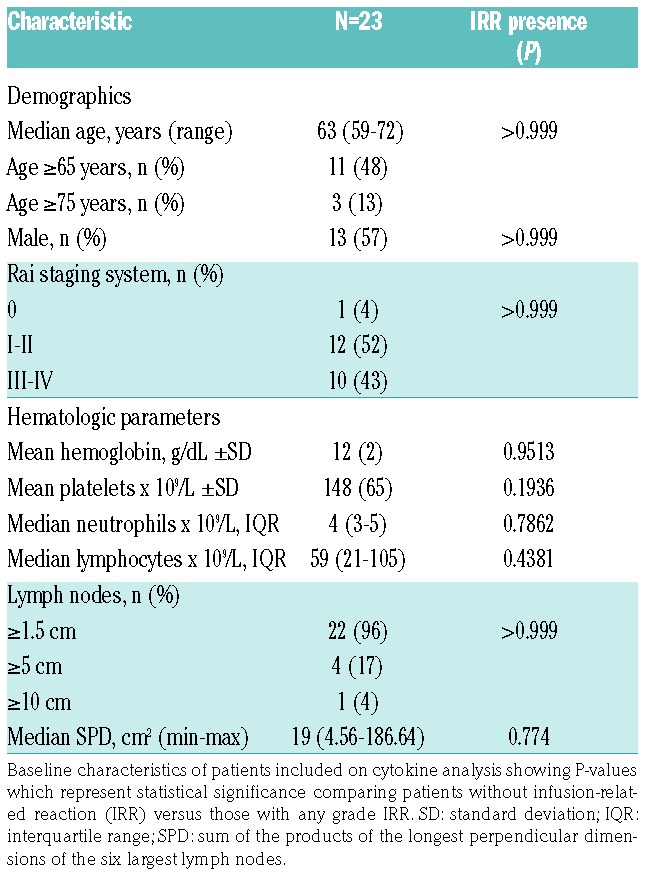
Age and disease characteristics at baseline were similar among patients with and without IRR (Table 1). At the time of entry to the study, patients had a median age of 63 years, and most of them had an acceptable performance status despite comorbidities. Three patients had a high tumor burden, defined as presenting lymph nodes with one axis measuring ≥10 cm, or ≥5 but <10 cm with lymphocytes in peripheral blood ≥25×109/L; nevertheless, none of them presented IRR.
Table 1.
Patients’ demographics and disease characteristics before treatment.
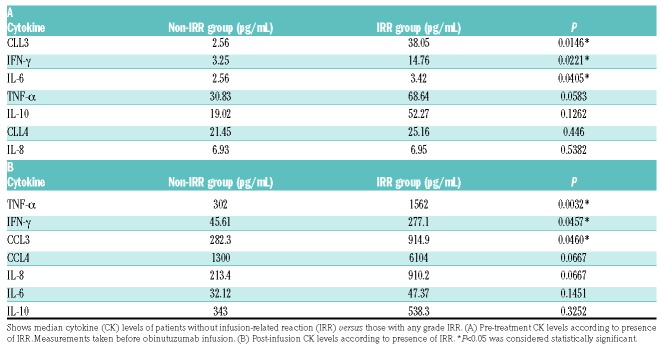
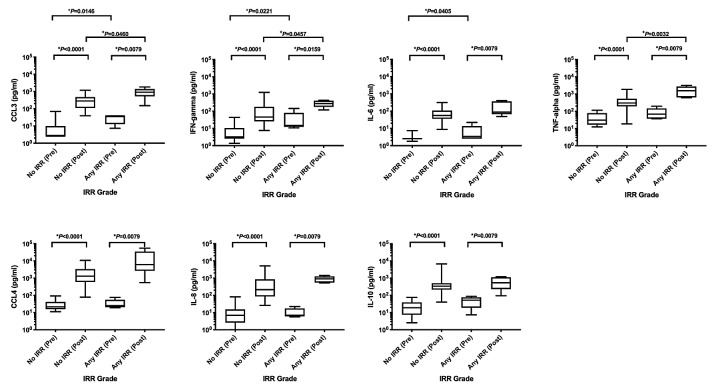
The majority of patients (22 of 23) showed maximum cytokine peak during Cycle 1 day 1 at the middle of obinutuzumab infusion (approximately 2 hours from the beginning of infusion) and this correlated with the onset of IRR-associated symptoms. Baseline levels of CCL3 (P=0.0146), IFN-γ (P=0.0221), and IL-6 (P=0.0405), prior to obinutuzumab infusion were statistically higher in patients that developed IRR, suggesting a possible predictive role in the development of IRR (Table 2). We observed a significant increase in all cytokines analyzed after obinutuzumab infusion, even in patients who did not develop IRR. However, when the post-infusion peaks were compared, only three cytokines, CCL3 (P=0.0460), IFN-γ (P=0.0457), and TNF-α (P=0.0032) showed levels with a significant increase in patients who developed IRR; suggesting that these cytokines could be associated with the clinical symptoms observed with IRR (Table 2 and Figure 1).
Table 2.
Cytokine levels in patients according to presence of infusion-related reactions.
Figure 1.
Infusion-related reaction (IRR) to biomarker. Values compare the C1D1 pre-infusion sample against the highest of all the eight subsequent measures (C1D1 mid-infusion sample on 22 of 23 patients) and are sorted by the presence or absence of any IRR. Statistical analyses were performed accordingly using Mann-Whitney test. This figure includes only statistically significant values. °Statistical significance between pre-infusion levels. +Statistical significance between post-infusion levels. *Statistical significance between pre- and post-infusion levels in each group.
In comparison, a similar study analyzed a subset of 38 patients with an underlying diagnosis of CLL, pooling the patients from two phase I/II trials (GAUSS: clinicaltrials.gov identifier: 00576758, and GAUGUIN: clinicaltrials.gov identifier: 00517530). All patients received treatment with obinutuzumab single agent. The studies report that 35 (92%) out of 38 of the patients developed IRR symptoms with the first infusion, and 28% of them were grade ≥3. The studies also found that three (8%) of the patients discontinued the treatment permanently due to IRR. In those patients, there was a consistent increase in proinflammatory cytokines IL-8, IL-6, TNF-α, and IFN-γ, with higher cytokine levels usually at the mid-infusion time point, similar to our observations in our study.7
Lastly, we also detected a much lower rate of IRR (19%), with only one patient developing a G3 IRR that resolved without further progression. The cytokine profile data showed that despite the low rate of clinical manifestations associated with IRR, all patients had an increase in all the cytokines that we tested. However, only CCL3, IFN-γ, and TNF-α showed a significant post-infusion increase that was observed exclusively in patients with clinical manifestations of IRR. Moreover, higher pre-infusion levels of CCL3, IFN-γ and IL-6 could predict those patients with the highest risk of developing obinutuzumab IRR when it is administered in combination with ibrutinib.
Taking together, our study shows that concurrent administration of ibrutinib (initiated on Cycle 1 day 1 before pre-medications) and obinutuzumab has a beneficial effect reducing the rates of IRR when compared with historical controls (obinutuzumab monotherapy), and this could have a significant impact, particularly in patients with advanced age or comorbidities. Similar results from ibrutinib combination have been found in previous studies, where the addition of ibrutinib to rituximab reduces the IRR rate from 16% to 1% in patients with Waldenström’s macroglobulinemia,13 supporting our findings that ibrutinib may help to reduce anti-CD20 IRR.
Even though the sample size is small, our observations provide additional insights into the biology of obinutuzumab-associated IRR and how to reduce those adverse events effectively using ibrutinib, while preserving the immune function needed for the activity of this monoclonal antibody. Additionally, our data suggest that B-cell receptor signaling modulation, such as BTK inhibition, using ibrutinib could modulate immune responses and adverse events associated with B-cell immunotherapies. This could have significant clinical relevance in patients treated with other types of immunotherapy, such as adoptive cellular therapy (i.e. chimeric antigen receptor T-cell treatment), who can develop significant, and sometimes lethal, cytokine release syndrome and neurotoxicities.14,15 However, additional studies are needed to understand the potential immune-modulatory role of ibrutinib and its applications in new immunotherapeutic protocols.
Footnotes
Information on authorship, contributions, and financial & other disclosures was provided by the authors and is available with the online version of this article at www.haematologica.org.
References
- 1.Sehn LH, Assouline SE, Stewart DA. A phase 1 study of obinu-tuzumab induction followed by 2 years of maintenance in patients with relapsed CD20-positive B-cell malignancies. Blood. 2012;119(22):5118–5125. [DOI] [PubMed] [Google Scholar]
- 2.Goede V, Fischer K, Busch R, et al. Obinutuzumab plus chlorambucil in patients with CLL and coexisting conditions. N Engl J Med. 2014;370(12):1101–1110. [DOI] [PubMed] [Google Scholar]
- 3.Tobinai K, Klein C, Oya N, Fingerle-Rowson G. A Review of Obinutuzumab (GA101), a Novel Type II Anti-CD20 Monoclonal Antibody, for the Treatment of Patients with B-Cell Malignancies. Adv Ther. 2017;34(2):324–356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lenz HJ. Management and preparedness for infusion and hypersensitivity reactions. Oncologist. 2007;12(5):601–609. [DOI] [PubMed] [Google Scholar]
- 5.Leblond V, Aktan M, Ferra Coll CM, et al. Safety of obinutuzumab alone or combined with chemotherapy for previously untreated or relapsed/refractory chronic lymphocytic leukemia in the Phase 3b GREEN study. Haematologica. 2018;103(11):1889–1898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Norin S, Björkstrand B, Rommel F, et al. Severe infusion-related reactions are uncommon in rituximab-treated CLL patients in clinical practice: Results from a Swedish national observational study. Leuk Res. 2015;39(1):33–37. [DOI] [PubMed] [Google Scholar]
- 7.Freeman C, Morschhauser F, Sehn L, et al. Cytokine release in patients with CLL treated with obinutuzumab and possible relationship with infusion-related reactions. Blood. 2015;12(24):2646–2649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fortner B, Viale PH. Health economic analysis of the burden of infusion reactions on patients, caregivers, and providers. Oncology (Williston Park). 2009;23(2 Suppl 1):31–36. [PubMed] [Google Scholar]
- 9.Schwartzberg LS, Stepanski EJ, Walker MS, Mathias S, Houts AC, Fortner BV. Implications of IV monoclonal antibody infusion reaction for the patient, caregiver, and practice: results of a multicenter study. Support Care Cancer. 2009;17(1):91–98. [DOI] [PubMed] [Google Scholar]
- 10.Casado LF, Burgos A, Gonzalez-Haba E, Loscertales J, et al. Economic evaluation of obinutuzumab in combination with chlorambucil in first-line treatment of patients with chronic lymphocytic leukemia in Spain. Clinicoecon Outcome Res. 2016;8:475–484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Soini E, Hautala A, Poikonen E, Becker U, Kyttälä M, Martikainen J. Cost-effectiveness of First-line Chronic Lymphocytic Leukemia Treatments When Full-dose Fludarabine Is Unsuitable. Clin Ther. 2016;38(4):889–904. [DOI] [PubMed] [Google Scholar]
- 12.Byrd JC, Flynn JM, Kipps TJ, et al. Randomized phase 2 study of obinutuzumab monotherapy in symptomatic, previously untreated chronic lymphocytic leukemia. Blood. 2016;127(1):79–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Brown JR, O’Brien S, Kingsley CD. Obinutuzumab plus fludarabine/cyclophosphamide or bendamustine in the initial therapy of CLL patients: the phase 1b GALTON trial. Blood. 2015;125(18):2779–2785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ruella M, Kenderian SS, Shestova O, et al. Kinase inhibitor ibrutinib to prevent cytokine-release syndrome after anti-CD19 chimeric antigen receptor T cells for B-cell neoplasms. Leukemia. 2017;31(1):246–248. [DOI] [PubMed] [Google Scholar]
- 15.Gauthier J, Hirayama AV, Hay KA, et al. Comparison of Efficacy and Toxicity of CD19-Specific Chimeric Antigen Receptor T-Cells Alone or in Combination with Ibrutinib for Relapsed and/or Refractory CLL. Blood. 2018;132(Suppl 1):299. [Google Scholar]