Abstract
The endosteal bone marrow niche and vascular endothelial cells provide sanctuaries for leukemic cells. In murine chronic myeloid leukemia (CML) CD44 on leukemia cells and E-selectin on bone marrow endothelium are essential mediators for the engraftment of leukemic stem cells. We hypothesized that non-adhesion of CML-initiating cells to E-selectin on the bone marrow endothelium may lead to superior eradication of leukemic stem cells in CML after treatment with imatinib than imatinib alone. Indeed, here we show that treatment with the E-selectin inhibitor GMI-1271 in combination with imatinib prolongs survival of mice with CML via decreased contact time of leukemia cells with bone marrow endothelium. Non-adhesion of BCR-ABL1+ cells leads to an increase of cell cycle progression and an increase of expression of the hematopoietic transcription factor and proto-oncogene Scl/Tal1 in leukemia-initiating cells. We implicate SCL/TAL1 as an indirect phosphorylation target of BCR-ABL1 and as a negative transcriptional regulator of CD44 expression. We show that increased SCL/TAL1 expression is associated with improved outcome in human CML. These data demonstrate the BCR-ABL1-specific, cell-intrinsic pathways leading to altered interactions with the vascular niche via the modulation of adhesion molecules – which could be exploited therapeutically in the future.
Introduction
The bone marrow (BM) microenvironment and in particular the endosteal BM niche,1 vascular endothelial cells,2 as well as secreted factors and mesenchymal stromal cells,3,4 protect leukemic stem cells (LSC) from eradication by various therapies, thereby leading to treatment resistance, disease relapse and disease progression. E-selectin, an adhesion molecule exclusively expressed on endothelial cells and activated by cytokines, is an essential component of the vascular niche in the BM microenvironment, where it promotes the proliferation of normal hematopoietic stem cells (HSC).5 E-selectin6 and one of its ligands,7 CD44,8 have been shown to be essential mediators of engraftment of chronic myeloid leukemia (CML)-initiating cells. However, the mechanism for overexpression of CD44 on leukemia-initiating cells (LIC) in CML mediating engraftment, as previously described by us,8 has not been established. CD44, known to mediate the transport of acute myeloid leukemia cells to stem cell-supportive ni ches,9 also acts as an E-selectin ligand on colon cancer10 and breast cancer cells.11
GMI-1271 is a specific small molecule antagonist of E-selectin with a dissociation constant of 0.54 mM. Co-administration of GMI-1271 was recently demonstrated to overcome resistance to bortezomib in E-selectin ligand-enriched multiple myeloma cells,12 and GMI-1271 is currently being tested in clinical trials in combination with chemotherapy in patients with acute myeloid leukemia. It is surmised that - similar to mobilization by granulocyte colony-stimulating factor13,14 - GMI-1271-mediated mobilization of LSC may break LSC dormancy and, thereby, lead to improved eradication by tyrosine kinase inhibitors or chemotherapy. We had previously shown that targeting the osteolineage compartment of the BM microenvironment can lead to successful reduction of LSC in CML.15 Imatinib, a tyrosine kinase inhibitor targeting BCR-ABL1, the oncoprotein causing CML, does not eradicate LSC.16,17 We hypothesized that treatment with GMI-1271 may lead to non-adhesion of CML-initiating cells to the BM endothelium and in combination with imatinib may be better at eliminating LSC in CML than imatinib alone.
Indeed, in this study we show that inhibition of E-selectin leads to a dissociation of BCR-ABL1+ cells from the endothelium. Concomitantly, this leads to increased leukemic cell proliferation and upregulation of the hematopoietic transcription factor and proto-oncogene Scl/Tal1, whose overexpression is frequently found in T-cell acute lymphoblastic leukemia.18 SCL/TAL1, itself phosphorylated by pAKT downstream of BCR-ABL1, is demonstrated to be a negative transcriptional regulator of CD44. Collectively, these data illustrate the reciprocal link between external cues from the BM microenvironment and LSC-intrinsic effects which, in turn, can influence LSC expression of adhesion molecules essential for interaction with the BM microenvironment and, thereby, modify therapy response. This is a concept which could influence future treatment strategies.
Methods
Additional methods are described in the Online Supplementary Material.
Mice
BALB/c or FVB mice were purchased from Charles River Laboratories (Wilmington, MA, USA). Rag-2−/− CD47−/− IL-2 receptor γ−/− (C57/Bl6 background) and Tie2-GFP mice (FVB background) were bred in our institute. All murine studies were approved by the local animal care committee (Regierungspräsidium Darmstadt).
Statistical analysis
Statistical significance between different treatment groups was assessed by the Student t-test or analysis of variance (ANOVA) test (with a Tukey test as a post-hoc test) using Prism Version 6 software (GraphPad, La Jolla, CA, USA). Differences in survival were assessed by Kaplan-Meier nonparametric tests (log-rank or Wilcoxon tests). Data are presented as the mean ± standard deviation and differences are considered statistically significant when P≤0.05.
Results
GMI-1271 decreases the contact time of human chronic myeloid leukemia cells with bone marrow endothelium
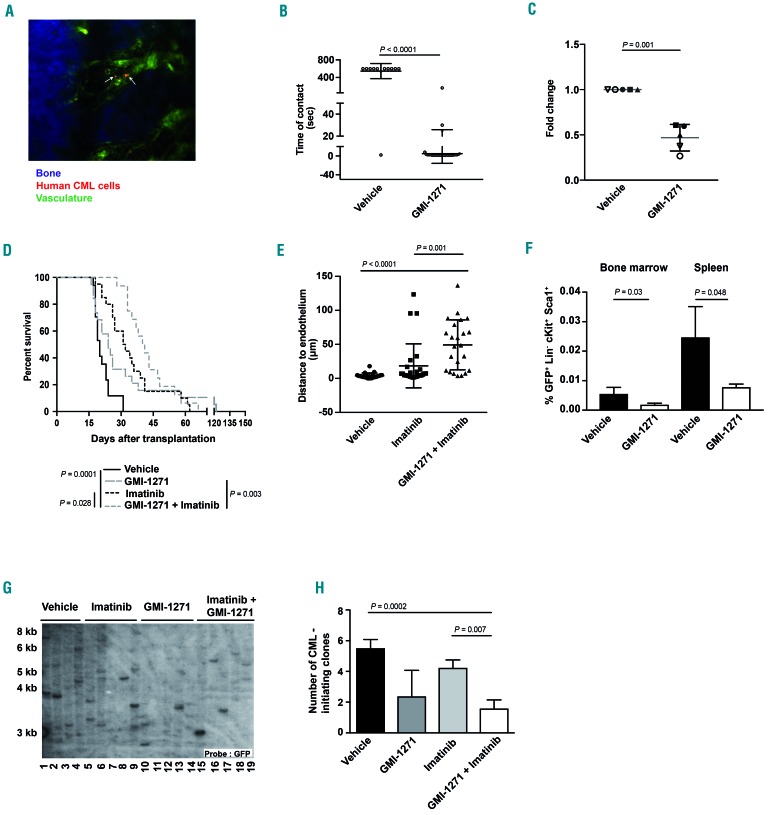
In order to test whether inhibition of E-selectin by GMI-1271 decreases adhesion of human CML cells to BM endothelium, we injected leukocytes from five different human CML patients into Rag-2−/−CD47−/−IL-2 receptor γ−/−mice19 treated with vehicle or GMI-1271 and imaged the calvarium of the injected mice by in vivo microscopy (Figure 1A and Online Supplementary Movie). This revealed a significantly reduced contact time of leukemia cells from four out of five patients with BM endothelium in mice treated with GMI-1271 (P<0.0001, Figure 1B and Online Supplementary Figure S1A-D). As anticipated, this result suggested that GMI-1271 leads to non-adhesion of human cells to the BM endothelium. Furthermore, in an in vitro adhesion assay of human CML cells plated on E-selectin, a smaller number of human CML cells adhered to E-selectin in the presence of GMI-1271 than in the presence of vehicle (P=0.0013) (Figure 1C).
Figure 1.
Inhibition of E-selectin affects survival in mice with chronic myeloid leukemia. (A) Representative two-photon in vivo microscopy image of the bone marrow (BM) calvarium of an unirradiated Rag-2−/−CD47−/−IL-2 receptor γ−/− mouse injected with 200,000-500,000 unsorted human chronic myeloid leukemia (CML) cells [from peripheral blood (PB) or BM], labeled with CMTMR (orange; white arrows), 2 h prior to in vivo microscopy. Vessels were visualized via the injection of dextran-FITC (1 mg per injection), while bones were visualized in blue due to second harmonic generation. The scale bar represents 50 mm. (B) Time of contact (seconds), determined by in vivo microscopy, between the calvarial endothelium and human unsorted CML cells from the PB of one patient labeled with CMTMR and injected into vehicle- or GMI-1271 (20 mg/kg/dose)-treated unirradiated Rag-2−/−CD47−/−IL-2 receptor γ−/− mice (P<0.0001, t-test). The mice had been treated with vehicle or GMI-1271 2 h before transplantation. (C) Adhesion (presented as fold change) of unsorted human CML cells from PB or BM to E-selectin-coated wells (50,000 CML cells/well) in the presence of vehicle vs. GMI-1271 (P=0.001, t-test) (n=5) after incubation for 6 h. Different symbols signify individual patients. The experiment was performed in duplicate. (D) Kaplan-Meier-style survival curve for Balb/c recipients of BCR-ABL1-transduced BM treated with vehicle (black solid line), 20 mg/kg/dose GMI-1271 intraperitoneally (long gray dashes), 100 mg/kg imatinib orally (black dots) and the combination of both imatinib and GMI-1271 (short gray dashes) beginning on day 9 after transplantation. Vehicle and imatinib were administered daily, while GMI-1271 was given twice daily. The difference in survival between mice treated with imatinib or imatinib plus GMI-1271 was statistically significant (P=0.028, log-rank test, n=15). (E) Distance (in mm) from endothelium of CMTMR-labeled GFP+ (BCR-ABL1+) Lin− cells (200,000) from FVB mice with established CML-like disease transplanted by intravenous injection into Tie2-GFP mice (FVB background) and imaged by in vivo microscopy 19 h after injection [P=0.001, analysis of variance/(ANOVA); Tukey test]. The imaging analysis was performed by ImageJ. (F) Percentage of GFP+ (BCR-ABL1+) Lin− c-Kit+ Sca-1+ cells of total leukocytes which homed to the BM (P=0.03, t-test) or spleen (P=0.048, t-test) of vehicle (black)- or GMI-1271 (white)-treated mice 18 h after transplantation (n=4). A total of 2.5 × 106 unsorted, BCR-ABL1-transduced cells had been injected. (G, H) Southern blot showing distinct proviral integration sites and, consequently, disease clonality in spleens (taken at the time of death, as shown in Figure 1D) of Balb/c recipients of BCR-ABL1-transduced BM treated with vehicle (lanes 1-4), imatinib (lanes 5-9), GMI-1271 (lanes 10-14) or imatinib plus GMI-1271 (lanes 15-19) (G) and disease clonality (H). The difference in disease clonality between imatinib- and imatinib plus GMI-1271-treated recipients as a measure of engraftment of leukemia-initiating clones is statistically significant (P=0.007, ANOVA; Tukey test). Data are expressed as mean ± standard deviation.
Inhibition of E-selectin affects survival in mice with chronic myeloid leukemia
Next, we tested the effect of inhibition of E-selectin in combination with imatinib mesylate, considered the standard of care for CML, on the survival of mice with CML. Treatment of murine recipients of BCR-ABL1-transduced BM in the retroviral transduction/transplantation model of CML-like myeloproliferative neoplasia15,20 with vehicle, imatinib, GMI-1271 or a combination of imatinib and GMI-1271 (Online Supplementary Figure S2A) revealed a modest, but significant prolongation of survival in mice that received the combination therapy compared to mice that received imatinib alone (P=0.028) (Figure 1D) with all mice succumbing to BCR-ABL1+ leukemia and pulmonary infiltration by mature neutrophils, as described elsewhere.8,15 Leukocyte counts (P=0.0008) (Online Supplementary Figure S2B), BCR-ABL1+ (GFP+) CD11b+ myeloid cells (P=0.031) (Online Supplementary Figure S2C) and spleen weights (P=0.024) (Online Supplementary Figure S2D) were significantly reduced in mice with CML treated with imatinib plus GMI-1271, when compared to vehicle-treated mice. However, the direct comparison of treatment with imatinib plus GMI-1271 vs. imatinib alone did not reveal a statistically significant difference. A significant reduction of GFP (BCR-ABL1)+ Lin− c-Kit+ (LK) cells, which harbor the LSC fraction in this model,21 was observed in mice treated with imatinib and GMI-1271, when compared to vehicle-treated mice (P=0.001) (Online Supplementary Figure S2E). Lin− GFP+ (BCR-ABL1+) cells from CML mice (P=0.001) (Figure 1E) or BCR-ABL1+ BaF3 cells (P=0.002) (Online Supplementary Figure S2F) injected into Tie2-GFP mice, which express green fluorescent protein (GFP) under the TEK receptor tyrosine kinase (Tie2)-promoter, were found significantly further away from the endothelium, but not the bone (Online Supplementary Figure S2G-H), when mice had been treated with GMI-1271 and imatinib than when they had been treated with vehicle or imatinib alone. Short-term homing of CML-initiating cells to BM (P=0.03) (Figure 1F) and spleen (P=0.048) (Figure 1F) of GMI-1271-treated recipients was impaired compared to that of vehicle-treated recipients. Long-term engraftment of CML-initiating clones, as measured by the enumeration of distinct proviral integration events in splenic tissue of diseased mice by Southern blotting, was significantly reduced in primary mice treated with imatinib plus GMI-1271 compared to those treated with imatinib alone (P=0.007) (Figure 1G, H).
In summary, these data suggest that in comparison to monotherapy with imatinib, inhibition of E-selectin in combination with imatinib mildly prolongs survival in mice with a CML-like myeloproliferative neoplasia via a reduction of homing and long-term engraftment of CML-initiating clones. Inhibition of E-selectin also reduces the number of human CML cells adhering to E-selectin.
Inhibition of E-selectin leads to an increase of Scl/Tal1 in BCR-ABL1+ leukemia-initiating cells
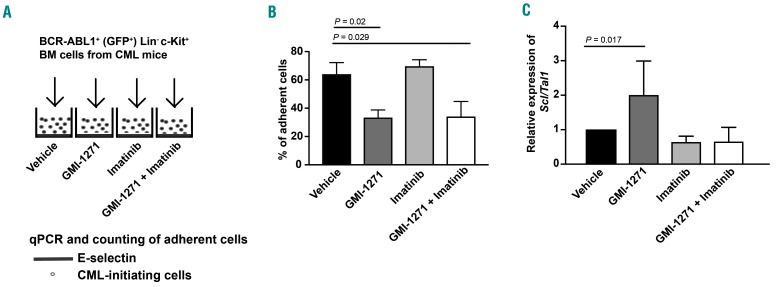
In order to explain the prolonged survival of mice treated with imatinib and GMI-1271, we tested the adhesion and gene expression of cell cycle-relevant genes and transcription factors in LIC in the presence of GMI-1271. To do so, we plated BCR-ABL1+ Lin− c-Kit+ BM cells from mice with CML on E-selectin-coated plates in the presence of vehicle, GMI-1271,22 imatinib23,24 or the combination of GMI-1271 plus imatinib (Figure 2A). As expected, this revealed that treatment with GMI-1271 (P=0.02) or GMI-1271 plus imatinib (P=0.029) significantly reduced the number of adherent cells (Figure 2B). In a competitive inhibition assay we plated BCR-ABL1+ BaF3 cells, which were used because sufficient numbers of LIC can only be retrieved from a large number of diseased mice, on plates pre-coated with E-selectin, while adding soluble E-selectin to the BCR-ABL1+ BaF3 cells treated with GMI-1271. This reversed the decreased adhesion of leukemia cells in the presence of GMI-1271 (P=0.021) (Online Supplementary Figure S3A) suggesting a specific role of E-selectin in this process. Furthermore, non-adhesion of primary murine BCR-ABL1+ LIC (P=0.017) (Figure 2C) or K562 cells (P=0.007) (Online Supplementary Figure S3B), which were tested in order to show the validity also in a human cell line, to E-selectin in the presence of GMI-1271 led to an increase of the transcription factor and cell cycle regulator25 stem cell leukemia/T-cell acute lymphocytic leukemia protein 1 (Scl/Tal1). Other transcription factors, however, did not significantly change in K562 cells (Online Supplementary Figure S3C-G, Online Supplementary Table S1). Conversely, addition of soluble E-selectin to BCR-ABL1+ BaF3 cells plated on E-selectin in the presence of GMI-1271 (as in Online Supplementary Figure S3A) decreased the expression of Scl/Tal1 (P=0.028) (Online Supplementary Figure S4A). In summary, these data suggest that E-selectin is involved in adhesion of CML cells and that non-adhesion leads to increased expression of Scl/Tal1.
Figure 2.
Inhibition of E-selectin leads to an increase of Scl/Tal1 in BCR-ABL1+ leukemia-initiating cells. (A) Schematic of an in vitro adhesion assay, in which 20,000 GFP+ (BCR-ABL1+) Lin− c-Kit+ bone marrow cells from mice with chronic myeloid leukemia treated with vehicle, GMI-1271, imatinib or the combination of GMI-1271 plus imatinib were plated on recombinant E-selectin in the presence of the respective drugs for 6 h. (B) Percentage of adherent GFP+ (BCR-ABL1+) Lin− c-Kit+ of total cells after 6 h of incubation on recombinant E-selectin in the presence of vehicle (black), 20 mM GMI-1271 (dark gray), 10 mM imatinib (light gray) or imatinib plus GMI-1271 (white) and several washing steps, normalized by the number of live cells. Twenty thousand cells per well were plated in triplicate. The percentage of adherent cells was reduced by treatment with GMI-1271 or imatinib plus GMI-1271 compared to vehicle [P=0.02 or P=0.029, respectively, analysis of variance (ANOVA); Tukey test, n=4]. (C) Relative expression of Scl/Tal1 in all GFP+ (BCR-ABL1+) Lin− c-Kit+ cells plated on recombinant E-selectin as in (A) and (B) in the presence of vehicle (black), GMI-1271 (dark gray), imatinib (light gray) or imatinib plus GMI-1271 (white). The expression of Scl/Tal1 in GMI-1271-treated cells was significantly increased compared to that of vehicle-treated cells (P=0.017, ANOVA; Tukey test, n=3). BM: bone marrow; CML: chronic myeloid leukemia; qPCR: real-time polymerase chain reaction.
Scl/Tal1 regulates CD44 expression
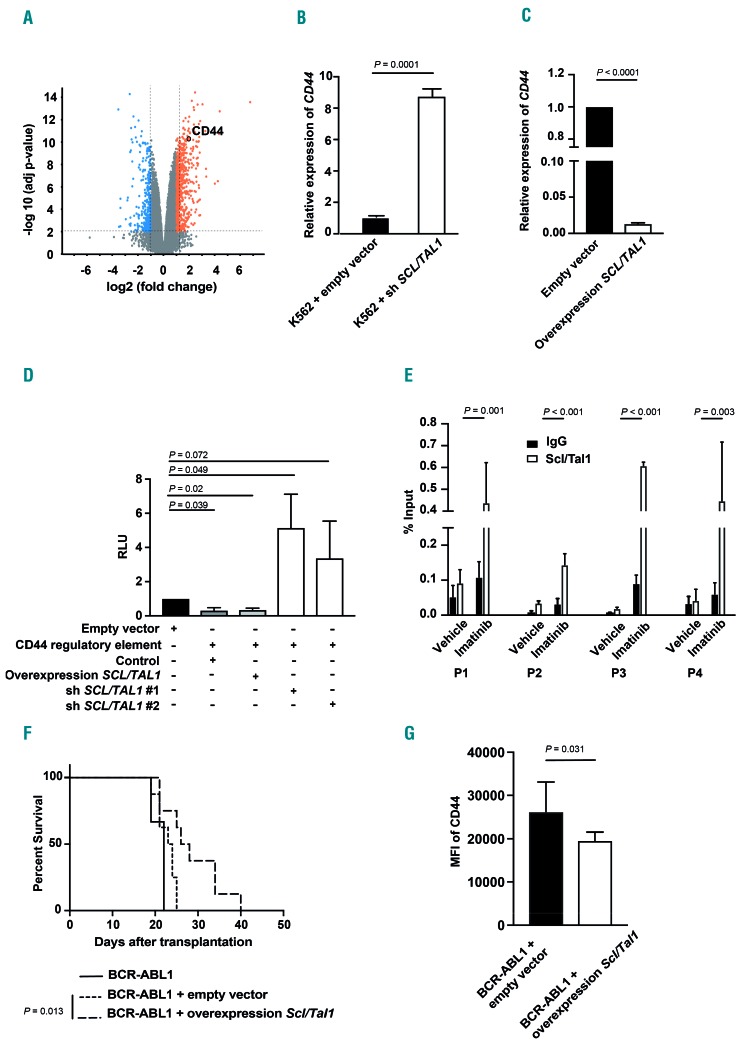
As SCL/TAL1 is a known transcriptional regulator involved in various hematologic malignancies and cell cycle progression,25 we performed a gene expression screen after knockdown of SCL/TAL1 in K562 cells (data deposited in the GEO-Expression database, GSE92251). This revealed that knockdown of SCL/TAL1 led to an upregulation of CD4426 (Figure 3A). As we had previously shown CD448 and its receptor E-selectin6,7 to be important mediators of the engraftment of LIC in CML, we hypothesized that SCL/TAL1 may regulate the expression of CD44 on CML cells, where CD44 is known to be overexpressed8 – albeit through an unknown mechanism. Indeed, specific knockdown of SCL/TAL1 in K562 cells led to upregulation of CD44 (P=0.0001) (Figure 3B and Online Supplementary Figure S5A) and, conversely, overexpression of SCL/TAL1 in K562 (P<0.0001) (Figure 3C and Online Supplementary Figure S5B, C) or in human CD34+ cells (P=0.0001) (Online Supplementary Figure S5D) led to decreased expression of CD44. We performed luciferase assays on K562 cells transfected with a control plasmid or a CD44 regulatory element with SCL/TAL1-binding sites and transduced with SCL/TAL1 shRNA- or SCL/TAL1-overexpressing lentivirus, in order to test activity of the CD44 regulatory element (Online Supplementary Figure S5E). This revealed decreased activity of the CD44 regulatory element when SCL/TAL1 was overexpressed (P=0.02) (Figure 3D and Online Supplementary Figure S5B, C) and increased activity when SCL/TAL1 was knocked down (P=0.049) (Figure 3D and Online Supplementary Figure S5A). Having shown the influence of SCL/TAL1 on the activity of the CD44 regulatory element, we tested the dependence of this on BCR-ABL1 in a chromatin immunoprecipitation assay using K562 cells treated with vehicle or imatinib. This revealed that imatinib treatment significantly increased the binding of SCL/TAL1 to the CD44 regulatory element (Figure 3E and Online Supplementary Figure S6A), but not to a control region (Online Supplementary Figure S6B). Furthermore, overexpression of SCL/TAL1 (Online Supplementary Figure S7A) in BCR-ABL1+ LIC in vivo significantly prolonged survival (P=0.013) (Figure 3F), led to decreased median fluorescence intensity of CD44 on BCR-ABL1+ CD11b+ myeloid cells (P=0.031) (Figure 3G) and a trend towards decreased disease clonality (Online Supplementary Figure S7B) in murine recipients in our CML model. This resembled what we had observed when using CD44-deficient BCR-ABL1+ donor BM,8 although the effect was not as pronounced. Overexpression of SCL/TAL1 in K562 cells (Online Supplementary Figure S5B) also decreased the median fluorescence intensity of CD44 (P=0.019) (Online Supplementary Figure S5C). Taken together, these data suggest that SCL/TAL1 negatively regulates the expression of CD44 and that overexpression of SCL/TAL1 on LIC leads to prolongation of survival in CML, similar to the effect of CD44 deficiency on CML-initiating cells.8
Figure 3.
Scl/Tal1 regulates CD44 expression. (A) Volcano plot showing up- or down-regulated genes in K562 cells after knockdown of SCL/TAL1 relative to knockdown of lacZ as a control. The x-axis indicates the fold change and the y-axis indicates the −log10 P value. The orange and blue highlighted regions show 2-fold up- and down-regulated genes, respectively, with a P value ≤0.05. CD44 has been circled. (B, C) Relative expression of CD44 in K562 cells after infection with a SCL/TAL1 shRNA-expressing or empty vector control (P=0.0001, t-test) (B) or an empty vector control- or a SCL/TAL1-overexpressing lentivirus (P<0.0001, t-test) (C). (D) Relative luminescence units (RLU) in a luciferase assay of K562 cells transfected with empty vector (control, black) or a plasmid expressing the CD44 regulatory element alone [P=0.039, analysis of variance (ANOVA); Tukey test], or the same CD44-regulatory element-transfected K562 cells transduced with a SCL/TAL1-overexpressing lentivirus (P=0.02, ANOVA; Tukey test) or transduced with two different shSCL/TAL1-expressing lentiviruses (P=0.049 and P=0.072, ANOVA; Tukey test, n=4). (E) Binding of SCL/TAL1 to the CD44 regulatory element in K562 cells treated with vehicle or imatinib as measured by a chromatin immunoprecipitation assay using an anti-SCL/TAL1 (white) or a control IgG (black) antibody and four different CD44 primer pairs (P1-P4). (F) Kaplan-Meier-style survival curve for Balb/c recipients of BCR-ABL1-transduced bone marrow (BM) (solid line) or BCR-ABL1-transduced BM cotransduced with empty vector (dotted line) or cotransduced with an Scl/Tal1-overexpressing lentivirus (dashed line) (P=0.013, log-rank test, n=8). (G) Median fluorescence intensity (MFI) of CD44 on BCR-ABL1+ CD11b+ myeloid cells from recipients of BCR-ABL1-transduced BM (black) or BCR-ABL1-transduced BM cotransduced with empty vector (black) or cotransduced with a Scl/Tal1-overexpressing vector (white) as in (F) (P=0.031, t-test, n=6).
Binding of CD44 to E-selectin influences the cell cycle of BCR-ABL1+ cells
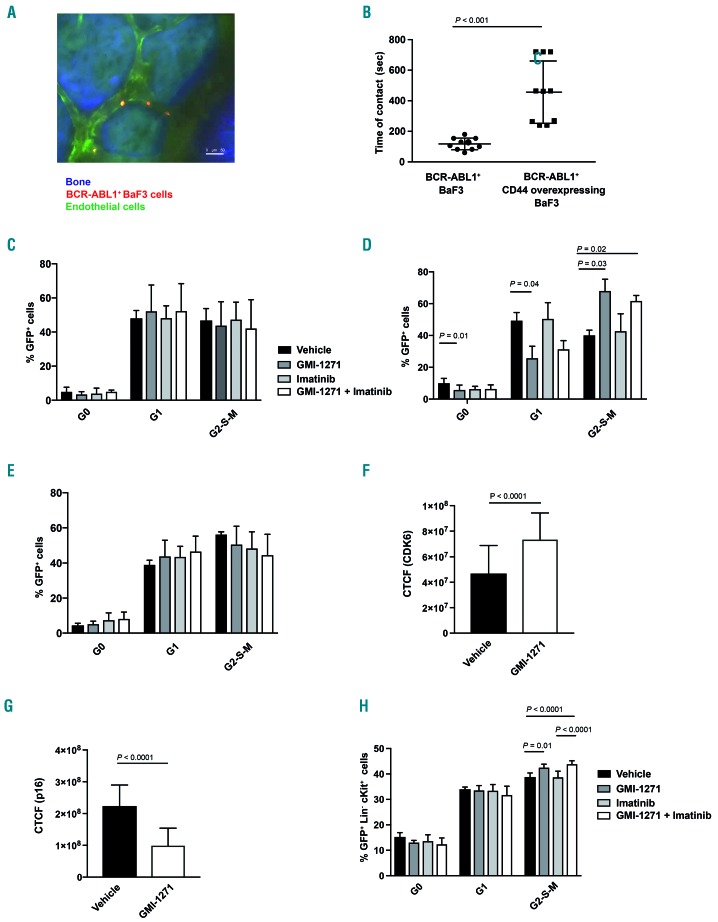
In order to test the nature of the interaction between CD44 and the BM endothelium directly, we performed in vivo imaging of the murine calvarium of live anesthetized mice as shown in Figure 1A, B and Online Supplementary Figure S1A-D. Indeed, in vivo imaging of CD44-overexpressing BCR-ABL1+ or BCR-ABL1+ BaF3 cells injected into Tie2-GFP mice revealed an increased contact time with the endothelium by CD44-overexpressing BCR-ABL1+ compared to BCR-ABL1+ BaF3 cells (P<0.001) (Figure 4A, B). In recapitulation of our findings with human CML cells (Figure 1B) treatment of mice with GMI-1271 decreased the contact time of BCR-ABL1+ BaF3 cells with BM endothelium (P=0.05) (Online Supplementary Figure S7C). Testing a possible influence of CD44, a marker for cancer stem cells,27 and SCL/TAL125 on cell cycle progression via binding to E-selectin, we plated BaF3 cells coexpressing BCR-ABL1 and CD44 on E-selectin in the presence of the four drugs or their combination. This demonstrated that GMI-1271 (with or without imatinib) did not alter the cell cycle of the non-adherent fraction, either when the cells were plated on E-selectin (Figure 4C and Online Supplementary Figure S8A), or on bovine serum albumin (Online Supplementary Figure S8B). In contrast, cell cycle analysis of the non-adherent fraction of BCR-ABL1+ BaF3 cells plated on E-selectin showed an increase of cells in the G2-S-M phase and a decrease in the G0 phase of the cell cycle in the presence of GMI-1271 (P=0.03 and P=0.01, respectively) (Figure 4D and, Online Supplementary Figure S8A) or GMI-1271 plus imatinib (P=0.02) (Figure 4D). No such differences were observed in the adherent fraction (Figure 4E and Online Supplementary Figure S8A) or when the BCR-ABL1+ BaF3 cells were plated on bovine serum albumin (Online Supplementary Figure S8C). BCR-ABL1+ BaF3 cells plated on E-selectin in the presence of GMI-1271 also exhibited increased expression of the cell cycle promoter cyclin dependent kinase (CDK) 6 (P<0.0001) (Figure 4F), a possible trend towards increased nuclear CDK4 (Online Supplementary Figure S8D, E), which has been associated with cell cycle progression, and decreased expression of the cell cycle inhibitor p16 (or cyclin dependent kinase inhibitor 2A) (P<0.0001) (Figure 4G). Consistently, GFP+ (BCR-ABL1+) Lin− c-Kit+ cells from mice with CML treated with GMI-1271 or GMI-1271 and imatinib had increased proportions of cells in the G2-S-M phase of the cell cycle compared to those treated with vehicle (P=0.01) or imatinib alone (P<0.0001) (Figure 4H). GFP+ (BCR-ABL1+) Lin− cells also expressed higher levels of SCL/TAL1 (P=0.04) (Online Supplementary Figure S8F) and lower levels of p16 (P=0.002) (Online Supplementary Figure S8G) when derived from CML mice treated with GMI-1271 compared to vehicle-treated animals. CD44 was significantly more highly expressed on most GFP+ (BCR-ABL1+) progenitor fractions or CD11b+ cells compared to GFP− (BCR-ABL1−) cells (Online Supplementary Figure S8H), in line with our previous results.8 Taken together, these data suggest that CD44 is involved in mediating contact with BM endothelium. Treatment with GMI-1271 leads to non-adhesion of BCR-ABL1+ cells to E-selectin, an increase in cell cycle and a concomitant increase of Scl/Tal1 in BCR-ABL1+ cells, which leads to downregulation of CD44. These data suggest a possible role for CD44 in cell cycle regulation of BCR-ABL1+ cells.
Figure 4.
Binding of CD44 to E-selectin influences the cell cycle of BCR-ABL1+ cells. (A) Representative two-photon in vivo microscopy image of the calvarial bone marrow of an unirradiated Tie2-GFP mouse injected with 1×106 sorted BCR-ABL1+ BaF3 cells, labeled with CMTMR (orange), 2 h prior to in vivo microscopy. Bones were visualized in blue due to second harmonic generation. The scale bar represents 50 mm. (B) Time of contact (seconds), determined by in vivo microscopy as in (A) between the calvarial endothelium and BCR-ABL1+ or BCR-ABL1+ CD44 overexpressing BaF3 cells labeled with CMTMR and injected into unirradiated Tie2-GFP mice (P<0.001, t-test, n=3). (C) Cell cycle analysis by Ki67-staining of serum-starved BaF3 cells coexpressing BCR-ABL1 and CD44 plated on E-selectin-coated plates and treated with vehicle, GMI-1271, imatinib or the combination of GMI-1271 plus imatinib. The percentages of GFP+ (BCR-ABL1+) cells of total in the G0, G1 or G2-S-M phases of the cell cycle are shown. The differences are not statistically significant. (D, E) Cell cycle analysis by Ki67-staining of serum-starved non-adherent (D) or adherent (E) BCR-ABL1+ BaF3 cells plated on E-selectin-coated plates and treated with vehicle, GMI-1271, imatinib or the combination of GMI-1271 plus imatinib. The percentages of GFP+ (BCR-ABL1+) cells of total in the different phases of the cell cycle are shown. Seventy thousand cells were plated and allowed to adhere for 6 h (n=3). (F, G) Corrected total cell fluorescence (CTCF) for CDK6 (F) and p16 (G) in BCR-ABL1+ BaF3 cells plated on E-selectin in the adhesion assay, performed in the presence of vehicle or GMI-1271. Seventy thousand cells were plated (n=3). (H) Cell cycle analysis by Ki67-staining of GFP+ (BCR-ABL1+) Lin− c-Kit+ cells from Balb/c mice with established chronic myeloid leukemia treated with vehicle, GMI-1271, imatinib or the combination of GMI-1271 plus imatinib on day 14 after transplantation. The percentages of GFP+ (BCR-ABL1+) Lin− c-Kit+ cells of total in the G0, G1 or G2-S-M phases of the cell cycle are shown (P values as indicated, analysis of variance; Tukey test, n=5).
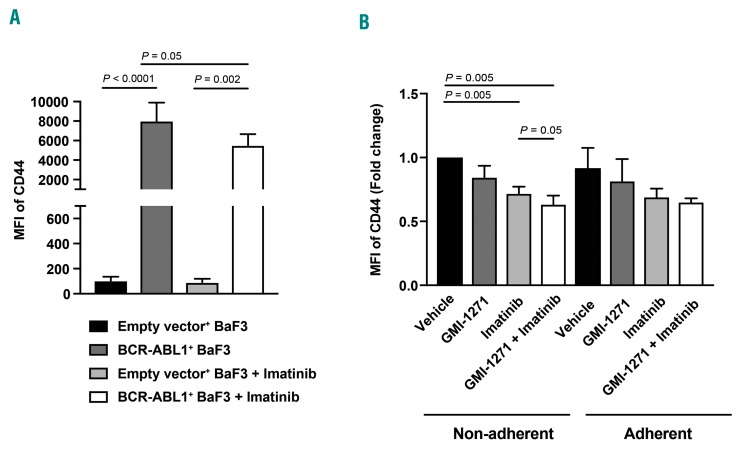
Expression of CD44 is synergistically influenced by SCL/TAL1 and BCR-ABL1
In order to test whether the expression of CD44 and/or SCL/TAL1 may be BCR-ABL1-dependent, we treated BaF3 cells transduced with empty vector or BCR-ABL1 with imatinib.23,24 This led to a significant decrease of the median fluorescence intensity of CD44, specifically on BCR-ABL1+ compared to empty vector-transduced BaF3 cells (P=0.05) (Figure 5A and Online Supplementary Figure S9A, B). Furthermore, in the non-adherent, but not the adherent fraction, of BCR-ABL1+ BaF3 cells plated on E-selectin the median fluorescence intensity of CD44 was synergistically reduced by combined treatment with GMI-1271 and imatinib compared to imatinib alone (P=0.05) (Figure 5B). Consistently, in non-adherent, but not adherent, BCR-ABL1+ BaF3 cells plated on E-selectin, expression of SCL/TAL1 and pSCL/TAL1 was significantly higher upon treatment with GMI-1271 or GMI-1271 and imatinib (Online Supplementary Figure S9C). These results suggest that the expression of CD44 is BCR-ABL1-dependent and that reduction of CD44 expression by GMI-1271 (via an increase in SCL/TAL1) is synergistically enhanced by cotreatment with imatinib.
Figure 5.
Expression of CD44 is influenced by BCR-ABL1. (A) Median fluorescence intensity (MFI) of CD44 on BaF3 cells transduced with empty vector or BCR-ABL1 plated on E-selectin after treatment with vehicle or 10 mM imatinib [P values as indicated, analysis of variance (ANOVA); Tukey test, n=3]. (B) MFI of CD44 (fold change) on the non-adherent and adherent fractions of BCR-ABL1+ BaF3 cells after treatment with vehicle, GMI-1271, imatinib or GMI-1271 and imatinib, normalized to vehicle (P=0.005 for vehicle vs. GMI-1271 plus imatinib, ANOVA; Tukey test, n=4).
CD44 expression influences adhesion and cell cycle status in imatinib-resistant chronic myeloid leukemia
Imatinib-resistance due to the point mutation BCR-ABL1T315I in CML is associated with worse clinical outcome28,29 and is due to altered interactions between BCR-ABL1T315I+ cells and the BM microenvironment.30 In support of this, the expression of CD44 was higher in BCR-ABL1T315I+ than in BCR-ABL1+ BaF3 cells (Online Supplementary Figure S10A, B) and also higher than in cells resistant to imatinib due to other point mutations (P=0.034) (Online Supplementary Figure S10B). Consistently, adhesion of BCR-ABL1T315I+ BaF3 cells to E-selectin was increased compared to that of BCR-ABL1+ cells (P=0.011) (Online Supplementary Figure S10C) and a larger percentage of adherent, but not non-adherent, BCR-ABL1T315I+ BaF3 cells were found in the G0 phase of the cell cycle (P=0.042) (Online Supplementary Figure S10D). These data support our findings on the role of CD44 in influencing the cell cycle in BCR-ABL1+ cells and suggest that increased CD44 expression and increased binding to E-selectin by BCR-ABL1T315I+ cells may contribute to dormancy and imatinib resistance.
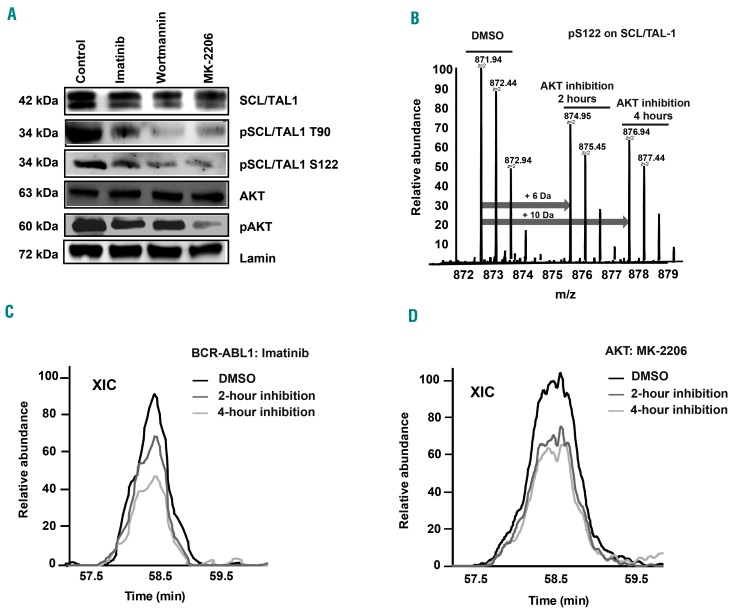
BCR-ABL1 modulates SCL/TAL1-binding to the CD44 regulatory element via pAKT
As SCL/TAL1 activity is regulated by phosphorylation at S122 and T90, we hypothesized that a kinase downstream of BCR-ABL1, such as AKT, may be phosphorylating SCL/TAL1.31 In confirmation of this, we demonstrated that treatment of K562 cells with imatinib, the phos-phoinositide-3-kinase (PI3K) inhibitor wortmannin, and in particular the AKT inhibitor MK-2206 reduced the expression of pTal1S122 and pTal1T90 (Figure 6A). Furthermore, quantitative mass spectrometry, performed using triple stable isotope labeling with amino acids in cell culture (SILAC)-labeling of K562 cells treated with vehicle, imatinib or MK-2206, demonstrated the mass increments resulting from medium (+6 Da) or heavy (+10 Da) SILAC-labeling on arginine on the pS122-bearing peptide “oxMVQLpSPPALAAPAAPGR” of SCL/TAL1 in AKT-inhibited cells (Figure 6B and Online Supplementary Figure S11A). More precise quantitative measurements by comparing the areas under the extracted ion chromatograms of individual SILAC triplets revealed the decrease of pTal1 S122 both 2 and 4 h after inhibition with imatinib (Figure 6C) or MK-2206 (Figure 6D). Taken together, these data suggest that SCL/TAL1 regulates the expression of CD44 via binding to the CD44 regulatory element in a BCR-ABL1- and AKT-dependent manner. BCR-ABL1 is known to activate AKT (Figure 6A),32,33 which phosphorylates SCL/TAL1 at S122 and T90, which in turn regulates the activity of the CD44 regulatory element by acting as a transcriptional repressor. Binding of SCL/TAL1 leads to decreased expression of CD44, decreased adhesion to the vascular niche in CML and an increase in cell cycling.
Figure 6.
BCR-ABL1 modulates SCL/TAL1-binding to the CD44 regulatory element via pAKT. (A) Immunoblot of lysates of K562 cells treated with vehicle, imatinib (10 mM), the PI3-kinase inhibitor wortmannin (20 mM) or the AKT-inhibitor MK-2206 (20 mM) and probed with antibodies to SCL/TAL1, pSCL/TAL1 T90, pSCL/TAL1S122, AKT, pAKT and lamin. Molecular weights are as indicated. The immunoblot is representative of three independent experiments. (B-D) Quantitative mass spectrometry (MS) of K562 cells treated with vehicle (dimethylsulfoxide, DMSO), imatinib or the AKT inhibitor MK-2206 for 0, 2 or 4 h using triple stable isotope labeling with amino acids in cell culture (SILAC) and light-, medium- or heavy-labeled cells. After treatment, differently labeled cells were mixed and followed by MS analysis. (B) An averaged MS1 spectrum showing the SILAC triplets of the pS122-bearing peptide “oxMVQLpSPPALAAPAAPGR” on SCL/TAL1 in AKT-inhibited cells. The arrows indicate the mass increments resulting from the medium (+6 Da) or heavy (+10 Da) SILAC-labeling on arginine. The relative intensities of the SILAC triplets revealed the changes of phosphorylation level on the peptide during the course of AKT inhibition. (C, D) Areas under the extracted ion chromatogram (XIC) after MS by triple SILAC labeling of the cells in (B) showing the relative abundance of pSCL/TAL1 S122 after inhibition with imatinib (10 mM) (C) and the AKT inhibitor MK-2206 (20 mM) (D).
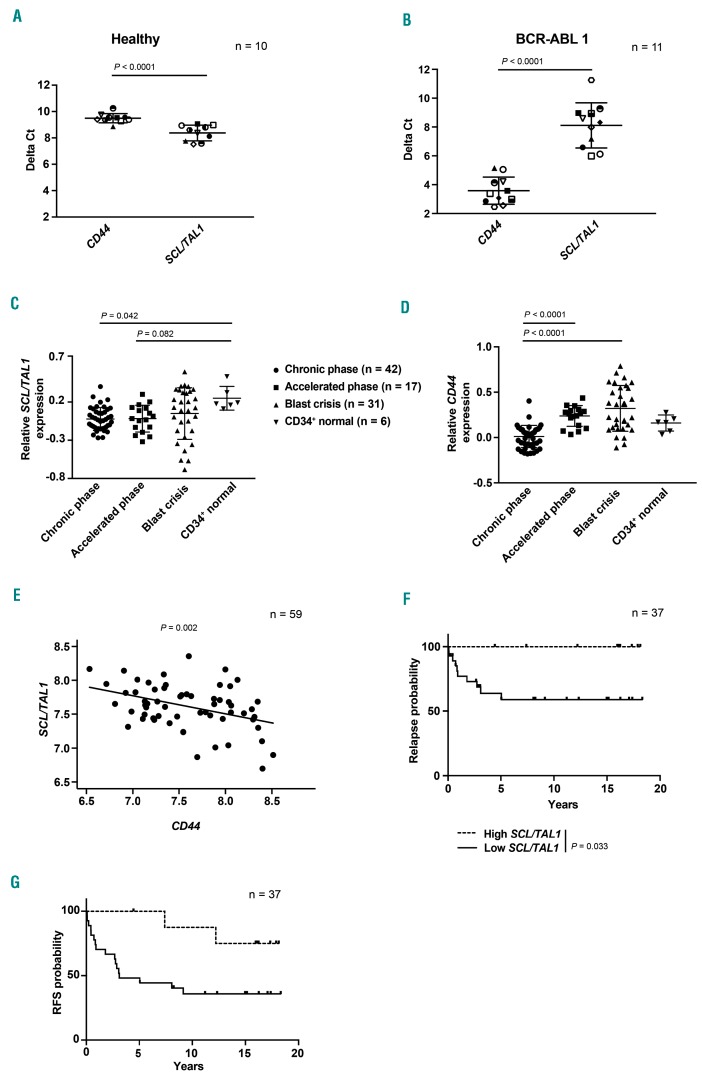
SCL/TAL1 influences outcome in human chronic myeloid leukemia
Given our observations in murine models and murine or human CML cells, we correlated levels of CD44 and SCL/TAL1 in leukocytes from healthy individuals and patients with CML. This revealed lower delta cycle threshold values for SCL/TAL1 and higher delta cycle threshold values for CD44 in healthy individuals (P<0.0001) (Figure 7A), while this relationship was reversed in human CML samples (P<0.0001) (Figure 7B), suggesting that SCL/TAL1 also acts as a transcriptional regulator in human CML cells. In order to test the significance of SCL/TAL1 and/or CD44 for phase of CML and disease course, we examined published datasets of SCL/TAL1 and CD44 gene expression in human CML samples34,35 and identified that SCL/TAL1 levels in patients in chronic phase (P=0.042) (Figure 7C) and – as a trend-accelerated phase were lower than in normal human CD34+ cells, as expected. CD44 expression increased with progression of CML from chronic to advanced phase CML with the highest CD44 expression found in samples from patients in blast crisis (P<0.0001) (Figure 7D).
Figure 7.
SCL/TAL1 influences outcome in human chronic myeloid leukemia. (A, B) Delta Ct values for CD44 and SCL/TAL1 in peripheral blood leukocytes of healthy individuals (n=10) (A) or patients with chronic myeloid leukemia (CML) (n=11) (B). (C, D) Relative SCL/TAL1 (C) and CD44 (D) expression in unsorted bone marrow cells of patients in chronic phase (CP) (circles), accelerated phase (AP) (squares) or blast crisis (BC) (triangles) of CML vs. normal human CD34+ cells (upside down triangles). Gene expression for each individual sample is normalized to mean expression in CP samples and is shown on a log10 scale. The expression of SCL/TAL1 in CP CML is significantly lower than that in human CD34+ cells from healthy individuals [P=0.042, analysis of variance (ANOVA); Tukey test]. (C) and the expression of CD44 is significantly higher in BC than in CP CML (P<0.0001, ANOVA; Tukey test. CP, n=42; AP, n=17; BC, n=31; normal CD34+ cells, n=6) (D). (E) Negative correlation between SCL/TAL1 and CD44 expression in CD34+ sorted samples from CP CML patients (n=59) from the study by McWeeney et al.34 (P=0.002, correlation coefficient −0.40). Normalized log2-transformed expression is shown. (F, G) Kaplan-Meier-style curve of the probability of relapse (P=0.033, log-rank test) (F) or relapse-free survival (P=0.024, log-rank test) (G) of CP CML patients grouped by low or high expression of SCL/TAL1 (n=37).
As we did not establish a clear inverse relationship between SCL/TAL1 and CD44 expression in samples from individual patients from this dataset, we examined expression of SCL/TAL1 and CD44 in CD34+-sorted samples from chronic phase CML patients (n=59). We observed a statistically significant negative correlation (P=0.002, correlation coefficient −0.40) between SCL/TAL1 and CD44 expression in these samples (Figure 7E). Lastly, we examined the association between SCL/TAL1 expression and outcomes after allogeneic transplantation in chronic phase CML patients (n=37),35 although this procedure is no longer the standard of care in CML. When dichotomizing SCL/TAL1 into high and low expression groups at the third quartile, we found that low SCL/TAL1 expression was statistically significantly associated with increased risk of relapse (P=0.033) (Figure 7F) and inferior relapse-free survival (P=0.024) (Figure 7G). In summary, these data suggest that expression of SCL/TAL1 (and, therefore, converse expression of CD44) may correlate with disease stage and survival in human CML patients, although larger cohorts would be needed to prove this definitively.
Discussion
In this study we show that inhibition of binding of BCR-ABL1+ cells to E-selectin in the vascular niche increases cell cycle progression and response to imatinib therapy. Furthermore, our data also imply that SCL/TAL1 is a regulator of the expression of CD44, whereby SCL/TAL1 is activated by AKT downstream of BCR-ABL1 (Online Supplementary Figure S12). In turn, CD44 influences the cell cycle of BCR-ABL1+ cells via its binding to E-selectin. The data connect the previously unknown mechanism of increased expression of CD44, a ligand for E-selectin and cancer stem cell marker, on BCR-ABL1+ cells with transcriptional regulation by SCL/TAL1, E-selectin in the vascular niche, engraftment in the BM microenvironment, cell cycle progression and response to therapy.
Our data on CML LSC contrast with findings in the normal HSC niche, in which a prominent role for E-selectin in promoting HSC proliferation has been described.5 This could be explained by our previous work demonstrating that interactions with the BM microenvironment differ drastically between normal HSC8 and LSC and even between oncogenes.15 However, in their study, Winkler et al. used GMI-1070, a pan-selectin antagonist and precursor to GMI-1271.5 In addition, a role for SCL/TAL1 in impe ding the transition of HSC from G0 to G1 has been demonstrated,36 which may support the pro-proliferative effects of E-selectin on normal HSC.5 Additionally, similar to our findings, CD44 inhibited cell cycle progression of vascular smooth muscle cells in response to binding of high molecular weight hyaluro-nan,37 another CD44 ligand in the extracellular matrix, and modulated the ERK and AKT pathways upon cell adhesion via CD44.38 In the present study, it cannot be excluded that the SCL/TAL1-mediated reduction of CD44 expression may also have led to decreased binding to hyaluronan and osteopontin, another extracellular matrix protein known to bind CD44.39 However, CML induction did not differ significantly between wildtype and osteopontin-knockout mice (unpublished data, DSK), suggesting that, unlike E-selectin, osteopontin is not essential for the engraftment of LIC in CML.
In agreement with our work it was demonstrated that inhibition of E- and P-selectin led to reduced rolling of neutrophils on endothelium, lowering the risk of neutrophil-mediated endothelial injury after xenotransplantation.40 Furthermore, in CML, we previously showed that deficiency of E-selectin in BM endothelium or deficiency of L-selectin, P-selectin glycoprotein ligand (PSGL)-1, enzymes involved in the synthesis of selectin ligands6 or CD448 on LIC were required for efficient engraftment of LIC, whereas P-selectin in the BM was not required.6 Therefore, emphasis in this work was laid on E-selectin and its ligands. Treatment with GMI-1271 also reverted the insensitivity of multiple myeloma cells overexpressing E-selectin ligands to bortezomib.12
Normal hematopoietic cells predominantly express the standard isoform of CD44 (CD44s).41 However, variant isoforms of CD44 (CD44v) are generated in cancers, including solid tumors42 and acute myeloid leukemia,43 but both forms, CD44s and CD44v, are cancer stem cell markers and both influence cancer cell stemness.42,44 In CML we found that the CD44s isoform has a role in homing and engraftment of LIC,8 while CD44v3 enhanced the replating capacity of CML progenitors.45
In summary, regulation of CD44 expression via SCL/TAL1, the AKT pathway and an oncogene, as well as the mechanism of cell cycle regulation of LSC upon non-adhesion to the niche, suggest a concept of how dislocation from the niche may alter LSC proliferation and response to therapy. This has, similarly, been hypothesized in the case of the concomitant use of granulocyte colony-stimulating factor46,47 or C-X-C motif chemokine receptor (CXCR) 4 inhibitors plus tyrosine kinase inhibitors or chemotherapy in leukemia,48 suggesting that these therapeutic strategies may be further exploitable in the future.
Acknowledgments
The authors thank M. Zörnig for helpful discussions and Glycomimetics Inc., in particular J. Magnani and W. Fogler, for provision of drugs and initial funding of this work. The authors also thank Stefanie Dimmeler for use of the in vivo microscope. This work was supported by the LOEWE Center for Cell and Gene Therapy Frankfurt (CGT) and institutional funds of the Georg-Speyer-Haus to DSK. The Georg-Speyer-Haus is funded jointly by the German Federal Ministry of Health (BMG) and the Ministry of Higher Education, Research and the Arts of the State of Hessen (HMWK). The LOEWE Center for Cell and Gene Therapy Frankfurt is funded by HMWK, reference number: III L 4-518/17.004 (2010). The project was also partly supported by Deutsche Krebshilfe (SyTASC / 70111969).
Footnotes
Check the online version for the most updated information on this article, online supplements, and information on authorship & disclosures: www.haematologica.org/content/105/1/136
References
- 1.Ishikawa F, Yoshida S, Saito Y, et al. Chemotherapy-resistant human AML stem cells home to and engraft within the bone-marrow endosteal region. Nat Biotechnol. 2007;25(11):1315–1321. [DOI] [PubMed] [Google Scholar]
- 2.Pitt LA, Tikhonova AN, Hu H, et al. CXCL12-producing vascular endothelial niches control acute T cell leukemia Maintenance. Cancer Cell. 2015;27(6):755–768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zhang B, Li M, McDonald T, et al. Microenvironmental protection of CML stem and progenitor cells from tyrosine kinase inhibitors through N-cadherin and Wnt-beta-catenin signaling. Blood. 2013;121(10):1824–1838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yamamoto-Sugitani M, Kuroda J, Ashihara E, et al. Galectin-3 (Gal-3) induced by leukemia microenvironment promotes drug resistance and bone marrow lodgement in chronic myelogenous leukemia. Proc Natl Acad Sci U S A. 2011;108(42): 17468–17473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Winkler IG, Barbier V, Nowlan B, et al. Vascular niche E-selectin regulates hematopoietic stem cell dormancy, self renewal and chemoresistance. Nat Med. 2012;18(11):1651–1657. [DOI] [PubMed] [Google Scholar]
- 6.Krause DS, Lazarides K, Lewis JB, von Andrian UH, Van Etten RA. Selectins and their ligands are required for homing and engraftment of BCR-ABL1+ leukemic stem cells in the bone marrow niche. Blood. 2014;123(9):1361–1371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dimitroff CJ, Lee JY, Rafii S, Fuhlbrigge RC, Sackstein R. CD44 is a major E-selectin ligand on human hematopoietic progenitor cells. J Cell Biol. 2001;153:1277–1286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Krause DS, Lazarides K, von Andrian UH, Van Etten RA. Requirement for CD44 in homing and engraftment of BCR-ABL-expressing leukemic stem cells. Nat Med. 2006;12(10):1175–1180. [DOI] [PubMed] [Google Scholar]
- 9.Jin L, Hope KJ, Zhai Q, Smadja-Joffe F, Dick JE. Targeting of CD44 eradicates human acute myeloid leukemic stem cells. Nat Med. 2006;12(10):1167–1174. [DOI] [PubMed] [Google Scholar]
- 10.Thomas SN, Zhu F, Schnaar RL, Alves CS, Konstantopoulos K. Carcinoembryonic antigen and CD44 variant isoforms cooperate to mediate colon carcinoma cell adhesion to E-and L-selectin in shear flow. J Biol Chem. 2008;283(23):15647–15655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Shirure VS, Liu T, Delgadillo LF, et al. CD44 variant isoforms expressed by breast cancer cells are functional E-selectin ligands under flow conditions. Am J Physiol Cell Physiol. 2015;308(1):C68–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Natoni A, Smith TAG, Keane N, et al. E-selectin ligands recognised by HECA452 induce drug resistance in myeloma, which is overcome by the E-selectin antagonist, GMI-1271. Leukemia. 2017;31(12):2642–2651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Saito Y, Uchida N, Tanaka S, et al. Induction of cell cycle entry eliminates human leukemia stem cells in a mouse model of AML. Nat Biotechnol. 2010;28(3):275–280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Drummond MW, Heaney N, Kaeda J, et al. A pilot study of continuous imatinib vs pulsed imatinib with or without G-CSF in CML patients who have achieved a complete cytogenetic response. Leukemia. 2009;23(6):1199–1201. [DOI] [PubMed] [Google Scholar]
- 15.Krause DS, Fulzele K, Catic A, et al. Differential regulation of myeloid leukemias by the bone marrow microenvironment. Nat Med. 2013;19(11):1513–1517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Graham SM, Jorgensen HG, Allan E, et al. Primitive, quiescent, Philadelphia-positive stem cells from patients with chronic myeloid leukemia are insensitive to STI571 in vitro. Blood. 2002;99(1):319–325. [DOI] [PubMed] [Google Scholar]
- 17.Bhatia R, Holtz M, Niu N, et al. Persistence of malignant hematopoietic progenitors in chronic myelogenous leukemia patients in complete cytogenetic remission following imatinib mesylate treatment. Blood. 2003;101(12):4701–4707. [DOI] [PubMed] [Google Scholar]
- 18.Chen B, Jiang L, Zhong ML, et al. Identification of fusion genes and characterization of transcriptome features in T-cell acute lymphoblastic leukemia. Proc Natl Acad Sci U S A. 2018;115(2):373–378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lavender KJ, Pang WW, Messer RJ, et al. BLT-humanized C57BL/6 Rag2-/-γc-/-CD47-/- mice are resistant to GVHD and develop B- and T-cell immunity to HIV infection. Blood. 2013;122(25):4013–4020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Li S, Ilaria RL, Million RP, Daley GQ, Van Etten RA. The P190, P210, and P230 forms of the BCR/ABL oncogene induce a similar chronic myeloid leukemia-like syndrome in mice but have different lymphoid leuke-mogenic activity. J Exp Med. 1999;189(9): 1399–1412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hu Y, Swerdlow S, Duffy TM, Weinmann R, Lee FY, Li S. Targeting multiple kinase pathways in leukemic progenitors and stem cells is essential for improved treatment of Ph+ leukemia in mice. Proc Natl Acad Sci U S A. 2006;103(45):16870–16875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chien S, Zhao X, Brown M, et al. A novel small molecule e-selectin inhibitor GMI-1271 blocks adhesion of AML blasts to e-selectin and mobilizes blood cells in nodscid IL2Rgc−/− mice engrafted with human AML. Blood. 2012;120(21):4092. [Google Scholar]
- 23.Copland M, Hamilton A, Elrick LJ, et al. Dasatinib (BMS-354825) targets an earlier progenitor population than imatinib in primary CML but does not eliminate the quiescent fraction. Blood. 2006;107(11):4532–4539. [DOI] [PubMed] [Google Scholar]
- 24.Chu S, Xu H, Shah NP, et al. Detection of BCR-ABL kinase mutations in CD34+ cells from chronic myelogenous leukemia patients in complete cytogenetic remission on imatinib mesylate treatment. Blood. 2005;105(5):2093–2098. [DOI] [PubMed] [Google Scholar]
- 25.Dey S, Curtis DJ, Jane SM, Brandt SJ. The TAL1/SCL transcription factor regulates cell cycle progression and proliferation in differentiating murine bone marrow monocyte precursors. Mol Cell Biol. 2010;30(9): 2181–2192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kolodziej S, Kuvardina ON, Oellerich T, et al. PADI4 acts as a coactivator of Tal1 by counteracting repressive histone arginine methylation. Nat Comm. 2014;5:3995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Senbanjo LT, Chellaiah MA. CD44: a multi functional cell surface adhesion receptor is a regulator of progression and metastasis of cancer cells. Front Cell Dev Biol. 2017;5:18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Branford S, Melo JV, Hughes TP. Selecting optimal second-line tyrosine kinase inhibitor therapy for chronic myeloid leukemia patients after imatinib failure: does the BCR-ABL mutation status really matter? Blood. 2009;114(27):5426–5435. [DOI] [PubMed] [Google Scholar]
- 29.Nicolini FE, Ibrahim AR, Soverini S, et al. The BCR-ABLT315I mutation compromises survival in chronic phase chronic myelogenous leukemia patients resistant to tyrosine kinase inhibitors, in a matched pair analysis. Haematologica. 2013;98(10): 1510–1516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kumar R, Merten M, Minciacchi V, et al. Specific and targetable interactions with the bone marrow microenvironment govern outcome in imatinib-resistant chronic myeloid leukemia. Blood. 2018;132(Suppl 1):936. [Google Scholar]
- 31.Palamarchuk A, Efanov A, Maximov V, Aqeilan RI, Croce CM, Pekarsky Y. Akt phosphorylates Tal1 oncoprotein and inhibits its repressor activity. Cancer Res. 2005;65(11):4515–4519. [DOI] [PubMed] [Google Scholar]
- 32.Skorski T, Kanakaraj P, Nieborowska-Skorska M, et al. Phosphatidylinositol 3-kinase activity is regulated by BCR/ABL and is required for the growth of Philadelphia chromosome-positive cells. Blood. 1995;86(2):726–736. [PubMed] [Google Scholar]
- 33.Atfi A, Abécassis L, Bourgeade MF. Bcr-Abl activates the AKT/Fox O3 signalling pathway to restrict transforming growth factor-beta-mediated cytostatic signals. EMBO Rep. 2005;6(10):985–991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.McWeeney SK, Pemberton LC, Loriaux MM, et al. A gene expression signature of CD34+ cells to predict major cytogenetic response in chronic-phase chronic myeloid leukemia patients treated with imatinib. Blood. 2010;115(2):315–325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Radich JP, Dai H, Mao M, et al. Gene expression changes associated with progression and response in chronic myeloid leukemia. Proc Natl Acad Sci U S A. 2006;103(8):2794–2799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lacombe J, Herblot S, Rojas-Sutterlin S, et al. Scl regulates the quiescence and the long-term competence of hematopoietic stem cells. Blood. 2010;115(4):792–803. [DOI] [PubMed] [Google Scholar]
- 37.Kothapalli D, Flowers J, Xu T, Puré E, Assoian RK. Differential activation of ERK and Rac mediates the proliferative and anti-proliferative effects of hyaluronan and CD44. J Biol Chem. 2008;283(46):31823–31829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Yu S, Cai X, Wu C, et al. Adhesion glycopro-tein CD44 functions as an upstream regulator of a network connecting ERK, AKT and Hippo-YAP pathways in cancer progression. Oncotarget. 2015;6(5):2951–2965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Rangaswami H, Bulbule A, Kundu GC. Osteopontin: role in cell signaling and cancer progression. Trends Cell Biol. 2006;16: 79–87. [DOI] [PubMed] [Google Scholar]
- 40.Laird CT, Hassanein W, O’Neill NA, et al. P-and E-selectin receptor antagonism prevents human leukocyte adhesion to activated porcine endothelial monolayers and attenuates porcine endothelial damage. Xenotransplantation. 2018;25(2):e12381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zöller M. CD44, hyaluronan, the hematopoietic stem cell, and leukemia-initiating cells. Front Immunol. 2015;6:235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Yan Y, Zuo X, Wei D. Concise review: emerging role of CD44 in cancer stem cells: a promising biomarker and therapeutic target. Stem Cells Transl Med. 2015;4(9):1033–1043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bendall LJ, Bradstock KF, Gottlieb DJ. Expression of CD44 variant exons in acute myeloid leukemia is more common and more complex than that observed in normal blood, bone marrow or CD34+ cells. Leukemia. 2000;14(7):1239–1246. [DOI] [PubMed] [Google Scholar]
- 44.Wang L, Zuo X, Xie K, Wei D. The role of CD44 and cancer stem cells. Methods Mol Biol. 2018;1692:31–42. [DOI] [PubMed] [Google Scholar]
- 45.Holm F, Hellqvist E, Mason CN, et al. Reversion to an embryonic alternative splicing program enhances leukemia stem cell self-renewal. Proc Natl Acad Sci U S A. 2015;112(50):15444–15449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Borthakur G, Kantarjian H, Wang X, et al. Treatment of core-binding-factor in acute myelogenous leukemia with fludarabine, cytarabine, and granulocyte colony-stimulating factor results in improved event-free survival. Cancer. 2008;113(11):3181–3185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Holtz M, Forman SJ, Bhatia R. Growth factor stimulation reduces residual quiescent chronic myelogenous leukemia progenitors remaining after imatinib treatment. Cancer Res. 2007;67:1113–1120. [DOI] [PubMed] [Google Scholar]
- 48.Uy GL, Rettig MP, Motabi IH, et al. A phase 1/2 study of chemosensitization with the CXCR4 antagonist plerixafor in relapsed or refractory acute myeloid leukemia. Blood. 2012;119(17):3917–3924. [DOI] [PMC free article] [PubMed] [Google Scholar]