Abstract
Allogeneic stem cell transplantation remains the only curative treatment for sickle cell anemia (SCA), but the place of myeloablative conditioning in the procedure remains to be defined. The aim of the present study was to analyze long-term outcomes, including chimerism, SCA-related events and biological data (hemoglobin, reticulocytes, HbS%), and fertility in a French series of 234 SCA patients under 30 years of age who, from 1988 to 2012, received a matched-sibling-donor stem cell transplantation following standardized myeloablative conditioning [busulfan, cyclophosphamide and rabbit antithymocyte globulin (ATG)]. Since the first report of the series (1988-2004), 151 new consecutive patients with SCA have been similarly transplanted. Considering death, non-engraftment or rejection (donor cells <5%) as events, the 5-year event-free survival was 97.9% (95% confidence interval: 95.5-100%), confirming, since the year 2000, an at least 95% chance of cure. In the overall cohort (n=234, median follow up 7.9 years), event-free survival was not associated with age, but chronic-graft-versus-host disease (cGvHD) was independently associated with recipient’s age >15 years (hazard ratio=4.37; P=0.002) and lower (5-15 vs. 20 mg/kg) ATG dose (hazard ratio=4.55; P=0.001). At one year, 44% of patients had mixed chimerism (5-95% donor cells), but those prepared with ATG had no graft rejection. No events related to SCA occurred in patients with mixed chimerism, even those with 15-20% donor cells, but hemolytic anemia stigmata were observed with donor cells <50%. Myeloablative transplantation with matched-sibling donor currently has a higher event-free survival (98%) in patients under 30 years of age than that reported for non-myeloablative conditioning (88%). Nevertheless, the risk of cGvHD in older patients and the need to preserve fertility might be indications for a non-myeloablative conditioning.
Introduction
Sickle cell anemia (SCA) represents a growing global health problem. Over 300,000 children are born each year with SCA worldwide, with 85% of these births occurring in sub-Saharan Africa.1 SCA is a severe recessive genetic disorder, resulting from a single nucleotide substitution in codon 6 of the beta-globin gene, producing abnormal hemoglobin (HbS) that is prone to polymer formation under deoxygenated conditions. Polymerized HbS leads to decreased red blood cell deformability and sickling within end arterioles, resulting in vaso-occlusive crisis and pain.
Despite significant progress in the management of SCA, such as the prevention of pneumococcal infection,2,3 the introduction of hydroxyurea therapy (HU),4–10 and early detection of cerebral vasculopathy with transcranial Doppler,11,12 followed by rapid establishment of transfusion programs for patients at risk of stroke,13,14 SCA remains a disease with a high risk of morbidity and early death.15–18
Allogeneic hematopoietic stem cell transplantation (SCT) is the only curative therapy for SCD19–26 as it can prevent SCA-related organ damage if the erythroid compartment is adequately replaced by donor erythropoiesis. However, barriers to SCT use include the risks of rejection, transplant-related mortality (TRM), chronic graft-versus-host disease (cGvHD), infertility, and the lack of matched-sibling donors (MSD). While at least 1,000 MSD-SCT have been performed so far worldwide, the number of transplanted SCA patients remains very low,23 especially in developing countries that have a large SCA population. We have previously reported studies in 87 consecutive myeloablative MSD-SCT for SCA patients performed in France between 1988 and December 2004.22 We showed that the addition of antithymocyte globulin (ATG) to the conditioning regimen (CR) allowed a significant reduction in the risk of rejection despite a higher prevalence of mixed chimerism. The myeloablative CR (MAC), consisting of busulfan, cyclophosphamide and rabbit ATG was well tolerated by these young patients (aged 2.2-22 years), as only one death occurred during aplasia and limited early toxicity was noted. Moreover, the outcome significantly improved with time, as event-free survival (EFS) was 95.3% at five years for the 44 patients transplanted between January 2000 and December 2004.22 These results led us to use the same MAC in SCA adults under the age of 30 years without major organ dysfunction and children with less severe cerebral vasculopathy.
Since that initial report, extremely interesting results have been obtained in adults using non-myeloablative (NMA) CR, resulting in 87% EFS with 13% rejection risk, but with preserved fertility and no GvHD with mixed chimerism.27–29 It is thus timely and warranted to report the long-term outcome of chimerism, cGvHD and fertility after MAC-MSD-SCT in order to provide evidence for making an informed decision about the use of MAC or NMA CR for SCA children and young adults. For this reason, we analyzed the results of MAC-MSD-SCT performed in France between 1988 and December 2012 in 234 SCA patients, ranging in age from 2.2 to 28.9 years with a minimum 5-year follow up for surviving patients, to evaluate long-term outcomes, especially in the context of incidence of cGvHD and chimerism.
Methods
Considering the better cure rate observed with MSD-SCT after MAC for SCA patients transplanted after 2000 (95.3%), the Société Française de Greffe de Moelle et de Thérapie Cellulaire (SFGM-TC) decided to continue the previously described protocol,22 and 151 new consecutive patients were transplanted between January 2005 and December 2012 for severe SCA. Thus, a total of 234 SCA patients had received MSD-SCT following MAC between November 1988 and December 2012. Informed consent was obtained from recipients, donors and their parents or guardians before transplantation. The data were obtained in the context of consensual treatment guidelines among French transplant centers in accordance with the Declaration of Helsinki, and the French laws and regulations protecting human subjects. To complement the data from the European Group for Blood and Marrow Transplantation (EBMT) registry, clinical and biological data including chimerism, eventual carcinogenic issues and fertility were recorded in a French database, and its use for this project was approved by the Créteil Institutional Review Board.
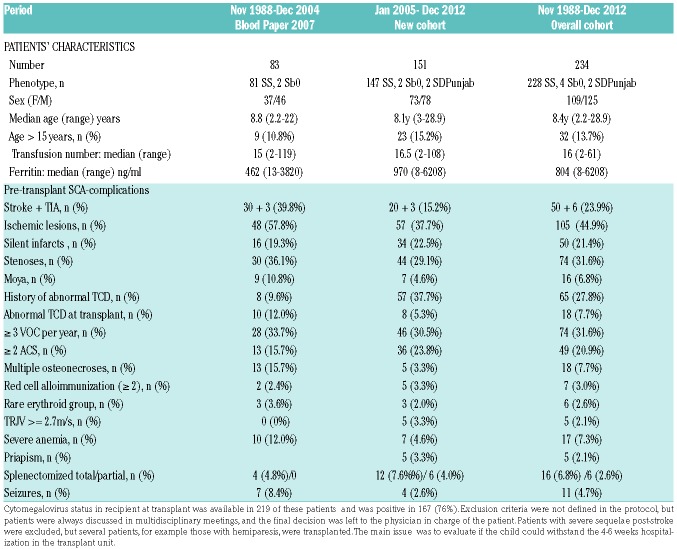
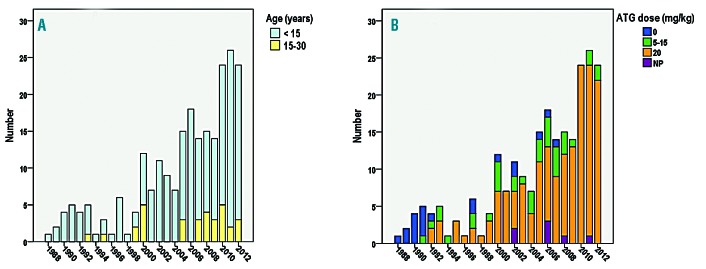
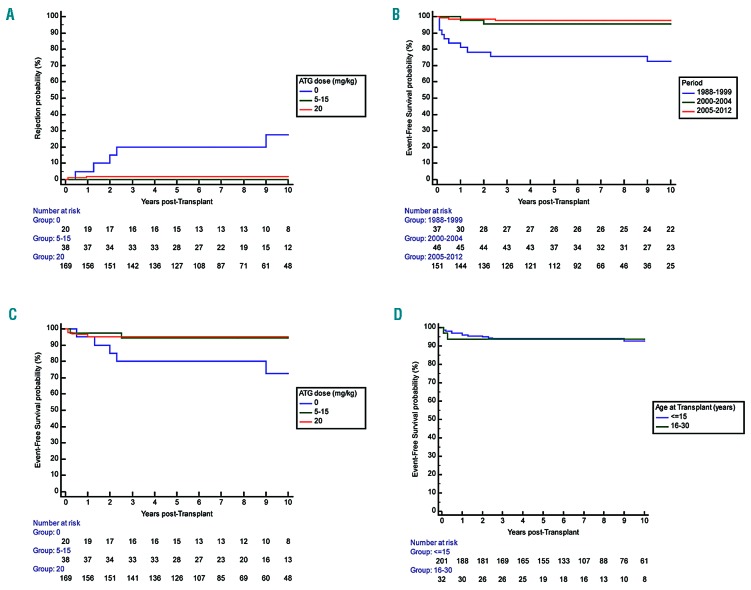
Patients’ characteristics are presented in Table 1. The overall median age at transplant was 8.4 years (range: 2.2-28.9) with 32 patients (13.7%) older than 15 years (Figure 1A). All donors were MSD and genotype, available in 208 of 234 patients, was heterozygous AS (n=127), AThal (n=2), AC (n=1), A/DPunjab (n=1), and homozygous AA (n=77). Median age of donors was 9.6 years (range 0-33.7), counting the donor’s age for isolated CBT as 0. The stem cell sources are shown in Table 2. All 234 consecutive patients were transplanted with an MSD following myeloablative CR using busulfan, cyclophosphamide at 200 mg/kg and rabbit ATG at different doses (Table 2 and Figure 1B).
Table 1.
Characteristics of sickle cell anemia patients transplanted in France with matched-sibling donors and myeloablative conditioning regimen.
Figure 1.
Matched-sibling donor hematopoietic stem cell transplantation (MSD-SCT) performed in France (1988-2012) after myeloablative conditioning regimen (n=234). Only 2% of patients were not prepared with anti-thymocyte globulin (ATG) in the second cohort versus 20.5% in the first cohort. The transplantation procedure and prophylaxis for graft-versus-host disease (GvHD) were as previously reported,22 except that busulfan has been administered intravenously since year 2001 (Busilvex® Pierre Fabre Médicaments, Boulogne-Billancourt, France) from day –10 to day –7 at the total dose of 12.8 mg/kg for patients weighing >34 kg, 15.2 mg/kg for patients weighing 23-34 kg, 17.6 mg/kg for patients weighing 16-23 kg, or 19.2 mg/kg for patients weighing 9-16 kg. Busulfan pharmacokinetics were not performed for the majority of patients (n=202). In addition, cyclosporine was replaced by mycophenolate mofetil after 2002 in case of GvHD requiring steroid therapy. (A) Proportion of patients younger or older than 15 years. (B) ATG doses. NP: not precise; these patients received ATG, but the exact dose was not recorded.
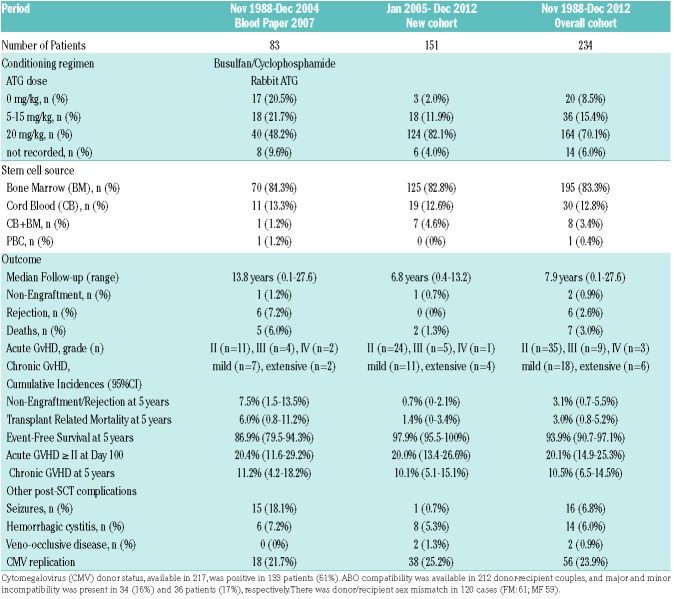
Table 2.
Matched-sibling donor hematopoietic stem cell transplantation (MSD-SCT) procedure and outcome in sickle cell anemia patients transplanted in France.
Chimerism was studied by analyzing various polymorphisms after polymerase chain reaction (PCR) amplification of DNA obtained from whole blood at 1, 3, 6, 9 and 12 months after transplantation and every year thereafter. Real-time (RT) quantitative PCR of insertion/deletion polymorphisms was used when the minority chimeric fraction was below 10% and short-tandem-repeat (STR)-PCR was performed when the minority fraction was over 10%. In both cases, analyses were performed according to the kit manufacturer’s recommendations (Promega; PowerPlex 16S assay for STR-PCR) and GenDex for Indel RT-PCR (KMRDX Chimerism Assay). When possible, peripheral-blood CD3+ T cells and CD3− cells were selected for analysis.
Exact Fisher tests were used to compare proportions and Wilcoxon rank sum tests for continuous distributions. Patients were censored on the date of death or last visit for Kaplan-Meier (KM) estimates of survival and on the date of event (death, non-engraftment or rejection) or last visit for KM estimates of EFS. Cumulative incidences of rejections, deaths, acute GvHD (aGvHD) grade ≥II and cGvHD were also estimated by the KM method because of the small number of deaths and rejections. Failure time data curves were compared by the Log rank test. Risk factors for EFS, acGvHD and cGvHD, were analyzed by Cox regression with estimated hazards ratio (HR) and 95% Confidence Interval (CI). Type 1 error was fixed at the 5% level. All tests were two-tailed. Univariate models were fitted and all variables associated with the outcome at the 10% level were retained for introduction into a multivariate model. Statistical analyses were performed on SPSS version 22, and MedCalc (Belgium).
Results
The median (range) follow up was of 7.9 years (0.1-27.6 years). All survivors had at least five years of follow up and results are presented in Table 2.
Engraftment
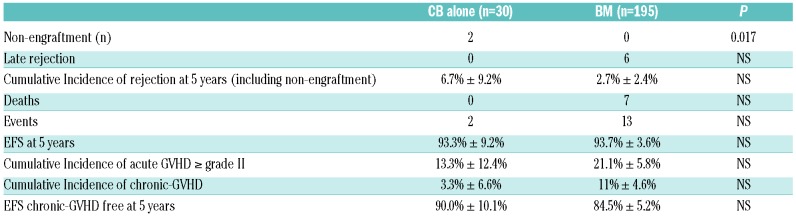
Two non-engraftments were observed, with rapid autologous reconstitution, in two patients transplanted with cord blood (CB) (Table 3). The first one, a patient who was transplanted 20 years ago, has not experienced any further SCA-related crisis since transplant, but still has 21% fetal Hb (HbF) (vs. <2% before SCT) and was still anemic (Hb 7 g/dL) at last visit. The second one, transplanted 12 years ago, is currently on HU therapy because of recurrent crises. For the other 232 patients, the time to absolute neutrophil count >0.5×109/L was significantly shorter after bone marrow transplantation (BMT) compared to CB transplantation (CBT) [mean±Standard Deviation (SD); 20.7±5.7 vs. 32.0±10.0, respectively; P<0.001]. Similarly, platelets reached 50×109/L sooner after BMT (day 26.5±12.2) than after CBT (day 44.6±18.3; P=0.001).
Table 3.
Rejection, transplant-related mortality, event-free survival as a function of the cell source, bone marrow (BM) versus cord blood (CB) alone.
Rejection
Rejection was defined as donor chimerism <5%. Despite initial successful donor engraftment, six rejections were observed 0.5, 0.9, 1.2, 2.0, 2.3 and 9-years post transplant in patients transplanted before year 2005, as previously reported.22 Five of them did not receive ATG as part of the preparative CR, and only one rejection occurred despite CR including ATG. Taking into account the two patients with non-engraftment and the six patients with rejection, the cumulative incidence of rejection for the overall cohort was 3.1% (95%CI: 0.7-5.5%) at five years; this was 20.0% (95%CI: 3.0-37.0%) in patients not prepared with ATG versus only 1.4% (95%CI: 0.0-3.0%) in those who received ATG (P<0.001). However, the ATG dose had no impact on the rejection risk (Figure 2A).
Figure 2.
Rejection probability and event-free-survival (EFS) in sickle cell anemia (SCA) patients transplanted with matched-sibling donor (MSD) after myeloablative conditioning regimen. (A) Probability of rejection according to the anti-thymocyte globulin (ATG) dose. Overall cumulative incidence of rejection at five years was 20.0% (95%CI: 3.0-37.0%) in patients prepared without ATG and only 1.4% (95%CI: 0.0-3.0%) (P<0.001) in those prepared with ATG. However, the risk of rejection was not associated with the ATG dose (5-15 mg/kg vs. 20 mg/kg). (B) EFS according to the ATG dose. EFS was similar after bone marrow transplantation (BMT) and cord blood transplantation (CBT), and in patients prepared with 5-15 mg/kg ATG than in those prepared with 20 mg/kg. (C) EFS in 234 patients depending on the period of transplant. EFS improved strongly as it was only 73.3% (95%CI: 58.7-87.9%) among the 38 patients transplanted before year 2000 and 97.4% (95%CI: 95.0 -99.8%) in the 196 patients transplanted after year 2000. (D) EFS according to age over or under 15 years. EFS was similar in patients younger or older than 15 at transplant.
Transplant-related mortality
Seven deaths occurred after MSD-SCT. Five occurred in patients transplanted before year 2005, as previously reported.22 Two deaths occurred at 0.5 and 2.5 years post transplant in the second cohort, because of adenoviral encephalitis30 and GvHD-related obliterans bronchiolitis, respectively. The cumulative incidence of TRM at five years was 3.0% (95%CI: 0.8-5.2%) and significantly decreased with time from 6.0% (95%CI: 0.8-11.2%) before January 2005 to only 1.4% (95%CI: 0-3.4%) for the 151 patients transplanted since January 2005 (P=0.045). There was no significant difference in TRM (P=0.490) between those prepared with 5-15 mg/kg ATG (5.5%; 95%CI: 0-13.1%) and those prepared with 20 mg/kg ATG (3.0%; 95%CI: 0.4-5.6%).
Event-free survival
Considering deaths (n=7), non-engraftments (n=2), and rejections (n=5) as events, the overall 5-year EFS was 93.9% (95%CI: 90.7-97.1%), but EFS strongly improved with time since it was 97.9% (95%CI: 95.5-100%) in the 151 patients of the second cohort versus 86.9% (79.5-94.3%) in the 83 patients of the first series (Figure 2B). Among the 190 patients transplanted after year 2000 and prepared with ATG, 5-year EFS was 97.8% (95%CI: 95.6-100%). EFS was similar in patients prepared with 5-15 mg/kg or with 20 mg/kg ATG (Figure 2C), in patients younger or older than 15 years (Figure 2D), and in patients transplanted from CB alone versus BM (Table 3). Cox regression analysis showed that EFS was not associated with the recipient’s or donor’s age or with the cell source, but was significantly associated (P<0.001) with the period of transplant (i.e. before or after year 2000) (HR=11.3; 95%CI: 3.9-33.4).
Graft-versus-host disease
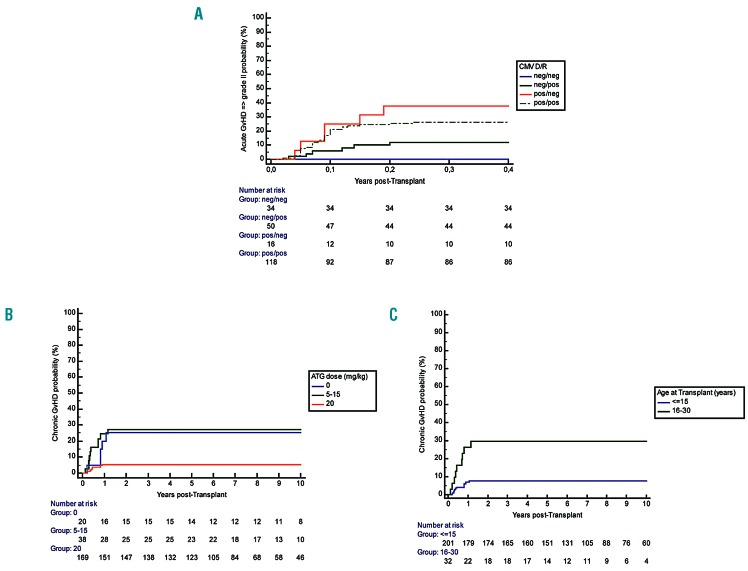
Acute GvHD was assessable in the 232 successfully engrafted patients. The overall cumulative incidence of aGvHD ≥II at day 100 was 20.1% (95%CI: 14.9-25.3%). The multivariate Cox regression analysis retained sex mismatch (HR=2.12, 95%CI: 1.15-3.90; P=0.016) and donor’s cytomegalovirus (CMV) positive status (HR=5.10, 95%CI: 2.15-12.05; P<0.001) as significant and independent risk factors for aGvHD ≥II (Figure 3A).
Figure 3.
Relationship of graft-versus-host disease (GvHD) and donor/recipient (D/R) cytomegalovirus (CMV) status, anti-thymocyte globulin (ATG) dose and recipient age. (A) Acute GvHD according to the D/R CMV status. Cumulative incidence of acute-GvHD at day 100 according to the D/R CMV status. (B) Chronic GvHD according to the ATG dose. Cumulative incidence of chronic GvHD at five years was significantly lower in the patients having received the high dose of ATG (20 mg/kg) (5.4%, 95%CI: 1.8-9.0%) versus those not prepared with ATG (25%, 95%CI: 5.6-44.4%) and those having received lower doses (5-15 mg/kg): 27.0%, 95%CI: 12.4-41.6%) (Log Rank P<0.001). (C) Chronic graft-versus-host disease (GvHD) according to recipient age at transplant. Cumulative incidence of chronic GvHD in children under 15 years of age was 7.6% (95%CI: 3.8-11.4%) versus 29.7% (95%CI: 13.1-46.3%) in those older than 15.
Chronic GvHD was assessable in 228 patients, and occurred in 24 patients. It was mostly mild in 18 patients, but extensive in six. Organ involvement included resolutive cutaneo-digestive (n=2), and obliterans bronchiolitis (n=4) which was responsible for death in two patients. The cumulative incidence of cGVHD at five years was 10.5% (95%CI: 6.5-14.5%), and was significantly lower (5.4%, 95%CI: 1.8-9.0%) in patients who received the highest ATG dose (20 mg/kg) than in those prepared without ATG (25%, 95%CI: 5.6-44.4%) and receiving lower doses (5-15 mg/kg) (27.0%, 95%CI: 12.4-41.6%) (Log Rank P<0.001). Moreover, 5-year chronic GvHD in children under 15 years of age was 7.6% (95%CI: 3.8-11.4%) versus 29.7% (95%CI: 13.1-46.3%) in those over 15 years of age. Multivariate Cox regression analysis retained only the recipient’s age (HR=1.098 per 1 year increase, 95%CI: 1.033-1.168; P=0.003) and the ATG dose (HR=0.91 per mg/kg increase, 95%CI: 0.86-0.96; P=0.001) as independent risk factors.
Other post-transplant complications
The number of seizures, hemorrhagic cystitis, and veno-occlusive disease are shown in Table 2. The proportion of patients experiencing seizures and/or posterior reversible leukoencephalopathy was significantly reduced in the second cohort (5.3% vs. 18.1%; P=0.002). CMV replication occurred in 23.9% of patients. No CMV-related disease occurred with pre-emptive therapy. Epstein-Barr virus (EBV) asymptomatic viral replications had not been systematically assessed in the first cohort, and were observed in 15 patients (6%) in the second cohort. Six of them required anti-CD20 treatment, but none developed post-transplant lymphoproliferative disease. Among the 234 patients, B-lymphoma occurred six years post transplant in one patient who had received long-term immunosuppressive therapy, but no other secondary malignancies were observed.
Gonadal function
All females who were post-pubertal at the time of transplantation (n=14) developed amenorrhea with low serum estradiol and elevated LH and FSH levels during the year following transplant, necessitating hormone replacement therapy. Thirty-two girls were pre-pubertal at transplantation, and most required hormonal therapy to develop secondary sexual characteristics at the bone age of 13 years. However, 9 of 32 of the pre-pubertal girls at SCT, who had reported to have spontaneously undergone normal puberty at the last visit, were significantly younger at transplant than those who required hormonal substitution for puberty induction [mean (SD) age 5.9 (2.6) vs. 10.1 (2.1); P=0.002]. At last visit, 20 females were older than 25, and four of them, who had been transplanted between 1988 and 1998 at 5.8, 6.1, 6.6 and 7.7 years of age, respectively, had had six spontaneous pregnancies and five children at approximately 20 years after SCT. Pre-transplant ovarian tissue cryopreservation has been systematically performed since year 1998. Unilateral oophorectomy was performed by laparoscopy under general anesthesia and ovarian cortical fragments were cryopreserved at a median age of 8.0 years (range: 3.0-26.4). Among the 93 girls trans planted since 1998, 11 were older than 25 years at the last visit; two wanted to become pregnant and requested auto-graft of ovarian fragments. The first of these was transplanted at 20 years of age and had received hormone replacement therapy because of post-transplant ovarian failure. Orthotopic ovarian fragment autograft was performed by laparoscopy 29 months after SCT, and the first signs of recovery of ovarian function were observed nine weeks after ovarian graft. Hormone replacement therapy was stopped at four months and the patient became pregnant six months after ovarian autograft, delivering a healthy girl at 38 weeks of gestation. She had another child three years later without requiring a new graft of ovarian fragments.31 In the second patient, transplanted at 22 years of age, autograft of ovarian fragments was performed 12 years post SCT, and recovery of ovarian function was observed after four months, but unfortunately no pregnancy occurred thereafter.
All the boys who were pre-pubertal at transplant and of pubertal age at the last visit developed normal puberty with normal testosterone, FSH and LH levels, in keeping with their bone age and pubertal status. At last visit, 19 males were over 25 years of age. Three of them who had been transplanted 11, 12 and 20 years ago fathered spontaneously. Pre-transplant sperm cryopreservation was performed in all pubertal males and pre-transplant testicular tissue cryopreservation has been systematically performed since year 2010 in prepubertal boys (n=25) (median age: 7.5 years; range: 3.4-10.2). However, so far, no patient has requested testicular fragment autografting (median age at last visit: 13.7 years; range: 5.8-21.2).
Chimerism studies
Chimerism was assessable in 208 patients and was categorized as full donor chimerism (>95% donor cells), rejection (<5% donor cells), and low (5-50% donor cells) or high (50-95% donor cells) mixed chimerism. At one year, full donor chimerism was present in 112 patients (54%), while 92 patients (44%) had mixed chimerism (83 high, and 9 low), and 4 (2%) had <5% donor cells (the 2 patients with no-engraftment and 2 who rejected the graft at 0.5 and 0.9 years).
Multivariate logistic regression analysis showed that the ATG dose (OR=1.06 per 1 mg/kg increase, 95%CI:1.01-1.12; P=0.017) and recipient’s age (OR=0.94 per year increase, 95%CI: 0.88-0.99; P=0.041) were independently inversely associated with the presence of mixed chimerism at one year. Moreover, cGvHD was significantly more frequent (P=0.018) in patients with full donor chimerism (18 of 112; 16.1%) than in those with mixed chimerism (5 of 92; 5.5%), These five patients all had donor cell% >75%. No significant association was found between ABO compatibility and chimerism.
Chimerism on separated populations (CD3+ and CD3−) was assessed in 103 patients. Whole blood donor cell chimerism was more significantly correlated with the CD3− (r=0.917, P<1×10−128) than with the CD3+ (r=0.418, P<1×10−15) population. During the first two years post transplant, CD3+ chimerism was significantly lower than CD3− and whole blood donor cell chimerism, but was similar thereafter.
Long-term outcome of chimerism
One-hundred and twelve patients remained stable with donor chimerism >70% at last visit. However, one girl with a rare erythroid group (U-), transplanted in 2000 from her brother’s CB (U+), had full donor chimerism at one and two years that remained stable for five years, but decreased thereafter with the appearance of hemolytic anemia. The patient was given donor lymphocyte infusions (DLI), which allowed the reversion to full donor chimerism along with anemia and hemolysis correction, but it induced cGvHD which was responsible for chronic pulmonary disease (currently well under control).
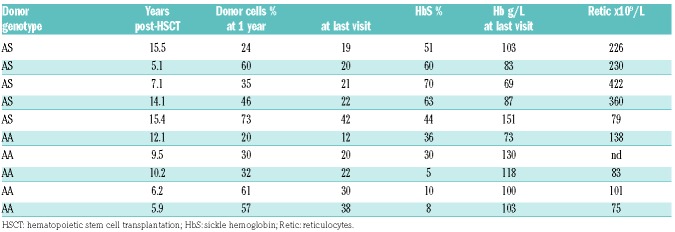
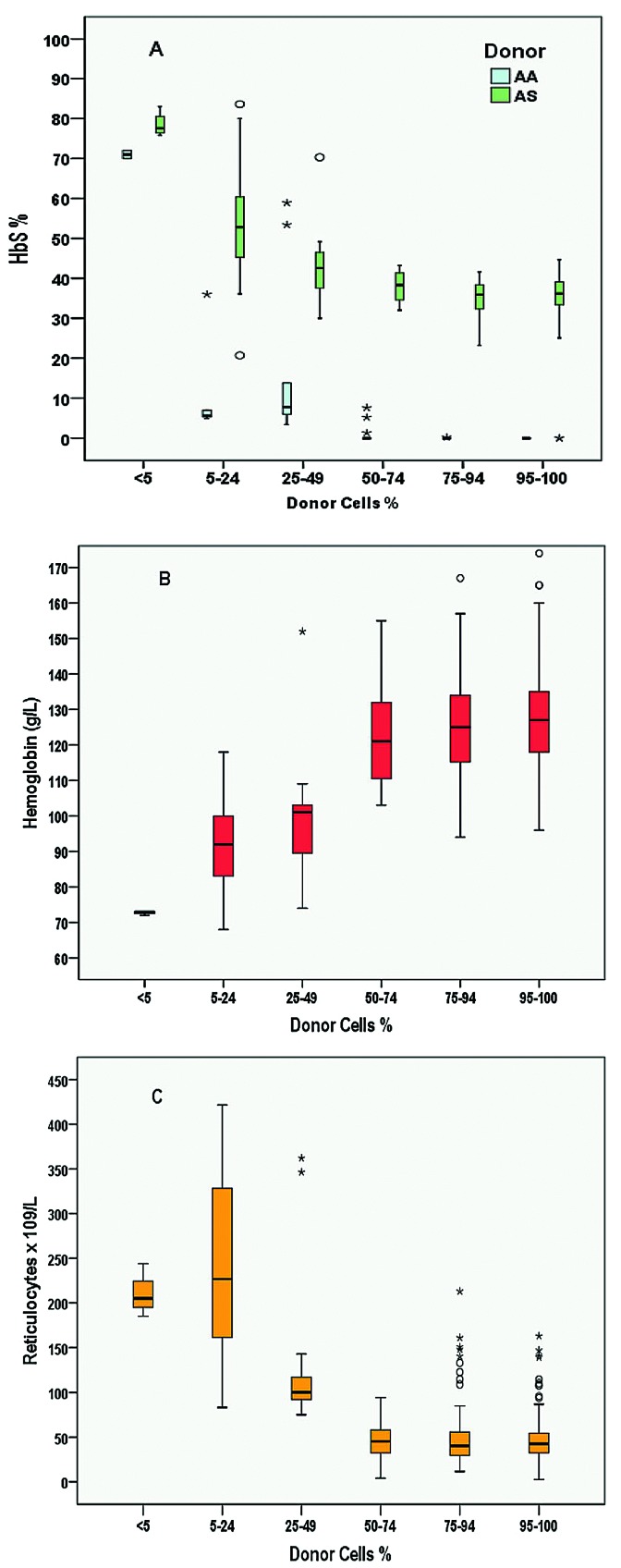
Among the 92 patients with mixed chimerism at one year, four rejected the graft (4.3%) at 1.5, 2, 2.3 and 9 years post transplant, but none had been prepared with ATG. Among the other 88 patients, none had rejected the graft at last visit, nine had switched to full donor chimerism, and 69 had high and 10 low mixed chimerism (Table 4). None of those with low mixed chimerism experienced SCA-related vaso-occlusive crisis (VOC) or acute-chest-syndrome (ACS), but five had hemoglobin <100 g/L or reticulocytes >150×109/L, considered as SCA-related symptoms. Thus, despite the absence of rejection and the absence of VOC or ACS occurrence, these patients were deemed as having SCA recurrence, resulting in an overall disease-free survival (DFS) of 95.5% at five years (95%CI: 92.7-98.3%). Three of the patients with low mixed chimerism and hemolytic anemia stigmata received DLI, which did not induce GvHD, but did not offer improvement of anemia and hemolysis. Figure 4 reports all biological data recorded at the same time as chimerism during the entire follow up. It can be seen that as long as donor chimerism remains higher than 50%, Hb and reticulocytes remain normal, resulting in no anemia, no hemolysis and similar HbS% as donor.
Table 4.
Biological data in patients with low mixed chimerism (5-49% donor cells) at last visit.
Figure 4.
Relationship between % donor cells and hemoglobin S percentage (HbS%), hemoglobin (Hb) level, and reticulocyte count. All biological data (HbS%, Hb and reticulocytes) recorded at the same time as the chimerism during the overall follow up were used for these box-plots. (A) Box-plot of HbS% according to the donor cell% category. This graph shows that HbS% is similar to that of the donor (AA or AS) as long as donor cell% is higher than 50%. (B) Box plot of Hb level according to donor cell% category. Hb level remains higher than 100g/L as long as donor cell% is higher than 50%. (C) Box plot of reticulocyte count according to % donor cells. Reticulocyte count remains lower than 100x109/L as long as % donor cells is higher than 50%.
Discussion
In this report, we confirm that SCA children and young adults transplanted after year 2000 (n=197) with an MSD and prepared with myeloablative-CR associating busulfan, cyclophosphamide and ATG have at least a 95% chance of cure. These results compare favorably with other published series using myeloablative19–26 and nonmyeloablative27–29 CR.
The TRM < 2% in this series must be interpreted in the context of mortality risk related to the SCA disease itself, which was reported to be 6.1% before the age of 18 years in the Dallas cohort,16 and 1% in the London17 and 2.5% in the Créteil14 newborn cohorts. Thus, MSD-SCT does not expose children to a higher risk of death than SCA itself, but offers improved quality of life. Moreover, it is important to remember that transition to adulthood carries a high risk of death,32 and that mortality in adults18 has increased during the last few years, whereas it has significantly decreased for children.
Except for frequent high fever during ATG infusion, myeloablative conditioning was well tolerated in the 234 young patients, with only one death during aplasia and two mild veno-occlusive diseases. High doses of ATG are known to increase the risk of viral complications. The occurrence of EBV reactivation, however well controlled with anti-CD20 treatment, and the case of fatal adenoviral meningoencephalitis30 are of concern, but the risk benefits between viral infections, which are manageable, and cGvHD, which is more difficult to control, need to be taken into account. Seizures and posterior reversible encephalopathy syndromes (PRES), which were highly frequent (16 of 83; 19%) before year 2005,22 despite preventive measures such as anticonvulsant prophylaxis with clonazepam during busulfan administration and cyclosporine therapy, strict control of arterial hypertension, prompt correction of magnesium deficiency, maintenance of the Hb concentration above 9 g/dL and platelet count above 50×109/L,33 were significantly (P=0.002) less frequent after 2005 (8 of 151; 5%), following the SFGM-TC recommendation to promptly replace cyclosporine with mycophenolate mofetil in case of GvHD requiring steroid therapy.22 However, the risk of seizures and PRES remains an adverse effect of cyclosporine and steroid therapy,34 and may warrant substituting cyclosporine for sirolimus27,28 that is less neurotoxic and induces good tolerance.35 Hemorrhagic cystitis occurred in 21 of 234 patients (9%) and was particularly severe in two patients. Cyclophosphamide is generally considered to be the most important predisposing factor for occurrence of hemorrhagic cystitis.36,37 Despite the absence of prospective randomized trials comparing busulfanfludarabine to busulfan-cyclophosphamide, several reports mention a lower risk of hemorrhagic cystitis with fludarabine.38,39 This complication may suggest replacing cyclophosphamide with fludarabine, as only a 2% risk of hemorrhagic cystitis was reported in children transplanted for non-malignant disease following busulfan-fludarabine versus 9% with busulfan-cyclophosphamide CR.39
The major long-term concern with busulfan is the ovarian failure observed in post-pubertal females and infertility risk in both genders.40,41 Despite the occurrence of spontaneous puberty in girls transplanted at a younger age and the birth without treatment of several babies from females transplanted 20-25 years ago during infancy, demonstrating some reversibility of ovarian dysfunction, it remains crucial to maximize the chances of fertility and to recommend pre-transplant cryopreservation of ovarian42–44 and testis45 tissues before SCT with myeloablative CR. In France, systematic ovarian and testicular tissue cryopreservation has been established since 1998 and 2010, respectively, and is free of charge. However, CR preserving fertility such as the one proposed by the National Institutes of Health (NIH) protocol using 3Gy total body irradiation with testis shielding in males and alemtuzumab27–29 should be preferred when gonadal cryopreservation is not easily accessible and for adults. It is also important to keep in mind that fertility is also compromised by SCA itself,46 and that this risk is lower when SCT is performed at a younger age.
Event-free survival in this series was similar for patients younger and older than 15 years, but the risk of cGvHD, not acceptable in this non-malignant disease, was much more frequent in older patients. We show that the high dose of ATG (20 mg/kg) significantly reduced the risk of cGvHD independently of the age at transplant by favoring mixed chimerism occurrence. In the 20 of 234 patients not prepared with ATG, mixed chimerism was unstable, and secondary graft failed in 5 of 20. In contrast, only one secondary rejection was observed before year-1 among the 214 patients prepared with ATG.
Mixed chimerism was present in 44% of patients at one year in this series. None of the patients prepared with ATG rejected the graft and none experienced VOC or acute chest syndrome. The absence of SCA-related symptoms in patients with mixed chimerism has been reported in other series.22,47–50 Low levels of donor erythroid engraftment were shown to result in donor cell predominance among erythroblasts and erythrocytes.48 This stimulated the development of non-myeloablative CR in order to reduce TRM and preserve fertility, and resulted in the recommendation of HSCT to older adult patients with organ dysfunction. While the first results after nonmyeloabla-tive SCT have been disappointing, very encouraging results were obtained by the NIH team in adults (17-64 years of age)27,28 using as stem cell source granulocyte-colony stimulating factor (G-CSF)-stimulated peripheral blood progenitors from HLA-matched sibling donors and sirolimus for GvHD prophylaxis, which was stopped after one year in those with >50% donor T-cell chimerism. The mean duration of immunosuppression was 2.1 years (range: 1.0-8.4). Among 30 patients transplanted, four experienced rejection (13%), which was responsible for one death in a patient with Moya Moya, but no TRM and no GvHD were observed. Mean donor T cell was 48%, mean myeloid chimerism was 86%, and 15 patients had stable chimerism despite sirolimus withdrawal. These results were reproduced in 13 adult patients by another team in Chicago.29 Thus, among the 43 patients reported in both series, 88% were alive, free of SCA and without GvHD. However, the limitations of the protocol are the long-term immunosuppression and the 300 cGy TBI that could favor a secondary malignancy. Other non-myeloablative or reduced intensity CR have been reported by several teams and fully described in a review,50 but the most successful results were obtained with the NIH protocol described above.
It is to be noted that the excellent results reported here mostly concern young patients and are less applicable to older patients with higher morbidities. Moreover, outcomes of cognitive performances, quality of life, costs and organ functioning are not reported here. This is an important limitation of our study but only prospective trials comparing MSD-SCT to standard-care would be able to define the true risk benefit balance for each procedure. Furthermore, this study does not allow the chances of fertility after ovarian or testicular reimplantation to be estimated. However, the present study is the first to report the very long-term outcome in patients with mixed chimerism and to show that the correction of hemolytic anemia requires a higher donor cell% than the prevention of SCA-related crises.
The issue now is to determine whether a protocol offering an EFS at 87% with no TRM and no GvHD should also be favored for children. In France, the myeloablative CR with 98% EFS with a very low risk of cGvHD is still currently used in children under 15 years of age, but we recommend the NIH protocol for patients older than 15.
However, we recognize that this is still a matter of debate and additional prospective studies may result in a change in course depending on the long-term results of MSD-SCT with non-myeloablative CR.
Acknowledgments
The authors would like to thank all the patients and their families for their participation in this study, all the nurses on the various transplantation units and outpatient clinics for their dedicated work and the doctors who referred patients for transplantation. We also thank Dr. Martine Torres for her critical reading and editing of the manuscript which was supported through an unrestricted grant from bluebird bio.
Footnotes
Check the online version for the most updated information on this article, online supplements, and information on authorship & disclosures: www.haematologica.org/content/105/1/91
References
- 1.Piel FB, Hay SI, Gupta S, Weatherall DJ, Williams TN. Global burden of sickle cell anaemia in children under five, 2010-2050: modelling based on demographics, excess mortality, and interventions. PLoS Med. 2013;10(7):e1001484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gaston MH, Verter JI, Woods G, et al. Prophylaxis with oral penicillin in children with sickle cell anemia. N Engl J Med. 1986;314(25):1593–1599. [DOI] [PubMed] [Google Scholar]
- 3.Adamkiewicz TV, Silk BJ, Howgate J, et al. Effectiveness of the 7-valent pneumococcal conjugate vaccine in children with sickle cell disease in the first decade of life. Pediatrics. 2008;121(3):562–569. [DOI] [PubMed] [Google Scholar]
- 4.Charache S, Terrin ML, Moore RD, et al. Effect of hydroxyurea on the frequency of painful crisis in sickle cell anemia. N Engl J Med. 1995;332(20):1317–1322. [DOI] [PubMed] [Google Scholar]
- 5.Ferster A, Vermylen C, Cornu G, et al. Hydroxyurea for treatment of severe sickle cell anemia: a pediatric clinical trial. Blood. 1996;88(6):1960–1964. [PubMed] [Google Scholar]
- 6.de Montalembert M, Belloy M, Bernaudin F, et al. Three-year follow-up of hydroxyurea treatment in severely ill children with sickle cell disease. The French Study Group on Sickle Cell Disease. J Pediatr Hematol/Oncol. 1997;19(4):313–318. [DOI] [PubMed] [Google Scholar]
- 7.Ware RE, Davis BR, Schultz WH, et al. Hydroxycarbamide versus chronic transfusion for maintenance of transcranial doppler flow velocities in children with sickle cell anaemia-TCD With Transfusions Changing to Hydroxyurea (TWiTCH): a multicentre, open-label, phase 3, non-inferiority trial. Lancet. 2016;387(10019):661–670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Thornburg CD, Files BA, Luo Z, et al. BABY HUG Investigators Impact of hydroxyurea on clinical events in the BABY HUG trial. Blood. 2012;120(22): 4304–4310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Steinberg MH, Barton F, Castro O, et al. Effect of hydroxyurea on mortality and morbidity in adult sickle cell anemia: risks and benefits up to 9 years of treatment. JAMA. 2003;289(13):1645–1651. [DOI] [PubMed] [Google Scholar]
- 10.Wang WC, Ware RE, Miller ST, et al. BABY HUG investigators. Hydroxycarbamide in very young children with sickle-cell anaemia: a multicentre, randomised, controlled trial (BABY HUG). Lancet. 2011; 377(9778):1663–1672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Adams RJ, McKie V, Nichols F, et al. The use of transcranial ultrasonography to pre dict stroke in sickle cell disease. N Engl J Med. 1992;326(9):605–610. [DOI] [PubMed] [Google Scholar]
- 12.Verlhac S, Bernaudin F, Tortrat D, et al. Detection of cerebrovascular disease in sickle cell disease children by transcranial Doppler sonography. Correlation with MRI and MRA and conventionnal angiog-raphy. Pediatr Radiol. 1995;25 Suppl 1:S14–S19. [PubMed] [Google Scholar]
- 13.Adams RJ, McKie VC, Hsu L, et al. Prevention of a first stroke by transfusions in children with sickle cell anemia and abnormal results on transcranial Doppler ultrasonography. N Engl J Med. 1998; 339(1):5–11. [DOI] [PubMed] [Google Scholar]
- 14.Bernaudin F, Verlhac S, Arnaud C, et al. Impact of early transcranial Doppler screening and intensive therapy on cerebral vasculopathy outcome in a newborn sickle cell anemia cohort. Blood. 2011; 117(4):1130–1140; quiz 1436. [DOI] [PubMed] [Google Scholar]
- 15.Platt OS, Brambilla DJ, Rosse WF, et al. Mortality in sickle cell disease: life expectancy and risk factors for early death. N Engl J Med. 1994;330(23):1639–1644. [DOI] [PubMed] [Google Scholar]
- 16.Quinn CT, Rogers ZR, Buchanan GR. Survival of children with sickle cell disease. Blood. 2004;103(11): 4023–4027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Telfer P, Coen P, Chakravorty S, et al. Clinical outcomes in children with sickle cell disease living in England: a neonatal cohort in East London. Haematologica. 2007;92(7):905–912. [DOI] [PubMed] [Google Scholar]
- 18.Lanzkron S, Carroll CP, Haywood C., Jr Mortality rates and age at death from sickle cell disease: U.S., 1979-2005. Public Health Rep. 2013;128(2):110–116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Vermylen C, Fernandez Robles E, Ninane J, Cornu G. Bone marrow transplantation in five children with sickle cell anaemia. Lancet. 1988;1(8600):1427–1428. [DOI] [PubMed] [Google Scholar]
- 20.Bernaudin F, Souillet G, Vannier JP, et al. Bone marrow transplantation (BMT) in 14 children with severe sickle cell disease (SCD): the French experience. GEGMO. Bone Marrow Transplant. 1993;12 Suppl 1:118–121. [PubMed] [Google Scholar]
- 21.Walters MC, Patience M, Leisenring W, et al. Bone marrow transplantation for sickle cell disease. N Engl J Med. 1996;335(6):369–376. [DOI] [PubMed] [Google Scholar]
- 22.Bernaudin F, Socie G, Kuentz M, et al. SFGM-TC. Long-term results of related myeloablative stem-cell transplantation to cure sickle cell disease. Blood. 2007; 110(7):2749–2756. [DOI] [PubMed] [Google Scholar]
- 23.Gluckman E, Cappelli B, Bernaudin F, et al. Sickle cell disease: an international survey of results of HLA-identical sibling hematopoietic stem cell transplantation. Blood. 2017;129(11):1548–1556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Vermylen C, Cornu G, Ferster A, et al. Haematopoietic stem cell transplantation for sickle cell anaemia: the first 50 patients transplanted in Belgium. Bone Marrow Transplant. 1998;22(1):1–6. [DOI] [PubMed] [Google Scholar]
- 25.Walters MC, Storb R, Patience M, et al. Impact of bone marrow transplantation for symptomatic sickle cell disease: an interim report. Multicenter investigation of bone marrow transplantation for sickle cell disease. Blood. 2000;95(6):1918–1924. [PubMed] [Google Scholar]
- 26.Kuentz M, Robin M, Dhedin N, et al. Is there still a place for myeloablative regimen to transplant young adults with sickle cell disease? Blood. 2011;118(16):4491–4492; author reply 4492-4493. [DOI] [PubMed] [Google Scholar]
- 27.Hsieh MM, Kang EM, Fitzhugh CD, et al. Allogeneic hematopoietic stem-cell transplantation for sickle cell disease. N Engl J Med. 2009;361(24):2309–2317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hsieh MM, Fitzhugh CD, Weitzel RP, et al. Nonmyeloablative HLA-matched sibling allogeneic hematopoietic stem cell transplantation for severe sickle cell phenotype. JAMA. 2014;312(1):48–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Saraf SL, Oh AL, Patel PR, et al. Nonmyeloablative Stem Cell Transplantation with Alemtuzumab/Low-Dose Irradiation to Cure and Improve the Quality of Life of Adults with Sickle Cell Disease. Biol Blood Marrow Transplant. 2016;22(3):441–448. [DOI] [PubMed] [Google Scholar]
- 30.Frange P, Peffault de Latour R, Arnaud C, et al. Adenoviral infection presenting as an isolated central nervous system disease without detectable viremia in two children after stem cell transplantation. J Clin Microbiol. 2011;49(6):2361–2364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Roux C, Amiot C, Agnani G, Aubard Y, Rohrlich PS, Piver P. Live birth after ovarian tissue autograft in a patient with sickle cell disease treated by allogeneic bone marrow transplantation. Fertil Steril. 2010; 93(7):2413.e15–19. [DOI] [PubMed] [Google Scholar]
- 32.Quinn CT, Rogers ZR, McCavit TL, Buchanan GR. Improved survival of children and adolescents with sickle cell disease. Blood. 2010;115(17):3447–3452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Walters MC, Sullivan KM, Bernaudin F, et al. Neurologic complications after allogeneic marrow transplantation for sickle cell anemia. Blood. 1995;85(4):879–884. [PubMed] [Google Scholar]
- 34.Gaziev J, Marziali S, Paciaroni K, et al. Posterior Reversible Encephalopathy Syndrome after Hematopoietic Cell Transplantation in Children with Hemoglobinopathies. Biol Blood Marrow Transplant. 2017;23(9):1531–1540. [DOI] [PubMed] [Google Scholar]
- 35.Powell JD, Lerner CG, Schwartz RH.Inhibition of cell cycle progression by rapamycin induces T cell clonal anergy even in the presence of costimulation. J Immunol. 1999;162(5):2775–2784. [PubMed] [Google Scholar]
- 36.Brugieres L, Hartmann O, Travagli JP, et al. Hemorrhagic Cystitis Following High-Dose Chemotherapy and Bone-Marrow Transplantation in Children with Malignancies - Incidence, Clinical Course, and Outcome. J Clin Oncol. 1989;7(2):194–199. [DOI] [PubMed] [Google Scholar]
- 37.Lunde LE, Dasaraju S, Cao Q, et al. Hemorrhagic cystitis after allogeneic hematopoietic cell transplantation: risk factors, graft source and survival. Bone Marrow Transplant. 2015;50(11):1432–1437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bartelink IH, van Reij EM, Gerhardt CE, et al. Fludarabine and exposure-targeted busulfan compares favorably with busul-fan/cyclophosphamide-based regimens in pediatric hematopoietic cell transplantation: maintaining efficacy with less toxicity. Biol Blood Marrow Transplant. 2014; 20(3):345–353. [DOI] [PubMed] [Google Scholar]
- 39.Harris AC, Boelens JJ, Ahn KW, et al. Comparison of pediatric allogeneic transplant outcomes using myeloablative busulfan with cyclophosphamide or fludarabine. Blood Adv. 2018;2(11):1198–1206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sanders JE, Hawley J, Levy W, et al. Pregnancies following high-dose cyclophosphamide with or without high-dose busulfan or total-body irradiation and bone marrow transplantation. Blood. 1996; 87(7):3045–3052. [PubMed] [Google Scholar]
- 41.Vatanen A, Wilhelmsson M, Borgström B, et al. Ovarian function after allogeneic hematopoietic stem cell transplantation in childhood and adolescence. Eur J Endocrinol. 2013;170(2):211–218. [DOI] [PubMed] [Google Scholar]
- 42.Poirot C, Abirached F, Prades M, Coussieu C, Bernaudin F, Piver P. Induction of puberty by autograft of cryopreserved ovarian tissue. Lancet. 2012;379(9815):588. [DOI] [PubMed] [Google Scholar]
- 43.Donnez J, Dolmans MM, Demylle D, et al. A. Livebirth after orthotopic transplantation of cryopreserved ovarian tissue. Lancet. 2004;364(9443):1405–1410. Erratum in: Lancet. 2004;364(9450):2020. [DOI] [PubMed] [Google Scholar]
- 44.Baert Y, Van Saen D, Haentjens P, In’t Veld P, Tournaye H, Goossens E. What is the best cryopreservation protocol for human testicular tissue banking? Hum Reprod. 2013;28(7):1816–1826. [DOI] [PubMed] [Google Scholar]
- 45.Smith-Whitley K. Reproductive issues in sickle cell disease. Blood. 2014; 124(24): 3538–3543. [DOI] [PubMed] [Google Scholar]
- 46.Walters MC, Patience M, Leisenring, et al. Stable mixed hematopoietic chimerism after bone marrow transplantation for sickle cell anemia. Biol Blood Marrow Transplant. 2001;7(12):665–673. [DOI] [PubMed] [Google Scholar]
- 47.Hsieh MM, Wu CJ, Tisdale JF. In mixed hematopoietic chimerism, the donor red cells win. Haematologica. 2011;96(1):13–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Fitzhugh CD, Cordes S, Taylor T, et al. At least 20% donor myeloid chimerism is necessary to reverse the sickle phenotype after allogeneic HSCT. Blood. 2017;130(17):1946–1948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Abraham A, Hsieh M, Eapen M, et al. Relationship between Mixed Donor-Recipient Chimerism and Disease Recurrence after Hematopoietic Cell Transplantation for Sickle Cell Disease. Biol Blood Marrow Transplant. 2017;23(12):2178–2183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Bernaudin F, Pondarré C, Galambrun C, Thuret I. Allogeneic/Matched Related Transplantation for β-Thalassemia and Sickle Cell Anemia. Adv Exp Med Biol. 2017;1013:89–122. [DOI] [PubMed] [Google Scholar]