Treatment for acute myeloid leukemia (AML) has not changed substantially in the past 40 years. We still largely rely on the substances (antimetabolites and topoisomerase inhibitors) that were introduced for AML treatment in the 1970s. Although treatment outcomes for AML, other than acute promyelocytic leukemia (APM), have improved over the years, these improvements have been slow and incremental and been largely achieved by optimizing dosing schedules and better supportive care.1 However, substantial improvements in treatment, with high cure rates, have been achieved in genetically well defined subgroups of leukemia like acute promyelocytic leukemia (driven by the PML/RARA fusion)2 and chronic myeloid leukemia (driven by the BCR/ABL fusion).3 AML, however, is a different beast. It is not a single disease but a genetically highly heterogeneous group of malignant diseases of the hematopoietic stem cell that have similar phenotypes. The great genetic heterogeneity of AML, which was recognizable even in the early cytogenetic studies, has become very apparent with the advent of next-generation sequencing which has allowed the analysis of AML genomes at base pair resolution.4,5
Considering that AML is a highly heterogeneous group of malignant diseases, it is not surprising that a ‘one size fits all’ standard treatment approach can not achieve satisfactory cure rates. One critical question is: how heterogenous is AML? How many different diseases are we dealing with? If we simply looked at the number of genes that have been shown to harbor somatic mutation in AML and the number of somatic mutations in each AML sample, the number of potential combinations, and thus AML subgroups, would be astronomical. However, this is most likely an overestimate of the heterogeneity and complexity of AML, and there is likely only a much smaller number of crucial pathways that are disrupted in AML pathogenesis. Identifying and targeting these crucial pathways should greatly improve AML therapy.
In their report in this issue of the Journal, Chen et al. present convincing evidence that homoharringtonine (HHT) (omacetaxine mepesuccinate), an alkaloid found in the Hainan plum yew,6 is targeting such a critical pathway in AML pathogenesis.7 HHT has been used in China for several years for the treatment of AML8,9 and was approved in 2012 by the US Food and Drug Administration (FDA) for the treatment of chronic myeloid leukemia (CML) with resistance to tyrosine kinase inhibitors.10 The mode of action of HHT in CML was reported to be through the inhibition of protein synthesis by binding to the A site of the ribosome.11
Building on the success of HHT in the treatment of AML patients in China, where a remission rate of 83% was achieved when HHT was used in combination with cytarabine and aclarubicin to treat de novo AML patients,9 Chen et al. explored the mechanism of HHT efficacy in murine and human AML cell lines as well as in murine and xenotransplant AML models. HHT treatment led to apoptosis and differentiation in cell lines and resulted in increased survival in murine AML transplantation models and in human AML xenograft models. Then the group set out to elucidate the molecular mechanism responsible for the observed effects of HHT treatment.
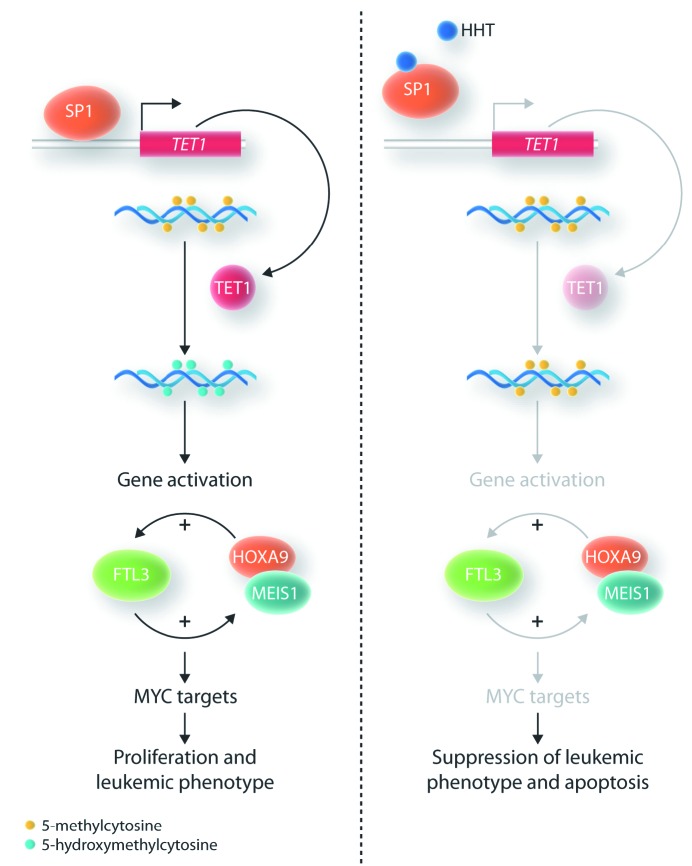
In a series of elegant experiments, Chen et al. showed that HHT binds to the transcription factor SP1, thereby inhibiting the binding of SP1 to its cognate DNA sequences. However, the route to this discovery was not straightforward. Initially, the group examined global 5-hydroxymethylcytosine (5hmC) levels in cells treated with HHT and noticed a remarkable reduction in global 5hmC levels. 5hmC is generated by the oxidation of methylcytosine groups in DNA through the action of the three dioxygenase enzymes TET1, 2 and 3.12 As only the expression of the TET1 gene, and not of TET2 or TET3, was significantly down-regulated after HHT treatment, the authors concluded that the reduction in TET1 expression, and hence a reduced TET1 enzymatic activity, was probably the cause of the reduced 5hmC levels. Examining the promoter region of TET1, they found multiple binding motifs for the transcription factor SP1 and reasoned that SP1 might be important for the HHT-induced downregulation of TET1 expression. And indeed, they could show that HHT treatment reduced binding of SP1 to its binding motifs in the TET1 promoter region. Furthermore, a drug affinity responsive target stability assay and a cellular thermal shift assay provided strong evidence that HHT is indeed binding to the SP1 protein and preventing SP1 from binding to its recognition sites in the TET1 promoter region.
What are the consequences of the HHT-induced reduction in TET1 transcription and, consequently, the global reduction in 5hmC levels? The 5hmC mark is associated with active transcription. Thus, the reduction in 5hmC levels was accompanied by a genome-wide reduction in gene transcription. More than 1,600 genes showed reduced expression. Very interestingly, one of the most strongly repressed genes was FLT3, which codes for the tyrosine kinase receptor FLT3. The authors confirmed that the FLT3 gene was one of the direct targets of TET1 action with chromatin immunoprecipiation-quantitative polymerase chain reaction (ChIP-qPCR). FLT3 signaling is critical in the maintenance of the leukemic phenotype and activates a number of genes, including HOXA9 and MEIS1, which are highly expressed in hematopoietic stem and progenitor cells. There is a positive feed-back loop between FLT3 and HOXA9 / MEIS1 expression. Enforced FLT3 expression leads to upregulation of HOXA9 and MEIS1, and enforced expression of HOXA9 and MEIS1 in turn results in upregulation of FLT3. In addition, looking at all of the genes that had reduced expression after HHT administration, the authors found a significant enrichment of MYC target genes. They were then able to confirm that MYC expression itself was reduced after HHT treatment. Myc is one of the master regulators of cellular growth, increasing ribosome biogenesis and protein synthesis.13
SP1 is a transcription factor which has many cognate binding sites in the promoter region of many genes. So what is the evidence that the HHT-mediated inhibition of SP1 binding to the TET1 promoter is critical for the phenotype observed in AML cells after HHT treatment and not the repression of a number of other genes? Very elegantly, the authors showed that repression of TET1 phenocopies the effect of HHT treatment.
In summary, by binding to the transcription factor SP1, HHT affects one of the core pathways that are responsible for growth (MYC and targets) and impaired differentiation (HOXA9 and MEIS1) of AML cells. The central players in this pathway are the dioxygenase TET1 and the receptor tyrosine kinase FLT3. FLT3-activating mutations are found in about 40% of AML cases and it is overexpressed in an even larger proportion of AML. Thus, HHT promises to be effective in the majority of AML cases. HHT might thus be efficacious in a larger number of AML patients than several of the more recently introduced targeted AML drugs like FLT3 inhibitors (e.g. midostaurin, gilteritinib), IDH1/2 inhibitors (enasidenib, evosidenib), or hedgehog pathway inhibitors (glasdegib).14 At the same time, one would hope that it is more specific for AML than conventional chemotherapeutic agents such as nucleoside analogs or anthracyclines, which affect very basic cellular functions like DNA metabolism and are thus highly toxic to normal cells. However, as HHT has been shown to bind to SP1, and SP1 is a widely expressed transcription factor whose expression is not restricted to AML cells, it is not surprising that HHT treatment can have considerable side effects, including myelosuppression. Another unanswered question is whether HHT only binds to SP1 or whether it also binds to other cellular proteins which, again, could cause side effects and adverse reactions.
Figure 1.
Diagram of the pathways affected by homoharringtonine (HHT). (A) Status of the pathways in the leukemic cells in the absence of HHT. (B) When HHT is present, it binds to SP1 which leads to a reduction of the activity of downstream pathways.
Although it is unlikely that HHT will provide a cure for AML, it could become an important component of ever more diversified and targeted treatment strategies in this disease. The effectiveness of HHT and its therapeutic window need to be assessed rigorously in controlled clinical trials. These should be accompanied by a detailed genetic work up to identify those AML subgroups that are especially responsive to HHT therapy.
References
- 1.Burnett A, Wetzler M, Löwenberg B. Therapeutic advances in acute myeloid leukemia. J Clin Oncol. 2011;29(5):487–494. [DOI] [PubMed] [Google Scholar]
- 2.Platzbecker U, Avvisati G, Cicconi L, et al. Improved Outcomes With Retinoic Acid and Arsenic Trioxide Compared With Retinoic Acid and Chemotherapy in Non-High-Risk Acute Promyelocytic Leukemia: Final Results of the Randomized Italian-German APL0406 Trial. J Clin Oncol. 2017;35(6):605–612. [DOI] [PubMed] [Google Scholar]
- 3.Bower H, Björkholm M, Dickman PW, Höglund M, Lambert PC, Andersson TM. Life Expectancy of Patients With Chronic Myeloid Leukemia Approaches the Life Expectancy of the General Population. J Clin Oncol. 2016;34(24):2851–2857. [DOI] [PubMed] [Google Scholar]
- 4.Cancer Genome Atlas Research Network Genomic and epigenomic landscapes of adult de novo acute myeloid leukemia. N Engl J Med. 2013;368(22):2059–2074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Papaemmanuil E, Gerstung M, Bullinger L, et al. Genomic Classification and Prognosis in Acute Myeloid Leukemia. N Engl J Med. 2016;374(23):2209–2221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Powell RG, Weisleder D, Smith CR. Antitumor alkaloids for Cephalataxus harringtonia: structure and activity. J Pharm Sci. 1972;61(8):1227–1230. [DOI] [PubMed] [Google Scholar]
- 7.Li C, Dong L, Su R, et al. Homoharringtonine exhibits potent antitumr effect and modulates DNA epigenome n acute myeloid leukemia by targeting SP1/TET1/5hmC. Haematologica. 2020; 105(1):148–160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jin J, Jiang DZ, Mai WY, et al. Homoharringtonine in combination with cytarabine and aclarubicin resulted in high complete remission rate after the first induction therapy in patients with de novo acute myeloid leukemia. Leukemia. 2006;20(8):1361–1367. [DOI] [PubMed] [Google Scholar]
- 9.Jin J, Wang JX, Chen FF, et al. Homoharringtonine-based induction regimens for patients with de-novo acute myeloid leukaemia: a multicentre, open-label, randomised, controlled phase 3 trial. Lancet Oncol. 2013;14(7):599–608. [DOI] [PubMed] [Google Scholar]
- 10.Nazha A, Kantarjian H, Cortes J, Quintás-Cardama A. Omacetaxine mepesuccinate (synribo) - newly launched in chronic myeloid leukemia. Expert Opin Pharmacother. 2013;14(14):1977–1986. [DOI] [PubMed] [Google Scholar]
- 11.Gürel G, Blaha G, Moore PB, Steitz TA. U2504 determines the species specificity of the A-site cleft antibiotics: the structures of tiamulin, homoharringtonine, and bruceantin bound to the ribosome. J Mol Biol. 2009;389(1):146–156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wu X, Zhang Y. TET-mediated active DNA demethylation: mechanism, function and beyond. Nat Rev Genet. 2017;18(9):517–534. [DOI] [PubMed] [Google Scholar]
- 13.van Riggelen J, Yetil A, Felsher DW. MYC as a regulator of ribosome biogenesis and protein synthesis. Nat Rev Cancer. 2010;10(4):301–309. [DOI] [PubMed] [Google Scholar]
- 14.Tiong IS, Wei AH. New drugs creating new challenges in acute myeloid leukemia. Genes Chromosomes Cancer. 2019;58(12):903–914. [DOI] [PubMed] [Google Scholar]