Abstract
The number of people living with HIV (PLWH) ≥65 years is increasing in the United States. By 2035, the proportion of PLWH in this age group is projected to be 27%. As PLWH live longer, they face age-related comorbidities. We compared non-HIV disease and medication burden among PLWH (n = 2359) and HIV-negative individuals (n = 2,010,513) ≥65 years using MarketScan® Medicare Supplemental health insurance claims from 2009 to 2015. Outcomes were common diagnoses and medication classes, prevalence of non-HIV conditions, number of non-HIV conditions, and daily non-antiretroviral therapy (ART) medications over a 1-year period. We examined age-standardized prevalence rates and prevalence ratios (PRs) and fit multivariable generalized linear models, stratified by sex. PLWH were younger (mean 71 vs. 76 years) and a larger proportion were men (81% vs. 45%). The most common diagnoses among both cohorts were hypertension and dyslipidemia. Most non-HIV conditions were more prevalent among PLWH. The largest absolute difference was in anemia (29.6 cases per 100 people vs.11.7) and the largest relative difference was in hepatitis C (PR = 22.0). Unadjusted mean number of non-HIV conditions and daily non-ART medications were higher for PLWH (4.61 conditions and 3.79 medications) than HIV-negative individuals (3.94 and 3.41). In models, PLWH had significantly more non-HIV conditions than HIV-negative individuals [ratios: men = 1.272, (95% confidence interval, 1.233–1.312); women = 1.326 (1.245–1.413)]. Among those with >0 daily non-ART medications, men with HIV had significantly more non-ART medications than HIV-negative men [ratio = 1.178 (1.133–1.226)]. The disease burden associated with aging is substantially higher among PLWH, who may require additional services to effectively manage HIV and comorbid conditions.
Keywords: HIV/AIDS, aging, Medicare, chronic disease, insurance claims, polypharmacy
Introduction
In the United States, there is a high prevalence of multimorbidity and polypharmacy, the presence of multiple comorbid conditions and the use of multiple medications, especially among older persons. An estimated 26.5% of people 18–44 years old have ≥1 chronic condition compared to 85.8% of people ≥65 years.1 Among individuals aged 20–59, 17.3% used more than 3 medications in the past month while among those aged ≥60 years, an estimated 27.3% used 3–4 medications in the past month and 36.7% used ≥5.2 Patients' multimorbidity and polypharmacy can pose challenges for health care providers, who must attempt to co-manage multiple conditions in a single patient while avoiding possible drug–drug interactions.
The introduction of effective and tolerable combination antiretroviral therapy (ART) has dramatically extended the lifespans of people living with HIV (PLWH).3 Older PLWH and their health care providers must manage HIV infection along with multiple other common age-related conditions. Evidence indicates that PLWH develop these conditions at higher rates and earlier ages than HIV-negative individuals, making elderly PLWH particularly vulnerable to the challenges associated with multimorbidity and polypharmacy.4–7 Potential mechanisms for this finding include the long-term effects of HIV infection itself, side effects of ART, or prevalence of risk factors for comorbidities.8,9
To date, much of the research on older PLWH has defined “older” as >50 years.5,7,10–12 Little research has focused on PLWH who would traditionally be considered elderly in the United States—those aged 65 and older insured through Medicare—who may have different comorbidity profiles than those 50+ years old.13 The number of PLWH who are ≥65 years is increasing in the United States. By 2035, the proportion of PLWH in this age group is projected to be nearly 27%.14 Additionally, most research has been limited to HIV+ men with analyses of HIV+ women focusing on specific conditions, such as heart disease and cognitive impairment,15–19 rather than the overall burden.
Our objective was to compare the non-HIV disease and non-ART medication burden among elderly PLWH to that of elderly HIV-negative individuals, overall and by sex. We evaluated the most common diagnoses and medication classes and the prevalence of non-HIV conditions within the two groups, and the number of non-HIV conditions and the number of daily non-ART medications.
Methods
Data source
This observational, cross-sectional analysis was conducted using the IBM Watson Health MarketScan® Medicare Supplemental and Coordination of Benefits Database, a proprietary administrative health care claims database, comprising health care claims for over 9 million covered lives from 1995 to 2015. Enrollees are insured through Medicare supplemental insurance plans paid for by employers. The data include enrollee demographic and enrollment information, inpatient and outpatient medical claims, and outpatient pharmacy claims. Patients enter the database when their enrollment begins and exit the database when they disenroll or die.
The CUNY SPH Institutional Review Board granted this study IRB exemption. The MarketScan database satisfies the conditions set forth in Sections 164.514 (a)-(b)1ii of the Health Insurance Portability and Accountability Act of 1996 privacy rule regarding the determination and documentation of statistically deidentified data. All data were previously collected.
Patient selection
PLWH ≥65 years and HIV-negative individuals ≥65 years were selected. The group of PLWH included individuals with (i) ≥2 claims with an International Classification of Diseases, Ninth Edition, Clinical Modification (ICD-9-CM) diagnosis of HIV (042, 795.71, V08, 079.53) in any position (primary or secondary) or (ii) one claim with an HIV diagnosis and ≥1 claim for ART between January 1, 2010 and September 30, 2015. The comparator group comprised individuals with no claims with an HIV diagnosis and no claims for ART from January 1, 2009 to September 30, 2015 (referred to as “HIV-negative individuals”).
The service date on a patient's most recent claim with an HIV diagnosis was referred to as the index date for PLWH, to evaluate diagnoses and medication use at an individual's oldest age in the database. A pseudo-index date for HIV-negative individuals was randomly assigned based on the distribution of calendar year for index dates among PLWH. Both groups were required to have 12 months of continuous enrollment in the Medicare Supplemental database before their index date to evaluate the study outcomes (evaluation period). Patients were required to be ≥65 years old at the start of the evaluation period.
Detailed information on the methods can be found in the Supplementary Data.
Exposure
The primary exposure of interest was HIV status.
Outcomes
The outcomes examined were as follows: (i) the most common non-HIV three-digit ICD-9-CM diagnosis codes appearing in the two groups, (ii) the most common non-ART therapeutic classes for filled medication prescriptions in the two groups, (iii) prevalence of non-HIV conditions in the two groups, (iv) the number of non-HIV health conditions diagnosed in individuals, and (v) the number of daily non-ART medications by individuals. All outcomes were based on health care claims with service dates in the 12 months before and including the index date.
The most common non-HIV health conditions were based on ICD-9-CM diagnosis codes on inpatient or outpatient claims, rolled up to the unique three-digit level to combine similar conditions. On outpatient pharmacy claims, therapeutic class was based on Redbook™, a database containing drug product information. All unique three-digit ICD-9-CM diagnosis codes present/unique therapeutic classes filled among the PLWH group were identified. This process was repeated for men with HIV, women with HIV, the overall HIV-negative group, HIV-negative men, and HIV-negative women.
The prevalence of the following non-HIV conditions was evaluated: ischemic heart disease (including myocardial infarction, angina, and atherosclerosis), congestive heart failure, cardiac dysrhythmia, hypertension, dyslipidemia, non-rheumatic heart valve disorders, peripheral vascular disease, dementia, cerebrovascular disease, rheumatologic disease, osteoarthritis, osteoporosis, peptic ulcer disease/esophageal reflux, liver disease, hepatitis C, diabetes, hemiplegia/paraplegia, kidney disease, non-AIDS defining cancer (cancers other than Kaposi's sarcoma, cervical cancer, and non-Hodgkin's lymphoma), benign prostate hyperplasia, anemia, unspecified acquired hypothyroidism, glaucoma, cataract, retinal disorders (including nondiabetic retinopathy, and retinal or macular degeneration), anxiety, depression, schizophrenia, bipolar disorder, substance abuse, and tobacco use disorder. This list was based on the conditions included in the Deyo Charlson Comorbidity Index,20 which is a claims-based measure of comorbidity score, the most common diagnoses in both PLWH and HIV-negative individuals in this dataset, and conditions included in other analyses of comorbidities.4,5,7,21–23 For each individual, the number of aforementioned non-HIV conditions present was counted (minimum of 0 and maximum of 32) as was the number of daily non-ART medications. AIDS-defining cancer did not contribute to the number of non-HIV conditions. To calculate the number of daily medications, the days' supplies of all non-ART outpatient pharmacy claims during the evaluation period were summed and the sum was divided by 366 days, in a method similar to the calculation for medication possession ratio.24
In a sensitivity analysis, the following conditions were not included when summing the number of non-HIV conditions because of their potential to be adverse events of ART25: hypertension, osteoporosis, ischemic heart disease, dyslipidemia, liver disease, depression, kidney disease, and anemia.
Covariates
The following demographic characteristics were based on enrollment information: age, United States Census Bureau region,26 and urbanicity based on residence in a Metropolitan Statistical Area.27 These variables were included as covariates because they may be risk factors for certain health conditions or affect risk factors.
Analyses
Demographic characteristics were stratified by HIV status and sex and compared using t-tests and chi-squared tests. The most common diagnoses and medication therapeutic classes were ranked. Among conditions that were present in ≥15% of PLWH or HIV-negative individuals, the rank of each condition was compared between PWLH and HIV-negative individuals. The same was done for HIV+ men and women with conditions present in ≥15% of either group. The rankings were descriptive in nature and unadjusted; therefore, no statistical comparisons were made. Ranks were used as a way of describing the most common conditions/medication classes in each group and comparing them between groups, regardless of the prevalence of those conditions/medication classes, because the top conditions could be the same between the two groups even while the proportion of people with each condition differed. Next, the prevalence of each non-HIV condition was compared between PLWH and HIV-negative individuals and also between sexes. To account for differences in age and sex, direct standardization was used to calculate prevalence rates per 100 people with 95% confidence intervals (CIs). Prevalence ratios (PRs) are also presented.
Finally, multivariable models were fit to determine the association between HIV status and the number of non-HIV conditions and daily non-ART medications. The number of non-HIV conditions was modeled using generalized linear models with a log link and negative binomial distribution. The number of daily non-ART medications was modeled in a two-stage process because of the proportion of individuals with 0 medications. The odds of having 0 non-ART medications was modeled using logistic regression models. Among patients who had a non-ART medication, the number of daily non-ART medications was modeled using generalized linear models with log link and gamma distribution. In all models, the primary exposure was HIV status and covariates were age group, region, and urbanicity. Separate models were fit for men and women. p-Values <0.05 were considered statistically significant. However, given the large size of the PLWH cohort in this analysis, the magnitude of the effect estimates should be considered in addition to the p-values.
Analyses were conducted with SAS 9.4 (Cary, NC).
Results
Patient selection and patient characteristics
Overall, 2359 individuals (1909 men and 450 women) met our criteria for being HIV+ and 2,010,513 people (895,123 men and 1,115,390 women) met our criteria for the HIV-negative comparison group (Supplementary Fig. S1). Mean age was significantly lower for PLWH than HIV-negative individuals (71.42 vs. 76.01 years, p < 0.001; Table 1). A significantly larger proportion of PLWH was men (80.92% vs. 44.52%, p < 0.001) and lived in an urban area than HIV-negative individuals (93.22% vs. 84.14%, p < 0.001). Similar trends were noted when comparing HIV+ men to HIV-negative men and HIV+ women to HIV-negative women (Supplementary Table S1). Among PLWH, men were significantly younger than women (p < 0.001) and the proportion of men living with HIV and residing in the South was significantly higher than women with HIV who more commonly lived in the Northeast or West (p < 0.001; Table 1).
Table 1.
Demographic Characteristics of People Living with HIV and HIV-Negative Individuals
| PLWH | HIV-negative individuals | PLWH: men | PLWH: women | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| N = 2359 | N = 2,010,513 | N = 1909 | N = 450 | |||||||
| N/mean | %/SD | N/mean | %/SD | p-Value | N/mean | %/SD | N/mean | %/SD | p-Value | |
| Age (mean, SD) | 71.42 | 5.19 | 76.01 | 7.56 | <0.001 | 71.26 | 4.91 | 72.12 | 6.20 | 0.001 |
| Age group (N, %) | ||||||||||
| 65–69 | 1084 | 45.95 | 498,688 | 24.80 | <0.001 | 884 | 46.31 | 200 | 44.44 | <0.001 |
| 70–74 | 770 | 32.64 | 501,139 | 24.93 | 628 | 32.90 | 142 | 31.56 | ||
| 75–79 | 301 | 12.76 | 383,888 | 19.09 | 253 | 13.25 | 48 | 10.67 | ||
| 80–84 | 122 | 5.17 | 306,351 | 15.24 | 92 | 4.82 | 30 | 6.67 | ||
| 85+ | 82 | 3.48 | 320,447 | 15.94 | 52 | 2.72 | 30 | 6.67 | ||
| Men (N, %) | 1909 | 80.92 | 895,123 | 44.52 | <0.001 | |||||
| Region (N, %) | ||||||||||
| Northeast | 653 | 27.68 | 460,866 | 22.92 | <0.001 | 503 | 26.35 | 150 | 33.33 | <0.001 |
| North Central | 350 | 14.84 | 640,903 | 31.88 | 287 | 15.03 | 63 | 14.00 | ||
| West | 720 | 30.52 | 598,610 | 29.77 | 543 | 28.44 | 177 | 39.33 | ||
| South | 615 | 26.07 | 300,761 | 14.96 | 560 | 29.33 | 55 | 12.22 | ||
| Unknown | 21 | 0.89 | 9373 | 0.47 | 16 | 0.84 | 5 | 1.11 | ||
| Urbanicity (N, %) | ||||||||||
| Urban | 2199 | 93.22 | 1,691,591 | 84.14 | <0.001 | 1793 | 93.92 | 406 | 90.22 | 0.019 |
| Rural | 143 | 6.06 | 310,512 | 15.44 | 104 | 5.45 | 39 | 8.67 | ||
| Unknown | 17 | 0.72 | 8410 | 0.42 | 12 | 0.63 | 5 | 1.11 | ||
PLWH, people living with HIV; SD, standard deviation.
Most common diagnoses and medications
The diagnoses ranked first, second, and third among both PLWH and HIV-negative individuals were essential hypertension (ICD-9-CM 401; PLWH = 47.9% vs. HIV-negative = 59.4%), disorders of lipid metabolism (ICD-9-CM 272; 35.7% vs. 46.9%), and symptoms involving respiratory system and other chest symptoms (ICD-9-CM 786; 31.3% vs. 26.3%; Table 2). Differences in rank were noted for diagnoses of symptoms involving the urinary or digestive symptoms (ICD-9-CM 788 and 787), which were more highly ranked and more common among PLWH (ranked 15 and 16th most common among PLWH vs. 27th and 28th among HIV-negative individuals). Other retinal disorders (ICD-9-CM 362) and osteoarthritis and allied disorders (ICD-9-CM 715) were ranked higher among HIV-negative individuals (ranked 15th and 11th most common among HIV-negative individuals vs. 28th and 29th among PLWH). For both PLWH and HIV-negative individuals, therapeutic medication classes related to heart conditions were commonly used (ranks first, second, fourth, and fifth most common among PLWH vs. ranks first through fourth among HIV-negative individuals). Non-HIV antiviral medications, including those used for influenza, viral hepatitis, cytomegalovirus, and herpes, were more common among PLWH than among HIV-negative individuals (ranked 11th most common among PLWH vs. outside of the top 20 conditions for HIV-negative individuals). Thyroid medications were ranked higher among HIV-negative individuals than PLWH (ranked 10th most common among HIV-negative individuals vs. 31st among PLWH).
Table 2.
Rank and Prevalence of Three-Digit Diagnoses and Therapeutic Classes Present in ≥15% of People Living with HIV or HIV-Negative Individuals
| PLWH | HIV-negative individuals | |||||
|---|---|---|---|---|---|---|
| Rank | N | % | Rank | N | % | |
| Most common three-digit ICD-9-CM diagnosis codes (N, %) | ||||||
| 401 Essential hypertension | 1 | 1129 | 47.9 | 1 | 1,195,074 | 59.4 |
| 272 Disorders of lipid metabolism | 2 | 842 | 35.7 | 2 | 942,840 | 46.9 |
| 786 Symptoms involving respiratory system and other chest symptoms | 3 | 738 | 31.3 | 3 | 529,172 | 26.3 |
| 780 General symptoms | 4 | 711 | 30.1 | 5 | 504,101 | 25.1 |
| 250 Diabetes mellitus | 5 | 611 | 25.9 | 6 | 484,451 | 24.1 |
| 366 Cataract | 6 | 523 | 22.2 | 4 | 505,396 | 25.1 |
| 729 Other disorders of soft tissues | 7 | 496 | 21.0 | 10 | 392,912 | 19.5 |
| 414 Other forms of chronic ischemic heart disease | 8 | 493 | 20.9 | 9 | 393,455 | 19.6 |
| 719 Other and unspecified disorder of joint | 9 | 479 | 20.3 | 7 | 430,153 | 21.4 |
| 427 Cardiac dysrhythmias | 10 | 433 | 18.4 | 13 | 363,473 | 18.1 |
| V04 Need for prophylactic vaccination and inoculation against certain viral diseases | 11 | 432 | 18.3 | 12 | 377,809 | 18.8 |
| 702 Other dermatoses | 12 | 423 | 17.9 | 8 | 398,251 | 19.8 |
| V58 Other and unspecified aftercare | 13 | 389 | 16.5 | 21 | 259,277 | 12.9 |
| 724 Other and unspecified disorders of back | 14 | 370 | 15.7 | 14 | 346,474 | 17.2 |
| 788 Symptoms involving urinary system | 15 | 366 | 15.5 | 27 | 224,619 | 11.2 |
| 787 Symptoms involving digestive system | 16 | 364 | 15.4 | 28 | 221,690 | 11.0 |
| 362 Other retinal disorders | 28 | 281 | 11.9 | 15 | 309,609 | 15.4 |
| 715 Osteoarthritis and allied disorders | 29 | 279 | 11.8 | 11 | 386,352 | 19.2 |
| Most common medication therapeutic classes (N, %) | ||||||
| Antihyperlipidemic drugs: not elsewhere classified | 1 | 1309 | 55.5 | 1 | 1,055,239 | 52.5 |
| Cardiac: beta blockers | 2 | 768 | 32.6 | 2 | 698,536 | 34.7 |
| Analgesics/antipyretics: opiate agonists | 3 | 745 | 31.6 | 5 | 494,203 | 24.6 |
| Cardiac: ACE inhibitors | 4 | 696 | 29.5 | 3 | 525,286 | 26.1 |
| Cardiac: calcium channel blockers | 5 | 576 | 24.4 | 4 | 505,870 | 25.2 |
| Psychotherapeutic agents: antidepressants | 6 | 575 | 24.4 | 8 | 404,924 | 20.1 |
| Gastrointestinal drugs miscellaneous: not elsewhere classified | 7 | 559 | 23.7 | 6 | 456,400 | 22.7 |
| Unclassified agents, not elsewhere classifieda | 8 | 481 | 20.4 | 17 | 284,251 | 14.1 |
| Quinolones: not elsewhere classified | 9 | 468 | 19.8 | 13 | 342,238 | 17.0 |
| Antibiotics: penicillins | 10 | 437 | 18.5 | 11 | 352,024 | 17.5 |
| Antivirals: not elsewhere classifieda | 11 | 437 | 18.5 | >23 | 82,416 | 4.1 |
| Anxiolytics, sedatives, and hypnotics: benzodiazepines | 12 | 413 | 17.5 | 19 | 253,656 | 12.6 |
| Analgesics/antipyretics: nonsteroid/anti-inflammatory | 13 | 408 | 17.3 | 12 | 347,236 | 17.3 |
| Adrenals and combinations: not elsewhere classifieda | 14 | 402 | 17.0 | 9 | 376,190 | 18.7 |
| Antibiotics: erythromycin and macrolide | 15 | 392 | 16.6 | 15 | 291,700 | 14.5 |
| Cardiac drugs: not elsewhere classified | 16 | 387 | 16.4 | 7 | 409,221 | 20.4 |
| Anti-inflammatory skin/mucous membrane agents and combinations: miscellaneous | 17 | 369 | 15.6 | 18 | 269,633 | 13.4 |
| Anti-inflammatory agents eye, ear, nose, and throat: not elsewhere classified | 18 | 364 | 15.4 | 14 | 325,192 | 16.2 |
| Thyroid/antithyroid, thyroid hormones | 31 | 223 | 9.5 | 10 | 371,322 | 18.5 |
Unclassified agents includes medications for benign prostate hyperplasia. Antivirals include medications for hepatitis, influenza, cytomegalovirus, and herpes. Adrenals include corticosteroids.
ICD-9-CM, International Classification of Diseases, Ninth Edition, Clinical Modification.
ACE, angiotensin-converting enzyme.
Diagnoses of other forms of chronic ischemic heart disease (ICD-9-CM 414, such as coronary atherosclerosis and aneurysm of heart or coronary vessels), of other dermatoses (ICD-9-CM 702, such as actinic or seborrheic keratosis), and of symptoms involving the urinary system (ICD-9-CM 788) were ranked higher and were more common among men living with HIV compared to women living with HIV, whereas special screening for malignant neoplasms (ICD-9-CM V76), other and unspecified anemias (ICD-9-CM 285), and osteoarthritis and allied disorders were ranked higher among women with HIV (Supplementary Table S2). Unclassified agents (which include medications for benign prostate hyperplasia) and non-HIV antivirals were more highly ranked among men living with HIV than women living with HIV.
Prevalence of non-HIV conditions
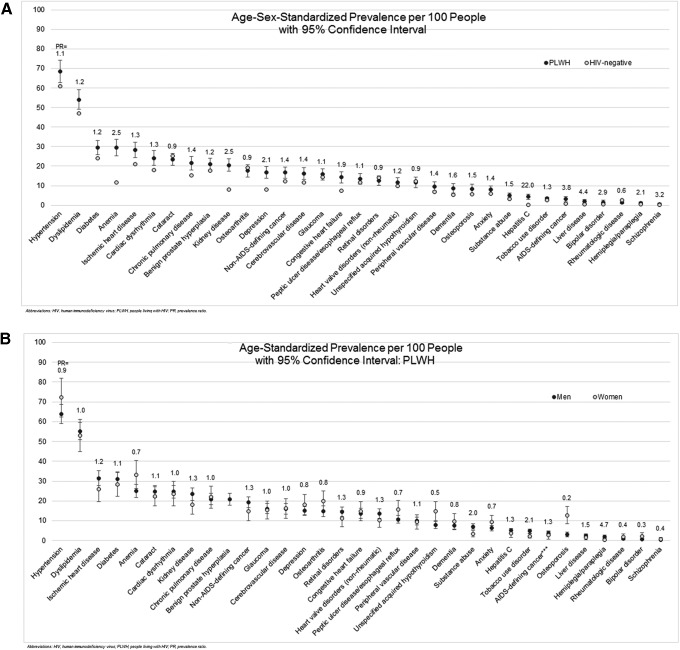
After age-sex standardization, hypertension and dyslipidemia remained the most prevalent non-HIV conditions for both PLWH and HIV-negative individuals (Fig. 1A). However, the rates were higher among PLWH than HIV-negative individuals [hypertension = 68.4 per 100 (95% CI 62.6–74.2) vs. 60.9 (60.8–61.0); dyslipidemia = 54.0 per 100 (95% CI 49.1–59.0) vs. 46.9 (46.8–47.0)]. Most conditions examined were more prevalent among PLWH. The largest absolute differences were seen in anemia [29.6 per 100 (95% CI 25.4–33.8) vs.11.7 (95% CI 11.6–11.7)] and kidney disease [20.5 per 100 (95% CI 17.4–23.7) vs. 8.1 (95% 8.0–8.1)]. The largest relative differences were found among less prevalent conditions: hepatitis C (PRPLWH vs. HIV-negative = 22.0), liver disease (PRPLWH vs. HIV-negative = 4.4), and schizophrenia (PRPLWH vs. HIV-negative = 3.2). AIDS-defining cancer was also more prevalent among PLWH (PRPLWH vs. HIV-negative = 3.8). Among PLWH, large differences in age-standardized prevalence rates were noted in the prevalence of osteoporosis that was more common among women [PLWH men = 3.0 per 100 (95% CI 1.9–4.2) vs. PLWH women = 12.9 (95% CI 8.5–17.2); PRmen vs. women = 0.2] and hemiplegia/paraplegia that was more common among men [PLWH men = 1.9 (95% CI 0.9–2.8) vs. PLWH women = 0.4 (95% CI 0–0.9); PRmen vs. women = 4.7] (Fig. 1B). Comparisons of prevalence rates between HIV+ men and HIV-negative men and HIV+ women and HIV-negative women were similar to the overall PLWH and HIV-negative comparisons (Supplementary Fig. S2A, B).
FIG. 1.
(A, B) Standardized prevalence of non-HIV conditions and AIDS-defining cancer per 100 people with 95% confidence intervals and prevalence ratios for (A) PLWH versus HIV-negative individuals and (B) HIV+ men versus HIV+ women. PLWH, people living with HIV; PR, prevalence ratio.
Multimorbidity and polypharmacy
In unadjusted analyses, the average number of non-HIV conditions and average number of daily non-ART medications were 4.61 conditions (median = 4) and 3.79 medications (median = 3.16) for PLWH compared to 3.94 conditions (median = 4) and 3.41 medications (median = 2.95) among HIV-negative individuals (Supplementary Table S3). While over three-quarters of the PLWH and HIV-negative cohorts had ≥2 non-HIV conditions and more than one-quarter had ≥5 daily non-ART medications, both were more common among PLWH. The number of comorbid conditions was similar between HIV+ men and HIV+ women; however, HIV+ men tended to use more non-ART medications.
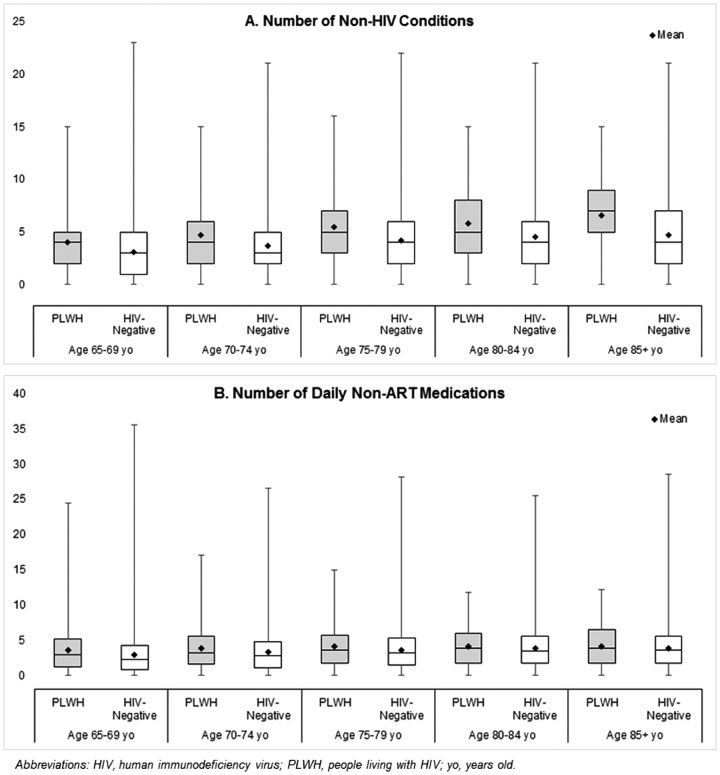
The number of non-HIV conditions and daily non-ART medications increased with age for both PLWH and HIV-negative individuals (Fig. 2A, B). Within each age stratum, the number of non-HIV conditions was higher for PLWH. The mean and median number of conditions for PLWH aged 65–69 was similar to those HIV-negative individuals aged 75–79 [PLWH mean = 4.02 (median = 4) vs. HIV-negative = 4.21 (4)] whereas PLWH 70–74 years old were similar to HIV-negative individuals ≥85 years [PLWH mean = 4.69 (median = 4) vs. HIV-negative = 4.70 (4)]. Trends were similar when evaluating daily non-ART medications with PLWH 65–69 years old resembling HIV-negative individuals 70–74 years old [PLWH mean = 3.58 (median = 2.92) vs. HIV-negative = 3.62 (3.21)] and PLWH 70–74 years old resembling HIV-negative individuals aged 80–84 [PLWH mean = 3.88 (median = 3.18) vs. HIV-negative = 3.83 (3.48)].
FIG. 2.
(A, B) Number of non-HIV conditions (A) and number of daily non-antiretroviral therapy medications per day (B), stratified by HIV status and age. yo, years old.
In adjusted models, men living with HIV had 27% (ratio = 1.272, 95% CI 1.233–1.312) more non-HIV conditions than HIV-negative men and women living with HIV had 33% (ratio = 1.326, 95% CI 1.245–1.413) more non-HIV conditions than HIV-negative women (Table 3). Results were similar when excluding conditions that may be side effects of ART. HIV+ men were 48% (ratio = 0.523, 95% CI 0.427–0.642) less likely to have no non-ART medications than HIV-negative men and among those with non-ART medications, had 18% more daily non-ART medications (ratio = 1.178, 95% CI 1.133–1.226) than HIV-negative men. Similar trends were found when comparing HIV+ women and HIV-negative women; however, the effect estimates were smaller and were not significant or were marginally significant (p = 0.043 in adjusted model). Age was found to be a strong positive predictor of both the number of non-HIV conditions and daily non-ART medications (Supplementary Tables S4A–C).
Table 3.
Modeling Results for Number of Non-HIV Conditions, No Non-ART Medications, and Number of Daily Non-ART Medications Among Those with Non-ART Medications Comparing PLWH and HIV-Negative Individuals, Stratified by Sex
| Model 1: crude | Model 2: adjusteda | |||||||
|---|---|---|---|---|---|---|---|---|
| Men: PLWH vs. HIV-negative Individuals | Women: PLWH vs. HIV-negative Individuals | Men: PLWH vs. HIV-negative Individuals | Women: PLWH vs. HIV-negative Individuals | |||||
| Ratio (95% CI) | p-Value | Ratio (95% CI) | p-Value | Ratio (95% CI) | p-Value | Ratio (95% CI) | p-Value | |
| Ratio for number of non-HIV conditionsb | 1.136 (1.100–1.173) | <0.001 | 1.214 (1.138–1.296) | <0.001 | 1.272 (1.233–1.312) | <0.001 | 1.326 (1.245–1.413) | <0.001 |
| Excluding potential ART side effects | 1.243 (1.199–1.288) | <0.001 | 1.221 (1.134–1.315) | <0.001 | ||||
| Ratio for odds of having no non-ART medicationsb | 0.579 (0.473–0.710) | <0.001 | 0.856 (0.598–1.227) | 0.398 | 0.523 (0.427–0.642) | <0.001 | 0.840 (0.586–1.203) | 0.341 |
| Ratio for number of daily non-ART medications, among individuals with non-ART medication usec | 1.089 (1.046–1.133) | <0.001 | 1.015 (0.936–1.100) | 0.726 | 1.178 (1.133–1.226) | <0.001 | 1.086 (1.003–1.176) | 0.043 |
Adjusted for age group, region, and urbanicity.
Ns for models of number of non-HIV conditions and odds of no non-ART medications were: men with HIV = 1909; HIV-negative men = 895,123; women with HIV = 450; and HIV-negative women = 1,115,390.
Ns for models of number of daily non-ART medications were: men with HIV = 1811; HIV-negative men = 818,618; women with HIV = 418; and HIV-negative women = 1,023,855.
CI, confidence interval; ART, antiretroviral therapy.
Discussion
We found that the most common diagnoses and medications used were similar among elderly PLWH and HIV-negative individuals in the United States. However, many common conditions were more prevalent among PLWH. PLWH tended to have a greater non-HIV disease burden and non-ART medication burden, each similar to that of HIV-negative individuals between 5 and 10 years older. Differences in non-ART medication use were larger when comparing HIV+ men to HIV-negative men, than when comparing HIV+ and HIV-negative women where the differences were only marginally significant.
Other analyses conducted in resource-rich countries and in younger patient populations have also shown that PLWH tend to have more non-HIV health conditions than similarly aged HIV-negative individuals, and that these differences become more pronounced after ages 40–50 years.4–6,28,29 PLWH have been found to be at higher risk for cardiovascular disease,15,30–35 stroke,33,36,37 renal disease,7,30,33,34 malignancies,30,33,38 chronic obstructive pulmonary disorder,39 bone fractures,7,33,34 and diabetes.7,29,34 Older PLWH also use more medications in total and more non-ART medications than older HIV-negative individuals.40–42 Similar to our analysis, a Spanish study of people 50–64 years old found that HIV+ men had more concomitant meds and more long-term polypharmacy than HIV-negative men.43 HIV+ women had similar numbers of concomitant medications but more long-term polypharmacy than HIV-negative women.43 Other comparative analyses conducted in the Netherlands and in Italy have also reported that PLWH resemble HIV-negative individuals 5–10 years older in terms of non-HIV disease and non-ART medication burden.4,7 Interestingly, small epigenetic studies of DNA methylation of PLWH and HIV-negative individuals have reported similar results.44,45
The proportion of PLWH in the United States ≥65 years is projected to increase from 5.4% in 2015 to 26.9% in 2035. In 2035, 44% of PLWH in this age group are projected to have ≥3 comorbid conditions, driven by hypertension, dyslipidemia, diabetes, and cancer.14 Additionally, multimorbidity appears to be increasing among PLWH over time regardless of age.46 Caring for these patients will involve managing multiple non-HIV conditions and multiple non-ART medications, in addition to HIV and ART.47
Multimorbidity and polypharmacy among elderly PLWH are of significant clinical concern. Comorbidities like chronic kidney disease and cardiovascular disease have been associated with increased 5-year mortality48 and a higher number of comorbidities has been associated with lower self-reported quality of life in both HIV+ and HIV-negative individuals.49 In the general population, polypharmacy has been associated with short-term mortality, frailty, disability, and adverse drug events.50–54 Many medications for comorbid conditions, like cardiovascular disease, may interact with certain types of ART55,56 and several studies have found that drug–drug interactions are common among PLWH.12,57–59 Both multimorbidity and polypharmacy have been associated with lower adherence to ART and other medications.60,61 Additionally, PLWH with comorbid conditions may prioritize ART over other medications, such as diabetes medications, potentially leading to worse outcomes for comorbid conditions.62
The potential mechanisms by which HIV infection may result in multimorbidity include chronic inflammation and immune compromise by the virus, side effects of ART that negatively influence ART adherence or that develop into chronic conditions, or the higher prevalence of traditional risk factors for certain chronic conditions among PLWH.8,9 Longer duration of HIV infection has been found to drive comorbidity prevalence independent of age.6 Even PLWH with successfully controlled HIV have higher mortality rates compared with age-matched HIV-controls.63 ART is also associated with adverse events, including decreases in bone density, dyslipidemia, hepatitis/liver disease, and chronic kidney disease;25 however, an increased disease burden was still associated with HIV when these conditions were excluded. Additionally, the increased number of comorbidities reported among PLWH may be due to higher prevalence of disease risk factors, such as smoking and substance abuse.28,39 Another potential factor is low rates of receipt of guideline-recommended care, specifically prescriptions for aspirin and statins for cardiovascular disease, among PLWH.64,65
The strengths of our analysis include examining a large, geographically diverse population of PLWH ≥65 years. Age 65 is the traditional cutoff used to define “older” in general population in the United States and is linked to eligibility for Medicare insurance. We also stratified our results by sex. Many previously published United States-based analyses have utilized data from the Veterans Administration health care system, where >90% of the sample are men. Lastly, we evaluated all diagnoses and medications recorded in the individuals' claims histories over a year-long period and included a wide range of non-HIV conditions, including both physical and mental health conditions.
This study also has limitations. First, claims are generated for billing purposes. There may be coding errors. Patients may receive a misdiagnosis that is recorded in claims and conditions for which they do not seek care will not appear in the database. Health care services covered by other insurance plans, such as Medicaid, or paid for out-of-pocket would also not appear in the database. HIV status was inferred from claims and misclassification may have occurred if patients in the HIV-negative cohort had undiagnosed or untreated HIV. However, the proportion of undiagnosed patients is likely very small relative to our total number of HIV-negative individuals and therefore, is unlikely to have affected our conclusions. Second, race, socioeconomic status, lifestyle behaviors, and HIV-related variables, such as disease duration, viral load, and CD4 counts, are not available in claims data. Because of this limitation, we could not evaluate potential causal relationships between HIV duration, ART use, or risk factors and multimorbidity or polypharmacy. Substance abuse and tobacco use disorder are likely under-coded.
Third, we assumed that patients took their medications as directed (e.g., a 30-day fill was taken for 30 days). The calculation of daily medications did not account for the length of time on the medication. Medications for short-term conditions, such as antibiotics for infection, contributed to the calculation for this analysis, while over-the-counter medications are not included. Fourth, the individuals analyzed had Medicare Supplemental and likely have higher socioeconomic status and more generous medical and pharmacy benefits than other elderly PLWH, limiting generalizability. Despite having more women than other studies, the number of HIV+ women was still small and more research is needed to evaluate multimorbidity and polypharmacy in women. Lastly, PLWH may be in more contact with health care providers than HIV-negative individuals, which may result in more frequent screenings for and earlier diagnosis of non-HIV conditions and more prescriptions.
In conclusion, elderly PLWH experience many of the same non-HIV conditions as elderly HIV-negative individuals; however, PLWH tend to have more non-HIV conditions and use more medications. Understanding the comorbidities facing elderly PLWH is key to the effective co-management of HIV and multiple comorbidities.
Supplementary Material
Author Disclosure Statement
Ms. A.M.K., Dr. A.P., and Dr. E.A.K. have no disclosures to report. Dr. D.N. reports the following grants from the National Institutes of Health: PROMISE—Program Refinements to Optimize Model Impact and Scalability based on Evidence; Costs, HIV Outcomes and Real World Determinants of Success (CHORDS) in HIV Care Coordination; IeDEA Central Africa; All-Africa IeDEA Meeting; Einstein, Rockefeller, CUNY Center for AIDS Research (CFAR); The HIV Center for Clinical and Behavioral Studies; and Together 5000. Dr. K.A. reports the following grants from the National Institutes of Health: Women's Interagency HIV Study (WIHS-V); IeDEA Central Africa; All-Africa IeDEA Meeting; and HIV/HPV Cancer Prevention, Treatment and Pathogenesis: Rwanda/Einstein Consortium.
Supplementary Material
References
- 1. Ward BW, Schiller JS, Goodman RA. Multiple chronic conditions among US adults: A 2012 update. Prev Chronic Dis 2014;11:E62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Gu Q, Dillon CF, Burt VL. Prescription Drug Use Continues to Increase: U.S. Prescription Drug Data From 2007–2008. NCHS Data Brief, No 42. Hyattsville MD: National Center for Health Statistics; 2010. Available at: http://cdc.gov/nchs/data/databriefs/db42pdf (Last accessed January15, 2019) [PubMed] [Google Scholar]
- 3. Antiretroviral Therapy Cohort Collaboration. Survival of HIV-positive patients starting antiretroviral therapy between 1996 and 2013: A collaborative analysis of cohort studies. Lancet HIV 2017;4:e349–e356 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Schouten J, Wit FW, Stolte IG, et al. Cross-sectional comparison of the prevalence of age-associated comorbidities and their risk factors between HIV-infected and uninfected individuals: The AGEhIV cohort study. Clin Infect Dis 2014;59:1787–1797 [DOI] [PubMed] [Google Scholar]
- 5. Rodriquez-Penney AT, Iudicello JE, Riggs PK, et al. Co-morbidities in persons infected with HIV: Increased burden with older age and negative effects on health-related quality of life. AIDS Patient Care STDS 2013;27:5–16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Guaraldi G, Zona S, Brothers TD, et al. Aging with HIV vs. HIV seroconversion at older age: A diverse population with distinct comorbidity profiles. PLoS One 2015;10:e0118531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Guaraldi G, Orlando G, Zona S, et al. Premature age-related comorbidities among HIV-infected persons compared with the general population. Clin Infect Dis 2011;53:1120–1126 [DOI] [PubMed] [Google Scholar]
- 8. Deeks SG. HIV Infection, inflammation, immunosenescene, and aging. Annu Rev Med 2011;62:141–155 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Escota GV, O'Halloran JA, Powderly WG, Presti RM. Understanding mechanisms to promote successful aging in persons living with HIV. Int J Infect Dis 2018;66:56–64 [DOI] [PubMed] [Google Scholar]
- 10. Havlik RJ, Brennan M, Karpiak SE. Comorbidities and depression in older adults with HIV. Sex Health 2011;8:551–559 [DOI] [PubMed] [Google Scholar]
- 11. Onen NF, Overton ET, Seyfried W, et al. Aging and HIV infection: A comparison between older HIV-infected persons and the general population. HIV Clin Trials 2010;11:100–109 [DOI] [PubMed] [Google Scholar]
- 12. Holtzman C, Armon C, Tedaldi E, et al. Polypharmacy and risk of antiretroviral drug interactions among the aging HIV-infected population. J Gen Intern Med 2013;28:1302–1310 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Vance DE, Mugavero M, Williq J, et al. Aging with HIV: A cross-sectional study of comorbidity prevalence and clinical characteristics across decades of life. J Assoc Nurses AIDS Care 2011;22:17–25 [DOI] [PubMed] [Google Scholar]
- 14. Smit M, Cassidy R, Cozzi-Lepri A, et al. Projections of non-communicable disease and health care costs among HIV-positive persons in Italy and the U.S.A.: A modeling study. PLoS One 2017;12:e0186638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Womack JA, Chang CC, So-Armah KA, et al. HIV infection and cardiovascular disease in women. J Am Heart Assoc 2014;3:e001035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Giesbrecht CJ, Thornton AE, Hall-Patch C, et al. Select neurocognitive impairment in HIV-infected women: Associated with HIV viral load, hepatitis C virus, and depression, but not leukocyte telomere length. PLoS One 2014;9:e89556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Valcour V, Maki P, Bacchetti P, et al. Insulin resistance and cognition among HIV-infected and HIV-uninfected adult women: The Women's Interagency HIV study. AIDS Res Hum Retroviruses 2012;28:447–453 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Baranoski AS, Harris A, Michaels D, et al. Relationship between poor physical function, inflammatory markers, and comorbidities in HIV-infected women on antiretroviral therapy. J Womens Health (Larchmt). 2014;23:69–76 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Cohen MH, Hotton AL, Hershow RC, et al. Gender-related risk factors improve mortality predictive ability of VACS Index among HIV-infected women. J Acquir Immune Defic Syndr 2015;70:538–544 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Deyo RA, Cherkin DC, Ciol MA. Adapting a clinical comorbidity index for use with ICD-9-CM administrative databases. J Clin Epidemiol 1992;45:613–619 [DOI] [PubMed] [Google Scholar]
- 21. Steinman MA, Lee SJ, Boscardin WJ, et al. Patterns of multimorbidity in elderly veterans. J Am Geriatr Soc 2012;60:1872–1880 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Lee TA, Shields AE, Vogeli C, et al. Mortality rate in veterans with multiple chronic conditions. J Gen Intern Med 2007;22:403–407 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Cornell JE, Pugh JA, Williams JW, et al. Multimorbidity clusters: Clustering binary data from multimorbidity clusters: Clustering binary data from a large clustering administrative medical database. Appl Multivariate Res 2007;12:163–182 [Google Scholar]
- 24. Leslie RS. Calculating medication Compliance, Adherence, and Persistence in Administrative Pharmacy Claims Databases. 2016. Available at: https://pharmasug.org/download/sde/sd2016/PharmaSUG_SD2016SDE_07_Leslie.pdf (Last accessed January15, 2019)
- 25. U.S. Department of Health and Human Services. Guidelines for the Use of Antiretroviral Agents in Adults and Adolescents Living with HIV: Limitations to Treatment Safety and Efficacy—Adverse Effects of Antiretroviral Agents. 2017. Available at: https://aidsinfo.nih.gov/guidelines/html/1/adult-and-adolescent-arv/31/adverse-effects-of-arv (Last accessed January15, 2019)
- 26. U.S. Department of Commerce, Economics and Statistics Administration U.S. Census Bureau. Census Regions and Divisions of the United States. 2015. Available at: https://www2.census.gov/geo/pdfs/maps-data/maps/reference/us_regdiv.pdf (Last accessed January15, 2019)
- 27. U.S. Department of Commerce, Economics and Statistics Administration U.S. Census Bureau. Metropolitan Areas. In Geographic Areas Reference Manual. 2014. Available at: https://www2.census.gov/geo/pdfs/reference/GARM/Ch13GARM.pdf (Last accessed January15, 2019)
- 28. Goulet JL, Fultz SL, Rimland D, et al. Aging and infectious diseases: Do patterns of comorbidity vary by HIV status, age, and HIV severity? Clin Infect Dis 2007;45:1593–1601 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Petoumenos K, Huang R, Hoy J, et al. Prevalence of self-reported comorbidities in HIV positive and HIV negative men who have sex with men over 55 years-The Australian Positive and Peers Longevity Evaluation Study (APPLES). PLoS One 2017;12:e0184583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Althoff KN, McGinnis KA, Wyatt CM, et al. Comparison of risk and age at diagnosis of myocardial infarction, end-stage renal disease, and non-AIDS-defining cancer in HIV-infected versus uninfected adults. Clin Infect Dis 2015;60:627–638 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Paisible AL, Chang CC, So-Armah KA, et al. HIV infection, cardiovascular disease risk factor profile, and risk for acute myocardial infarction. J Acquir Immune Defic Syndrom 2015;68:209–216 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Freiberg MS, Chang CC, Skanderson M, et al. The risk of incident coronary heart disease among veterans with and without HIV and hepatitis C. Circ Cardiovasc Qual Outcomes 2011;4:425–432 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Rasmussen LD, May MT, Kronborg G, et al. Time trends for risk of severe age-related diseases in individuals with and without HIV infection in Denmark: A nationwide population-based cohort study. Lancet HIV 2015;2:e288–e298 [DOI] [PubMed] [Google Scholar]
- 34. Gallant J, Hsue PY, Shreay S, Meyer N. Comorbidities among US patients with prevalent HIV infection–a trend analysis. J Infect Dis 2017;216:1525–1533 [DOI] [PubMed] [Google Scholar]
- 35. Shah ASV, Stelzle D, Lee KK, et al. Global burden of atherosclerotic cardiovascular disease in people living with HIV. Circulation 2018;138:1100–1112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Sico JJ, Chang CC, So-Armah K, et al. HIV status and the risk of ischemic stroke among men. Neurology 2015;84:1933–1940 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Tripathi A, Liese AD, Winniford MD, et al. Impact of clinical and therapeutic factors on incident cardiovascular and cerebrovascular events in a population-based cohort of HIV-infected and non-HIV-infected adults. Clin Cardiol 2014;37:517–522 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Park LS, Tate JP, Rodriguez-Barradas MC, et al. Cancer incidence in HIV-infected versus uninfected veterans: Comparison of cancer registry and ICD-9 code diagnoses. J AIDS Clin Res 2014;5:1000318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Crothers K, Butt AA, Gibert CL, et al. Increased COPD among HIV-positive compared to HIV-negative veterans. Chest 2006;130:1326–1333 [DOI] [PubMed] [Google Scholar]
- 40. Greene M, Steinman MA, McNicholl IR, Valcour V. Polypharmacy, drug-drug interactions, and potentially inappropriate medications in older adults with human immunodeficiency virus infection. J Am Geriatr Soc 2014;62:447–453 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Moore HN, Mao L, Oramasionwu CU. Factors associated with polypharmacy and the prescription of multiple medications among persons living with HIV (PLWH) compared to non-PLWH. AIDS Care 2015;27:1443–1448 [DOI] [PubMed] [Google Scholar]
- 42. Ware D, Palella FJ, Jr, Chew KW, et al. Prevalence and trends of polypharmacy among HIV-positive and -negative men in the Multicenter AIDS Cohort Study from 2004 to 2016. PLoS One 2018;13:e0203890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Gimeno-Gracia M, Crusells-Canales MJ, Armesto-Gómez FJ, et al. Polypharmacy in older adults with human immunodeficiency virus infection compared with the general population. Clin Interv Aging 2016;11:1149–1157 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Gross AM, Jaeger PA, Kreisberg JF, et al. Methylome-wide analysis of chronic HIV infection reveals five-year increase in biological age and epigenetic targeting of HLA. Mol Cell 2016;62:157–168 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Rickabaugh TM, Baxter RM, Sehl M, et al. Acceleration of age-associated methylation patterns in HIV-1-infected adults. PLoS One 2015;10:e0119201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Guaraldi G, Palella FJ., Jr Clinical implications of aging with HIV infection: Perspectives and the future medical care agenda. AIDS 2017;31 Suppl 2:S129–S135 [DOI] [PubMed] [Google Scholar]
- 47. Wong C, Gange SJ, Moore RD, et al. Multimorbidity among persons living with HIV in the U.S. Clin Infect Dis 2018:66:1230–1238 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Hentzien M, Dramé M, Allavena C, et al. Impact of age-related comorbidities on five-year overall mortality among elderly HIV-infected patients in the late HAART era—role of chronic renal disease. J Nutr Health Aging 2016;20:408–414 [DOI] [PubMed] [Google Scholar]
- 49. Langebeek N, Kooij KW, Wit FW, et al. Impact of co-morbidity and aging on health-related quality of life in HIV-positive and HIV-negative individuals. AIDS 2017;31:1471–1481 [DOI] [PubMed] [Google Scholar]
- 50. Richardson K, Ananou A, Lafortune L, et al. Variation over time in the association between polypharmacy and mortality in the older population. Drugs Aging 2011;28:547–560 [DOI] [PubMed] [Google Scholar]
- 51. Gnijdic D, Hilmer SN, Blyth FM, et al. Polypharmacy cutoff and outcomes: Five or more medicines were used to identify community-dwelling older men at risk of different adverse outcomes. J Clin Epidemiol 2012;65:989–995 [DOI] [PubMed] [Google Scholar]
- 52. Jyrkka J, Enlund H, Korhonen MJ, et al. Polypharmacy status as an indicator of mortality in an elderly population. Drugs Aging 2009;26:1039–1048 [DOI] [PubMed] [Google Scholar]
- 53. Espino DV, Bazaldua OV, Palmer RF, et al. Suboptimal medication use and mortality in an older adult community-based cohort: Results from the Hispanic EPESE Study. J Gerontol A Biol Sci Med Sci 2006;61:170–175 [DOI] [PubMed] [Google Scholar]
- 54. Gandhi TK, Weingart SN, Borus J, et al. Adverse drug events in ambulatory care. N Engl J Med 2003;348;1556–1564 [DOI] [PubMed] [Google Scholar]
- 55. Smith JM, Flexner C. The challenge of polypharmacy in an aging population and implications for future antiretroviral therapy development. AIDS 2017;31:S173–S184 [DOI] [PubMed] [Google Scholar]
- 56. Burgess MJ, Zeuli JD, Kasten MJ. Management of HIV/AIDS in older patients–drug/drug interactions and adherence to antiretroviral therapy. HIV AIDS (Auckl) 2015;7:251–264 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. McNicholl IR, Gandhi M, Hare CB, et al. A pharmacist-led program to evaluate and reduce polypharmacy and potentially inappropriate prescribing in older, HIV-positive patients. Pharmacotherapy 2017;37:1498–1506 [DOI] [PubMed] [Google Scholar]
- 58. Marzolini C, Back D, Weber H, et al. Ageing with HIV: Medication use and risk for potential drug-drug interactions. J Antimicrob Chemother 2011;66:2107–2111 [DOI] [PubMed] [Google Scholar]
- 59. Tseng A, Szadkowski L, Walmsley S, et al. Association of age with polypharmacy and risk of drug interactions with antiretroviral medications in HIV-positive patients. Ann Pharmacother 2013;47:1429–1439 [DOI] [PubMed] [Google Scholar]
- 60. Abara WE, Adekeye OA, Xu J, et al. Correlates of combination antiretroviral adherence among recently diagnosed older HIV-infected adults between 50 and 64 years. AIDS Behav 2016;20:2674–2681 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Krentz HB, Gill MJ. The impact of non-antiretroviral polypharmacy on the continuity of antiretroviral therapy (ART) among HIV patients. AIDS Patient Care STDS 2016;30:11–17 [DOI] [PubMed] [Google Scholar]
- 62. Batchelder AW, Gonzalez JS, Berg KM. Differential medication nonadherence and illness beliefs in co-morbid HIV and type 2 diabetes. J Behav Med 2014;37:266–275 [DOI] [PubMed] [Google Scholar]
- 63. de Coninck Z, Hussain-Alkhateeb L, Bratt G, et al. Non-AIDS mortality is higher among successfully treated people living with HIV compared with matched HIV-negative control persons: A 15-year follow-up cohort study in Sweden. AIDS Patient Care STDS 2018;32:297–305 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Ladapo JA, Richards AK, DeWitt CM, et al. Disparities in the quality of cardiovascular care between HIV-infected versus HIV-uninfected adults in the United States: A cross-sectional study. J Am Heart Assoc 2017;6:pii: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Levy ME, Greenberg AE, Magnus M, et al. Evaluation of statin eligibility, prescribing practices, and therapeutic responses using ATP III, ACC/AHA, and NLA dyslipidemia treatment guidelines in a large urban cohort of HIV-infected outpatients. AIDS Patient Care STDS 2018;32:58–69 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.