Abstract
Sphingosine 1-phosphate (S1P) lyase is an intracellular enzyme that catalyzes the irreversible degradation of S1P and has been suggested as a therapeutic target for the treatment of psoriasis vulgaris. Because S1P induces differentiation of keratinocytes, we examined whether modulation of S1P lyase and altered intracellular S1P levels regulate proliferation and differentiation of human neonatal epidermal keratinocyte (HEKn) cells. To identify the physiological functions of S1P lyase in skin, we inhibited S1P lyase in HEKn cells with an S1P lyase-specific inhibitor (SLI) and with S1P lyase 1 (SGPL1)-specific siRNA (siSGPL1). In HEKn cells, pharmacological treatment with the SLI caused G1 arrest by upregulation of p21 and p27 and induced keratin 1, an early differentiation marker. Similarly, genetic suppression by siSGPL1 arrested the cell cycle at the G1 phase and activated differentiation. In addition, enzyme suppression by siSGPL1 upregulated keratin 1 and differentiation markers including involucrin and loricrin. When hyperproliferation of HEKn cells was induced by interleukin (IL)-17 and IL-22, pharmacologic inhibition of S1P lyase by SLI decreased proliferation and activated differentiation of HEKn cells simultaneously. In addition, SLI administration ameliorated imiquimod-induced psoriatic symptoms including erythema, scaling, and epidermal thickness in vivo. We thus demonstrated that S1P lyase inhibition reduces cell proliferation and induces keratinocyte differentiation, and that inhibition may attenuate psoriasiform changes. Collectively, these findings suggest that S1P lyase is a modulating factor for proliferation and differentiation, and support its potential as a therapeutic target for psoriasis in human keratinocytes.
Keywords: cell cycle, sphingolipid, psoriasis, cell differentiation
Sphingosine 1-phosphate (S1P) is a bioactive sphingolipid metabolite that regulates many aspects of cell growth and differentiation. Sphingosine kinases (Sphks) catalyze the formation of S1P from sphingosine (SO) and ATP (1–4). Because S1P is an important regulator of proliferation and differentiation of human keratinocytes, S1P has been considered as an inducer of differentiation after inhibition of cell proliferation (5–7). As a signaling molecule that initiates differentiation of keratinocytes, S1P binding to the S1P receptor on the plasma membrane leads to calcium (Ca2+) mobilization from internal storage such as the ER. S1P signaling via the G protein-coupled receptor (S1P3) activates the phospholipase C (PLC)-dependent pathway. PLC activation produces inositol triphosphate (IP3) that binds to the inositol trisphosphate receptor (IP3R) on the ER, and releases Ca2+ ions from the ER. Elevated intracellular Ca2+ levels induce differentiation of keratinocytes via the PLC-dependent pathway (8, 9). In addition, intracellular S1P generated by Sphk is released out of the cell via sphingolipid transporter 2 (Spns2), and binds to S1P3 via an autocrine loop (10).
Epidermal differentiation of keratinocytes is initiated by the repetitive mitotic division of keratinocytes followed by exiting the cell cycle (11). Thus, cell cycle arrest is the first step of keratinocyte differentiation. Differentiation of keratinocytes occurs sequentially with early, late, and terminal differentiation stages (12). Deficiency of cell-to-cell contact formation via adherence junctions and desmosomes, which tighten each epithelium, results in the typical morphology of undifferentiated cells (13), and cells that leave the basal layer begin to differentiate gradually and proliferation is halted (14). Calcium is required to initiate differentiation, which is an important regulator of growth and differentiation of keratinocytes. Increased intracellular Ca2+ mediated by S1P upregulates the genes involved in keratinocyte differentiation and induces adherens junctions such as E-cadherin. In contrast, the cell cycle inhibitors, p21 and p27, are activated and cell proliferation is halted. This alteration causes the cells to proceed to the differentiation process (8, 10, 15–18).
Cellular S1P is tightly regulated by Sphk-mediated synthesis and S1P lyase-mediated degradation. S1P lyase is a membrane-bound enzyme, which irreversibly degrades S1P to hexadecanal and phosphoethanolamine, resulting in the reduction of intracellular S1P. Substrates of S1P lyase are phosphorylated forms of SO, dihydro-sphingosine, sphingadiene, and phytosphingosine (4, 19–21). S1P lyase-deficient mice have shown extremely increased S1P levels in circulation and in tissues, together with various phenotypes and increased pro-inflammatory response, but defective neutrophil migration (22). Especially in skin, heterozygous S1P lyase-deficient mice developed mild epidermal hyperplasia with orthokeratotic hyperkeratosis (23). Because S1P is modulated, S1P lyase can be an important regulatory point for proliferation of keratinocytes. However, the role of S1P lyase in cell growth and differentiation of human keratinocytes has not been examined in detail.
In this study, we examined whether differentiation and proliferation of human epidermal keratinocytes are regulated by inhibition of S1P lyase and altered S1P levels. We found that keratinocytes with suppressed S1P lyase had elevated S1P levels and induced G1 growth arrest with the suppression of cyclin D1, cyclin D3, and cyclin-dependent kinase (CDK)2, CDK4, and CDK6. This is mediated mainly by the induction of p21 and p27 and is finally followed by keratinocyte differentiation. Collectively, these findings suggest that S1P lyase could be a modulating point of proliferation in human keratinocytes.
MATERIALS AND METHODS
Cell culture and reagents
Human neonatal epidermal keratinocyte (HEKn) cells were cultured in EpiLife basal medium supplemented with human keratinocyte growth supplement (Gibco) at 37°C with 5% CO2. In order to prevent cells from differentiating spontaneously, cells were cultured not exceeding 80% confluence and the experiments were performed with only second or third passage cells. For induction of keratinocyte differentiation, HEKn cells in growth supplement-free medium were treated with S1P (Avanti Polar Lipids) or S1P lyase-specific inhibitor (SLI), [(R)-6-(4-(4-benzyl-7-chloronaphthalen-1-yl)-2-methylpiperazin-1-yl] nicotine nitrile, synthesized in-house. To induce keratinocyte differentiation by calcium treatment, HEKn cells were incubated for 5 days in medium either with low Ca2+ (60 μM) to normal growth conditions or high Ca2+ (2 mM) concentration to induce keratinocyte differentiation (24).
Psoriatic model in mice
Eight-week-old BALB/c mice (Orient Bio, Sungnam, Korea) were maintained in a specific pathogen-free facility with 12:12 h light/dark cycle and provided with food and water. After 1 week of acclimation, the mice were divided into six groups (n = 5 mice per group): group 1, vehicle cream treated; group 2, 5% imiquimod (IMQ) cream (Aldara cream) treated only (DONG-A ST, Seoul, Korea); group 3, cyclosporin A (CyA; Sigma-Aldrich) injected; group 4, 2 mg/kg of SLI injected; group 5, 5 mg/kg of SLI injected; group 6, 10 mg/kg of SLI injected. Groups 3–6 were co-treated with IMQ at a daily topical dose of 62.5 mg in a cream on the shaved back and the right ear for 8 days. To induce psoriasis-like skin inflammation and skin lesions, IMQ was applied onto the shaved back skin and the right ear of the mice (25). CyA or SLI was administered by subcutaneous injection on the shaved back skin. The control mice were treated only with a vehicle cream (Vaseline Lanette cream; Fargon, The Netherlands). To score the severity of skin lesions, an objective scoring system was applied according to the Psoriasis Area Severity Index (25). Scores of 0 (none), 1 (mild), 2 (moderate), 3 (severe), and 4 (very severe) were given for each of the three symptoms: erythema (redness), scaling, and skin thickness on a scale of 0–4 were checked daily. Scoring of psoriatic effects on the back skin was performed by independent observers blinded to treatment.
Ear thickness of mice was checked using a micrometer (Mitutoyo, Kanagawa, Japan) every 2 days. All experimental procedures were approved by Gachon University IACUC.
Keratinocyte growth rate assay
Cell growth rate was measured using a cell proliferation assay kit (Welgene, Gyeongsan, Korea) using 2,3-Bis(2-methoxy-4-nitro-5-sulfophenyl)-2H-tetrazolium-5-carboxanilide (XTT). To induce hyperproliferation of keratinocytes, cells were treated with 100 ng/ml of IL-17 or IL-22 (R&D Systems), which promote the proliferation of keratinocytes. Prior to stimulation with ILs, cells were starved for 24 h in growth supplement-free medium.
RNA preparation and real-time PCR
Total RNA from cells was extracted using an Easy-Spin total RNA extraction kit (Intron Biotechnology, Sungnam, Korea) in accordance with the manufacturer’s protocol. cDNA was synthesized using the iScript™ cDNA synthesis kit (Bio-Rad). Real-time PCR analysis was performed in StepOnePlus equipment (Applied Biosystems) using 2× SYBR Green Master Mix (Takara, Shiga, Japan). Expression levels of mRNA were measured as a ratio with β-actin, which was the normalization control. Primer sequences used in this study are stated in supplemental Table S1.
Western blot analysis
Cells were lysed in RIPA buffer (Biosesang, Sungnam, Korea) containing protease inhibitor. Proteins were quantified using a BCA protein assay kit (Pierce). Protein lysates were subjected to SDS-PAGE and transferred onto PVDF membrane. The membrane was blocked with 5% skim milk in TBST (TBS with 0.1% Tween 20) for 1 h and probed with the following primary antibodies: alkaline ceramidase 1 (Acer1) (OriGene), Sphk1, S1P lyase, β-actin (Millipore), keratin 1, involucrin, loricrin (Invitrogen), keratin 10 (Abcam), cell cycle regulation antibody sampler, and phospho-protein kinase C (PKC) antibody sampler kit (Cell Signaling Technology). The blots were subsequently incubated with HRP-conjugated secondary antibody for 1 h. Protein signals were detected using SuperSignal West Pico Chemiluminescent substrate (Pierce) and image analysis of Western blots was performed using a Chemiluminescence image analyzer (Vilber Lourmat, Collégien, France). Bands were quantified using the ImageJ program. Western blot analysis for the protein expression levels of control groups were normalized to β-actin along with histograms presenting average densitometric values.
Sphingolipid analysis by LC/MS/MS
For quantitative analysis of sphingoid bases in keratinocytes, cells were harvested and lysed in PBS. C17:0 ceramide as an internal standard was added to each cell extract (1 mg of protein), and sphingolipids were extracted by methanol/CHCl3 (1:2, v/v) including 0.01% butylated hydroxytoluene. To saponify phospholipids, KOH was added and incubated at 37°C for 2 h. Lipid extracts were neutralized by the addition of acetic acid and the organic phase was separated and evaporated under N2. SO and sphinganine (SA) were separated by HPLC with C18 column (XTerra C18; 3.5 μm, 2.1 × 50 mm) and ionized in positive ESI mode as described previously (26). [M+]/product ions from corresponding sphingolipid metabolites were monitored for multiple reaction monitoring quantification by a tandem mass spectrometer, API 4000Q-trap (Applied Biosystems), interfacing with an ESI source.
Cell cycle analysis by flow cytometry
Cells were treated with SLI or transfected with siRNA and were washed with PBS. The cells were then centrifuged and fixed in 70% ethanol in PBS at 4°C. After washing in PBS, the cells were stained with propidium iodide (PI) containing RNase at room temperature for 45 min. Cell cycle distribution of keratinocytes was analyzed using the flow cytometer, Cytomics FC 500 (Beckman Coulter). One hundred thousand events were counted during data collection. The percentage of cells in G1, S, and G2/M phase was determined using CXP analysis software (Beckman Coulter).
siRNA-mediated inhibition of SGPL1 expression
Silencer Select Predesigned siRNA against human S1P lyase 1 (SGPL1) and scrambled siRNA were purchased from Ambion (Life Technologies). Cells were cultured for 24 h before transfection, and when 50% confluence was reached in growth supplement-free medium, cells were transfected with either scrambled siRNA or siRNA against SGPL1, overnight. Lipofectamine 3000 reagent (Invitrogen) was used for all siRNA transfections. Then, cells were harvested to analyze the expression of mRNA and protein.
Statistical analysis
Differences between groups were tested by two-tailed t-test, one-way or two-way ANOVA test within multiple groups, followed Dunnett’s or Tukey’s multi-comparison tests. Statistically significant differences were indicated as *P < 0.05, **P < 0.01, and ***P < 0.001.
RESULTS
Human epidermal keratinocyte differentiation is associated with increased S1P and downregulated S1P lyase
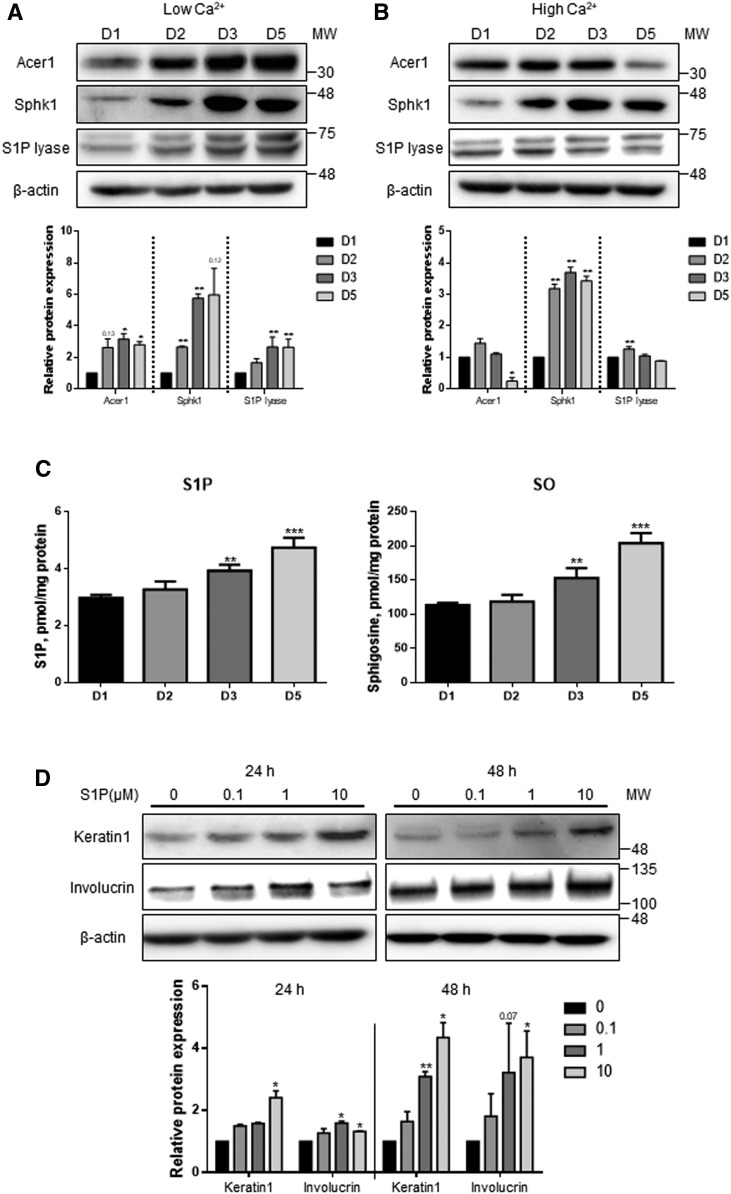
S1P is associated with inhibition of cell proliferation and induction of differentiation in human keratinocytes (5). To evaluate the expression of the genes involved in the sphingolipid biosynthetic pathway in human epidermal keratinocytes, their protein levels were measured during proliferation and differentiation of keratinocytes. Because Ca2+ is known as an important inducer of keratinocyte differentiation, HEKn cells were incubated for 5 days in medium either with low Ca2+ (60 μM) to maintain normal cell growth conditions or high Ca2+ (2 mM) concentrations to induce differentiation (24). To measure protein expression of the enzymes, Western blotting was performed. The protein levels of Acer1 and Sphk1, enzymes that catalyze the production of S1P concomitantly, were increased under low Ca2+ conditions as cells proliferated. The expression of S1P lyase was also increased in a time-dependent manner (Fig. 1A). In contrast, the high Ca2+ condition downregulated Acer1 but upregulated Sphk1. No change was found in S1P lyase (Fig. 1B). To examine whether altered expression of S1P metabolizing enzymes under high Ca2+ conditions changes cellular S1P levels, we measured the S1P levels by LC/MS/MS. The levels of cellular S1P and SO were increased in a time-dependent manner (Fig. 1C). These results support that the high calcium condition induced an increase of S1P level in keratinocytes during differentiation. To examine whether S1P induces differentiation of HEKn cells, cells were treated with extracellular S1P at various concentrations. We found that the protein levels of keratin 1, the marker of early differentiation, and involucrin, the marker of late differentiation, were increased in the cells by extracellular S1P treatment (Fig. 1D). These results suggest that S1P metabolism is directly associated with keratinocyte differentiation.
Fig. 1.
Human epidermal keratinocyte differentiation is associated with increased S1P and downregulated S1P lyase. A, B: HEKn cells were treated with low Ca2+ (60 μM) and high Ca2+ (2 mM). Lysates from the cells at each different day (days 1, 2, 3, and 5) were analyzed by Western blot for Acer1, Sphk1, and S1P lyase [mean ± SEM; *P < 0.05, **P < 0.01 vs. day 1 (D1) treatment; n = 3]. MW, molecular weight. C: HEKn cells were treated with high Ca2+ for 5 days. Analyses of sphingoid base lipids, S1P and SO, were performed by LC/MS/MS (Mean ± SEM; **P < 0.01, ***P < 0.001 vs. day 1 treatment; n = 3). D: Keratin 1 and involucrin, as markers of keratinocyte differentiation, induced by extracellular S1P, were measured by Western blot. All protein expression levels were normalized to β-actin (mean ± SEM; *P < 0.05, **P < 0.01 vs. no treatment with S1P; n = 3). All Western blots shown are representative of three independent experiments.
SLI elevates cellular S1P levels in HEKn cells by inhibiting S1P lyase
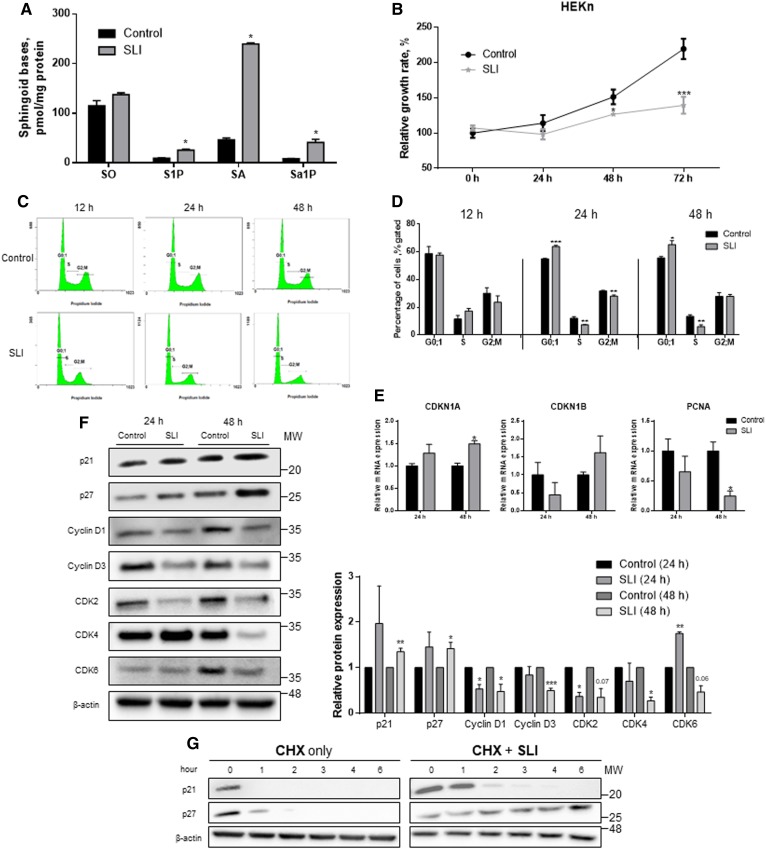
SLI was reported to alleviate the development of multiple sclerosis in experimental autoimmune encephalomyelitis (EAE) mouse models (27). SLI inhibits catalytic activity of S1P lyase by binding to the active site of the enzyme (supplemental Fig. S1A, B). To identify whether SLI alters cellular S1P, sphingoid bases in HEKn cells were measured by LC/MS/MS after treatment with SLI. While the level of S1P was increased by 2.5-fold, the SO level was not changed. In addition, the degrees of alteration in SA and sphinganine 1-phosphate (Sa1P) levels were higher than those of SO or S1P (Fig. 2A). Based on the finding that SLI increased the levels of S1P in the cells, we questioned whether the proliferation of HEKn cells is altered by S1P lyase inhibition. Notably, treatment with SLI reduced the growth rate of HEKn cells in a time-dependent manner (Fig. 2B). This result suggested that inhibition of S1P lyase could be associated with the regulation of cell growth, possibly by cell cycle arrest. To examine whether reduced proliferation by SLI leads to cell cycle arrest, cells were treated with SLI and cell cycle distribution was quantified by flow cytometry at various time points. While the DNA histogram of the control group did not change at each incubation time, SLI-treated cells showed that the proportion of the G0/1 phase increased at 24 h and 48 h. Interestingly, the proportion of S phase was decreased when treated with SLI at 24 and 48 h (Fig. 2C, D). On the other hand, G2 proportion was not changed regardless of SLI treatment except for the 24 h time point that showed a slightly decreased G2 proportion. These changes might be due to the induction of G1 phase growth arrest. To analyze the expression of regulators that are involved in the G1 phase, we measured the mRNA levels of CDK inhibitor (CDKN)1A encoding p21 and proliferating cell nuclear antigen (PCNA), a cell cycle regulator in DNA replication. We found that CDKN1A was upregulated but PCNA was downregulated. In contrast, the expression of CDKN1B encoding p27 was not altered (Fig. 2E). Activation of p21 and p27 signaling is a crucial mechanism to prevent the G1 phase from entering S phase. While SLI elevated the protein levels of p21 and p27, the protein levels of cyclin D1, cyclin D3, CDK2, CDK4, and CDK6 were decreased after 24 or 48 h posttreatment with SLI (Fig. 2F). To confirm that S1P lyase inhibition by SLI is associated with maintaining the effects of p21 and p27 by inhibiting degradation or blocking synthesis, cells were treated with SLI and cycloheximide for various times. In the presence of SLI, p21 and p27 stayed longer than in the presence of only cycloheximide. Therefore, SLI treatment prevented the clearance of both p21 and p27 in HEKn cells (Fig. 2G). These results suggest that pharmacological inhibition of S1P lyase induces G1 arrest via induction and increased half-life of p21 and p27, and contributes to the differentiation of keratinocytes.
Fig. 2.
Accumulation of S1P by SLI not only suppressed cell proliferation but also induced G1 arrest via p21 and p27 induction. A: HEKn cells were treated with SLI or not treated for 48 h. Analyses of sphingoid base lipids, SO, S1P, SA, and Sa1P, were performed by LC/MS/MS (mean ± SEM; *P < 0.05 vs. control group; n = 3). B: The growth rate of HEKn cells was confirmed by XTT assay (mean ± SEM; *P < 0.05, ***P < 0.001 vs. control group of each time; n = 3). C, D: Cell cycle distributions in G0;1, S, and G2/M were analyzed at 12, 24, and 48 h posttreatment with SLI or not treated using PI staining. Curve fitting analysis performed to compare the distribution of the control and treated HEKn cells, these data are representative of three independent experiments. The analysis to monitor cell cycle distribution was normalized to the percentages among G1, S, and G2/M phases (mean ± SEM; *P < 0.05, **P < 0.01, ***P < 0.001 vs. control group of each phase; n = 3). HEKn cells were treated with or without 10 μM of SLI for 24 and 48 h. E: Cells were harvested and mRNA were isolated. Total mRNA was measured by real-time PCR for cell cycle regulator genes. F: Whole cell lysates were harvested to Western blot for cell cycle regulators (n = 3). MW, molecular weight. G: HEKn cells were treated with 10 μg/ml of cycloheximide and SLI for 0–6 h. All protein expression levels were normalized to β-actin. Data are presented as the mean ± SEM; *P < 0.05, **P < 0.01, ***P < 0.001 versus the control group of each time. All Western blot data shown are representative of three independent experiments. CHX, cycloheximide. Q23
SLI upregulates the expression of differentiation marker proteins and alters cell morphology with reduced cell proliferation
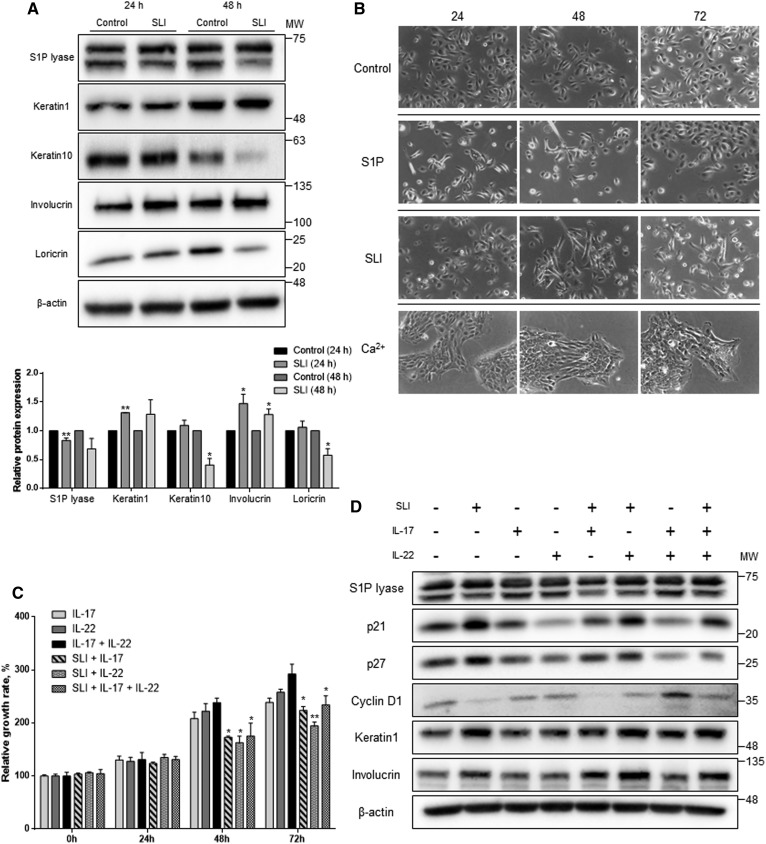
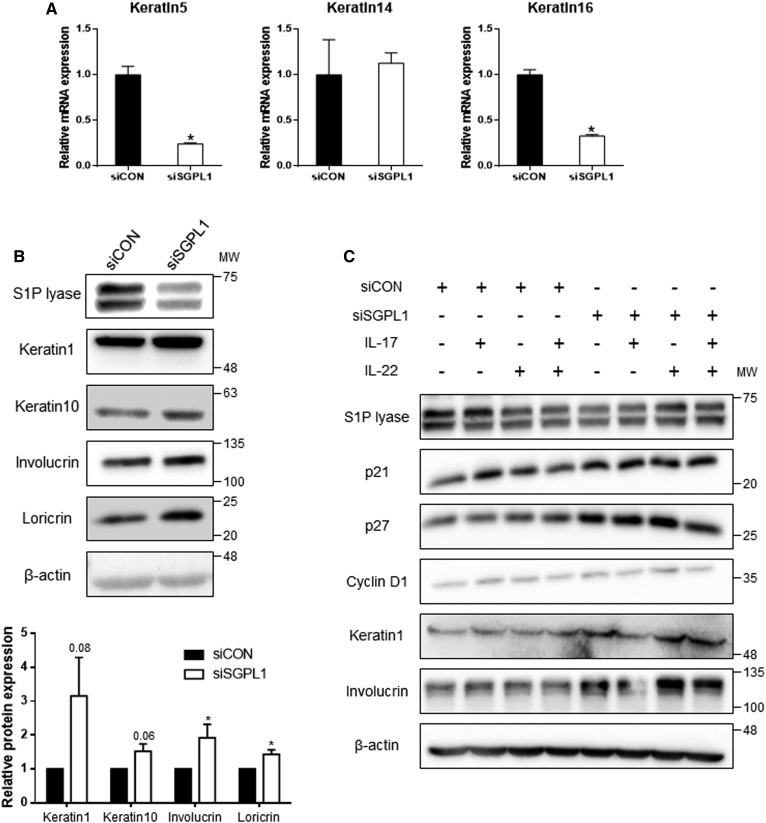
Cell cycle arrest is required to initiate keratinocyte differentiation (28, 29). Because inhibition of S1P lyase by SLI led to G1 arrest via p21 and p27, we investigated to determine whether the growth arrest determines induction of keratinocytes. To verify the changes at the protein level, we measured the protein levels of differentiation markers (Fig. 3A). The protein levels of keratin 1 and involucrin increased, although the level of keratin 10, an early differentiation marker of keratinocytes, was reduced. These results demonstrated that not all differentiation marker proteins were increased by altered S1P. However, the protein level of loricrin, a terminal differentiation marker, was decreased only at the 48 h time point. Taken together, S1P lyase inhibition by SLI caused activation of early and late keratinocyte differentiation. Next, we found that the cell morphology was altered when cells were treated with SLI. To compare with fully differentiated HEKn cells, the cells were treated with SLI, S1P, and Ca2+ and cell morphology was observed (Fig. 3B). While cells incubated in a medium without any treatment showed proliferative cell morphology, cells treated with either S1P or SLI showed an extended cell shape and did not indicate proliferation. However, S1P-treated keratinocytes were restored with morphology like proliferative cells after 72 h of incubation, whereas cells incubated with SLI were differentiated at 48 h. These data indicate that SLI induced morphological changes similar to S1P-treated cells, but not to the levels of fully differentiated cells as observed by Ca2+ treatment. We also measured the cell proliferation rate after treatment with IL-17 and IL-22 to induce hyperproliferation and mimic the pathological condition of psoriasis. Under this condition, we examined whether inhibition of S1P lyase reduces the growth rate of keratinocytes and induces differentiation. Compared with cells of the control group, the sustained treatment with IL-17 or IL-22 promoted proliferation (supplemental Fig. S2). Interestingly, co-treatment with both SLI and ILs reduced the growth rate when compared with the cells treated only with each cytokine (Fig. 3C). In addition, p21 and p27 were equally increased in HEKn cells treated with both SLI and ILs when compared with the cells with no SLI treatment. Reduction of cyclin D1 expression was found in SLI-treated cells in the presence of ILs. Both keratin 1 and involucrin were increased when cells were treated with SLI regardless of the presence of ILs (Fig. 3D). These results indicate that proliferation of keratinocytes induced by ILs is suppressed, and differentiation is induced by S1P lyase inhibition.
Fig. 3.
SLI induces keratinocyte differentiation as well as reduces the proliferation in the hyperproliferative condition. A: Whole cell protein lysates were harvested to Western blot for keratinocyte differentiation markers including keratin 1, keratin 10, involucrin, and loricrin. All protein expression levels were normalized to β-actin (mean ± SEM; *P < 0.05, **P < 0.01 vs. the control group of each time; n = 3). MW, molecular weight. B: HEKn cells were cultured in medium containing 10 μM of S1P, 10 μM of SLI, 2 mM of Ca2+, or not treated for 24, 48, and 72 h. These data are representative of three independent experiments. C: Cells treated with 100 ng/ml of IL-17 and IL-22 and SLI for 24, 48, and 72 h. The growth rate of HEKn cells was confirmed by XTT assay. Data are presented as the mean ± SEM; *P < 0.05, **P < 0.01 versus the groups not treated with SLI of each time; n = 3. D: Total protein lysates after treatment with SLI or ILs in HEKn cells were analyzed for protein levels. Cell lysates were Western blotted to assess differentiation markers and cell cycle regulators (n = 2).
S1P lyase suppression by siRNA induces G1 arrest and keratinocyte differentiation
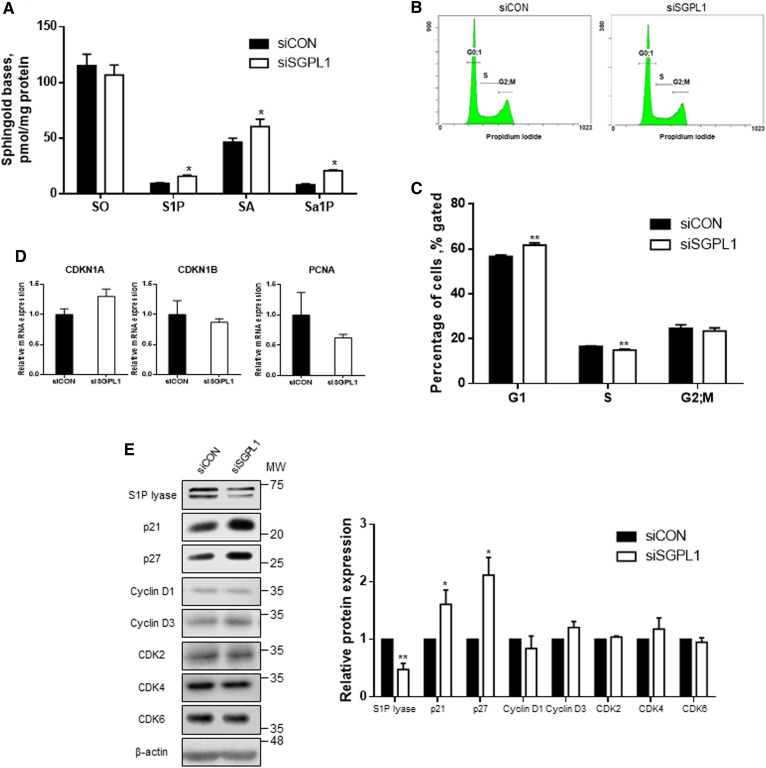
Inhibition of S1P lyase by SLI induced the differentiation of keratinocytes. To verify whether induction of differentiation in human keratinocytes by S1P lyase suppression is not compound-specific, we genetically suppressed S1P lyase expression by SGPL1-specific siRNA (siSGPL1). siSGPL1 suppressed the expression of SGPL1 at both mRNA and protein levels (supplemental Fig. S3A, B). To examine whether genetic suppression of SGPL1 affects the sphingolipid profile, we measured cellular levels of sphingoid bases in siSGPL1-treated HEKn cells by LC/MS/MS (Fig. 4A). Similar to the results observed in the pharmacological study, which showed that SLI treatment did not alter SO levels, in siSGPL1-transfected cells, S1P, SA, and Sa1P levels were elevated when compared with those of siCON controls. We investigated whether S1P lyase suppression induces growth arrest by induction of p21 or p27 as observed in SLI-treated cells. Cells transfected with siRNA were analyzed for cell cycle distributions using flow cytometry (Fig. 4B, C). Compared with siCON, the siSGPL1-transfected cells in the G0/1 phase were increased to 61.73 ± 0.96%, and cells in the S phase were reduced to 14.98 ± 0.38%. In contrast, the percentage of cells in the G2 phase was not different between the transfected groups of cells. Additionally, the expression of cell cycle regulators was measured in HEKn cells to verify the effect of S1P lyase suppression-induced G1 growth arrest on keratinocytes. The mRNA levels of the cell cycle regulators did not change (Fig. 4D). Although the transcriptional levels of these genes were comparable to those of the control groups, the protein levels of these regulators were altered (Fig. 4E). The protein levels of p21 and p27 were both induced. However, no significant change was found in the levels of other regulators, including cyclin D1, cyclin D3, CDK2, and CDK6. These findings suggest that S1P lyase suppression by siRNA induced G1 growth arrest via p21 and p27 induction and increased cellular S1P levels.
Fig. 4.
siSGPL1 induces G1 arrest via p21 and p27 by increase of S1P in HEKn cells. A: siRNA oligomers were transfected to HEKn cells. After 48 h posttransfection, cells were harvested to measure lipid accumulation of SO, S1P, SA, and Sa1P by LC/MS/MS (mean ± SEM; *P < 0.05 vs. the control group; n = 3). B, C: After siRNA transfection for 48 h, cell cycle distributions in G0;1, S, and G2/M were analyzed using PI staining. Curve fitting analysis was performed to compare the distribution of the control and SGPL1-transfected HEKn cells; these data are representative of three independent experiments. The analysis to monitor cell cycle distribution is normalized to the percentages of G1, S, and G2 phases (mean ± SEM; *P < 0.05, **P < 0.01 vs. the control of each phase; n = 3). D: Total mRNA was measured by real-time PCR for cell cycle regulator genes. The amount of mRNA was normalized by β-actin (mean ± SEM; n = 3). E: Cell lysates were Western blotted to assess cell cycle regulators. All protein expression levels were normalized to β-actin (mean ± SEM; *P < 0.05, **P < 0.01 vs. siCON; n = 3). MW, molecular weight. All Western blot data shown are representative of three independent experiments.
We next assessed whether suppression of S1P lyase induces differentiation of HEKn cells. Suppression of S1P lyase markedly downregulated keratin 5 and keratin 16 (Fig. 5A). In contrast, the protein levels of keratin 1, keratin 10, involucrin, and loricrin were elevated at all differentiation phases including early, late, and terminal steps (Fig. 5B). Specifically, unlike in SLI-treated cells in which only keratin 1 and involucrin were increased, keratin 10 and loricrin were both elevated in siSGPL1-treated cells. Compared with the SLI effect, the expression of differentiation markers was enhanced after siRNA transcription. In addition, cell cycle inhibitors and keratinocyte differentiation markers were increased even in the presence of ILs (Fig. 5C). These results demonstrate that SGPL1 suppression in keratinocytes induces growth arrest and cell differentiation despite the hyperproliferative environment induced by ILs.
Fig. 5.
S1P lyase knockdown by siSGPL1 and expression of genes involved in cell cycle progression and keratinocyte differentiation. A: Expression of keratins in HEKn cells. Total mRNA was synthesized cDNA. Real-time PCR was performed with proliferative markers (keratin 5, keratin 14, and keratin 16). The amount of mRNA was normalized by β-actin. Mean ± SEM, *P < 0.05 vs the control, n = 3. B: Western blots for keratinocyte differentiation markers including keratin 1, keratin 10, involucrin, and loricrin were performed on cell protein lysates. All protein expression levels were normalized to β-actin (mean ± SEM; *P < 0.05 vs. siCON; n = 3). MW, molecular weight. Data shown are representative of three independent experiments. C: Total protein lysates after transfection with siRNA or treatment with ILs in HEKn cells were analyzed for protein levels. Cell lysates were Western blotted to assess differentiation markers and cell cycle regulators (n = 2).
S1P lyase inhibition by SLI ameliorates IMQ-induced psoriasis skin lesions in mice
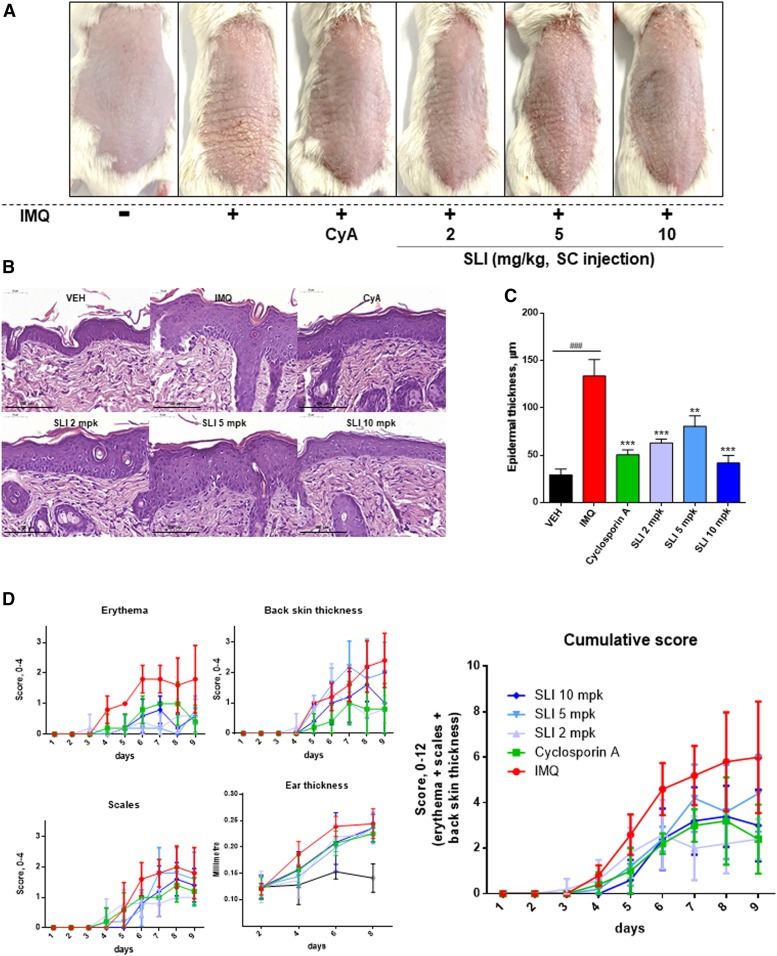
To examine whether SLI could improve psoriasis in vivo, we induced psoriatic effect on BALB/c mice by administering IMQ. Back skin treated with IMQ showed pathological changes on the epidermis. After treatment for 8 days, IMQ treatment induced psoriasis-like symptoms including the development of erythema and scaling (Fig. 6A). We found that CyA, a positive control (30), reduced the degree of erythema and scaling, and SLI alleviated the psoriasis lesion on the back skin as compared with the IMQ-treated controls (Fig. 6A). Histological analysis demonstrated that the epidermis of the skin lesions was thickened by IMQ when compared with the vehicle-treated controls (Fig. 6B). In contrast, CyA and SLI at all doses reduced the epidermal thickness significantly (Fig. 6B, C). Cumulative scores combining the degree of erythema, scaling, and skin thickness according to the Psoriasis Area Severity Index demonstrated that SLI treatment significantly ameliorated pathological conditions of IMQ-induced psoriasis (Fig. 6D, Table 1). However, we could not find the changes in ear thickness among the groups. These results suggest that SLI effectively ameliorates IMQ-induced psoriasis in mice.
Fig. 6.
S1P lyase inhibition ameliorates IMQ-induced psoriasis in mice. BALB/c mice (n = 5) were treated daily with IMQ cream or vehicle cream on the shaved back skin and right ear for 8 days. Mice were administered 2, 5, or 10 mg/kg of SLI or 10 mg/kg of cyclosporin A with IMQ together. A: Phenotypical presentation of mouse back skin after 9 days of treatment. B: H&E staining showing the difference in epidermal thickening of back skin after 9 days of treatment. Scale bar length at the bottom = 100 μm. C: The epidermal thickness of the back skin was measured after H&E staining. ###P < 0.001 compared with the vehicle cream control group. D: The cumulative score was measured and expressed as the sum of erythema, scaling, and back skin thickness in each group. The experiments shown are representative of five experiments. The significance between IMQ only and IMQ with SLI treatment are indicated in Table 1. Data are expressed as the mean ± SD; **P < 0.01 and ***P < 0.001 versus the IMQ only-treated mouse model. VEH, vehicle cream; CyA, cyclosporin A.
TABLE 1.
Scoring of psoriasis severity of the IMQ-treated mice with SLI administration
| Day | IMQ | CyA | SLI 2 | SLI 5 | SLI 10 |
| 5 | 2.6 ± 0.89 | 1 ± 1 | 1.8 ± 1.1 | 1.2 ± 0.84 | 0.6 ± 0.89a |
| 6 | 4.6 ± 1.14 | 2.2 ± 0.45a | 2.6 ± 1.52a | 2.4 ± 0.55a | 2.4 ± 1.34a |
| 7 | 5.2 ± 1.3 | 3 ± 0.71a | 2 ± 1.41b | 4.2 ± 1.48 | 3.2 ± 1.48a |
| 8 | 5.8 ± 2.17 | 3.2 ± 1.92a | 2.2 ± 1.3b | 3.6 ± 2.07a | 3.4 ± 1.34 |
| 9 | 6 ± 2.45 | 2.4 ± 1.52b | 2.4 ± 0.89b | 4.4 ± 1.67 | 3 ± 1.58c |
Data are expressed as the mean ± SD. CyA, cyclosporin A.
P < 0.05 versus IMQ only-treated mouse model, n = 5.
P < 0.0001 versus IMQ only-treated mouse model, n = 5.
P < 0.001 versus IMQ only-treated mouse model, n = 5.
DISCUSSION
S1P has been reported to be involved in diverse cellular functions including cell proliferation, immune suppression, and cardiovascular functions (31). Subsequently, it has recently been stated that S1P lyase that regulates S1P levels by degradation is associated with various S1P-mediated cellular functions (19). S1P lyase can be attributed to regulate intracellular pools of S1P. It terminates sphingolipid metabolism by catalyzing S1P to hexadecanal and phosphoethanolamine. Inhibition of S1P lyase can increase cellular S1P levels in various cell types. The S1P degradation pathway caused by S1P lyase occurs ubiquitously in various cell types. S1P lyase-deficient mice exhibited considerably elevated S1P levels in circulation and liver (21, 32) as well as in the immune system (1, 22). In skin, heterozygous S1P lyase-deficient mice developed acanthosis/orthokeratotic hyperkeratosis because S1P inhibits epidermal acanthosis and regulates nonallergic skin inflammation in a mouse model (23). However, the effects of S1P lyase inhibition, which increases S1P levels, on keratinocyte proliferation and differentiation in animal models have not been studied in detail.
In this study, we demonstrated that: 1) S1P elevation by pharmacologic and genetic inhibition of S1P lyase decreased cell proliferation by arresting the cell cycle at the G1 phase; 2) increased S1P levels induced differentiation of keratinocytes by upregulation of genes involved in differentiation; 3) inhibition of S1P lyase induced differentiation and inhibited growth rate in hyperproliferative keratinocytes; and 4) SLI ameliorated IMQ-induced psoriasis in a mouse model. Our results suggest that S1P lyase could be an important regulation point for keratinocyte differentiation and proliferation in the hyperproliferative skin disease, psoriasis.
A variety of signaling pathways relating to keratinocyte differentiation have been discovered. Differentiation of human epidermal keratinocytes by intracellular Ca2+ forms a physical or chemical barrier on both the outside and the inside of the skin. S1P has been known to regulate keratinocyte proliferation and differentiation as a signaling molecule, inducing Ca2+ release from the ER and Golgi apparatus (31, 33). Although the receptor that binds to S1P directly on the surface of ER has been reported, the exact mechanism of Ca2+ release from the ER by S1P has not been elucidated yet (8, 15, 34, 35).
S1P functions both as an extracellular ligand for G protein-coupled receptors (S1P1–5) and as an intracellular second messenger. Unlike the PLC-dependent pathway via S1P3, S1P causes transient activation of ERK and inactivation of Akt/protein kinase B via S1P2 and inhibits cell proliferation (33). S1P phosphatase, encoded by SGPP1, is another enzyme that regulates cellular S1P by catalyzing the dephosphorylation of S1P to form SO. A recent report demonstrated that keratinocytes isolated from the skin of sgpp1−/− mice had elevated levels of intracellular S1P and increased expression of genes associated with differentiation (36). Additionally, accumulation of S1P by the Sphk1 activator induced differentiation of keratinocytes (37). Thus, modulation of the enzymes metabolizing S1P levels leads to keratinocyte differentiation.
SLI was originally recommended for the treatment of multiple sclerosis to induce peripheral lymphopenia in experimental autoimmune encephalomyelitis (27). Pharmacological inhibition attenuated cell proliferation in HEKn cells. Consistent with the results of reduced growth rate, FACS analysis demonstrated that cell growth is arrested at the G1 phase when treated with SLI or siRNA. G1 arrest was caused by the CDK inhibitors p21 and p27, followed by reduced CDKs and cyclin D expression. These results suggest that S1P elevation by SGPL1 inhibition/suppression inhibits cell proliferation by arresting the cell cycle at the G1 phase, which is a first step in the differentiation of keratinocytes. Moreover, keratinocyte differentiation is associated with PKC levels. PKC activity is dependent on intracellular calcium concentration. PKC expression could be a major regulator determining proliferation and differentiation of keratinocytes. Downregulation of phospho-PKCα mediates growth arrest via induction of p21 (24, 34, 35, 38). Unlike phospho-PKCα, when cells were treated with SLI for 48 h, the expression of other isoforms of the PKC family did not change except for PKCζ/λ (supplemental Fig. S4). The reason for the change in expression of PKCζ/λ in keratinocyte differentiation is not clear and it needs to be studied further. Thus, the role of PKC in p21 upregulation during G1 arrest due to S1P lyase inhibition could be a finding to indicate differentiation in human keratinocytes.
Previously, the findings that growth arrest by upregulation of p21 and p27 induces squamous differentiation were reported (28, 29). Inconsistent with these reports, we found that pharmacological and genetic inhibition of S1P lyase upregulated p21 and p27 and ultimately arrested the cell cycle (Figs. 2, 4). In contrast, there are conflicting results showing that epidermal differentiation does not necessarily have to undergo growth arrest prior to differentiation (39, 40). Even with these conflicting results, both opinions agreed that regulation of the cell cycle is importantly associated with differentiation. This controversial point could be due to the degree or phase of growth arrest, but it is not clear at this moment.
Although our results demonstrated that SLI treatment and siRNA transfection induce an increase in the levels of S1P in HEKn cells, the resulting expression pattern of differentiation markers was different. While SLI only increased the protein levels of keratin 1 and involucrin, which are the markers of early and late differentiation, respectively, suppression of S1P lyase by SGPL1-specific siRNA showed upregulation of keratin 1, keratin 10, involucrin, and loricrin. This inconsistency could be due to higher cellular S1P levels with SLI treatment than those found with SGPL1 suppression. However, the exact reason is not clear at this moment, and we cannot exclude the possibility of the compound-specific effect.
Because cell confluence can initiate the differentiation of keratinocytes, cells could be differentiated even in low calcium concentration, which is a normal growth condition. To exclude this possibility, we grew the cells not to reach confluence until day 5 and tried to distinguish the normal growth condition (low Ca2+) from the differentiating condition (high Ca2+). We found alteration of expression of S1P-metabolizing genes represented by upregulated synthetic Sphk1 and no change in degrading S1P lyase (Fig. 1B). Indeed, altered S1P metabolism led to increased cellular S1P. These results suggest that S1P lyase could be a regulation point for S1P during differentiation.
We examined the effects of S1P on cell proliferation under hyperproliferative conditions, which is one of the characteristics of psoriasis. Lesions on psoriatic skin activate T cells, secreting pro-inflammatory cytokines, IL-17, and IL-22 that have a synergistic effect in the pathogenesis of psoriasis (41–44). We found that the inhibition of S1P lyase induced the expression of p21 and p27, as well as keratin 1 and involucrin, via the induction of p21 and p27, when compared with the hyperproliferative control. In cells co-treated with IL-17 and IL-22, the decrease in p21 and p27 was confirmed with increased expression of cyclin D1 and also the upregulation of differentiation. As a result, inhibition of S1P lyase reduced the growth rate of keratinocytes under hyperproliferative state and possibly activated differentiation. To verify this point, we performed an in vivo study using an IMQ-induced psoriasis mouse model. IMQ induced epidermal expression of IL-23, IL-17A, and IL-17F as well as an increase in splenic Th17 cells (25). In addition, the skin regularly treated with IMQ caused skin lesions showing abnormal keratinocyte proliferation and differentiation similar to psoriasis in humans. Administration of SLI at various doses resulted in ameliorated symptoms of psoriasis, such as erythema, scaling, and epidermal thickness in mouse skin. In contrast, there was no effect on ear thickness by SLI. Because ear thickness is the parameter to indicate the extent of severity of inflammation, SLI has no effect on the anti-inflammatory effect. Previous reports demonstrated that skin irritation and hyperplasia were induced in heterozygous S1P lyase-deficient mice via increased S1P (45). This conflicting result could be due to a systemic S1P increase by heterozygous whole-body SGPL1 deficiency and possible inter-organ interaction. However, the exact mechanism is not clear and deserves further study. Therefore, inhibition of S1P lyase not only arrested the cell cycle but improved the pathophysiological aspects of psoriasis. Whether modulation of S1P lyase is directly involved in psoriasis therapy is not clear at this moment and deserves further study.
Taken together, this study showed that the differentiation of keratinocytes is induced by the inhibition of S1P lyase and elevated cellular S1P. We found that the cell cycle was arrested at the G1 phase and led to the differentiation of keratinocytes. Cytokine-mediated hyperproliferation of keratinocytes was inhibited by S1P lyase inhibition. Consequently, our study suggests that inhibition of S1P lyase could be an important regulator of psoriasis.
Supplementary Material
Footnotes
Abbreviations:
- Acer1
- alkaline ceramidase 1
- CDK
- cyclin-dependent kinase
- CDKN
- cyclin-dependent kinase inhibitor
- CyA
- cyclosporin A
- HEKn
- human neonatal epidermal keratinocyte
- IL
- interleukin
- IMQ
- imiquimod
- PCNA
- proliferating cell nuclear antigen
- PI
- propidium iodide
- PKC
- protein kinase C
- PLC
- phospholipase C
- SA
- sphinganine
- Sa1P
- sphinganine 1-phosphate
- SGPL1
- sphingosine 1-phosphate lyase 1
- siSGPL1
- sphingosine 1-phosphate lyase 1-specifc siRNA
- SLI
- sphingosine 1-phosphate lyase-specific inhibitor
- SO
- sphingosine
- S1P
- sphingosine 1-phosphate
- Sphk
- sphingosine kinase
- XTT
- 2,3-Bis(2-methoxy-4-nitro-5-sulfophenyl)-2H-tetrazolium-5-carboxanilide
This work was supported by the Bio and Medical Technology Development Program through the National Research Foundation of Korea (NRF), funded by the Korean government (MSIP) (NRF-2014M3A9B6069338 and 2018M3A9F3020970). The authors declare that they have no conflicts of interest with the contents of this article.
The online version of this article (available at http://www.jlr.org) contains a supplement.
REFERENCES
- 1.Rivera J., Proia R. L., and Olivera A.. 2008. The alliance of sphingosine-1-phosphate and its receptors in immunity. Nat. Rev. Immunol. 8: 753–763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Fyrst H., and Saba J. D.. 2010. An update on sphingosine-1-phosphate and other sphingolipid mediators. Nat. Chem. Biol. 6: 489–497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hannun Y. A., and Obeid L. M.. 2008. Principles of bioactive lipid signalling: lessons from sphingolipids. Nat. Rev. Mol. Cell Biol. 9: 139–150. [DOI] [PubMed] [Google Scholar]
- 4.Bartke N., and Hannun Y. A.. 2009. Bioactive sphingolipids: metabolism and function. J. Lipid Res. 50 (Suppl.): S91–S96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sauer B., Vogler R., von Wenckstern H., Fujii M., Anzano M. B., Glick A. B., Schafer-Korting M., Roberts A. B., and Kleuser B.. 2004. Involvement of Smad signaling in sphingosine 1-phosphate-mediated biological responses of keratinocytes. J. Biol. Chem. 279: 38471–38479. [DOI] [PubMed] [Google Scholar]
- 6.Feingold K. R. 2009. The outer frontier: the importance of lipid metabolism in the skin. J. Lipid Res. 50(Suppl): S417–S422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Holleran W. M., Takagi Y., and Uchida Y.. 2006. Epidermal sphingolipids: metabolism, function, and roles in skin disorders. FEBS Lett. 580: 5456–5466. [DOI] [PubMed] [Google Scholar]
- 8.Celli A., Mackenzie D. S., Zhai Y., Tu C. L., Bikle D. D., Holleran W. M., Uchida Y., and Mauro T. M.. 2012. SERCA2-controlled Ca(2)+-dependent keratinocyte adhesion and differentiation is mediated via the sphingolipid pathway: a therapeutic target for Darier’s disease. J. Invest. Dermatol. 132: 1188–1195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bikle D. D., Xie Z., and Tu C. L.. 2012. Calcium regulation of keratinocyte differentiation. Expert Rev. Endocrinol. Metab. 7: 461–472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Young K. W., and Nahorski S. R.. 2002. Sphingosine 1-phosphate: a Ca2+ release mediator in the balance. Cell Calcium. 32: 335–341. [DOI] [PubMed] [Google Scholar]
- 11.Missero C., Di Cunto F., Kiyokawa H., Koff A., and Dotto G. P.. 1996. The absence of p21Cip1/WAF1 alters keratinocyte growth and differentiation and promotes ras-tumor progression. Genes Dev. 10: 3065–3075. [DOI] [PubMed] [Google Scholar]
- 12.Zhu S., Oh H. S., Shim M., Sterneck E., Johnson P. F., and Smart R. C.. 1999. C/EBPbeta modulates the early events of keratinocyte differentiation involving growth arrest and keratin 1 and keratin 10 expression. Mol. Cell. Biol. 19: 7181–7190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wise-Draper T. M., Morreale R. J., Morris T. A., Mintz-Cole R. A., Hoskins E. E., Balsitis S. J., Husseinzadeh N., Witte D. P., Wikenheiser-Brokamp K. A., Lambert P. F., et al. 2009. DEK proto-oncogene expression interferes with the normal epithelial differentiation program. Am. J. Pathol. 174: 71–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Blanpain C., and Fuchs E.. 2009. Epidermal homeostasis: a balancing act of stem cells in the skin. Nat. Rev. Mol. Cell Biol. 10: 207–217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tu C. L., Oda Y., Komuves L., and Bikle D. D.. 2004. The role of the calcium-sensing receptor in epidermal differentiation. Cell Calcium. 35: 265–273. [DOI] [PubMed] [Google Scholar]
- 16.Young K. W., and Nahorski S. R.. 2001. Intracellular sphingosine 1-phosphate production: a novel pathway for Ca2+ release. Semin. Cell Dev. Biol. 12: 19–25. [DOI] [PubMed] [Google Scholar]
- 17.Bikle D. D., Oda Y., and Xie Z.. 2004. Calcium and 1,25(OH)2D: interacting drivers of epidermal differentiation. J. Steroid Biochem. Mol. Biol. 89–90: 355–360. [DOI] [PubMed] [Google Scholar]
- 18.Houben E., Holleran W. M., Yaginuma T., Mao C., Obeid L. M., Rogiers V., Takagi Y., Elias P. M., and Uchida Y.. 2006. Differentiation-associated expression of ceramidase isoforms in cultured keratinocytes and epidermis. J. Lipid Res. 47: 1063–1070. [DOI] [PubMed] [Google Scholar]
- 19.Serra M., and Saba J. D.. 2010. Sphingosine 1-phosphate lyase, a key regulator of sphingosine 1-phosphate signaling and function. Adv. Enzyme Regul. 50: 349–362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gault C. R., Obeid L. M., and Hannun Y. A.. 2010. An overview of sphingolipid metabolism: from synthesis to breakdown. Adv. Exp. Med. Biol. 688: 1–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Saddoughi S. A., Song P., and Ogretmen B.. 2008. Roles of bioactive sphingolipids in cancer biology and therapeutics. Subcell. Biochem. 49: 413–440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Allende M. L., Bektas M., Lee B. G., Bonifacino E., Kang J., Tuymetova G., Chen W., Saba J. D., and Proia R. L.. 2011. Sphingosine-1-phosphate lyase deficiency produces a pro-inflammatory response while impairing neutrophil trafficking. J. Biol. Chem. 286: 7348–7358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Schümann J., Grevot A., Ledieu D., Wolf A., Schubart A., Piaia A., Sutter E., Cote S., Beerli C., Pognan F., et al. 2015. Reduced activity of sphingosine-1-phosphate lyase induces podocyte-related glomerular proteinuria, skin irritation, and platelet activation. Toxicol. Pathol. 43: 694–703. [DOI] [PubMed] [Google Scholar]
- 24.Denning M. F., Dlugosz A. A., Williams E. K., Szallasi Z., Blumberg P. M., and Yuspa S. H.. 1995. Specific protein kinase C isozymes mediate the induction of keratinocyte differentiation markers by calcium. J. Cell Growth Differ. 6: 149–157. [PubMed] [Google Scholar]
- 25.van der Fits L., Mourits S., Voerman J. S., Kant M., Boon L., Laman J. D., Cornelissen F., Mus A. M., Florencia E., Prens E. P., et al. 2009. Imiquimod-induced psoriasis-like skin inflammation in mice is mediated via the IL-23/IL-17 axis. J. Immunol. 182: 5836–5845. [DOI] [PubMed] [Google Scholar]
- 26.Yoo H. H., Son J., and Kim D. H.. 2006. Liquid chromatography-tandem mass spectrometric determination of ceramides and related lipid species in cellular extracts. J. Chromatogr. B Analyt. Technol. Biomed. Life Sci. 843: 327–333. [DOI] [PubMed] [Google Scholar]
- 27.Weiler S., Braendlin N., Beerli C., Bergsdorf C., Schubart A., Srinivas H., Oberhauser B., and Billich A.. 2014. Orally active 7-substituted (4-benzylphthalazin-1-yl)-2-methylpiperazin-1-yl]nicotinonitriles as active-site inhibitors of sphingosine 1-phosphate lyase for the treatment of multiple sclerosis. J. Med. Chem. 57: 5074–5084. [DOI] [PubMed] [Google Scholar]
- 28.Harvat B. L., Wang A., Seth P., and Jetten A. M.. 1998. Up-regulation of p27Kip1, p21WAF1/Cip1 and p16Ink4a is associated with, but not sufficient for, induction of squamous differentiation. J. Cell Sci. 111: 1185–1196. [DOI] [PubMed] [Google Scholar]
- 29.Cho Y. S., Bae J. M., Chun Y. S., Chung J. H., Jeon Y. K., Kim I. S., Kim M. S., and Park J. W.. 2008. HIF-1alpha controls keratinocyte proliferation by up-regulating p21(WAF1/Cip1). Biochim. Biophys. Acta. 1783: 323–333. [DOI] [PubMed] [Google Scholar]
- 30.Kovalik M., Thoday K. L., Handel I. G., Bronsvoort B. M., Evans H., van den Broek A. H., and Mellanby R. J.. 2011. Ciclosporin A therapy is associated with disturbances in glucose metabolism in dogs with atopic dermatitis. Vet. Dermatol. 22: 173–180. [DOI] [PubMed] [Google Scholar]
- 31.Spiegel S., and Kolesnick R.. 2002. Sphingosine 1-phosphate as a therapeutic agent. Leukemia. 16: 1596–1602. [DOI] [PubMed] [Google Scholar]
- 32.Bektas M., Allende M. L., Lee B. G., Chen W., Amar M. J., Remaley A. T., Saba J. D., and Proia R. L.. 2010. Sphingosine 1-phosphate lyase deficiency disrupts lipid homeostasis in liver. J. Biol. Chem. 285: 10880–10889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kim D. S., Kim S. Y., Kleuser B., Schafer-Korting M., Kim K. H., and Park K. C.. 2004. Sphingosine-1-phosphate inhibits human keratinocyte proliferation via Akt/protein kinase B inactivation. Cell. Signal. 16: 89–95. [DOI] [PubMed] [Google Scholar]
- 34.Dlugosz A. A., and Yuspa S. H.. 1993. Coordinate changes in gene expression which mark the spinous to granular cell transition in epidermis are regulated by protein kinase C. J. Cell Biol. 120: 217–225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Cabodi S., Calautti E., Talora C., Kuroki T., Stein P. L., and Dotto G. P.. 2000. A PKC-eta/Fyn-dependent pathway leading to keratinocyte growth arrest and differentiation. Mol. Cell. 6: 1121–1129. [DOI] [PubMed] [Google Scholar]
- 36.Allende M. L., Sipe L. M., Tuymetova G., Wilson-Henjum K. L., Chen W., and Proia R. L.. 2013. Sphingosine-1-phosphate phosphatase 1 regulates keratinocyte differentiation and epidermal homeostasis. J. Biol. Chem. 288: 18381–18391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hong J. H., Youm J. K., Kwon M. J., Park B. D., Lee Y. M., Lee S. I., Shin D. M., and Lee S. H.. 2008. K6PC-5, a direct activator of sphingosine kinase 1, promotes epidermal differentiation through intracellular Ca2+ signaling. J. Invest. Dermatol. 128: 2166–2178. [DOI] [PubMed] [Google Scholar]
- 38.Chae M., Jung J. Y., Bae I. H., Kim H. J., Lee T. R., and Shin D. W.. 2016. Lipin-1 expression is critical for keratinocyte differentiation. J. Lipid Res. 57: 563–573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zanet J., Freije A., Ruiz M., Coulon V., Sanz J. R., Chiesa J., and Gandarillas A.. 2010. A mitosis block links active cell cycle with human epidermal differentiation and results in endoreplication. PLoS One. 5: e15701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Gandarillas A. 2012. The mysterious human epidermal cell cycle, or an oncogene-induced differentiation checkpoint. Cell Cycle. 11: 4507–4516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Guilloteau K., Paris I., Pedretti N., Boniface K., Juchaux F., Huguier V., Guillet G., Bernard F. X., Lecron J. C., and Morel F.. 2010. Skin inflammation induced by the synergistic action of IL-17A, IL-22, oncostatin M, IL-1{alpha}, and TNF-{alpha} recapitulates some features of psoriasis. J. Immunol. 184: 5263–5270. [DOI] [PubMed] [Google Scholar]
- 42.Ma W. Y., Jia K., and Zhang Y.. 2016. IL-17 promotes keratinocyte proliferation via the downregulation of C/EBPalpha. Exp. Ther. Med. 11: 631–636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Liang S. C., Tan X. Y., Luxenberg D. P., Karim R., Dunussi-Joannopoulos K., Collins M., and Fouser L. A.. 2006. Interleukin (IL)-22 and IL-17 are coexpressed by Th17 cells and cooperatively enhance expression of antimicrobial peptides. J. Exp. Med. 203: 2271–2279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Bernard F. X., Morel F., Camus M., Pedretti N., Barrault C., Garnier J., and Lecron J. C.. 2012. Keratinocytes under fire of proinflammatory cytokines: bona fide innate immune cells involved in the physiopathology of chronic atopic dermatitis and psoriasis. J. Allergy (Cairo). 2012: 718725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Billich A., Baumruker T., Beerli C., Bigaud M., Bruns C., Calzascia T., Isken A., Kinzel B., Loetscher E., Metzler B., et al. 2013. Partial deficiency of sphingosine-1-phosphate lyase confers protection in experimental autoimmune encephalomyelitis. PLoS One. 8: e59630. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.