Summary
Extracellular bacterial cellulose contributes to biofilm stability and to the integrity of the bacterial cell envelope. In Gram-negative bacteria, cellulose is synthesized and secreted by a multi-component cellulose synthase complex. The BcsA subunit synthesizes cellulose and also transports the polymer across the inner membrane. Translocation across the outer membrane occurs through the BcsC porin, which extends into the periplasm via 19 tetra-tricopeptide repeats (TPR). We present the crystal structure of a truncated BcsC, encompassing the last TPR repeat and the complete outer membrane channel domain, revealing a 16-stranded, β-barrel pore architecture. The pore is blocked by an extracellular gating loop, while the extended C terminus inserts deeply into the channel and positions a conserved Trp residue near its extracellular exit. The channel is lined with hydrophilic and aromatic residues suggesting a mechanism for facilitated cellulose diffusion based on aromatic stacking and hydrogen bonding.
Graphical Abstract
eTOC
Acheson et al. investigated the crystal structure of an outer membrane porin implicated in biofilm cellulose secretion. The structure reveals a large transmembrane channel lined with hydrophilic and hydrophobic residues. The porin closely associates with a periplasmic TPR domain, thereby providing insights into the interaction with inner membrane cellulose synthase components.
Introduction
Biofilms are sessile bacterial communities in which the cells are encased in a 3-dimensional matrix consisting of proteinaceous fibers, polysaccharides, and nucleic acids (McCrate et al., 2013). The biofilm matrix forms a protective structure that significantly reduces the efficacy of antimicrobial agents. Accordingly, biofilms are responsible for the majority of nosocomial infections by, for example, contaminating catheters, implants, or surgical devices (Bryers, 2008; Römling and Balsalobre, 2012; Stewart and Costerton, 2001). Hence, there is considerable interest in elucidating the mechanisms of biofilm biogenesis.
A common biofilm component of Gram-negative Enterobacteriaceae (e.g. Escherichia coli, Salmonella typhimurium, and Klebsiella pneumonia) is phosphoethanolamine-cellulose (pEtN-cellulose) (Thongsomboon et al., 2018), a linear polymer of glucose and glucose-6-phosphoethanolamine units, synthesized by a cellulose synthase complex (Romling and Galperin, 2015). Cellulose is assembled from cytosolic nucleotide-activated (UDP) glucose units and translocated to the cell surface across the inner- and outer-membranes (IM and OM, respectively) (Whitney and Howell, 2013; Zimmer, 2019).
In Enterobacteria, the cellulose synthase complex contains at least three IM subunits. The catalytically active BcsA subunit, a processive family-2 glycosyltransferase (Cantarel et al., 2009), is a membrane-embedded enzyme that synthesizes the cellulose polymer. During synthesis, BcsA translocates cellulose across the IM through a channel formed by its own membrane-spanning domain. Catalysis requires the presence of the BcsB subunit, which is anchored to the inner membrane via a C-terminal TM helix, while its periplasmic part contains two copies of a repeating unit, each containing a carbohydrate-binding and a flavodoxin-like domain (McNamara et al., 2015; Morgan et al., 2013; Morgan et al., 2016). The third subunit associated with the IM is BcsG, a membrane-integrated phosphoethanolamine transferase (Sun et al., 2018; Thongsomboon et al., 2018). This subunit transfers a pEtN group most likely from phosphatidyl-ethanolamine lipids to the C6 position of every second glucose unit of the polymer to produce pEtN-cellulose (Thongsomboon et al., 2018). Recent negative stain electron microscopy studies showed that the E. coli IM-associated cellulose synthase assembles into large complexes containing multiple BcsA-B-G sub-complexes, and possibly two other subunits, BcsF, a small integral membrane protein required for BcsG function, and BcsR, a small cytosolic protein of unknown function (Krasteva et al., 2017).
Finally, nascent pEtN-cellulose must cross the OM to reach its final destination. Exopolysaccharides synthesized by Gram-negative bacteria each have their own dedicated channel: e.g. alginate and poly N-acetylglucosamine (PNAG) are transported by the OM-integrated porins AlgE and PgaA, respectively (Whitney and Howell, 2013). pEtN-cellulose is secreted through the β-barrel porin BcsC (McNamara et al., 2015; Romling and Galperin, 2015). Efficient polymer translocation is contingent upon direct interactions of the OM subunits with the biosynthetic machineries in the IM. To that effect, the OM-channels often contain or associate with tetra-tricopeptide repeats (TPR), which are common protein interaction modules (Lamb, 1995; Zeytuni and Zarivach, 2012). A basic TPR repeat consists of two short antiparallel α-helices, and multiple repeats frequently assemble into solenoid structures serving as docking platforms (Jinek et al., 2004). The BcsC porin is a 1157 residues long protein containing two predicted domains: an N-terminal domain with 19 TPRs, and a C-terminal pore-forming β-barrel made up of ~400 residues. Interestingly, while in vivo BcsC is essential for cellulose secretion, in vitro the heterodimeric BcsA-BcsB complex is fully functional without it (Matthysse et al., 1995; Omadjela et al., 2013).
Here, we present the crystal structure of a truncated BcsC protein, encompassing the 19th TPR repeat (TPR19), a linker region, and the complete β-barrel pore. BcsC forms a large channel blocked by a short extracellular gating loop. A conserved C-terminal oligopeptide inserts deeply into the channel and positions an invariant aromatic residue near the extracellular channel exit, suggesting a role in polysaccharide secretion. Further, the channel contains a number of conserved aromatic and polar residues that likely facilitate pEtN-cellulose diffusion by aromatic stacking and hydrogen bonding.
Results
Purification of recombinant full-length E. coli BcsC identified a proteolytic fragment, residues 710 to 1157, suitable for crystallization. This fragment was cloned with an N-terminal PelB signal sequence, followed by a deca-histidine tag and tobacco etch virus (TEV) cleavage site (Figure 1A). The recombinant protein was purified by metal affinity and gel filtration chromatography and crystallized using sitting drop vapor diffusion (Figure S1A). Experimental phases were determined using a crystal of Se-Met labeled protein by single-wavelength anomalous diffraction (SAD) and a complete model, containing all residues from 710 to 1157, was built semi-automatically and refined using data to a resolution of 1.85 Å (see STAR METHODS and Table S1)
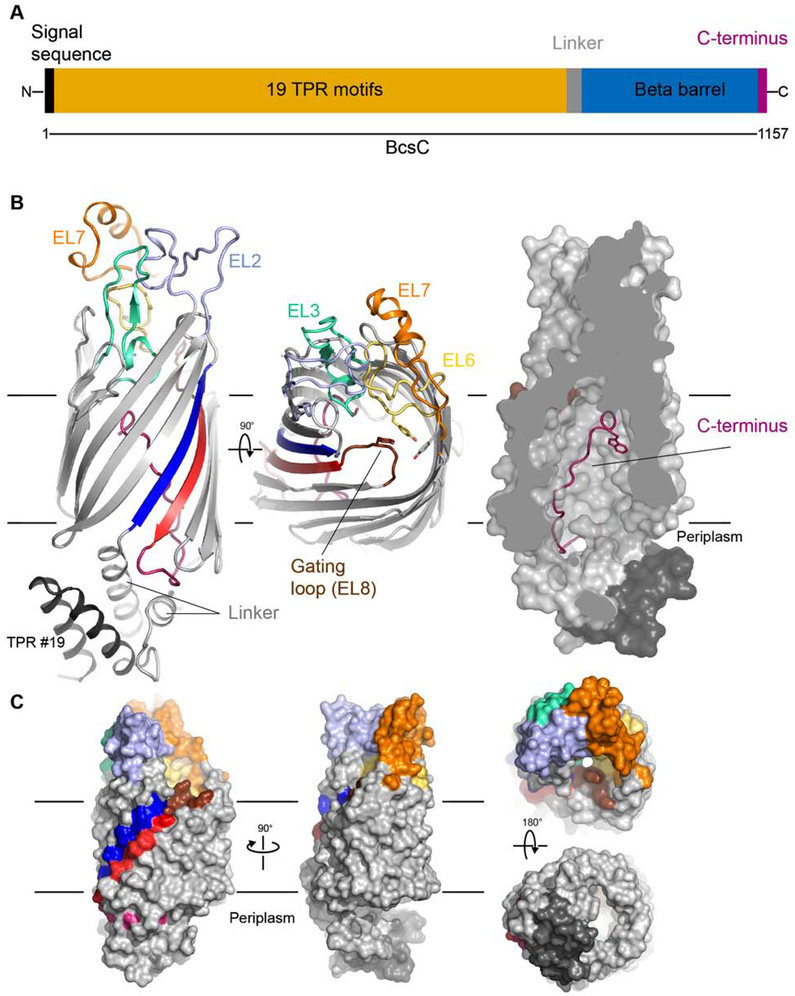
Figure 1. Architecture of the cellulose synthase outer membrane pore.
(A) Illustration of the BcsC cellulose synthase subunit containing an N-terminal signal sequence and C-terminal β-barrel pore.
(B) Structure of the E. coli BcsC2 construct containing the β-barrel porin preceded by the linker region and TPR domain #19. The TPR helices are colored black, the linker region is shown in light gray, and the β-barrel is colored gray with the exception of its N- and C-terminal strands (blue and red, respectively). The main extracellular loops EL2,3,6,7, and 8, are colored and labeled and the C-terminal periplasmic extension is shown in magenta. (See also Figure S2)
(C) Surface representation of BcsC2 colored as in panel B.
The architecture of the BcsC β-barrel porin domain
The membrane-integrated porin domain of BcsC forms a kidney-shaped 16-stranded β-barrel with a curved face formed by β-strands 2–11, and a flat face formed by strand 1 together with strands 12–16 (Figure 1B). Eight extracellular loops (EL1–8) create a dome-shaped structure that covers approximately half of the extracellular channel opening. EL8 between β-strands 15 and 16 (hereafter referred to as ‘gating loop’) interacts with EL6 (between β-strands 11 and 12) and blocks the extracellular exit of the channel.
A number of conserved aromatic residues line the lumen of the BcsC channel (Figure 2A). In particular, Trp962, Tyr988, Trp1008, Tyr1025, Tyr1030, Tyr1124, as well as the C-terminal Trp1157, form multiple potential aromatic docking sites for the translocating glucan, in a manner similar to that described for the IM BcsA channel (Morgan et al., 2013; Morgan et al., 2016). BcsC forms a large pore with ~15 Å diameter within the membrane plane, and an equally wide opening towards the periplasm (Figure 3). Well-resolved water molecules inside the BcsC channel demonstrate that the entire pore volume is accessible to solvent (Figure S3). Thus, pEtN-cellulose probably remains hydrated when inside the channel, as the pore dimensions are too wide to efficiently displace coordinating water molecules. This stands in contrast to translocation through the IM BcsA channel, where water molecules are excluded from the channel to maintain the permeability barrier of the plasma membrane.
Figure 2. Coordination of BcsC’s C-terminus inside the channel and sequence conservation.
(A) Interactions of BcsC’s C-terminus with residues forming the OM channel. Residues belonging to the C terminal extension are colored magenta, other residues are shown as ‘ball-and-sticks’ in gray for carbon atoms. Conserved residues likely to coordinate the translocating PE-cellulose are shown as ‘ball-and-sticks’ in gray and blue for the carbon atoms for polar and aromatic residues, respectively.
(B) Sequence conservation of BcsC based on conservation among PE-cellulose synthesizing cellulose synthases shown from blue (lowest) to red (highest) conservation. Calculated in CONSURF (Ashkenazy et al., 2010).
Figure 3. Properties of the OM channel.
(A) Surface rendering of the transmembrane channel calculated in HOLE. The gating loop is colored red. Green sections of the channel surface represent pore radii between 1.15 and 2.3Å. Right panel: Graph of the pore radii along the channel axis. (See also Figure S2)
(B) Electrostatic potential of BcsC2 calculated in Pymol using the Adaptive Poisson-Boltzmann Solver (APBS) plugin from −5 to 5 kT (red and blue, respectively) (Jurrus et al., 2018). (See also Figure S3)
pEtN-cellulose is a zwitterionic polymer carrying positive and negative charges at its amino and phosphate groups, respectively. Strikingly, the electrostatic potential of the channel interior is highly electronegative, due to numerous acidic residues exposed to the channel lumen (Figure 3B). It is possible that electrostatic interactions facilitate the initial insertion of the polymer into the pore. Similarly, the presence of acidic residues inside the PNAG OM channel (formed by PgaA) has been shown to be crucial for PNAG secretion in vivo (Wang et al., 2016). Here, PNAG is partially deacetylated in the periplasm (and thus positively charged) before PgaA entry (Little et al., 2014) (Figure S4). Accordingly, electrostatic interactions could be a significant driving force for pEtN-cellulose entry into the OM channel.
An intriguing unique feature of the structure is the location of the C-terminal ~15 residues (1142–1157), which are inserted into the lumen of the channel in an extended conformation, and traverse almost the entire length of the pore, reaching the extracellular EL3 loop (Figure 1B). This position of the C-terminal oligopeptide is stabilized by a network of interactions, mostly hydrogen bonds (H-bonds), involving primarily the backbone carbonyl groups of the C-terminus, and a number of lumen-lining amino acids that are conserved among cellulose synthase complexes producing pEtN-cellulose (Figures 2A and S1B). The base of the C-terminal oligopeptide is anchored by two H-bonds involving carbonyls of Asp1143 and Met1144, and Arg780 (linker helix) and Arg1042, respectively (Figure S2). Similarly, the carbonyl groups of Gln1149 and Ile1152 accept H-bonds from Arg1134 and Arg825, respectively (Figure 2). Leu1151 is oriented so that both its carbonyl and amide groups are H-bonded to the side chain carboxyl of Glu789, which must be protonated on Oε2. Finally, two H-bonds involve the side chains of the C-terminal fragment: the hydroxyl group of the conserved Tyr1154 with the carboxylate of Asp881, while the carboxylate of Asp1156 accepts an H-bond from the hydroxyl of Ser920 in the EL3 loop. In addition to this elaborate H-bond network, there is one intimate hydrophobic interaction with the side chain of Leu1151 embedded in a pocket formed by Thr787 and Glu789 from β-strand 1, and Met810 and Gln812 from β-strand 2. These interactions stabilize the conserved C-terminal Trp1157 midway across the channel. At this position, the pore is sufficiently wide to accommodate a glucan chain without displacing the C terminus (Figure 3A), thus Trp1157 likely interacts with the translocating cellulose polymer (Figures 1B and 2).
Putative channel gating mechanism
Most of the conserved β-barrel loops are located on the extracellular side and form a rigid dome-shaped structure (Figure 1). The channel exit is constricted by the inward tilting of the gating loop, which packs against the invariant Tyr1025 and Tyr1030 of the EL6 loop (Figures 1B and 3). In this position, the gating loop essentially prevents permeation of solutes larger than a water molecule. Upon insertion of the pEtN-cellulose, the gating loop likely moves away from the channel axis to allow polymer permeation (Figure 4A). During translocation, the conserved Tyr1124 near the tip of the gating loop together with Tyr1025 in the EL6 loop could coordinate the polymer, thereby facilitating translocation. The sequence conservation of the gating and EL6 loops supports this hypothesis (Figure S1B). A similar gating mechanism has been suggested for BcsA, where loops connecting TM helices 5–6 and 7–8 likely close the channel’s periplasmic exit in the absence of the cellulose polymer (Morgan et al., 2013).
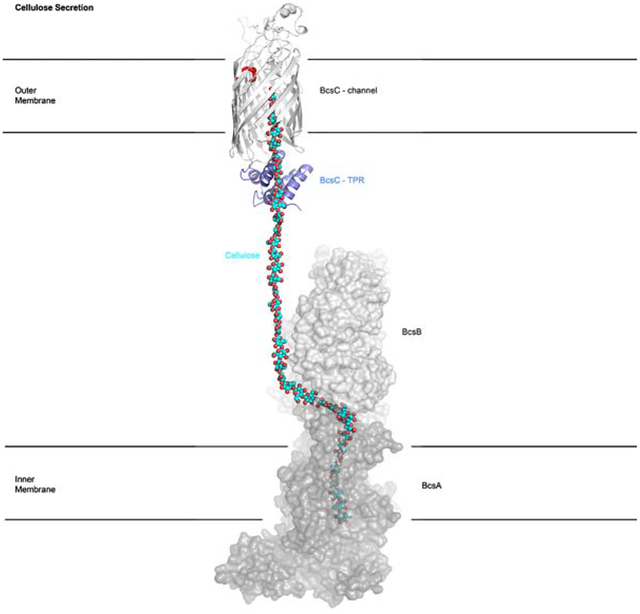
Figure 4. Model of channel opening and association with other synthase subunits.
(A) Model of BcsC’s channel opening during cellulose translocation. The extracellular gating and EL6 loops are shown in red and yellow, respectively. The periplasmic domain of BcsC has been omitted, for clarity.
(B) Bacterial cellulose synthase components span the cell envelope. BcsA, -B, -C, and –Z are conserved in Gram-negative cellulose synthase complexes. BcsG, together with additional subunits, is prevalent among Enterobacteria. PDB coordinates of the depicted structures are: 4P00 (BcsAB), 5OJH (BcsG), 3QXF (BcsZ), and 5XW7 (TRP1–6). IM/OM: Inner and Outer Membrane.
The structure and coordination of the TPR19
The periplasmic domain of BcsC contains nineteen TPRs that are expected to mediate interactions with the IM components (Figure 4A). In bacterial alginate synthase, the BcsC homolog is formed by two proteins, AlgE for the β-barrel (Tan et al., 2014; Whitney et al., 2011) and AlgK for the periplasmic domains (Keiski et al., 2010). While AlgK is not essential for alginate secretion in vivo, in its absence, the insertion of AlgE into the OM is dramatically reduced and bacteria secrete low molecular weight uronic acids, instead of high molecular weight alginate polymers (Keiski et al., 2010). Hence, the periplasmic TPR domains in alginate and cellulose synthase complexes are necessary for proper assembly of a functional synthase. In our crystal structure, the TPR19 (residues 710–744) packs against a short helical linker leading into β-strand 1 of the β-barrel (Figure 1B and S2). This TPR19-linker interaction mimics the canonical solenoid arrangement of TPR repeats, i.e. one helix packs into a groove formed by the α-helices of the preceding TPR motif. A similar packing of TPRs has been observed in several other proteins, including the isolated six N-terminal TPRs of BcsC from Enterobacter sp. CJF-220, a close homolog of E. coli BcsC, as well as O-GlcNAc transferase (Jinek et al., 2004; Nojima et al., 2017).
Discussion
The secretion of pEtN-cellulose by Gram-negative bacteria requires several steps. First, a cellulose polymer is synthesized by the BcsA-BcsB heterodimer in the IM and concomitantly translocated across the IM (Figure 4B). This translocation occurs through a channel-formed by BcsA where the cellulose polymer is coordinated by polar and CH-π stacking interactions, frequently observed in protein-carbohydrate complexes (Kumari et al., 2012; Morgan et al., 2013; Morgan et al., 2016).
Next, BcsG covalently modifies some glucose units with pEtN (Thongsomboon et al., 2018). This reaction must occur near the periplasmic side of the inner membrane, because BcsG is membrane anchored and the reaction likely requires phosphatidylethanolamine lipids serving as substrates (Figure 4B). The attachment of pEtN increases cellulose’s hydrophilicity, although the polymer remains an amphipathic macromolecule prone to aggregation (Hollenbeck et al., 2018). Hence, transport across the periplasm and the OM must minimize nonspecific interactions with the peptidoglycan, as well as other cellulose polymers (Whitney and Howell, 2013). The cellulose synthase macro-complex likely achieves this task by closely associating BcsC with the BcsA-BcsB-BcsG complex. BcsB contains two carbohydrate-binding domains that are positioned directly above BcsA’s periplasmic channel exit and extend ~65 Å into the periplasmic space. Although the binding properties of these domains have not yet been characterized, it is possible that BcsB directs the nascent glucan toward the OM.
A direct interaction of BcsC with BcsB could provide sufficient shielding of the glucan from other periplasmic components, thus preventing aggregation prior to secretion. BcsC extends into the periplasmic space with 19 predicted TPRs, of which the N-terminal six repeats of a close homolog have been characterized structurally (Nojima et al., 2017). Although the arrangement of the TPRs in the cellulose synthase macro-complex could be substantially different, the crystallized repeats span a distance of about 75 Å (Figure 4B). Considering that full-length BcsC includes >3-times the number of TPRs and that BcsB protrudes into the periplasm by about 65 Å (Morgan et al., 2013), hence a direct BcsB-BcsC interaction spanning the periplasm is possible. It is currently unknown whether the TPRs also interact with the translocating pEtN-cellulose polymer, yet the presence of several conserved aromatic residues would allow glucan coordination similar to within the membrane channels.
BcsC’s C-terminal TPR packs against the linker region, which in turn fits into an electropositive pocket formed by the barrel’s periplasmic PL5–7 loops (connecting β-strands 10/11, 12/13 and 14/15, respectively) and C-terminus (Figure S4). Similar pockets exist in PgaA and AlgE, which translocate PNAG and alginate across the OM, respectively. While PgaA contains a linker region connecting 8 periplasmic TPRs with the barrel, AlgE associates with AlgK (Rehman et al., 2013) containing 10 TPRs flanked on both sides by α-helical regions (Keiski et al., 2010), (Figure S4). In these cases, the periplasmic TPRs not only interact with the OM porin but also with polymer modifying periplasmic enzymes (Rehman et al., 2013; Wang et al., 2016). We hypothesize, that the close association of the OM pore with the periplasmic TPRs is a conserved architectural feature among exopolysaccharide synthases. This, in turn, is likely important for efficient polymer translocation across the periplasm and the OM.
Cellulose transport across the IM is induced and energized by substrate binding to BcsA’s active site (Morgan et al., 2016). Biosynthesis is sufficient to translocate the polymer one glucose unit at a time through BcsA’s TM channel and likely also across the periplasm and the OM (Figure 4B). Translocation could further be facilitated by the alignment or aggregation of the secreted glucans on the cell surface, yet this contribution would only exist after a portion of the polymer has been released on the extracellular side. Thus, translocation energy barriers due to non-specific interactions with the glucan must be avoided. It has been shown that the OM maltodextrin channel minimizes such energy barriers by precisely positioning CH-π stacking and hydrophilic interactions with the oligosaccharide along its translocation path (Meyer and Schulz, 1997). A similar coordination pattern is feasible in BcsC, but at this moment structural information on a translocation intermediate is lacking.
Recent negative stain electron microscopy analyses of cellulose synthases’ IM components suggest a supra-molecular assembly of the BcsA-BcsB-BcsG components (possibly stabilized by the additional BcsF, BcsR, and BcsQ subunits) (Krasteva et al., 2017). Assuming that every BcsA-BcsB heterodimer interacts with one BcsC subunit in the OM, oligomerization or at least close proximity of multiple BcsC subunits in the OM is likely. BcsC displays a cluster of conserved residues including Thr884, Asp893, Arg915, Asn974, and Tyr1096 on its membrane-facing surface (Figure 2B), which could facilitate interactions with a neighboring BcsC subunit. However, in a detergent-solubilized state, BcsC purifies and crystallizes as a monomer.
Our data provide no evidence that multiple BcsC channels fuse to form a pore large enough to translocate a pre-assembled cellulose fiber, as recently discussed (Krasteva et al., 2017). Instead, we hypothesize that a cellulose macro-complex likely secretes multiple glucan chains individually, which can interact on the cell surface to form fibrous structures frequently observed in biofilms. A similar process is assumed to underlie cellulose microfibril formation in plant cell walls (Purushotham et al., 2016).
STAR METHODS
LEAD CONTACT AND DATA AVAILABILITY
Further information and requests for resources and reagents should be directed to and will be fulfilled by the lead contact: Jochen Zimmer, (jochen_zimmer@virginia.edu).
EXPERIMENTAL MODEL AND SUBJECT DETAILS
Recombinant EcBcsC2 was expressed in E. coli C41(λDE3) using a pET20 plasmid encoding a PelB secretion signal sequence, deca-histidine tag, TEV cleavage site, and EcBcsC2 (EcBcsC residues 710 – 1167).
METHOD DETAILS
Constructs
EcBcsC2 was produced by removing the first 18 TPRs of the full-length protein cloned into the pET20b expression vector harboring an N-terminal PelB signal sequence followed by eight His residues. Primer deletion was used to remove the coding region while simultaneously adding an additional two histidines and a TEV protease cleavage site, thus creating a pET20-PelB-10xHis-TEV-EcBcsC2 construct.
Protein Expression
BcsC2 was expressed in E. coli C41(λDE3) using modified Lysogeny Broth as follows: 10 g/L yeast extract, 10 g/L tryptone, NaCl was replaced by M salts (50 mM NH4Cl, 25 mM KH2PO4, 25 mM Na2HPO4, 5 mM Na2SO4), and 80155 autoinduction solution (0.8% glycerol, 0.15% glucose, 0.5% lactose) was used for expression. Cells were grown in 1 L of media in 3 L baffled flasks at 28 °C for 24 hours.
Cells were collected, pelleted, and ruptured using a Microfluidics M110L microfluidizer. The extract was clarified of unbroken cells by slow speed centrifugation for 20 min at 15,000 g. The resultant supernatant was further pelleted for 2 hr at 100,000 g to isolate the EcBcsC2 containing membranes, flash frozen in liquid N2, and stored at −80 °C until purification. Selenomethionine-derivatized BcsC2 was expressed in E. coli C41(λDE3) cells as described above using feedback inhibition of the methionine biosynthetic pathway. Briefly, cells were grown in 125 mL starter cultures in lysogeny broth to an OD of ~3. In the evening 6 L of M9 minimal media supplemented with 2 mg/L thiamine, 0.1 mM CaCl2, and 2 mM MgSO4 was inoculated and grown at 37°C overnight. The next morning OD was monitored until it reached ~0.5. At this time, the temperature was reduced to 25 °C, and 10 mL of a 100× stock of 100 mg/mL Lys, Thr, Phe, 50 mg/mL Leu, Iso and Val, and 60 mg/mL selenomethionine were added. After 30 minutes, protein expression was induced by addition of 500 μM IPTG and 0.5% lactose. Expression was carried out for ~5 – 6 hours.
Protein purification
The thawed membrane pellet was solubilized in 25 mM Tris 8.5, 300 mM NaCl, 35 mM imidazole, 5% glycerol, 30 mM LDAO (lauryl-dimethylamine N-oxide) and 3 mM DDM (dodecyl-β-D-maltopyranoside) (buffer A) for 1 hr, after which insoluble material was removed by centrifugation for 30 min at 100,000 g. 5 mL of Ni NTA agarose beads (ThermoFisher) were prepared by washing with buffer A without detergent, were added to the supernatant, and allowed to rock at 4 °C for 1 hr. The sample was added to a gravity flow column and washed with buffer A without LDAO substituted with 40 mM imidazole and 1 mM DDM. EcBcsC2 was released from the beads using 25 mM Tris pH 8.5 100 mM NaCl, 300 mM imidazole and 0.6 % C8E4 (tetraethylene glycol monooctyl ether) (buffer B). The eluent was concentrated in a 50 MWCO Amicon spin column and applied to 10/30 S200 size exclusion column equilibrated with buffer B without imidazole for further purification. A single peak was analyzed by SDS-PAGE and fractions were pooled and either concentrated to ~15 mg/mL based on an extinction coefficient of 144 mM−1cm−1 or diluted to 1 mg/mL in Buffer B for His-tag removal. TEV cleavage was carried out at 1:100 mass ratio of EcBcsC2 and TEV protease. Cleaved tag, non-cleaved EcBcs2 and TEV protease were removed by applying to a 5 mL HisTrap column equilibrated with buffer B. Protein was immediately used for crystallization with the remainder flash frozen and stored at −80 °C. Purity was assessed by SDS-PAGE, Fig. S1A, and folding was assessed by PAGE gel mobility of boiled and non-boiled samples.
Crystallization and data collection
Crystallization screening was carried out with a TPP Labtech Mosquito liquid handling robot using sitting drop Intelliplate 3 with commercial screens MemGold 1 and 2, JCSG+, PEGs Suite and 2. MemGold produced several hits where 100 mM Tris 8.5, 200 mM CaCl2 and 45% PEG 400 were expanded upon. Iterative additive and concentration screening led to final condition of 5 – 10 mg/mL EcBcsC2 diluted in ddH2O combined 1:1 with 100 mM Tris 8.5, 200 mM CaCl2 and 30 – 40% PEG 400, 3% 1,6-hexanediol. Non-cleaved His_TEV_BcsC2 produced the highest quality crystals, as removal of the tag resulted in rapid nucleation and small crystal clusters. Crystals grew in sitting drop VDX plates in 1 to 3 days after which they were cryo-protected by directly adding 20% glycerol in crystallization solution to the drop, harvested and flash cooled in liquid nitrogen. Data were collected at the Argonne and Brookhaven National Labs, beamlines SER-CAT and AMX, and reduced in XDS (Kabsch, 2010).
Structure Determination
Crystallization produced crystals in space groups P21 and P21212. Crystals of selenomethionine-derivatized BcsC2 (P21) were used for SAD phasing in Phenix.autobuild without automatic model building (Adams et al., 2010) and based on 4 out of 12 identified selenomethionine sites. In P21, the crystallographic asymmetric unit contains two BcsC2 protomers. The initial experimental phases were improved by 2-fold non-crystallographic symmetry averaging in DM and PARROT using manually built solvent and averaging masks (Cowtan, 2010; Winn et al., 2011). The resulting electron density was of sufficient quality to build a partial poly-alanine model containing 8 β-strands and no periplasmic or extracellular loops, which was iteratively extended and partly refined in ARP/wARP as implemented in the HKL3000 software suite (Minor et al., 2006). The resultant model was then used for molecular replacement phasing of the highest resolution dataset in space group P21212 by Phaser (McCoy et al., 2007), manually completed in COOT (Emsley and Cowtan, 2004), and refined in Phenix.refine using TLS parameters towards the end of refinement (Painter, 2006). The resultant model contains BcsC residues 710–1157 and Phe, Gln, and Ser of the TEV cleavage site, the remaining tag residues were disordered and not included in the final model. The final model was refined to Rwork and Rfree values of 17 and 19%, respectively, with 97.1 % and 2.9 % residues in the preferred and allowed regions of the Ramachandran diagram. Validation of the final model was assessed by MolProbity as part of the Phenix.refine package, and the PDB Validation Service.
QUANTIFICATION AND STATISTICAL ANALYSIS
Data collection and refinement statistics for X-ray crystallography can be found in Supplemental Table S1.
DATA AND CODE AVAILABILITY
The atomic coordinates and structure factors of EcBcsC2 have been deposited into the PDB with accession code PDB ID: 6TZK.
Supplementary Material
KEY RESOURCES TABLE
| REAGENT or RESOURCE | SOURCE | IDENTIFIER |
|---|---|---|
| Bacterial Strains | ||
| E. coli C41(λDE3) | Lucigen | 60442 |
| Chemicals, Peptides, and Recombinant Proteins | ||
| Selenomethionine | Anatrace | S2000 |
| LDAO | Anatrace | D360S |
| DDM | Anatrace | T350 |
| C8E4 | Anatrace | D310S |
| Nis-Pur Ni-NTA Resin | Thermo-Fisher | 88221 |
| Superdex S200 10/30 | GE | 17-1043-10 |
| Critical Commercial Assays | ||
| MemGold | Molecular Dimensions | MD-139 |
| Additive Screen | Hampton Research | HR2–428 |
| Deposited Data | ||
| E. coli BcsC2 | This paper | PDB: 6TZK |
| E. coli PgaA | (Wang etal., 2016) | PDB:4Y25 |
| P. aeruginosa AlgE | (Whitney et al., 2011) | PDB:3RBH |
| Oligonucleotides | ||
| EcBCS_PelB_10His_TEV_C2_F GAAAACCTGTACTTCCAGAGTGACAAGTTAATACACACGAGC |
IDT | N/A |
| Unverisal_PelB_10His_TEV_R TGGAAGTACAGGTTTTCATGATGGTGATGATGATGGTGATGGTGG |
IDT | N/A |
| Recombinant DNA | ||
| pET20_PelB_8His_BcsC | This paper | N/A |
| pET20_PelB_10His_TEV_BcsC2 | This paper | N/A |
| Software and Algorithms | ||
| HKL3000 | (Minor et al, 2006) | https://sbgrid.org/ |
| Phenix 1.9 | (Adams et al., 2010) | https://sbgrid.org/ |
| Coot | (Emsley and Cowtan 2004) | https://sbgrid.org/ |
| Pymol 2.2 | Schrodinger, LLC | https://sbgrid.org/ |
| CCP4 7.0 | Winn et al., 2011 | https://sbgrid.org/ |
| Other | ||
Highlights:
Crystal structure of an outer membrane cellulose channel
Model of cellulose translocation across the outer membrane
Polysaccharide transport based on facilitated diffusion
Acknowledgements
We thank the beamline staffs at the NSLS-II AMX and APS SER-CAT beamlines. The AMX beamline of the National Synchrotron Light Source II is a U.S. Department of Energy (DOE) Office of Science User Facility operated for the DOE Office of Science by Brookhaven National Laboratory under Contract No. DE-SC0012704. C.C. SER-CAT used resources of the Advanced Photon Source, a U.S. Department of Energy (DOE) Office of Science User Facility operated for the DOE Office of Science by Argonne National Laboratory under Contract No. DE-AC02-06CH11357. JFA was supported in part by NIH grant 1F32GM126647-01 and JZ acknowledges support by NIH grant 5R01GM101001.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Declaration of Interests
The authors declare no competing interests.
Bibliography
- Adams P, Afonine P, Bunkoczi G, Chen V, Davis I, Echols N, Headd J, Hung L, Kapral G, Grosse-Kunstleve R, et al. (2010). PHENIX: a comprehensive Python-based system for macromolecular structure solution. Acta Crystallogr D Biol Crystallogr 66, 213–221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashkenazy H, Erez E, Martz E, Pupko T, and Ben-Tal N (2010). ConSurf 2010: calculating evolutionary conservation in sequence and structure of proteins and nucleic acids. Nucleic Acids Res 38, W529–533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bryers JD (2008). Medical biofilms. Biotechnol Bioeng 100, 1–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cantarel B, Coutinho P, Rancurel C, and Bernard T (2009). The Carbohydrate-Active EnZymes database (CAZy): an expert resource for Glycogenomics. Nucleic Acids Res 37, D233–238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cowtan K (2010). Recent developments in classical density modification. Acta Crystallogr D Biol Crystallogr 66, 470–478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emsley P, and Cowtan K (2004). Coot: model-building tools for molecular graphics. Acta Crystallogr D Biol Crystallogr 60, 2126–2132. [DOI] [PubMed] [Google Scholar]
- Hollenbeck EC, Antonoplis A, Chai C, Thongsomboon W, Fuller GG, and Cegelski L (2018). Phosphoethanolamine cellulose enhances curli-mediated adhesion of uropathogenic Escherichia coli to bladder epithelial cells. Proc Natl Acad Sci U S A 115, 10106–10111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jinek M, Rehwinkel J, Lazarus BD, Izaurralde E, Hanover JA, and Conti E (2004). The superhelical TPR-repeat domain of O-linked GlcNAc transferase exhibits structural similarities to importin alpha. Nat Struct Mol Biol 11, 1001–1007. [DOI] [PubMed] [Google Scholar]
- Jurrus E, Engel D, Star K, Monson K, Brandi J, Felberg LE, Brookes DH, Wilson L, Chen J, Liles K, et al. (2018). Improvements to the APBS biomolecular solvation software suite. Protein science : a publication of the Protein Society 27, 112–128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kabsch W (2010). Xds. Acta Crystallogr D Biol Crystallogr 66, 125–132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keiski C-L, Harwich M, Jain S, Neculai AM, Yip P, Robinson H, Whitney JC, Riley L, Burrows LL, Ohman DE, et al. (2010). AlgK is a TPR-containing protein and the periplasmic component of a novel exopolysaccharide secretin. Structure 18, 265–273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krasteva PV, Bernal-Bayard J, Travier L, Martin FA, Kaminski PA, Karimova G, Fronzes R, and Ghigo JM (2017). Insights into the structure and assembly of a bacterial cellulose secretion system. Nat Commun 8, 2065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumari M, Sunoj RB, and Balaji PV (2012). Exploration of CH···π mediated stacking interactions in saccharide: aromatic residue complexes through conformational sampling. Carbohydrate Res 361, 133–140. [DOI] [PubMed] [Google Scholar]
- Lamb J, Tugendreich S, Hieter P (1995). Tetratrico peptide repeat interactions: to TPR or not to TPR. TIBS 20, 257–259. [DOI] [PubMed] [Google Scholar]
- Little DJ, Li G, Ing C, DiFrancesco BR, Bamford NC, Robinson H, Nitz M, Pomès R, and Howell PL (2014). Modification and periplasmic translocation of the biofilm exopolysaccharide poly-β−1,6-N-acetyl-D-glucosamine. Proc Natl Acad Sci U S A 111, 11013–11018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matthysse AG, White S, and Lightfoot R (1995). Genes required for cellulose synthesis in Agrobacterium tumefaciens. J Bacteriol 177, 1069–1075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCoy A, Grosse-Kunstleve R, Adams P, Winn M, Storoni L, and Read R (2007). Phaser crystallographic software. J Appl Crystallogr 40, 658–674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCrate OA, Zhou X, Reichhardt C, and Cegelski L (2013). Sum of the Parts: Composition and Architecture of the Bacterial Extracellular Matrix. J Mol Biol. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McNamara JT, Morgan JLW, and Zimmer J (2015). A molecular description of cellulose biosynthesis. Annu Rev Biochem 84, 17.11–17.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer JE, and Schulz GE (1997). Energy profile of maltooligosaccharide permeation through maltoporin as derived from the structure and from a statistical analysis of saccharide-protein interactions. Protein Sci 6, 1084–1091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minor W, Cymborowski M, Otwinowski Z, and Chruszcz M (2006). HKL-3000: the integration of data reduction and structure solution--from diffraction images to an initial model in minutes. Acta Crystallogr D Biol Crystallogr 62, 859–866. [DOI] [PubMed] [Google Scholar]
- Morgan J, Strumillo J, and Zimmer J (2013). Crystallographic snapshot of cellulose synthesis and membrane translocation. Nature 493, 181–186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan JL, McNamara JT, Fischer M, Rich J, Chen HM, Withers SG, and Zimmer J (2016). Observing cellulose biosynthesis and membrane translocation in crystallo. Nature 531, 329–334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nojima S, Fujishima A, Kato K, Ohuchi K, Shimizu N, Yonezawa K, Tajima K, and Yao M (2017). Crystal structure of the flexible tandem repeat domain of bacterial cellulose synthesis subunit C. Sci Rep 7, 13018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Omadjela O, Narahari A, Strumillo J, Mélida H, Mazur O, Bulone V, and Zimmer J (2013). BcsA and BcsB form the catalytically active core of bacterial cellulose synthase sufficient for in vitro cellulose synthesis. Proc Natl Acad Sci U S A 110, 17856–17861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Painter J (2006). TLSMD web server for the generation of multi-group TLS models. J Appl Cryst 39, 109–111. [Google Scholar]
- Purushotham P, Cho SH, Diaz-Moreno SM, Kumar M, Nixon BT, Bulone V, and Zimmer J (2016). A single heterologously expressed plant cellulose synthase isoform is sufficient for cellulose microfibril formation in vitro. Proc Natl Acad Sci U S A 113, 11360–11365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rehman ZU, Wang Y, Moradali MF, Hay ID, and Rehm BHA (2013). Insights into the Assembly of the Alginate Biosynthesis Machinery in Pseudomonas aeruginosa. Appl Environ Microbiol 79, 3264–3272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Römling U, and Balsalobre C (2012). Biofilm infections, their resilience to therapy and innovative treatment strategies. J Intern Med 272, 541–561. [DOI] [PubMed] [Google Scholar]
- Romling U, and Galperin MY (2015). Bacterial cellulose biosynthesis: diversity of operons, subunits, products, and functions. Trends Microbiol 23, 545–557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stewart P, and Costerton J (2001). Antibiotic resistance of bacteria in biofilms. Lancet 358, 135–138. [DOI] [PubMed] [Google Scholar]
- Sun L, Vella P, Schnell R, Polyakova A, Bourenkov G, Li F, Cimdins A, Schneider TR, Lindqvist Y, Galperin MY, et al. (2018). Structural and Functional Characterization of the BcsG Subunit of the Cellulose Synthase in Salmonella typhimurium. J Mol Biol 430, 3170–3189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan J, Rouse SL, Li D, Pye VE, Vogeley L, Brinth AR, El Arnaout T, Whitney JC, Howell PL, Sansom MS, et al. (2014). A conformational landscape for alginate secretion across the outer membrane of Pseudomonas aeruginosa. Acta Crystallogr D Biol Crystallogr 70, 2054–2068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thongsomboon W, Serra DO, Possling A, Hadjineophytou C, Hengge R, and Cegelski L (2018). Phosphoethanolamine cellulose: A naturally produced chemically modified cellulose. Science 359, 334–338. [DOI] [PubMed] [Google Scholar]
- Wang Y, Andole Pannuri A, Ni D, Zhou H, Cao X, Lu X, Romeo T, and Huang Y (2016). Structural Basis for Translocation of a Biofilm-supporting Exopolysaccharide across the Bacterial Outer Membrane. J Biol Chem 291, 10046–10057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitney JC, Hay ID, Li C, Eckford PD, Robinson H, Amaya MF, Wood LF, Ohman DE, Bear CE, Rehm BH, et al. (2011). Structural basis for alginate secretion across the bacterial outer membrane. Proc Natl Acad Sci U S A 108, 13083–13088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitney JC, and Howell PL (2013). Synthase-dependent exopolysaccharide secretion in Gram-negative bacteria. Trends Microbiol 21, 63–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winn MD, Ballard CC, Cowtan KD, Dodson EJ, Emsley P, Evans PR, Keegan RM, Krissinel EB, Leslie AG, McCoy A, et al. (2011). Overview of the CCP4 suite and current developments. Acta Crystallogr D Biol Crystallogr 67, 235–242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeytuni N, and Zarivach R (2012). Structural and functional discussion of the tetra-trico-peptide repeat, a protein interaction module. Structure 20, 397–405. [DOI] [PubMed] [Google Scholar]
- Zimmer J (2019). Structural features underlying recognition and translocation of extracellular polysaccharides. Interface Focus 9, 20180060. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The atomic coordinates and structure factors of EcBcsC2 have been deposited into the PDB with accession code PDB ID: 6TZK.