Abstract
Herpes simplex virus (HSV)-1 and HSV-2 cause painful blisters and shallow ulcers in exposed skin and mucosae during primary or recurrent infection. In addition, recurrent and potentially blinding HSV-1 infections of the eye afflict nearly half a million persons in the U.S. Current clinical therapies rely on nucleoside analog drugs such as acyclovir (ACV) or ganciclovir to ameliorate primary infections and reduce the frequency and duration of reactivations. However, these treatments do not fully suppress viral shedding and drug-resistant mutants develop in the eye and in vulnerable, immunosuppressed patients. Herpesvirus DNA replication requires several enzymes in the nucleotidyl transferase superfamily (NTS) that have recombinase and nuclease activities. We previously found that compounds which block NTS enzymes efficiently inhibit replication of HSV-1 and HSV-2 by up to 1 million-fold in Vero and human foreskin fibroblasts. Among the compounds with potent suppressive effects in culture is the anti-fungal drug ciclopirox. Here we report that topical application of ciclopirox olamine to the eyes of mice infected with HSV-1 reduced virus shed from the corneal epithelium compared with saline control, and reduced development of blepharitis to the level of mice treated with ACV. Results were dose-dependent. In addition, treatment with ciclopirox olamine significantly reduced acute and latent HSV-1 infection of the peripheral nervous system. These results support further development of ciclopirox olamine as a repurposed topical agent for HSV infections.
Keywords: herpes simplex virus, ciclopirox, corneal, replication, antiviral
1. Introduction
Herpes simplex virus (HSV)-1 and HSV-2 cause much suffering and morbidity linked to a variety of diseases they perpetrate in infected persons (Whitley, 2001). Painful blisters and shallow ulcers form in skin and mucosal surfaces of the mouth and genital tract at the site of penetrance and reoccur periodically throughout life from a latent reservoir in nerve ganglia innervating the initial site infection. Rarely, HSV may travel retrogradely into the central nervous system during primary or recurrent infection to cause clinically critical encephalitis. Recurrent HSV-1 infections of the eye afflict 400,000 persons in the U.S. (Farooq & Shukla, 2012; Lairson et al., 2003), causing eyelid disease (blepharitis) and potentially blinding corneal keratitis (Azher et al., 2017). Nucleoside analogs such as ACV or ganciclovir are in current clinical use to ameliorate primary infections with HSV-1, and decrease the duration and frequency of reactivations (Field & Vere Hodge, 2013; James & Prichard, 2014; King, 1988). However, these treatments do not fully suppress viral shedding (Johnston et al., 2012; Vere Hodge & Field, 2013). Furthermore, drug-resistant mutants develop in immunosuppressed patients (Andrei et al., 2000; Andrei et al., 2013; Field & Vere Hodge, 2013; Gilbert et al., 2002; James & Prichard, 2014; Schmit & Boivin, 1999), and also in immunocompetent persons with recurrent eye infections (Burrel et al., 2013; Duan et al., 2008; Duan et al., 2009; van Velzen et al., 2013), and in healthy children (Wang et al., 2011). A therapeutic option with a different mechanism of action than nucleoside analogs would be clinically useful.
HSVs replicate in epithelial cells at the site of initial penetrance before spreading into the nervous system. Herpesvirus DNA replication requires numerous viral enzymes (Weller & Coen, 2012). Several of these enzymes have recombinase and nuclease activities consistent with members of the nucleotidyl transferase superfamily (NTS), including the pUL30 polymerase, pUL15 terminase, and the pUL12 nuclease which works in concert with the ICP8 single-stranded annealing protein (Bryant et al., 2012; Schumacher et al., 2012; Selvarajan et al., 2013; Weller & Coen, 2012). NTS enzymes share a similar protein fold and enzymatic mechanisms including four conserved carboxylates that coordinate two divalent cations required to promote a hydroxyl-mediated nucleophilic scission reaction (Nowotny, 2009; Nowotny & Yang, 2009). These enzymatic activities represent new potential targets for antiviral therapies. We previously found that compounds which block NTS enzymes efficiently inhibit replication of HSV-1 and HSV-2 by up to 1-million fold in Vero cells and human foreskin fibroblasts (Tavis et al., 2014). Among the compounds with potent suppressive effects on HSVs in culture is the FDA-approved antifungal drug ciclopirox. Ciclopirox has an EC50 against HSV-1 of 0.27 μM in Vero cells and a therapeutic index of >185; these values are similar to ACV’s EC50 of 0.16 μM and therapeutic index of >625 (Tavis et al., 2014). Ciclopirox suppresses ACV-resistant (thymidine kinase-deficient) viruses, and time of addition experiments further suggest a different mechanism of action than ACV (Tavis et al., 2014).
Ciclopirox olamine, the ethanolamine salt of ciclopirox, has long been known as an effective antimycotic, with broad-spectrum activity against dermatophytes, yeast, filamentous fungi (Abrams et al., 1991; Bohn & Kraemer, 2000; Dittmar & Lohaus, 1973; Jue et al., 1985). It gained FDA approval for topical applications such as dandruff, seborrhoeic dermatitis and onychomycosis (Subissi et al., 2010). The potential to repurpose a drug already FDA-approved for topical use in a new antiviral indication is an exciting prospect. Here we provide evidence that topically applied ciclopirox olamine can reduce HSV replication and disease in a mouse model of corneal infection with HSV-1.
2. Materials and methods
2.1. Viruses, cells and compounds
Vero cells were maintained in Dulbecco’s modified Eagle’s medium (DMEM) supplemented with 3% newborn calf serum and 3% bovine growth serum (BGS), 2 mM L-glutamine and 1× penicillin-streptomycin (P/S) (Invitrogen). Stocks of HSV-1 KOS (Smith, 1964) and strain 17 (Brown et al., 1973) were propagated on Vero cells and titers were determined by standard plaque assay (Knipe & Spang, 1982). Acyclovir (Sigma Aldrich) and ciclopirox olamine (Toronto Research Chemicals) were reconstituted in normal saline and working concentrations were prepared by dilution in normal saline just prior to use.
2.2. Animal studies
BALB/c mice, 6 wk of age, were purchased from Taconic Farms. They were handled in strict accordance with good animal practice as defined by Institutional and Public Health Service guidelines, and with work approved by the Institutional Animal Care and Use Committee. The animals were housed and bred in the Saint Louis University School of Medicine Department of Comparative Medicine. Corneas of anesthetized mice were scarified as previously described (Morrison & Knipe, 1994) and infected by dropwise application of 1×104 pfu/eye HSV-1. Swabs of the corneal surface were performed at various times post-infection and virus collected on them was quantified as previously described (Knipe & Spang, 1982). At 4 d post-infection (acute) or 28 d post-infection (latent), mice were euthanized and trigeminal ganglia (TG) were dissected. For determination of latent viral genome load, DNA was isolated from TG using a QIAamp DNA Mini Kit (Qiagen) according to the manufacturer’s instructions.
2.3. Drug treatment
Mice were lightly anesthetized prior to each treatment using isofluorane. Drug was applied dropwise to the corneal surface in 4 μl vol and mice were maintained under isofluorane anesthesia another 3 min before being returned to their cage. Final concentrations of ciclopirox olamine were 3 μg/eye/dose and 1 μg/eye/dose; ACV was administered at 4.5 μg/eye/dose and 1.5 μg/eye/dose. Treatments were administered thrice per day for 4 d beginning 4 h post-infection.
2.4. Quantitative real-time PCR
The primers and Taqman probe for real-time PCR are specific for the HSV-1 LAT region. The primer sequences in ICP0 were: forward 5’-AGCCCCGTCTCGAACAGT-3’; reverse 5’-ACCACCATGACGACGACTC-3’; and Universal Probe Library probe #56 was 5’-FAM-GGAGGTG-dark quencher-3’ (Roche). PCR reactions (25 μl vol) contained FastStart Universal Probe master mix containing Rox (Roche), and 1 μM forward and reverse primers, 250 μM probe, and 100 ng DNA template. Quantification was performed in triplicate using an ABI 7500 genetic analyzer (Applied Biosystems) with 1 cycle at 50°C for 2 min; 1 cycle at 95°C for 10 min; 40 cycles at 95°C for 20 s and 60°C for 1 min. Forty-five to 1.8×105 copies of cesium chloride gradient-purified HSV-1 DNA were used to generate a standard curve. Data were analyzed using Sequence Detection System software (ABI) and expressed as HSV-1 genome equivalents per microgram total DNA.
2.5. Statistical analyses
Statistical significance of differences in titer of virus in TG, or of virus shed from the corneal surface was determined on individual days by ANOVA. The Kruskal-Wallis non-parametric test was used to analyze differences in blepharitis scores on individual days with Tukey’s test for multiple comparisons. We compared qPCR results among groups using ANOVA with Bonferonni correction.
3. Results
3.1. Effect of ciclopirox on HSV-1 infection of the eye
Ciclopirox and ciclopirox olamine have equivalent EC50 values against HSV in cultured cells (Tavis et al., 2014), and unpublished observation). Before conducting in vivo efficacy studies, toxicity of ciclopirox olamine for the corneal epithelium was evaluated. Various doses of ciclopirox olamine were applied to the corneas of mice three times per day for 5 d, followed by inspection with an ophthalmoscope. Ciclopirox olamine at 3 μg/eye/dose was selected as a non-toxic maximal dose and a lower dose of 1 μg/eye/dose was also used to assess dose-responsiveness. ACV at 4.5 μg/eye/dose was used as a positive control because it was previously demonstrated to be effective in reducing HSV-1 replication in the cornea (Willey et al., 1991). A 3-fold lower dose of 1.5 μg/eye was also used.
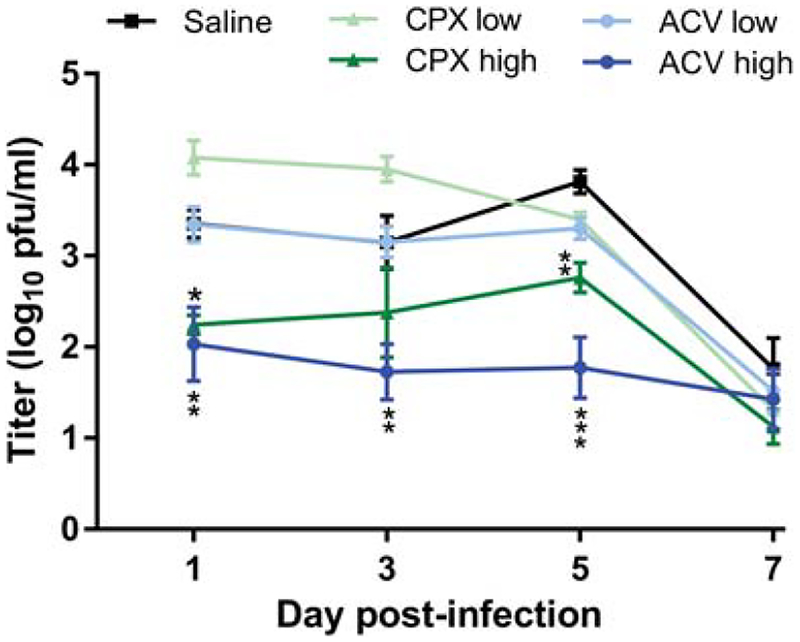
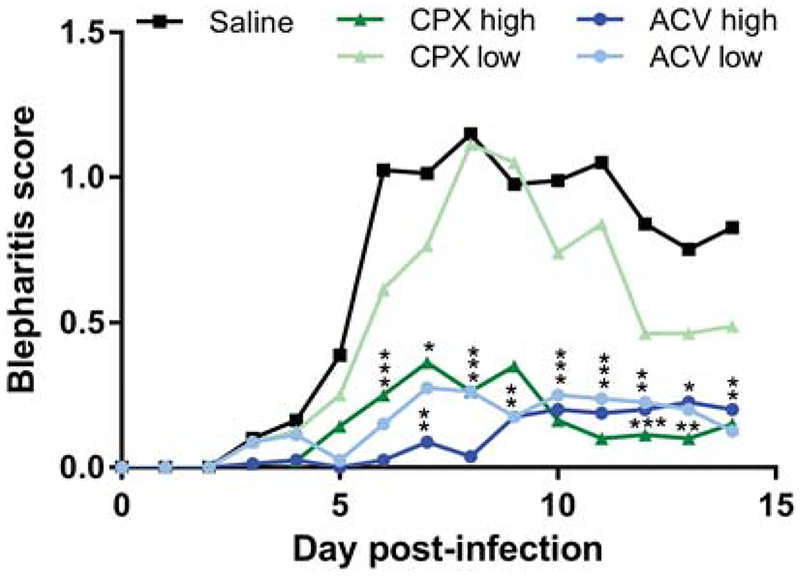
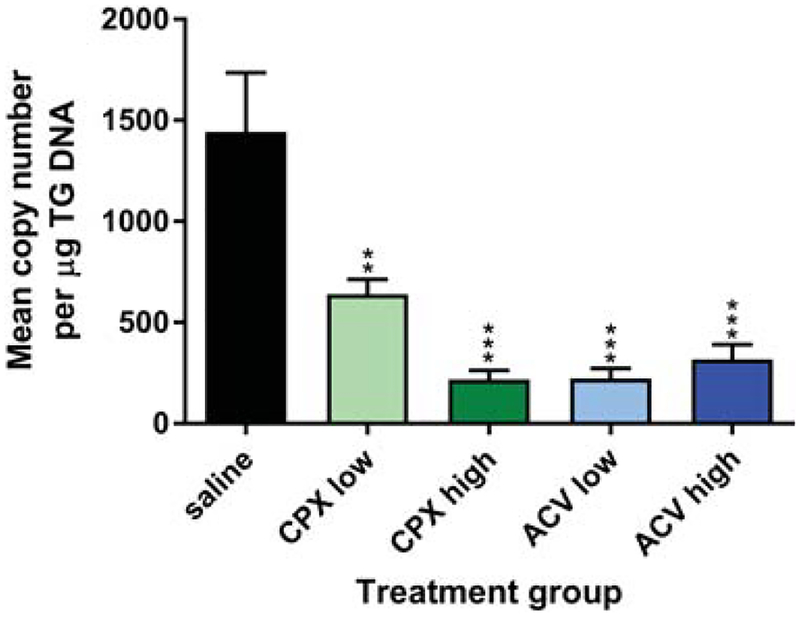
Groups of BALB/c mice were infected on the scarified corneas with 1×104 pfu of HSV-1 KOS. Ciclopirox olamine, ACV or saline was then applied to the corneal surface three times per day for 5 d beginning 4 h post-infection. Corneal swabs taken between doses of drug revealed that the higher doses of ACV and ciclopirox controlled virus replication in the corneal epithelium better than application of saline days 1 through 5 post-infection (Fig. 1). The suppressive effect was dose-dependent because neither the lower dose of ciclopirox olamine nor ACV reduced HSV-1 replication compared with saline control. By day 7 virus replication in all groups had declined. Signs of blepharitis over time post-infection were extremely mild in mice treated with either 4.5 or 1.5 μg/eye ACV compared with saline. Similarly, the higher dose of ciclopirox olamine provided significant protection from blepharitis (Fig. 2). However, the protective effect was lost at the lower dose of ciclopirox. Differences in keratitis could not be assessed because this dose of HSV-1 KOS caused minimal corneal disease even in mice treated with saline (data not shown). Weight of the mice was also assessed daily post-infection as an indicator of overall health, and although all treatment groups lost less weight than the control group no significant differences between treatment groups was observed (data not shown). Lastly, the effect of drugs on latent infection of the TG was assessed 28 d post-infection by qPCR. TG of KOS-infected mice treated with saline contained large numbers of latent viral genomes (Fig. 3). Markedly fewer viral genomes were detected in TG of mice treated with low and high doses of ciclopirox olamine or ACV, though the lower dose of ciclopirox was slightly less effective. Thus, ciclopirox olamine treatment of the cornea can reduce HSV-1 replication in the epithelium, eyelid disease, and latent infection.
Figure 1. Ciclopirox olamine (CPX) can suppress HSV-1 replication in the cornea.
Mice were infected on the corneas with 1×104 pfu HSV-1 KOS, and subsequently treated thrice daily for 5 d with saline, or with CPX or ACV at the indicated doses per eye. Virus shed from the corneal epithelium over time post-infection was quantified by titration of virus collected on eye swabs. Data represent the mean ± SEM of swabs from a total of 12 mice per group and are the combined results of two experiments. *P=0.0358, **P=0.0077 to 0.0012, ***P<0.0001 for ciclopirox (3 μg) compared with saline control.
Figure 2. Ciclopirox olamine (CPX) can suppress HSV-1-induced blepharitis.
Groups of 10 mice were infected on the corneas with 1×104 pfu HSV-1 KOS, and subsequently treated thrice daily for 5 d with saline, or with CPX or ACV at the indicated doses per eye. Blepharitis was assessed daily in masked fashion for 14 d post-infection. Data represent the mean ± SD of 40 mice and are the combined results of two experiments. *, P = 0.05–0.0183; **, P = 0.0065–0.0014; ***, P < 0.0005 for treatment group compared with saline control.
Figure 3. Ciclopirox olamine (CPX) reduces latent infection.
Viral genomes in the TG of mice described in Fig. 2 were assessed 28 d post-infection by qPCR by comparison to a standard curve. Data represent the mean ± SEM of 39 to 40 TG per group and are the combined results of 2 experiments. P≤0.0004 for all groups compared with saline control by ANOVA.
3.2. Effect of ciclopirox treatment on infection with a more virulent HSV-1 strain
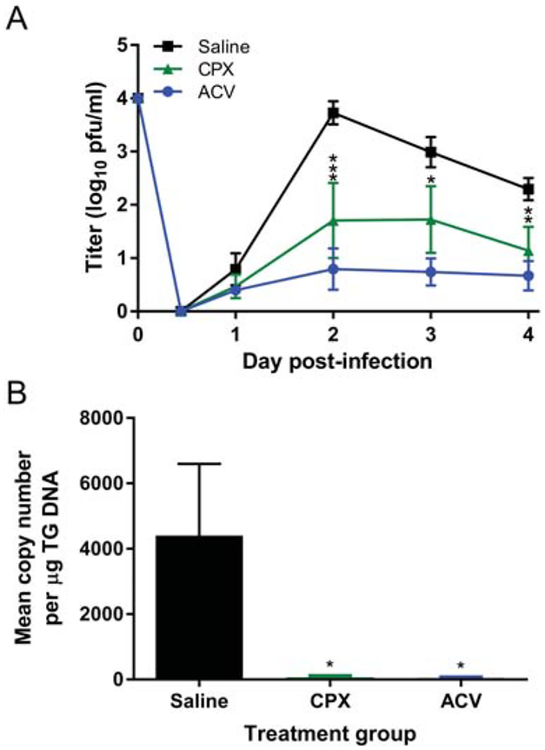
HSV-1 KOS is considered a strain of relatively low virulence (Dix et al., 1983; Luker et al., 2006). To determine whether ciclopirox olamine would inhibit replication of a different strain of HSV-1, we infected groups of mice with 1×104 pfu of HSV-1 strain 17 and subsequently treated them with the higher dose of ciclopirox olamine, ACV, or with saline only. Both drugs again significantly suppressed HSV-1 replication in the cornea compared with the control group (Fig. 4A). To determine whether acute infection of the nervous system was affected by drug treatment, we quantified viral genomes in the TG 4 d post-infection. Mice treated with saline contained a large number of viral genomes in the TG (Fig. 4B). In contrast, ciclopirox and ACV markedly reduced the number of viral genomes detectable at this early time post-infection. Thus, ciclopirox treatment also protects mice against acute infection with a more virulent HSV-1 strain.
Figure 4. Ciclopirox olamine (CPX) treatment suppresses HSV-1 strain 17 infection.
Groups of 5 mice were infected on the scarified corneas with 1×104 pfu of HSV-1 strain 17, and subsequently treated thrice per day with saline or the indicated drug. A) Virus shed from the corneal epithelium over time post-infection. *, P = 0.0156; **, P = 0.004; ***, P = 0.0008 for ciclopirox compared with saline control by ANOVA. Acyclovir compared with saline control, P < 0.0001 for days 2–4. B) Quantity of viral genomes in the TG 4 d post-infection. *, P = 0.0408–.0396 for both treatment groups compared with saline control by ANOVA. Data represent the mean ± SEM of 10 mice (20 TG) and are the combined results of 2 experiments.
4. Discussion
We have demonstrated that topical treatment with ciclopirox olamine reduces HSV-1 replication in the cornea, acute and latent infection of the nervous system, and development of blepharitis. The effect of both ACV and ciclopirox on replication of HSV-1 KOS is dose-dependent. Whether ciclopirox reduces infection of the nervous system solely because of its impact on replication in the cornea is unknown, but this is unlikely given that low dose ciclopirox appeared to have a disproportionately large effect on accumulation of latent genomes (compare Fig. 1 and 3). Pharmacokinetic experiments have demonstrated that ciclopirox olamine can be detected in the corneas and TG of mice treated orally with a single high dose and is maintained there for 4 h post-treatment (data not shown). The finding that ciclopirox enters the peripheral nervous system suggests the possibility that ciclopirox may directly suppress virus replication in the TG, either after uptake into axons from the treated cornea or via the bloodstream. Thus, ciclopirox may have the potential, even when applied topically, to ameliorate HSV infection in the nervous system.
The reduction in blepharitis observed in drug-treated mice is likely due to ciclopirox suppression of acute infection in the eye and TG because blepharitis is largely the result of virus spread from the TG to the eyelid (Summers et al., 2001). This reported mechanism underlying development of blepharitis is consistent with our observation that disease did not occur until 5 d or more post-infection, a time when infection of the cornea is declining rapidly. In contrast to the higher dose of ciclopirox, the lower dose had no effect on acute replication in the cornea. Although the differential impact of high dose versus low dose ciclopirox on subsequent infection of the nervous system is small (Fig. 3), the difference may become magnified as virus mounts a return trip to the eyelid.
Anti-inflammatory effects of ciclopirox were demonstrated in a topical application to the mouse ear (Abrams et al., 1991; Hanel et al., 1991; Liebel et al., 2006). Consistent with this report, we did not observe an increase in angiogenesis in the cornea (data not shown). However, HSV-1 KOS caused only mild effects on the cornea, including induction of keratitis, which prevents us from drawing a definitive conclusion in this regard. It will be of interest to test ciclopirox formulated in ophthalmic ointment for effectiveness and anti-inflammatory effects, and conceivably a higher non-toxic dose of ciclopirox could be achieved.
The mechanism by which ciclopirox/ciclopirox olamine reduces HSV replication is not yet known. Its antifungal activity appears to result from disruption of cell membrane integrity or function (Bohn & Kraemer, 2000; Jue et al., 1985). In bacteria, ciclopirox potently inhibits respiration (Bohn & Kraemer, 2000; Jue et al., 1985). Ciclopirox reduces HIV replication (Hanauske-Abel et al., 2013) by inhibiting synthesis of the rare amino acid hypusine, thereby preventing formation of mature, hypusine-containing eIF5A needed for HIV transcription initiation (Hoque et al., 2009). Ciclopirox also chelates iron and has been shown to inhibit the iron-dependent ribonucleotide reductase enzyme (Eberhard et al., 2009). Although HSV also expresses a ribonucleotide reductase, the enzymatic function is not essential in dividing cells and thus its potential suppression is unlikely to be the sole contributor to inhibition of HSV replication. More likely, ciclopirox interferes with the function of one or more viral NTS enzymes. Thus, the inhibitory effects of ciclopirox on HSV replication may be via direct antiviral or indirect activities.
Microbial resistance to existing drugs is a growing clinical concern. Nucleos(t)ide analog-resistant HSV mutants develop in up to 6.4% of immunocompetent individuals (Piret & Boivin, 2011) and up to 36% of immunocompromised individuals with herpetic eye disease (Andrei et al., 2000; Field & Vere Hodge, 2013; Gilbert et al., 2002; James & Prichard, 2014; Schmit & Boivin, 1999). We have found that ciclopirox olamine powerfully suppresses ACV-resistant mutants of HSV-1 and HSV-2 in tissue culture (Tavis et al., 2014). It will be interesting to determine whether ciclopirox synergize with ACV since it has a different apparent mechanism of action (Tavis et al., 2014). The fact that ciclopirox olamine has FDA approval for topical use suggests exciting potential for repurposing this antifungal drug as an anti-herpesviral medication.
Highlights.
The antifungal drug ciclopirox olamine was tested for in vivo activity against HSV-1.
Topical application of ciclopirox olamine to mouse eyes reduced HSV-1 shedding.
Ciclopirox olamine decreased eyelid disease comparable to treatment with acyclovir.
Ocular treatment with ciclopirox olamine reduced acute and latent HSV-1 infection of the nervous system.
Ciclopirox olamine shows promise as a topical treatment repurposed for HSV infections.
Acknowledgements
We thank Tiffany Edwards for helpful advice and Maria Korom for initial work establishing drug treatment in the mouse eye model.
This work was supported by the National Institutes of Health Institute of Clinical and Translational Sciences [grant UL1 TR002345] from the National Center for Advancing Translational Sciences (NCATS) to LM.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Declaration of interests: LM is an inventor on a patent application covering use of ciclopirox and ciclopirox olamine for HSV-1 and HSV-2, and is a scientific advisor to Casterbridge Pharmaceuticals, Inc.
References
- Abrams BB, Hanel H, & Hoehler T (1991). Ciclopirox olamine: a hydroxypyridone antifungal agent. Clin Dermatol, 9(4), 471–477. [DOI] [PubMed] [Google Scholar]
- Andrei G, Fiten P, De Clercq E, Snoeck R, & Opdenakker G (2000). Monitoring drug resistance for herpesviruses. Methods Mol Med, 24, 151–169. doi: 10.1385/1-59259-2457:151 [DOI] [PubMed] [Google Scholar]
- Andrei G, Georgala A, Topalis D, Fiten P, Aoun M, Opdenakker G, & Snoeck R (2013). Heterogeneity and evolution of thymidine kinase and DNA polymerase mutants of herpes simplex virus type 1: implications for antiviral therapy. J Infect Dis, 207(8), 1295–1305. doi: 10.1093/infdis/jit019 [DOI] [PubMed] [Google Scholar]
- Azher TN, Yin XT, Tajfirouz D, Huang AJ, & Stuart PM (2017). Herpes simplex keratitis: challenges in diagnosis and clinical management. Clin Ophthalmol, 11, 185–191. doi: 10.2147/opth.s80475 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bohn M, & Kraemer KT (2000). Dermatopharmacology of ciclopirox nail lacquer topical solution 8% in the treatment of onychomycosis. J Am Acad Dermatol, 43(4 Suppl), S57–69. [DOI] [PubMed] [Google Scholar]
- Brown SM, Ritchie DA, & Subak-Sharpe JH (1973). Genetic studies with herpes simplex virus type 1. The isolation of temperature-sensitive mutants, their arrangement into complementation groups and recombination analysis leading to a linkage map. J Gen Virol, 18(3), 329–346. doi: 10.1099/0022-1317-18-3-329 [DOI] [PubMed] [Google Scholar]
- Bryant KF, Yan Z, Dreyfus DH, & Knipe DM (2012). Identification of a divalent metal cation binding site in herpes simplex virus 1 (HSV-1) ICP8 required for HSV replication. J Virol, 86(12), 6825–6834. doi: 10.1128/jvi.00374-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burrel S, Boutolleau D, Azar G, Doan S, Deback C, Cochereau I, Agut H, Gabison EE (2013). Phenotypic and genotypic characterization of acyclovir-resistant corneal HSV-1 isolates from immunocompetent patients with recurrent herpetic keratitis. J Clin Virol, 58(1), 321–324. doi: 10.1016/j.jcv.2013.05.001 [DOI] [PubMed] [Google Scholar]
- Dittmar W, & Lohaus G (1973). HOE 296, a new antimycotic compound with a broad antimicrobial spectrum. Laboratory results. Arzneimittelforschung, 23(5), 670–674. [PubMed] [Google Scholar]
- Dix RD, McKendall RR, & Baringer JR (1983). Comparative neurovirulence of herpes simplex virus type 1 strains after peripheral or intracerebral inoculation of BALB/c mice. Infect Immun, 40(1), 103–112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duan R, de Vries RD, Osterhaus AD, Remeijer L, & Verjans GM (2008). Acyclovir-resistant corneal HSV-1 isolates from patients with herpetic keratitis. J Infect Dis, 198(5), 659–663. doi: 10.1086/590668 [DOI] [PubMed] [Google Scholar]
- Duan R, de Vries RD, van Dun JM, van Loenen FB, Osterhaus AD, Remeijer L, & Verjans GM (2009). Acyclovir susceptibility and genetic characteristics of sequential herpes simplex virus type 1 corneal isolates from patients with recurrent herpetic keratitis. J Infect Dis, 200(9), 1402–1414. doi: 10.1086/606028 [DOI] [PubMed] [Google Scholar]
- Eberhard Y, McDermott SP, Wang X, Gronda M, Venugopal A, Wood TE, Hurren R, Datti A, Batey RA, Wrana J, Antholine WE, Dick JE, Schimmer AD (2009). Chelation of intracellular iron with the antifungal agent ciclopirox olamine induces cell death in leukemia and myeloma cells. Blood, 114(14), 3064–3073. doi: 10.1182/blood-2009-03-209965 [DOI] [PubMed] [Google Scholar]
- Farooq AV, & Shukla D (2012). Herpes simplex epithelial and stromal keratitis: an epidemiologic update. Surv Ophthalmol, 57(5), 448–462. doi: 10.1016/j.survophthal.2012.01.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Field HJ, & Vere Hodge RA (2013). Recent developments in anti-herpesvirus drugs. Br Med Bull, 106, 213–249. doi: 10.1093/bmb/ldt011 [DOI] [PubMed] [Google Scholar]
- Gilbert C, Bestman-Smith J, & Boivin G (2002). Resistance of herpesviruses to antiviral drugs: clinical impacts and molecular mechanisms. Drug Resist Updat, 5(2), 88–114. [DOI] [PubMed] [Google Scholar]
- Hanauske-Abel HM, Saxena D, Palumbo PE, Hanauske AR, Luchessi AD, Cambiaghi TD, Hoque M, Spino M, D’Alliessi Gandolfi D, Heller DS, Singh S, Park MH, Cracchiolo BM, Tricta F, Connelly J, Popowicz AM, Cone RA, Holland B, Pe’ery T, & Mathews MB (2013). Drug-induced reactivation of apoptosis abrogates HIV-1 infection. PLoS One, 8(9), e74414. doi: 10.1371/journal.pone.0074414 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanel H, Smith-Kurtz E, & Pastowsky S (1991). [Therapy of seborrheic eczema with an antifungal agent with an antiphlogistic effect]. Mycoses, 34 Suppl 1, 91–93. [PubMed] [Google Scholar]
- Hoque M, Hanauske-Abel HM, Palumbo P, Saxena D, D’Alliessi Gandolfi D, Park MH, Pe’ery T & Mathews MB (2009). Inhibition of HIV-1 gene expression by Ciclopirox and Deferiprone, drugs that prevent hypusination of eukaryotic initiation factor 5A. Retrovirology, 6, 90. doi: 10.1186/1742-4690-6-90 [DOI] [PMC free article] [PubMed] [Google Scholar]
- James SH, & Prichard MN (2014). Current and future therapies for herpes simplex virus infections: mechanism of action and drug resistance. Curr Opin Virol, 8, 54–61. doi: 10.1016/j.coviro.2014.06.003 [DOI] [PubMed] [Google Scholar]
- Johnston C, Saracino M, Kuntz S, Magaret A, Selke S, Huang ML, Schiffer JT, Koelle DM, Corey L & Wald A (2012). Standard-dose and high-dose daily antiviral therapy for short episodes of genital HSV-2 reactivation: three randomised, open-label, cross-over trials. Lancet, 379(9816), 641–647. doi: 10.1016/s0140-6736(11)61750-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jue SG, Dawson GW, & Brogden RN (1985). Ciclopirox olamine 1% cream. A preliminary review of its antimicrobial activity and therapeutic use. Drugs, 29(4), 330–341. [DOI] [PubMed] [Google Scholar]
- King DH (1988). History, pharmacokinetics, and pharmacology of acyclovir. J Am Acad Dermatol, 18(1 Pt 2), 176–179. [DOI] [PubMed] [Google Scholar]
- Knipe DM, & Spang AE (1982). Definition of a series of stages in the association of two herpesviral proteins with the cell nucleus. Journal of Virology, 43, 314–324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lairson DR, Begley CE, Reynolds TF, & Wilhelmus KR (2003). Prevention of herpes simplex virus eye disease: a cost-effectiveness analysis. Arch Ophthalmol, 121(1), 108–112. [DOI] [PubMed] [Google Scholar]
- Liebel F, Lyte P, Garay M, Babad J, & Southall MD (2006). Anti-inflammatory and antiitch activity of sertaconazole nitrate. Arch Dermatol Res, 298(4), 191–199. doi: 10.1007/s00403-006-0679-8 [DOI] [PubMed] [Google Scholar]
- Luker KE, Schultz T, Romine J, Leib DA, & Luker GD (2006). Transgenic reporter mouse for bioluminescence imaging of herpes simplex virus 1 infection in living mice. Virology, 347(2), 286–295. doi: 10.1016/j.virol.2005.12.016 [DOI] [PubMed] [Google Scholar]
- Morrison LA, & Knipe DM (1994). Immunization with replication-defective mutants of herpes simplex virus type 1: sites of immune intervention in pathogenesis of challenge virus infection. J Virol, 68(2), 689–696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nowotny M (2009). Retroviral integrase superfamily: the structural perspective. EMBO Rep, 10(2), 144–151. doi: 10.1038/embor.2008.256 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nowotny M, & Yang W (2009). Structural and functional modules in RNA interference. Curr Opin Struct Biol, 19(3), 286–293. doi: 10.1016/j.sbi.2009.04.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piret J, & Boivin G (2011). Resistance of herpes simplex viruses to nucleoside analogues: mechanisms, prevalence, and management. Antimicrob Agents Chemother, 55(2), 459–472. doi: 10.1128/aac.00615-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmit I, & Boivin G (1999). Characterization of the DNA polymerase and thymidine kinase genes of herpes simplex virus isolates from AIDS patients in whom acyclovirand foscarnet therapy sequentially failed. J Infect Dis, 180(2), 487–490. doi: 10.1086/314900 [DOI] [PubMed] [Google Scholar]
- Schumacher AJ, Mohni KN, Kan Y, Hendrickson EA, Stark JM, & Weller SK (2012). The HSV-1 exonuclease, UL12, stimulates recombination by a single strand annealing mechanism. PLoS Pathog, 8(8), e1002862. doi: 10.1371/journal.ppat.1002862 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selvarajan Sigamani S, Zhao H, Kamau YN, Baines JD, & Tang L (2013). The structure of the herpes simplex virus DNA-packaging terminase pUL15 nuclease domain suggests an evolutionary lineage among eukaryotic and prokaryotic viruses. J Virol, 87(12), 7140–7148. doi: 10.1128/jvi.00311-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith KO (1964). Relationship between the envelope and the infectivity of herpes simplex virus. Proc Soc Exp Biol Med, 115, 814–816. [DOI] [PubMed] [Google Scholar]
- Subissi A, Monti D, Togni G, & Mailland F (2010). Ciclopirox: recent nonclinical and clinical data relevant to its use as a topical antimycotic agent. Drugs, 70(16), 2133–2152. doi: 10.2165/11538110-000000000-00000 [DOI] [PubMed] [Google Scholar]
- Summers BC, Margolis TP, & Leib DA (2001). Herpes simplex virus type 1 corneal infection results in periocular disease by zosteriform spread. J Virol, 75(11), 5069–5075. doi: 10.1128/jvi.75.11.5069-5075.2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tavis JE, Wang H, Tollefson AE, Ying B, Korom M, Cheng X, Cao F, Davis KL, Wold WS & Morrison LA (2014). Inhibitors of nucleotidyltransferase superfamily enzymes suppress herpes simplex virus replication. Antimicrob Agents Chemother, 58(12), 7451–7461. doi: 10.1128/aac.03875-14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Velzen M, van de Vijver DA, van Loenen FB, Osterhaus AD, Remeijer L, & Verjans GM (2013). Acyclovir prophylaxis predisposes to antiviral-resistant recurrent herpetic keratitis. J Infect Dis, 208(9), 1359–1365. doi: 10.1093/infdis/jit350 [DOI] [PubMed] [Google Scholar]
- Vere Hodge RA, & Field HJ (2013). Antiviral agents for herpes simplex virus. Adv Pharmacol, 67, 1–38. doi: 10.1016/B978-0-12-405880-4.00001-9 [DOI] [PubMed] [Google Scholar]
- Wang Y, Wang Q, Zhu Q, Zhou R, Liu J, & Peng T (2011). Identification and characterization of acyclovir-resistant clinical HSV-1 isolates from children. J Clin Virol, 52(2), 107–112. doi: 10.1016/j.jcv.2011.06.009 [DOI] [PubMed] [Google Scholar]
- Weller SK, & Coen DM (2012). Herpes simplex viruses: mechanisms of DNA replication. Cold Spring Harb Perspect Biol, 4(9), a013011. doi: 10.1101/cshperspect.a013011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitley RJ (2001). Herpes Simplex Viruses In Knipe D, Howley P (Ed.), Fields Virology (Fourth Edition ed, Vol. Volume 2, pp. 2461–2509). Philadelphia, PA: Lippincott Williams & Wilkins. [Google Scholar]
- Willey DE, Williams I, Faucett C, & Openshaw H (1991). Ocular acyclovir delivery by collagen discs: a mouse model to screen anti-viral agents. Curr Eye Res, 10 Suppl, 167–169. [DOI] [PubMed] [Google Scholar]