Abstract
Although many people think of aggression as a negative or undesirable emotion, it is a normal part of many species’ repertoire of social behaviors. Purposeful and controlled aggression can be adaptive in that it warns other individuals of perceived breaches in social contracts with the goal of dispersing conflict before it escalates into violence. Aggression becomes maladaptive, however, when it escalates inappropriately or impulsively into violence. Despite ample data demonstrating that impulsive aggression and violence occurs in both men and women, aggression has historically been considered a uniquely masculine trait. As a result, the vast majority of studies attempting to model social aggression in animals, particularly those aimed at understanding the neural underpinnings of aggression, have been conducted in male rodents. In this review, we summarize the state of the literature on the neurobiology of social aggression in female rodents, including social context, hormonal regulation and neural sites of aggression regulation. Our goal is to put historical research in the context of new research, emphasizing studies using ecologically valid methods and modern sophisticated techniques.
Keywords: social aggression, neural circuitry, sex hormones, ecological context, animal models
1. Introduction
Aggression is a natural component of social interactions among all higher-order animals (Bjorkvist and Niemela, 1992; Blanchard and Blanchard, 2003; de Waal, 2000; Potegal, 2012) that functions to optimize evolutionary fitness (Archer, 1988). Given the complexity of social systems, it is not surprising that aggression is not a unitary behavior, but one that is displayed in different circumstances (e.g., defense of territories, resources, and offspring) and for different purposes (Archer, 1988). Further, these different forms of aggression have separate neural circuitries and cellular mechanisms (e.g., Haller, 2013).
Social, or interpersonal, aggression is the most prevalent form of aggression among people and has been modeled extensively in animals. Perhaps due to sociocultural factors or gender stereotyping, the prevailing view has been that social aggression is largely a male-specific behavior. This archaic idea is at odds with literature demonstrating that females readily engage in social, interpersonal aggression (Björkqvist and Niemelä, 1992; Campbell, 2007). Indeed, female aggression is a major problem in mental health and criminal justice settings and presents as a symptom of numerous psychiatric disorders. For example, in recent years, arrests for assault have increased among adolescent girls (Campbell, 2013; FBI, 2012) and diagnoses for conduct disorder have likewise increased in girls (Collishaw et al., 2004; Freitag et al., 2018).
Most instances of aggression among human females are against other females, whereas human males most often are aggressive towards other males (Campbell, 2013). However, one realm in which women are aggressive towards men comes from studies of domestic violence in which physical assaults occur in millions of families every year (Reviewed by Kimmel, 2002). A surprising discovery from the 1985 Second National Family Violence Survey revealed that approximately 12% of women and 11% of men reported being physically aggressive toward their spouses at least once in the previous year. A more recent longitudinal survey found that within a year and a half of being married about a third of both husbands and wives were physically aggressive towards their spouse (O’Leary et al., 1994). Instances of interpersonal aggression are reported across many social and ethnic groups in the US and around the world (Falk et al., 2017; Gaman et al., 2017; Harwell et al., 2003; Lown and Vega, 2001; Ramos et al., 2004). It is clear then that there is an acute need for a better understanding of the neurobiology of female aggression.
An unfortunate consequence of the textbook view of aggression being primarily in the domain of male social behaviors is that progress in our understanding of the neurobiology regulating aggression in females has been slow to gain traction. Attempts to bring female aggression into the mainstream have largely been ignored. DeBold and Miczek wrote in 1984: “More correctly, male and female rats are sexually dimorphic in terms of the stimuli which elicit aggression and not in terms of males being aggressive and females nonaggressive.” (p.185). Rather than becoming a landmark volume, the book “Of Mice and Women: Aspects of Female Aggression” edited by Kaj Bjorkqvist and Pirkko Niemela (1992), has languished in obscurity, receiving only a single (though enthusiastic) rating on Amazon. This state of affairs has left our understanding of the neurobiology of female aggression in the dark ages, especially in comparison to the sophisticated application of modern molecular tools to the analysis of inter-male aggression (Golden et al., 2011; Lin et al., 2011).
In this narrative review, we wish to complement the excellent overview provided by Duque-Wilckens and Trainor (2017) highlighting sex similarities and differences in aggression in animal models. Here, we will focus on social aggression in females (typically modeled in the resident-intruder paradigm) and omit studies of maternal aggression that represent an important, but less broadly applicable, example of female aggression. In taking this approach, we will emphasize common principles for the contextual, hormonal and neural control of aggression across rodent models of female aggression (Table 1). The goal here is to inspire ecologically-valid investigations into mechanisms of female aggression paralleling the advances made in our understanding of aggression in males.
Table 1.
Brain Regions and Neural Mechanisms Involved in Regulation of Female Aggression
| Brain Region | Manipulation | Effect on Aggression | References |
|---|---|---|---|
| VMH | |||
| lesion | ↑ aggression | Albert et al, 1992a; Malsbury et al., 1977 | |
| estradiol implant (plus systemic P) | ↓ aggression | Meisel and Sterner, 1990; Sterner et al., 1992; Takahashi and Lisk, 1985 | |
| progesterone implant (with prior estradiol) | ↓ aggression | Meisel and Sterner, 1990; Takahashi and Lisk, 1985a | |
| various | estrogen receptors critical for aggression | Hashikawa et al., 2017a | |
| AH | |||
| lesion | ↑ aggression | Hammond and Rowe, 1976 | |
| AVP infusion | ↓ aggression | Gutzler et al., 2010 | |
| AVP antagonist | ↑ aggression | Gutzler et al., 2010 | |
| serotonin agonist | ↑ aggression | Terranova et al., 2016 | |
| MPOA | |||
| lesion | ↓ aggression | Hammond and Rowe, 1976 | |
| OT infusion | ↓ aggression | Harmon et al., 2002 | |
| OT antagonist | ↑ aggression | Harmon et al., 2002 | |
| Amygdala | |||
| corticomedial lesion | ↓ aggression | Potegal et al., 1996 | |
| ↑ pERK labeling | ↑ aggression | Silva et al., 2010 | |
| BNST | |||
| ↑c-Fos labeling | ↑ aggression | Davis and Marler, 2004 | |
| ↑ pERK labeling | ↑ aggression | Silva et al., 2010 | |
| NAc | |||
| mGluR5 signaling | ↑ aggression | Been et al., 2016 | |
| VTA | |||
| progesterone antagonist | ↑ aggression | Frye et al., 2014 | |
| Ventricles | |||
| ventricular serotonin agonist | no effect | Joppa et al., 1997 | |
| ventricular OT | ↓ aggression | McCarthy, 1990 | |
2. Social Context
Among rodents, there is immense variation is social ecology and behavior. Rats and mice, the most commonly studied laboratory rodents, tend to live in multigenerational polygamous social groups and are considered highly social animals. Mice experience more variation in their adaptability to different living situations, which manifests in their social structures; mice that have access to excess food, such as in urban areas, tend to live under a social hierarchy, whereas those that live in open areas, such as fields or shrubs, tend to be more territorial. Nonetheless, the majority of mice and rat species are social and polygamous. Prairie voles, another well-studied laboratory rodent, are also social and live in colonies, although they may maintain social monogamy or polygamy within those colonies. Finally, Syrian hamsters are territorial and solitary (Gattermann et al., 2001). Djungarian hamsters (P. campbelli) may have a monogamous mating system with overlapping territories with other females, yet for the closely related Siberian hamster (P. sungorus) females maintain exclusive home territories (Wynne-Edwards and Lisk, 1987). These differences in social ecology ultimately impact many realms of behavioral interactions among these species, including aggression in females. As such, trying to draw broad conclusions about aggressive behavior in females, or its underlying neurobiology, from any one species can lead to misleading conclusions.
Although female aggression is understudied in a laboratory setting, enough research exists to draw conclusions about the impact of different social ecologies on this behavior. The differences among genera and species described above not only impact levels of aggression but also how this behavior changes under different living conditions and stimuli induced in a laboratory. Thus, female aggression manifests differently in rodent species based on their social ecology and these differences are prominent when examining social context and aggression in a laboratory setting.
When considering the impact of social context on aggression, research can be divided into two main subtypes: first, differences in aggression based on the context of encounters regardless of their natural history and second, differences based on the natural history of the animal, such that the behavioral ecology of that species impacts how each species reacts to social conditions. Although the first refers to proximate social context while the second refers to ultimate natural history, both are of interest when determining the factors that impact and predict female aggression.
Irrespective of baseline female aggression between species, the size of the intruder, typically a reflection of age, is often predictive of whether or not an attack will occur. For example, female mice will attack younger intruders longer than older intruders (Ayer and Whitsett, 1980). Similarly, the body weight of female Syrian hamsters is predictive of social dominance, such that higher body weight is positively correlated with dominance; when matched with males of equal weight, females are always dominant, but when matched with a heavier male, dominance is mixed (Drickamer and Vandenbergh, 1973; Marques and Valenstein, 1977). Interestingly, whereas this high correlation of weight with dominance is present in both sexes of Syrian hamsters, it is more pronounced in females (Payne and Swanson, 1970). Similarly, most species tend to be more aggressive in their home as compared to an unfamiliar place, a phenomenon especially studied in rats (Albert et al., 1988). Thus, the size of the intruder and the place of an encounter are two general contexts that can vastly change a female’s aggressive response, regardless of the natural history of the animal.
Though the differences described above may seem obvious, differences that exist based on the social ecology of specific species are much more complex. Cohabitation, housing with a conspecific during or prior to testing, has profoundly different species-specific effects based on social ecology. In rats, females housed with a gonadally-intact male are significantly more aggressive towards unfamiliar females than those housed with a gonadectomized male. Females housed with either an intact or a gonadectomized male are still more aggressive towards an unfamiliar female intruder than those housed with an intact female (Albert et al., 1988; Albert et al., 1993). Further, females housed in mix-sex colonies are less aggressive towards male intruders than female intruders, but when housed as a single-sex colony, a dominant female will emerge that will willingly attack any male intruder (Blanchard et al., 2001). Comparably, male-female housed pairs of mice are significantly more aggressive than are uni-sex pairs, and a positive correlation exists between population size and aggression, such that mice housed in a larger mixed population express more aggression (Ayer and Whitsett, 1980). The effect of housing in isolation versus within a group is less clear; female Rockland-Swiss albino mice are quicker to fight after being housed in isolation than within a group, while the opposite effect is seen in ICR laboratory house mice (Gray, 1979; Howard et al., 1981). Generally speaking, these data suggest that cohabitation with a male conspecific, rather than a female or a sterile male, increases female aggression in both rats and mice.
Cohabitation has also been studied in prairie voles and hamsters. As in mice and rats, cohabitation with males produces a significant increase in aggression in female prairie voles over time, specifically against another female intruder (Bowler et al., 2002). However, this female-female aggression is altered through the relationship of the two females; when a trio of two females and a male are housed together, significantly less female-female aggression and more prosocial behaviors occur if the two females are siblings (Firestone et al., 1991). In Syrian hamsters, group housing causes a significant decrease in aggression, specifically toward young intruders (Brain, 1972). Further, comparison of different species of hamsters reveals species-specific aggression patterns; Female Turkish hamsters seem to exhibit equal aggression towards both conspecific and heterospecific species, whereas female Syrian hamsters exhibit significantly more aggression towards heterospecific intruders (delBarco-Trillo and Johnston, 2012).
Similarly, hormones often play a role in sex-specific aggressive responses, such that alterations in a female’s hormonal state can change her response to a male, versus a female, intruder. In rats, females primarily attack female intruders, regardless of the hormonal status of the intruder (DeBold and Miczek, 1984). In mice, however, the effects of hormonal status are less concrete; there are reports that females are equally aggressive towards male and female intruders, and that females who are treated with estradiol, as opposed to testosterone, remain non-aggressive. In comparison, testosterone-treated females readily attack intact males, a response characteristic of males, while they remain relatively unaggressive towards females (Ayer and Whitsett, 1980; Barkley and Goldman, 1978).
A variety of prior experiences can impact female aggression across species (de Jonge, 1986). In both Syrian hamsters and prairie voles, sexual experience seems to increase the female’s aggressive response (Bales and Carter, 2003; Bowler et al., 2002; Carter, 1973). A comparable effect of previous aggressive experience is seen in hamsters. Female hamsters who experience an aggressive encounter are “primed” for further aggression in the short term, such that they will attack a second intruder significantly faster than the first within a 30 minute period, a change that is associated with transient neurophysiological changes in the amygdala (Potegal et al., 1996; Potegal, 1992). In the long-term, the effect of aggressive experience depends on the outcome of the aggressive encounter for the female. When female hamsters are given brief repeated encounters with a non-aggressive intruder, their aggressive behavior escalates such that the onset of aggressive behavior is faster in future encounters, even in the absence of recent aggressive experience. This type of aggression escalation is marked by increased dendritic spine density in the nucleus accumbens (Staffend and Meisel, 2012). In contrast, female hamsters who experience repeated defeats show a long-term decrease in aggressive behavior, a phenomenon known as conditioned defeat (Huhman et al., 2003; Potegal, Huhman, Moore, & Meyerhoff, 1993). This effect is most pronounced during diestus 2 and proestrus (Solomon, Karom, & Huhman, 2007), when aggression levels are relatively low. Rats who experience peri-pubertal stress express more aggression when confronted with ovariectomized females and intact males, regardless of the aggressor-female’s stage of estrus (Cordero et al., 2013). However, female hamsters are more capable of adapting to repeated social stress than males; exposure to stress on a repetitive basis leads to similar increases in serum cortisol levels in males with each exposure, while these levels decrease in females over time (Taravosh-Lahn and Delville, 2004). These results suggest that previous stress-related experience, whether it be in the form of induced peri-pubertal stress or a recent aggressive-encounter, increases aggression levels. Hamsters, however, are capable of adapting to repeated social stress, suggesting a difference between rats and hamsters. While these differences alone are interesting, the neurobiological substrates underlying these differences may be more important in predicting and diffusing female aggression.
3. Genetic Selection
Essentially nothing is known of the genetics of inter-female aggression though this is certainly an area worth studying. Ebert and Hyde (1976) captured wild house mice (Mus musculus) and tested them in a resident-intruder paradigm with female mice as stimulus animals. Of the wild-caught mice, approximately 25% were aggressive towards the female intruder. Given the behavioral variability of the captured sample, Ebert and Hyde (1976) proposed the behavioral variability might be reflected in genetic variability among the individual mice. To test this they selected the female mice that displayed high or low levels of aggression, breeding the high or low aggression offspring for 11 generations (Hyde and Sawyer, 1980). The selection process produced a divergent lines of mice that differed from randomly selected mice bred over the same number of generations.
Several interesting conclusions emerged from this simple behavioral genetics approach. The first was that selection on female offspring did not affect levels of aggression in the male littermates (Ebert and Hyde, 1976). A second was that decreases in aggression emerged more quickly and were of a greater magnitude than did increased aggression in the selected lines. What is important about this latter finding is that C57BL/6 mice are less aggressive than their wild-caught ancestors. As suggested by Ebert and Hyde (1976) the relative absence of aggression in C57BL/6 mice probably emerged as a byproduct of the generation of this mouse strain. As C57BL/6 strains are both commonly used in mouse research, and as this strain forms the genetic background for many knockout and transgenic lines, it will be important to identify other mouse strains if modern genetic approaches are to be applied to studying female aggression.
4. Photoperiod
Syrian and Siberian hamsters breed seasonally such that they are reproductively active during the long photoperiods of late spring and summer, with a collapse of the reproductive system as fall and winter approach (reviewed in Goldman, 2001). These seasonal changes in physiology and behavior can be mimicked in the laboratory by altering the light cycle in the animals’ vivarium. A daily regimen in which the lights are on for 14 hours per day produce reproductive competence, whereas reducing the amount of light per day to perhaps 6 or 8 hours induces the involution of reproductive physiology. Hamsters are interesting in that the induction of short light photoperiods impairs reproduction, though the spring-like recovery of the system (recrudescence) occurs spontaneously, even if the hamsters are kept in short light. The effects of light are mediated through a neural pathway, ultimately affecting pineal secretion of melatonin. As light inhibits melatonin secretion, high daily levels of melatonin mediate the effects of short day lighting on reproduction.
These experimental approaches taken to examine seasonal changes in reproduction have been applied to developing an understanding of the seasonal regulation of aggression in females. In general, these studies show that short (winter) photoperiods increase the levels of aggression in female hamsters. As with reproduction, maintaining female Syrian hamsters in a short photoperiod produced a longitudinal increase in aggression, first seen after 14 weeks in short days (Honrado, Paclik, and Fleming, 1991). Interestingly, with continued housing in short photoperiods there was a spontaneous reduction of aggression to summer levels by 20 weeks (Honrado et al., 1991). Thus, the increase in aggression induced by short photoperiods is light mediated, while the circannual recovery is light independent.
Generally, studies of Syrian and Siberian hamsters support the idea that short photoperiods enhance aggression in females (Badura and Nunez, 1989; Caldwell and Albers, 2004; Fleming et al, 1988; Gutzler et al., 2009; Honrado et al., 1991; Scotti et al., 2007). For most of these studies the change was registered as a straightforward increase in offensive attacks. One study by Fleming et al. (1988) did not measure any photoperiod induced changes in offensive aggression, but instead noted a reduction in defensive postures with the introduction of short-day lighting. The Fleming et al. (1988) study was the only one to use a neutral arena (rather than a conventional resident-intruder paradigm) to test the animals, which might account for the higher levels of defensive behaviors.
The natural expectation was that the effects of short-day lighting on aggression were mediated vial the pineal gland. Indeed, female hamsters with their pineal gland surgically removed showed no change in aggression when maintained on short days (Badura and Nunez, 1989; Fleming et al., 1988).The results from the converse experiment, giving extended exposure to melatonin in females housed in long-day photoperiods were more mixed. In one case there was no effect of melatonin administration (Badura and Nunez, 1989), whereas another study melatonin decreased defensive behaviors like that seen in short-day hamsters (Fleming et al., 1988).
Because changes in light cycles classically affect reproductive physiology, the sensitivity of female Syrian hamsters kept in short days to the inhibitory effects of ovarian hormones was studied (Badura and Nunez, 1989). As with long day photoperiods, estradiol alone had no effect on aggression in females maintained in short days. High doses of progesterone in conjunction with estradiol inhibited aggression regardless of photoperiod. The ability of photoperiod to affect sensitivity to hormones was detected with low doses of progesterone. Here, progesterone was without effect on short day Syrian hamsters. A similar approach was taken with Siberian hamsters (Scotti et al., 2007). For Siberian hamsters short days also accentuated aggression, but as noted neither estrous cycle nor hormone treatments modulated aggression in animals maintained in either long or short photoperiods.
The general hypothesis going into these studies was based on the notion that during the breeding season the activation of female sexual responsiveness inhibits aggression. Consequently, with the suppression of reproduction in winter photoperiods there should be a corresponding release of aggression. The basic phenomenon of enhanced aggression by short photoperiods is supported, albeit given a sparse literature. With a limited number of exceptions (Caldwell and Albers, 2004; Gutzler et al., 2009) there have been no real attempts to investigate mechanisms mediating the increases in aggression with short photoperiods. Interest in this question is timely given a technically sophisticated study by Todd et al. (2018) demonstrating a neural circuit mediating a circadian rhythm in aggression in male mice. At the same time, this state of the literature is unlikely to change as the economics of laboratory research make such long-term studies cost-prohibitive.
5. Hormonal Control
Historically, the prevailing belief that females are not normally aggressive skewed the way in which the endocrine basis of female aggression was primarily studied. The approach taken (reviewed by Gandelman, 1980) was to provide female rodents, in particular house mice, with hormone treatments that activated aggression in males. These hormone treatments took two forms. One was to ovariectomize female mice, for example, and give them chronic administration of testosterone or estradiol [based on the ‘aromatazation hypothesis’ of testosterone actions (Shinoda, 1994)]. The results of these studies were that given a high enough dose and extended duration of exposure, female mice would indeed attack stimulus males, though perhaps at lower levels of aggression than do normal males. The conclusion from these studies was that females had the neural circuitry for aggression, though they were less sensitive to the activational effects of gonadal steroids than were males (Gandelman, 1980). In the second paradigm, female mice were treated neonatally with testosterone or estradiol during the sensitive period of sexual differentiation to direct the development of neural pathways in a male-like direction. These early hormone treatments made the females more responsive to adult hormone treatments with these females displaying levels of aggression equivalent to that of normal males (Gandelman, 1980).
Fortunately, others have taken a more ecological approach to the question of hormonal regulation of aggression in female rodents. Aggression is of course antithetical to affiliative social interaction, a necessary condition for mating. Consequently, it made sense to assume that females would be less aggressive during the phase of their reproductive (i.e., estrous) cycle during which copulation occurred. With a few exceptions reported in rats (Cordero et al., 2013; de Jong et al., 2014) aggression fluctuated with the estrous cycle demonstrating the lowest levels during the stage of the female’s height of sexual responsiveness (corresponding to vaginal proestrous cytology) for rats (Ho et al, 2001), house mice (Koonce and Frye, 2014). Syrian hamsters (Ciaccio et al., 1979; Floody and Pfaff, 1977; Kislak and Beach, 1955; Wise, 1974) and deer mice (Davis and Marler, 2004).
The estrous cycle variations in aggressiveness to intruders logically suggested that fluctuations in ovarian hormone secretion formed the underlying basis for the expression of aggression. Spontaneously ovulating rodents often have a brief (estrous) cycle lasting about 4 days. During this cycle there are sequential surges in estradiol followed by progesterone leading to a period of sexual responsiveness by the female that is associated with the proestrous phase of vaginal cytology (See Figure 1). As we noted, there is a decrease in aggression in cycling female rodents corresponding to the proestrus phase, naturally suggesting that the sequence of estradiol and progesterone secretion activating female sexual behavior also is the hormonal basis for the inhibition of aggression. A similar pattern is observed for induced ovulators (e.g., prairie voles) in which females exhibit high levels of aggression without ovarian hormones (Bowler et al., 2002; Dluzen and Carter, 1979). As a result, much of the focus of research on the hormonal control of female aggression has manipulated estradiol and/or progesterone levels to see the effects of these manipulations on aggression.
Figure 1. Aggression varies across the estrous cycle.
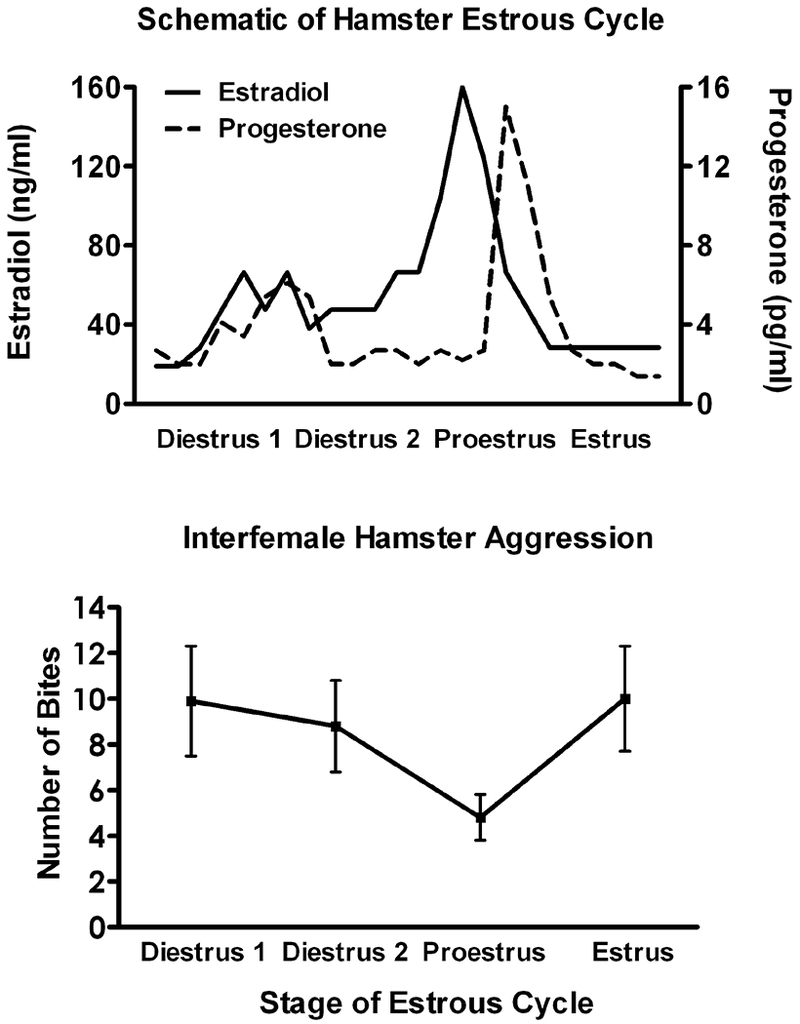
Fluctuations in aggression across the estrous cycle are commonly reported, though effects vary in magnitude among rodent species. Syrian hamsters are a species in which consistent variation in aggression is reported. Top: Schematic of hamster estrous cycle illustrating fluctuations in blood levels of the ovarian hormones estradiol and progesterone. Estradiol and progesterone levels both rise during Diestrus 2, peak surrounding Proestrus, and are lowest during Estrus and Diestrus 1. Bottom: Number of bites as a function of estrous cycle. During a five-minute resident-intruder test, female hamsters are most aggressive when estradioland progesterone levels are lowest. In contrast, during Proestus, when estradiol and progesterone levels are highest, the number of bites decreases.
In general, female rodents are aggressive following removal of their ovaries (DeBold and Miczek, 1984; Kislak and Beach, 1955; Lisk and Nachtigall, 1988; McDermott and Carter, 1980; Razzoli et al., 2003; Suchowsky et al., 1971) and show more extreme differences in levels of aggression across hormonal treatments than across the estrous cycle (e.g., Figure 1). Treatment of ovariectomized females with estradiol alone does not affect the levels of aggression regardless of the rodent species (Bowler et al., 2002; Ciaccio et al., 1979; Dluzen and Carter, 1979; Floody and Pfaff, 1977; Frank and Fraps, 1945; Kislak and Beach, 1955; Meisel and Sterner, 1990; Meisel et al., 1988; Razzoli et al., 2003; Sterner et al., 1992). Short term progesterone on its own, mimicking the duration of the estrous cycle surge, also does not alter levels of aggression (Floody, 1977; Meisel et al., 1988; Meisel et al., 1990; Sterner et al., 1992). However if the estrous cycle sequence of estradiol followed by progesterone is simulated, aggression is reliably reduced (Ciaccio et al., 1979; Floody and Pfaff, 1977; Frank and Fraps, 1945; Kislak and Beach, 1955; Koonce and Frye, 2014; McDermott and Carter, 1980; Meisel and Sterner, 1990; Ogawa et al., 1998; Razzoli et al., 2003; Sterner et al., 1992).
The inhibiting effects of progesterone on aggression wane within a day or two after administration (eg. Ho et al., 2001; McDermott and Carter, 1980). More interestingly are observations that progesterone has a biphasic action on aggression. The spontaneous surge in progesterone during the estrous cycle can be followed by mating-induced progesterone release. This second release of progesterone is thought to induce the female’s progestational state; preparing the uterus to accept the fertilized ovum (Morin, 1977). Ciaccio et al. (1979) first demonstrated this biphasic action of progesterone in which the first administration inhibited aggression, whereas continued progesterone administration elevated aggression for as long as the progesterone was present. The Ciaccio et al. (1979) experiment utilized extended periods of estradiol and progesterone treatment, yet the interpretation of these extended treatments is not problematic as Meisel et al. (1990) demonstrated the same biphasic actions of progesterone with a more discrete estradiol and progesterone regimen.
The general conclusion from these studies is that female rodents are aggressive except for those periods in which sexual behavior is induced; a conclusion we support. What is important to note is that sexual receptivity and aggression are not mutually exclusive neurally as context can come to play in terms of whether sex or aggression is the prominent behavior displayed. Though an example of a study taking an artificial approach to ovarian hormone replacement, Meisel et al. (1988) implanted ovariectomized female hamsters with continuous release Silastic capsules containing estradiol. Over the 14 days of treatment the female hamsters showed increasing levels of female sexual behavior towards male hamsters, yet at the same time exhibiting high levels of aggression to female hamsters when tested on the same day. An injection of progesterone at the end of this extended estradiol treatment eliminated aggression towards intruder female hamsters as well.
6. Neurotransmitters and Signaling
6.1. Serotonin
It is well established that serotonin impacts aggression in male rodents across a number of species (Olivier and Mos, 1986). Generally, activation of the serotonin system in males decreases aggressive behavior, and several drugs that facilitate serotonin signaling have been termed “serenics” for their anti-aggressive properties (Takahashi et al., 2011). For example, when fluprazine, a serotonergic agonist, was administered to either wild-caught or a laboratory strain of house mice intrasexual aggression was inhibited (Ferrari et al., 1996). In agreement with this, suppression of monoamine oxidase-A, an enzyme which breaks down synaptic serotonin, caused an increase in aggression in female mice (Wallian et al., 1993). In female rats, short-term administration of a serotonin reuptake inhibitor significantly reduced estrous-dependent aggression (Ho et al., 2001).
In other studies the effects of manipulating serotonin on female aggression are not as clear. For example a mixed set of findings were reported by Haug et al. (1990) in which a serotonin agonist reduced aggression against a lactating female intruder, whereas an SSRI did not. In prairie voles, there was no impact of SSRIs on aggressive behavior in females, although the SSRIs significantly decreased male aggression (Villalba et al., 1997). Similarly, in female hamsters, serotonin agonists that effectively suppressed aggression in males either had no effect on aggression in females (Joppa et al., 1997) or further enhanced aggression (Terranova et al., 2016). This set of findings points to the importance of translational models taking note of sex as the development of pharmaceutics may have differential effectiveness on males and females.
6.2. GABA
Despite the substantial literature on the role of gamma-aminobutryic acid (GABA) in regulating aggression in males, much less is known about its role in females. In female hamsters, the relationship between aggression and GABA has mainly been investigated by correlating GABA binding with individual differences in aggressive behaviors. Using this paradigm, it was found that significantly higher levels of GABA binding occurs in the mid-brain regions of female hamsters selected for aggressiveness, regardless of their endocrine state (Potegal et al., 1982). Interestingly, a GABA reuptake inhibitor ethyl (R,S)-nipecotate suppresses aggressive behaviors in these more aggressive hamsters, but not in their less aggressive counterparts (Potegal, 1986). These results suggest that within-species differences in aggression may be due to intrinsic variances in endogenous GABA activity. Relatedly, alcohol, a GABA receptor agonist, has also been implicated in female aggression; in rats, low doses of ethanol increases aggression towards small intruder as compared to saline, or high doses of ethanol (Blanchard et al., 1987). Thus, there seems to be a positive relationship between GABA and aggression.
6.3. Oxytocin/Vasopressin
The roles of oxytocin and vasopressin in regulating aggression have been studied extensively in male rodents (e.g., Ferris and Potegal, 1988; Delville et al., 1996), as well as in female rodents who are pregnant or lactating (e.g., Bosch and Neumann, 2012). Far less attention, however, has been paid to the role of these neuropeptides in regulating non-maternal female aggression.
Contrary to the ample evidence suggesting that vasopressin facilitates aggression in male rodents, vasopressin may have an inhibitory effect on aggression in females. In hamsters, injection of Manning compound, a potent VIA receptor antagonist, into the anterior hypothalamus reduces attack latency and increases the duration of aggressive behavior (Gutzler et al., 2010). Injecting AVP itself into the AH of female hamsters has mixed results depending on the social context in which animals are tested. In the absence of conspecifics, AVP injection into the AH increases flank marking, a territorial scent marking behavior (Hennessey et al., 1994). However, in the presence of a conspecific female intruder, AVP injection into the AH has no effect on flank marking. Following social defeat, female California mice have significantly more activated AVP neurons in the SON and PVN (Steinman et al., 2015), suggesting that perhaps AVP signaling plays a part in the inhibition of aggressive behavior following social defeat. In contrast, in mandarin voles, another rodent species in which females show high levels of aggression, dominant females have significantly more vasopressin-immunoreactive neurons in the lateral hypothalamus and anterior hypothalamus than do subordinates (Qiao et al., 2014), suggesting that there may be species differences in how vasopressin regulates non-maternal female aggression. Research on the role of vasopressin in regulating aggression in female rats and mice is notably absent.
Research on the role of oxytocin in regulating female aggression is focused primarily on aggression in a maternal context rather than offensive or territorial aggression. Despite this, there is some research to suggest that, like vasopressin, oxytocin has an inhibitory role on aggression in female rodents. In female hamsters, injecting OT into the MPOA/AH significantly reduces the duration of aggressive behavior towards a non-aggressive female conspecific intruder, whereas blocking oxytocin receptors in this brain region increases the duration of aggression under the same behavioral conditions (Harmon et al., 2002). In female mice, injections of OT in the cerebral ventricles inhibits aggression towards unfamiliar juveniles (McCarthy, 1990). Finally, when compared to wildtype mice, OT knockout mice are more aggressive than their wild type counterparts (Ragnauth et al., 2005). Together, these data suggest that oxytocin inhibits female aggression outside of the peripartum period.
7. Neural Circuitry
Several key brain regions are involved in the neural circuitry underlying female aggression. As outlined in recent reviews (Duque-Wilckens and Trainor, 2017; Hashikawa et al., 2016), these brain regions overlap with and differ from the neural circuitry underlying male aggression in several ways. It is worth noting that the majority of the research on the neurobiology of female aggression is dated, both chronologically and methodologically. We would assert this is due to the widespread, but incorrect, dogma regarding sex differences in aggression (i.e., females are not aggressive), perceived difficulty in working with female research subjects, or a general lack of interest in female biology. While several labs are currently using modern, sophisticated methods (Golden et al., 2011, 2016, 2017; Falkner et al., 2014, 2016; Lin et al., 2011) to dissect the neural circuitry underlying male social aggression, few (e.g., Hashikawa, et al., 2017a) have made parallel strides in females. Here, we attempt to summarize the literature on the neural regulation of female social aggression, highlighting when possible contemporary studies that are using modern approaches.
7.1. Hypothalamus
In females, the most thoroughly-studied brain region underlying aggressive behavior is the hypothalamus. Early electrical stimulation studies found that, like in males, stimulation of several hypothalamic areas elicits aggression in female rats that is not affected by ovariectomy or estrogen replacement (Kruk et al., 1984). Although several hypothalamic nuclei have been shown to contribute to the neural circuitry controlling female aggression, the ventromedial hypothalamus (VMH) has the largest body of literature supporting its role.
It is well-established that VMH lesions increase aggressive behaviors in female Syrian hamsters (Malsbury et al., 1977) and rats (Albert et al, 1992a). Closer investigation reveals that antereolateral VMH lesions are sufficient to induce this change, while postereolateral lesions have little to no effect (Malsbury et al., 1978). More recently, single-unit recordings in freely behaving female mice have shown that a large portion of VMH neurons are activated in the presence of conspecifics, and this neural activity is enhanced in response to male, but not female, conspecifics only when females are sexually receptive (Nomoto and Lima, 2015). This suggests that VMH activity drives sex-specific and reproductive state-dependent social behaviors, such as aggression.
Given its role in choosing the appropriate behavioral response in many social contexts, it not surprising that activation of the VMH for female aggression is mediated in part by ovarian hormones. Estradiol implants into the VMH in conjunction with systemic progesterone injections significantly decrease aggressive behavior; this finding has been reported a number of times in golden hamsters (Meisel and Sterner, 1990; Sterner et al., 1992; Takahashi and Lisk, 1985b). Likewise, progesterone implants into the VMH, following systemic estradiol, significantly decrease female hamster aggression towards conspecific females and males (Meisel and Sterner, 1990; Takahashi and Lisk, 1985a). Thus, estradiol and progesterone likely mediate aggressive behavior through a negative feedback mechanism on the VMH.
Recent work has demonstrated that estrogen receptor alpha expressing (esr+) cells in the ventrolateral portion of the VMH (VMHvl) are critical for aggression in female mice (Hashikawa et al., 2017a), as was previously shown for aggression in male mice (Lee et al., 2014; Lin et al., 2011; Yang et al., 2013). Using a behavior-centered, technically-sophisticated paradigm, they showed that Esr+ VMHvl cells are are activated during aggression, acute inhibition of these neurons decreases aggressive behavior, and acute activation elicits aggressive behavior in female mice. What’s more, using c-Fos mapping and optical recording, they discovered that the VMHvl contains anatomically-distinct populations of cells that are responsible for aggression or sexual behavior in female mice. Consistent with the early behavior genetics work summarized in Section 3, aggression was seen primarily in female mice from a Swiss-Webster background and not from C57BL/6 mice.
The anterior hypothalamus (AH), lying rostral to the VMH, has also been implicated in the circuitry of aggression. Here, however, the literature is more variable. Generally speaking, AH lesions lead to increased female aggression, but this effect is reduced by ovariectomy, suggesting that ovarian hormones act on the AH to activate aggressive behavior (Hammond and Rowe, 1976). However, neither estradiol nor progesterone injection into the AH has an effect on female aggression (Meisel and Sterner, 1990; Takahashi and Lisk, 1985a, 1985b; Sterner et al., 1992). Despite the lack of effect of estradiol in the AH on aggression, estradiol injection into the AH increases the association between vaginal marking and aggression in female hamsters, a relationship which is typically inverse. This effect is diminished by simultaneous injection of estradiol into the VMH (Takahashi and Lisk, 1985b), suggesting that the effect of ovarian hormones is not specific to the AH. Instead, arginine vasopressin (AVP) may mediate the reported role of the AH in aggression. Indeed, AVP regulates female aggression and social communication in a number of species. For example, dominant female prairie voles display significantly more vasopressin-immunoreactive neurons in the AH than subordinates (Qiao et al., 2014). In female hamsters, injection of AVP into the AH inhibits female aggression (Gutzler et al., 2010). Specifically, AVP acts on V1a receptors to exert its effect on social behaviors; injection of a V1a antagonist into the AH increases female aggression (Gutzler et al., 2010). Taken together these data suggest that it is primarily vasopressin, rather than ovarian hormones, acting in the AH to inhibit female aggression.
The AH is often considered as part of a continuum with its close neighbor, the medial preoptic area (mPOA) and, when considered together, provide additional insight into the neural regulation of female aggression. For example, when oxytocin is injected into the mPOA-AH complex of female hamsters directly before testing, there is a significant decrease in the duration of female-female aggression (Harmon et al., 2002). Similarly, when an oxytocin antagonist is injected into the MPOA-AH 30 minutes prior to testing, there is a significant increase in aggression towards a non-aggressive female intruder (Harmon et al., 2002). Interestingly, when a cholinergic agonist is injected into the mPOA-AH of female rats, females are more aggressive towards male intruders (Floody, 2009). Because female hamsters readily attack both male and female intruders, this suggests a differential effect of oxytocin versus acetylcholine on the mPOA/AH complex. In cases where the mPOA and AH are examined separately, there is still a clear role of the mPOA in the circuitry of female aggression. Bilateral lesions of the mPOA reduce aggression and increase submissive responding in female hamsters (Hammond and Rowe, 1976). This effect may be mediated at least in part by progesterone, as progesterone injection into the mPOA is highly effective in inhibiting female aggression towards male intruders (Takahashi and Lisk, 1985a).
7.2. Extended Amygdala
In contrast to the hypothalamus, the amygdala appears to facilitate aggression in females. Radiofrequency lesions to the amygdala in hamsters reduce aggression, an effect that is greatest when lesions are placed in the anterior amygdala (Potegal et al., 1996). Further, attack priming in hamsters, in which latency to attack intruders is decreased with repeated aggressive exposure, is linked to increased c-Fos expression in the amygdala (Potegal et al., 1996). In female California mice, increases in aggression associated with short-day photoperiod are concomitant with increased pERK expression in the medial amygdala (Silva et al., 2010). As with the hypothalamus, vasopressin is likely involved in regulated aggression via the amygdala; this same short-day photoperiod aggression is also associated with lower levels of AVP-receptor binding within the amygdala (Caldwell and Albers, 2004). In female mice, aromatase-expressing neurons in the posterodorsal medial amygdala are specifically required for some components of maternal aggression, but not for aggressive behavior outside of a maternal context (Unger et al., 2015). Aggression is not, however, affected by estradiol in the amygdala, suggesting that certain forms of aggression may involve the amygdala, while estrous-dependent aggression likely does not (Sterner et al., 1992).
Often considered an extension of the amygdala, the bed nucleus of the stria terminalis (BNST), also plays a role in female aggression (Lebow and Chen, 2016). Due to its close relationship with the amygdala, both neuroanatomically and functionally (Lebow and Chen, 2016) it is not surprising that it is implicated in the same types of aggression. For example, the BNST is implicated in the aggression priming plasticity described previously in female hamsters (Potegal et al., 1996). Furthermore, short-photoperiod aggression increases are linked to increased pERK expression in the BNST (Silva et al., 2010) and lower levels of AVP-receptor binding in the BNST of female hamsters (Caldwell and Albers, 2004). Interestingly, the BNST also has a role in estrous-induced aggressive behavior, such that c-Fos expression increases in the BNST of female California mice with estrous-associated increases in aggression (Davis and Marler, 2004). So, while the BNST is closely related to the amygdala, is it implicated not only in the aggression circuitry of short-day photoperiod and transient priming, but it also is likely involved in estrous-induced aggression.
7.3. Mesolimbic Circuitry
As compared to the short, transient-like priming effect described above, a long-term model of escalated aggression has also been described in female hamsters. In this model, an adult male stimulus hamster was placed into a female subject’s home cage for 5 minutes on each of 5 consecutive days. Across repeated days, females show a decrease in attack latency, indicating an experience-dependent escalation of aggression. This form of aggression is associated with increased dendritic spine density on medium spiny neurons in the nucleus accumbens (NAc) (Staffend and Meisel, 2012). Using a combination of pharmacology and molecular biology, it was demonstrated that this experience-dependent structural plasticity relies on mGluR5 receptors in the NAc, which regulate the Fragile X Mental Retardation Protein (FMRP) signaling pathway (See Figure 2). Specifically, this repeated exposure to aggression results in a rapid, transient decrease in the phosphorylation of FMRP in the NAc. This, in turn, leads to an increase in synaptic scaffolding proteins, such as PSD95 and SAPAP3, that are likely responsible for the observed increase in dendritic spine density (Been et al., 2016). Thus, while the amygdala and BNST likely play a role in the short-term, transient model of aggression priming, longer-term escalated aggression plasticity is the result of alterations in the FMRP signaling pathway in the NAc. These data are among few studies addressing neuroplastic changes at the cellular/molecular that contribute to behavioral changes that accompany aggressive experience.
Figure 2. Proposed model for molecular signaling underlying the escalation of aggression in female hamsters.
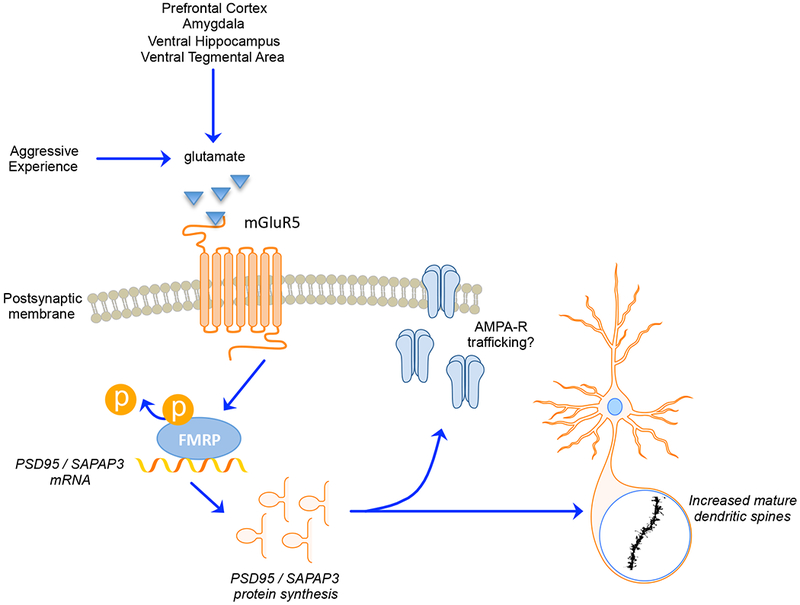
Interfemale aggressive encounters activate metabotropic glutamate receptors (e.g., mGluR5) in the nucleus accumbens (NAc). Although the source of glutamatergic input is unknown, it is likely to orginate from the prefrontal cortex, amygdala, ventral hippocampus, or ventral tegmental area. This activation leads to Gq-mediated signaling that activates PP2A, resulting in the rapid dephosphorylation of FMRP. The dephosphorylation of FMRP allows for de-repression of local translation of synaptic scaffolding proteins, such as PSD-95 and SAPAP-3 in dendrites. Repeated aggressive experience produces a long-term increase in this translation and the resulting increase in synaptic scaffolding proteins is associated with the proliferation of mature dendritic spines in the NAc core. This structural plasticity in the NAc core likely reflects an increase in excitatory inputs to the cell and presumably mediates a heightened response to future aggressive encounters, leading to escalated aggressive behavior. Reprinted with permission from Been et al. (2016).
The last major brain region likely involved in the neural circuitry of female aggression is the ventral tegmental area (VTA). Here, progesterone exerts an anxiolytic effect, resulting in a decrease in aggressive behaviors. In female mice, blocking progesterone receptors in the VTA results in a significant increase in aggression and male rejection (Frye et al., 2014). Further, infusions of a progesterone metabolite into the VTA in mice leads to increase anti-aggression behaviors, regardless of estradiol priming. These infusions also enhance the level of this metabolite throughout the midbrain, hippocampus, striatum, and cortex, suggesting that the progesterone metabolite in the VTA may trigger biosynthesis of it in other regions, increasing anti-aggressive behaviors (Frye et al., 2008). Notably, the VTA is not only involved in the neural circuitry of aggression but also projects to several other regions upon progesterone activation associated with decreased aggression.
7.4. Developing a Neuroanatomical Circuit
We do not have a very good understanding of the neural circuits regulating aggression in females. Figure 3 illustrates that the neural modules involved in female aggression have connections that integrate with the BNST (Yamamoto et al., 2018). Going forward this neuroanatomical literature can be used to form testable hypotheses to delineate functional circuits regulating female aggression.
Figure 3. Towards a neurocircuit of female aggression.
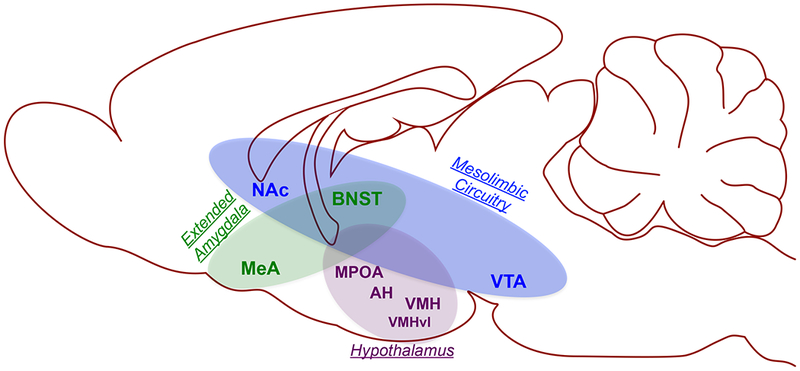
Compared to our knowledge of the neurobiology of aggression in males, we know much less about the neural substrates of aggression in females. Still, from the existing data, a circuit can be generated in which the hypothalamus, extended amygdala, and mesolimbic circuitry are key nodes. Within the hypothalamus, the medial preoptic area (MPOA) facilitates the expression of aggressive behavior, whereas the anterior hypothalamus (AH) and ventromedial hypothalamus (VMH) play an inhibory role. Interestingly, recent data suggests that the ventrolateral portion of the VMH (VMHvl) is an excitatory sub-population within the VMH, perhaps due to its glutamatergic projections to the mesolimbic reward circuitry. Within the mesolimbic circuitry, the role of the ventral tegmental area (VTA) is largely unknown. In contrast, the primary efferent target of the VTA, the nucleus accumbens (NAc), motivates aggressive behavior and confers long-term escalation of aggressive behavior with repeated experience via a molecular signaling pathway that depends on activation of metabotropic glutamate receptors. The NAc interacts structurally and functionally with the extended amygdala, including the medial amygdala (MeA) and bed nucleus of the stria terminalis (BNST), both of which play a excitatory role in female aggression. It is interesting to note that the BNST in particular seems well-positioned to interact with each of the identified neural substrates of female aggression. Future work should take advantage of modern functional neuroanatomical methods to investigate the BNST as a potential coordinator of female aggressive behavior.
Within our diagram the VMH represents a paradox in which it is both excitatory and inhibitory in regulating female aggression. Embedded in this paradox may be an important insight into the female aggression circuitry. Hashikawa et al. (2017a) elegantly demonstrated that optogenetic activation of estrogen receptor containing neurons in the ventrolateral portion of the VMH (i.e., VMHvl) elicits aggression in female mice. There is some evidence that neurons in the VMHvl are local GABAergic neurons (Murphy and Renaud, 1968), although more recent studies suggest that most VMHvl neurons are glutamatergic (Hashikawa et al., 2017; Yamamoto et al., 2018). If VMH glutamatergic projection neurons include the VTA as one of their targets (Geisler et al., 2007), this would provide an anatomical link between hypothalamic circuits controlling the expression of aggression with its rewarding consequences. This hypothesis has yet to be tested, however, and is not strongly supported by neuroanatomical data (Canteras, Simerly, & Swanson, 1994). Extended estrogen treatment both elicits female sexual behavior towards a male and aggression towards a female in female hamsters (Meisel et al., 1988) supporting the idea that circuits underlying female aggression and sexual behavior are distinct. This behavioral conclusion is consistent with Hashikawa et al.’s (2017a) demonstration that there are separate groups of VMHvl neurons functionally linked to aggression or sexual behavior in female mice. The work by Hashikawa et al. (2017a) serves as a lesson on the importance of selecting the appropriate behavioral context when measuring female aggression, but also provides a template for future investigations of aggression-related neural circuitry.
8. Conclusions
Research on human aggression has long noted that physical aggression is more common in boys and men than in girls and women. As noted in Section 1, it is clear that this sex difference in human aggression is shrinking. Conduct disorder is on the rise in young girls and physical attacks initiated by teenage girls are similarly becoming more prevalent. Most notable are the results of surveys showing that among newly married couples wives and husbands are equally likely to initiate physical aggression towards their spouse, a finding that cuts across demographics. One conclusion emerging from these trends is that developmental, social and/or cultural factors previously restrained physical aggression in girls and women, and that the easing of these restrictions has revealed a common potential for aggression in women and men, given appropriate environmental and contextual triggers.
The results of animal research have been used to support the historical dogma of sex differences in human aggression. A goal of this review is to highlight a bias in animal studies that contributed to this conclusion and to present decades of research documenting and characterizing the biological regulation of aggression in females, primarily in rodent models. Although there are certainly sex differences, it is clear that female rodents, like males, exhibit robust intraspecific aggression (DeBold and Miczek., 1984). Instead, females and males differ in the contexts in which aggression occurs (females tend to attack other females, whereas males attack other males), the hormonal control of aggression (gonadal hormones tend to inhibit aggression in females, whereas gonadal hormones promote the potential for aggression in males), in the neurochemistry (serotonin agonists diminish aggression in males though not to the same extent in females), and also perhaps in neurocircuitry.
Our review highlights the need to develop animal models that emerge from a comprehensive and programmatic neurobiological understanding of female aggression that utilizes modern cellular and molecular tools that have dramatically advanced our understanding of aggression in male models (Aleyasin et al., 2018; Hashikawa et al., 2017b). Unfortunately this will not be as simple as it sounds. Based on an identified genome and several embryological advantages, house mice are the primary mammalian species in which gene knockout and transgenic lines can be generated. The ancestors of these mice readily exhibited aggression, though decades of inbreeding all but eliminated aggression in the primary genetic background (C57BL/6 mice) used to generate knockout and transgenic lines (Ebert and Hyde 1976; Gatewood et al., 2006; Ogawa et al., 1998). As a result house mice may not be the basis for an effective animal model for female aggression.
Syrian hamsters are the rodent species in which we know the most regarding physiological and neurobiological mechanisms underlying female aggression. The original selection of Syrian hamsters as models of female aggression resulted from their social structure in which both males and females live in and defend individual territories in the wild. Because of this social structure, aggression in female hamsters is directed towards both male and female intruders and is not confounded by dominance hierarchies. Virally mediated gene delivery is very effective in hamsters (Been et al., 2013; Hedges et al., 2009) and can be the basis for genetic manipulations, though this approach can be inefficient for complex gene strategies. A ray of hope comes from the development of GFP transgenic hamsters (Gao et al., 2014), providing a proof of principle for this type of genetic approach (see also Wang et al., 2016). Future efforts to develop transgenic approaches in species that naturally display aggressive behavior will be vital to developing animal models of aggression that highlight both sexes.
It is clear that the study of aggression in females is of interest both from a basic biology perspective as well as the basis for identifying potential therapeutic targets for drug development directed at treating pathological aggression and violence in girls and women, a growing problem worldwide.
Highlights.
Contrary to archaic beliefs, females readily engage in social aggression.
Aggression has been modeled in female rodents, but to a lesser extent than in males.
From existing data we can outline a basic neural circuitry of female aggression.
The hypothalamus, extended amygdala, and mesolimbic circuitry are key neural nodes.
Modern experiments are needed to understand the neurobiology of female aggression.
Acknowledgements
This review and some of the research described were supported by the National Institutes of Health Grant DA013680 (RLM), the National Science Foundation Grant IOS 1256799 (RLM), and the Koshland Integrated Natural Sciences Center at Haverford College (LEB).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflicts of interest
None declared.
References
- Albert DJ, Dyson EM, Petrovic DM, Walsh ML, 1988. Activation of aggression in female rats by normal males and by castrated males with testosterone implants. Physiol. Behav 44, 9–13. [DOI] [PubMed] [Google Scholar]
- Albert DJ, Jonik RH, Walsh ML, 1993. Influence of combined estradiol and testosterone implants on the aggressiveness of nonaggressive female rats. Physiol. Behav 53, 709–713. [DOI] [PubMed] [Google Scholar]
- Albert DJ, Jonik RH, Walsh ML, 1992. Hormone-dependent aggression in male and female rats: experiential, hormonal, and neural foundations. Neurosci. Biobehav. Rev 16, 177–192. [DOI] [PubMed] [Google Scholar]
- Aleyasin H, Flanigan ME, Russo SJ, 2018. Neurocircuitry of aggression and aggression seeking behavior: nose poking into brain circuitry controlling aggression. Curr. Opin. Neurobiol 49, 184–191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Archer J, 1988. The Behavioural Biology of Aggression. Cambridge: University Press. [Google Scholar]
- Ayer ML, Whitsett JM, 1980. Aggressive behaviour of female prairie deer mice in laboratory populations. Anim. Behav 28, 763–771. [DOI] [PubMed] [Google Scholar]
- Badura LL, Nunez AA, 1989. Photoperiodic modulation of sexual and aggressive behavior in female golden hamsters (Mesocricetus auratus): role of the pineal gland. Horm. Behav 23, 27–42. [DOI] [PubMed] [Google Scholar]
- Bales KL, Carter CS, 2003. Sex differences and developmental effects of oxytocin on aggression and social behavior in prairie voles (Microtus ochrogaster). Horm. Behav 44, 178–184. [DOI] [PubMed] [Google Scholar]
- Barkley MS, Goldman BD, 1978. Studies on opponent status and steroid mediation of aggression in female mice. Behav. Biol 23, 118–123. [DOI] [PubMed] [Google Scholar]
- Been LE, Hedges VL, Vialou V, Nestler EJ, Meisel RL, 2013. ΔJunD overexpression in the nucleus accumbens prevents sexual reward in female Syrian hamsters: ΔJunD prevents sexual reward. Genes Brain Behav 12, 666–672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Been LE, Moore KM, Kennedy BC, Meisel RL, 2016. Metabotropic glutamate receptor and fragile X signaling in a female model of escalated aggression. Biol Psychiatry 79, 685–692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Björkqvist K, Niemelä P, 1992. New Trends in the Study of Female Aggression, in: Of Mice and Women. pp. 3–16. [Google Scholar]
- Blanchard DC, Blanchard RJ, 2003. What can animal aggression research tell us about human aggression? Horm. Behav 44, 171–177. [DOI] [PubMed] [Google Scholar]
- Blanchard RJ, Dulloog L, Markham C, Nishimura O, Nikulina Compton J, Jun A, Blanchard DC, 2001. Sexual and aggressive interactions in a visible burrow system with provisioned burrows. Physiol. Behav 72, 245–254. [DOI] [PubMed] [Google Scholar]
- Blanchard RJ, Hori K, Blanchard DC, Hall J, 1987. Ethanol effects on aggression of rats selected for different levels of aggressiveness. Pharmacol. Biochem. Behav 27, 641–644. [DOI] [PubMed] [Google Scholar]
- Bosch OJ, Neumann ID, 2012. Both oxytocin and vasopressin are mediators of maternal care and aggression in rodents: from central release to sites of action. Horm. Behav 61, 293–303. [DOI] [PubMed] [Google Scholar]
- Bowler CM, Cushing BS, Carter CS, 2002. Social factors regulate female-female aggression and affiliation in prairie voles. Physiol. Behav 76, 559–566. [DOI] [PubMed] [Google Scholar]
- Brain PF, 1972. Effects of isolation/grouping on endocrine function and fighting behavior in male and female golden hamsters. (Mesocricetus auratus Waterhouse). Behav. Biol 7, 349–357. [DOI] [PubMed] [Google Scholar]
- Caldwell HK, Albers HE, 2004. Photoperiodic regulation of vasopressin receptor binding in female Syrian hamsters. Brain Res 1002, 136–141. [DOI] [PubMed] [Google Scholar]
- Campbell A, 2007. Sex Differences in Aggression, in: Oxford Handbook of Evolutionary Psychology. pp. 365–381. [Google Scholar]
- Campbell A, 2013. The evolutionary psychology of women’s aggression. Philos. Trans. R. Soc. Lond. B Biol. Sci 368, 20130078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carter CS, 1973. Stimuli contributing to the decrement in sexual receptivity of female golden hamsters (Mesocricetus auratus). Anim. Behav 21, 827–834. [DOI] [PubMed] [Google Scholar]
- Ciaccio LA, Lisk RD, Reuter LA, 1979. Prelordotic behavior in the hamster: a hormonally modulated transition from aggression to sexual receptivity. J. Comp. Physiol. Psychol 93, 771–780. [DOI] [PubMed] [Google Scholar]
- Collishaw S, Maughan B, Goodman R, Pickles A, 2004. Time trends in adolescent mental health. J. Child Psychol. Psychiatry. 45, 1350–1362. [DOI] [PubMed] [Google Scholar]
- Cordero MI, Ansermet F, Sandi C, 2013. Long-term programming of enhanced aggression by peripuberty stress in female rats. Psychoneuroendocrinology 38, 2758–2769. [DOI] [PubMed] [Google Scholar]
- Davis ES, Marler CA, 2004. c-fos Changes following an aggressive encounter in female California mice: a synthesis of behavior, hormone changes and neural activity. Neuroscience 127, 611–624. [DOI] [PubMed] [Google Scholar]
- de Jong FH, Eerland EMJ, van de Poll NE, 1986. Sex-specific interactions between aggressive and sexual behavior in the rat: Effects of testosterone and progesterone. Horm. Behav 20, 432–444. [DOI] [PubMed] [Google Scholar]
- de Jong TR, Beiderbeck DI, Neumann ID, 2014. Measuring virgin female aggression in the female intruder test (FIT): effects of oxytocin, estrous cycle, and anxiety. PloS One 9, 91701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Waal FB, 2000. Primates–a natural heritage of conflict resolution. Science 289, 586–590. [DOI] [PubMed] [Google Scholar]
- DeBold JF, Miczek KA, 1984. Aggression persists after ovariectomy in female rats. Horm. Behav 18, 177–190. [DOI] [PubMed] [Google Scholar]
- delBarco-Trillo J, Johnston RE, 2012. Asymmetric learning to avoid heterospecific males in Mesocricetus hamsters. Zoology 115, 270–274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delville Y, Mansour KM, Ferris CF, 1996. Testosterone facilitates aggression by modulating vasopressin receptors in the hypothalamus. Physiol. Behav 60, 25–29. [DOI] [PubMed] [Google Scholar]
- Dluzen DE, Carter CS, 1979. Ovarian hormones regulating sexual and social behaviors in female prairie voles, Microtus ochrogaster. Physiol. Behav 23, 597–600. [DOI] [PubMed] [Google Scholar]
- Drickamer LC, Vandenbergh JG, 1973. Predictors of social dominance in the adult female golden hamster (Mesocricetus auratus). Anim. Behav 21, 564–570. [DOI] [PubMed] [Google Scholar]
- Duque-Wilckens N, Trainor B, 2017. Behavioral Neuroendocrinology of Female Aggression. Oxford Research Encyclopedia of Neuroscience. [Google Scholar]
- Ebert PD, Hyde JS, 1976. Selection for agonistic begavior in wild female Mus musculus. Behav. Genetics 6, 291–304. [DOI] [PubMed] [Google Scholar]
- Falk Ö, Sfendla A, Brändström S, Anckarsäter H, Nilsson T, Kerekes N, 2017. Personality and trait aggression profiles of male and female prison inmates. Psychiatry Res 250, 302–309. [DOI] [PubMed] [Google Scholar]
- Falkner AL, Dollar P, Perona P, Anderson DJ, Lin D, 2014. Decoding ventromedial hypothalamic neural activity during male mouse aggression. J. Neurosci 34, 5971–5984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Falkner AL, Grosenick L, Davidson TJ, Deisseroth K, Lin D, 2016. Hypothalamic control of male aggression-seeking behavior. Nat. Neurosci 19, 596–604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FBI, 2012. United States Department of Justice; Crime in the United States. Retrieved from http://www.fbi.gov/about-us/cjis/ucr/crime-in-the-u.s/2011/crime-in-the-u.s.-2011 [Google Scholar]
- Ferrari PF, Palanza P, Rodgers RJ, Mainardi M, Parmigiani S, 1996. Comparing different forms of male and female aggression in wild and laboratory mice: an ethopharmacological study. Physiol. Behav 60, 549–553. [DOI] [PubMed] [Google Scholar]
- Ferris CF, Potegal M, 1988. Vasopressin receptor blockade in the anterior hypothalamus suppresses aggression in hamsters. Physiol. Behav 44, 235–239. [DOI] [PubMed] [Google Scholar]
- Firestone KB, Thompson KV, Carter CS, 1991. Female-female interactions and social stress in prairie voles. Behav. Neural Biol 55, 31–41. [DOI] [PubMed] [Google Scholar]
- Fleming AS, Phillips A, Rydall A, Levesque L, 1988. Effects of photoperiod, the pineal gland and the gonads on agonistic behavior in female golden hamsters (Mesocricetus auratus). Physiol. Behav 44, 227–234. [DOI] [PubMed] [Google Scholar]
- Floody OR, 2009. Effects on hamster vocalization and aggression of carbachol injections into the MPOA/AH. Physiol. Behav 96, 294–299. [DOI] [PubMed] [Google Scholar]
- Floody OR, Pfaff DW, 1977. Aggressive behavior in female hamsters: the hormonal basis for fluctuations in female aggressiveness correlated with estrous state. J. Comp. Physiol. Psychol 91, 443–464. [DOI] [PubMed] [Google Scholar]
- Frank AH, Fraps RM, 1945. Induction of estrus in the ovariectomized golden hamster. Endocrinology 37, 357–361. [DOI] [PubMed] [Google Scholar]
- Freitag CM, Konrad K, Stadler C, De Brito SA, Popma A, Herpertz SC, Fairchild G, 2018. Conduct disorder in adolescent females: current state of research and study design of the FemNAT-CD consortium. Eur. Child Adolesc. Psychiatry 27, 1077–1093. [DOI] [PubMed] [Google Scholar]
- Frye CA, Paris JJ, Rhodes ME, 2008. Estrogen is necessary for 5alpha-pregnan-3alpha-ol-20-one (3alpha,5alpha-THP) infusion to the ventral tegmental area to facilitate social and sexual, but neither exploratory nor affective behavior of ovariectomized rats. Pharmacol. Biochem. Behav 91, 261–270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frye CA, Walf AA, Kohtz AS, Zhu Y, 2014. Progesterone-facilitated lordosis of estradiolprimed mice is attenuated by knocking down expression of membrane progestin receptors in the midbrain. Steroids 81, 17–25 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaman A, McAfee S, Homel P, Jacob T, 2017. Understanding patterns of intimate partner abuse in male-male, male-female, and female-female couples. Psychiatr. Q 88, 335–347. [DOI] [PubMed] [Google Scholar]
- Gandelman R, 1980. Gonadal hormones and the induction of intraspecific fighting in mice. Neurosci. Biobehav. Rev 4, 133–140. [DOI] [PubMed] [Google Scholar]
- Gao M, Zhang B, Liu J, Guo X, Li H, Wang T, Liu G, 2014. Generation of transgenic golden Syrian hamsters. Cell Res 24, 380–382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gatewood JD, Wills A, Shetty S, Xu J, Arnold AP, Burgoyne PS, Rissman EF, 2006. Sex chromosome complement and gonadal sex influence aggressive and parental behaviors in mice. J. Neurosci 26, 2335–2342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gattermann R, Fritzsche P, Neumann K, Al-Hussein I, Kayser A, Abiad M, Yakti R, 2001. Notes on the current distribution and the ecology of wild golden hamsters (Mesocricetus auratus). J. Zool. Lond 254, 359–365. [Google Scholar]
- Geisler S, Derst C, Veh RW, Zahm DS, 2007. Glutamatergic afferents of the ventral tegmental area in the rat. J. Neurosci 27, 5730–5743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golden SA, Aleyasin H, Heins R, Flanigan M, Heshmati M, Takahashi A, Shaham Y, 2017. Persistent conditioned place preference to aggression experience in adult male sexuallyexperienced CD-1 mice: Persistent aggression conditioned place preference in CD-1 mice. Genes Brain Behav 16, 44–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golden SA, Covington HE, Berton O, Russo SJ, 2011. A standardized protocol for repeated social defeat stress in mice. Nat. Protoc 6, 1183–1191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golden SA, Heshmati M, Flanigan M, Christoffel DJ, Guise K, Pfau ML, Russo SJ, 2016. Basal forebrain projections to the lateral habenula modulate aggression reward. Nature 534, 688–692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldman BD, 2001. Mammalian photoperiodic system: formal properties and neuroendocrine mechanisms of photoperiodic time measurement. J. Biol. Rhythms 16, 283–301. [DOI] [PubMed] [Google Scholar]
- Gray LE, 1979. The effects of the reproductive status and prior housing conditions on the aggressiveness of female mice. Behav. Neural Biol 26, 508–513. [DOI] [PubMed] [Google Scholar]
- Gutzler SJ, Karom M, Erwin WD, Albers HE, 2010. Arginine-vasopressin and the regulation of aggression in female Syrian hamsters (Mesocricetus auratus): V1a receptors and female aggression. Eur. J. Neurosci 31, 1655–1663. [DOI] [PubMed] [Google Scholar]
- Gutzler SJ, Karom M, Erwin WD, Albers HE, 2009. Photoperiodic regulation of adrenal hormone secretion and aggression in female Syrian hamsters. Horm. Behav 56, 481–489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haller J, 2013. The neurobiology of abnormal manifestations of aggression–a review of hypothalamic mechanisms in cats, rodents, and humans. Brain Res. Bull 93, 97–109. [DOI] [PubMed] [Google Scholar]
- Hammond MA, Rowe FA, 1976. Medial preoptic and anterior hypothalamic lesions: influences on aggressive behavior in female hamsters. Physiol. Behav 17, 507–513. [DOI] [PubMed] [Google Scholar]
- Harmon AC, Huhman KL, Moore TO, Albers HE, 2002. Oxytocin inhibits aggression in female Syrian hamsters. J. Neuroendocrinol 14, 963–969. [DOI] [PubMed] [Google Scholar]
- Harwell TS, Moore KR, Spence MR, 2003. Physical violence, intimate partner violence, and emotional abuse among adult American Indian men and women in Montana. Prevent. Med 37, 297–303. [DOI] [PubMed] [Google Scholar]
- Hashikawa K, Hashikawa Y, Falkner A, Lin D, 2016. The neural circuits of mating and fighting in male mice. Curr. Opin. Neurobiol 38, 27–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hashikawa K, Hashikawa Y, Tremblay R, Zhang J, Feng JE, Sabol A, Lin D, 2017a. Esr1+ cells in the ventromedial hypothalamus control female aggression. Nat. Neurosci 20, 1580–1590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hashikawa Y, Hashikawa K, Falkner AL, Lin D, 2017b. Ventromedial hypothalamus and the generation of aggression, Front. Syst. Neurosci 11, 94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haug M, Wallian L, Brain PF, 1990. Effects of 8-OH-DPAT and fluoxetine on activity and attack by female mice towards lactating intruders. Gen. Pharmacol 21, 845–849. [DOI] [PubMed] [Google Scholar]
- Hedges VL, Chakravarty S, Nestler EJ, Meisel RL, 2009. Δ FosB overexpression in the nucleus accumbens enhances sexual reward in female Syrian hamsters. Genes Brain Behav 8, 442–449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hennessey AC, Huhman KL, Albers HE, 1994. Vasopressin and sex differences in hamster flank marking. Physiol. Behav 55, 905–911. [DOI] [PubMed] [Google Scholar]
- Ho HP, Olsson M, Westberg L, Melke J, Eriksson E, 2001. The serotonin reuptake inhibitor fluoxetine reduces sex steroid-related aggression in female rats: an animal model of premenstrual irritability? Neuropsychopharmacology 24, 502–510. [DOI] [PubMed] [Google Scholar]
- Honrado GI, Paclik L, Fleming AS, 1991. The effects of short day exposure on seasonal and circadian reproductive rhythms of female golden hamsters. Physiol. Behav 50, 357–363. [DOI] [PubMed] [Google Scholar]
- Howard SM, Gandelman R, Rosenthal C, 1981. Isolation potentiates the aggression-activating property of testosterone in female mice. Physiol. Behav 26, 971–972. [DOI] [PubMed] [Google Scholar]
- Hyde JS, Ebert PD, 1976. Correlated response in selection for aggressiveness in female mice. I. Male aggressiveness. Behav. Genetics 6, 421–427. [DOI] [PubMed] [Google Scholar]
- Hyde JS, Sawyer TF, 1980. Selection for agonistic behavior in wild female mice. Behav. Genetics 10, 349–359. [DOI] [PubMed] [Google Scholar]
- Joppa MA, Rowe RK, Meisel RL, 1997. Effects of serotonin 1A or 1B receptor agonists on social aggression in male and female Syrian hamsters. Pharmacol. Biochem. Behav 58, 349–353. [DOI] [PubMed] [Google Scholar]
- Kimmel MS, 2002. “Gender Symmetry” in domestic violence: A substantive and methodological research review. Viol. Against Women 8, 1332–1363. [Google Scholar]
- Kislak JW, Beach FA, 1955. Inhibition of aggressiveness by ovarian hormones. Endocrinology 56, 684–692. [DOI] [PubMed] [Google Scholar]
- Koonce CJ, Frye CA, 2014. Female mice with deletion of Type One 5α-reductase have reduced reproductive responding during proestrus and after hormone-priming. Pharmacol. Biochem. Behav 122, 20–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lebow MA, Chen A, 2016. Overshadowed by the amygdala: the bed nucleus of the stria terminalis emerges as key to psychiatric disorders. Molec. Psychiatry 21, 450–463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee H, Kim D-W, Remedios R, Anthony TE, Chang A, Madisen L, Anderson DJ, 2014. Scalable control of mounting and attack by Esr1+ neurons in the ventromedial hypothalamus. Nature 509, 627–632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin D, Boyle MP, Dollar P, Lee H, Lein ES, Perona P, Anderson DJ, 2011. Functional identification of an aggression locus in the mouse hypothalamus. Nature 470, 221–226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lisk RD, Nachtigall MJ, 1988. Estrogen regulation of agonistic and proceptive responses in the golden hamster. Horm. Behav 22, 35–48. [DOI] [PubMed] [Google Scholar]
- Lown EA, Vega WA, 2001. Intimate partner violence and health: self-assessed health, chronic health, and somatic symptoms among Mexican American women. Psychosomat. Med 63, 352–360. [DOI] [PubMed] [Google Scholar]
- Malsbury CW, Kow L-M, Pfaff DW, 1977. Effects of medial hypothalamic lesions on the lordosis response and other behaviors in female golden hamsters. Physiol. Behav 19, 223–237. [DOI] [PubMed] [Google Scholar]
- Malsbury CW, Strull D, Daood J, 1978. Half-cylinder cuts antero-lateral to the ventromedial nucleus reduce sexual receptivity in female golden hamsters. Physiol. Behav 21, 79–87. [DOI] [PubMed] [Google Scholar]
- Marques DM, Valenstein ES, 1977. Individual differences in aggressiveness of female hamsters: response to intact and castrated males and to females. Anim. Behav 25, 131–139. [DOI] [PubMed] [Google Scholar]
- McCarthy MM, 1990. Oxytocin inhibits infanticide in female house mice (Mus domesticus). Horm. Behav 24, 365–375. [DOI] [PubMed] [Google Scholar]
- McDermott JL, Carter CS, 1980. Ovarian hormones, copulatory stimuli, and female sexual behavior in the Mongolian gerbil. Horm. Behav 14, 211–223. [DOI] [PubMed] [Google Scholar]
- Meisel RL, Fraile IG, Pfaff DW, 1990. Hypothalamic sites of progestin action on aggression and sexual behavior in female Syrian hamsters. Physiol. Behav 47, 219–223. [DOI] [PubMed] [Google Scholar]
- Meisel RL, Sterner MR, 1990. Progesterone inhibition of sexual behavior is accompanied by an activation of aggression in female Syrian hamsters. Physiol. Behav 47, 415–417. [DOI] [PubMed] [Google Scholar]
- Meisel RL, Sterner MR, Diekman MA, 1988. Differential hormonal control of aggression and sexual behavior in female Syrian hamsters. Horm. Behav 22, 453–466. [DOI] [PubMed] [Google Scholar]
- Morin LP, 1977. Progesterone: Inhibition of rodent sexual behavior. Physiol. Behav 18, 701–715. [DOI] [PubMed] [Google Scholar]
- Murphy JT, Renaud LP, 1968. Inhibitory interneurons in the ventromedial nucleus of the hypothalamus. Brain Res 9, 385–389. [DOI] [PubMed] [Google Scholar]
- Nomoto K, Lima SQ, 2015. Enhanced male-evoked responses in the ventromedial hypothalamus of sexually receptive female mice. Curr. Biol 25, 589–594. [DOI] [PubMed] [Google Scholar]
- Ogawa S, Eng V, Taylor J, Lubahn DB, Korach KS, Pfaff DW, 1998. Roles of estrogen receptor-alpha gene expression in reproduction-related behaviors in female mice. Endocrinology 139, 5070–5081. [DOI] [PubMed] [Google Scholar]
- O’Leary KD, Malone J, Tyree A, 1994. Physical aggression in early marriage: prerelationship and relationship effects. Consult. Clin. Psychol 62, 594–602. [DOI] [PubMed] [Google Scholar]
- Olivier B, Mos J, 1986. Serenics and aggression. Stress Med 2, 197–209. [Google Scholar]
- Payne AP, Swanson HH, 1970. Agonistic behaviour between pairs of hamsters of the same and opposite sex in a neutral observation area. Behaviour 36, 260–269. [PubMed] [Google Scholar]
- Potegal M, 2012. Temporal and frontal lobe initiation and regulation of the top-down escalation of anger and aggression. Behav. Brain Res 231, 386–395. [DOI] [PubMed] [Google Scholar]
- Potegal M, 1992. Time course of aggressive arousal in female hamsters and male rats. Behav. Neural Biol 58, 120–124. [DOI] [PubMed] [Google Scholar]
- Potegal M, 1986. Differential effects of ethyl (R,S)-nipecotate on the behaviors of highly and minimally aggressive female golden hamsters. Psychopharmacology 89, 444–448. [DOI] [PubMed] [Google Scholar]
- Potegal M, Ferris CF, Hebert M, Meyerhoff J, Skaredoff L, 1996. Attack priming in female Syrian golden hamsters is associated with a c-fos-coupled process within the corticomedial amygdala. Neuroscience 75, 869–880. [DOI] [PubMed] [Google Scholar]
- Potegal M, Perumal AS, Barkai AI, Cannova GE, Blau AD, 1982. GABA binding in the brains of aggressive and non-aggressive female hamsters. Brain Res 247, 315–324. [DOI] [PubMed] [Google Scholar]
- Qiao X, Yan Y, Wu R, Tai F, Hao P, Cao Y, Wang J, 2014. Sociality and oxytocin and vasopressin in the brain of male and female dominant and subordinate mandarin voles. J. Comp. Physiol. A 200, 149–159. [DOI] [PubMed] [Google Scholar]
- Ragnauth AK, Devidze N, Moy V, Finley K, Goodwillie A, Kow L-M, Pfaff DW, 2005. Female oxytocin gene-knockout mice, in a semi-natural environment, display exaggerated aggressive behavior. Genes Brain, Behav 4, 229–239. [DOI] [PubMed] [Google Scholar]
- Ramos BM, Carlson BE, McNutt L-A, 2004. Lifetime Abuse, Mental Health, and African American Women. J. Fam. Viol 19, 153–164. [Google Scholar]
- Razzoli M, Cushing BS, Carter CS, Valsecchi P, 2003. Hormonal regulation of agonistic and affiliative behavior in female mongolian gerbils (Meriones unguiculatus). Horm. Behav 43, 549–553. [DOI] [PubMed] [Google Scholar]
- Scotti M-AL, Place NJ, Demas GE, 2007. Short-day increases in aggression are independent of circulating gonadal steroids in female Siberian hamsters (Phodopus sungorus). Horm. Behav 52, 183–190. [DOI] [PubMed] [Google Scholar]
- Shinoda K, 1994. Brain aromatization and its associated structures. Endocrine Journal 41, 115–138. [DOI] [PubMed] [Google Scholar]
- Silva AL, Fry WHD, Sweeney C, Trainor BC, 2010. Effects of photoperiod and experience on aggressive behavior in female California mice. Behav. Brain Res 208, 528–534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staffend NA, Meisel RL, 2012. Aggressive experience increases dendritic spine density within the nucleus accumbens core in female Syrian hamsters. Neuroscience 227, 163–169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sterner MR, Meisel RL, Diekman MA, 1992. Forebrain sites of estradiol-17!b action on sexual behavior and aggression in female Syrian hamsters. Behav. Neurosci 106, 162–171. [DOI] [PubMed] [Google Scholar]
- Suchowsky GK, Pegrassi L, Bonsignori A, 1971. Steroids and aggressive behaviour. Psychopharmacologia 21, 32–38. [DOI] [PubMed] [Google Scholar]
- Takahashi A, Quadros IM, de Almeida RMM, Miczek KA, 2011. Behavioral and pharmacogenetics of aggressive behavior. Behav. Neurogenetics 12, 73–138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi LK, Lisk RD, 1985a. Diencephalic sites of progesterone action for inhibiting aggression and facilitating sexual receptivity in estrogen-primed golden hamsters. Endocrinology 116, 2393–2399. [DOI] [PubMed] [Google Scholar]
- Takahashi LK, Lisk RD, 1985b. Estrogen action in anterior and ventromedial hypothalamus and the modulation of heterosexual behavior in female golden hamsters. Physiol. Behav 34, 233–239. [DOI] [PubMed] [Google Scholar]
- Takahashi LK, Lisk RD, Burnett AL, 1985. Dual estradiol action in diencephalon and the regulation of sociosexual behavior in female golden hamsters. Brain Res 359, 194–207. [DOI] [PubMed] [Google Scholar]
- Taravosh-Lahn K, Delville Y, 2004. Aggressive behavior in female golden hamsters: development and the effect of repeated social stress. Horm. Behav 46, 428–435. [DOI] [PubMed] [Google Scholar]
- Terranova JI, Song Z, Larkin TE, Hardcastle N, Norvelle A, Riaz A, Albers HE, 2016. Serotonin and arginine-vasopressin mediate sex differences in the regulation of dominance and aggression by the social brain, in: Proc. Natl. Acad. Sci. U.S.A 113, 13233–13238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Todd WD., Fenselau H, Wang JL, Zhang R, Machado NL, Venner A, Broadhurst RY, Kaur S, Lynagh T, Olson DP, Lowell BB, Fuller PM, Saper CB, 2018. A hypothalamic circuit for the circadian control of aggression. Nat. Neurosci 21, 717–724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villalba C, Boyle PA, Caliguri EJ, De Vries GJ, 1997. Effects of the selective serotonin reuptake inhibitor fluoxetine on social behaviors in male and female prairie voles (Microtus ochrogaster). Horm. Behav 32, 184–191. [DOI] [PubMed] [Google Scholar]
- Wallian L, Brain PF, Haug M, 1993. Aggression in female mice: contrasting effects of amiflamine (FLA 336), a selective and reversible MAO-type A inhibitor. Physiol. Behav 54, 411–414. [DOI] [PubMed] [Google Scholar]
- Wang Y, Kayoumu A, Lu G, Xu P, Qiu X, Chen L, Liu G, 2016. Experimental models in Syrian golden hamster replicate human acute pancreatitis. Sci. Rep 6, 28014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wise DA, 1974. Aggression in the female golden hamster: Effects of reproductive state and social isolation. Horm. Behav 5, 235–250. [DOI] [PubMed] [Google Scholar]
- Wynne-Edwards KE, Lisk RD, 1987. Behavioral interactions differentiate Djungarian (Phodopus campbelli) and Siberian (Phodopus sungorus) hamsters. Can. J. Zool 65, 2229–2235. [Google Scholar]
- Yamamoto R, Ahmed N, Ito T, Gungor NZ, Pare D, 2018. Optogenetic study of anterior BNST and basomedial amygdala projections to the ventromedial hypothalamus. eNeuro 5, e0204–18.2018 1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang CF, Chiang MC, Gray DC, Prabhakaran M, Alvarado M, Juntti SA, Shah NM, 2013. Sexually dimorphic neurons in the ventromedial hypothalamus govern mating in both sexes and aggression in males. Cell 153, 896–909. [DOI] [PMC free article] [PubMed] [Google Scholar]


