Abstract
Influenza (flu) is a serious disease for older adults, with increased severity of infection and greater risk for hospitalization and death. Flu infection is limited to pulmonary epithelial cells, yet there are many systemic symptoms and older adults are more susceptible to flu-related complications. In older adults, flu rarely comes without additional complications and there is a perfect storm for enhanced disease due to multiple factors including existing co-morbidities, plus impaired lung function and dysregulated immune responses that occur with even healthy aging. Commonly, opportunistic secondary bacterial infections prosper in damaged lungs. Intensified systemic inflammation with aging can cause dysfunction in extra-pulmonary organs and tissues such as cardiovascular, musculoskeletal, neuropathologic, hepatic, and renal complications. Often overlooked is the underappreciated connections between many of these conditions, which exacerbate one another when in parallel. This review focuses on flu infection and the numerous complications in older adults associated with diminished immune responses.
Keywords: Influenza, Aging, Immunology, Cardiovascular, Musculoskeletal, Neuropathologic, Hepatic, Renal, Secondary Bacteria Infection
1.0. Introduction
Influenza viruses belong to the Orthomyxoviridae family and are enveloped, negative-sense, single-stranded RNA viruses with segmented genomes [1]. Influenza A is most often associated with human illness with H1N1 subtypes more often resulting in milder illness when compared to H3N2 subtypes, which are responsible for 80% of flu-related deaths [2]. Rapid mutations leave us with an ineradicable disease that has frequent epidemics. Although most influenza virus infections result in an acute, self-limiting illness, severe and fatal infections are associated with hemorrhagic bronchitis, bronchiolitis, and alveolitis with pulmonary edema and hemorrhage [3]. There are also several comorbidities that increase vulnerability to influenza infection, including underlying chronic respiratory and cardiovascular conditions, diabetes mellitus and immunosuppression [4, 5]. Immunocompromised individuals also have increased risk of enhanced disease after flu infection with prolonged viral shedding that can last for months [6]. There are severe age-related immune declines that lead to increased susceptibility and severity of flu infection in older adults. Additionally, older individuals often experience fewer clinical signs and symptoms of classical influenza infection, while also experiencing more severe complications of the disease [4–6].
It is well established that immune function declines with aging. Age-related declines of both the innate and adaptive immune systems result in increased susceptibility to infection, as well as increased severity of infection in older adults [7, 8]. Influenza (flu) is problematic in older adults with increased risk for serious complications and hospitalization. In addition, approximately 90% of flu-related deaths occur in this population [9], with influenza and pneumonia being the eighth leading cause of death among persons over 65 years of age in the United States [10]. Even when death is avoided, older adults have an increased risk for secondary complications and morbidities from flu infection. The average annual infection rate of seasonal flu is 10–20 percent of the world population with 3–5 million hospitalizations and estimated United States economic burden of $87.1 billion USD [11, 12].
Flu infections in older adults often cause hospitalization due to prolonged illness and secondary infections, such as bacterial pneumonia. Hospitalizations attributable to flu vary depending on the flu season length and circulating strains [2]. Multiple models demonstrate a common trend for increased flu-related mortality over the past 30 years [13, 14], seemingly parallel to the increased older adult population and the growing aged population is at greatest risk. The mortality rate for adults over 85 is 16x greater than individuals 65–69 years of age [2]. Additionally, the average length of a hospital stay for flu-related complications is 2 days longer in patients over 75 compared to adults 50–64 years of age [2].
In addition to common flu symptoms, flu infection leaves older adults more susceptible to secondary infection and causes dysfunction in many other tissues. These complications are often both caused by and lead to other co-morbidities, which increases mortalities. For example, secondary bacterial infection following flu is responsible for five million cases of severe illness, which results in 250,000–500,000 deaths worldwide. Recently, these have been categorized together by the CDC as influenza/pneumonia deaths [14, 15]. Due to both immunosenescence and overall senescence that occurs with aging, the body is more prone to complications resulting from delayed and weakened responses to maintain homeostatic function. Extrapulmonary and other complications in older adults can include cardiovascular dysfunction, musculoskeletal atrophy, neurological degeneration, and worsening of preexisting diseases. Importantly, many of these effects may be delayed so the association with flu infection may be initially masked.
Age-related declines in immune responses have been well-documented in both murine and human systems. Here, we will briefly review age-related immune changes and how they relate to the impaired response to flu infection, focusing more on the clinical significance of common and uncommon flu-associated complications in older adults. Identifying mechanisms of common and uncommon flu-associated complications in older adults can provide initial insight for future therapeutic investigations to enhance age-related immune declines and determine effective ways to prevent flu-related morbidity and mortalities.
1.1. Age-Related Immune Deficits
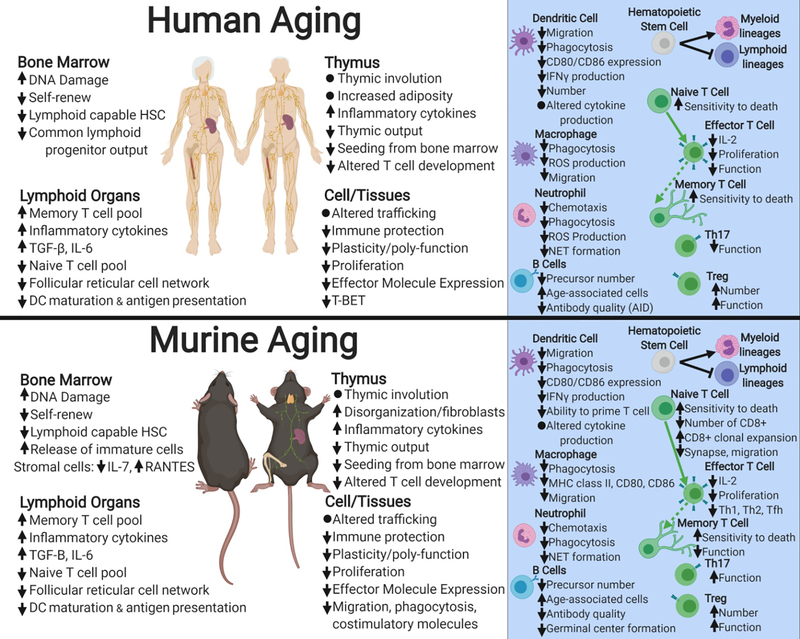
The overall aging process leads to a decreased ability to respond to stressors and maintain homeostasis. Commonly, murine models of aging are utilized to investigate age-related changes in the immune system and the C57BL/6 mouse is considered to be one of the most studied animal models. Indeed, this mouse shares many important processes with the human immune system and in aging biology (Figure 1 [16–1]). Briefly, during the biological process of aging many immune system components are impacted. Age-related DNA damage in the bone marrow is associated with increased release of immature cells into the blood and a bias for differentiation toward myeloid lineage cells over lymphoid lineage cells from hematopoietic stem cells [22, 39, 16, 40, 41]. The innate immune system (specifically neutrophils, macrophages, and dendritic cells [DCs]) exhibits decreased migration and chemotaxis [29, 31, 37, 42], as well as decreased phagocytosis [33, 36, 39, 42, 46] and changes in population frequencies [28, 39, 40, 59] in both aging humans and mice. Similarly, the adaptive immune system exhibits decreased function with aging. In both mice and humans, thymic involution and dysfunction lead to decreased naïve T cell output with aging [58, 60]. In addition, T cell function and memory T cell generation are negatively impacted by aging [18, 19, 27, 30, 64, 65, 69] and antibody quality is also reduced [77, 78]. In summary, the aged immune systems of both humans and mice exhibit delayed and reduced responsiveness with regards to both innate and adaptive aspects resulting in an overall poor response to an infectious challenge.
Figure 1. Summary of age-related changes to the immune systems of humans and mice [11–76].
Increases or decreases in cell numbers or particular functions are indicated by arrows pointing upward or downward. Figure made with biorender.com.
1.2. Impact of Aging on Immune Responses to Influenza Infection
Along with immunosenescence, or the deleterious age-associated changes in immunity described above [81], “inflammaging” leads to cytokine imbalance, tipping towards constant inflammation both within tissues and systemically. Indeed, immunosenescence and inflammaging play a large role in exacerbating flu infection in older adults. Immunosenescence is associated with decreased phagocyte function, altered cellular migration, changes in cell population numbers, and reduced antibody production [45]. Further, we would expect the same trends during flu infection, which would impair adaptive immunity and hinder the immune responsiveness to infection.
Innate immune cells, specifically DCs, produce less interferon I/III in older adults, while showing decreasing phagocytic capacity [82]. Further, phagocytes and DCs have altered receptor signal transduction and systemic over population of neutrophils (neutrophilia) in older adults can promote increased serum levels of IL-6 and c-reactive protein that increases death risk [82]. Larger issues arise during a secondary infection (bacterial or viral) since the increased inflammatory/infection damage leaves the system both frail and exhausted [82]. DCs play a large role in generating innate and adaptive immune responses, but aged mouse studies have observed that the number of DCs recovered from spleens of aged mice is 50–70% of that found in young mice during flu infection [83]. Further studies indicated that during flu infection, DCs in aged mice had significant differences in maturation, migration, and recruitment [83–86], as well as low expression of the co-stimulatory molecules CD40 and CD86 [87].
Age-related declines in immune function contribute significantly and lead to multiple manifestations with regards to the CD4 T cell response to flu antigens [88]. Importantly, the proper functioning of CD4 T cells is essential for a robust CD8 T cell response to flu infection. Changes in T cell responses during flu infection with aging include impaired T cell receptor signaling via reduced immunological synapse formation [88–90] and deficits in CD4 T cell activation, differentiation, and proliferation [19]. With aging there is also an increase in FoxP3+ regulatory CD4 T cells (Tregs), which produce anti-inflammatory molecules, resulting in reduced expression of co-stimulatory molecules CD40 and CD86 [87].
CD8 T cells are indispensable for clearance of flu infected cells. Influenza virus-specific CD8 T cells from older adults exhibit decreased functionality with corresponding increases in CD57 and KLRG1 expression, indicating a senescent phenotype [91]. These same CD8 T cells also have reduced expression of inhibitory receptors such as PD-1, resulting in higher frequencies of flu-specific CD8 T cells that exhibit reduced lytic capacity and decreased migration and chemotaxis ability during flu infection. The overall result of these age-related changes is slower viral clearance [83, 92]. Increasing age also has a significant impact on the memory CD8 T cell response to flu infection due to the loss of protective effector memory cells from peripheral tissues over time [93, 94, 95].
Similarly, aging impacts B cell and antibody responses to flu infection. Declines in B cell precursors in old age are associated with preferential loss of lymphoid-biased hematopoietic stem cells [58]. With aging, there is decreased germinal center formation during flu infection and B cells produce lower quality antibody [77,78] in both humans and mice [77]. In addition, the increase in senescent B cells with aging is negatively associated with a protective response following flu vaccination. Importantly, many of these age-related declines in humoral responses following influenza infection and vaccination are partially due to declines in CD4 T cell function with aging [20, 71].
Related to these immune cell specific alterations, lymph node stromal cells and particularly fibroblastic reticular cells, are impacted by aging. Lymph node fibroblastic reticular cells are not significantly different between young and aged mice at steady state but have delayed proliferation and expansion in aged mediastinal lymph nodes during flu infection [96]. Further, in aged lymph nodes, mouse fibroblastic reticular cells exhibit notably reduced homeostatic chemokine production, which is associated with reduced T cell homing to these lymph nodes during flu infection. Ultimately, this is associated with delayed and impaired immune responses in aged mice [96].
Another factor to be considered regarding the immune response to flu infection is the gut microbiota, which is also important in regulating immune responses both locally and systemically [97]. Pulmonary immunity is directly related to gut microbiota, which mediates flu infection-related responses, including dendritic cell migration, T cell priming, cytokine secretion, and overall viral clearance [98]. Dysbiosis of the gut microbiota induced by antibiotic administration during flu infection influences helper T cell responses and can negatively impact flu outcomes and recovery [99]. This is of particular interest with aging, as older adults are more likely to have concurrent infections and may be on antibiotics at the time of flu infection itself or due to a secondary infection. Adequate probiotic intervention after antibiotic treatment may improve the intestinal ecosystem and prevent Th2-shifted immunity [100]. Indeed, without probiotic treatment there is further dysregulation of cytokines and cell mediated regeneration related to flu infection [100].
2.0. Common complications in older adults
Influenza infection of the upper respiratory tract leads to complications in other tissues and organs (see Figure 2). These complications are made worse by the diminished immune response in older adults. In this section, we will describe the common complications seen in older adults with influenza (flu) infection.
Figure 2. Influenza infection of the upper respiratory tract leads to complications in other tissues and organs.
These complications are made worse by the diminished immune response in older adults. Figure made with biorender.com.
2.1. Secondary bacterial infections
The most frequent concurrent bacterial infections with flu are pneumopathogens: Staphylococcus aureus, Streptococcus pneumoniae, Streptococcus pyogenes, Haemophilus influenzae, and occasionally other gram-negative bacilli [101, 102]. Opportunistic bacterial infections, which are common in older adults, occur during and post influenza through many mechanisms. For example, necrosis of airway epithelial cells can result in loss of mucociliary clearance, which then leads to increased colonization of the upper respiratory tract due to loss of physical barriers [103]. In fact, exposure of bacterial attachment sites can result from damaged surfaces of airways with associated fibrin deposition, tissue repair, regenerative process, and sialidase activity of the viral neuraminidase [104, 105]. Similar to humans, mice infected with flu have suppressed Th17 responses and macrophage function leading to inhibited NADPH-oxidase-dependent phagocytic bacterial clearance [106,107].
Secondary bacterial infection can cause physical damage resulting in blockage or small airway functional loss from cellular debris. Additionally, proteinaceous edema fluid and development of exudative transmural pleuritis can severely compromise the respiratory system [108, 109]. In older adults, there is a loss of respiratory epithelial progenitor basal cells resulting in epithelial cell damage, bacterial invasion and decreased lung repair [80]. During enhanced disease states the generation of a robust inflammatory response can lead to subsequent increase in anti-inflammatory responses, which restricts adaptive immune responses [110, 111]. Human studies examining the effects of the immunosuppressive cytokine interleukin (IL)-10 demonstrate that the ratio of interferon gamma to IL-10 (IFNγ:IL-10) produced by ex vivo stimulation with flu virus and IL-7 of peripheral blood T cells is reduced in old age due to increases in IL-10 production [112] and this significantly correlates with patients suffering from laboratory diagnosed flu infection [113]. These studies also associated high IL-10 in the blood with increased longevity, but also greater susceptibility to flu infection in older adults. Thus, IL-10 seems to be important in longevity to control overall age-related inflammation, but it can also have a negative impact on the adaptive immune response to infection, leaving older individuals more susceptible to severe infection.
Conversely, lack of control of proinflammatory cytokines can also aggravate disease in older adults and has been described in cases of flu and Streptococcus pneumoniae [114]. In some cases, there has been strong evidence of excessive neutrophil infiltration and early mortality [114]. In aged mice, susceptibility to S. pneumoniae infection was correlated with increased systemic proinflammatory cytokines, including TNF-alpha, prior to S. pneumoniae infection [114]. Indeed, we and others have shown that old mice have a prolonged and elevated inflammatory response following flu infection [24, 96, 115, 116, 117].
In summary, bacterial infection following flu leads to weakened lung efficiency, blood oxygenation, and multiple organ system disfunction. In fact, hospitalizations and mortality resulting from flu infections are most commonly due to these complications, rather than the flu infection itself.
2.2. Acute Respiratory Distress Syndrome
In addition to secondary bacterial infections, acute respiratory distress syndrome (ARDS) is another common complication of flu infection, especially in the context of human aging [118, 119, 120]. ARDS is severe respiratory failure characterized by diffuse inflammation of alveolar and vascular lung structures which produces progressive hypoxemia [121]. ARDS is associated with the rapid onset of cyanosis, delirium, incontinence, and lungs filled with frothy blood-tinged sputum, all of which can be attributed to primary viral flu pneumonia [122, 123]. The rapid progression from viral pneumonia to ARDS in previously healthy adults suggests that the body’s reaction to infection leads to advanced disease states rather than flu-associated damage [124]. When combined with flu infection, ARDS can be devastating and can often result in death. In an ARDS study independent of flu, patients had a 67% survival rate at 5 years post hospital discharge and suffered decreased physical quality of life, increased costs and use of health care services, and irrecoverable severe lung injury [125, 126]. The H1N1 pandemic of 2009 caused severe hypoxemic respiratory failure defined as ARDS, independent of bacterial infection, but still with multi-organ involvement [127, 128]. Heart failure independent of myocarditis can also occur during flu infection and has higher prevalence in ARDS patients when compared to the general population [129]. In theory, older adults would not have the stem cell potential to repair the hypoxemic damage from this advanced disease nor the efficiency to clear damage quickly. Indeed, ARDS following flu leads to weakened lung efficiency, blood oxygenation, and disrupts multiple organ systems.
3.0. Extrapulmonary and Other Uncommon Complications in Older Adults
In this section, we will examine the extrapulmonary and uncommon complications of flu infection in older adults (see Figure 2). These complications are often overlooked due to the distal nature from the primary site of infection and often times delayed onset.
3.1. Cardiac Complications
Cardiovascular complications during influenza infection are common in older adults. Myocarditis, defined as inflammation of the heart muscle affecting its ability to pump blood, is diagnosed with documented flu infection in 0.4–13% of cases, varying on the flu season [130, 131]. Cellular infiltration and myocyte necrosis have been found in 30–50% of flu and flu-related deaths at autopsy, despite no previous cardiac complications or involvement [132, 133, 134]. Further, myocarditis was not dependent on secondary pneumonia or other infections and was evident in cases of flu infection alone [135]. Age-related concerns arose when 69% of fatal cases, with both bacterial pneumonia and cardiac complications present post-flu, were in those over 18 years of age [132]. During the influenza A H1N1 pandemic of 2009, 70% (31/44) of studied patients with myocarditis were flu-related and, interestingly, only 32% were in people over 40 years of age [130, 131, 132, 133, 134, 135].
Ischemic heart disease (IHD), defined as the narrowing of the heart arteries, is also associated with flu infection and believed to stem from inflammation that plays a critical role in acute coronary syndrome [136]. This stems from systemic pro-inflammatory responses triggered by flu infection that are coupled with pro-coagulant effects [137]. No human study or autopsy results currently suggest the theory of direct virus invasion of the vascular bed. In rare cases, viremia in pandemic influenza strains has occurred [138, 139], but not typically in most patients [140]. In a mouse model of atherosclerosis, mice over 24 months of age with this condition and flu infection develop more prominent inflammatory and thrombotic effects on atherosclerotic plaques, with subendothelial and smooth muscle immune infiltration [141]. This suggests that older adults with atherosclerosis are at greater risk for increased severity of flu infection and potential cardiac complications.
Similar to ischemic heart disease are ischemic cerebral vascular accidents (CVAs), which also significantly increase in incidence after respiratory tract infection [142]. A study of adults (>18 years) found transient increases in adverse vascular events following an acute flu infection, wherein the median age at myocardial infarction was 72.3 years of age and the median age at first stroke following acute flu infection was 78.3 years of age [142]. Thus, suggesting an age-related at-risk population even though the direct link between flu infection and stroke events is unclear. A common theory is that increased damage and clotting factors in the blood during infection leads to greater risks of clotting in the arteries and the brain. Notably, reduced levels of these clotting factors and reduced stroke incidence are present in vaccinated patients with most significant vaccine effects in men >65 years [143]. Similarly, in patients treated with oseltamivir, a common flu anti-viral, there is a 34% stroke risk reduction after 6 months in patients <65 years [144]. Interestingly, those who get an annual flu vaccine have a lesser likelihood of cardiac complications [145–147] and the largest treatment effect was seen among the highest risk patients (>65 years), with more active coronary disease. Indeed, vaccinated older adults were at a 2.9% risk for composite cardiovascular events versus 4.7% in those who were unvaccinated in a recent meta-analysis [145].
3.2. Musculoskeletal
Primary flu infection is limited to pulmonary epithelial cells, yet myalgias are a common symptom and older adults have increased risk for physical disability following flu infection. Skeletal muscle wasting in patients with lung diseases, including COPD, acute respiratory distress syndrome, and cystic fibrosis, contributes to worse outcomes and is associated with increased morbidity following flu infection [148]. Diaphragm dysfunction has also been reported in patients during flu infection and other related lung complications [149–151]. Importantly, skeletal muscle dysfunction plays a role in the pathophysiology of ARDS and is associated with prolonged mechanical ventilation, weaning failure (atrophy), and markedly elevated risk of returning to ICU post discharge. The median age of patients with ARDS who survived to be discharged from the ICU was 45 years (n=117) and the median age of those who died in ICU was 50 years (n = 78) [125]. This results in a vicious cycle of increased hospitalization and impaired quality of life associated with greater morbidity and mortality [126, 152]. These studies found age was significantly associated with physical performance post ARDS hospital discharge, in those >52 years [126]. In fact, critically ill patients with ARDS from flu exhibit weakness, fatigue, reduced exercise capacity, and lose muscle mass even after ICU discharge and after lung recovery [125].
Interestingly, skeletal muscle more distal to the site of infection also is affected by flu infection. Despite the fact that cases of viremia are rare and direct skeletal muscle infection does not occur in influenza [153–155], the clinical association of flu infection and disability with aging has been repeatedly shown especially in those >65 years of age [156–158]. Indeed, following flu infection older adults (>65 years) are at increased risk to lose activities of daily living and develop disabilities [158, 159]. Our laboratory was the first to demonstrate a molecular link for this interaction by characterizing the impact of flu infection on muscle health in mice. Overall, flu leads to mobility impairments with induction of inflammatory and muscle degradation genes and downregulation of positive regulators of muscle growth. These effects are prolonged with aging (mice >21 months), suggesting a direct link between flu infection and increased risk of disability in older adults [115]. Others demonstrated in adult (12–16 week) mice that muscle wasting during/post flu infections is through IL-6 regulation of the E3 ubiquitin ligase atrogin-1 pathway [160], further supporting increased risk with aging as IL-6 is already implicated in aging.
In more serious situations, though much less common, extreme muscle wasting during flu infection has resulted in rhabdomyolysis, where extreme muscle breakdown causes leaking of muscle components into the blood stream that can lead to renal failure [155, 161–173]. Myositis and rhabdomyolysis have been reported during influenza A & B infection [168]. Additionally, a higher incidence of myositis has been observed in older adults (observed cases in 70–86 year old patients) [169]. About 50% of adult patients hospitalized with influenza A had elevated serum creatine phosphokinase (CK), an indicator of muscle damage that can potentially lead to renal failure [174]. Between a quarter and half of people older than 85 years are estimated to be frail [175]. Frailty has also been associated with both an impaired antibody response to influenza and increased sarcopenia following flu infection [175]. Loss of muscle integrity and function is clinically important, since flu infection has been associated with a greater incidence of falls in men and women over 70 years of age [176, 177]. A clinical study of 650 adults with confirmed flu cases and a mean age of 63 reported that two thirds of the subjects were at risk for flu-related morbidity and self-reported disability related to muscle pain/fatigue around 20.4% [178]. Thus, despite not commonly considered a complication of flu infection, skeletal muscle wasting during flu infection is more prevalent with aging [177] and leads to long term disability and loss of resilience.
3.3. Central and Peripheral Nervous System Complications
There is a link between influenza and CNS manifestations. Neurologic complications from the flu include Reye’s syndrome, encephalopathy, encephalomyelitis, transverse myelitis, aseptic meningitis, focal neurologic disorders, and Guillain-Barre syndrome [179]. While these complications are rare, they occur more frequently in children and older adults [179, 180]. Additionally, it seems that immunogenetics play a role in neurological associated influenza complications.
Influenza-associated encephalitis (IAE)/encephalopathy can be extremely severe and can affect all age groups, but the majority of patients were under 5 years of age and have 30% fatality rate [181, 182]. In a 21-person study of acute encephalopathy associated with flu infection, the ages spanned from 4–78 years with 5 patients >60 years, 8 patients 40–59 years, 2 patients 18–25 years, 6 patients <12 years [183]. Impaired consciousness with IAE can put patients in a dreamlike state and leave them confused or faint. Acute necrotizing encephalopathy (ANE) has severe and sudden onset and is characterized by multiple brain lesions, frequently involving the thalami. In Japan there are about 10 cases of ANE per year, 28% are fatal and age-associated prevalence occurs in children under 5 years of age [181]. Other forms of encephalitis associated with influenza include, acute encephalopathy with biphasic seizures (AESD), febrile seizures, and subcortical white matter lesions. Flu can trigger genetic predispositions for AESD in children <2 years old and has a low fatality rate, but high probability of recurring neurological issues including intellectual, motor deficits and epilepsy [181]. In less severe cases there is only mild encephalitis/encephalopathy with a reversible lesion (MERS) of the splenium of the corpus callosum, which is often associated with good clinical outcome. MERS usually resolves in about 10 days and is most common type of encephalopathy among children aged 3–8 years [181]. Similar to MERS there is Posterior Reversible Encephalopathy Syndrome (PRES), where areas of edema via MRI are detected days to weeks after initial viral symptoms. In adult patient cases of PRES, the brain edema is often malignant, with elevated cytokines IL-8 and IL-10, but no detectable virus in the cerebral spinal fluid [184]. More severe conditions also arise from flu-associated CNS disease such as acute hemorrhagic leukoencephalopathy (AHLE), which affects adults and children. Gonzalez et al observes a 46-year old mother and her 11-year old daughter both with AHLE [182]. AHLE is characterized by rapid and fulminant demyelination and inflammation of the white matter with possible links to flu-associated multiple sclerosis flair ups [181, 182].
Flu-associated and multifaceted disease complications (observed in patients 4–78 years) do not occur in singlet, in fact, patients with IAE more frequently experience concurrent hepatic and renal function dysfunction, which could suggest a component of metabolic encephalopathy coexisting severe influenza illness rather than as a direct effect of virus itself [183]. Dysregulated immune responses have also been posited to drive neurologic complications in influenza. Serum levels of IL-6, TNF-alpha, and IL-10 were found to be significantly elevated in pediatric patients with IAE as compared to influenza-infected patients without neurologic involvement [185, 186]. Interestingly, elevated Il-6, TNF-alpha, and IL-10 has also been observed in the serum of older adults [187], suggesting that may also be at higher risk for these CNS events. It is possible that CNS dysfunction in older adults may be over looked due to pre-existing cognitive impairment, such as Alzheimer’s Disease or dementia, or may not associated with the flu infection itself due to confounding aging factors [187].
A correlative study on H3N2 flu virus-associated encephalopathy examined cytokine levels and NF-kB activation in peripheral blood mononuclear cells from 30 children with influenza virus-associated encephalopathy at the acute stage of flu infection [185]. This study linked serum levels of IL-6, soluble tumor necrosis factor receptor-1 (sTNFR1), and IL-10 levels with flu virus-associated encephalopathy. Higher NF-kB activation in peripheral blood mononuclear cells (PBMCs) in flu-associated encephalopathic patients’ groups was higher than patients with uncomplicated H3N2 influenza infection [185]. In young mice, A/Puerto Rico/8/1934 H1N1 flu virus induced neuroinflammatory responses paralleled loss of neurotrophic and glial regulatory factors in the hippocampus, and deficits in cognitive function. This suggests that flu-induced neuropathy that is observed clinically is likely due to neuroinflammation and can lead to cognitive declines [188]. Indeed, neuropathology with influenza is a real threat and it affects both mind and body resulting in observed loss of motor skills, reflexes, cognition, and more. Older adults are at greater risk for these complications due to many factors including elevated basal inflammation, pre-existing cognitive conditions, and other co-morbidities.
3.4. Gastrointestinal and gut microbiota complications
Flu infection can also be accompanied by gastrointestinal symptoms and other complications. Murine studies have supported these clinical associations as well. Flu infection itself induces gut microbiota dysbiosis through type I interferons (IFN-I) favoring proteobacteria overgrowth [189]. Such shifts can also impact T cell trafficking, differentiation, and even inflammatory responses. In this instance there is a greater chance of secondary salmonella infection localized to the gut during or post flu infection [189].
Common gastroenteritis-like symptoms in many flu infected patients include abdominal pain, nausea vomiting, and diarrhea [190–192]. In 6–10 week old male mice, lymphocytes derived from the respiratory mucosa migrated into the intestinal mucosa during flu lung infection via CCL25-CCR9 chemokine signaling, which destroyed the intestinal microbiota homeostasis in the small intestine and led to intestinal injury [193]. Respiratory influenza virus infection can also induce intestinal immune injury via microbiota-mediated Th17 cell-dependent inflammation [193]. Age-related changes, whether direct or indirect due to antibiotic treatment, may impair flu responses in older adults. In 8–16 week old CBA/J female mouse models, respiratory influenza virus infection induces intestinal immune injury via microbiota-mediated Th17 cell-dependent inflammation [117, 194].
Flu RNA has been found in stools of patients with confirmed flu infection and this ranged from 3 to 71% in studies with children and 7.2% to 47% in studies on adults [192,195–202]. Altogether, the prevalence of flu virus in stool was 20.9% for patients in the above-mentioned studies [203]. Gastrointestinal symptoms, most commonly vomiting, were reported in 30.9% of the pandemic 2009 H1N1 flu infections, 2.8% prevalent in seasonal H1N1 infection, 25.3% of influenza B virus infections and 21.9% for influenza A(H3N2) [203–205]. In theory, the already weakened immune system of older adults would be less prepared for these types of infections.
3.5. Hepatic Complications
Liver injury, from secondary systemic inflammation, mediated by viral infection is also an uncommon complication during flu infection, however this is observed in some older patients [120, 206]. Papic et al. examined pandemic 2009 flu patients (mean age of 46.79) compared to seasonal flu patients (mean age of 47.06) and determined both illicit significant immune responses to infection leading to hepatocellular injury [206]. Additionally, Polakos et al. infected humans aged 18–45 years with 107 TCID50 influenza A/Kawasaki/86 (H1N1) virus to observe the flu-induced changes to the human liver [207]. Indeed, severe cases of flu have reported liver damage evident through elevated blood transaminases [alanine transaminase (AST) and aspartate transaminase (ALT)], bilirubin, and Gamma-glutamyl transferase (GGT) [206, 207]. Additionally, these elevations were associated with duration of hospitalization, hypoxia, and serum C-reactive protein (CRP) levels [206, 207]. Others have even noted centrizonal hepatic hemorrhagic necrosis in deceased pandemic flu patients ages 24–65 years upon autopsy [208, 209], while Avian A(H5N1) and A(H7N9) were specifically connected with transaminase elevations [209, 210]. Beyond these threats, the liver is also susceptible to clots, hemorrhage, and less severely metabolic dysfunction [208, 209]. It is likely since there is increased systemic inflammation in older adults during flu infection, and that they may have pre-existing hepatic deficiencies, older adults may be more susceptible to hepatic complications during flu infection. In the context of future directions, more research should focus on the metabolic impact of flu infection on organs such as the liver, particularly in the context of aging.
3.6. Renal Complications
As with other complications, flu-associated renal complications are worse in those with pre-existing risk factors, including obesity, chronic kidney disease, and increased age [211, 212]. Indeed, older adults are more likely to suffer from pre-existing renal impairment. Flu infection, even without severe complications such as rhabdomyolysis, can cause acute kidney injuries. Incidence of acute kidney injuries ranged from 18–66% in ICU admitted flu patients with the average age of 43 years and 13% of patients over 60 years [213–215]. Further, in the 2009 pandemic influenza A (H1N1) studies observed acute tubular necrosis (ATN), acute kidney injury was reported in up to 66% of critically ill adults (aged 25–50 years) and was associated with higher mortality rates [216–218]. These renal injuries are speculated to be due to decreased renal perfusion secondary to hypovolemia or a vasodilatory state of sepsis during severe flu infection. Importantly, renal function is regularly monitored in ICU admitted older adults for almost all infections. However, renal function and filtration declines with aging and the decrease of repair capacity leaves the organ vulnerable in older adults. In the context of future directions, there should be more research into the relation between kidney injury and flu infection so that more preventative action can be taken.
4.0. Conclusions
Diminished immune responses in older adults sets this population aside from others with pre-existing vulnerability to influenza infection and its complications. In addition to weakened and delayed immune responses, older adults often struggle with recovery post infection due to extenuating complications. Indeed, the dysregulation of immune responses and increased systemic inflammation contribute to multiorgan complications that negatively impact older adults. The most common complication in older adults is secondary bacterial infection, such as bacterial pneumonia. Indeed, secondary bacterial infection is implicated in the majority of flu-associated deaths in older adults. While commonly overlooked, uncommon complications, such as ARDS, often times follow pneumonia and can lead to advanced disease states. Extrapulmonary flu complications are also more common in older adults. The severity of flu-associated myocarditis spans a wide spectrum ranging from asymptomatic to severe heart disease, though the relation to age is not clear. Interestingly, skeletal muscle more distal to the site of infection also is affected by flu infection with older adults more at risk for weakness and muscle atrophy, often resulting in declines in physical function. Surprisingly, even separate from these instances of flu virus in the intestine, there are microbiota altering events during lung flu infection, which shift T cell population frequencies and induce dysbiosis. Adverse neurological and central nervous system complications with flu, mainly seen in pediatric and older adults, have associations with demyelination, encephalopathy, seizures, and cognitive declines. Other commonly overlooked complications from systemic inflammation and multiorgan disfunction include hepatic and renal systems which are already weakened in older adults from pre-existing complications. While vaccination reduces some of these common and uncommon complications, the negative impacts of flu infection are still evident in older adults. Thus, older adults need to be monitored more closely for flu-associated complications to prevent flu-associated morbidities and disabilities.
Highlights.
Older adults have preexisting vulnerability to flu infection and its complications
Weakened and delayed immune responses lead to increased systemic inflammation
Dysregulated immune responses and inflammation leads to multiorgan complications
Vaccination reduces severity and duration of flu complications in older adults
Older adults need closer monitoring for flu complications to prevent morbidities
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
5.0 References
- 1.Wright P, Neumann G, Kawaoka Y G. Orthomyxoviruses, Fields Virology. Vol 2 5th ed. Philadelphia: Lippincott Williams & Wilkins, (2007). [Google Scholar]
- 2.Thompson WW, Shay DK, Weintraub E, Brammer L, Bridges CB, Cox NJ, Fukuda K, Influenza-associated hospitalizations in the United States, Jama, 292 (2004) 1333–1340. 10.1001/jama.292.11.1333 [DOI] [PubMed] [Google Scholar]
- 3.Taubenberger JK, Morens DM, The pathology of influenza virus infections, Annu. Rev. Pathmechdis. Mech. Dis, 3 (2008) 499–522. 10.1146/annurev.pathmechdis.3.121806.154316 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hulme KD, Gallo LA, Short KR, Influenza virus and glycemic variability in diabetes: a killer combination?, Frontiers in microbiology, 8 (2017) 861 10.3389/fmicb.2017.00861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Neidich SD, Green WD, Rebeles J, Karlsson EA, Schultz-Cherry S, Noah TL, Chakladar S, Hudgens MG, Weir SS, Beck MA, Increased risk of influenza among vaccinated adults who are obese, International journal of obesity, 41 (2017) 1324 10.1038/ijo.2017.131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Memoli MJ, Athota R, Reed S, Czajkowski L, Bristol T, Proudfoot K, Hagey R, Voell J, Fiorentino C, Ademposi A, The natural history of influenza infection in the severely immunocompromised vs nonimmunocompromised hosts, Clinical infectious diseases, 58 (2013) 214–224. 10.1093/cid/cit725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Maue AC, Haynes L, CD4+ T cells and immunosenescence–a mini-review, Gerontology, 55 (2009) 491–495. 10.1159/000214842 [DOI] [PubMed] [Google Scholar]
- 8.Agarwal S, Busse PJ, Innate and adaptive immunosenescence, Annals of Allergy, Asthma & Immunology, 104 (2010) 183–190. 10.1016/j.anai.2009.11.009. [DOI] [PubMed] [Google Scholar]
- 9.Heron M, Deaths: leading causes for 2010. Natl Vital Statistics Rep. 2013. December 20; 62 (6): 1–96. Hyattsville, MD: National Center for Health Statistics https://www.cdc.gov/nchs/data/nvsr/nvsr62/nvsr62_06.pdf (last accessed on July 21, 2019). [PubMed] [Google Scholar]
- 10.Nichols H, The top 10 leading causes of death in the United States, Medical news today, (2017). http://www.medicalnewstoday.com/articles/282929.php (last accessed on July 21, 2019).
- 11.Fischer II WA, Chason KD, Brighton M, Jaspers I, Live attenuated influenza vaccine strains elicit a greater innate immune response than antigenically-matched seasonal influenza viruses during infection of human nasal epithelial cell cultures, Vaccine, 32 (2014) 1761–1767. 10.1016/j.vaccine.2013.12.069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Molinari N-AM, Ortega-Sanchez IR, Messonnier ML, Thompson WW, Wortley PM, Weintraub E, Bridges CB, The annual impact of seasonal influenza in the US: measuring disease burden and costs, Vaccine, 25 (2007) 5086–5096. 10.1016/j.vaccine.2007.03.046 [DOI] [PubMed] [Google Scholar]
- 13.Simonsen L, Reichert TA, Viboud C, Blackwelder WC, Taylor RJ, Miller MA, Impact of influenza vaccination on seasonal mortality in the US elderly population, Archives of internal medicine, 165 (2005) 265–272. 10.1001/archinte.165.3.265 [DOI] [PubMed] [Google Scholar]
- 14.Dushoff J, Plotkin JB, Viboud C, Earn DJ, Simonsen L, Mortality due to influenza in the United States—an annualized regression approach using multiple-cause mortality data, American journal of epidemiology, 163 (2005) 181–187. 10.1093/aje/kwj024 [DOI] [PubMed] [Google Scholar]
- 15.Heron MP, Deaths: leading causes for 2013, (2016). https://www.cdc.gov/nchs/data/nvsr/nvsr65/nvsr65_02.pdf (last accessed on July 21, 2019).
- 16.Morris DE, Cleary DW, Clarke SC, Secondary bacterial infections associated with influenza pandemics, Frontiers in microbiology, 8 (2017) 1041 10.3389/fmicb.2017.01041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gubbels Bupp MR, Potluri T, Fink AL, Klein SL, The confluence of sex hormones and aging on immunity, Frontiers in Immunology, 9 (2018) 1269 10.3389/fimmu.2018.01269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Haynes L, Eaton SM, Burns EM, Randall TD, Swain SL, CD4 T cell memory derived from young naive cells functions well into old age, but memory generated from aged naive cells functions poorly, Proceedings of the National Academy of Sciences, 100 (2003) 15053–15058. 10.1073/pnas.2433717100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Haynes L, The effect of aging on cognate function and development of immune memory, Current opinion in immunology, 17 (2005) 476–479. 10.1016/j.coi.2005.07.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Eaton SM, Burns EM, Kusser K, Randall TD, Haynes L, Age-related defects in CD4 T cell cognate helper function lead to reductions in humoral responses, Journal of Experimental Medicine, 200 (2004) 1613–1622. 10.1084/jem.20041395 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Reber AJ, Chirkova T, Kim JH, Cao W, Biber R, Shay DK, Sambhara S, Immunosenescence and challenges of vaccination against influenza in the aging population, Aging and disease, 3 (2012) 68 https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3320806/pdf/ad-3-1-68.pdf (last accessed on July 21, 2019). [PMC free article] [PubMed] [Google Scholar]
- 22.Nikolich-Žugich J, Aging of the T cell compartment in mice and humans: from no naive expectations to foggy memories, The Journal of Immunology, 193 (2014) 2622–2629. 10.4049/jimmunol.1401174 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Haynes L, Eaton SM, Swain SL, The defects in effector generation associated with aging can be reversed by addition of IL-2 but not other related γc-receptor binding cytokines, Vaccine, 18 (2000) 1649–1653. 10.1016/S0264-410X(99)00501-0 [DOI] [PubMed] [Google Scholar]
- 24.Haynes L, Eaton SM, Burns EM, Rincon M, Swain SL, Inflammatory cytokines overcome age-related defects in CD4 T cell responses in vivo, The Journal of Immunology, 172 (2004) 5194–5199. 10.4049/jimmunol.172.9.5194 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Messaoudi I, Warner J, Fischer M, Park B, Hill B, Mattison J, Lane MA, Roth GS, Ingram DK, Picker LJ, Delay of T cell senescence by caloric restriction in aged long-lived nonhuman primates, Proceedings of the National Academy of Sciences, 103 (2006) 19448–19453. 10.1073/pnas.0606661103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Uhrlaub JL, Brien JD, Widman DG, Mason PW, Nikolich-Žugich J, Repeated in vivo stimulation of T and B cell responses in old mice generates protective immunity against lethal West Nile virus encephalitis, The Journal of Immunology, 186 (2011) 3882–3891. 10.4049/jimmunol.1002799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Agrawal A, Tay J, Ton S, Agrawal S, Gupta S, Increased reactivity of dendritic cells from aged subjects to self-antigen, the human DNA, The Journal of Immunology, 182 (2009) 1138–1145. 10.4049/jimmunol.182.2.1138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jing Y, Shaheen E, Drake RR, Chen N, Gravenstein S, Deng Y, Aging is associated with a numerical and functional decline in plasmacytoid dendritic cells, whereas myeloid dendritic cells are relatively unaltered in human peripheral blood, Human immunology, 70 (2009) 777–784. 10.1016/j.humimm.2009.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wenisch C, Patruta S, Daxböck F, Krause R, Hörl W, Effect of age on human neutrophil function, Journal of leukocyte biology, 67 (2000) 40–45. 10.1002/jlb.67.1.40 [DOI] [PubMed] [Google Scholar]
- 30.Haynes L, Eaton SM, Burns EM, Randall TD, Swain SL, CD4 T cell memory derived from young naive cells functions well into old age, but memory generated from aged naive cells functions poorly, Proceedings of the National Academy of Sciences, 100 (2003) 15053–15058. 10.1073/pnas.2433717100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hearps AC, Martin GE, Angelovich TA, Cheng WJ, Maisa A, Landay AL, Jaworowski A, Crowe SM, Aging is associated with chronic innate immune activation and dysregulation of monocyte phenotype and function, Aging cell, 11 (2012) 867–875. 10.1111/j.1474-9726.2012.00851.x. [DOI] [PubMed] [Google Scholar]
- 32.Puchta A, Naidoo A, Verschoor CP, Loukov D, Thevaranjan N, Mandur TS, Nguyen P.-s., Jordana M, Loeb M, Xing Z, TNF drives monocyte dysfunction with age and results in impaired anti-pneumococcal immunity, PLoS pathogens, 12 (2016) e1005368 10.1371/journal.ppat.1005368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Verschoor CP, Johnstone J, Millar J, Parsons R, Lelic A, Loeb M, Bramson JL, Bowdish DM, Alterations to the frequency and function of peripheral blood monocytes and associations with chronic disease in the advanced-age, frail elderly, PLoS One, 9 (2014) e104522 10.1371/journal.pone.0104522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Agrawal A, Agrawal S, Cao J-N, Su H, Osann K, Gupta S, Altered innate immune functioning of dendritic cells in elderly humans: a role of phosphoinositide 3-kinase-signaling pathway, The Journal of Immunology, 178 (2007) 6912–6922. 10.4049/jimmunol.178.11.6912. [DOI] [PubMed] [Google Scholar]
- 35.Moretto MM, Lawlor EM, Khan IA, Aging mice exhibit a functional defect in mucosal dendritic cell response against an intracellular pathogen, The Journal of Immunology, 181 (2008) 7977–7984. 10.4049/jimmunol.181.11.7977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Stout-Delgado HW, Yang X, Walker WE, Tesar BM, Goldstein DR, Aging impairs IFN regulatory factor 7 up-regulation in plasmacytoid dendritic cells during TLR9 activation, The Journal of Immunology, 181 (2008) 6747–6756. 10.4049/jimmunol.181.10.6747 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Pereira LF, de Souza APD, Borges TJ, Bonorino C, Impaired in vivo CD4+ T cell expansion and differentiation in aged mice is not solely due to T cell defects: decreased stimulation by aged dendritic cells, Mechanisms of ageing and development, 132 (2011) 187–194. 10.1016/j.mad.2011.03.005. [DOI] [PubMed] [Google Scholar]
- 38.Chougnet CA, Thacker RI, Shehata HM, Hennies CM, Lehn MA, Lages CS, Janssen EM, Loss of phagocytic and antigen cross-presenting capacity in aging dendritic cells is associated with mitochondrial dysfunction, The Journal of Immunology, 195 (2015) 2624–2632. 10.4049/jimmunol.1501006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Grolleau-Julius A, Garg MR, Mo R, Stoolman LL, Yung RL, Effect of aging on bone marrow-derived murine CD11c+ CD4− CD8α− dendritic cell function, The Journals of Gerontology Series A: Biological Sciences and Medical Sciences, 61 (2006) 1039–1047. 10.1093/gerona/61.10.1039 [DOI] [PubMed] [Google Scholar]
- 40.Jones V. Brahmakshatriya, Huston G, Dibble J, Swain SL, TLR-activated dendritic cells enhance the response of aged naive CD4 T cells via an IL-6–dependent mechanism, The Journal of Immunology, 185 (2010) 6783–6794. 10.4049/jimmunol.0901296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sapey E, Greenwood H, Walton G, Mann E, Love A, Aaronson N, Insall RH, Stockley RA, Lord JM, Phosphoinositide 3-kinase inhibition restores neutrophil accuracy in the elderly: toward targeted treatments for immunosenescence, Blood, 123 (2014) 239–248. 10.1182/blood-2013-08-519520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Boehmer ED, Goral J, Faunce DE, Kovacs EJ, Age‐dependent decrease in Toll‐like receptor 4‐mediated proinflammatory cytokine production and mitogen‐activated protein kinase expression, Journal of leukocyte biology, 75 (2004) 342–349. 10.1189/jlb.0803389. [DOI] [PubMed] [Google Scholar]
- 43.Boehmer ED, Meehan MJ, Cutro BT, Kovacs EJ, Aging negatively skews macrophage TLR2-and TLR4-mediated pro-inflammatory responses without affecting the IL-2-stimulated pathway, Mechanisms of ageing and development, 126 (2005) 1305–1313. 10.1016/j.mad.2005.07.009. [DOI] [PubMed] [Google Scholar]
- 44.Montgomery RR, Shaw AC, Paradoxical changes in innate immunity in aging: recent progress and new directions, Journal of leukocyte biology, 98 (2015) 937–943. 10.1189/jlb.5MR0315-104R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Nyugen J, Agrawal S, Gollapudi S, Gupta S, Impaired functions of peripheral blood monocyte subpopulations in aged humans, Journal of clinical immunology, 30 (2010) 806–813. 10.1007/s10875-010-9448-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Van Duin D, Mohanty S, Thomas V, Ginter S, Montgomery RR, Fikrig E, Allore HG, Medzhitov R, Shaw AC, Age-associated defect in human TLR-1/2 function, The Journal of Immunology, 178 (2007) 970–975. 10.4049/jimmunol.178.2.970. [DOI] [PubMed] [Google Scholar]
- 47.Molony RD, Nguyen JT, Kong Y, Montgomery RR, Shaw AC, Iwasaki A, Aging impairs both primary and secondary RIG-I signaling for interferon induction in human monocytes, Sci. Signal, 10 (2017) eaan2392 10.1126/scisignal.aan2392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Seidler S, Zimmermann HW, Bartneck M, Trautwein C, Tacke F, Age-dependent alterations of monocyte subsets and monocyte-related chemokine pathways in healthy adults, BMC immunology, 11 (2010) 30 10.1186/1471-2172-11-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Chara L, Sánchez-Atrio A, Pérez A, Cuende E, Albarrán F, Turrión A, Chevarria J, Sánchez MA, Monserrat J, de la Hera A, Monocyte populations as markers of response to adalimumab plus MTX in rheumatoid arthritis, Arthritis research & therapy, 14 (2012) R175 10.1186/ar3928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Xia L, Lu J, Xiao W, Blockage of TNF-α by infliximab reduces CCL2 and CCR2 levels in patients with rheumatoid arthritis, Journal of Investigative Medicine, 59 (2011) 961–963. 10.2310/JIM.0b013e31821c0242. [DOI] [PubMed] [Google Scholar]
- 51.Arnardottir HH, Dalli J, Colas RA, Shinohara M, Serhan CN, Aging delays resolution of acute inflammation in mice: reprogramming the host response with novel nano-proresolving medicines, The Journal of Immunology, 193 (2014) 4235–4244. 10.4049/jimmunol.1401313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Albright JW, Albright JF, Ageing alters the competence of the immune system to control parasitic infection, Immunology letters, 40 (1994) 279–285. 10.1016/0165-2478(94)00066-2 [DOI] [PubMed] [Google Scholar]
- 53.Bradley SF, Kauffman CA, Aging and the response to Salmonella infection, Experimental gerontology, 25 (1990) 75–80. 10.1016/0531-5565(90)90012-Q [DOI] [PubMed] [Google Scholar]
- 54.Aprahamian T, Takemura Y, Goukassian D, Walsh K, Ageing is associated with diminished apoptotic cell clearance in vivo, Clinical & Experimental Immunology, 152 (2008) 448–455. 10.1111/j.1365-2249.2008.03658.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Boelen E, Stassen FR, Steinbusch HW, Borchelt DR, Streit WJ, Ex vivo cultures of microglia from young and aged rodent brain reveal age-related changes in microglial function, Neurobiology of aging, 33 (2012) 195. e191–195. e112. 10.1016/j.neurobiolaging.2010.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Swift ME, Burns AL, Gray KL, DiPietro LA, Age-related alterations in the inflammatory response to dermal injury, Journal of Investigative Dermatology, 117 (2001) 1027–1035. 10.1046/j.0022-202x.2001.01539.x. [DOI] [PubMed] [Google Scholar]
- 57.Shimazu T, Iida R, Zhang Q, Welner RS, Medina KL, Alberola-lla J, Kincade PW, CD86 is expressed on murine hematopoietic stem cells and denotes lymphopoietic potential, Blood, 119 (2012) 4889–4897. 10.1182/blood-2011-10-388736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Riley RL, Impaired B lymphopoiesis in old age: a role for inflammatory B cells?, Immunologic research, 57 (2013) 361–369. 10.1007/s12026-013-8444-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Berent-Maoz B, Montecino-Rodriguez E, Dorshkind K, Genetic regulation of thymocyte progenitor aging, Seminars in immunology, Elsevier, 2012, pp. 303–308. 10.1016/j.smim.2012.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Labrie JE, Sah AP, Allman DM, Cancro MP, Gerstein RM, Bone marrow microenvironmental changes underlie reduced RAG-mediated recombination and B cell generation in aged mice, Journal of Experimental Medicine, 200 (2004) 411–423. 10.1084/jem.20040845 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Palmer DB, The effect of age on thymic function, Frontiers in immunology, 4 (2013) 316 10.3389/fimmu.2013.00316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Alter-Wolf S, Blomberg BB, Riley RL, Deviation of the B cell pathway in senescent mice is associated with reduced surrogate light chain expression and altered immature B cell generation, phenotype, and light chain expression, The Journal of Immunology, 182 (2009) 138–147. 10.4049/jimmunol.182.1.138 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Sansoni P, Cossarizza A, Brianti V, Fagnoni F, Snelli G, Monti D, Marcato A, Passeri G, Ortolani C, Forti E, Lymphocyte subsets and natural killer cell activity in healthy old people and centenarians [see comments], Blood, 82 (1993) 2767–2773. http://www.bloodjournal.org/content/82/9/2767 (last accessed on July 21, 2019). [PubMed] [Google Scholar]
- 64.Mattoo H, Faulkner M, Kandpal U, Das R, Lewis V, George A, Rath S, Durdik JM, Bal V, Naive CD4 T cells from aged mice show enhanced death upon primary activation, International immunology, 21 (2009) 1277–1289. 10.1093/intimm/dxp094 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.van der Geest KS, Abdulahad WH, Tete SM, Lorencetti PG, Horst G, Bos NA, Kroesen B-J, Brouwer E, Boots AM, Aging disturbs the balance between effector and regulatory CD4+ T cells, Experimental gerontology, 60 (2014) 190–196. 10.1016/j.exger.2014.11.005. [DOI] [PubMed] [Google Scholar]
- 66.Bruunsgaard H, Pedersen AN, Schroll M, Skinhøj P, Pedersen B, Proliferative responses of blood mononuclear cells (BMNC) in a cohort of elderly humans: role of lymphocyte phenotype and cytokine production, Clinical & Experimental Immunology, 119 (2000) 433–440. 10.1046/j.1365-2249.2000.01146.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Aggarwal S, Gupta S, Increased apoptosis of T cell subsets in aging humans: altered expression of Fas (CD95), Fas ligand, Bcl-2, and Bax, The Journal of Immunology, 160 (1998) 1627–1637. https://www.jimmunol.org/content/160/4/1627.long (last accessed on July 21, 2019). [PubMed] [Google Scholar]
- 68.Aggarwal S, Gollapudi S, Gupta S, Increased TNF-α-induced apoptosis in lymphocytes from aged humans: changes in TNF-α receptor expression and activation of caspases, The Journal of Immunology, 162 (1999) 2154–2161. https://www.jimmunol.org/content/162/4/2154 (last accessed on July 21, 2019). [PubMed] [Google Scholar]
- 69.Haynes L, Linton P-J, Eaton SM, Tonkonogy SL, Swain SL, Interleukin 2, but not other common γ chain–binding cytokines, can reverse the defect in generation of CD4 effector T cells from naive T cells of aged mice, Journal of Experimental Medicine, 190 (1999) 1013–1024. 10.1084/jem.190.7.1013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Lefebvre JS, Maue AC, Eaton SM, Lanthier PA, Tighe M, Haynes L, The aged microenvironment contributes to the age‐related functional defects of CD4 T cells in mice, Aging cell, 11 (2012) 732–740. 10.1111/j.1474-9726.2012.00836.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Maue AC, Eaton SM, Lanthier PA, Sweet KB, Blumerman SL, Haynes L, Proinflammatory adjuvants enhance the cognate helper activity of aged CD4 T cells, The Journal of Immunology, 182 (2009) 6129–6135. 10.4049/jimmunol.0804226 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Lefebvre JS, Masters AR, Hopkins JW, Haynes L, Age-related impairment of humoral response to influenza is associated with changes in antigen specific T follicular helper cell responses, Scientific reports, 6 (2016) 25051 10.1038/srep25051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Lages CS, Suffia I, Velilla PA, Huang B, Warshaw G, Hildeman DA, Belkaid Y, Chougnet C, Functional regulatory T cells accumulate in aged hosts and promote chronic infectious disease reactivation, The Journal of Immunology, 181 (2008) 1835–1848. 10.4049/jimmunol.181.3.1835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Raynor J, Karns R, Almanan M, Li K-P, Divanovic S, Chougnet CA, Hildeman DA, IL-6 and ICOS antagonize Bim and promote regulatory T cell accrual with age, The Journal of Immunology, 195 (2015) 944–952. 10.4049/jimmunol.1500443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Huang M-C, Liao J-J, Bonasera S, Longo DL, Goetzl EJ, Nuclear factor-κB-dependent reversal of aging-induced alterations in T cell cytokines, The FASEB Journal, 22 (2008) 2142–2150. 10.1096/fj.07-103721. [DOI] [PubMed] [Google Scholar]
- 76.Kieper WC, Burghardt JT, Surh CD, A role for TCR affinity in regulating naive T cell homeostasis, The Journal of Immunology, 172 (2004) 40–44. 10.4049/jimmunol.172.1.40. [DOI] [PubMed] [Google Scholar]
- 77.Johnson SA, Cambier JC, Ageing, autoimmunity and arthritis: senescence of the B cell compartment–implications for humoral immunity, Arthritis Res Ther, 6 (2004) 131 10.1186/ar1180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Nicoletti C, Yang X, Cerny J, Repertoire diversity of antibody response to bacterial antigens in aged mice. III. Phosphorylcholine antibody from young and aged mice differ in structure and protective activity against infection with Streptococcus pneumoniae, The Journal of Immunology, 150 (1993) 543–549. https://www.jimmunol.org/content/150/2/543 (last accessed on July 21, 2019). [PubMed] [Google Scholar]
- 79.Hao Y, O’Neill P, Naradikian M, Scholz J, Cancro M, AB cell subset uniquely responsive to innate stimuli accumulates in aged mice (45.7), Am Assoc Immnol, 2011. 10.1182/blood-2011-01-330530 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Kash JC, Walters K-A, Davis AS, Sandouk A, Schwartzman LM, Jagger BW, Chertow DS, Qi L, Kuestner RE, Ozinsky A, Lethal synergism of 2009 pandemic H1N1 influenza virus and Streptococcus pneumoniae coinfection is associated with loss of murine lung repair responses, MBio, 2 (2011) e00172–00111. 10.1128/mBio.00172-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Caruso C, Buffa S, Candore G, Colonna-Romano G, Dunn-Walters D, Kipling D, Pawelec G, Mechanisms of immunosenescence, Immunity & Ageing, 6 (2009) 10 10.1186/1742-4933-6-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Solana R, Tarazona R, Gayoso I, Lesur O, Dupuis G, Fulop T, Innate immunosenescence: effect of aging on cells and receptors of the innate immune system in humans, Seminars in immunology, Elsevier, 2012, pp. 331–341. 10.1016/j.smim.2012.04.008. [DOI] [PubMed] [Google Scholar]
- 83.Linton PJ, Li SP, Zhang Y, Bautista B, Huynh Q, Trinh T, Intrinsic versus environmental influences on T‐cell responses in aging, Immunological reviews, 205 (2005) 207–219. 10.1038/ni1033 [DOI] [PubMed] [Google Scholar]
- 84.Lanzavecchia A, Sallusto F, Regulation of T cell immunity by dendritic cells, Cell, 106 (2001) 263–266. 10.1016/s0092-8674(01)00455-x [DOI] [PubMed] [Google Scholar]
- 85.Sallusto F, Lanzavecchia A, The instructive role of dendritic cells on T-cell responses, Arthritis Research & Therapy, 4 (2002) S127 10.1186/ar567 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Iwasaki A, Mucosal dendritic cells, Annu. Rev. Immunol, 25 (2007) 381–418. 10.1146/annurev.immunol.25.022106.141634. [DOI] [PubMed] [Google Scholar]
- 87.Chiu B-C, Stolberg VR, Zhang H, Chensue SW, Increased Foxp3+ Treg cell activity reduces dendritic cell co-stimulatory molecule expression in aged mice, Mechanisms of ageing and development, 128 (2007) 618–627. 10.1016/j.mad.2007.09.002. [DOI] [PubMed] [Google Scholar]
- 88.Garcia GG, Miller RA, Single-cell analyses reveal two defects in peptide-specific activation of naive T cells from aged mice, The Journal of Immunology, 166 (2001) 3151–3157. 10.4049/jimmunol.166.5.3151. [DOI] [PubMed] [Google Scholar]
- 89.Tamir A, Eisenbraun MD, Garcia GG, Miller RA, Age-dependent alterations in the assembly of signal transduction complexes at the site of T cell/APC interaction, The Journal of Immunology, 165 (2000) 1243–1251. 10.4049/jimmunol.165.3.1243. [DOI] [PubMed] [Google Scholar]
- 90.Garcia GG, Miller RA, Age-dependent defects in TCR-triggered cytoskeletal rearrangement in CD4+ T cells, The Journal of Immunology, 169 (2002) 5021–5027. 10.4049/jimmunol.169.9.5021. [DOI] [PubMed] [Google Scholar]
- 91.Dolfi DV, Mansfield KD, Polley AM, Doyle SA, Freeman GJ, Pircher H, Schmader KE, Wherry EJ, Increased T‐bet is associated with senescence of influenza virus‐specific CD8 T cells in aged humans, Journal of leukocyte biology, 93 (2013) 825–836. 10.1189/jlb.0912438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Jiang J, Bennett AJ, Fisher E, Williams-Bey Y, Shen H, Murasko DM, Limited expansion of virus-specific CD8 T cells in the aged environment, Mechanisms of ageing and development, 130 (2009) 713–721. 10.1016/j.mad.2009.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Yager EJ, Ahmed M, Lanzer K, Randall TD, Woodland DL, Blackman MA, Age-associated decline in T cell repertoire diversity leads to holes in the repertoire and impaired immunity to influenza virus, Journal of Experimental Medicine, 205 (2008) 711–723. 10.1084/jem.20071140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Decman V, Laidlaw BJ, DiMenna LJ, Abdulla S, Mozdzanowska K, Erikson J, Ertl HC, Wherry EJ, Cell-intrinsic defects in the proliferative response of antiviral memory CD8 T cells in aged mice upon secondary infection, The Journal of Immunology, 184 (2010) 5151–5159. 10.4049/jimmunol.0902063. [DOI] [PubMed] [Google Scholar]
- 95.Ely KH, Roberts AD, Kohlmeier JE, Blackman MA, Woodland DL, Aging and CD8+ T cell immunity to respiratory virus infections, Experimental gerontology, 42 (2007) 427–431. 10.1016/j.exger.2006.11.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Masters AR, Hall A, Bartley JM, Keilich SR, Lorenzo EC, Jellison ER, Puddington L, Haynes L, Assessment of Lymph Node Stromal Cells as an Underlying Factor in Age-Related Immune Impairment, The Journals of Gerontology: Series A, (2019). 10.1093/gerona/glz029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Samuelson DR, Welsh DA, Shellito JE, Regulation of lung immunity and host defense by the intestinal microbiota, Frontiers in microbiology, 6 (2015) 1085 10.3389/fmicb.2015.01085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Ichinohe T, Pang IK, Kumamoto Y, Peaper DR, Ho JH, Murray TS, Iwasaki A, Microbiota regulates immune defense against respiratory tract influenza A virus infection, Proceedings of the National Academy of Sciences, 108 (2011) 5354–5359. 10.1073/pnas.1019378108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Yu B, Dai C.-q., Chen J, Deng L, Wu X.-l., Wu S, Zhao C.-l., Jiang Z.-y., Chen X.-y., Dysbiosis of gut microbiota induced the disorder of helper T cells in influenza virus-infected mice, Human vaccines & immunotherapeutics, 11 (2015) 1140–1146. 10.1080/21645515.2015.1009805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Sudo N, Yu XN, Aiba Y, Oyama N, Sonoda J, Koga Y, Kubo C, An oral introduction of intestinal bacteria prevents the development of a long‐term Th2‐skewed immunological memory induced by neonatal antibiotic treatment in mice, Clinical & Experimental Allergy, 32 (2002) 1112–1116. 10.1046/j.1365-2222.2002.01430.x. [DOI] [PubMed] [Google Scholar]
- 101.Morens DM, Taubenberger JK, Fauci AS, Predominant role of bacterial pneumonia as a cause of death in pandemic influenza: implications for pandemic influenza preparedness, The Journal of infectious diseases, 198 (2008) 962–970. 10.1086/591708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Schwarzmann SW, Adler JL, Sullivan RJ, Marine WM, Bacterial pneumonia during the Hong Kong influenza epidemic of 1968–1969: experience in a city-county hospital, Archives of internal medicine, 127 (1971) 1037–1041. 10.1001/archinte.1971.00310180053006. [DOI] [PubMed] [Google Scholar]
- 103.McCullers JA, Insights into the interaction between influenza virus and pneumococcus, Clinical microbiology reviews, 19 (2006) 571–582. 10.1128/CMR.00058-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.DeLeo FR, Musser JM, Axis of coinfection evil, The Journal of infectious diseases, 201 (2010) 488–490. 10.1086/650304. [DOI] [PubMed] [Google Scholar]
- 105.McCullers JA, Bartmess KC, Role of neuraminidase in lethal synergism between influenza virus and Streptococcus pneumoniae, The Journal of infectious diseases, 187 (2003) 1000–1009. 10.1086/368163. [DOI] [PubMed] [Google Scholar]
- 106.Robinson KM, McHugh KJ, Mandalapu S, Clay ME, Lee B, Scheller EV, Enelow RI, Chan YR, Kolls JK, Alcorn JF, Influenza A virus exacerbates Staphylococcus aureus pneumonia in mice by attenuating antimicrobial peptide production, The Journal of infectious diseases, 209 (2013) 865–875. 10.1093/infdis/jit527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Sun K, Metzger DW, Influenza infection suppresses NADPH oxidase–dependent phagocytic bacterial clearance and enhances susceptibility to secondary methicillin-resistant Staphylococcus aureus infection, The Journal of Immunology, 192 (2014) 3301–3307. 10.4049/jimmunol.1303049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Segel GB, Halterman MW, Lichtman MA, The paradox of the neutrophil’s role in tissue injury, Journal of leukocyte biology, 89 (2011) 359–372. 10.1189/jlb.0910538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Kostrzewska K, Massalski W, Narbutowicz B, Zielinski W, Pulmonary staphylococcal complications in patients during the influenza epidemic in 1971–1972, Materia medica Polona. Polish journal of medicine and pharmacy, 6 (1974) 207. [PubMed] [Google Scholar]
- 110.Chiu B-C, Stolberg VR, Freeman CM, Chensue SW, Mononuclear phagocyte-derived interleukin-10 suppresses the innate pulmonary granuloma cytokine response in aged mice, The American journal of pathology, 171 (2007) 829–837. 10.2353/ajpath.2007.061122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Opal SM, DePalo VA, Anti-inflammatory cytokines, Chest, 117 (2000) 1162–1172. 10.1378/chest.117.4.1162. [DOI] [PubMed] [Google Scholar]
- 112.Skowronski DM, Hottes TS, McElhaney JE, Janjua NZ, Sabaiduc S, Chan T, Gentleman B, Purych D, Gardy J, Patrick DM, Immuno-epidemiologic correlates of pandemic H1N1 surveillance observations: higher antibody and lower cell-mediated immune responses with advanced age, Journal of Infectious Diseases, 203 (2011) 158–167. 10.1093/infdis/jiq039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.McElhaney JE, Xie D, Hager WD, Barry MB, Wang Y, Kleppinger A, Ewen C, Kane KP, Bleackley RC, T cell responses are better correlates of vaccine protection in the elderly, The Journal of Immunology, 176 (2006) 6333–6339. 10.4049/jimmunol.176.10.6333. [DOI] [PubMed] [Google Scholar]
- 114.Hinojosa E, Boyd AR, Orihuela CJ, Age-associated inflammation and toll-like receptor dysfunction prime the lungs for pneumococcal pneumonia, The Journal of infectious diseases, 200 (2009) 546–554. 10.1086/600870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Bartley JM, Pan SJ, Keilich SR, Hopkins JW, Al-Naggar IM, Kuchel GA, Haynes L, Aging augments the impact of influenza respiratory tract infection on mobility impairments, muscle-localized inflammation, and muscle atrophy, Aging (Albany NY), 8 (2016) 620 10.18632/aging.100882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Lefebvre JS, Lorenzo EC, Masters AR, Hopkins JW, Eaton SM, Smiley ST, Haynes L, Vaccine efficacy and T helper cell differentiation change with aging, Oncotarget, 7 (2016) 33581 10.18632/oncotarget.9254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Bartley JM, Zhou X, Kuchel GA, Weinstock GM, Haynes L, Impact of age, caloric restriction, and influenza infection on mouse gut microbiome: an exploratory study of the role of age-related microbiome changes on influenza responses, Frontiers in immunology, 8 (2017) 1164 10.3389/fimmu.2017.01164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Rothberg MB, Haessler SD, Complications of seasonal and pandemic influenza, Critical care medicine, 38 (2010) e91–e97. 10.1097/CCM.0b013e3181c92eeb. [DOI] [PubMed] [Google Scholar]
- 119.Rothberg MB, Haessler SD, Brown RB, Complications of viral influenza, The American journal of medicine, 121 (2008) 258–264. 10.1016/j.amjmed.2007.10.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Sellers SA, Hagan RS, Hayden FG, Fischer WA, The hidden burden of influenza: A review of the extra‐pulmonary complications of influenza infection, Influenza and other respiratory viruses, 11 (2017) 372–393. 10.1111/irv.12470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Salihefendic N, Zildzic M, Ahmetagic S, Acute respiratory distress syndrome (ARDS) from endemic influenza A/H1N1: prehospital management, Medical Archives, 69 (2015) 62 10.5455/medarh.2015.69.62-63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Morens DM, Fauci AS, The 1918 influenza pandemic: insights for the 21st century, The Journal of infectious diseases, 195 (2007) 1018–1028. 10.1086/511989. [DOI] [PubMed] [Google Scholar]
- 123.Starr I, Influenza in 1918: recollections of the epidemic in Philadelphia, Annals of Internal Medicine, 85 (1976) 516–518. 10.7326/0003-4819-85-4-516. [DOI] [PubMed] [Google Scholar]
- 124.Fedson DS, Confronting the next influenza pandemic with anti‐inflammatory and immunomodulatory agents: why they are needed and how they might work, Influenza and other respiratory viruses, 3 (2009) 129–142. 10.1111/j.1750-2659.2009.00090.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Herridge MS, Cheung AM, Tansey CM, Matte-Martyn A, Diaz-Granados N, Al-Saidi F, Cooper AB, Guest CB, Mazer CD, Mehta S, One-year outcomes in survivors of the acute respiratory distress syndrome, New England Journal of Medicine, 348 (2003) 683–693. 10.1056/NEJMoa022450. [DOI] [PubMed] [Google Scholar]
- 126.Herridge MS, Tansey CM, Matté A, Tomlinson G, Diaz-Granados N, Cooper A, Guest CB, Mazer CD, Mehta S, Stewart TE, Functional disability 5 years after acute respiratory distress syndrome, New England Journal of Medicine, 364 (2011) 1293–1304. 10.1056/NEJMoa1011802. [DOI] [PubMed] [Google Scholar]
- 127.Rewar S, Mirdha D, Rewar P, Treatment and prevention of pandemic H1N1 influenza, Annals of global health, 81 (2015) 645–653. 10.1016/j.aogh.2015.08.014. [DOI] [PubMed] [Google Scholar]
- 128.Peek GJ, Mugford M, Tiruvoipati R, Wilson A, Allen E, Thalanany MM, Hibbert CL, Truesdale A, Clemens F, Cooper N, Efficacy and economic assessment of conventional ventilatory support versus extracorporeal membrane oxygenation for severe adult respiratory failure (CESAR): a multicentre randomised controlled trial, The Lancet, 374 (2009) 1351–1363. 10.1016/S0140-6736(09)61069-2. [DOI] [PubMed] [Google Scholar]
- 129.Brown SM, Pittman J, Miller III RR, Horton KD, Markewitz B, Hirshberg E, Jones J, Grissom CK, Right and left heart failure in severe H1N1 influenza A infection, European Respiratory Journal, 37 (2011) 112–118. 10.1183/09031936.00008210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Kodama M, Influenza myocarditis, Circulation Journal, 74 (2010) 2060–2061. 10.1253/circj.CJ-10-0833. [DOI] [PubMed] [Google Scholar]
- 131.Karjalainen J, Nieminen MS, Heikkilä J, Influenza Al myocarditis in conscripts, Acta Medica Scandinavica, 207 (1980) 27–30. 10.1111/j.0954-6820.1980.tb09670.x. [DOI] [PubMed] [Google Scholar]
- 132.Paddock CD, Liu L, Denison AM, Bartlett JH, Holman RC, DeLeon-Carnes M, Emery SL, Drew CP, Shieh W-J, Uyeki TM, Myocardial injury and bacterial pneumonia contribute to the pathogenesis of fatal influenza B virus infection, Journal of Infectious Diseases, 205 (2012) 895–905. 10.1093/infdis/jir861. [DOI] [PubMed] [Google Scholar]
- 133.Oseasohn R, Adelson L, Kaji M, Clinicopathologic study of thirty-three fatal cases of Asian influenza, New England Journal of Medicine, 260 (1959) 509–518. 10.1056/NEJM195903122601101. [DOI] [PubMed] [Google Scholar]
- 134.Ukimura A, Izumi T, Matsumori A, A national survey on myocarditis associated with the 2009 influenza A (H1N1) pandemic in Japan, Circulation Journal, (2010) 1008040838–1008040838. 10.1253/circj.CJ-10-0452. [DOI] [PubMed] [Google Scholar]
- 135.Ukimura A, Ooi Y, Kanzaki Y, Inomata T, Izumi T, A national survey on myocarditis associated with influenza H1N1pdm2009 in the pandemic and postpandemic season in Japan, Journal of Infection and Chemotherapy, 19 (2013) 426–431. 10.1007/s10156-012-0499-z. [DOI] [PubMed] [Google Scholar]
- 136.Corrales-Medina VF, Madjid M, Musher DM, Role of acute infection in triggering acute coronary syndromes, The Lancet infectious diseases, 10 (2010) 83–92. 10.1016/S1473-3099(09)70331-7. [DOI] [PubMed] [Google Scholar]
- 137.Madjid M, Aboshady I, Awan I, Litovsky S, Casscells SW, Influenza and cardiovascular disease: is there a causal relationship?, Texas Heart Institute Journal, 31 (2004) 4 https://www.ncbi.nlm.nih.gov/pmc/articles/PMC387426/ (last accessed July 21, 2019). [PMC free article] [PubMed] [Google Scholar]
- 138.Tse H, To KK, Wen X, Chen H, Chan K-H, Tsoi H-W, Li IW, Yuen K-Y, Clinical and virological factors associated with viremia in pandemic influenza A/H1N1/2009 virus infection, PloS one, 6 (2011) e22534 10.1371/journal.pone.0022534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Likos AM, Kelvin DJ, Cameron CM, Rowe T, Kuehnert MJ, Norris PJ, National Heart L, Blood Institute Retrovirus Epidemiology Donor Study‐II, Influenza viremia and the potential for blood‐borne transmission, Transfusion, 47 (2007) 1080–1088. 10.1111/j.1537-2995.2007.01264.x. [DOI] [PubMed] [Google Scholar]
- 140.BAINTON D, JONES GR, HOLE D, Influenza and ischaemic heart disease-a possible trigger for acute myocardial infarction?, International Journal of Epidemiology, 7 (1978) 231–239. 10.1093/ije/7.3.231. [DOI] [PubMed] [Google Scholar]
- 141.Naghavi M, Wyde P, Litovsky S, Madjid M, Akhtar A, Naguib S, Siadaty MS, Sanati S, Casscells W, Influenza infection exerts prominent inflammatory and thrombotic effects on the atherosclerotic plaques of apolipoprotein E–deficient mice, Circulation, 107 (2003) 762–768.. 10.1161/01.cir.0000048190.68071.2b. [DOI] [PubMed] [Google Scholar]
- 142.Smeeth L, Thomas SL, Hall AJ, Hubbard R, Farrington P, Vallance P, Risk of myocardial infarction and stroke after acute infection or vaccination, New England Journal of Medicine, 351 (2004) 2611–2618. 10.1056/NEJMoa041747 [DOI] [PubMed] [Google Scholar]
- 143.Grau AJ, Fischer B, Barth C, Ling P, Lichy C, Buggle F, Influenza vaccination is associated with a reduced risk of stroke, Stroke, 36 (2005) 1501–1506. 10.1161/01.STR.0000170674.45136.80. [DOI] [PubMed] [Google Scholar]
- 144.Madjid M, Curkendall S, Blumentals WA, The influence of oseltamivir treatment on the risk of stroke after influenza infection, Cardiology, 113 (2009) 98–107. 10.1159/000172796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Udell JA, Zawi R, Bhatt DL, Keshtkar-Jahromi M, Gaughran F, Phrommintikul A, Ciszewski A, Vakili H, Hoffman EB, Farkouh ME, Association between influenza vaccination and cardiovascular outcomes in high-risk patients: a meta-analysis, Jama, 310 (2013) 1711–1720. 10.1001/jama.2013.279206. [DOI] [PubMed] [Google Scholar]
- 146.Estabragh ZR, Mamas MA, The cardiovascular manifestations of influenza: a systematic review, International journal of cardiology, 167 (2013) 2397–2403. 10.1016/j.ijcard.2013.01.274. [DOI] [PubMed] [Google Scholar]
- 147.Udell JA, Farkouh ME, Solomon SD, Vardeny O, Does influenza vaccination influence cardiovascular complications?, Taylor & Francis, 2015. 10.1586/14779072.2015.1044439. [DOI] [PubMed] [Google Scholar]
- 148.Barreiro E, Sznajder JI, Nader GA, Budinger GS, Muscle dysfunction in patients with lung diseases. A growing epidemic, American journal of respiratory and critical care medicine, 191 (2015) 616–619. 10.1164/rccm.201412-2189OE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Doucet M, Russell AP, Léger B, Debigaré R, Joanisse DR, Caron M-A, LeBlanc P, Maltais F, Muscle atrophy and hypertrophy signaling in patients with chronic obstructive pulmonary disease, American journal of respiratory and critical care medicine, 176 (2007) 261–269. 10.1164/rccm.200605-704OC [DOI] [PubMed] [Google Scholar]
- 150.Mador MJ, Bozkanat E, Skeletal muscle dysfunction in chronic obstructive pulmonary disease, Respiratory research, 2 (2001) 216 10.1186/rr60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Hooijman PE, Beishuizen A, Witt CC, de Waard MC, Girbes AR, Spoelstra-de Man AM, Niessen HW, Manders E, van Hees HW, van den Brom CE, Diaphragm muscle fiber weakness and ubiquitin–proteasome activation in critically ill patients, American journal of respiratory and critical care medicine, 191 (2015) 1126–1138. 10.1164/rccm.201412-2214OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Puthucheary ZA, Rawal J, McPhail M, Connolly B, Ratnayake G, Chan P, Hopkinson NS, Phadke R, Dew T, Sidhu PS, Acute skeletal muscle wasting in critical illness, Jama, 310 (2013) 1591–1600. 10.1001/jama.2013.278481. [DOI] [PubMed] [Google Scholar]
- 153.Kessler HA, Trenholme GM, Vogelzang NJ, Patterson R, Semel JD, Harris AA, Levin S, Elevated creatine phosphokinase levels associated with influenza A/Texas/1/77 infection, Scandinavian journal of infectious diseases, 15 (1983) 7–10. 10.3109/inf.1983.15.issue-1.02. [DOI] [PubMed] [Google Scholar]
- 154.Farrell MK, Partin JC, Bove KE, Jacobs R, Hilton PK, Epidemic influenza myopathy in Cincinnati in 1977, The Journal of pediatrics, 96 (1980) 545–551. 10.1016/S0022-3476(80)80864-X. [DOI] [PubMed] [Google Scholar]
- 155.Kessler HA, Trenholme GM, Harris AA, Levin S, Acute myopathy associated with influenza A/Texas/1/77 infection: isolation of virus from a muscle biopsy specimen, JAMA, 243 (1980) 461–462. 10.1001/jama.1980.03300310049022. [DOI] [PubMed] [Google Scholar]
- 156.Falsey AR, Hennessey PA, Formica MA, Cox C, Walsh EE, Respiratory syncytial virus infection in elderly and high-risk adults, New England Journal of Medicine, 352 (2005) 1749–1759. 10.1056/NEJMoa043951. [DOI] [PubMed] [Google Scholar]
- 157.Gozalo PL, Pop‐Vicas A, Feng Z, Gravenstein S, Mor V, Effect of influenza on functional decline, Journal of the American Geriatrics Society, 60 (2012) 1260–1267. 10.1111/j.1532-5415.2012.04048.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Ferrucci L, Guralnik JM, Pahor M, Corti MC, Havlik RJ, Hospital diagnoses, Medicare charges, and nursing home admissions in the year when older persons become severely disabled, Jama, 277 (1997) 728–734. 10.1001/jama.1997.03540330050034. [DOI] [PubMed] [Google Scholar]
- 159.Kuiken T, Taubenberger JK, Pathology of human influenza revisited, Vaccine, 26 (2008) D59–D66. 10.1016/j.vaccine.2008.07.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Radigan KA, Nicholson TT, Welch LC, Chi M, Amarelle L, Angulo M, Shigemura M, Shigemura A, Runyan CE, Morales-Nebreda L, Influenza A Virus Infection Induces Muscle Wasting via IL-6 Regulation of the E3 Ubiquitin Ligase Atrogin-1, The Journal of Immunology, 202 (2019) 484–493. 10.4049/jimmunol.1701433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Singh U, Scheld WM, Infectious etiologies of rhabdomyolysis: three case reports and review, Clinical Infectious Diseases, 22 (1996) 642–649. 10.1093/clinids/22.4.642. [DOI] [PubMed] [Google Scholar]
- 162.Annerstedt M, Herlitz H, Mölne J, Oldfors A, Westberg G, Rhabdomyolysis and acute renal failure associated with influenza virus type A, Scandinavian journal of urology and nephrology, 33 (1999) 260–264. 10.1080/003655999750015880. [DOI] [PubMed] [Google Scholar]
- 163.Unverdi S, Akay H, Ceri M, Inal S, Altay M, Demiroz AP, Duranay M, Acute kidney injury due to rhabdomyolysis in H1N1 influenza infection, Renal failure, 33 (2011) 450–451. 10.3109/0886022X.2011.565137. [DOI] [PubMed] [Google Scholar]
- 164.Naderi ASA, Palmer BF, Rhabdomyolysis and acute renal failure associated with influenza virus type B infection, The American journal of the medical sciences, 332 (2006) 88–89. 10.1097/00000441-200608000-00007. [DOI] [PubMed] [Google Scholar]
- 165.Cunningham E, Kohli R, Venuto RC, Influenza-associated myoglobinuric renal failure, JAMA, 242 (1979) 2428–2429. 10.1001/jama.1979.03300220040021. [DOI] [PubMed] [Google Scholar]
- 166.Wakabayashi Y, Nakano T, Kikuno T, Ohwada T, Kikawada R, Massive rhabdomyolysis associated with influenza A infection, Internal Medicine, 33 (1994) 450–453. 10.2169/internalmedicine.33.450. [DOI] [PubMed] [Google Scholar]
- 167.Berry L, Braude S, Influenza A infection with rhabdomyolysis and acute renal failure--a potentially fatal complication, Postgraduate medical journal, 67 (1991) 389–390. 10.1136/pgmj.67.786.389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Foulkes W, Rees J, Sewry C, Influenza A and rhabdomyolysis, Journal of Infection, 21 (1990) 303–304. 10.1016/0163-4453(90)94077-D. [DOI] [PubMed] [Google Scholar]
- 169.Yoshino M, Suzuki S, Adachi K, FUKAYAMA M, INAMATSU T, High incidence of acute myositis with type A influenza virus infection in the elderly, Internal medicine, 39 (2000) 431–432. 10.2169/internalmedicine.39.431. [DOI] [PubMed] [Google Scholar]
- 170.Ayala E, Kagawa FT, Wehner JH, Tam J, Upadhyay D, Rhabdomyolysis associated with 2009 influenza A (H1N1), Jama, 302 (2009) 1863–1864. 10.1001/jama.2009.1582. [DOI] [PubMed] [Google Scholar]
- 171.Oba K, Nishihara A, Okamura K, Ajiro Y, Yamaguchi Y, Okazaki K, Sato S, Suzuki T, Nakano H, Metori S, Two cases of acute myositis associated with influenza A virus infection in the elderly, Journal of Nippon Medical School, 67 (2000) 126–129. 10.1272/jnms.67.126. [DOI] [PubMed] [Google Scholar]
- 172.Parikh M, Dolson G, Ramanathan V, Sangsiraprapha W, Novel H1N1‐associated rhabdomyolysis leading to acute renal failure, Clinical Microbiology and Infection, 16 (2010) 330–332. 10.1111/j.1469-0691.2010.03185.x. [DOI] [PubMed] [Google Scholar]
- 173.Kaida K.-i., Kamakura K, Masaki T, Okano M, Nagata N, Inoue K, Painful small-fibre multifocal mononeuropathy and local myositis following influenza B infection, Journal of the neurological sciences, 151 (1997) 103–106. 10.1016/S0022-510X(97)00085-3. [DOI] [PubMed] [Google Scholar]
- 174.Napolitano L, Park P, Sihler K, Papadimos T, Chenoweth C, Cinti S, Zalewski C, Sharangpani R, Somsel P, Wells E, Intensive-care patients with severe novel influenza A (H1N1) virus infection-Michigan, June 2009, Morbidity and Mortality Weekly Report, 58 (2009) 749–752. https://www.cdc.gov/mmwr/preview/mmwrhtml/mm58d0710a1.htm (last accessed on July 21, 2019). [PubMed] [Google Scholar]
- 175.Clegg A, Young J, Iliffe S, Rikkert MO, Rockwood K, Frailty in elderly people, The lancet, 381 (2013) 752–762. 10.1016/S0140-6736(12)62167-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Dukas L, Bischoff HA, Lindpaintner LS, Schacht E, Birkner‐binder D, Damm TN, Thalmann B, Stähelin HB, Alfacalcidol reduces the number of fallers in a community‐dwelling elderly population with a minimum calcium intake of more than 500 mg daily, Journal of the American Geriatrics Society, 52 (2004) 230–236. 10.1111/j.15325415.2004.52060.x. [DOI] [PubMed] [Google Scholar]
- 177.Morley JE, Haren MT, Rolland Y, Kim MJ, Frailty, Medical Clinics, 90 (2006) 837–847. 10.1016/j.mcna.2006.05.019. [DOI] [PubMed] [Google Scholar]
- 178.Margolis KL, Poland GA, Nichol KL, MacPherson DS, Meyer JD, Korn JE, Lofgren RP, Frequency of adverse reactions after influenza vaccination, The American journal of medicine, 88 (1990) 27–30. 10.1016/0002-9343(90)90123-U. [DOI] [PubMed] [Google Scholar]
- 179.Studahl M, Influenza virus and CNS manifestations, Journal of Clinical Virology, 28 (2003) 225–232. 10.1016/S1386-6532(03)00119-7. [DOI] [PubMed] [Google Scholar]
- 180.Glaser CA, Winter K, DuBray K, Harriman K, Uyeki TM, Sejvar J, Gilliam S, Louie JK, A population-based study of neurologic manifestations of severe influenza A (H1N1) pdm09 in California, Clinical infectious diseases, 55 (2012) 514–520. 10.1093/cid/cis454. [DOI] [PubMed] [Google Scholar]
- 181.Mizuguchi M, Influenza encephalopathy and related neuropsychiatric syndromes, Influenza and other respiratory viruses, 7 (2013) 67–71. 10.1111/irv.12177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Gonzalez BE, Brust DG, Novel influenza A (H1N1) presenting as an acute febrile encephalopathy in a mother and daughter, Clinical infectious diseases, 49 (2009) 1966–1967. 10.1086/649014. [DOI] [PubMed] [Google Scholar]
- 183.Steininger C, Popow-Kraupp T, Laferl H, Seiser A, Gödl I, Djamshidian S, Stöckl EP, Acute encephalopathy associated with influenza A virus infection, Clinical infectious diseases, 36 (2003) 567–574. 10.1086/367623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Akins PT, Belko J, Uyeki TM, Axelrod Y, Lee KK, Silverthorn J, H1N1 encephalitis with malignant edema and review of neurologic complications from influenza, Neurocritical care, 13 (2010) 396–406. 10.1007/s12028-010-9436-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Ichiyama T, Morishima T, Isumi H, Matsufuji H, Matsubara T, Furukawa S, Analysis of cytokine levels and NF-κB activation in peripheral blood mononuclear cells in influenza virus-associated encephalopathy, Cytokine, 27 (2004) 31–37. 10.1016/j.cyto.2004.03.012. [DOI] [PubMed] [Google Scholar]
- 186.Kawada J.-i., Kimura H, Ito Y, Hara S, Iriyama M, Yoshikawa T, Morishima T, Systemic cytokine responses in patients with influenza-associated encephalopathy, The Journal of infectious diseases, 188 (2003) 690–698. 10.1086/377101. [DOI] [PubMed] [Google Scholar]
- 187.Hasegawa S, Matsushige T, Inoue H, Takahara M, Kajimoto M, Momonaka H, Ishida C, Tanaka S, Morishima T, Ichiyama T, Serum soluble CD163 levels in patients with influenza-associated encephalopathy, Brain and Development, 35 (2013) 626–629. 10.1016/j.braindev.2012.10.005. [DOI] [PubMed] [Google Scholar]
- 188.Jurgens HA, Johnson RW, Environmental enrichment attenuates hippocampal neuroinflammation and improves cognitive function during influenza infection, Brain, behavior, and immunity, 26 (2012) 1006–1016. 10.1016/j.bbi.2012.05.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Deriu E, Boxx GM, He X, Pan C, Benavidez SD, Cen L, Rozengurt N, Shi W, Cheng G, Influenza virus affects intestinal microbiota and secondary salmonella infection in the gut through type I interferons, PLoS pathogens, 12 (2016) e1005572 10.1371/journal.ppat.1005572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Baden LR, Drazen JM, Kritek PA, Curfman GD, Morrissey S, Campion EW, H1N1 influenza A disease—information for health professionals, Mass Medical Soc, 2009. 10.1056/NEJMe0903992. [DOI] [PubMed] [Google Scholar]
- 191.Shinde V, Bridges CB, Uyeki TM, Shu B, Balish A, Xu X, Lindstrom S, Gubareva LV, Deyde V, Garten RJ, Triple-reassortant swine influenza A (H1) in humans in the United States, 2005–2009, New England Journal of Medicine, 360 (2009) 2616–2625. 10.1056/NEJMoa0903812. [DOI] [PubMed] [Google Scholar]
- 192.Dilantika C, Sedyaningsih ER, Kasper MR, Agtini M, Listiyaningsih E, Uyeki TM, Burgess TH, Blair PJ, Putnam SD, Influenza virus infection among pediatric patients reporting diarrhea and influenza-like illness, BMC infectious diseases, 10 (2010) 3 10.1186/1471-2334-10-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Wang J, Li F, Wei H, Lian Z-X, Sun R, Tian Z, Respiratory influenza virus infection induces intestinal immune injury via microbiota-mediated Th17 cell–dependent inflammation, Journal of Experimental Medicine, 211 (2014) 2397–2410. 10.1084/jem.20140625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.McDermott MR, Bienenstock J, Evidence for a common mucosal immunologic system: I. Migration of B immunoblasts into intestinal, respiratory, and genital tissues, The Journal of Immunology, 122 (1979) 1892–1898. https://www.jimmunol.org/content/122/5/1892.long (last accessed on July 21, 2019). [PubMed] [Google Scholar]
- 195.Wootton SH, Scheifele DW, Mak A, Petric M, Skowronski DM, Detection of human influenza virus in the stool of children, The Pediatric infectious disease journal, 25 (2006) 1194–1195. 10.1097/01.inf.0000245097.95543.11 [DOI] [PubMed] [Google Scholar]
- 196.Tamura D, Fujino M, Ozawa M, Iwatsuki-Horimoto K, Goto H, Sakai-Tagawa Y, Horimoto T, Nirasawa M, Kawaoka Y, Significance of seasonal influenza viruses in the stool of pediatric patients, The Pediatric infectious disease journal, 29 (2010) 578–579. 10.1097/INF.0b013e3181dab225. [DOI] [PubMed] [Google Scholar]
- 197.Wootton SH, Aguilera EA, Wanger A, Jewell A, Patel K, Murphy JR, Piedra PA, Detection of NH1N1 influenza virus in nonrespiratory sites among children, The Pediatric infectious disease journal, 33 (2014) 95–96. 10.1097/INF.0b013e3182a09de7. [DOI] [PubMed] [Google Scholar]
- 198.Chan MC, Lee N, Chan PK, To K, Wong RY, Ho W-S, Ngai KL, Sung JJ, Seasonal influenza A virus in feces of hospitalized adults, Emerging infectious diseases, 17 (2011) 2038 10.3201/eid1711.110205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Yoo SJ, Moon SJ, Kuak E-Y, Yoo HM, Kim CK, Chey M-J, Shin B-M, Frequent detection of pandemic (H1N1) 2009 virus in stools of hospitalized patients, Journal of clinical microbiology, 48 (2010) 2314–2315. 10.1128/JCM.00325-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.To KK, Chan KH, Li IW, Tsang TY, Tse H, Chan JF, Hung IF, Lai ST, Leung CW, Kwan YW, Viral load in patients infected with pandemic H1N1 2009 influenza A virus, Journal of medical virology, 82 (2010) 1–7. 10.1002/jmv.21664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Suryaprasad A, Morgan OW, Peebles P, Warner A, Kerin TK, Esona MD, Bowen MD, Sessions W, Xu X, Cromeans T, Virus detection and duration of illness among patients with 2009 pandemic influenza A (H1N1) virus infection in Texas, Clinical Infectious Diseases, 52 (2011) S109–S115. 10.1093/cid/ciq014. [DOI] [PubMed] [Google Scholar]
- 202.Chan MC, Lee N, Chan PK, Leung T, Sung JJ, Fecal detection of influenza A virus in patients with concurrent respiratory and gastrointestinal symptoms, Journal of Clinical Virology, 45 (2009) 208–211. 10.1016/j.jcv.2009.06.011. [DOI] [PubMed] [Google Scholar]
- 203.Minodier L, Charrel RN, Ceccaldi P-E, Van Der Werf S, Blanchon T, Hanslik T, Falchi A, Prevalence of gastrointestinal symptoms in patients with influenza, clinical significance, and pathophysiology of human influenza viruses in faecal samples: what do we know?, Virology journal, 12 (2015) 215 10.1186/s12985-015-0448-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.de Jong MD, Cam BV, Qui PT, Hien VM, Thanh TT, Hue NB, Beld M, Phuong LT, Khanh TH, Chau NVV, Fatal avian influenza A (H5N1) in a child presenting with diarrhea followed by coma, New England Journal of Medicine, 352 (2005) 686–691. 10.1056/NEJMoa044307. [DOI] [PubMed] [Google Scholar]
- 205.Wiwanitkit V, Diarrhoea as a presentation of bird flu infection: a summary on its correlation to outcome in Thai cases, Gut, 54 (2005) 1506–1506. 10.1136/gut.2005.072488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Papic N, Pangercic A, Vargovic M, Barsic B, Vince A, Kuzman I, Liver involvement during influenza infection: perspective on the 2009 influenza pandemic, Influenza and other respiratory viruses, 6 (2012) e2–e5. 10.1111/j.1750-2659.2011.00287.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Polakos NK, Cornejo JC, Murray DA, Wright KO, Treanor JJ, Crispe IN, Topham DJ, Pierce RH, Kupffer cell-dependent hepatitis occurs during influenza infection, The American journal of pathology, 168 (2006) 1169–1178. 10.2353/ajpath.2006.050875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Bal A, Suri V, Mishra B, Bhalla A, Agarwal R, Abrol A, Ratho RK, Joshi K, Pathology and virology findings in cases of fatal influenza A H1N1 virus infection in 2009–2010, Histopathology, 60 (2012) 326–335. 10.1111/j.1365-2559.2011.04081.x. [DOI] [PubMed] [Google Scholar]
- 209.Yuen K, Chan P, Peiris M, Tsang D, Que T, Shortridge K, Cheung P, To W, Ho E, Sung R, Clinical features and rapid viral diagnosis of human disease associated with avian influenza A H5N1 virus, The Lancet, 351 (1998) 467–471. 10.1016/s0140-6736(98)01182-9 [DOI] [PubMed] [Google Scholar]
- 210.Gao H-N, Lu H-Z, Cao B, Du B, Shang H, Gan J-H, Lu S-H, Yang Y-D, Fang Q, Shen Y-Z, Clinical findings in 111 cases of influenza A (H7N9) virus infection, New England Journal of Medicine, 368 (2013) 2277–2285. 10.1056/NEJMoa1305584. [DOI] [PubMed] [Google Scholar]
- 211.Bagshaw SM, Sood MM, Long J, Fowler RA, Adhikari NK, Acute kidney injury among critically ill patients with pandemic H1N1 influenza A in Canada: cohort study, BMC nephrology, 14 (2013) 123 10.1186/1471-2369-14-123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Jung JY, Park BH, Hong S-B, Koh Y, Suh GY, Jeon K, Koh SO, Kim JY, Cho JH, Choi HS, Acute kidney injury in critically ill patients with pandemic influenza A pneumonia 2009 in Korea: a multicenter study, Journal of critical care, 26 (2011) 577–585. 10.1016/j.jcrc.2011.02.012. [DOI] [PubMed] [Google Scholar]
- 213.Pettilä V, Webb SA, Bailey M, Howe B, Seppelt IM, Bellomo R, Acute kidney injury in patients with influenza A (H1N1) 2009, Intensive care medicine, 37 (2011) 763–767. 10.1007/s00134-011-2166-8 [DOI] [PubMed] [Google Scholar]
- 214.Nin N, Lorente J, Soto L, Ríos F, Hurtado J, Arancibia F, Ugarte S, Echevarría E, Cardinal P, Saldarini F, Acute kidney injury in critically ill patients with 2009 influenza A (H1N1) viral pneumonia: an observational study, Intensive care medicine, 37 (2011) 768–774. 10.1007/s00134-011-2167-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Martin-Loeches I, Papiol E, Rodríguez A, Diaz E, Zaragoza R, Granada RM, Socias L, Bonastre J, Valverdú M, Pozo JC, Acute kidney injury in critical ill patients affected by influenza A (H1N1) virus infection, Critical Care, 15 (2011) R66 10.1186/cc10046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Carmona F, Carlotti AP, Ramalho LN, Costa RS, Ramalho FS, Evidence of renal infection in fatal cases of 2009 pandemic influenza A (H1N1), American journal of clinical pathology, 136 (2011) 416–423. 10.1309/AJCP1Y6LLHWSKYHW. [DOI] [PubMed] [Google Scholar]
- 217.Chen C-I, Kao P-F, Wu M-Y, Fang Y-A, Miser JS, Liu J-C, Sung L-C, Influenza vaccination is associated with lower risk of acute coronary syndrome in elderly patients with chronic kidney disease, Medicine, 95 (2016). 10.1097/MD.0000000000002588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Mauad T, Hajjar LA, Callegari GD, da Silva LF, Schout D, Galas FR, Alves VA, Malheiros DM, Auler JO Jr, Ferreira AF, Lung pathology in fatal novel human influenza A (H1N1) infection, American journal of respiratory and critical care medicine, 181 (2010) 72–79. 10.1164/rccm.200909-1420OC. [DOI] [PubMed] [Google Scholar]