Abstract
This study sought to compare lead (Pb) concentrations in toenails and blood and to investigate the association of each biomarker with children’s cognitive function. Toenails and whole blood samples were collected from 224 twelve-year-old children, and their full-scale intelligence quotient (FSIQ) was assessed using the Wechsler Intelligence Scale for Children–4th edition. Inductively coupled plasma-mass spectrometry was used to determine blood (BPb) and toenail (TPb) Pb concentrations. Log BPb and Log TPb were significantly correlated (r2 = 0.49, p < 0.001). In unadjusted analyses, both log-transformed BPb and TPb were significantly associated with decreased FSIQ, but BPb accounted for approximately quadruple the FSIQ scores’ variability than log-transformed TPb (model R2 = 0.12 and R2 = 0.03, respectively). After adjusting for neighborhood deprivation, caregiver intelligence (assessed with the Wechsler Abbreviated Scale of Intelligence–2nd edition), and child BMI, BPb remained significantly associated with decreased FSIQ, while TPb did not (p = 0.16). These results suggest that while concentrations of Pb in blood and toenails are correlated, TPb does not predict cognitive outcomes at these exposure levels. With caution and in conjunction with BPb, TPb may be used as a population-based biomarker of Pb exposure.
Keywords: biomarker, lead, toenail, children’s exposure
Introduction
Exposure to Pb, even at low-levels, is toxic to humans and has been consistently associated with worsened cognitive function in children (Baghurst et al., 1992; Bellinger et al., 1991; Bellinger et al., 1987; Bellinger et al., 1992; Dietrich et al., 1993; Dietrich et al., 1991; Reuben et al., 2017; Wasserman et al., 1997). In the early 1990s, both the Centers for Disease Control and Prevention (CDC) and World Health Organization (WHO) set their levels of concern at a blood Pb (BPb) concentration of 10 μg/dL (CDC, 2012; WHO, 1995), but more recent evidence suggests that blood concentrations below this level are associated with cognitive deficits (Adler, 2005; Barbosa et al., 2005; Koller et al., 2004; Lanphear et al., 2005). This, along with the continuing decline in pediatric BPb levels, motivated both organizations to lower their definition of an elevated BPb level to ≥5 μg/dL (CDC, 2012; WHO, 2010). Indeed, in an international pooled analysis the largest fraction of IQ deficits were observed below 10 μg/dL (Adler, 2005) and suggested that there is no safe threshold for Pb exposure (Barbosa et al., 2005; Koller et al., 2004).
Recent widespread Pb exposure due to corrosion of the drinking water distribution system in Flint, MI, USA has highlighted the need for tools for surveillance of children’s Pb exposure to aid in public health interventions (Hanna-Attisha et al., 2016). BPb is considered the gold-standard for assessing Pb exposure and is the most commonly used biomarker for population studies and as a clinical diagnostic test (Barbosa et al., 2005). In adults, the half-life of BPb is ~35 days (Rabinowitz et al., 1976; WHO, 1995), but it can persist in bone for decades (Lidsky and Schneider, 2003). Children undergo bone remodeling as they grow, resulting in the release of Pb into their blood (Gwiazda et al., 2005). Pb is repartitioned from bone to the blood more rapidly in children than adults. Additionally, it has been reported that the half-life of Pb in children’s blood may be much longer than that in adults, at ~10 months (Manton et al., 2000). Thus, in children, BPb is a widely accepted biomarker for assessing total body burden of Pb.
Toenails have also been evaluated as a biological matrix to determine Pb exposure given that nails are formed in the nailbed, which has a blood supply. Pb concentrations in the nailbed blood supply are likely a major determinant of toenail Pb (TPb) concentrations and, therefore, may serve as a potential biomarker of Pb exposure (Rabinowitz et al., 1976). While there are relatively few studies of toenail growth rates, one small (n = 22) study found the average adult great toenail (whole nail) can reflect up to 10 months of exposure to some trace-elements (Yaemsiri et al., 2010). Another study indicates that collecting clippings from all 10 toenails represents an integrated period of exposure over 3–12 months (Longnecker et al., 1993). Toenails can be self-collected and are non-invasive compared to BPb, which requires venipuncture (or in some situations capillary puncture) to be performed by a healthcare professional.
One previous study (Gulson, 1996), compared blood, urine, and toenail Pb in an adult man over a period of six months. The author concluded that toenails, compared to blood and urine, contained too much variation to serve as a reliable biomarker, and concluded that nails are unfit Pb biomarkers for non-exposed populations (Gulson, 1996). However, This study was limited in sample size (n=1) and its use of an adult. Due to the differing biological pathway of Pb in children (Gwiazda et al., 2005) and their susceptibility to worsened cognitive function as a result of Pb exposure (Baghurst et al., 1992; Bellinger et al., 1991; Bellinger et al., 1987; Bellinger et al., 1992; Dietrich et al., 1993; Dietrich et al., 1991; Wasserman et al., 1997), age is potentially a pertinent factor in evaluating toenails’ effectiveness as a Pb biomarker. Although a comprehensive review (Sukumar, 2006) of nails as biomarkers found that age is not a significant factor in nail element levels, broadly, only one study (Hayashi et al., 1993) in the review investigated Pb specifically. This study found Pb levels varied considerably between individuals, sometimes due to age, sex, and season of collection (Hayashi et al., 1993). Despite concern over variability in nail Pb both within and across individuals, there are still relatively few studies concerning the efficacy of nails as a biomarker in children. Multiple studies have evaluated children’s blood (Bellinger et al., 1991; Lanphear et al., 2005; Wasserman et al., 1997) and nail (Hussein Were et al., 2008; Oyoo-Okoth et al., 2010; Wilhelm et al., 1991) samples for Pb separately, but we are only aware of one previous study (Sanders et al., 2014) that has compared the Pb concentrations of blood and toenails in children. However, this study (Sanders et al., 2014) had a limited sample size (n=20), was conducted among exposed populations, and did not use cognitive function as a measure of actual impact. To our knowledge, our study is the only other one to compare blood and toenail Pb in children, and the only to correlate toenail Pb to cognitive function. Therefore, the objectives of this study were to compare TPb and BPb concentrations and examine the association of each biomarker with cognitive scores in a cohort of 12-year-old children.
Methods
Study design and subjects
Study participants were drawn from the Cincinnati Childhood Allergy and Air Pollution Study (CCAAPS), a prospective cohort study of children and their caregivers residing in the Greater Cincinnati, Ohio metropolitan region (LeMasters et al., 2006; Ryan et al., 2005). Children enrolled in the CCAAPS cohort completed study visits at ages 1, 2, 3, 4, 7, and 12 years. The study was approved by the Institutional Review Boards of the University of Cincinnati, Cincinnati Children’s Hospital Medical Center, and the New York State Department of Health. Participants and caregivers provided informed assent and consent, respectively, prior to participating in the age 12-year visit.
Cognitive testing and biospecimen collection and testing
All specimens and data included in the present analyses were collected at the age 12 study visit including direct assessment of child full-scale IQ using the Wechsler Intelligence Scale for Children—4th edition (WISC-IV) and collection of whole blood and toenail samples. The Wechsler Intelligence Scale for Children-IV (WISC-IV) is a standardized, individually administered instrument for assessing the cognitive abilities of children ages 6–16 (Wechsler, 2003). Details regarding the test can be found in Wechsler (2003). Trained study staff administered the assessment in private rooms. A subset of CCAAPS participants completed a portion of the age 12 evaluation by mail and provided toenail samples.
Methods for blood lead analysis by ICP-MS have been described in detail previously (Palmer et al., 2006). Briefly, children’s whole-blood samples were collected in 3 mL, pre-certified BPb collection tubes containing K2EDTA, stored at 5° C, and shipped to the Wadsworth Center (New York State Department of Health, Albany, New York, USA) for analysis within six weeks of collection. Samples were analyzed for Pb using a Thermo Scientific XSeries 2 inductively coupled plasma-mass spectrometer (ICP-MS), with matrix-matched calibration (Palmer et al., 2006). Four levels of internal quality control (IQC) materials covering the range of human BPb levels expected were analyzed in each analytical run. All IQC samples were prepared in-house from whole blood obtained from Pb-dosed animals. Method accuracy was assessed throughout the study by analyzing National Institute of Standards and Technology (NIST) Standard Reference Material (SRM) 955c – Toxic Metals in Caprine Blood (NIST, Gaithersburg, MD). Method performance was monitored through successful participation in six external quality assessment schemes for trace elements that included BPb. The analysis was repeated for any BPb value >5 μg/dL to confirm the measurements. In addition, 2.5% of all blood specimens were randomly selected for re-analysis. The limit of detection (LOD), calculated according to IUPAC recommendations, reported for BPb was 0.072 μg/dL. The Wadsworth Center also provided a limit of quantitation (LOQ), which is calculated according to EPA guidelines (EPA, 1998), and is 0.240 µg/dL.
Children’s toenails were collected with scissors and placed into envelopes. Nails were prepared and analyzed as we have previously described in detail (Unrine et al., 2019). Briefly, the nails were ultrasonicated for 10 minutes in a bath sonicator (Fisher Scientific, FS30) in polypropylene microcentrifuge tubes containing 1 mL trace-metal grade acetone, followed by 10 minutes in 1% (v/v) Citranox® acidic detergent, which is very low in metal content, followed by two repetitions with 18.2 MΩ cm−1 deionized (DI) water. The nails were then placed in an oven at 60°C and dried to a constant mass. Nails that weighed more than 10 mg were digested in 15 mL metal-free polypropylene centrifuge tubes in a MARSXpress microwave digestion system (CEM, Matthews, NC, USA) by heating in 750 µL of ultra-pure HNO3 (Aristar Ultra, BDH Chemicals) to 90°C and holding at that temperature for 10 minutes. This heating step was repeated with the addition of 250 µL of trace-metal grade H2O2 to each sample. Samples were then brought up to 15 mL with DI water and analyzed using ICP-MS (Agilent 7500cx, Santa Clara, CA, USA). Samples with masses below 10 mg were digested in 75 µL of nitric acid in fluoropolymer microcentrifuge tubes at 90 C for 2 days, then brought to 1.5 mL with DI water and transferred into a 15 mL metal-free centrifuge tubes for analysis. We used 10 μg/L Bi as an internal standard, and analyzed method blanks, duplicates, spike recovery samples, and standard reference materials (SRM; CRM #13, Human Hair, National Institute of Environmental Studies, Tsukuba, Japan). Hair is the closest matrix match to nails currently available as an SRM, both being composed largely of keratin.
Statistical analysis
Both TPb and BPb were found to be log-normally distributed and transformed prior to additional analyses. We initially conducted descriptive analyses including calculating the Pearson correlation between TPb and BPb. Next, we examined the relationship between TPb and BPb using a linear regression model. Finally, we estimated the association between both measures of Pb exposure (TPb and BPb) and child full scale IQ in separate linear regression models, adjusting for a priori selected covariates including caregiver full-scale IQ assessed with the Wechsler Abbreviated Scale of Intelligence–2nd edition (WASI-2), body mass index (BMI) at age 12, and a measure of neighborhood-level deprivation based on census-tract level socioeconomic indicators (Brokamp et al., 2019) (https://github.com/cole-brokamp/dep_index). Descriptive statistics are expressed as mean and standard deviation. Data analysis was conducted using SAS version 9.4 (SAS Institute Inc., Cary, NC) and R (3.5.1; R Foundation for Statistical Computing).
Results
Baseline Characteristics
A total of 344 children and caregivers completed the age 12 study visit in-person and an additional 10 participants completed a portion of the age 12 visit by mail. Of these, 252 children provided toenail samples (247 in-person, 5 by mail) for analysis. An overview of the demographic, exposure, and other characteristics of the 252 participants with available toenail data is provided in Table 1. The majority of children who provided toenail samples were male (58.5%) and white (77.8%). Median (IQR) concentrations of Pb in toenails and blood were 0.66 (0.28) µg/g and toenails 0.46 (0.49) µg/dL, respectively.
Table 1.
Descriptive statistics broken down by gender and race. SD = standard deviation.
| Overall | Male | Female | ||
|---|---|---|---|---|
| n1 | Mean (SD) | Mean (SD) | Mean (SD) | |
| Age (years) | 247 | 12.1 (0.80) | 12.1 (0.80) | 12.2 (0.80) |
| BPb (µg/dL) | 224 | 0.57 (0.37) | 0.64 (0.43) | 0.47 (0.21) |
| TPb (µg/g) | 252 | 0.66 (1.17) | 0.71 (1.30) | 0.57 (0.95) |
| Full-scale IQ (WISC-IV) | 241 | 101.6 | 101.0 (15.5) | 102.4 (14.2) |
| Caregiver IQ (WASI-2) | 239 | 105.4 (13.4) | 104.7 (14.1) | 106.4 (12.3) |
| BMI | 244 | 20.7 (5.2) | 20.1 (5.2) | 21.6 (5.2) |
| Overall | White | Black / Mixed Race | ||
| n1 | Mean (SD) | Mean (SD) | Mean (SD) | |
| Age (years) | 247 | 12.1 (0.80) | 12.2 (0.82) | 12.1 (0.73) |
| BPb (µg/dL) | 224 | 0.57 (0.37) | 0.51 (0.30) | 0.76 (0.5) |
| TPb (µg/g) | 252 | 0.66 (1.17) | 0.55 (0.92) | 1.00 (1.75) |
| Full-scale IQ (WISC-IV) | 241 | 101.6 | 105.3 (13.4) | 89.0 (13.0) |
| Caregiver IQ (WASI-2) | 239 | 105.4 (13.4) | 108.7 (11.5) | 94.1 (13.6) |
| BMI | 244 | 20.7 (5.2) | 19.9 (4.8) | 23.5 (5.7) |
Number of participants with available information. Total does not sum to 252 due to incomplete data available for some participants. 224 individuals had both BPb and TPb measurements.
Association Between TPb and BPb
Concentrations of Pb in toenails and blood were significantly and positively correlated (r2 = 0.49, p < 0.001). Parameter estimates obtained from the linear regression model examining the association between log-transformed TPb and BPb also indicate that TPb is significantly associated with BPb (β = 1.33, 95% CI = 1.02 – 1.66) with approximately 24% of the variability in TPb explained by BPb concentrations (model R2 = 0.24) (Figure 1).
Figure 1.
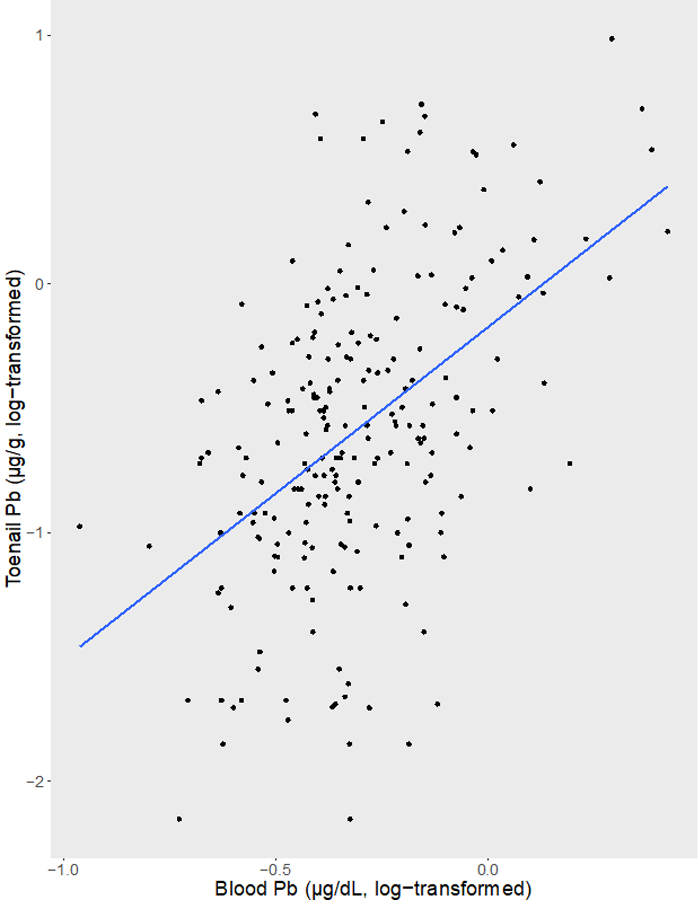
Linear Association Between log-transformed Blood Pb and Toenail Pb Concentrations.
Association between Pb Measured in Blood and Toenails and Childhood IQ
Both TPb and BPb were significantly associated with child FSIQ in unadjusted models (p = 0.005 and p < 0.001, respectively) (Table 2). After adjusting for caregiver IQ, community deprivation, and BMI, the association between BPb and FSIQ was attentuated but remained statistically significant (p < 0.001) gender was not statistically signficant (β =−13.15, 95% CI = −20.42 – 5.88). After covariate adjustment the association between TPb and child IQ was no longer statistically significant (p = 0.192) (Table 2). Parameter estimates for the co-variates used in the model are in Table 3.
Table 2.
Unadjusted and adjusted associations between Pb measured in blood and toenails and child IQ.
| Matrix | Unadjusted Association (β2, 95% CI) | Adjusted1Association (β2, 95% CI) |
|---|---|---|
| Blood | −23.45 (−31.92 –14.98) | −13.15 (−20.42 – 5.88) |
| Toenail | −4.47 (−7.57 – 1.37) | −1.70 (−4.27 – 0.862) |
Adjusted for caregiver IQ, community deprivation index, and BMI
Parameter estimates for a log10 change in exposure (i.e. a 10-fold increase in Pb concentrations).
Table 3.
Parameter estimates for co-variates in the multiple regression model.
| Matrix | Covariate | Parameter Estimate | P-value |
|---|---|---|---|
| Blood | Caregiver IQ | 0.325 | <0.001 |
| Deprivation Index | −17.7 | 0.001 | |
| BMI | −0.849 | <0.001 | |
| Toenail | Caregiver IQ | 0.395 | <0.001 |
| Deprivation Index | −17.6 | 0.001 | |
| BMI | −0.745 | <0.001 |
Discussion
The results of our analyses demonstrate that, while Pb measured in toenails is correlated with BPb levels, the latter is more robust with respect to associated cognitive health effects. Few studies have evaluated using children’s toenails as a biomarker for Pb exposure. A study in China with 317 children found a poor correlation between BPb and TPb with an r2 value of 0.03, approximately ten times lower than in the present study (Wang et al., 2009). Interestingly, in the same study, the r2 value for the relationship between BPb and spot urine Pb was 0.26, which is similar to our relationship between BPb and TPb. The BPb levels in that study were as high as about 4 μg/dL. A study in Vietnam(Sanders et al., 2014) of 20 children found a statistically significant correlation between TPb and BPb, with a Spearman’s correlation coefficient of 0.65. Participants in that study included a population who lived near a site where used batteries were smelted to obtain Pb ingots. The children’s BPb values ranged from 6.2 to 62.3 μg/dL, thus the study may not be relevant to typical exposures typically observed in the United States. Other studies have analyzed children’s toenails for Pb, but did not compare it to other biomarkers for reference (Carneiro et al., 2011; Hussein Were et al., 2008; Oyoo-Okoth et al., 2010; Wickre et al., 2004), or did not compare it to blood, the standard biomarker for Pb (Priya and Geetha, 2011; Wilhelm et al., 1991; Wilhelm et al., 1994).
There is a need for tools to evaluate chemical exposures in large populations and help identify geographic areas with the greatest exposure for public health interventions (Hanna-Attisha et al., 2016). Toenails offer several potential advantages as a biomatrix including self-collection, shipped to labs by regular mail, and stored at room temperature. In contrast, collecting blood is more invasive, requires phlebotomy (or capillary puncture), and shipping must follow protocol that include biohazards. However, in our study TPb explained less than 25% of the variation in concentrations measured using the gold-standard biomarker, BPb. One possible explanation for this discordance is the variable time-frame of exposure integrated by the biomarkers. Toenails take approximately 10 months to grow in adults(Yaemsiri et al., 2010) and therefore clippings reflect the Pb body burden from up to a year prior to blood collection. In contrast, BPb levels reflects body burden at the time of collection. Although not conducted with Pb, one study found the reproducibility of toenail Se and As, for which toenails reflect exposure levels, have Spearman correlation coefficients of 0.48 and 0.54 for repeated specimens collected from the same subject, respectively. Other elements’ within subject correlations varied from 0.26 (Cu) to 0.58 (Zn)(Garland et al., 1993). Although toenails may be a useful biomarker for some trace elements, random within-person variability may result in attenuation in measures of association in health studies. This is possibly reflected in the variance of the structure of the data as the coefficient of variation for BPb and TPb was 64% and 180%, respectively.
Interestingly, we did observe a significant decrease in IQ scores associated with BPb with the majority of the blood concentraitons below 3.5 µg/dL, however the association with TPb levels failed to reach statistical significance. These findings add to the literature suggesting BPb levels < 3.5 μg/dL, which has been suggested as the new definition of an elevated BPb level for young children in the US, are associated with decreased cognitive function in children. For example, the least squares mean FSIQ score for the lowest exposure concentrations of around 0.1 μg/dL was approximately 118, while the least squares mean FSIQ score for the 3.16 μg/dL was only approximately 90.
Conclusions, Limitations and Future Directions
There are some limitations to our study, including the cross-sectional nature of this analysis. To further evaluate the utility of TPb as a surveillance tool for research purposes, future studies should have a longitudinal design to account for differences in integrated exposure time between BPb and TPb. It is possible that TPb reflects exposure up to a year prior to sample collection. The growth rate of children’s toenails has not been evaluated and would help to refine the exposure window for which TPb could be indicative. A larger sample size may also result in a statistically significant correlation between TPb and cognitive scores. In conclusion, our study suggests that Pb is deposited and measurable in toenails, though the uncertainty regarding within-person variability, toenail growth rates, and other factors requires additional research before TPb is considered a reliable biomarker for use in Pb exposure studies.
Highlights.
Toenails may be useful for surveillance of children’s lead exposure because the can be self-collected.
Information on utility of toenails as a biomarker of children’s lead exposure is limited.
Blood and toenail lead were correlated in a cohort of 12 year old children from Cincinnati.
Blood lead was correlated with cognitive scores but toenail lead was not.
Acknowledgements
The authors gratefully acknowledge S. Shrestha and H. Swanson. This study was funded by the National Institutes of Health under 5R25ES027684 and P30ES026529. Funding for the CCAAPS cohort was provided by the National Institute of Environmental Health Sciences under R01ES019890 and R01ES11170. The authors would like to thank Chris Wolfe, Rachel Wolf, and Zana Percy for assisting with data collection and study coordination.
This study was funded by the National Institutes of Health under 5R25ES027684 and P30ES026529. Funding for the CCAAPS cohort was provided by the National Institute of Environmental Health Sciences under R01ES019890 and R01ES11170. The authors thank S. Shrestha.
Footnotes
Conflict of Interest.
The authors had no competing financial interests with relation to this work.
References
- Adler T. Questioning lead standards: even low levels shave points off IQ. Environmental Health Perspectives 2005; 113: A473. [Google Scholar]
- Baghurst PA, McMichael AJ, Wigg NR, Vimpani GV, Robertson EF, Roberts RJ, et al. Environmental Exposure to Lead and Children’s Intelligence at the Age of Seven Years. New England Journal of Medicine 1992; 327: 1279–1284. [DOI] [PubMed] [Google Scholar]
- Barbosa F, Tanus-Santos JE, Gerlach RF, Parsons PJ. A Critical Review of Biomarkers Used for Monitoring Human Exposure to Lead: Advantages, Limitations, and Future Needs. Environmental Health Perspectives 2005; 113: 1669–1674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bellinger D, Leviton A, Sloman J, Rabinowitz M, Needleman HL, Waternaux C. Low-level lead exposure and children’s cognitive function in the preschool years. Pediatrics 1991; 87: 219–227. [PubMed] [Google Scholar]
- Bellinger D, Leviton A, Waternaux C, Needleman H, Rabinowitz M. Longitudinal analyses of prenatal and postnatal lead exposure and early cognitive development. New England journal of medicine 1987; 316: 1037–1043. [DOI] [PubMed] [Google Scholar]
- Bellinger DC, Stiles KM, Needleman HL. Low-Level Lead Exposure, Intelligence and Academic Achievement: A Long-term Follow-up Study. Pediatrics 1992; 90: 855–861. [PubMed] [Google Scholar]
- Brokamp C, Beck AF, Goyal NK, Ryan P, Greenberg JM, Hall ES. Material community deprivation and hospital utilization during the first year of life: an urban population–based cohort study. Annals of epidemiology 2019; 30: 37–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carneiro MFH, Grotto D, Batista BL, Rhoden CR, Barbosa F. Background values for essential and toxic elements in children’s nails and correlation with hair levels. Biological trace element research 2011; 144: 339–350. [DOI] [PubMed] [Google Scholar]
- CDC. What Do Parents Need to Know to Protect Their Children? 2018. CDC, 2012. [Google Scholar]
- Dietrich KN, Berger OG, Succop PA, Hammond PB, Bornschein RL. The developmental consequences of low to moderate prenatal and postnatal lead exposure: Intellectual attainment in the cincinnati lead study cohort following school entry. Neurotoxicology and Teratology 1993; 15: 37–44. [DOI] [PubMed] [Google Scholar]
- Dietrich KN, Succop PA, Berger OG, Hammond PB, Bornschein RL. Lead exposure and the cognitive development of urban preschool children: The cincinnati lead study cohort at age 4 years. Neurotoxicology and Teratology 1991; 13: 203–211. [DOI] [PubMed] [Google Scholar]
- EPA. Method 6020a: Inductively coupled plasma - mass spectrometry United States Environmental Protection Agency, Washington, DC USA, 1998. [Google Scholar]
- Garland M, Morris JS, Rosner BA, Stampfer MJ, Spate VL, Baskett CJ, et al. Toenail trace element levels as biomarkers: reproducibility over a 6-year period. Cancer Epidemiology and Prevention Biomarkers 1993; 2: 493–497. [PubMed] [Google Scholar]
- Gulson BL. Nails: concern over their use in lead exposure assessment. Science of the total environment 1996; 177: 323–327. [Google Scholar]
- Gwiazda R, Campbell C, Smith D. A Noninvasive Isotopic Approach to Estimate the Bone Lead Contribution to Blood in Children: Implications for Assessing the Efficacy of Lead Abatement. Environmental Health Perspectives 2005; 113: 104–110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanna-Attisha M, LaChance J, Sadler RC, Schnepp AC. Elevated Blood Lead Levels in Children Associated With the Flint Drinking Water Crisis: A Spatial Analysis of Risk and Public Health Response. American Journal of Public Health 2016; 106: 283–290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayashi M, Yamamoto K, Yoshimura M, Hayashi H, Shitara A. Cadmium, lead, and zinc concentrations in human fingernails. Bulletin of environmental contamination and toxicology 1993; 50: 547–553. [DOI] [PubMed] [Google Scholar]
- Hussein Were F, Njue W, Murungi J, Wanjau R. Use of human nails as bio-indicators of heavy metals environmental exposure among school age children in Kenya. Science of The Total Environment 2008; 393: 376–384. [DOI] [PubMed] [Google Scholar]
- Koller K, Brown T, Spurgeon A, Levy L. Recent Developments in Low-Level Lead Exposure and Intellectual Impairment in Children. Environmental Health Perspectives 2004; 112: 987–994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lanphear BP, Hornung R, Khoury J, Yolton K, Baghurst P, Bellinger DC, et al. Low-Level Environmental Lead Exposure and Children’s Intellectual Function: An International Pooled Analysis. Environmental Health Perspectives 2005; 113: 894–899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LeMasters GK, Wilson K, Levin L, Biagini J, Ryan P, Lockey JE, et al. High prevalence of aeroallergen sensitization among infants of atopic parents. J Pediatr 2006; 149: 505–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lidsky TI, Schneider JS. Lead neurotoxicity in children: basic mechanisms and clinical correlates. Brain 2003; 126: 5–19. [DOI] [PubMed] [Google Scholar]
- Longnecker MP, Stampfer MJ, Morris JS, Spate V, Baskett C, Mason M, et al. A 1-y trial of the effect of high-selenium bread on selenium concentrations in blood and toenails. The American journal of clinical nutrition 1993; 57: 408–413. [DOI] [PubMed] [Google Scholar]
- Manton WI, Angle CR, Stanek KL, Reese YR, Kuehnemann TJ. Acquisition and Retention of Lead by Young Children. Environmental Research 2000; 82: 60–80. [DOI] [PubMed] [Google Scholar]
- Oyoo-Okoth E, Admiraal W, Osano O, Ngure V, Kraak MHS, Omutange ES. Monitoring exposure to heavy metals among children in Lake Victoria, Kenya: Environmental and fish matrix. Ecotoxicology and Environmental Safety 2010; 73: 1797–1803. [DOI] [PubMed] [Google Scholar]
- Palmer CD, Lewis ME Jr, Geraghty CM, Barbosa F Jr, Parsons PJ. Determination of lead, cadmium and mercury in blood for assessment of environmental exposure: A comparison between inductively coupled plasma–mass spectrometry and atomic absorption spectrometry. Spectrochimica Acta Part B: Atomic Spectroscopy 2006; 61: 980–990. [Google Scholar]
- Priya MDL, Geetha A. Level of trace elements (copper, zinc, magnesium and selenium) and toxic elements (lead and mercury) in the hair and nail of children with autism. Biological trace element research 2011; 142: 148–158. [DOI] [PubMed] [Google Scholar]
- Rabinowitz MB, Wetherill GW, Kopple JD. Kinetic analysis of lead metabolism in healthy humans. The Journal of Clinical Investigation 1976; 58: 260–270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reuben A, Caspi A, Belsky DW, Broadbent J, Harrington H, Sugden K, et al. Association of Childhood Blood Lead Levels With Cognitive Function and Socioeconomic Status at Age 38 Years and With IQ Change and Socioeconomic Mobility Between Childhood and AdulthoodChildhood Blood Lead Levels, Cognitive Function, and Socioeconomic Status in AdulthoodChildhood Blood Lead Levels, Cognitive Function, and Socioeconomic Status in Adulthood. JAMA 2017; 317: 1244–1251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryan PH, LeMasters G, Biagini J, Bernstein D, Grinshpun SA, Shukla R, et al. Is it traffic type, volume, or distance? Wheezing in infants living near truck and bus traffic. Journal of Allergy and Clinical Immunology 2005; 116: 279–284. [DOI] [PubMed] [Google Scholar]
- Sanders AP, Miller SK, Nguyen V, Kotch JB, Fry RC. Toxic metal levels in children residing in a smelting craft village in Vietnam: a pilot biomonitoring study. BMC Public Health 2014; 14: 114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sukumar A. Human nails as a biomarker of element exposure. Reviews of environmental contamination and toxicology. Springer, 2006, pp. 141–177.
- Unrine JM, Slone SA, Sanderson W, Johnson N, Durbin EB, Shrestha S, et al. A case-control study of trace-element status and lung cancer in Appalachian Kentucky. PLOS ONE 2019; 14: e0212340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Q, Zhao HH, Chen JW, Gu KD, Zhang YZ, Zhu YX, et al. Adverse health effects of lead exposure on children and exploration to internal lead indicator. Science of The Total Environment 2009; 407: 5986–5992. [DOI] [PubMed] [Google Scholar]
- Wasserman GA, Liu X, Lolacono NJ, Factor-Litvak P, Kline JK, Popovac D, et al. Lead exposure and intelligence in 7-year-old children: the Yugoslavia Prospective Study. Environmental Health Perspectives 1997; 105: 956–962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wechsler D. WISC-IV: Wechsler Intelligence Scale for Children. . Pearson Education Inc, Psychological Corporation, San Antionio, TX, USA, 2003. [Google Scholar]
- WHO. Environmental Health Criteria 165. Geneva: International Programme on Chemical Safety. World Health Organization; 1995. [Google Scholar]
- WHO. Exposure to Lead: A Major Public Health Concern. 2010.
- Wickre JB, Folt CL, Sturup S, Karagas MR. Environmental Exposure and Fingernail Analysis of Arsenic and Mercury in Children and Adults in a Nicaraguan Gold Mining Community. Archives of Environmental Health: An International Journal 2004; 59: 400–409. [DOI] [PubMed] [Google Scholar]
- Wilhelm M, Hafner D, Lombeck I, Ohnosorge FK. Monitoring of cadmium, copper, lead and zinc status in young children using toenails: comparison with scalp hair. Science of The Total Environment 1991; 103: 199–207. [DOI] [PubMed] [Google Scholar]
- Wilhelm M, Lombeck I, Ohnesorge FK. Cadmium, copper, lead and zinc concentrations in hair and toenails of young children and family members: a follow-up study. Science of The Total Environment 1994; 141: 275–280. [DOI] [PubMed] [Google Scholar]
- Yaemsiri S, Hou N, Slining MM, He K. Growth rate of human fingernails and toenails in healthy American young adults. Journal of the European Academy of Dermatology and Venereology 2010; 24: 420–3. [DOI] [PubMed] [Google Scholar]


