Abstract
Primary sclerosing cholangitis (PSC) is a chronic, cholestatic, idiopathic liver disease characterized by fibro-obliterative inflammation of the hepatic bile ducts. In a clinically significant proportion of patients, PSC progresses to cirrhosis, end-stage liver disease, and in some cases, cholangiocarcinoma (CCA). The development of CCA in PSC is unpredictable, its surveillance and diagnosis complex, and its treatment options limited unless detected early. Herein we provide a focused review of the current literature regarding CCA surveillance in patients with PSC and discuss the diagnostic and management challenges that exist. Where evidence is limited, we present our perspective and approach as well as directions for future research.
Keywords: Neoplasm, bile duct neoplasms, bile duct diseases, biological tumor marker, magnetic resonance imaging, ultrasonography, cytological techniques, fluorescence in situ hybridization, early detection of cancer, mass screening, imaging, cholangiography
INTRODUCTION
Primary sclerosing cholangitis (PSC) is a chronic, cholestatic liver disease of unknown etiology characterized by inflammation and concentric fibrosis of intrahepatic and/or extrahepatic bile ducts. It affects approximately 30,000 individuals in the United States (U.S.)—with a disease prevalence of 10 per 100,000—and is most frequently diagnosed in the 4th and 5th decade of life, although its onset may be much earlier or later [1–3]. Several hypotheses exist regarding the etiopathogenesis of PSC, all of which culminate in progressive biliary injury, hepatobiliary fibrosis, and hepatic decompensation [4, 5]. In addition, at any point during the course of disease, individuals with PSC are at increased risk (>1,500 fold over the general population) of developing superimposed bile duct cancer, i.e. cholangiocarcinoma (CCA).
Clinically, concern for CCA arises when a patient with PSC shows rapid progression of liver disease, including worsening serum levels of bilirubin or other biochemical liver tests with or without pain, weight loss, and/or anorexia. Although the predictors of CCA in PSC are currently not well understood, CCA development is believed to be a sequela of the chronic biliary tract inflammation [2]. With an incidence of approximately 0.5 – 1% per year, CCA is a leading cause of death in patients with PSC, in approximately half of whom CCA is detected within the first year of PSC diagnosis [6, 7]. Because of these reasons, as well as the dismal prognosis of CCA in all but the earliest stages, annual surveillance for CCA is recommended in patients with PSC.
In this article, we provide a synopsis of the current literature regarding CCA surveillance in patients with PSC and discuss the diagnostic and management challenges that exist therein. Where evidence is limited, we propose our perspective and approach as well as directions for future investigative study.
BARRIERS TO RELIABLE DETECTION
There are numerous factors in PSC that can hinder accurate and timely detection of CCA. Such factors include, but are not limited to the: 1) Unpredictability of which PSC patients will develop CCA and when; 2) Frequent lack of clinical forewarning until CCA is advanced; 3) Similarity between the cholangiographic appearance of a benign biliary stricture of PSC and a stricture due to CCA; 4) Elusive nature of submucosal or periductal (as opposed to radial, mass forming) growth of some CCA subtypes [8]; 5) Confounding of cytological assessment due to the inflammatory changes inherent to PSC; 6) Desmoplastic reaction associated with CCA leading to acellular biliary samples.
Given the challenges that exist with CCA surveillance and detection in PSC, a combination of tests is often used in clinical practice to maximize yield [7, 9]. An overview of these tests is provided below, and their diagnostic performance based on a recent large clinical study is summarized in Table 1 [7].
Table 1:
Diagnostic Performance of Various Modalities for Detecting Cholangiocarcinoma in Patients with Primary Sclerosing Cholangitis
| AUC (95% CI) |
Sensitivity (95% CI) |
Specificity (95% CI) |
Accuracy (95% CI) |
|
|---|---|---|---|---|
| Invasive imaging | ||||
| ERC | 0.79 | 91% (0.73–0.98) | 66% (0.59–0.72) | 69% (0.62–0.74) |
| ERC-obtained specimens | ||||
| Cytology* | 0.78 | 46% (0.23–0.71) | 97% (0.91–0.99) | 91% (0.84–0.95) |
| FISH | ||||
| Non-invasive imaging** | ||||
| Ultrasound | 0.76 | 57% (0.37–0.76) | 94% (0.90–0.97) | 90% (0.86–0.94) |
| MRCP | 0.82 | 89% (0.57–0.98) | 75% (0.67–0.83) | 76% (0.68–0.83) |
| CA 19–9 | ||||
| ≥20 U/mL | 0.79 | 78% (0.58–0.90) | 67% (0.60–0.73) | 68% (0.62–0.74) |
| ≥40 U/mL | 0.71 | 57% (0.37–0.74) | 84% (0.78–0.88) | 81% (0.75–0.86) |
| ≥129 U/mL | 0.57 | 13% (0.05–0.32) | 100% (0.99–1) | 90% (0.86–0.94) |
Key: AUC, area under the curve; CA 19–9, Carbohydrate antigen 19–9; ERC, endoscopic retrograde cholangiography; FISH, fluorescence in-situ hybridization (polysomy only); MRCP, magnetic resonance cholangiopancreatography.
Includes positive and suspicious cytology results.
Reflects overall performance, which includes definite, probable, and possible findings.
Adapted from Charatcharoenwitthaya et al. [7].
DIAGNOSTIC TESTS
Endoscopic Retrograde Cholangiography (ERC)
ERC has traditionally been an important tool for diagnosis of PSC, anatomic characterization of strictures, and therapy of biliary obstruction. While ERC is still effective and commonly used as a therapeutic intervention to relieve obstruction, its use as a first-line diagnostic tool in PSC has been decreasing due to improved non-invasive cholangiography (to be discussed) [10]. With respect to characterization of biliary strictures, ERC compares favorably with percutaneous cholangiography; it is also less invasive and has fewer complications [11, 12].
In addition to the functionalities mentioned above, ERC also allows for specimen acquisition in the form of biliary epithelial cells (cholangiocytes) harvested via ductal brushings and also intraductal tissue biopsies. These specimens can be submitted for microscopic analysis and represent valuable diagnostic data, as will be described below. Of note, endoscopic ultrasound and intraductal ultrasound may provide complementary information in select cases; however, endoscopic ultrasound typically has satisfactory performance for only distal strictures (≤2 cm proximal to the hilum), [8] and intraductal ultrasound is limited by poor interobserver variability and lack of formal criteria, among other issues [8, 13]. Thus, these modalities are not discussed further herein.
Biliary Cytology and Intraductal Biopsies
Microscopic evaluation of biliary epithelial brushing and biopsy specimens offers specificity that approaches 100% but is limited by low sensitivity, which is reported to range between 18%-40% [7, 14, 15]. Factors contributing to low sensitivity include those mentioned above, i.e. periductal (or submucosal) rather than radial growth of some CCAs, confounding of cytological features due to the inflammation associated with PSC, and the desmoplastic reaction seen in many CCAs, as well as others, such as insufficient flexibility of endoscopic devices (e.g. biopsy forceps) in some cases and sampling under indirect visualization [8]. There are limited data which suggest that repeat brushings within an ERC can significantly improve the diagnostic yield [16], but there is no consensus on the optimal number of brushes or to-and-fro passes per brush [8, 13].
Direct visualization via cholangioscopy represents an adjunct modality which may help increase the diagnostic utility of ERC as well as the yield of biliary sampling performed therein [17]. This promising technology has been recently shown to have high accuracy with regard to ruling in or out CCA in patients with indeterminate biliary lesions [18]. However, before direct cholangioscopy can be recommended widely in patients with PSC, more data are needed in this population, ideally in the form of larger prospective and randomized studies [14, 19].
Fluorescence In-Situ Hybridization (FISH)
FISH is a molecular diagnostic technique that has been relatively recently adapted for use on biliary epithelial brushing specimens. This technique uses fluorescently labeled DNA probes that hybridize to four chromosomal loci (CEP 3, CEP 7, CEP 17, and 9p21) to identify nuclear aneusomy (numerical gain or loss of selected chromosomes or chromosomal loci) [2, 20]. Using fluorescence microscopy, nuclei with abnormal probe signal numbers (i.e. aneusomic nuclei) can be identified; five or more cells showing gains of two or more of the four probes (i.e. polysomy) in brushing specimens is associated with a >50% chance of having CCA or developing it during follow up [9].
FISH has complementary value to routine cytology and improves its sensitivity by 14%-24% when the latter is negative [8, 13]. FISH appears to also have high specificity, comparable to that of routine cytology [8]. Recently, it has been shown that PSC patients with an initial FISH polysomy result followed by another polysomy result (i.e. serial polysomy) are significantly more likely to develop CCA during follow-up as compared to patients without serial polysomy (75 % vs. 18 % at 3 years). These findings suggest that PSC patients with serial polysomy should be monitored particularly closely for development of CCA.
Non-Invasive Imaging
A variety of non-invasive imaging modalities exist to assess for CCA in patients with PSC. These include ultrasound, magnetic resonance imaging (MRI) with magnetic resonance cholangiopancreatography (MRCP), computerized tomography, and positron emission tomography (PET). Due to a number of reasons, including radiation exposure, requisite need for iodinated contrast dye, and/or false positives and negatives, the latter two modalities are less favorable options and are seldom necessary [2, 14, 21].
Definite imaging features of CCA include a typical signal intensity and enhancement of a mass with delayed venous phase enhancement on MRI, or visualization of a duct-based mass on ultrasound. Thickening of the bile duct wall with proximal biliary dilatation, which can be visualized by magnetic resonance or by ultrasound, is consistent with “possible” CCA. With MRCP, overall sensitivity and specificity are 89% and 75%, respectively, and overall accuracy is 76%. Interestingly, in one study, abdominal ultrasound—a more readily available and less costly technique—had an overall sensitivity and specificity of 57% and 94%, respectively, and an overall accuracy of 90% [7].
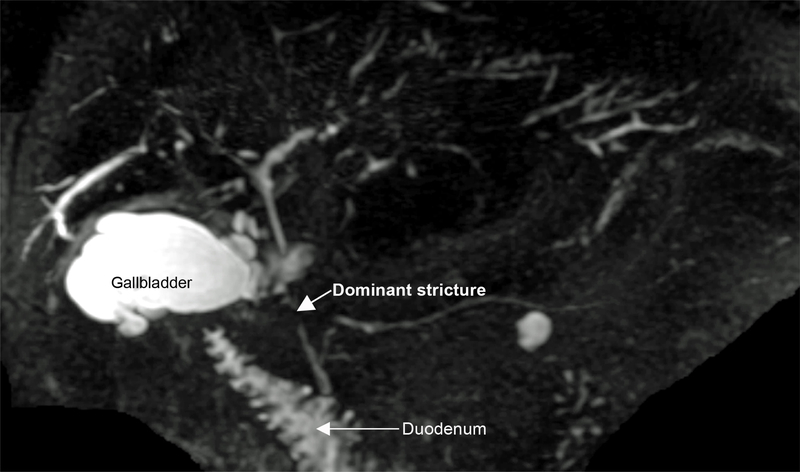
As alluded to above, CCA can be difficult to distinguish from the stricturing process that is inherent to PSC (Figure 1). Although concern for malignancy is appropriately high with dominant strictures (defined as a stenosis with a diameter of ≤1.5 mm in the common bile duct or of ≤1 mm in a hepatic duct), only a minority of dominant strictures in PSC are due to CCA [7]. In addition, CCA can be particularly difficult to detect by both non-invasive imaging as well as ERC when it is of the infiltrating (i.e. periductal), non-mass forming morphological subtype. With these limitations in mind, imaging modalities have a central complementary role in CCA surveillance and diagnosis, but if the suspicion for cancer is high based on other clinical features and data, they do not sufficiently exclude CCA.
Figure 1:
Magnetic resonance cholangiopancreatography (MRCP) maximal intensity projection (MIP) demonstrating diffuse intrahepatic changes of primary sclerosing cholangitis (PSC) and a mid-common bile duct dominant stricture concerning (but not definitively diagnostic) for cholangiocarcinoma in PSC patient who presented with abdominal pain and rising serum bilirubin. The patient went on to have endoscopic retrograde cholangiography with biliary sampling for further evaluation of the dominant extrahepatic stricture.
Carbohydrate Antigen 19–9 (CA 19–9)
In addition to imaging studies, a complementary approach to surveillance fo CCA in patients with PSC is the use of serum biomarkers. The best studied and only clinically available serum biomarker for CCA is CA 19–9. This tumor marker was first described in 1979 by Koprowski et al., [22] who by using hybridoma technology, generated a panel of antibodies against a colorectal carcinoma cell line, one of which was designated “1116 NS 19–9”. This monoclonal antibody was subsequently found to react with a carbohydrate antigenic determinant (CA 19–9) produced by CCAs, which was ultimately identified as being sialylated lacto-N-fucopentaose II, an oligosaccharide biochemically related to the Lewisa blood group antigen [23].
CA 19–9, as with the other tests mentioned thus far, is not exempt of limitations. It can be elevated in patients with bacterial cholangitis, pancreatic malignancy, or non-malignant biliary obstruction, and it is not synthesized in individuals negative for the Lewisa blood antigen, which represents 6% and 22% of U.S. Whites and Blacks, respectively [14, 24]. Nonetheless, it does improve sensitivity for CCA detection when combined with other tests, such as imaging. The optimal cut-off value for CA 19–9 value for CCA in PSC has been investigated in several studies [14]; a cut-off of 130 U/mL (normal<55 U/mL), for example, is associated with a sensitivity and specificity of 79% and 98%, respectively [25]. Lower values are associated with improved sensitivity at the expense of specificity. In cases where it is unclear whether CA 19–9 elevation is attributable to benign biliary obstruction or to CCA, such as in the setting of a new dominant stricture, persistent elevation of CA 19–9 after biliary decompression suggests malignancy and should prompt further evaluation and management.
CURRENT SURVEILLANCE STRATEGIES AND FUTURE DIRECTIONS
Surveillance strategies are predicated on the availability of a highly sensitive and cost-effective diagnostic test(s), effective treatment strategies, and the agreeability of the diagnostic test and subsequent treatment to both patients and providers [14]. Once these criteria have been successfully met, studies must demonstrate a reduction in disease-related mortality. In the case of CCA in PSC, we are approaching but have not yet reached this point. Current treatment strategies for CCA include surgical resection and a specialized liver transplantation protocol. Surgical resection with negative tumor margins can be considered for early stage CCA in patients with preserved liver function and little hepatic fibrosis, but this option is associated with a 3-year survival of less than 20% [26]. A newer and potentially curative treatment, consisting of high dose neoadjuvant radiotherapy with chemosensitization followed by living donor liver transplantation, is currently the best treatment option (albeit available at very few institutions) and is associated with a 5-year survival of approximately 70%. Unfortunately, this survival rate applies only to those patients who complete the treatment protocol, which itself can only be offered to only a small minority of patients at very few institutions [26]. Therefore, the benefits of early diagnosis of CCA as a result of CCA surveillance in patients with PSC are at least in part dependent on the availability of specialized liver transplantation centers.
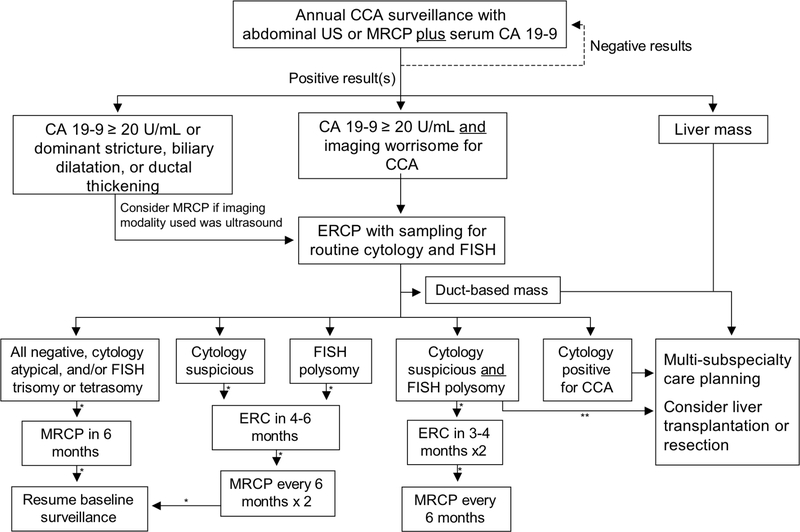
Based on existing literature and experience, a reasonable approach to CCA surveillance, and one that we practice, is annual CA 19–9 testing coupled with imaging evaluation with ultrasound or MRI/MRCP. In some instances, this may require referral to tertiary centers where patients with PSC are managed regularly and familiarity with the nuances of PSC is relatively high. Patients with concerning findings on either test merit further investigation to rule out CCA, and we propose a management algorithm for this (Figure 2). Future research should validate this algorithm and determine the utility of incorporating new technologies such as cholangioscopy and associated advanced techniques (e.g., endomicroscopy, narrow band imaging) as well as the cost-effectiveness of the existing algorithm and potential additions.
Figure 2:
Proposed cholangiocarcinoma surveillance algorithm for patients with primary sclerosing cholangitis.
*Assumes stable findings. CA 19–9 and liver biochemistries to also be checked along with cholangiography. In addition, cytology and FISH to be checked with ERC.
**Consider early subspecialist referral if worsening in serum tests or other signs or symptoms.
Footnotes
DISCLOSURES, CONFLICTS OF INTEREST
None.
REFERENCES
- [1].Aron JH, Bowlus CL. The immunobiology of primary sclerosing cholangitis. Semin Immunopathol [Review] 2009; 31(3): 383–97. 10.1007/s00281-009-0154-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Razumilava N, Gores GJ, Lindor KD. Cancer surveillance in patients with primary sclerosing cholangitis. Hepatology [Review] 2011; 54(5): 1842–52. 10.1002/hep.24570 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Kaplan GG, Laupland KB, Butzner D, Urbanski SJ, Lee SS. The burden of large and small duct primary sclerosing cholangitis in adults and children: a population-based analysis. Am J Gastroenterol 2007; 102(5): 1042–9. 10.1111/j.1572-0241.2007.01103.x [DOI] [PubMed] [Google Scholar]
- [4].Pollheimer MJ, Halilbasic E, Fickert P, Trauner M. Pathogenesis of primary sclerosing cholangitis. Best Pract Res Clin Gastroenterol [Research Support, Non-U.S. Gov’t] 2011; 25(6): 727–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Tabibian JH, O’Hara SP, Larusso NF. Primary Sclerosing Cholangitis: The Gut-Liver Axis. Clin Gastroenterol Hepatol [Letter] 2012. 10.1016/j.cgh.2012.01.024 [DOI] [PubMed] [Google Scholar]
- [6].Fevery J, Verslype C, Lai G, Aerts R, Van Steenbergen W. Incidence, diagnosis, and therapy of cholangiocarcinoma in patients with primary sclerosing cholangitis. Dig Dis Sci [Case Reports] 2007; 52(11): 3123–35. 10.1007/s10620-006-9681-4 [DOI] [PubMed] [Google Scholar]
- [7].Charatcharoenwitthaya P, Enders FB, Halling KC, Lindor KD. Utility of serum tumor markers, imaging, and biliary cytology for detecting cholangiocarcinoma in primary sclerosing cholangitis. Hepatology [Research Support, Non-U.S. Gov’t] 2008; 48(4): 1106–17. [DOI] [PubMed] [Google Scholar]
- [8].Cote GA, Sherman S. Biliary stricture and negative cytology: what next? Clin Gastroenterol Hepatol [Case Reports] 2011; 9(9): 739–43. 10.1016/j.cgh.2011.04.011 [DOI] [PubMed] [Google Scholar]
- [9].Bangarulingam SY, Bjornsson E, Enders F, et al. Long-term outcomes of positive fluorescence in situ hybridization tests in primary sclerosing cholangitis. Hepatology 2010; 51(1): 174–80. 10.1002/hep.23277 [DOI] [PubMed] [Google Scholar]
- [10].Dave M, Elmunzer BJ, Dwamena BA, Higgins PD. Primary sclerosing cholangitis: meta-analysis of diagnostic performance of MR cholangiopancreatography. Radiology [Meta-Analysis] 2010; 256(2): 387–96. [DOI] [PubMed] [Google Scholar]
- [11].Winick AB, Waybill PN, Venbrux AC. Complications of percutaneous transhepatic biliary interventions. Tech Vasc Interv Radiol [Review] 2001; 4(3): 200–6. 10.1016/S1089-2516(01)90026-5 [DOI] [PubMed] [Google Scholar]
- [12].Park JS, Kim MH, Lee SK, et al. Efficacy of endoscopic and percutaneous treatments for biliary complications after cadaveric and living donor liver transplantation. Gastrointest Endosc [Comparative Study] 2003; 57(1): 78–85. 10.1067/mge.2003.11 [DOI] [PubMed] [Google Scholar]
- [13].Levy MJ, Baron TH, Clayton AC,, et al. Prospective evaluation of advanced molecular markers and imaging techniques in patients with indeterminate bile duct strictures. Am J Gastroenterol [Comparative Study Research Support, N.I.H., Extramural] 2008; 103(5): 1263–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Chapman R, Fevery J, Kalloo A, et al. Diagnosis and management of primary sclerosing cholangitis. Hepatology [Practice Guideline] 2010; 51(2): 660–78. 10.1002/hep.23294 [DOI] [PubMed] [Google Scholar]
- [15].Siqueira E, Schoen RE, Silverman W, et al. Detecting cholangiocarcinoma in patients with primary sclerosing cholangitis. Gastrointest Endosc [Research Support, Non-U.S. Gov’t] 2002; 56(1): 40–7. [DOI] [PubMed] [Google Scholar]
- [16].de Bellis M, Fogel EL, Sherman S, et al. Influence of stricture dilation and repeat brushing on the cancer detection rate of brush cytology in the evaluation of malignant biliary obstruction. Gastrointest Endosc [Research Support, Non-U.S. Gov’t] 2003; 58(2): 176–82. [DOI] [PubMed] [Google Scholar]
- [17].Tischendorf JJ, Kruger M, Trautwein C, et al. Cholangioscopic characterization of dominant bile duct stenoses in patients with primary sclerosing cholangitis. Endoscopy 2006; 38(7): 665–9. 10.1055/s-2006-925257 [DOI] [PubMed] [Google Scholar]
- [18].Ramchandani M, Reddy DN, Gupta R, et al. Role of single-operator peroral cholangioscopy in the diagnosis of indeterminate biliary lesions: a single-center, prospective study. Gastrointest Endosc [Clinical Trial] 2011; 74(3): 511–9. 10.1016/j.gie.2011.04.034 [DOI] [PubMed] [Google Scholar]
- [19].Chen YK, Pleskow DK. SpyGlass single-operator peroral cholangiopancreatoscopy system for the diagnosis and therapy of bile-duct disorders: a clinical feasibility study (with video). Gastrointest Endosc [Research Support, Non-U.S. Gov’t] 2007; 65(6): 832–41. [DOI] [PubMed] [Google Scholar]
- [20].Barr Fritcher EG, Kipp BR, Voss JS, et al. Primary sclerosing cholangitis patients with serial polysomy fluorescence in situ hybridization results are at increased risk of cholangiocarcinoma. Am J Gastroenterol 2011; 106(11): 2023–8. 10.1038/ajg.2011.272 [DOI] [PubMed] [Google Scholar]
- [21].Blechacz B, Gores GJ. Positron emission tomography scan for a hepatic mass. Hepatology [Research Support, N.I.H., Extramural Research Support, Non-U.S. Gov’t] 2010; 52(6): 2186–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Koprowski H, Steplewski Z, Mitchell K, Herlyn M, Herlyn D, Fuhrer P. Colorectal carcinoma antigens detected by hybridoma antibodies. Somatic Cell Genet [Research Support, U.S. Gov’t, P.H.S.] 1979; 5(6): 957–71. [DOI] [PubMed] [Google Scholar]
- [23].Magnani JL, Nilsson B, Brockhaus M, et al. A monoclonal antibody-defined antigen associated with gastrointestinal cancer is a ganglioside containing sialylated lacto-N-fucopentaose II. J Biol Chem [Research Support, Non-U.S. Gov’t Research Support, U.S. Gov’t, P.H.S.] 1982; 257(23): 14365–9. [PubMed] [Google Scholar]
- [24].Brecher M. ABO H, and Lewis Blood Groups and Structurally Related Antigens. Technical Manual Of The American Association of Blood Banks. 15 ed. Bethesda: American Association of Blood Banks; 2005; pp. 305–6. [Google Scholar]
- [25].Levy C, Lymp J, Angulo P, Gores GJ, Larusso N, Lindor KD. The value of serum CA 19–9 in predicting cholangiocarcinomas in patients with primary sclerosing cholangitis. Dig Dis Sci [Clinical Trial] 2005; 50(9): 1734–40. 10.1007/s10620-005-2927-8 [DOI] [PubMed] [Google Scholar]
- [26].Rosen CB, Heimbach JK, Gores GJ. Liver transplantation for cholangiocarcinoma. Transpl Int [Review] 2010; 23(7): 692–7. 10.1111/j.1432-2277.2010.01108.x [DOI] [PubMed] [Google Scholar]