Abstract
Blockage or restriction of blood flow through conduit arteries results in tissue ischemia downstream of the disturbed area. Local tissues can adapt to this challenge by stimulating vascular remodeling through angiogenesis and arteriogenesis thereby restoring blood perfusion and removal of wastes. Multiple molecular mechanisms of vascular remodeling during ischemia have been identified and extensively studied. However, therapeutic benefits from these findings and insights are limited due to the complexity of various signaling networks and a lack of understanding central metabolic regulators governing these responses. The gasotransmitters NO and H2S have emerged as master regulators that influence multiple molecular targets necessary for ischemic vascular remodeling. In this review, we discuss how NO and H2S are individually regulated under ischemia, what their roles are in angiogenesis and arteriogenesis, and how their interaction controls ischemic vascular remodeling.
Keywords: hypoxia, ischemia, oxygen, endothelium, blood flow
Introduction
Reduction of blood flow through conduit arteries to different organs including the brain contributes to ischemic tissue disease, such as peripheral and coronary artery disease and stroke. In order to restore blood supply, ischemic tissue responds by forming new vasculature and remodeling preexisting ones. This combination of vascular network alterations is often collectively referred to as ischemic vascular remodeling[8,90]. Vascular remodeling responses under ischemic conditions consists of both arteriogenesis and angiogenesis[90]. Physiologically, two conduit arteries connected by a collateral vessel typically maintain similar pressures, resulting in low or little net flow across the collateral. However, occlusion in the proximal side of either conduit artery disrupts this balance and initiates blood flow across connecting collateral vessels thereby stimulating arteriogenesis. The change in hemodynamics experienced by the collateral vessel stimulates outward vascular remodeling to increase lumen diameter and wall thickness (figure 1A). On the other hand, angiogenesis is attributed to microvascular growth in general, with the strict definition that it is the outgrowth of preexisting capillaries that includes tip cell sprouting, stalk cell elongation, and vessel maturation (figure 1B).
Figure 1-. Vascular remodeling due to arteriogenesis or angiogenesis.
Remodeling of macro- and microvasculature occurs through arteriogenesis and angiogenesis, respectively. Panel A illustrates blood flow across two arterial branches resulting in equivalent pressure (P1 ≅ P2) with minimal flow across intervening collateral arteries. Upon occlusion of one branch, typically due to atherosclerosis, a pressure differential occurs (P1’>P2’) leading to increased flow and shear across collateral vessels resulting in increased luminal diameter (D1<D2) and vascular wall thickness (T1<T2). Panel B shows key steps in angiogenesis involving endothelial tip cell sprouting upon extracellular matrix degradation, progressive vessel stalk elongation, and subsequent pericyte recruitment that lead to formation of a mature microvessel structure.
Although therapeutic approaches based on stimulating these various angiogenesis and arteriogenesis responses show promise in animal models, translation of successful therapies have not been realized in the clinic. One important cause of this disconnection is the complexity of multiple molecular mechanisms regulating ischemic vascular remodeling. For example, although VEGF is an essential proangiogenic and remodeling factor, a patient may have VEGF signaling dysregulation versus expression deficiencies. Therefore, simply manipulating a single molecular target, such as VEGF, in a complex network of growth and remodeling responses may not be effective as a therapeutic approach targeting comprehensive changes involved in ischemic vascular remodeling [91]. Therefore, it is important to understand master molecular regulators that fine-tune ischemic vascular changes involving both angiogenesis and arteriogenesis. In this regard, the recently termed ‘gasotransmitter’ molecules may be promising master targets for ischemic vascular remodeling.
As a growing concept over the past decade, gasotransmitters have been recognized as a necessary part of signaling networks. By definition, gasotransmitters are a group of gaseous signaling molecules. However, they may also exert effects in ionic forms as well[95]. Analogous to neurotransmitters, gasotransmitters are endogenously synthesized by enzymes, may be stored in a biochemical ‘pool’ and released under certain permissive conditions to stimulate responses. But unlike neurotransmitters, gasotransmitters are regulated in a different manner[108]. Firstly, gasotransmitters may be widely reactive and more diffusive. Therefore, they might not be easily compartmentalized within the cell. Instead, they are metabolized to inactive forms that can be mobilized under specific biological conditions when needed. Secondly, gasotransmitters are not entirely mediated by ligand-receptor interaction. Rather, their specificity depends on their bioavailability in the proximity of a target molecule as well as the chemical reactivity of the target molecule under certain conditions. Two well-studied gasotransmitters exemplifying these features are nitric oxide (NO) and hydrogen sulfide (H2S). Importantly, both of these molecules critically regulate angiogenesis and arteriogenesis under physiological and hypoxic/ischemic conditions.
In mammalian cells, three isoforms of NO synthase (NOS) generate NO in an L-arginine and oxygen dependent manner[37]. NO is well known to interact with transition metals to exert its functions. A well-characterized receptor is soluble guanylate cyclase (sCG). By activating vascular smooth muscle sCG-cGMP, endothelial derived NO works as a paracrine factor to control vascular tone. Additionally, formation of S-nitrosothiols allows NO regulation of protein function through cysteine post-translational modification. During ischemic vascular remodeling, NO enhances both angiogenesis and arteriogenesis, while also interacting with reactive oxygen species (ROS), which may also influence vascular remodeling responses. Together, this makes NO a critical, central regulator of ischemic vascular remodeling.
Different from NO, H2S synthesis is controlled enzymatically by proteins including cystathionine β-synthase (CBS), cystathionine γ-lyase (CSE), and 3-mercaptopyruvate sulfurtransferase (3-MST)[108]. Properties and actions of H2S and NO may seem similar at first glance as H2S also binds to transition metals and post-translationally modifies protein cysteines. However, H2S significantly differs from NO in its stability, chemical reactivity, target molecule selectivity, and molecular regulatory mechanisms. For example, although H2S has also been reported to regulate vascular tone, its mechanism involves alteration of potassium channels and phosphodiesterase activity rather than sCG activity [16,73]. On the other hand, H2S and NO have the ability to differentially regulate protein function when they do share the same molecular target [4,72]. Moreover, accumulating studies clearly demonstrate regulatory roles of H2S on the vasculature. Both angiogenesis and arteriogenesis are regulated by H2S metabolism along with the understanding that cooperative interaction between NO and H2S is important for ischemic vascular remodeling. In this review, we focus on how NO and H2S are individually regulated, their role in ischemic vascular remodeling, and how they may interact in this process.
NO in Vascular Growth and Remodeling
Regulation of NO Synthesis
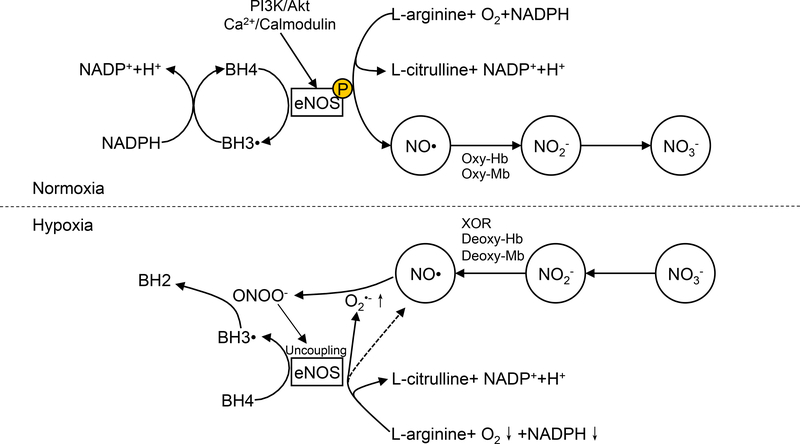
NO was first defined as the endothelial derived relaxing factor (NO), which clearly illustrates its importance for cardiovascular health[46,64]. In the cardiovascular system, NO is derived primarily from endothelial NOS (eNOS or NOS3). Other two members of the NOS family, neural NOS (nNOS or NOS1) and inducible NOS (iNOS or NOS2), share similar structures but show differential tissue expression and enzyme regulation. All NOS isoforms are homodimers, each monomer of which has a C-terminal reductase domain, an N-terminal oxidase domain and a central heme domain[37]. The reductase domain binds to NADPH, flavin mononucleotide (FMN) and flavin adenine dinucleotide (FAD). An electron is transferred from NADPH, via FMN and FAD, to the heme where oxygen (O2) is reduced. The heme also receives a second electron from tetrahydrobiopterin (BH4) in the oxidase domain, resulting in O2 production and BH4 oxidation[36]. BH4 oxidation can be recycled back to the reduced form by receiving another electron from the NOS reductase domain. To couple this reduction, NOS hydroxylates and oxidizes L-arginine to L-citrulline and NO. In this way, NOS utilizes O2 and L-arginine to produce NO and L-citrulline (Figure 2).
Figure 2-. NO bioavailabilty and eNOS activity under normoxia and hypoxia.
NO generation in the vasculature under normoxic conditions occurs through activation of the eNOS enzyme involving L-arginine substrate conversion to L-citrulline plus NO. eNOS generation of NO under hypoxic conditions can become dysfunctional due to enzyme uncoupling and ROS formation. Alternative non-enzymatic generation of NO under hypoxia can occur through nitrite/nitrate reduction back to NO via different molecular pathways.
Regulation of NOS enzyme activity is complex and regulated in multiple ways. Calmodulin association with the enzyme accelerates electron flow from NADPH to the heme and conversion of L-arginine and O2 to L-citrulline and NO[36]. In eNOS and nNOS, calmodulin binding affinity is increased by elevated cytosolic Ca2+, while the binding affinity is already high in iNOS at low Ca2+ concentration due to structural differences[37]. Therefore, eNOS and nNOS are activated by an increase of cytosolic Ca2+, but iNOS is constitutively active once expressed. Additionally, eNOS and nNOS activity can be regulated by phosphorylation. Most importantly, phosphorylation of eNOS at S1177 stimulates electron flow and increases enzyme sensitivity to Ca2+ resulting in eNOS activation[34,68]. On the contrary, eNOS phosphorylation at T495 may interfere with the calmodulin binding site and negatively regulates eNOS activity[34]. Similarly, nNOS can be activated by phosphorylation at S1412/S1417 and inhibited by phosphorylation at S847[83,85,86].
NO Regulation of Vascular Development
NO is a major regulator for vascular growth in development. Vascular endothelial growth factor (VEGF) and other proangiogenic factors, such as basic fibroblast growth factor (bFGF) and transforming growth factor β (TGFβ), increase eNOS activity by eNOS activation via PI3K/Akt dependent S1177 phosphorylation or PLC dependent increase of cytosolic Ca2+[39,66]. Inhibiting NO synthesis neutralizes proangiogenic effects of these factors, suggesting a predominant downstream role of NO[5,76]. NO increases angiogenesis by soluble guanylate cyclase (sGC) activation. The downstream cGMP/PKG/MAPK pathway increases endothelial cell proliferation and migration, resulting in increased angiogenesis[11]. Reciprocally, NO can also increase growth factor expression (e.g. VEGF, bFGF) in a cGMP/MAPK dependent manner[32,111].
NOS deficiency in the vasculature may result in defective vascular development. As the predominant source of NO, eNOS plays a major role in NO mediated angiogenesis[38]. eNOS deficient mice develop alveolar capillary dysplasia[43]. Incidences of abnormal aortic valves and limb deficiency are also observed in eNOS knockout mice, which may be caused by vascular defects[42,59]. However, eNOS genetic deficiency in mice is not lethal, suggesting complimentary roles of nNOS and iNOS in vascular development or alternative modes of generating NO [1,38].
NO has an intriguing role in collateral formation. VEGF is known to be important for native collateral development[25]. Considering that VEGF induced angiogenesis heavily depends on NO availability, it is easy to speculate that NO serves as a crucial promoter for collateral growth. However, a study comparing wild-type versus eNOS knockout mice found no differences in collateral density or diameter at embryonic and neonatal stages, when collaterals are initially formed[30]. However, in adult mice (6 months old), the native collateral number was 25% lower in eNOS knockout mice, suggesting that eNOS/NO may be more important for maintaining collateral density during growth to adulthood[30]. It is important to note that the collateral circulation under healthy conditions is different from diseased or ischemic conditions where NO/eNOS bioavailability and function would clearly be affected.
NO Regulation of Ischemic Vascular Remodeling
Reduction of conduit artery function results in major changes of local tissue involving two primary aspects: blood flow and oxygen tension. Importantly, the components of vascular remodeling, angiogenesis and arteriogenesis, have different sensitivity to these changes. Under healthy conditions, collaterals have oscillatory flow with little net flow across them. As previously mentioned, occlusion of one feed artery results in directional net flow across collaterals. This change of hemodynamic force is the primary stimulation for collateral remodeling[18,22]. Increased laminar flow stimulates signaling pathways, such as RhoA, PI3K and PKA, to activate eNOS. eNOS derived NO elicits collateral dilation as an initial response to attenuate ischemia. Additionally, shear stress also increases eNOS expression via the c-Src/Ras/ERK pathway that can further reinforce remodeling responses[30]. Finally, eNOS/NO also contributes to arteriogenesis by inducing endothelial cell proliferation and mediates mural cell recruitment important for remodeling [30] [107]. During this process, eNOS expression is increased in a growing collateral, but then decreases once the collateral is matured[19].
Unlike arteriogenesis, hypoxia signaling largely controls angiogenesis under ischemia. Under hypoxia, cells respond by activating hypoxia-inducible factor (HIF). HIF is a transcription factor composed of α and β subunits. Under normoxic conditions, prolyl hydroxylase domain containing proteins (PHD1, 2, 3) hydroxylate HIF-α on the oxygen-dependent degradation domain, which is then recognized by von Hippel-Lindau protein (pVHL) and subjected to degradation[48,90]. Under reduced oxygen tension, HIF-α hydroxylation is decreased leading to its accumulation and translocation into the nucleus, where it dimerizes with HIF-β to activate expression of various hypoxia responsive genes, such as VEGF and its receptors[13,51,89]. Similar to the role of VEGF signaling in development, activation of VEGF receptors also cause eNOS phosphorylation at S1179 to increase NO production, via the PI3K/Akt pathway. Expression of another HIF target gene CXCL12 recruits endothelial progenitor cells (EPCs) involving activation of its ligand CXCR4[106]. EPCs are characterized by eNOS expression, which is important for their recruitment under ischemia and may contribute to tissue NO bioavailability[80,97].
HIF stabilization is also controlled by S-nitrosation. S-nitrosation is the binding of a nitroso group to a cysteine thiol, forming an S-nitrosothiol. S-nitrosation usually requires nitrosating agents, such as N2O3 and peroxynitrite (ONOO−)[108]. Although NO itself cannot provide the nitroso group (a common misconception), it does contribute by forming N2O3 via oxidation and ONOO- by reaction with superoxide (O2•−). Alternatively, a nitroso group can be transferred from another s-nitrosothiol or a metal nitrosyl. S-nitrosation modifies various components of the HIF pathway. Firstly, HIF-1α can be S-nitrosated on a cysteine residue (mouse Cys533 and human Cys520) in the oxygen dependent degradation domain, resulting in decreased pVHL binding[60]. Secondly, S-nitrosation of pVHL at Cys162 also reduces its binding affinity[75]. Thirdly, PHD2 can also be S-nitrosated at several cysteine residues, although the biological relevance of these modifications requires further investigation[24]. While S-nitrosation of HIF signaling components are thought to be important in tumorigenesis and inflammatory response, it may also be involved during chronic tissue ischemia. Ischemia itself and a number of cardiovascular risk factors increase O2•− and peroxide (H2O2) production from the mitochondrial respiratory chain, NADPH oxidase (NOX), and xanthine oxidase (XO)[35,61]. An increase in reactive oxygen species (ROS) in the presence of NO may in turn increase S-nitrosation that activates HIF transcription activity.
Increased ROS may also regulate eNOS activity. As discussed above, eNOS activity relies on electron flow and coupling of reduction and oxidation of substrates. The disruption of this electron flow causes eNOS uncoupling and production of O2•− instead of NO. During ischemia, increased O2•− from various sources can react with NO to produce ONOO−,, which can reduce BH4 bioavailability due to oxidation and also directly disrupt the BH4 binding site (Cys99) on eNOS[36]. ONOO− oxidizes a zinc cluster at Cys99, decreasing eNOS dimer stability and function[112]. Therefore, although eNOS is up regulated in ischemia, eNOS may fail to increase NO bioavailability in ischemic tissue due to these and other reasons, at least in the initial response to ischemia (Figure 2). Therefore, alternative sources of NO are likely necessary for effective ischemic vascular remodeling.
Although NO is freely diffusive, its diffusion distance is well limited by heme protein scavenging and rapid oxidation[27]. In the cell, NO can be oxidized by cytochrome P450 enzymes and oxygenated myoglobin[14,40]. Oxygenated hemoglobin in the red cell also efficiently oxidizes NO to nitrite and nitrate[41,52]. It is important to understand that NO equivalents can be obtained from selective reduction of nitrate back to nitrite and subsequent nitrite reduction back to NO. Moreover, these substrates, in particular nitrate, may be obtained in large amounts through the diet. Together, this helps explains the observation that nitrate concentrations in human plasma is approximately 20 μM[71]. Although the majority of circulating nitrate is eventually excreted by the kidneys, up to 25% of it is concentrated in salivary glands and secreted into the oral cavity[65]. Once in the oral cavity, commensal bacterial flora is able to reduce nitrate to nitrite, which is then swallowed. It has been known for some time that nitrite can be reduced to NO under acidic conditions. Therefore, ingested nitrite may be rapidly reduced to NO in the stomach (pH ~3) or reabsorbed back into the systemic circulation constituting the enterosalivary system of nitrate/nitrite metabolism. Consistent with these facts, it has been shown that loss of nitrate reducing bacteria decreases plasma nitrite and elevates blood pressure in healthy individuals, suggesting that this pathway is important for nitrite recycling and regulation of vascular tone [99]. Acidic pH is also commonly seen in ischemic or elevated metabolic conditions. Under hypoxia or acidosis, a number of proteins can also reduce nitrite back to NO, including xanthine oxidase, deoxyhemoglobin, deoxymyoglobin, and others[21,52]. Therefore, both nitrite and nitrate act as NO biological reservoirs that can be mobilized under physiological and ischemic conditions (Figure 2). This can be critical for vascular remodeling during tissue ischemia and for patients with peripheral vascular disease who have decreased nitrite levels[2,3]. In the next section, we review the therapeutic approaches for ischemic disease based on NO donors, including nitrite and nitrate.
NO Therapies for Ischemic Disease
NO has been implemented in clinical medicine for a long time, even before it was realized that NO was responsible for beneficial effects of certain drugs. A classical example is nitroglycerin which is an organic nitrate used to mitigate angina and myocardial infarction. In the modern era, NO donors have been extensively investigated for mechanisms of hypertension and ischemic disease. They have also been used to study the role of NO for vascular growth and remodeling. Sodium nitroprusside (SNP) is an endothelium-independent NO• donor, which releases NO by cell metabolism and stimulates angiogenesis after ischemic injury in the kidney and the brain [44,109]. S-nitrosothiols, as discussed earlier, are another class of NO donors with –SNO moieties, such as S-nitroso-glutathione (GSNO) and S-nitroso-N-acetylpenicillamine (SNAP) and have been used in experiments demonstrating increased angiogenesis through NO formation. However, S-nitrosothiols may also generate nitrosonium ion (NO+) and induce protein S-nitrosation. Another group of NO donors often used in experiments are diazeniumdiolates (NONOates). NONOates are compounds with a diolate group bound to a nucleophile adduct. They decompose spontaneously in solution and generate NO. The proangiogenic effect of NONOates has been examined in various in vitro and in vivo models[12,28,67,84,88]. NONOates may also increase arteriogenesis in ischemic tissue models[96]. Moreover, NONOates are able to rescue vascular defect due to lack of eNOS[29]. However, their clinical use for ischemic tissue disease is limited by intravenous administration and light sensitivity[69]. In summary, while these various NO donors have been useful for research studies, they have been of little clinical utility due to several limitations including problematic administration, light sensitivity, drug resistance, lack of tissue specificity, and substantial side effects.
As previously described above, nitrite can act as an endocrine factor to regulate vascular responses in response to ischemia[27,33,98]. Using the femoral artery ligation model in mice, our group reported that chronic treatment of sodium nitrite selectively restored limb blood flow and increased vascular density only in ischemic tissue[55]. Importantly, the endothelial cell proliferation effect of nitrite was limited to ischemic tissue where it was also found that nitrite concentrations increased. The number of collateral arterial branches and diameter was also increased by nitrite treatment, highlighting increased arteriogenesis [9]. It is worth noting that both nitrite induced angiogenesis and arteriogenesis are dependent on NO formation as the NO scavenger cPTIO blunted the beneficial effects of nitrite therapy. Importantly, delayed sodium nitrite treatment (3 days post ligation) also facilitated collateral remodeling in an NO dependent manner after femoral artery ligation illustrating the ability for this approach to resolve established chronic ischemia [9]. We have also reported that delayed sodium nitrite therapy protects against ischemia induced by femoral arterial ligation in aged diabetic Db/Db mice involving both angiogenesis and arteriogenesis[10]. This beneficial effect was not only NO dependent but also blunted by febuxostat (a specific xanthine oxidase inhibitor), indicating that nitrite reduction by xanthine oxidase is a key mechanism of NO generation during diabetic tissue ischemia. Using a genome-wide gene expression profile approach, we found a large spectrum of genes regulating vascular remodeling, immune responses, and tissue repair that were altered by sodium nitrite treatment only in ischemic tissue, further highlighting tissue specific and broad spectrum molecular effects of nitrite therapy for ischemic vascular remodeling [78]. Lastly, a multi-center, double blinded, randomized, placebo controlled clinical trial of long term sodium nitrite therapy in diabetic PAD patients revealed increased flow-mediated dilation of the brachial artery compared to placebo suggesting a possible clinical benefit of this therapy [70]. However, larger clinical trials for longer durations are needed to better understand the spectrum of clinical effects of nitrite therapy for ischemic vascular remodeling.
Similarly, dietary supplement of sodium nitrate is able to increase plasma nitrite level that may protect against tissue ischemic injury[15,82]. A long-term supplement of dietary nitrate was reported to increase perfusion in ischemic tissue after femoral artery ligation in mice, involving mobilization of endothelial progenitor cells from the bone marrow into ischemic tissue[45]. However, we reported that intraperitoneal administration of nitrate at equivalent successful dosages of nitrite therapy failed to increase ischemic tissue perfusion and angiogenesis using the femoral artery ligation model, reinforcing the concept that high concentrations of nitrate metabolized through the enterosalivary endocrine system are important for beneficial effects of nitrate therapy[55]. Thus, use of nitrite or dietary nitrate reveals differential therapeutic effects depending on dosage and route of administration.
H2S in Vascular Growth and Remodeling
H2S Regulation of Vascular Development
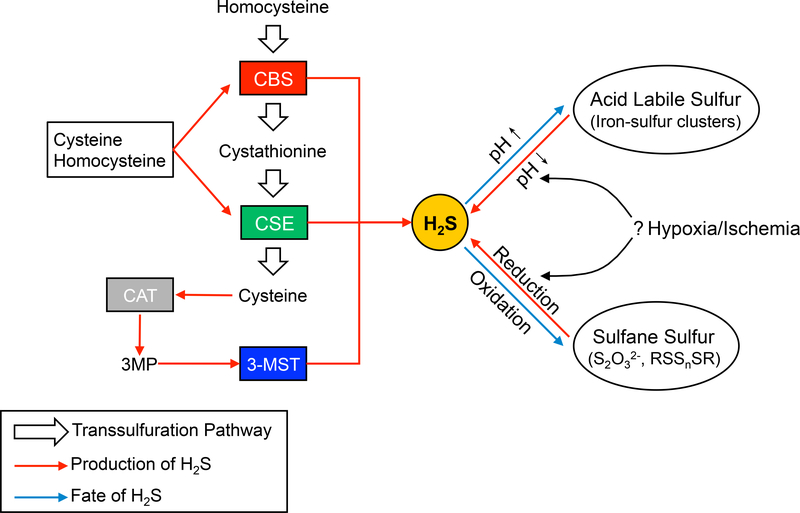
Before being recognized as gasotransmitter, H2S was simply regarded as a minor byproduct of the transsulfuration pathway (Figure 3). Two enzymes in this pathway, cystathionine β-synthase (CBS) and cystathionine γ-lyase (CSE) both regulate cysteine synthesis[108]. However, they may also use sulfur containing ammino acids, such as homocysteine and cysteine, to generate H2S. Alternatively, cysteine aminotransferase (CAT) can convert cysteine to 3-mercaptopyruvate (3-MP), where 3-mercaptopyruvate sulfurtransferase (3-MST) in the mitochondria uses 3-MP to produce H2S. Once produced, excessive H2S is quickly removed by mitochondrial oxidation. Although detailed steps of H2S oxidation in the mitochondria still requires additional investigation, several enzymes are known to contribute to this process including sulfide quinone reducatase (SQR), persulfide dioxidase (ETHE1), rhodanase, and sulfite oxidase. The oxidation of H2S may also form persulfides and polysulfides that are often collectively referred to as sulfane sulfur[62]. It is possible that sulfane sulfur represents a biological pool of H2S, as nitrite is to NO, which can be reduced back to H2S when needed[74]. Sulfane sulfur may also cause protein cysteine modification by transsulfuration (also referred to as sulfhydration) that alters protein function[108]. Additionally, H2S might also be mobilized from iron sulfur clusters, contained within a biochemical acid labile sulfur pool under acidic conditions as known to occur during tissue ischemia. However, it remains unclear how H2S equivalents are precisely generated and metabolized during ischemia, which requires further study (Figure 3).
Figure 3-. H2S synthesis and bioavailability.
H2S generation occurs through enzymes regulating the transsulfuration pathway. CSE and CBS are primary synthesis enzymes that produce H2S as a result of homocysteine or cystathionine metabolism. Cysteine aminotransferase (CAT) can also convert cysteine to 3-mercaptopyruvate that can be converted to H2S by the enzyme 3-MST located in the mitochondria. Once H2S is formed it is able to interact with numerous other molecules entering biological reservoir pools of acid labile sulfur or sulfane sulfur.
Both in vitro siRNA silencing and genetic deletion of CSE in vivo suppress VEGF-induce angiogenesis, indicating an important proangiogenic role of CSE[77]. Vasodilation by acetylcholine, VEGF and NO is also reduced in CSE deficient mice [26,105]. CBS genetic deficiency also leads to compromised vasodilation and angiogenesis[6,102]. However, lack of CBS results in severe homocysteinemia and oxidative stress. Therefore, compromised vasodilation and angiogenesis cannot be solely attributed to H2S deficiency alone in these mice.
Similar to NO, H2S may act as a physiological vasodilator. Genetic deletion of CSE has been reported to result in hypertension in mice[105]. The effect of CSE and H2S deficiency was reported to be due to loss of KATP channel activation in vascular smooth muscle cells[31,110]. Further studies found that H2S induced vasodilation also involves activating Cl−/HCO3− exchanger (intercellular activation)[58], inhibiting phosphodiesterase activity[16], and activating voltage-dependent potassium channels[23,87].
H2S may also serve as a proangiogenic factor in a non-classical manner. H2S has been reported to increase endothelial cell proliferation and migration using in vitro wound healing, transwell migration, matrigel tube formation, and matrigel vessel plug assays[20,77]. The angiogenic activity of H2S may be mediated by various signaling pathways, including PI3K/Akt, KATP, ERK1/2 and p38 MAPK[93]. Interestingly, although VEGF protein expression was not increased by exogenous H2S, VEGF signaling appears to be intimately involved with endogenous H2S production[77]. VEGF receptor 2 contains a disulfide bridge that can be reduced by H2S, which facilitates receptor signal activation[95]. These data highlight the multifaceted ways in which H2S can act to augment signaling responsible for vascular growth.
H2S Regulation of Ischemic Vascular Remodeling
Under ischemic conditions, H2S bioavailability is typically thought to be increased due decreased tissue oxygen tension. However, this may also occur through altering H2S producing enzymes. We have recently reported that after permanent ischemic insult due to femoral artery ligation, CSE activity and H2S levels increase in both plasma and ischemic muscle, suggesting systemic and local H2S production via CSE[54]. The increased CSE activity in the plasma may be attributed to increased excretion of extracellular CSE and recruitment of circulating monocytes[7,54]. However, CSE expression and activity was selectively increased in ischemic muscle and remained unchanged in non-ischemic tissue[54]. The increase in plasma H2S level was completely blunted in CSE knockout and largely decreased in ischemic CSE deficient tissue, highlighting CSE as a prominent source of H2S under ischemia[54].
H2S bioavailability is critical for vascular remodeling during ischemia. In the femoral artery ligation model, CSE knockout mice have decreased angiogenesis in the ischemic muscle compared to wild-type mice. Moreover, the number and diameter of collaterals are also reduced by CSE deficiency[54]. Importantly, CSE mice have less basal NO bioavailability and compromised ability to mobilize NO in tissues after ischemia[53,54]. Consistent with a defect in NO metabolism, nitrite therapy is able to rescue NO bioavailability and ischemic vascular remodeling in CSE gene deficient mice [54]. The decrease of NO availability in CSE knockout mice can be explained by eNOS deactivation and dysfunction. In the heart, CSE knockout mice show increased oxidative stress and decreased BH4/BH2 ratio, which can cause eNOS uncoupling and dysfunction[53]. eNOS activity is also decreased by reduced phosphorylation at S1177 and increased phosphorylation at T495 in CSE knockout mice, independent of Akt activation[53]. Interestingly, eNOS can be post-translationally modified by H2S, which promotes eNOS phosphorylation and dimer formation[4].
Other H2S producing enzymes may also contribute to its bioavailability during ischemia. CBS expression can be up regulated by HIF when cells are exposed to ischemia[94]. The roles of CSE and CBS may be tissue specific as kidney unilateral occlusion reduces H2S bioavailability due to decreased CBS expression, while CSE expression increases in an apparent attempt to compensate[50]. Moreover, H2S may also be mobilized through non-enzymatic pathways under ischemia (Figure 3). Mitochondria respiration is inhibited by ischemia/hypoxia, resulting in decreased H2S oxidation in mitochondria[92], and mitochondrial function is known to be selectively reduced under ischemia[101]. These intracellular conditions favor reduction of the sulfane sulfur pool, especially thiosulfate, contributing to H2S availability[74]. Additionally, ischemia decreases local tissue pH, which might possibly trigger the release of H2S from acid labile sulfur.
H2S may also affect HIF signaling. HIF-1α activity can be increased by H2S, which may also contribute to its proangiogenic effects[17,63]. We have reported that exogenous H2S augments HIF-1α expression and activity during hypoxia in primary endothelial cell cultures [8]. However, this effect may be to be limited to culture conditions or cell specificity. Contrary to our findings, it has been reported that H2S suppresses HIF-1 translation and increases HIF-1 degradation under hypoxic conditions in hepatoma, cervical carcinoma, neuroblastoma, and EA.hy926 hybridoma cell lines [49,103]. These differences aside, the physiological and pathological relevance of this complex regulation of HIF-1 by endogenous H2S remains poorly understood and highlights the importance of carefully considering model systems to study.
H2S as a Therapeutic Approach for Ischemic Disease
Na2S and NaHS are the most commonly used H2S donors reported in the literature. As sulfide salts, its important to understand that they release H2S without cell metabolism, therefore providing a simple approach to study exogenous H2S. In the mouse and rat femoral artery ligation models, intraperitoneal or intravenous administration of Na2S and NaHS were reported to restore blood flow and increase revascularization in the ischemic muscle[8,57,100]. Additionally, intraperitoneal injection of NaHS increases angiogenesis in a transient focal cerebral I/R injury in rats[47]. NaHS supplement in drinking water also protects left anterior descending artery (LAD) ligation induced myocardial infarction in mice[81]. However, it is difficult to envision how sulfide therapy given via routes in these studies are effective given the short half life of NaHS in solution (i.e. seconds to minutes). In summary, the use of sulfide salts as a therapeutic approach is limited. Firstly, Na2S and NaHS immediately release H2S in bolus amount and do not support a sustained plasma H2S level. Secondly, plasma H2S peaks very shortly after a single intravenous injection of Na2S in mice, and is quickly diminished by 30 minutes. Thirdly, the concentration of sulfide salts is difficult to control due to their volatile and auto-oxidation properties. Thus, several investigators have sought to identify or develop long-lasting H2S donors such as diallyl trisulfide (DATS), diallyl disulfide (DADS), GYY4137, and others.
Diallyl trisulfide (DATS), a natural extract from garlic, has been reported and used as a long-term H2S donor. Different from Na2S/NaHS, DATS is a polysulfide compound representing an oxidized form of sulfide. Due to its chemical nature, DATS may act in dual ways as a polysulfide or H2S donor. Early studies reported that DATS inhibited angiogenesis using in vitro endothelial cell proliferation, migration, matrigel tube formation assays, and the in vivo chick chorioallantoic membrane assay[56,104]. This inhibitory feature was attributed to decreased FAK and VEGF signaling, inactivated Akt and increased apoptosis. However, vascular remodeling under ischemic conditions is different. We have found that systematic administration of DATS through either intraperitoneal or intravenous routes is able to increase H2S and sulfane sulfur levels in serum and tissue, with the intravenous route being superior [54,79]. In the femoral artery ligation model, intravenous DATS increased arteriogenesis and angiogenesis selectively in ischemic tissue. DATS was also able to rescue a decrease in NO bioavailability and impaired vascular remodeling in CSE knockout mice[54]. Mechanistically, we found that DATS stimulated expression of inflammatory and proangiogenic factors, such as interleukin 16 (IL-16), basic fibrolast growth factor (bFGF) and VEGF, associated with increased HIF-1α activation[54]. Similarly, intraperitoneal injection of DATS increased myocardial angiogenesis in a model of transverse aortic constriction induced heart failure, where VEGF-A and bFGF expression were also increased[79]. Together, these data demonstrate the many potential benefits of using a long lasting H2S donor that requires further study to determine systemic effects on blood pressure and other vascular responses.
NO and H2S Interaction in Ischemia
As discussed above, our study and others have demonstrated an integral role of NO in H2S mediated arteriogenesis and angiogenesis, highlighting potential interactions between NO and H2S metabolism for ischemic vascular remodeling[8,54,79]. The regulation of NO and H2S on angiogenesis and vasodilation has been shown to be mutually dependent[26]. Whereas, H2S has been reported to activate eNOS via PI3K/Akt, while also inhibiting phosphodiesterase 5 (PDE5). Importantly, a deficiency of either NO or H2S bioavailability impairs angiogenesis and vasodilation under ischemia.
How then are NO and H2S related in regulating ischemic vascular remodeling? In mice, Na2S or DATS treatments increase NO bioavailability in both the systemic circulation and skeletal muscle, whereas CSE genetic deficiency decreased it [8,54]. Although Na2S therapy can increase NOS (eNOS and iNOS) expression in ischemic tissue [8], NOS may not necessarily be the main source of NO without proper oxygen, substrate, and cofator supply. Indeed, the absence of eNOS or inhibition of NOS activity does not affect Na2S increased NO bioavailability or the recovery of blood flow in ischemic tissue. Na2S therapy can also increase NO levels by enhancing xanthine oxidase mediated nitrite reduction back to NO[8]. On the other hand, the proangiogenic effect of DATS was blunted by either NOS inhibition or NO scavenging in wild type mice, while nitrite therapy was able to rescue angiogenic defects due to genetic deficiency of CSE, highlighting a complex reciprocal relationship between these two molecules [54].
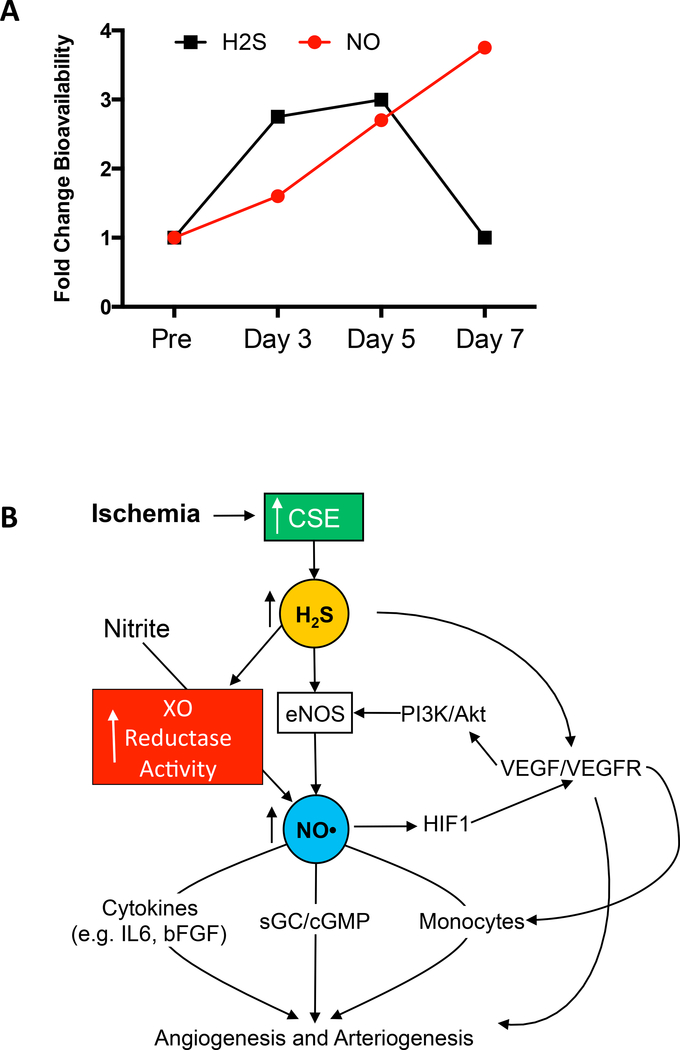
While the importance of H2S and NO is becoming clearer for ischemic vascular remodeling, figure 4A illustrates the temporal changes in these metabolites plasma bioavailability in response to femoral artery ligation gleaned from our studies. As a result of tissue ischemia, H2S levels quickly increase in response to elevated CSE expression and activity, followed by a later increase in NO bioavailability. The increase in NO can come from either increased NOS or nitrite reduction that initiates vascular remodeling to increase blood flow to ischemic tissue. Figure 4B summarizes key events and signaling pathways involved in H2S and NO regulation of ischemic vascular remodeling and growth. However, much more work is needed to understand cell specific roles for H2S and NO generation and metabolism during ischemia, identification of distinct molecular targets and how they are chemically modified, what gene expression changes are elicited by these molecules and signaling pathways, and eventually what clinical benefits they might provide for therapeutic vascular remodeling.
Figure 4-. Cooperation between H2S and NO in regulating ischemic vascular remodeling.
Panel A illustrates temporal changes in plasma H2S and NO levels after femoral artery ligation (adapted from references 8 & 53). Plasma H2S levels were found to quickly increase after femoral artery ligation preceding an increase in plasma NO levels, which subsequently return to normal levels after NO bioavailability is increased. Panel B shows molecular and biochemical pathways activated after tissue ischemia that results in activation of arteriogenesis and angiogenesis responses in the femoral artery ligation model.
Acknowledgements
This work was support by NIH Grant HL113303 to C.G.K. C.G.K. also has intellectual property regarding nitrite therapy and H2S measurement chemistries and methods, and is a founder and scientific advisor for Theravasc, Inc. and Innolyzer LLC. S.Y. is funded by a fellowship from the Malcolm Feist Cardiovascular Research Endowment, LSU Health Sciences Center-Shreveport.
Bibliography
- 1.Al-Shabrawey M, El-Remessy A, Gu X, Brooks SS, Hamed MS, Huang P, Caldwell RB. Normal vascular development in mice deficient in endothelial NO synthase: possible role of neuronal NO synthase. Mol Vis 9: 549–558, 2003. [PubMed] [Google Scholar]
- 2.Allen JD, Miller EM, Schwark E, Robbins JL, Duscha BD, Annex BH. Plasma nitrite response and arterial reactivity differentiate vascular health and performance. Nitric Oxide-Biol Ch 20: 231–237, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Allen JD, Stabler T, Kenjale A, Ham KL, Robbins JL, Duscha BD, Dobrosielski DA, Annex BH. Plasma nitrite flux predicts exercise performance in peripheral arterial disease after 3months of exercise training. Free Radic Biol Med 49: 1138–1144, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Altaany Z, Ju Y, Yang G, Wang R. The coordination of S-sulfhydration, S-nitrosylation, and phosphorylation of endothelial nitric oxide synthase by hydrogen sulfide. Sci Signal 7: ra87, 2014. [DOI] [PubMed] [Google Scholar]
- 5.Babaei S, Teichert-Kuliszewska K, Monge JC, Mohamed F, Bendeck MP, Stewart DJ. Role of nitric oxide in the angiogenic response in vitro to basic fibroblast growth factor. Circ Res 82: 1007–1015, 1998. [DOI] [PubMed] [Google Scholar]
- 6.Beard RS Jr., Bearden SE. Vascular complications of cystathionine beta-synthase deficiency: future directions for homocysteine-to-hydrogen sulfide research. Am J Physiol Heart Circ Physiol 300: H13–26, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bearden SE, Beard RS Jr., Pfau JC. Extracellular transsulfuration generates hydrogen sulfide from homocysteine and protects endothelium from redox stress. Am J Physiol Heart Circ Physiol 299: H1568–1576, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bir SC, Kolluru GK, McCarthy P, Shen X, Pardue S, Pattillo CB, Kevil CG. Hydrogen sulfide stimulates ischemic vascular remodeling through nitric oxide synthase and nitrite reduction activity regulating hypoxia-inducible factor-1alpha and vascular endothelial growth factor-dependent angiogenesis. J Am Heart Assoc 1: e004093, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bir SC, Pattillo CB, Pardue S, Kolluru GK, Docherty J, Goyette D, Dvorsky P, Kevil CG. Nitrite anion stimulates ischemic arteriogenesis involving NO metabolism. Am J Physiol Heart Circ Physiol 303: H178–188, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bir SC, Pattillo CB, Pardue S, Kolluru GK, Shen X, Giordano T, Kevil CG. Nitrite anion therapy protects against chronic ischemic tissue injury in db/db diabetic mice in a NO/VEGF-dependent manner. Diabetes 63: 270–281, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bir SC, Xiong Y, Kevil CG, Luo J. Emerging role of PKA/eNOS pathway in therapeutic angiogenesis for ischaemic tissue diseases. Cardiovasc Res 95: 7–18, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Blecher K, Martinez LR, Tuckman-Vernon C, Nacharaju P, Schairer D, Chouake J, Friedman JM, Alfieri A, Guha C, Nosanchuk JD, Friedman AJ. Nitric oxide-releasing nanoparticles accelerate wound healing in NOD-SCID mice. Nanomedicine 8: 1364–1371, 2012. [DOI] [PubMed] [Google Scholar]
- 13.Bosch-Marce M, Okuyama H, Wesley JB, Sarkar K, Kimura H, Liu YV, Zhang H, Strazza M, Rey S, Savino L, Zhou YF, McDonald KR, Na Y, Vandiver S, Rabi A, Shaked Y, Kerbel R, Lavallee T, Semenza GL. Effects of aging and hypoxia-inducible factor-1 activity on angiogenic cell mobilization and recovery of perfusion after limb ischemia. Circ Res 101: 1310–1318, 2007. [DOI] [PubMed] [Google Scholar]
- 14.Brunori M, Giuffre A, Forte E, Mastronicola D, Barone MC, Sarti P. Control of cytochrome c oxidase activity by nitric oxide. Biochim Biophys Acta 1655: 365–371, 2004. [DOI] [PubMed] [Google Scholar]
- 15.Bryan NS, Calvert JW, Elrod JW, Gundewar S, Ji SY, Lefer DJ. Dietary nitrite supplementation protects against myocardial ischemia-reperfusion injury. Proc Natl Acad Sci U S A 104: 19144–19149, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bucci M, Papapetropoulos A, Vellecco V, Zhou Z, Pyriochou A, Roussos C, Roviezzo F, Brancaleone V, Cirino G. Hydrogen sulfide is an endogenous inhibitor of phosphodiesterase activity. Arterioscler Thromb Vasc Biol 30: 1998–2004, 2010. [DOI] [PubMed] [Google Scholar]
- 17.Budde MW, Roth MB. Hydrogen sulfide increases hypoxia-inducible factor-1 activity independently of von Hippel-Lindau tumor suppressor-1 in C. elegans. Mol Biol Cell 21: 212–217, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cai W, Schaper W. Mechanisms of arteriogenesis. Acta Biochim Biophys Sin (Shanghai) 40: 681–692, 2008. [PubMed] [Google Scholar]
- 19.Cai WJ, Kocsis E, Luo X, Schaper W, Schaper J. Expression of endothelial nitric oxide synthase in the vascular wall during arteriogenesis. Mol Cell Biochem 264: 193–200, 2004. [DOI] [PubMed] [Google Scholar]
- 20.Cai WJ, Wang MJ, Moore PK, Jin HM, Yao T, Zhu YC. The novel proangiogenic effect of hydrogen sulfide is dependent on Akt phosphorylation. Cardiovasc Res 76: 29–40, 2007. [DOI] [PubMed] [Google Scholar]
- 21.Cantu-Medellin N, Kelley EE. Xanthine oxidoreductase-catalyzed reduction of nitrite to nitric oxide: insights regarding where, when and how. Nitric Oxide-Biol Ch 34: 19–26, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Carmeliet P Mechanisms of angiogenesis and arteriogenesis. Nat Med 6: 389–395, 2000. [DOI] [PubMed] [Google Scholar]
- 23.Cheang WS, Wong WT, Shen B, Lau CW, Tian XY, Tsang SY, Yao X, Chen ZY, Huang Y. 4-aminopyridine-sensitive K+ channels contributes to NaHS-induced membrane hyperpolarization and relaxation in the rat coronary artery. Vascul Pharmacol 53: 94–98, 2010. [DOI] [PubMed] [Google Scholar]
- 24.Chowdhury R, Flashman E, Mecinovic J, Kramer HB, Kessler BM, Frapart YM, Boucher JL, Clifton IJ, McDonough MA, Schofield CJ. Studies on the reaction of nitric oxide with the hypoxia-inducible factor prolyl hydroxylase domain 2 (EGLN1). J Mol Biol 410: 268–279, 2011. [DOI] [PubMed] [Google Scholar]
- 25.Clayton JA, Chalothorn D, Faber JE. Vascular endothelial growth factor-A specifies formation of native collaterals and regulates collateral growth in ischemia. Circ Res 103: 1027–1036, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Coletta C, Papapetropoulos A, Erdelyi K, Olah G, Modis K, Panopoulos P, Asimakopoulou A, Gero D, Sharina I, Martin E, Szabo C. Hydrogen sulfide and nitric oxide are mutually dependent in the regulation of angiogenesis and endothelium-dependent vasorelaxation. Proc Natl Acad Sci U S A 109: 9161–9166, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Corti P, Gladwin MT. Is nitrite the circulating endocrine effector of remote ischemic preconditioning? Circ Res 114: 1554–1557, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cui X, Chen J, Zacharek A, Roberts C, Savant-Bhonsale S, Chopp M. Treatment of stroke with (Z)-1-[N-(2-aminoethyl)-N-(2-ammonioethyl) amino] diazen-1-ium-1, 2-diolate and bone marrow stromal cells upregulates angiopoietin-1/Tie2 and enhances neovascularization. Neuroscience 156: 155–164, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cui X, Chopp M, Zacharek A, Zhang C, Roberts C, Chen J. Role of endothelial nitric oxide synthetase in arteriogenesis after stroke in mice. Neuroscience 159: 744–750, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Dai X, Faber JE. Endothelial nitric oxide synthase deficiency causes collateral vessel rarefaction and impairs activation of a cell cycle gene network during arteriogenesis. Circ Res 106: 1870–1881, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Dombkowski RA, Russell MJ, Schulman AA, Doellman MM, Olson KR. Vertebrate phylogeny of hydrogen sulfide vasoactivity. Am J Physiol Regul Integr Comp Physiol 288: R243–252, 2005. [DOI] [PubMed] [Google Scholar]
- 32.Dulak J, Jozkowicz A, Dembinska-Kiec A, Guevara I, Zdzienicka A, Zmudzinska-Grochot D, Florek I, Wojtowicz A, Szuba A, Cooke JP. Nitric oxide induces the synthesis of vascular endothelial growth factor by rat vascular smooth muscle cells. Arterioscler Thromb Vasc Biol 20: 659–666, 2000. [DOI] [PubMed] [Google Scholar]
- 33.Elrod JW, Calvert JW, Gundewar S, Bryan NS, Lefer DJ. Nitric oxide promotes distant organ protection: evidence for an endocrine role of nitric oxide. Proc Natl Acad Sci U S A 105: 11430–11435, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Fleming I, Busse R. Molecular mechanisms involved in the regulation of the endothelial nitric oxide synthase. Am J Physiol Regul Integr Comp Physiol 284: R1–12, 2003. [DOI] [PubMed] [Google Scholar]
- 35.Forstermann U Nitric oxide and oxidative stress in vascular disease. Pflugers Arch 459: 923–939, 2010. [DOI] [PubMed] [Google Scholar]
- 36.Forstermann U, Munzel T. Endothelial nitric oxide synthase in vascular disease: from marvel to menace. Circulation 113: 1708–1714, 2006. [DOI] [PubMed] [Google Scholar]
- 37.Forstermann U, Sessa WC. Nitric oxide synthases: regulation and function. Eur Heart J 33: 829–837, 837a-837d, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Fukumura D, Gohongi T, Kadambi A, Izumi Y, Ang J, Yun CO, Buerk DG, Huang PL, Jain RK. Predominant role of endothelial nitric oxide synthase in vascular endothelial growth factor-induced angiogenesis and vascular permeability. Proc Natl Acad Sci U S A 98: 2604–2609, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Fukumura D, Kashiwagi S, Jain RK. The role of nitric oxide in tumour progression. Nat Rev Cancer 6: 521–534, 2006. [DOI] [PubMed] [Google Scholar]
- 40.Giuffre A, Forte E, Brunori M, Sarti P. Nitric oxide, cytochrome c oxidase and myoglobin: competition and reaction pathways. Febs Lett 579: 2528–2532, 2005. [DOI] [PubMed] [Google Scholar]
- 41.Gow AJ, Stamler JS. Reactions between nitric oxide and haemoglobin under physiological conditions. Nature 391: 169–173, 1998. [DOI] [PubMed] [Google Scholar]
- 42.Gregg AR, Schauer A, Shi O, Liu Z, Lee CG, O’Brien WE. Limb reduction defects in endothelial nitric oxide synthase-deficient mice. Am J Physiol 275: H2319–2324, 1998. [DOI] [PubMed] [Google Scholar]
- 43.Han RN, Stewart DJ. Defective lung vascular development in endothelial nitric oxide synthase-deficient mice. Trends Cardiovasc Med 16: 29–34, 2006. [DOI] [PubMed] [Google Scholar]
- 44.Heineke J, Auger-Messier M, Xu J, Oka T, Sargent MA, York A, Klevitsky R, Vaikunth S, Duncan SA, Aronow BJ, Robbins J, Crombleholme TM, Molkentin JD. Cardiomyocyte GATA4 functions as a stress-responsive regulator of angiogenesis in the murine heart. J Clin Invest 117: 3198–3210, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hendgen-Cotta UB, Luedike P, Totzeck M, Kropp M, Schicho A, Stock P, Rammos C, Niessen M, Heiss C, Lundberg JO, Weitzberg E, Kelm M, Rassaf T. Dietary nitrate supplementation improves revascularization in chronic ischemia. Circulation 126: 1983–1992, 2012. [DOI] [PubMed] [Google Scholar]
- 46.Ignarro LJ, Buga GM, Wood KS, Byrns RE, Chaudhuri G. Endothelium-derived relaxing factor produced and released from artery and vein is nitric oxide. Proceedings of the National Academy of Sciences 84: 9265–9269, 1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Jang H, Oh MY, Kim YJ, Choi IY, Yang HS, Ryu WS, Lee SH, Yoon BW. Hydrogen sulfide treatment induces angiogenesis after cerebral ischemia. J Neurosci Res 92: 1520–1528, 2014. [DOI] [PubMed] [Google Scholar]
- 48.Jeffrey Man HS, Tsui AK, Marsden PA. Nitric oxide and hypoxia signaling. Vitam Horm 96: 161–192, 2014. [DOI] [PubMed] [Google Scholar]
- 49.Kai S, Tanaka T, Daijo H, Harada H, Kishimoto S, Suzuki K, Takabuchi S, Takenaga K, Fukuda K, Hirota K. Hydrogen sulfide inhibits hypoxia- but not anoxia-induced hypoxia-inducible factor 1 activation in a von hippel-lindau- and mitochondria-dependent manner. Antioxid Redox Signal 16: 203–216, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kasinath BS. Hydrogen sulfide to the rescue in obstructive kidney injury. Kidney Int 85: 1255–1258, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kelly BD, Hackett SF, Hirota K, Oshima Y, Cai Z, Berg-Dixon S, Rowan A, Yan Z, Campochiaro PA, Semenza GL. Cell type-specific regulation of angiogenic growth factor gene expression and induction of angiogenesis in nonischemic tissue by a constitutively active form of hypoxia-inducible factor 1. Circ Res 93: 1074–1081, 2003. [DOI] [PubMed] [Google Scholar]
- 52.Kim-Shapiro DB, Schechter AN, Gladwin MT. Unraveling the reactions of nitric oxide, nitrite, and hemoglobin in physiology and therapeutics. Arterioscler Thromb Vasc Biol 26: 697–705, 2006. [DOI] [PubMed] [Google Scholar]
- 53.King AL, Polhemus DJ, Bhushan S, Otsuka H, Kondo K, Nicholson CK, Bradley JM, Islam KN, Calvert JW, Tao YX, Dugas TR, Kelley EE, Elrod JW, Huang PL, Wang R, Lefer DJ. Hydrogen sulfide cytoprotective signaling is endothelial nitric oxide synthase-nitric oxide dependent. Proc Natl Acad Sci U S A 111: 3182–3187, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kolluru GK, Bir SC, Yuan S, Shen X, Pardue S, Wang R, Kevil CG. Cystathionine gamma-lyase regulates arteriogenesis through NO dependent monocyte recruitment. Cardiovasc Res, 107: 590–600, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kumar D, Branch BG, Pattillo CB, Hood J, Thoma S, Simpson S, Illum S, Arora N, Chidlow JH Jr., Langston W, Teng X, Lefer DJ, Patel RP, Kevil CG. Chronic sodium nitrite therapy augments ischemia-induced angiogenesis and arteriogenesis. Proc Natl Acad Sci U S A 105: 7540–7545, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Lai KC, Hsu SC, Yang JS, Yu CC, Lein JC, Chung JG. Diallyl trisulfide inhibits migration, invasion and angiogenesis of human colon cancer HT-29 cells and umbilical vein endothelial cells, and suppresses murine xenograft tumour growth. J Cell Mol Med 19: 474–484, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Langston JW, Toombs CF. Defining the minimally effective dose and schedule for parenteral hydrogen sulfide: long-term benefits in a rat model of hindlimb ischemia. Med Gas Res 5: 5, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Lee SW, Cheng Y, Moore PK, Bian JS. Hydrogen sulphide regulates intracellular pH in vascular smooth muscle cells. Biochem Biophys Res Commun 358: 1142–1147, 2007. [DOI] [PubMed] [Google Scholar]
- 59.Lee TC, Zhao YD, Courtman DW, Stewart DJ. Abnormal aortic valve development in mice lacking endothelial nitric oxide synthase. Circulation 101: 2345–2348, 2000. [DOI] [PubMed] [Google Scholar]
- 60.Li F, Sonveaux P, Rabbani ZN, Liu S, Yan B, Huang Q, Vujaskovic Z, Dewhirst MW, Li CY. Regulation of HIF-1alpha stability through S-nitrosylation. Mol Cell 26: 63–74, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Li JM, Shah AM. Endothelial cell superoxide generation: regulation and relevance for cardiovascular pathophysiology. Am J Physiol Regul Integr Comp Physiol 287: R1014–1030, 2004. [DOI] [PubMed] [Google Scholar]
- 62.Libiad M, Yadav PK, Vitvitsky V, Martinov M, Banerjee R. Organization of the human mitochondrial hydrogen sulfide oxidation pathway. J Biol Chem 289: 30901–30910, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Liu XH, Pan LL, Zhuo Y, Gong QH, Rose P, Zhu YZ. Hypoxia-Inducible Factor-1 alpha Is Involved in the Pro-angiogenic Effect of Hydrogen Sulfide under Hypoxic Stress. Biol Pharm Bull 33: 1550–1554, 2010. [DOI] [PubMed] [Google Scholar]
- 64.Loscalzo J The identification of nitric oxide as endothelium-derived relaxing factor. Circ Res 113: 100–103, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Lundberg JO, Weitzberg E, Gladwin MT. The nitrate-nitrite-nitric oxide pathway in physiology and therapeutics. Nat Rev Drug Discov 7: 156–167, 2008. [DOI] [PubMed] [Google Scholar]
- 66.Luque Contreras D, Vargas Robles H, Romo E, Rios A, Escalante B. The role of nitric oxide in the post-ischemic revascularization process. Pharmacol Ther 112: 553–563, 2006. [DOI] [PubMed] [Google Scholar]
- 67.Majumder S, Sinha S, Siamwala JH, Muley A, Reddy Seerapu H, Kolluru GK, Veeriah V, Nagarajan S, Sridhara SR, Priya MK, Kuppusamy M, Srinivasan S, Konikkat S, Soundararajan G, Venkataraman S, Saran U, Chatterjee S. A comparative study of NONOate based NO donors: spermine NONOate is the best suited NO donor for angiogenesis. Nitric Oxide-Biol Ch 36: 76–86, 2014. [DOI] [PubMed] [Google Scholar]
- 68.McCabe TJ, Fulton D, Roman LJ, Sessa WC. Enhanced electron flux and reduced calmodulin dissociation may explain “calcium-independent” eNOS activation by phosphorylation. J Biol Chem 275: 6123–6128, 2000. [DOI] [PubMed] [Google Scholar]
- 69.Miller MR, Megson IL. Recent developments in nitric oxide donor drugs. Br J Pharmacol 151: 305–321, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Mohler ER 3rd, Hiatt WR, Gornik HL, Kevil CG, Quyyumi A, Haynes WG, Annex BH. Sodium nitrite in patients with peripheral artery disease and diabetes mellitus: safety, walking distance and endothelial function. Vasc Med 19: 9–17, 2014. [DOI] [PubMed] [Google Scholar]
- 71.Moshage H, Kok B, Huizenga JR, Jansen PL. Nitrite and nitrate determinations in plasma: a critical evaluation. Clin Chem 41: 892–896, 1995. [PubMed] [Google Scholar]
- 72.Mustafa AK, Gadalla MM, Sen N, Kim S, Mu W, Gazi SK, Barrow RK, Yang G, Wang R, Snyder SH. H2S signals through protein S-sulfhydration. Sci Signal 2: ra72, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Mustafa AK, Sikka G, Gazi SK, Steppan J, Jung SM, Bhunia AK, Barodka VM, Gazi FK, Barrow RK, Wang R, Amzel LM, Berkowitz DE, Snyder SH. Hydrogen sulfide as endothelium-derived hyperpolarizing factor sulfhydrates potassium channels. Circ Res 109: 1259–1268, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Olson KR, Deleon ER, Gao Y, Hurley K, Sadauskas V, Batz C, Stoy GF. Thiosulfate: a readily accessible source of hydrogen sulfide in oxygen sensing. Am J Physiol Regul Integr Comp Physiol 305: R592–603, 2013. [DOI] [PubMed] [Google Scholar]
- 75.Palmer LA, Doctor A, Chhabra P, Sheram ML, Laubach VE, Karlinsey MZ, Forbes MS, Macdonald T, Gaston B. S-nitrosothiols signal hypoxia-mimetic vascular pathology. J Clin Invest 117: 2592–2601, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Papapetropoulos A, Garcia-Cardena G, Madri JA, Sessa WC. Nitric oxide production contributes to the angiogenic properties of vascular endothelial growth factor in human endothelial cells. J Clin Invest 100: 3131–3139, 1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Papapetropoulos A, Pyriochou A, Altaany Z, Yang G, Marazioti A, Zhou Z, Jeschke MG, Branski LK, Herndon DN, Wang R, Szabo C. Hydrogen sulfide is an endogenous stimulator of angiogenesis. Proc Natl Acad Sci U S A 106: 21972–21977, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Pattillo CB, Fang K, Pardue S, Kevil CG. Genome expression profiling and network analysis of nitrite therapy during chronic ischemia: possible mechanisms and interesting molecules. Nitric Oxide-Biol Ch 22: 168–179, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Polhemus DJ, Kondo K, Bhushan S, Bir SC, Kevil CG, Murohara T, Lefer DJ, Calvert JW. Hydrogen sulfide attenuates cardiac dysfunction after heart failure via induction of angiogenesis. Circ Heart Fail 6: 1077–1086, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Qiao W, Niu LY, Liu Z, Qiao T, Liu CJ. Endothelial Nitric Oxide Synthase as A Marker for Human Endothelial Progenitor Cells. Tohoku J Exp Med 221: 19–27, 2010. [DOI] [PubMed] [Google Scholar]
- 81.Qipshidze N, Metreveli N, Mishra PK, Lominadze D, Tyagi SC. Hydrogen sulfide mitigates cardiac remodeling during myocardial infarction via improvement of angiogenesis. Int J Biol Sci 8: 430–441, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Raat NJ, Noguchi AC, Liu VB, Raghavachari N, Liu D, Xu X, Shiva S, Munson PJ, Gladwin MT. Dietary nitrate and nitrite modulate blood and organ nitrite and the cellular ischemic stress response. Free Radic Biol Med 47: 510–517, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Rameau GA, Chiu LY, Ziff EB. Bidirectional regulation of neuronal nitric-oxide synthase phosphorylation at serine 847 by the N-methyl-D-aspartate receptor. J Biol Chem 279: 14307–14314, 2004. [DOI] [PubMed] [Google Scholar]
- 84.Ridnour LA, Isenberg JS, Espey MG, Thomas DD, Roberts DD, Wink DA. Nitric oxide regulates angiogenesis through a functional switch involving thrombospondin-1. Proc Natl Acad Sci U S A 102: 13147–13152, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Sanchez-Blazquez P, Rodriguez-Munoz M, Garzon J. Mu-opioid receptors transiently activate the Akt-nNOS pathway to produce sustained potentiation of PKC-mediated NMDAR-CaMKII signaling. PLoS One 5: e11278, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Sanchez-Ruiloba L, Aicart-Ramos C, Garcia-Guerra L, Pose-Utrilla J, Rodriguez-Crespo I, Iglesias T. Protein kinase D interacts with neuronal nitric oxide synthase and phosphorylates the activatory residue serine 1412. PLoS One 9: e95191, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Schleifenbaum J, Kohn C, Voblova N, Dubrovska G, Zavarirskaya O, Gloe T, Crean CS, Luft FC, Huang Y, Schubert R, Gollasch M. Systemic peripheral artery relaxation by KCNQ channel openers and hydrogen sulfide. J Hypertens 28: 1875–1882, 2010. [DOI] [PubMed] [Google Scholar]
- 88.Shabani M, Pulfer SK, Bulgrin JP, Smith DJ. Enhancement of wound repair with a topically applied nitric oxide-releasing polymer. Wound Repair Regen 4: 353–362, 1996. [DOI] [PubMed] [Google Scholar]
- 89.Shweiki D, Itin A, Soffer D, Keshet E. Vascular endothelial growth factor induced by hypoxia may mediate hypoxia-initiated angiogenesis. Nature 359: 843–845, 1992. [DOI] [PubMed] [Google Scholar]
- 90.Silvestre JS, Smadja DM, Levy BI. Postischemic revascularization: from cellular and molecular mechanisms to clinical applications. Physiol Rev 93: 1743–1802, 2013. [DOI] [PubMed] [Google Scholar]
- 91.Simons M, Alitalo K, Annex BH, Augustin HG, Beam C, Berk BC, Byzova T, Carmeliet P, Chilian W, Cooke JP, Davis GE, Eichmann A, Iruela-Arispe ML, Keshet E, Sinusas AJ, Ruhrberg C, Woo YJ, Dimmeler S, American Heart Association Council on Basic Cardiovascular S, Council on Cardiovascular S, Anesthesia. State-of-the-Art Methods for Evaluation of Angiogenesis and Tissue Vascularization: A Scientific Statement From the American Heart Association. Circ Res 116: e99–132, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Stein A, Bailey SM. Redox Biology of Hydrogen Sulfide: Implications for Physiology, Pathophysiology, and Pharmacology. Redox Biol 1: 32–39, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Szabo C, Papapetropoulos A. Hydrogen sulphide and angiogenesis: mechanisms and applications. Br J Pharmacol 164: 853–865, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Takano N, Peng YJ, Kumar GK, Luo W, Hu H, Shimoda LA, Suematsu M, Prabhakar NR, Semenza GL. Hypoxia-inducible factors regulate human and rat cystathionine beta-synthase gene expression. Biochem J 458: 203–211, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Tao BB, Liu SY, Zhang CC, Fu W, Cai WJ, Wang Y, Shen Q, Wang MJ, Chen Y, Zhang LJ, Zhu YZ, Zhu YC. VEGFR2 functions as an H2S-targeting receptor protein kinase with its novel Cys1045-Cys1024 disulfide bond serving as a specific molecular switch for hydrogen sulfide actions in vascular endothelial cells. Antioxid Redox Signal 19: 448–464, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Troidl K, Tribulova S, Cai WJ, Ruding I, Apfelbeck H, Schierling W, Troidl C, Schmitz-Rixen T, Schaper W. Effects of endogenous nitric oxide and of DETA NONOate in arteriogenesis. J Cardiovasc Pharmacol 55: 153–160, 2010. [DOI] [PubMed] [Google Scholar]
- 97.Urbich C, Dimmeler S. Endothelial progenitor cells: characterization and role in vascular biology. Circ Res 95: 343–353, 2004. [DOI] [PubMed] [Google Scholar]
- 98.Venkatesh PK, Pattillo CB, Branch B, Hood J, Thoma S, Illum S, Pardue S, Teng X, Patel RP, Kevil CG. Dipyridamole enhances ischaemia-induced arteriogenesis through an endocrine nitrite/nitric oxide-dependent pathway. Cardiovasc Res 85: 661–670, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Vikas K, Syed H, Vanessa P, Jon OL, Eddie W, Amrita A. Physiological role for nitrate-reducing oral bacteria in blood pressure control. Free Radical Biology and Medicine 55: 93–100, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Wang MJ, Cai WJ, Li N, Ding YJ, Chen Y, Zhu YC. The hydrogen sulfide donor NaHS promotes angiogenesis in a rat model of hind limb ischemia. Antioxid Redox Signal 12: 1065–1077, 2010. [DOI] [PubMed] [Google Scholar]
- 101.Waypa GB, Marks JD, Guzy R, Mungai PT, Schriewer J, Dokic D, Schumacker PT. Hypoxia triggers subcellular compartmental redox signaling in vascular smooth muscle cells. Circ Res 106: 526–535, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Weiss N, Heydrick S, Zhang YY, Bierl C, Cap A, Loscalzo J. Cellular redox state and endothelial dysfunction in mildly hyperhomocysteinemic cystathionine beta-synthase-deficient mice. Arterioscler Thromb Vasc Biol 22: 34–41, 2002. [DOI] [PubMed] [Google Scholar]
- 103.Wu B, Teng H, Yang G, Wu L, Wang R. Hydrogen sulfide inhibits the translational expression of hypoxia-inducible factor-1alpha. Br J Pharmacol 167: 1492–1505, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Xiao D, Li M, Herman-Antosiewicz A, Antosiewicz J, Xiao H, Lew KL, Zeng Y, Marynowski SW, Singh SV. Diallyl trisulfide inhibits angiogenic features of human umbilical vein endothelial cells by causing Akt inactivation and down-regulation of VEGF and VEGF-R2. Nutr Cancer 55: 94–107, 2006. [DOI] [PubMed] [Google Scholar]
- 105.Yang G, Wu L, Jiang B, Yang W, Qi J, Cao K, Meng Q, Mustafa AK, Mu W, Zhang S, Snyder SH, Wang R. H2S as a physiologic vasorelaxant: hypertension in mice with deletion of cystathionine gamma-lyase. Science 322: 587–590, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Yellowley C CXCL12/CXCR4 signaling and other recruitment and homing pathways in fracture repair. Bonekey Rep 2: 300, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Yu J, deMuinck ED, Zhuang Z, Drinane M, Kauser K, Rubanyi GM, Qian HS, Murata T, Escalante B, Sessa WC. Endothelial nitric oxide synthase is critical for ischemic remodeling, mural cell recruitment, and blood flow reserve. Proc Natl Acad Sci U S A 102: 10999–11004, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Yuan S, Patel RP, Kevil CG. Working with nitric oxide and hydrogen sulfide in biological systems. Am J Physiol Lung Cell Mol Physiol 308: L403–415, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Zhang F, Iadecola C. Nitroprusside improves blood flow and reduces brain damage after focal ischemia. Neuroreport 4: 559–562, 1993. [DOI] [PubMed] [Google Scholar]
- 110.Zhao W, Zhang J, Lu Y, Wang R. The vasorelaxant effect of H(2)S as a novel endogenous gaseous K(ATP) channel opener. EMBO J 20: 6008–6016, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Ziche M, Parenti A, Ledda F, Dell’Era P, Granger HJ, Maggi CA, Presta M. Nitric oxide promotes proliferation and plasminogen activator production by coronary venular endothelium through endogenous bFGF. Circ Res 80: 845–852, 1997. [DOI] [PubMed] [Google Scholar]
- 112.Zou MH, Shi C, Cohen RA. Oxidation of the zinc-thiolate complex and uncoupling of endothelial nitric oxide synthase by peroxynitrite. J Clin Invest 109: 817–826, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]