Abstract
Objective
To systematically quantify the prevalence, severity, and nature of preventable patient harm across a range of medical settings globally.
Design
Systematic review and meta-analysis.
Data sources
Medline, PubMed, PsycINFO, Cinahl and Embase, WHOLIS, Google Scholar, and SIGLE from January 2000 to January 2019. The reference lists of eligible studies and other relevant systematic reviews were also searched.
Review methods
Observational studies reporting preventable patient harm in medical care. The core outcomes were the prevalence, severity, and types of preventable patient harm reported as percentages and their 95% confidence intervals. Data extraction and critical appraisal were undertaken by two reviewers working independently. Random effects meta-analysis was employed followed by univariable and multivariable meta regression. Heterogeneity was quantified by using the I2 statistic, and publication bias was evaluated.
Results
Of the 7313 records identified, 70 studies involving 337 025 patients were included in the meta-analysis. The pooled prevalence for preventable patient harm was 6% (95% confidence interval 5% to 7%). A pooled proportion of 12% (9% to 15%) of preventable patient harm was severe or led to death. Incidents related to drugs (25%, 95% confidence interval 16% to 34%) and other treatments (24%, 21% to 30%) accounted for the largest proportion of preventable patient harm. Compared with general hospitals (where most evidence originated), preventable patient harm was more prevalent in advanced specialties (intensive care or surgery; regression coefficient b=0.07, 95% confidence interval 0.04 to 0.10).
Conclusions
Around one in 20 patients are exposed to preventable harm in medical care. Although a focus on preventable patient harm has been encouraged by the international patient safety policy agenda, there are limited quality improvement practices specifically targeting incidents of preventable patient harm rather than overall patient harm (preventable and non-preventable). Developing and implementing evidence-based mitigation strategies specifically targeting preventable patient harm could lead to major service quality improvements in medical care which could also be more cost effective.
Introduction
Patient harm during healthcare is a leading cause of morbidity and mortality internationally.1 2 The World Health Organization defines patient harm as “an incident that results in harm to a patient such as impairment of structure or function of the body and/or any deleterious effect arising there from or associated with plans or actions taken during the provision of healthcare, rather than an underlying disease or injury, and may be physical, social or psychological (eg, disease, injury, suffering, disability and death).”3 The health burden and patient experiencing healthcare-related patient harm has been reported to be comparable to chronic diseases such as multiple sclerosis and cervical cancer in developed countries, and tuberculosis and malaria in developing countries.4 5 Harmful patient incidents are also a major financial burden for healthcare systems across the globe. It is estimated that 10-15% of healthcare expenditure is consumed by the direct sequelae of healthcare-related patient harm.6 7
Early detection and prevention of patient harm in healthcare is an international policy priority.8 In principle, zero harm would be the ideal goal. However, this goal is not feasible because some harms cannot be avoided in clinical practice. For example, some adverse drug reactions which occur in the absence of any error in the prescription process and without the possibility of detection are less likely to be preventable. In recent years, the recognition that a proportion of patient harm is not preventable has increased attention to the notion of preventable patient harm.9 Most studies classify patient harm as preventable if it occurs as a result of an identifiable modifiable cause, and its future recurrence can be avoided by reasonable adaptation to a process, or adherence to guidelines, although universal consensus has not been established.10 Key sources of preventable patient harm could include the actions of healthcare professionals (errors of omission or commission), healthcare system failures, or involve a combination of errors made by individuals, system failures, and patient characteristics.11 12 13 14 Strengthening the focus on preventable patient harm has the potential to lead to greater tangible clinical benefits and improved translation of patient safety research findings into clinical practice. Patient safety improvement strategies underpinned by better understanding of the nature of preventable patient harm have greater prospects of efficiency (because they are more specific) and implementation (because clinicians can readily recognise their value).10
There are several systematic reviews on overall patient harm across different medical settings, but none of these have focused on preventable patient harm.1 15 16 17 We undertook a systematic review and meta-analysis to estimate the prevalence of preventable patient harm across medical settings including hospitals, various specialties, and in primary care. We also examined the severity and most commonly occurring types of preventable patient harm.
Methods
This systematic review was conducted and reported in accordance with the Reporting Checklist for Meta-analyses of Observational Studies (MOOSE).18 The completed MOOSE checklist is available in eTable 1.
Eligibility criteria
We included quantitative observational studies such as cohort (prospective or retrospective) and cross sectional studies in any geographical area in any medical care setting (primary, secondary, and tertiary care) published from January 2000 onwards. We selected this start date because it coincides with when the published patient safety research began to increase in volume after the publication of the landmark report To Err is Human: Building a Safer Health System in 1999.1 5 19
The primary outcome was the prevalence of preventable patient harm. Patient harm (which is synonymous with adverse events in healthcare) is defined as unanticipated, unforeseen accidents (eg, patient injuries, care complications, or death) which are a direct result of the care dispensed rather than the patient's underlying disease. Patient harm is preventable firstly, when occurring as a result of an identifiable and modifiable cause and secondly, when the prevention of future recurrence of the patient harm is possible with reasonable adaptation to a process and adherence to guidelines.10
The secondary outcomes were the severity and types of preventable patient harm. In accordance to the reporting format of the eligible studies, severity of preventable patient harm was classified into mild, moderate, and severe. Key types of preventable harm were drug-related, diagnostic, medical procedure-related, and healthcare-acquired infections (definitions are presented in eTable 1).
We excluded the following: studies reporting data on harm but not on preventable patient harm; studies with an exclusive focus on a specific type of harm only (only drug-related harm) or a specific severity level of harm only (incidents which only resulted in readmissions or extended length of stay) because such estimates would differ from estimates based on any type or any severity level of preventable patient harm; and studies focused on specific patient populations (eg, patients with a particular disease) because such estimates could differ from estimates in the general population.
Searches
We searched five electronic bibliographic databases from January 2000 to 27 January 2019: Medline, Cinahl, Embase, Pubmed, and PsycINFO. We supplemented these searches by screening grey literature sources including three databases (WHOLIS, Google Scholar, SIGLE), relevant reports, and conference abstracts. We also screened existing systematic reviews and checked the reference lists of eligible studies. The search strategy is available in eTable 3.
Study selection and extraction
We exported the results of the searches to Endnote X8 and removed duplicates. We completed screening in two stages. Initially, the titles and abstracts of the studies were screened for eligibility. Afterwards, the full texts of studies initially assessed as relevant for the review were retrieved and checked against our inclusion or exclusion criteria. We devised a data extraction spreadsheet, after being piloted, to extract descriptive data on key study characteristics (eg, number and age of participants, research design, data collection, assessment of preventability) and quantitative outcomes (prevalence, types, and severity of preventable patient harm). Two independent researchers (KK and MP) performed the screening and data extraction with disagreements resolved by discussion within the wider team (AA, DA, RH, RK). The inter-rater reliability was excellent (kappa=0.88 and 0.90).
Risk of bias assessment
We evaluated the risk of bias in the studies by using an adapted form of the Newcastle Ottawa scale for cross sectional and cohort studies.20 This assessed the representativeness of the sample, sample size, response rate, ascertainment of the exposure, control of confounding variables, assessment of preventability, and appropriate statistical analysis, which provided a score ranging from 0 (lowest grade) to 9 (highest grade). A higher grade indicated a lower risk of bias. For our analyses, studies scoring 7 or above were considered as low risk, whereas studies scoring below 7 were considered as high risk.
Analyses
Our primary outcome was the prevalence of preventable patient harm expressed as the proportion of patients with at least one preventable patient harmful incident and stratified according to different medical services. We also calculated and reported the median prevalence of preventable patient harm and interquartile ranges across all medical care settings. Our secondary outcomes were the severity and types of preventable patient harm expressed as proportions of the total number of preventable patient harmful incidents. We pooled all data in Stata 15 by using the metaprop command.21 To improve the meaning and interpretation of our findings in relation to the prevalence, severity, and common types of preventable patient harm, we also present data on the prevalence, severity, and common types of overall harm (preventable and non-preventable) by using the same pool of studies in all analyses.
We conducted univariable and multivariable meta regression to test the influence of study level moderators on the prevalence of preventable patient harm using the metareg command.22 Consistent with the recommendations of Thompson and Higgins,23 eight prespecified study level moderators were hypothesised to have an effect on the prevalence of preventable patient harm (medical setting, population, research design, assessment method of harm, assessment of preventability, sample size, risk of bias, WHO region). Moderators were selected and coded following consensus procedures and each moderator value was based on a minimum of eight studies.23 Covariates meeting our significance criterion (P<0.10) were entered into a multivariable meta regression model. The P<0.10 threshold was conservative, to avoid prematurely discounting potentially important explanatory variables. Because proportions were often expected to be small, we used Freeman-Tukey Double Arcsine transformation to stabilise the variances and then performed a random effects meta-analysis implementing the DerSimonian-Laird method.24 25
Random effects models were used in all analyses because they are more conservative and have better properties in the presence of heterogeneity.26 27 Heterogeneity was quantified by using the I2 statistic. Conventionally, I2 values of 25%, 50%, and 75% indicate low, moderate, and high heterogeneity, respectively.28 We inspected the symmetry of the funnel plots and used Egger’s test to examine for publication bias.29 Funnel plots were constructed using the metafunnel command,30 and the Egger test was computed using the metabias command.31
Patient and public involvement
Two patient partners, who were members of our research advisory panel, were involved in the development of our research questions and in selecting the outcome measures of this study. The two patients also provided critical feedback to the protocol of the systematic review and advised on the interpretation and dissemination of results.
Results
The searches yielded 7313 citations. After we removed duplicates and reviewed the titles and abstracts, 6522 articles were excluded. Of the remaining 307 studies, 241 were excluded after reviewing the full article. A total of 66 studies reporting 70 independent samples were included in the review.17 32 33 34 35 36 37 38 39 40 41 42 43 44 45 46 47 48 49 50 51 52 53 54 55 56 57 58 59 60 61 62 63 64 65 66 67 68 69 70 71 72 73 74 75 76 77 78 79 80 81 82 83 84 85 86 87 88 89 90 91 92 93 94 95 96 97 98 Figure 1 shows the study flow for the selection process.
Fig 1.
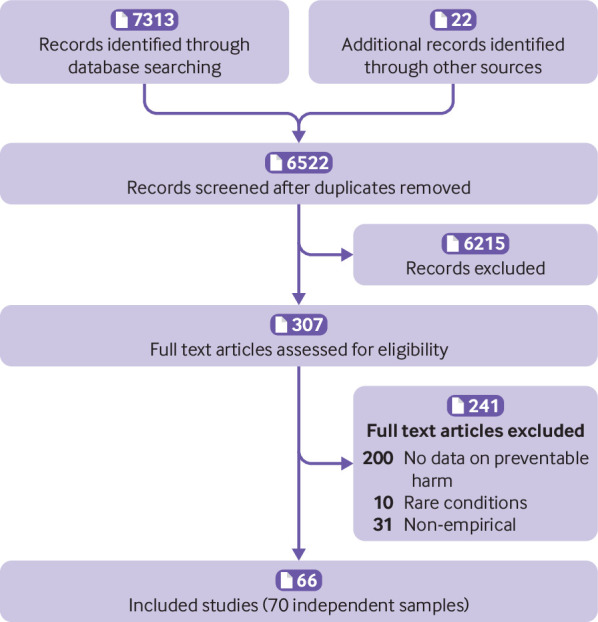
Flowchart of the inclusion of studies in the review
Descriptive characteristics
This review is based on a pooled sample of 337 025 patients, 28 150 of who experienced harmful incidents and 15 419 experienced preventable harmful incidents. A total of 47 148 harmful incidents were identified in the pooled sample, 25 977 (55%) of which were preventable. The sample sizes ranged widely across studies (median 1440 patients, range 128-96 047). Thirty three studies (47%) were conducted in the US, 27 (39%) in Europe, and 10 (14%) elsewhere. The most common study design was retrospective or cross-sectional (n=50; 71%) followed by prospective (20; 29%). Fifty three studies (76%) reviewed the medical charts of patients to detect harm, whereas 17 studies (24%) monitored patients over time or were based on self reports (eg, interviews with patients). All included studies assess the preventability of patient harm by using consensus procedures between two or more trained reviewers (physicians or teams of physicians and nurses). Fifty studies (71%) used a standardised Likert scale to facilitate the consensus decisions for the preventability of patient harm among the reviewers (harmful incidents assigned a score of four out of six and over were considered preventable).99 The remaining 20 studies (29%) used implicit agreed criteria to reach consensus regarding the preventability of patient harm among the reviewers. Most studies were conducted in general hospitals involving patients from a range of specialties (45 studies; 64%). Twelve studies (17%) were conducted in advanced care specialties (intensive care 6 studies; surgery 6 studies), six studies (8%) in emergency department, four in obstetrics (6%), and three in primary care (4%). Except for six studies (9%), which were based on children and adolescents, and five studies on older adults (7%), the remaining 59 studies (84%) were mainly based on adults. Further details of the descriptive characteristics of the included studies are available in eTable 2.
All 70 studies reported data on the prevalence of preventable patient harm and overall patient harm. One third of the studies (20 studies, 29%) reported data on the severity of preventable patient harm. Forty three studies (60%) reported proportions of at least two of the following six types of preventable patient harm: drug management, non-drug therapeutic management, diagnosis, invasive medical procedures, surgical procedures, and infections acquired during healthcare.
Risk of bias results
The Newcastle Ottawa scores for the studies ranged from three to nine (maximum 9, a higher score indicating a lower risk of bias). Twenty nine studies (41%) scored eight or above and were considered to be at low risk of bias (see full assessment in eTable 3).
Meta-analysis of the prevalence of preventable patient harm stratified by medical settings
Table 1 shows that the pooled prevalence of preventable patient harm was 6% (95% confidence interval 5% to 7%, I2=99%) and the median prevalence was 5% (interquartile range 3-9%). In comparison, the pooled prevalence of overall harm (preventable and non-preventable) was 12% (95% confidence interval 9% to 14%, I2=99%; table 1) and the median was 10% (interquartile range 7-15%). The highest pooled prevalence estimate of preventable patient harm was reported in intensive care (18%, 95% confidence interval 12% to 26%, I2=96%) and surgery (10%, 7% to 13%, I2=97%) and the lowest in obstetrics (2%, 0% to 4%, I2=95%). Figure 2 presents the forest plot of the prevalence of preventable patient harm across medical care settings.
Table 1.
Proportions of types of preventable patient harm and overall patient harm
| Outcome | No | Preventable harm | Overall harm | |||||
|---|---|---|---|---|---|---|---|---|
| % (95% CI) | I2 | Median (IQR) | % (95% CI) | I2 | Median (IQR) | |||
| Prevalence | ||||||||
| Overall | 70 | 6 (5 to 7) | 99 | 5 (3-9) | 12 (9 to 14) | 99 | 10 (7-15) | |
| Emergency department | 6 | 3 (2 to 4) | 78 | 3 (3-4) | 5 (3 to 6) | 84 | 5 (4-6) | |
| Hospitals | 45 | 5 (4 to 6) | 99 | 5 (3-7) | 10 (9 to 12) | 99 | 10 (7-12) | |
| Intensive care | 6 | 18 (12 to 26) | 96 | 14 (10-28) | 34 (19 to 50) | 99 | 29 (20-59) | |
| Obstetrics | 4 | 2 (0 to 4) | 95 | NA | 4 (2 to 6) | 92 | NA | |
| Primary care | 3 | 3 (0 to 9) | 0 | NA | 7 (3 to 10) | 0 | NA | |
| Surgery | 6 | 10 (7 to 13) | 97 | 9 (9-10) | 20 (14 to 27) | 99 | 22 (15-30) | |
| Severity of patient harm | ||||||||
| Mild | 20 | 49 (43 to 56) | 97 | 45 (40-55) | 50 (41 to 59) | 98 | 49 (43-58) | |
| Moderate | 20 | 36 (31 to 42) | 96 | 38 (30-50) | 36 (28 to 44) | 98 | 36 (27-47) | |
| Severe | 20 | 12 (9 to 15) | 94 | 10 (8-19) | 12 (8 to 15) | 95 | 13 (6-17) | |
| Types of patient harm | ||||||||
| Drugs | 25 | 25 (16 to 34) | 98 | 20 (9-35) | 26 (19 to 34) | 99 | 21 (17-30) | |
| Other therapeutic | 17 | 24 (21 to 30) | 98 | 22 (16-30) | 20 (9 to 31) | 98 | 21 (12-32) | |
| Procedure | 20 | 23 (13 to 33) | 98 | 18 (6-28) | 24 (17 to 31) | 98 | 19 (14-32) | |
| Surgical procedure | 18 | 23 (9 to 38) | 98 | 21 (8-36) | 31 (20 to 42) | 98 | 27 (16-41) | |
| Diagnosis | 20 | 16 (11 to 21) | 98 | 12 (5-22) | 9 (6 to 12) | 98 | 10 (6-11) | |
| Healthcare infections | 14 | 16 (11 to 22) | 98 | NA | 21 (15 to 28) | 98 | NA | |
The proportions for types of preventable or overall harm do not add to 100% because each figure in the table is the pooled proportion which has been calculated by combining (after assigning appropriate weights) proportions extracted from several independent studies using meta-analysis. Moreover, not all studies reported all types of preventable or overall harm and therefore it is not appropriate to assume they add up to 100%.
NA=not applicable.
Fig 2.
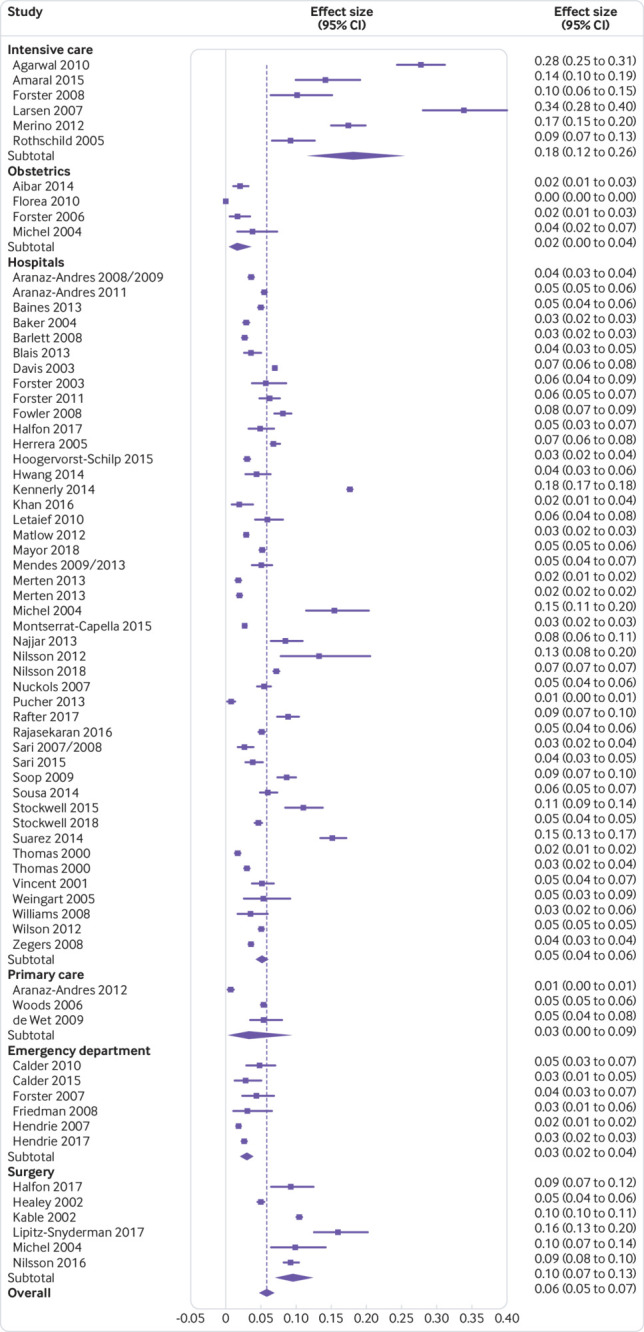
Forest plot of the pooled prevalence of preventable patient harm across medical care settings
Meta-analysis of the severity and types of preventable patient harm
Table 1 shows the pooled proportions of the severity and types of preventable patient harm. The pooled proportion of mild harm was 49% (95% confidence interval 43% to 56%, I2=97%), moderate harm was 36% (31% to 42%, I2=96%), and severe harm was 12% (9% to 15%, I2=94%).
Drug management incidents (25%, 95% confidence interval 16% to 34%, I2=98%), and other therapeutic management incidents (24%, 21% to 30%, I2=98%), accounted for the highest proportion of preventable patient harm followed by incidents related to surgical procedures (23%, 9% to 38%, I2=98%), healthcare infections (16%, 11% to 22%, I2=98%), and diagnosis (16%, 11% to 21%, I2=98%).
Meta-regressions exploring the variance in the prevalence of preventable patient harm
Table 2 shows the results of the univariable and multivariable analyses. The univariable analyses showed that the prevalence of preventable patient harm was higher across studies based in advanced specialties such as surgery and intensive care (b=0.08, 95% confidence interval 0.05 to 0.11), in studies with relatively small sample sizes (b=0.03, 0.01 to 0.06), and in studies on children and older adults (b=0.03, −0.01 to 0.05). These three variables (medical care setting, population group, and sample size) were therefore eligible for inclusion in the multivariable regression analysis. All the other variables (research design, assessment method of harm, assessment of preventability, risk of bias, and WHO region) were ineligible for inclusion in multivariable analyses because none of them influenced the prevalence of preventable patient harm in unvariable analyses (P>0.10).
Table 2.
Univariable and multivariable predictors of the prevalence of preventable patient harm (n=70)
| Variable | No | Univariable | Multivariable | |||||
|---|---|---|---|---|---|---|---|---|
| Regression coefficient (95% CI) | SE | P value | Regression coefficient (95% CI) | SE | P value | |||
| WHO region: | ||||||||
| US | 33 | 1 | — | — | — | — | — | |
| Europe | 27 | −0.01 (−0.03 to 0.01) | 0.01 | 0.59 | NA | NA | NA | |
| Asia or other | 10 | −0.01 (−0.02 to 0.04) | 0.02 | 0.54 | NA | NA | NA | |
| Medical setting: | ||||||||
| General hospitals and obstetrics | 49 | 1 | — | — | 1 | — | — | |
| Primary care and emergency department | 9 | −0.02 (−0.05 to 0.01) | 0.02 | 0.18 | −0.03 (−0.06 to 0.01) | 0.02 | 0.12 | |
| Advanced hospital specialties | 12 | 0.08 (0.05 to 0.11) | 0.02 | <0.001 | 0.07 (0.04 to 0.10) | 0.01 | <0.001 | |
| Research design: | ||||||||
| Retrospective or cross sectional | 50 | 1 | — | — | — | — | — | |
| Prospective | 20 | 0.01 (−0.01 to 0.04) | 0.01 | 0.31 | NA | NA | NA | |
| Sample size: | ||||||||
| Large (n>1000) | 43 | 1 | — | — | 1 | — | — | |
| Small (n<1000) | 27 | 0.03 (0.01 to 0.06) | 0.01 | 0.02 | 0.02 (−0.01 to 0.04) | 0.01 | 0.12 | |
| Population: | ||||||||
| Adults | 59 | 1 | — | — | — | — | — | |
| Children or older adults | 11 | 0.03 (−0.01 to 0.05) | 0.02 | 0.09 | 0.02 (−0.01 to 0.05) | 0.01 | 0.09 | |
| Assessment method: | ||||||||
| Medical record review | 53 | 1 | — | — | — | — | — | |
| Surveys with patients and health providers | 17 | −0.01 (−0.04 to 0.02) | 0.01 | 0.58 | NA | NA | NA | |
| Preventability by consensus among reviewers using: | ||||||||
| Standardised Likert scale | 43 | 1 | — | — | 1 | — | — | |
| Implicit criteria | 27 | 0.01 (−0.01 to 0.04) | 0.01 | 0.36 | NA | NA | NA | |
| Risk of bias: | ||||||||
| High (<7 score) | 41 | 1 | — | — | — | — | — | |
| Low (>7 score) | 29 | −0.01 (−0.03 to 0.02) | 0.01 | 0.89 | NA | NA | NA | |
SE=standard error; NA=not applicable.
The overall multivariable model was statistically significant (χ2 (4)=33.98, P<0.001) and reduced the I2 statistic from 79% to 31%. Only the medical care setting (b=0.07, 95% confidence interval 0.04 to 0.10) remained a significant predictor of the prevalence of preventable patient harm in multivariable analyses suggesting that the prevalence of preventable patient harm is higher in advanced medical specialties (surgery and primary care) compared with studies in general hospitals. The population group and sample size were not significantly associated with the prevalence of preventable patient harm after controlling for the medical care setting in the multivariable analyses.
Small study bias
Figure 3 shows some evidence of publication bias as indicated by visual inspections of the funnel plots and by the Egger test for small study effects for the primary outcome (bias coefficient for the main analysis 1.20, 95% confidence interval 0.24 to 2.15, P=0.02).
Fig 3.
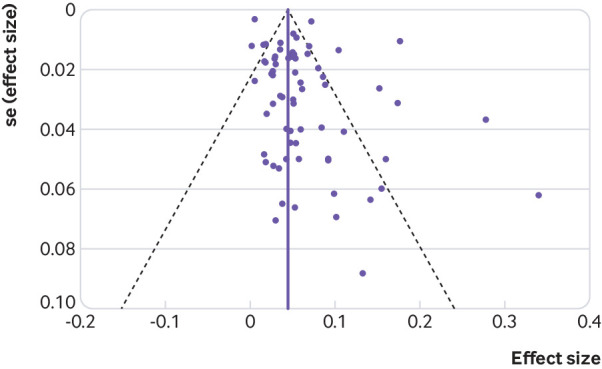
Funnel plot of studies included in analysis with pseudo 95% confidence intervals (se=standard error)
Discussion
Understanding and mitigating preventable patient harm is a major public health challenge across the globe. We conducted a systematic review and meta-analysis to understand the prevalence, severity, and common types of preventable patient harm across medical care settings. We pooled data from 70 studies and we found that preventable patient harm occurs in 6% of patients across medical care settings. Considering that a pooled prevalence of 12% for overall harm was found, we conclude that half of patient harm is preventable. The proportion of severe preventable patient harm causing prolonged, permanent disability or death was 12%. The most common types of preventable patient harm were related to drugs, other therapeutic management, and invasive medical and surgical procedures. The most extensive evidence on preventable patient harm comes from hospitals (45 studies) but less evidence is available for specific medical specialties. Preventable patient harm was more prevalent in patients treated in surgical and intensive care units compared with patients treated within across general hospitals. None of the other method variations which we examined across the studies influenced the pooled prevalence of preventable patient harm (population group, research design, assessment method of harm, assessment of preventability, sample size, risk of bias, or WHO region).
Strengths and limitations of the study
Despite the unique focus on preventable patient harm and several method strengths, this review has also limitations. Firstly, the prevalence of preventable patient harm varied considerably across studies and this variation was only partly explained in meta regression analyses. Other relevant factors likely accounted for the unexplained heterogeneity. For example, variations in the timeframe used to detect harm might be important when interpreting the differences in the prevalence estimates,1 alongside variations in the implementation of quality assurance programmes and the quality of the documentation used for detecting preventable patient harm. For example, quality assurance programmes have possibly been implemented in parallel with some of the reviewed studies which might account for some proportion of the heterogeneity that we observed in this meta-analysis.
Secondly, a critical eligibility criterion to ensure feasibility of this review was that data on preventable patient harm were available in the published reports of the studies. Studies which did not report data on preventable patient harm were excluded from the analyses. However, most studies focused primarily on overall patient harm, reported preventable patient harm as a secondary outcome, and only one third of the studies provided an analysis of severity and types of preventable patient harm.100
Thirdly, preventability rankings are likely to evolve over time especially after new technological advancements in healthcare. Consequently, some patient harms which are now considered non-preventable might be preventable in the future.10 However, the studies we reviewed consistently found that about 50% of patient harm was preventable and we did not observe any different patterns over the past 19 years.
Fourthly, over half of the reviewed studies employed retrospective case record reviews to investigate the prevalence, nature, and severity of preventable patient harm. Although case record reviews are the most universally used method for assessing patient harm to date, patients and healthcare providers have repeatedly expressed concerns that data contained in case records do not capture the full range of harms that they experience during their healthcare encounters.101 102 On the other hand, self reporting of patient harms (either by patients or healthcare providers) relies on recall and has its own limitations. Combining methods (such as prospective case record reviews with surveys with patients and healthcare providers)103 with the parallel engagement of patients as partners in identifying medical errors and mitigating preventable patient harm are promising approaches for enhancing patient safety.104 105
Comparison with other studies
Our headline finding is that preventable patient harm is a highly prevalent international healthcare challenge which causes unnecessary patient suffering and can result in several avoidable deaths. As this review is specifically designed to understand patterns of preventable patient harm, comparisons with existing reviews focused on overall harm is problematic.1 15 106 107 108 Although we concur that examining the nature of overall harm is important, increasing the emphasis on preventable patient harm (which is the most amenable form of patient harm) is critical in terms of designing efficient patient safety strategies.
There is also evidence that preventable patient harm is not only a public health concern but incurs a considerable opportunity cost. The excess length of hospital stays attributable to medical errors is estimated to be 2.4 million hospital days, which accounts for $9.3 billion (£7.3bn; €8.2bn) excess charges in the US.7 Similarly, only six selected types of preventable patient harms in English hospitals result in 934 excell bed days per 100 000 population, which is equivalent to over 3500 salaried hospital nurses each year.109 Thus, investments in developing and evaluating mitigation strategies for preventable patient harm are urgently needed and are strongly supported by our findings.
Policy implications
Our findings provide a useful agenda of priority areas for mitigating preventable patient harm. When exploring the nature of preventable patient harm, drug related and therapeutic incidents comprise the majority. This finding echoes recommendations from international patient safety policy initiatives in the past decade including the recent WHO’s third global patient safety challenge “medication without harm.”106 110 Thus, it would be logical to prioritise efforts on developing and testing evidence-based mitigation strategies for these specific types of preventable patient harm. As this study establishes the scale of preventable patient harm in medical care settings, the need to gain better insight about the systemic and cultural circumstances under which preventable patient harm occurs is highlighted as a priority area. Several studies have sought to explain patient harms by reference to their sociotechnical context. For example, Vincent and colleagues proposes that patient harm occur because of contributory factors (which include “active” and “latent” failures) in the healthcare system.111 These failures correspond to characteristics of the system such as the tasks that are undertaken, the people, technology, and tools that are involved, and the organisational values and structures in which the system operates.112 The studies included in our review, however, did not provide much insight into the way in which such factors might have contributed to the instances of preventable harm identified. Retrospective examination of patient harm often does not capture the myriad ways in which contributory factors could combine to produce—or avert—a preventable incident of patient harm.113 Mixed method approaches, which connect the occurrence of patient harm to the presence of specific contributory factors and engage patients as partners in establishing these connections, have excellent prospects to achieve an in depth understanding of possible pathways to patient harm.114 115 116 117 118
A thorough understanding of the nature of preventable patient harm and its determinants could offer useful, evidence-based directions for designing efficient mitigation strategies. A combination of individual-level measures (eg, educational interventions for practitioners), system-level measures (eg, human-centred design of healthcare tasks and work environments), and organisational-level measures (eg, introducing quality monitoring and improvement processes) are likely to be a promising strategy for mitigating preventable patient harm,119 120 but scalable evaluations of these interventions are needed to support wider implementation. Furthermore, the interventions depend on the presence of an organisational context that supports their implementation.121 122
Another important finding is that preventable patient harm appears to be a serious concern in advanced medical specialties including intensive care and surgical units. Patients treated in these specialties were more likely to experience preventable patient harm compared with patients treated in general hospitals. Surgical harm is a sizeable part of the overall in-hospital harm,15 123 but our estimates are higher than anticipated. The underlying causes of these figures warrant further investigation because current safety standards could “be failing to rescue” many high risk patients treated in advanced specialties.124 Moreover, clinicians in these specialties are often exposed to work pressures and are expected to deliver life-changing decisions quickly which might negatively impact on their personal wellbeing, a well known risk factor for preventable medical incidents.125 On the other hand, surgery and intensive care units deal with high risk patients to whom complex medical procedures are implemented. Patient harm therefore might be more detectable in these settings because of its immediate, serious, or cumulative impact on patients’ health or because better surveillance systems for detecting patient harm are implemented in these settings. Additionally, it is not always clear from the study designs that some proportion of the preventable patient harm has not occurred in the transition between general hospital care and advanced specialty care.108
Another major contribution of our synthesis is that it highlights key gaps in the literature on preventable patient harm. Only two studies were based in primary care, where over 80% of healthcare service is delivered internationally,8 126 and no evidence was identified in psychiatry. Certain types of preventable harms which tend to occur in primary care and psychiatry might remain undetected or untargeted by quality and safety improvement programmes. For example, we found that diagnostic harm is a common preventable type of harm but our understanding of its nature needs to be improved. A likely explanation is that diagnostic harm is directly or indirectly linked with the provision of services in primary care where research on preventable patient harm is sparse.127 128 Obtaining more precise estimates of the types and sources of preventable diagnostic harm occurring in primary care or in transitions from primary care to hospital care could lay the foundation for implementing efficient interventions for diagnostic harm. Systemic interventions, enhanced patient involvement in decision making for diagnoses, use of electronic tools, and emotion-cognitive interventions for boosting practitioners’ confidence or certainty in making diagnoses are potentially fruitful intervention areas for reducing diagnostic harm but have not been systematically evaluated or implemented in practice.104 127 128 129 130
Less than a handful of studies focused on children and older adults, groups increasingly viewed as vulnerable to low quality or unsafe care. Furthermore, only a fraction of the included studies were conducted in developing countries, as many studies from developing countries failed to provide data on preventability of harm which rendered them ineligible. Thus, despite the evidence showing that the prevalence of overall harm is higher in developing countries compared with developed countries, we did not find such difference for preventable patient harm.
Commissioning research to understand the prevalence, nature, and impact of preventable patient harm in primary care and psychiatry, among vulnerable patient groups (eg, young children, older adults, or marginalised groups of the society such prison healthcare) and in developing countries has the potential to advance policy guidance and practice for mitigating preventable patient harm.
Conclusion
Our findings affirm that preventable patient harm is a serious problem across medical care settings. Priority areas are the mitigation of major sources of preventable patient harm (such as drug incidents) and greater focus on advanced medical specialties. It is equally imperative to build evidence across specialties such as primary care and psychiatry, vulnerable patient groups, and developing countries. Improving the assessment and reporting standards of preventability in future studies is critical for reducing patient harm in medical care settings.
What is already known on this topic
A better understanding of the nature of preventable patient harm has the potential to impact on international healthcare policy and practice
The prevalence of overall patient harm has been established by systematic reviews but the prevalence of preventable patient harm has received less attention
What this study adds
A meta-analysis that quantifies the prevalence, nature, and severity of preventable patient harm in a range of medical care settings
At least one in 20 patients are affected by preventable patient harm in medical care settings
Approximately 12% of preventable patient harm causes permanent disability or patient death and is mostly related to drug incidents, therapeutic management, and invasive clinical procedures
Web Extra.
Extra material supplied by the author
Supplementary materials: Searches and eTable 1-4
Contributors: The original idea for the research was developed by MP, DMA, RNK, DP, PB, AJA. MP conducted the analysis with input from KK, EK, DMA, RNK, DP, AA, PB, and AJA. MP and KK conducted the searches, study selection, quality assessments, and other data extraction. MP, KK, and DMA wrote the paper. All authors interpreted the findings and contributed to critical revision of the manuscript. All authors had full access to the data in the study and can take responsibility for the integrity of the data and the accuracy of the data analysis. MP is the guarantor. The corresponding author attests that all listed authors meet authorship criteria and that no others meeting the criteria have been omitted.
Funding: This study was funded by the UK General Medical Council (RMS 113361). The NIHR Greater Manchester Patient Safety Translational Research Centre (GMPSTRC-2012-1) funded the corresponding author’s time spent in this project. MP, EK, and PB are also co-investigators in the Evidence Synthesis Working Group (project 390), which is supported by the NIHR School for Primary Care Research. The research team members were independent from the funding agencies. The views expressed in this manuscript are those of the authors and not necessarily those of the General Medical Council, the National Health Service, the NIHR, or the Department of Health. The funders had no role in the design and conduct of the study; the collection, management, analysis, and interpretation of the data; and the preparation, review, or approval of the manuscript.
Competing interests: All authors have completed the ICMJE uniform disclosure form at www.icmje.org/coi_disclosure.pdf and all other authors declare no support from any organisation for the submitted work; no financial relationships with any organisations that might have an interest in the submitted work in the previous three years; and no other relationships or activities that could appear to have influenced the submitted work.
Ethical approval: Not required.
Data sharing: No additional data are available.
The manuscript’s guarantor (MP) affirms that the manuscript is an honest, accurate, and transparent account of the study being reported; and that no important aspects of the study have been omitted; and that any discrepancies from the study as planned have been explained.
References
- 1. de Vries EN, Ramrattan MA, Smorenburg SM, Gouma DJ, Boermeester MA. The incidence and nature of in-hospital adverse events: a systematic review. Qual Saf Health Care 2008;17:216-23. 10.1136/qshc.2007.023622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Jha AK, Larizgoitia I, Audera-Lopez C, Prasopa-Plaizier N, Waters H, Bates DW. The global burden of unsafe medical care: analytic modelling of observational studies. BMJ Qual Saf 2013;22:809-15. 10.1136/bmjqs-2012-001748. [DOI] [PubMed] [Google Scholar]
- 3. The Conceptual Framework for the International Classification for Patient Safety World Alliance for Patient Safety Taxonomy. World Health Organisation, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Makary MA, Daniel M. Medical error-the third leading cause of death in the US. BMJ 2016;353:i2139. 10.1136/bmj.i2139. [DOI] [PubMed] [Google Scholar]
- 5. Stelfox HT, Palmisani S, Scurlock C, Orav EJ, Bates DW. The “To Err is Human” report and the patient safety literature. Qual Saf Health Care 2006;15:174-8. 10.1136/qshc.2006.017947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Slawomirski L, Auraaen A, Klazinga N. The economics of patient safety. Organisation for Economic Co-operation and Development, 2017. [Google Scholar]
- 7. Zhan C, Miller MR. Excess length of stay, charges, and mortality attributable to medical injuries during hospitalization. JAMA 2003;290:1868-74. 10.1001/jama.290.14.1868. [DOI] [PubMed] [Google Scholar]
- 8. Sheikh A, Panesar SS, Larizgoitia I, Bates DW, Donaldson LJ. Safer primary care for all: a global imperative. Lancet Glob Health 2013;1:e182-3. 10.1016/S2214-109X(13)70030-5. [DOI] [PubMed] [Google Scholar]
- 9. Pronovost PJ, Colantuoni E. Measuring preventable harm: helping science keep pace with policy. JAMA 2009;301:1273-5. 10.1001/jama.2009.388. [DOI] [PubMed] [Google Scholar]
- 10. Nabhan M, Elraiyah T, Brown DR, et al. What is preventable harm in healthcare? A systematic review of definitions. BMC Health Serv Res 2012;12:128. 10.1186/1472-6963-12-128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Chang A, Schyve PM, Croteau RJ, O’Leary DS, Loeb JM. The JCAHO patient safety event taxonomy: a standardized terminology and classification schema for near misses and adverse events. Int J Qual Health Care 2005;17:95-105. 10.1093/intqhc/mzi021. [DOI] [PubMed] [Google Scholar]
- 12. Elder NC, Dovey SM. Classification of medical errors and preventable adverse events in primary care: a synthesis of the literature. J Fam Pract 2002;51:927-32. [PubMed] [Google Scholar]
- 13. Loeb JM, Chang A. Patient safety: Reduction of adverse events through common understanding and common reporting tools. Towards an international patient safety taxonomy. World Health Organization, 2003. [Google Scholar]
- 14. Brennan TA, Leape LL, Laird NM, et al. Harvard Medical Practice Study I Incidence of adverse events and negligence in hospitalized patients: results of the Harvard Medical Practice Study I. 1991. Qual Saf Health Care 2004;13:145-51, discussion 151-2. 10.1136/qshc.2002.003822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Anderson O, Davis R, Hanna GB, Vincent CA. Surgical adverse events: a systematic review. Am J Surg 2013;206:253-62. 10.1016/j.amjsurg.2012.11.009. [DOI] [PubMed] [Google Scholar]
- 16. Long SJ, Brown KF, Ames D, Vincent C. What is known about adverse events in older medical hospital inpatients? A systematic review of the literature. Int J Qual Health Care 2013;25:542-54. 10.1093/intqhc/mzt056. [DOI] [PubMed] [Google Scholar]
- 17. Nilsson L, Pihl A, Tågsjö M, Ericsson E. Adverse events are common on the intensive care unit: results from a structured record review. Acta Anaesthesiol Scand 2012;56:959-65. 10.1111/j.1399-6576.2012.02711.x. [DOI] [PubMed] [Google Scholar]
- 18. Stroup DF, Berlin JA, Morton SC, et al. Meta-analysis of observational studies in epidemiology: a proposal for reporting. Meta-analysis Of Observational Studies in Epidemiology (MOOSE) group. JAMA 2000;283:2008-12. 10.1001/jama.283.15.2008. [DOI] [PubMed] [Google Scholar]
- 19. Lilford R, Stirling S, Maillard N. Citation classics in patient safety research: an invitation to contribute to an online bibliography. Qual Saf Health Care 2006;15:311-3. 10.1136/qshc.2005.017178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Luchini C, Stubbs B, Solmi M, et al. Assessing the quality of studies in meta-analyses: Advantages and limitations of the Newcastle Ottawa Scale. World J Metaanal 2017;5:80-4. 10.13105/wjma.v5.i4.80. [DOI] [Google Scholar]
- 21. Kontopantelis E, Reeves D. metaan: Random-effects meta-analysis. Stata J 2010;10:395-407. 10.1177/1536867X1001000307. [DOI] [Google Scholar]
- 22. Harbord RM, Higgins JPT. Meta-regression in Stata. Stata J 2008;8:493-519. 10.1177/1536867X0800800403. [DOI] [Google Scholar]
- 23. Thompson SG, Higgins JPT. How should meta-regression analyses be undertaken and interpreted? Stat Med 2002;21:1559-73. 10.1002/sim.1187. [DOI] [PubMed] [Google Scholar]
- 24. Freeman MF, Tukey JW. Transformations Related to the Angular and the Square Root. Ann Math Stat 1950;21:607-11. 10.1214/aoms/1177729756. [DOI] [Google Scholar]
- 25. DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials 1986;7:177-88. 10.1016/0197-2456(86)90046-2. [DOI] [PubMed] [Google Scholar]
- 26. Brockwell SE, Gordon IR. A comparison of statistical methods for meta-analysis. Stat Med 2001;20:825-40. 10.1002/sim.650. [DOI] [PubMed] [Google Scholar]
- 27. Kontopantelis E, Reeves D. Performance of statistical methods for meta-analysis when true study effects are non--normally distributed: a comparison between DerSimonian-Laird and restricted maximum likelihood. Stat Methods Med Res 2012;21:657-9. 10.1177/0962280211413451. [DOI] [PubMed] [Google Scholar]
- 28. Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ 2003;327:557-60. 10.1136/bmj.327.7414.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ 1997;315:629-34. 10.1136/bmj.315.7109.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Sterne JAC, Harbord RM. Funnel plots in meta-analysis. Stata J 2004;4:127-41. 10.1177/1536867X0400400204. [DOI] [Google Scholar]
- 31. Harbord RM, Harris RJ, Sterne JAC. Updated tests for small-study effects in meta-analyses. Stata J 2009;9:197-210. 10.1177/1536867X0900900202. [DOI] [Google Scholar]
- 32. Agarwal S, Classen D, Larsen G, et al. Prevalence of adverse events in pediatric intensive care units in the United States. Pediatr Crit Care Med 2010;11:568-78. 10.1097/PCC.0b013e3181d8e405. [DOI] [PubMed] [Google Scholar]
- 33. Aibar L, Rabanaque MJ, Aibar C, Aranaz JM, Mozas J. Patient safety and adverse events related with obstetric care. Arch Gynecol Obstet 2015;291:825-30. 10.1007/s00404-014-3474-3. [DOI] [PubMed] [Google Scholar]
- 34. Amaral ACKB, McDonald A, Coburn NG, et al. Expanding the scope of Critical Care Rapid Response Teams: a feasible approach to identify adverse events. A prospective observational cohort. BMJ Qual Saf 2015;24:764-8. 10.1136/bmjqs-2014-003833. [DOI] [PubMed] [Google Scholar]
- 35. Aranaz-Andrés JM, Aibar-Remón C, Vitaller-Murillo J, Ruiz-López P, Limón-Ramírez R, Terol-García E, ENEAS work group Incidence of adverse events related to health care in Spain: results of the Spanish National Study of Adverse Events. J Epidemiol Community Health 2008;62:1022-9. 10.1136/jech.2007.065227. [DOI] [PubMed] [Google Scholar]
- 36. Aranaz-Andrés JM, Aibar C, Limón R, et al. A study of the prevalence of adverse events in primary healthcare in Spain. Eur J Public Health 2012;22:921-5. 10.1093/eurpub/ckr168. [DOI] [PubMed] [Google Scholar]
- 37. Aranaz-Andrés JM, Aibar-Remón C, Limón-Ramírez R, et al. IBEAS team Prevalence of adverse events in the hospitals of five Latin American countries: results of the ‘Iberoamerican Study of Adverse Events’ (IBEAS). BMJ Qual Saf 2011;20:1043-51. 10.1136/bmjqs.2011.051284. [DOI] [PubMed] [Google Scholar]
- 38. Aranaz-Andrés JM, Aibar-Remón C, Vitaller-Burillo J, et al. ENEAS work group Impact and preventability of adverse events in Spanish public hospitals: results of the Spanish National Study of Adverse Events (ENEAS). Int J Qual Health Care 2009;21:408-14. 10.1093/intqhc/mzp047. [DOI] [PubMed] [Google Scholar]
- 39. Baines RJ, Langelaan M, de Bruijne MC, et al. Changes in adverse event rates in hospitals over time: a longitudinal retrospective patient record review study. BMJ Qual Saf 2013;22:290-8. 10.1136/bmjqs-2012-001126. [DOI] [PubMed] [Google Scholar]
- 40. Baker GR, Norton PG, Flintoft V, et al. The Canadian Adverse Events Study: the incidence of adverse events among hospital patients in Canada. CMAJ 2004;170:1678-86. 10.1503/cmaj.1040498 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Bartlett G, Blais R, Tamblyn R, Clermont RJ, MacGibbon B. Impact of patient communication problems on the risk of preventable adverse events in acute care settings. CMAJ 2008;178:1555-62. 10.1503/cmaj.070690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Blais R, Sears NA, Doran D, et al. Assessing adverse events among home care clients in three Canadian provinces using chart review. BMJ Qual Saf 2013;22:989-97. 10.1136/bmjqs-2013-002039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Calder L, Pozgay A, Riff S, et al. Adverse events in patients with return emergency department visits. BMJ Qual Saf 2015;24:142-8. 10.1136/bmjqs-2014-003194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Calder LA, Forster A, Nelson M, et al. Adverse events among patients registered in high-acuity areas of the emergency department: a prospective cohort study. CJEM 2010;12:421-30. 10.1017/S1481803500012574. [DOI] [PubMed] [Google Scholar]
- 45. Davis P, Lay-Yee R, Briant R, Scott A. Preventable in-hospital medical injury under the “no fault” system in New Zealand. Qual Saf Health Care 2003;12:251-6. 10.1136/qhc.12.4.251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. de Wet C, Bowie P. The preliminary development and testing of a global trigger tool to detect error and patient harm in primary-care records. Postgrad Med J 2009;85:176-80. 10.1136/pgmj.2008.075788. [DOI] [PubMed] [Google Scholar]
- 47. Florea A, Caughey SS, Westland J, et al. The Ottawa hospital quality incident notification system for capturing adverse events in obstetrics. J Obstet Gynaecol Can 2010;32:657-62. 10.1016/S1701-2163(16)34569-8. [DOI] [PubMed] [Google Scholar]
- 48. Forster AJ, Fung I, Caughey S, et al. Adverse events detected by clinical surveillance on an obstetric service. Obstet Gynecol 2006;108:1073-83. 10.1097/01.AOG.0000242565.28432.7c. [DOI] [PubMed] [Google Scholar]
- 49. Forster AJ, Kyeremanteng K, Hooper J, Shojania KG, van Walraven C. The impact of adverse events in the intensive care unit on hospital mortality and length of stay. BMC Health Serv Res 2008;8:259. 10.1186/1472-6963-8-259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Forster AJ, Murff HJ, Peterson JF, Gandhi TK, Bates DW. The incidence and severity of adverse events affecting patients after discharge from the hospital. Ann Intern Med 2003;138:161-7. 10.7326/0003-4819-138-3-200302040-00007. [DOI] [PubMed] [Google Scholar]
- 51. Forster AJ, Rose NGW, van Walraven C, Stiell I. Adverse events following an emergency department visit. Qual Saf Health Care 2007;16:17-22. 10.1136/qshc.2005.017384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Forster AJ, Worthington JR, Hawken S, et al. Using prospective clinical surveillance to identify adverse events in hospital. BMJ Qual Saf 2011;20:756-63. 10.1136/bmjqs.2010.048694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Fowler FJ, Jr, Epstein A, Weingart SN, et al. Adverse events during hospitalization: results of a patient survey. Jt Comm J Qual Patient Saf 2008;34:583-90. 10.1016/S1553-7250(08)34073-2. [DOI] [PubMed] [Google Scholar]
- 54. Friedman SM, Provan D, Moore S, Hanneman K. Errors, near misses and adverse events in the emergency department: what can patients tell us? CJEM 2008;10:421-7. 10.1017/S1481803500010484. [DOI] [PubMed] [Google Scholar]
- 55. Halfon P, Staines A, Burnand B. Adverse events related to hospital care: a retrospective medical records review in a Swiss hospital. Int J Qual Health Care 2017;29:527-33. 10.1093/intqhc/mzx061. [DOI] [PubMed] [Google Scholar]
- 56. Healey MA, Shackford SR, Osler TM, Rogers FB, Burns E. Complications in surgical patients. Arch Surg 2002;137:611-7, discussion 617-8. 10.1001/archsurg.137.5.611. [DOI] [PubMed] [Google Scholar]
- 57. Hendrie J, Sammartino L, Silvapulle MJ, Braitberg G. Experience in adverse events detection in an emergency department: incidence and outcome of events. Emerg Med Australas 2007;19:16-24. 10.1111/j.1742-6723.2006.00896.x. [DOI] [PubMed] [Google Scholar]
- 58. Hendrie J, Yeoh M, Richardson J, et al. Case-control study to investigate variables associated with incidents and adverse events in the emergency department. Emerg Med Australas 2017;29:149-57. 10.1111/1742-6723.12736. [DOI] [PubMed] [Google Scholar]
- 59. Herrera-Kiengelher L, Chi-Lem G, Báez-Saldaña R, et al. Frequency and correlates of adverse events in a respiratory diseases hospital in Mexico city. Chest 2005;128:3900-5. 10.1378/chest.128.6.3900. [DOI] [PubMed] [Google Scholar]
- 60. Hoogervorst-Schilp J, Langelaan M, Spreeuwenberg P, de Bruijne MC, Wagner C. Excess length of stay and economic consequences of adverse events in Dutch hospital patients. BMC Health Serv Res 2015;15:531. 10.1186/s12913-015-1205-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Hwang J-I, Chin HJ, Chang Y-S. Characteristics associated with the occurrence of adverse events: a retrospective medical record review using the Global Trigger Tool in a fully digitalized tertiary teaching hospital in Korea. J Eval Clin Pract 2014;20:27-35. 10.1111/jep.12075. [DOI] [PubMed] [Google Scholar]
- 62. Kable AK, Gibberd RW, Spigelman AD. Adverse events in surgical patients in Australia. Int J Qual Health Care 2002;14:269-76. 10.1093/intqhc/14.4.269 [DOI] [PubMed] [Google Scholar]
- 63. Kennerly DA, Kudyakov R, da Graca B, et al. Characterization of adverse events detected in a large health care delivery system using an enhanced global trigger tool over a five-year interval. Health Serv Res 2014;49:1407-25. 10.1111/1475-6773.12163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Khan A, Furtak SL, Melvin P, Rogers JE, Schuster MA, Landrigan CP. Parent-Reported Errors and Adverse Events in Hospitalized Children. JAMA Pediatr 2016;170:e154608. 10.1001/jamapediatrics.2015.4608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Larsen GY, Donaldson AE, Parker HB, Grant MJ. Preventable harm occurring to critically ill children. Pediatr Crit Care Med 2007;8:331-6. 10.1097/01.PCC.0000263042.73539.99. [DOI] [PubMed] [Google Scholar]
- 66. Lehmann LS, Puopolo AL, Shaykevich S, Brennan TA. Iatrogenic events resulting in intensive care admission: frequency, cause, and disclosure to patients and institutions. Am J Med 2005;118:409-13. 10.1016/j.amjmed.2005.01.012. [DOI] [PubMed] [Google Scholar]
- 67. Lipitz-Snyderman A, Pfister D, Classen D, et al. Preventable and mitigable adverse events in cancer care: Measuring risk and harm across the continuum. Cancer 2017;123:4728-36. 10.1002/cncr.30916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Matlow AG, Baker GR, Flintoft V, et al. Adverse events among children in Canadian hospitals: the Canadian Paediatric Adverse Events Study. CMAJ 2012;184:E709-18. 10.1503/cmaj.112153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Mayor S, Baines E, Vincent C, et al. Health Services and Delivery Research. Measuring harm and informing quality improvement in the Welsh NHS: the longitudinal Welsh national adverse events study. NIHR Journals Library, 2017. [PubMed] [Google Scholar]
- 70. Mendes W, Martins M, Rozenfeld S, Travassos C. The assessment of adverse events in hospitals in Brazil. Int J Qual Health Care 2009;21:279-84. 10.1093/intqhc/mzp022. [DOI] [PubMed] [Google Scholar]
- 71. Mendes W, Pavão ALB, Martins M, Moura MdeL, Travassos C. The feature of preventable adverse events in hospitals in the State of Rio de Janeiro, Brazil. Rev Assoc Med Bras (1992) 2013;59:421-8. 10.1016/j.ramb.2013.03.002. [DOI] [PubMed] [Google Scholar]
- 72. Merino P, Álvarez J, Cruz Martín M, Alonso Á, Gutiérrez I, SYREC Study Investigators Adverse events in Spanish intensive care units: the SYREC study. Int J Qual Health Care 2012;24:105-13. 10.1093/intqhc/mzr083. [DOI] [PubMed] [Google Scholar]
- 73. Merten H, Zegers M, de Bruijne MC, Wagner C. Scale, nature, preventability and causes of adverse events in hospitalised older patients. Age Ageing 2013;42:87-93. 10.1093/ageing/afs153. [DOI] [PubMed] [Google Scholar]
- 74. Michel P, Quenon JL, de Sarasqueta AM, Scemama O. Comparison of three methods for estimating rates of adverse events and rates of preventable adverse events in acute care hospitals. BMJ 2004;328:199. 10.1136/bmj.328.7433.199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Montserrat-Capella D, Suárez M, Ortiz L, Mira JJ, Duarte HG, Reveiz L, AMBEAS Group Frequency of ambulatory care adverse events in Latin American countries: the AMBEAS/PAHO cohort study. Int J Qual Health Care 2015;27:52-9. 10.1093/intqhc/mzu100. [DOI] [PubMed] [Google Scholar]
- 76. Najjar S, Hamdan M, Euwema MC, et al. The Global Trigger Tool shows that one out of seven patients suffers harm in Palestinian hospitals: challenges for launching a strategic safety plan. Int J Qual Health Care 2013;25:640-7. 10.1093/intqhc/mzt066. [DOI] [PubMed] [Google Scholar]
- 77. Nilsson L, Borgstedt-Risberg M, Soop M, et al. Incidence of adverse events in Sweden during 2013-2016: a cohort study describing the implementation of a national trigger tool. BMJ Open 2018;8:e020833 10.1136/bmjopen-2017-020833 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Nilsson L, Risberg MB, Montgomery A, Sjödahl R, Schildmeijer K, Rutberg H. Preventable Adverse Events in Surgical Care in Sweden: A Nationwide Review of Patient Notes. Medicine (Baltimore) 2016;95:e3047. 10.1097/MD.0000000000003047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Nuckols TK, Bell DS, Liu H, Paddock SM, Hilborne LH. Rates and types of events reported to established incident reporting systems in two US hospitals. Qual Saf Health Care 2007;16:164-8. 10.1136/qshc.2006.019901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Pucher PH, Aggarwal R, Twaij A, Batrick N, Jenkins M, Darzi A. Identifying and addressing preventable process errors in trauma care. World J Surg 2013;37:752-8. 10.1007/s00268-013-1917-9. [DOI] [PubMed] [Google Scholar]
- 81. Rafter N, Hickey A, Conroy RM, et al. The Irish National Adverse Events Study (INAES): the frequency and nature of adverse events in Irish hospitals-a retrospective record review study. BMJ Qual Saf 2017;26:111-9. 10.1136/bmjqs-2015-004828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Rajasekaran S, Ravi S, Aiyer SN. Incidence and preventability of adverse events in an orthopaedic unit: a prospective analysis of four thousand, nine hundred and six admissions. Int Orthop 2016;40:2233-8. 10.1007/s00264-016-3282-4. [DOI] [PubMed] [Google Scholar]
- 83. Rothschild JM, Landrigan CP, Cronin JW, et al. The Critical Care Safety Study: The incidence and nature of adverse events and serious medical errors in intensive care. Crit Care Med 2005;33:1694-700. 10.1097/01.CCM.0000171609.91035.BD. [DOI] [PubMed] [Google Scholar]
- 84. Sari AA, Doshmangir L, Torabi F, et al. The incidence, nature and consequences of adverse events in Iranian hospitals. Arch Iran Med 2015;18:811-15. http://www.aimjournal.ir/Article/920 [PubMed] [Google Scholar]
- 85. Sari ABA, Cracknell A, Sheldon TA. Incidence, preventability and consequences of adverse events in older people: results of a retrospective case-note review. Age Ageing 2008;37:265-9. 10.1093/ageing/afn043. [DOI] [PubMed] [Google Scholar]
- 86. Sari AB-A, Sheldon TA, Cracknell A, et al. Extent, nature and consequences of adverse events: results of a retrospective casenote review in a large NHS hospital. Qual Saf Health Care 2007;16:434-9. 10.1136/qshc.2006.021154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Soop M, Fryksmark U, Köster M, Haglund B. The incidence of adverse events in Swedish hospitals: a retrospective medical record review study. Int J Qual Health Care 2009;21:285-91. 10.1093/intqhc/mzp025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Sousa P, Uva AS, Serranheira F, Nunes C, Leite ES. Estimating the incidence of adverse events in Portuguese hospitals: a contribution to improving quality and patient safety. BMC Health Serv Res 2014;14:311. 10.1186/1472-6963-14-311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Stockwell DC, Bisarya H, Classen DC, et al. A trigger tool to detect harm in pediatric inpatient settings. Pediatrics 2015;135:1036-42. 10.1542/peds.2014-2152. [DOI] [PubMed] [Google Scholar]
- 90. Stockwell DC, Landrigan CP, Toomey SL, et al. GAPPS Study Group Adverse Events in Hospitalized Pediatric Patients. Pediatrics 2018;142:e20173360. 10.1542/peds.2017-3360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Suarez C, Menendez MD, Alonso J, Castaño N, Alonso M, Vazquez F. Detection of adverse events in an acute geriatric hospital over a 6-year period using the Global Trigger Tool. J Am Geriatr Soc 2014;62:896-900. 10.1111/jgs.12774. [DOI] [PubMed] [Google Scholar]
- 92. Thomas EJ, Brennan TA. Incidence and types of preventable adverse events in elderly patients: population based review of medical records. BMJ 2000;320:741-4. 10.1136/bmj.320.7237.741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Vincent C, Neale G, Woloshynowych M. Adverse events in British hospitals: preliminary retrospective record review. BMJ 2001;322:517-9. 10.1136/bmj.322.7285.517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Weingart SN, Pagovich O, Sands DZ, et al. What can hospitalized patients tell us about adverse events? Learning from patient-reported incidents. J Gen Intern Med 2005;20:830-6. 10.1111/j.1525-1497.2005.0180.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Williams DJ, Olsen S, Crichton W, et al. Detection of adverse events in a Scottish hospital using a consensus-based methodology. Scott Med J 2008;53:26-30. 10.1258/RSMSMJ.53.4.26. [DOI] [PubMed] [Google Scholar]
- 96. Wilson RM, Michel P, Olsen S, et al. WHO Patient Safety EMRO/AFRO Working Group Patient safety in developing countries: retrospective estimation of scale and nature of harm to patients in hospital. BMJ 2012;344:e832. 10.1136/bmj.e832. [DOI] [PubMed] [Google Scholar]
- 97. Woods D, Thomas E, Holl J, Altman S, Brennan T. Adverse events and preventable adverse events in children. Pediatrics 2005;115:155-60. 10.1542/peds.2004-0410. [DOI] [PubMed] [Google Scholar]
- 98. Zegers M, de Bruijne MC, Wagner C, et al. Adverse events and potentially preventable deaths in Dutch hospitals: results of a retrospective patient record review study. Qual Saf Health Care 2009;18:297-302. 10.1136/qshc.2007.025924. [DOI] [PubMed] [Google Scholar]
- 99. Brennan TA, Leape LL, Laird NM, et al. Incidence of adverse events and negligence in hospitalized patients. Results of the Harvard Medical Practice Study I. N Engl J Med 1991;324:370-6. 10.1056/NEJM199102073240604. [DOI] [PubMed] [Google Scholar]
- 100. Garrouste-Orgeas M, Philippart F, Bruel C, Max A, Lau N, Misset B. Overview of medical errors and adverse events. Ann Intensive Care 2012;2:2. 10.1186/2110-5820-2-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Schwappach DLB. Review: engaging patients as vigilant partners in safety: a systematic review. Med Care Res Rev 2010;67:119-48. 10.1177/1077558709342254. [DOI] [PubMed] [Google Scholar]
- 102. Ricci-Cabello I, Avery AJ, Reeves D, Kadam UT, Valderas JM. Measuring Patient Safety in Primary Care: The Development and Validation of the “Patient Reported Experiences and Outcomes of Safety in Primary Care” (PREOS-PC). Ann Fam Med 2016;14:253-61. 10.1370/afm.1935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Bjertnaes O, Deilkås ET, Skudal KE, Iversen HH, Bjerkan AM. The association between patient-reported incidents in hospitals and estimated rates of patient harm. Int J Qual Health Care 2015;27:26-30. 10.1093/intqhc/mzu087. [DOI] [PubMed] [Google Scholar]
- 104. McDonald KM, Bryce CL, Graber ML. The patient is in: patient involvement strategies for diagnostic error mitigation. BMJ Qual Saf 2013;22(Suppl 2):ii33-9. 10.1136/bmjqs-2012-001623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Khan A, Spector ND, Baird JD, et al. Patient safety after implementation of a coproduced family centered communication programme: multicenter before and after intervention study. BMJ 2018;363:k4764. 10.1136/bmj.k4764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Laatikainen O, Miettunen J, Sneck S, Lehtiniemi H, Tenhunen O, Turpeinen M. The prevalence of medication-related adverse events in inpatients-a systematic review and meta-analysis. Eur J Clin Pharmacol 2017;73:1539-49. 10.1007/s00228-017-2330-3. [DOI] [PubMed] [Google Scholar]
- 107. Stang AS, Wingert AS, Hartling L, Plint AC. Adverse events related to emergency department care: a systematic review. PLoS One 2013;8:e74214. 10.1371/journal.pone.0074214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Vlayen A, Verelst S, Bekkering GE, Schrooten W, Hellings J, Claes N. Incidence and preventability of adverse events requiring intensive care admission: a systematic review. J Eval Clin Pract 2012;18:485-97. 10.1111/j.1365-2753.2010.01612.x. [DOI] [PubMed] [Google Scholar]
- 109. Hauck KD, Wang S, Vincent C, Smith PC. Healthy Life-Years Lost and Excess Bed-Days Due to 6 Patient Safety Incidents: Empirical Evidence From English Hospitals. Med Care 2017;55:125-30. 10.1097/MLR.0000000000000631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Donaldson LJ, Kelley ET, Dhingra-Kumar N, Kieny MP, Sheikh A. Medication Without Harm: WHO’s Third Global Patient Safety Challenge. Lancet 2017;389:1680-1. 10.1016/S0140-6736(17)31047-4. [DOI] [PubMed] [Google Scholar]
- 111. Vincent C, Taylor-Adams S, Stanhope N. Framework for analysing risk and safety in clinical medicine. BMJ 1998;316:1154-7. 10.1136/bmj.316.7138.1154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. Hignett S, Lang A, Pickup L, et al. More holes than cheese. What prevents the delivery of effective, high quality and safe health care in England? Ergonomics 2018;61:5-14. 10.1080/00140139.2016.1245446. [DOI] [PubMed] [Google Scholar]
- 113. Reason J. The contribution of latent human failures to the breakdown of complex systems. Philos Trans R Soc Lond B Biol Sci 1990;327:475-84. 10.1098/rstb.1990.0090. [DOI] [PubMed] [Google Scholar]
- 114. Howard R, Avery A, Bissell P. Causes of preventable drug-related hospital admissions: a qualitative study. Qual Saf Health Care 2008;17:109-16. 10.1136/qshc.2007.022681. [DOI] [PubMed] [Google Scholar]
- 115. Neale G, Woloshynowych M, Vincent C. Exploring the causes of adverse events in NHS hospital practice. J R Soc Med 2001;94:322-30. 10.1177/014107680109400702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116. Jylhä V, Saranto K, Bates DW. Preventable adverse drug events and their causes and contributing factors: the analysis of register data. Int J Qual Health Care 2011;23:187-97. 10.1093/intqhc/mzq085. [DOI] [PubMed] [Google Scholar]
- 117. Sujan MA, Ingram C, McConkey T, Cross S, Cooke MW. Hassle in the dispensary: pilot study of a proactive risk monitoring tool for organisational learning based on narratives and staff perceptions. BMJ Qual Saf 2011;20:549-56. 10.1136/bmjqs.2010.048348. [DOI] [PubMed] [Google Scholar]
- 118. Phipps DL, Walshe K, Parker D, Noyce PR, Ashcroft DM. Job characteristics, well-being and risky behaviour amongst pharmacists. Psychol Health Med 2016;21:932-44. 10.1080/13548506.2016.1139142. [DOI] [PubMed] [Google Scholar]
- 119. Huiskes VJ, Burger DM, van den Ende CH, van den Bemt BJ. Effectiveness of medication review: a systematic review and meta-analysis of randomized controlled trials. BMC Fam Pract 2017;18:5. 10.1186/s12875-016-0577-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120. Khalil H, Bell B, Chambers H, et al. Professional, structural and organisational interventions in primary care for reducing medication errors. Cochrane Db Syst Rev 2017;10:CD003942 10.1002/14651858.CD003942.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121. Dixon-Woods M, McNicol S, Martin G. Ten challenges in improving quality in healthcare: lessons from the Health Foundation’s programme evaluations and relevant literature. BMJ Qual Saf 2012;21:876-84. 10.1136/bmjqs-2011-000760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122. Phipps DL, Jones CEL, Parker D, Ashcroft DM. Organizational conditions for engagement in quality and safety improvement: a longitudinal qualitative study of community pharmacies. BMC Health Serv Res 2018;18:783. 10.1186/s12913-018-3607-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123. Ahmed AH, Giri J, Kashyap R, et al. Outcome of adverse events and medical errors in the intensive care unit: a systematic review and meta-analysis. Am J Med Qual 2015;30:23-30. 10.1177/1062860613514770. [DOI] [PubMed] [Google Scholar]
- 124. Ghaferi AA, Birkmeyer JD, Dimick JB. Variation in hospital mortality associated with inpatient surgery. N Engl J Med 2009;361:1368-75. 10.1056/NEJMsa0903048. [DOI] [PubMed] [Google Scholar]
- 125. Shanafelt TD, Balch CM, Bechamps G, et al. Burnout and medical errors among American surgeons. Ann Surg 2010;251:995-1000. 10.1097/SLA.0b013e3181bfdab3. [DOI] [PubMed] [Google Scholar]
- 126. Panesar SS, deSilva D, Carson-Stevens A, et al. How safe is primary care? A systematic review. BMJ Qual Saf 2016;25:544-53. 10.1136/bmjqs-2015-004178. [DOI] [PubMed] [Google Scholar]
- 127. Zwaan L, Schiff GD, Singh H. Advancing the research agenda for diagnostic error reduction. BMJ Qual Saf 2013;22(Suppl 2):ii52-7. 10.1136/bmjqs-2012-001624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128. Riches N, Panagioti M, Alam R, et al. The Effectiveness of Electronic Differential Diagnoses (DDX) Generators: A Systematic Review and Meta-Analysis. PLoS One 2016;11:e0148991. 10.1371/journal.pone.0148991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129. Singh H, Graber ML, Kissam SM, et al. System-related interventions to reduce diagnostic errors: a narrative review. BMJ Qual Saf 2012;21:160-70. 10.1136/bmjqs-2011-000150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130. McDonald KM, Matesic B, Contopoulos-Ioannidis DG, et al. Patient safety strategies targeted at diagnostic errors: a systematic review. Ann Intern Med 2013;158:381-9. 10.7326/0003-4819-158-5-201303051-00004. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary materials: Searches and eTable 1-4


