Abstract
Tryptamines are monoamine alkaloids with hallucinogenic properties and are widely abused worldwide. To hasten the regulations of novel substances and predict their abuse potential, we designed and synthesized four novel synthetic tryptamine analogs: Pyrrolidino tryptamine hydrochloride (PYT HCl), Piperidino tryptamine hydrochloride (PIT HCl), N,N-dibutyl tryptamine hydrochloride (DBT HCl), and 2-Methyl tryptamine hydrochloride (2-MT HCl). Then, we evaluated their rewarding and reinforcing effects using the conditioned place preference (CPP) and self-administration (SA) paradigms. We conducted an open field test (OFT) to determine the effects of the novel compounds on locomotor activity. A head-twitch response (HTR) was also performed to characterize their hallucinogenic properties. Lastly, we examined the effects of the compounds on 5-HTR1a and 5-HTR2a in the prefrontal cortex using a quantitative real-time polymerase chain reaction (qRT-PCR) assay. None of the compounds induced CPP in mice or initiated SA in rats. PYT HCl and PIT HCl reduced the locomotor activity and elevated the 5-HTR1a mRNA levels in mice. Acute and repeated treatment with the novel tryptamines elicited HTR in mice. Furthermore, a drug challenge involving a 7-day abstinence from drug use produced higher HTR than acute and repeated treatments. Both the acute treatment and drug challenge increased the 5-HTR2a mRNA levels. Ketanserin blocked the induced HTR. Taken together, the findings suggest that PYT HCl, PIT HCl, DBT HCl, and 2-MT HCl produce hallucinogenic effects via 5-HTR2a stimulation, but may have low abuse potential.
Keywords: Novel synthetic tryptamine analogs, Abuse potential, Conditioned place preference, Self-administration, Head-twitch response, 5-HT2 receptors
INTRODUCTION
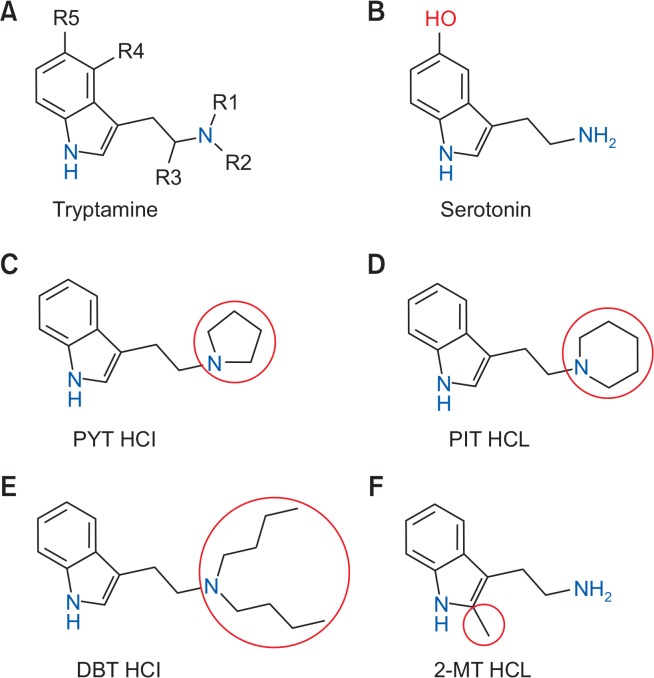
Tryptamines are a group of monoamine alkaloids (Fig. 1) that are synthesized through decarboxylation of the amino acid tryptophan (Tittarelli et al., 2015). They contain an indole ring structure: a bicyclical combination of a benzene ring and a pyrrole ring, with amino group attached to a 2-carbon side chain (Hill and Thomas, 2011). These substances are reported to induce euphoria and hallucinations (visual and auditory) and have been used by people for recreation (Peden et al., 1981). There are also reports of intoxications leading to hospitalization and deaths in the European Union, the US, and Japan (Araújo et al., 2015). In animal studies, tryptamines have been found to substitute fully and shared stimulus effects with various abused hallucinogens (Gatch et al., 2011), suggesting that tryptamines and hallucinogens share common pharmacological effects. In response to these observations, tryptamines are now included into Schedule I of the Controlled Substances Act (Drug Enforcement Administration (DEA), Department of Justice, 2003). However, in spite of the regulatory measures, novel tryptamines are continuously synthesized by introducing chemical modifications to circumvent drug abuse legislation, leaving a difficult problem for legal authorities.
Fig. 1.
The general chemical structure of (A) tryptamine with marked substitution sites, (B) serotonin, and the novel synthetic tryptamine analogs, (C) pyrrolidino tryptamine hydrochloride (PYT HCl), (D) piperidino tryptamine hydrochloride (PIT HCl), (E) N,N-dibutyl tryptamine hydrochloride (DBT HCl) which are produced by the addition of a cyclopentane, cyclohexane, and two butyl groups to the terminal nitrogen atom attached to R1/R2 instead of the hydrogen, and (F) 2-methyl tryptamine hydrochloride (2-MT HCl) which has a 2-methyl in the indole ring.
Previous studies show that tryptamines have an affinity for 5-HT1a, 5-HT2a, and 5-HT2c receptors (Nagai et al., 2007; Ray, 2010) and can inhibit reuptake and increase the release of serotonin, which have attributed to their hallucinogenic effects (Gibbons, 2012; Tittarelli et al., 2015). An experimental approach widely used to determine the hallucinogenic potential of a drug is the drug-induced head-twitch response (HTR) in rodents (Canal and Morgan, 2012). The HTR is a brief paroxysmal head rotation in rodents that is thought to be mediated via the 5-HT2a receptor (Corne et al., 1963; Corne and Pickering, 1967; Canal and Morgan, 2012). Tryptamine hallucinogens and 5-HTR2a agonists like 5-MeO-DIPT, 5-MeO-AMT, and 5-MeO-DMT have been reported to elicit head twitches in rodents (Fantegrossi et al., 2006; Halberstadt et al., 2011; Abiero et al., 2019). Pretreatment with 5-HTR2a antagonists, such as ketanserin (KS), block the drug-induced HTR (Fantegrossi et al., 2010). Based on this information, the HTR is a useful behavioral model to examine the hallucinogenic properties of a drug, especially a novel compound.
As part of the continuing efforts of the Drug Abuse Research Institute of Korea (DARC) to hasten the regulations of novel synthetic tryptamines and to predict the abuse potential of future synthetic tryptamine compounds with similar modifications, we synthesized four novel synthetic tryptamine analogs, (C) pyrrolidino tryptamine hydrochloride (PYT HCl), (D) piperidino tryptamine hydrochloride (PIT HCl), (E) N,N-dibutyl tryptamine hydrochloride (DBT HCl), and (F) 2-methyl tryptamine hydrochloride (2-MT HCl) (Fig. 1), and examined their abuse potential. We performed the conditioned place preference (CPP) in mice and self-administration (SA) test in rats to assess their rewarding and reinforcing effects. We also examined the effects of the new tryptamines on locomotor activity and 5-HTR1a mRNA expression in the prefrontal cortex (PFC). In addition, we investigated the effects of the acute and repeated treatment as well as the challenge in the HTR paradigm on the expression of 5-HTR2a in the PFC. We chose the PFC because it has been implicated in motor activity and the HTR (Fuster, 1991; Willins and Meltzer, 1997; Erberk and Rezaki, 2007). Another cohort of mice was pretreated with KS during the HTR test to determine the involvement of 5-HTR2a receptors in the hallucinogenic effects of the compounds.
MATERIALS AND METHODS
Animals
All animals were purchased from Hanlim Animal Laboratory Co. (Hwasung, Korea) and were housed in a temperature- and humidity-controlled (22 ± 2°C, 55 ± 5%) room with a 12-h light/dark (07:00–19:00 h light) schedule. Male C57BL/6 mice, aged 6 weeks and weighing 20–25 g, were maintained at 6 per cage, and Sprague Dawley rats, aged 8 weeks weighing 250–300 g, were maintained at 4 per cage. The mice were used for the open field, CPP, and HTR assays. The rats were used for the SA test. Before the experiments, all animals were habituated to the laboratory settings for five days. They had free access to food and water during habituation and experiments; however, this was not the case for rats during the lever training and actual SA sessions. Animal care and maintenance were carried out in compliance with the Principles of Laboratory Animal Care (NIH publication No. 85–23 revised 1985) and the Animal Care and Use Guidelines of Sahmyook University, Korea. All efforts were made to minimize the number of animals used and their suffering.
Drugs
PYT HCl, PIT HCl, DBT HCl, and 2-MT HCl powders were synthesized and generously provided by the Laboratory of Medicinal Chemistry of Kyunghee University (Seoul, Korea), whereas KS and methamphetamine (METH) were purchased from Sigma-Aldrich (St. Louis, MO, USA). 2-MT HCl was diluted with 5% dimethyl sulfoxide, 5% polysorbate 80, and normal saline (0.9% w/v NaCl). PYT HCl, PIT HCl, DBT HCl, KS, and METH were diluted with normal saline. All drugs were administered either intraperitoneally (open field test [OFT], CPP and HTR) or intravenously (SA), and the doses were based on prior experiments that addressed abuse potential and tryptamine induced-HTR (Darmani et al., 1990b; Fantegrossi et al., 2010; Gatch et al., 2011; Canal and Morgan, 2012; Creehan et al., 2015; Botanas et al., 2017; Abiero et al., 2019).
N,N-dialkyltryptamines, DBT, PYT, and PIT HCl: N,N-dialkyltryptamines were synthesized from indole-3-glyoxylyl chloride as described previously (Gribble and Pelcman, 1992). In briefly, indole-3-glyoxylyl chloride was treated with three different dialkylamines, such as N,N-dibutylamine, pyrrolidine or piperidine to give indole-3-(N,N-dimethyl)glyoxylamides. These amides were reduced with LiAlH4 to give N,N-dialkyltryptamines. The resulting amines were treated with HCl to afford them as an HCl salt. Their structures were confirmed by the following spectroscopic analyses.
DBT HCl: 1H NMR (400 MHz, D2O) δ 7.58 (d, J=7.7 Hz, 1H), 7.45 (d, J=7.9 Hz, 1H), 7.26–7.17 (m, 2H), 7.12 (t, J=6.7 Hz, 1H), 3.38 (t, J=7.0 Hz, 2H), 3.13 (t, J=7.0 Hz, 2H), 3.04–3.16 (m, 4H), 1.48–1.60 (m, 4H), 1.17–1.31 (m, 4H), 0.80 (t, J=6.9 Hz, 6H); 13C NMR (100 MHz, DMSO-d6) δ 136.68, 127.17, 123.77, 121.66, 118.94, 118.72, 112.01, 109.75, 52.59, 51.80(2C), 25.29(2C), 19.97(2C), 19.87, 14.03(2C); High-Resolution-MS, calcd for C18H29N2[M+H]+ 273.2325, found 273.2326.
3-(2-Pyrrolidin-1-ylethyl)-1H-indole hydrochloride (PYT HCl): 1H NMR (400 MHz, D2O) δ 7.57 (d, J=8.1 Hz, 1H), 7.43 (d, J=8.1 Hz, 1H), 7.19 (s, 1H), 7.18 (t, J=7.8 Hz, 1H), 7.09 (t, J=7.8 Hz, 1H), 3.49 (m, 2H), 3.37 (t, J=7.3 Hz, 2H), 3.08 (t, J=7.3 Hz, 2H), 2.95 (m, 2H), 1.99 (m, 2H), 1.83 (m, 2H); 13C NMR (100 MHz, DMSO-d6) δ 136.69, 127.15, 123.65, 121.67, 118.91, 118.78, 112.01, 109.79, 54.55, 53.11(2C), 23.21(2C), 21.84; High-Resolution-MS, calcd for C14H19N2[M+H]+ 215.1543, found 215.1542.
3-(2-Piperidin-1-ylethyl)-1H-indole hydrochloride (PIT HCl): 1H NMR (400 MHz, D2O) δ 7.58 (d, J=7.9 Hz, 1H), 7.44 (d, J=8.2 Hz, 1H), 7.22-7.16 (m, 2H), 7.10 (t, J=7.4 Hz, 1H), 3.47 (d, J=12.0 Hz, 2H), 3.28 (t, J=7.8 Hz, 2H), 3.12 (t, J=7.8 Hz, 2H), 2.85 (t, J=12.0 Hz, 2H), 1.83 (m, 2H), 1.70 (m, 1H), 1.60 (m, 2H), 1.38 (m, 1H); 13C NMR (100 MHz, DMSO-d6) δ 136.70, 127.18, 123.54, 121.67, 118.90, 118.83, 111.99, 109.82, 56.44, 52.23(2C), 22.82(2C), 22.01, 19.97; High-Resolution-MS, calcd for C15H21N2[M+H]+ 229.1699, found 229.1705.
2-MT HCl: 2-methyltryptamine was synthesized from 2-methylindole as described previously (Vallejos et al., 2005). In briefly, 2-methylindole was condensed with nitromethane and then reduced with LiAlH4 to give 2-methyltryptamine. The resulting amine was treated with HCl to afford 2-MT as an HCl salt. Its structure was confirmed by the following spectroscopic analyses. 1H NMR (400 MHz, CDCl3) δ 8.10 (bs, 1H), 7.50 (d, J=7.1 Hz, 1H), 7.23 (d, J=7.2 Hz, 1H), 7.12–7.03 (m, 2H), 2.96 (t, J=6.3 Hz, 2H), 2.84 (t, J=6.3 Hz, 2H), 2.35 (s, 3H), 1.41 (s, 2H); 13C NMR (100 MHz, DMSO-d6) δ 135.34, 131.90, 128.78, 120.95, 119.13, 118.00, 110.28, 109.02, 42.63, 28.26, 11.76; High-Resolution-MS, calcd for C11H15N2[M+H]+ 175.1230, found 175.1239.
Conditioned place preference test
The place preference apparatus that was used consisted of two Plexiglas compartments (17.4×12.7×12.7 cm3) made of polyvinylchloride and separated by a guillotine door. To provide a visual and tactile difference between the compartments, one compartment was white with a 6.352-mm stainless steel mesh floor, while the other was black with a stainless-steel grid floor (3.2-mm diameter rods placed 7.9 mm apart); the compartments were covered and illuminated. Behavior was recorded and analyzed using a computer-based video tracking system (Ethovision, Noldus, Netherlands).
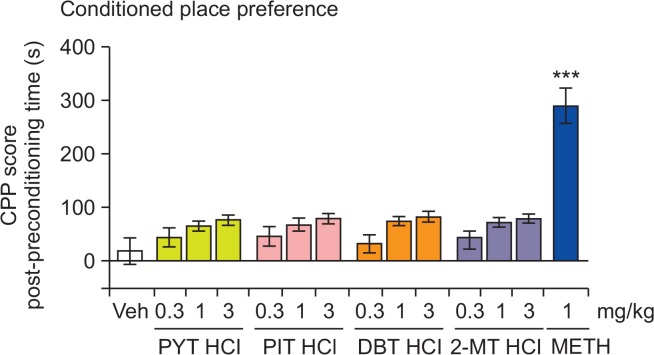
The CPP test consisted of three phases: habituation and pre-conditioning, conditioning, and post-conditioning. During habituation, the mice were allowed to explore both compartments for 20 min once a day for two days. On the day after habituation, the time spent on each side was recorded (pre-conditioning). To eliminate side-preference bias, the data from the pre-conditioning phase were used to group the animals that spent similar amounts of time in each compartment. Mice that spent over 840 s in one compartment were excluded from the test (n=5). In the conditioning phase, mice (n=10 per group) received PYT HCl, PIT HCl, DBT HCl, 2-MT HCl (0.3, 1, or 3 mg/kg), METH (1 mg/kg), or vehicle (veh) and were confined to a randomly designated compartment for 45 min. Parallel experiments were also performed with METH because this drug has been demonstrated to induce conditioned place preference (Berry et al., 2012). On alternate days, the mice received the veh and were put in the non-drug-paired compartment. The conditioning lasted for 8 sessions (4 drug and 4 veh conditioning sessions). The post-conditioning phase started 24 h after the final conditioning session. In this phase, the drug-free mice were allowed to freely access both compartments for 20 min. The CPP score was calculated as the difference in the time spent in the drug-paired compartment between the post- and pre-conditioning phases.
Self-administration test
For the drug SA test, rats were placed in standard operant conditioning chambers (Coulbourn Instruments, Allen-town, PA, USA) with ventilation fans to mask the external noise. Each operant chamber had a food pellet dispenser, two 4.5-cm-wide response levers (left and right), a stimulus light located 6 cm above the left lever, and a centrally positioned house light (2.5 W, 24 V) at the top of the chamber. Downward pressure (approximately equivalent to 25 g) on the levers resulted in a programmed response (described below). Located beside the operant chamber was a motor-driven syringe pump that delivered solutions at 0.01 mL/s. Solutions flowed through polytetrafluoroethylene tubing connected to a swivel, which was mounted on a counterbalanced arm at the top of the chamber, allowing free movement of the animals within the chamber. The tubing was connected to an intravenous catheter implanted in the animal. Graphic State Notation software (Coulbourn Instruments) was used to control experimental parameters and collect data.
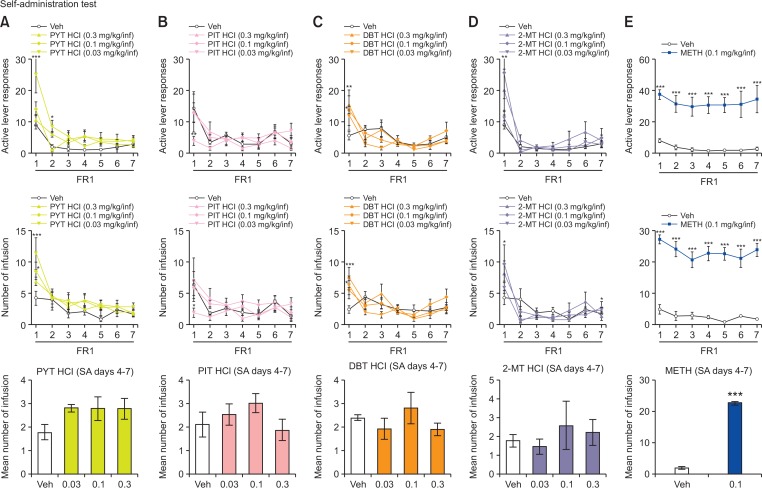
Initially, rats were trained to press the active (drug-paired) lever (30 min/day for 3 days) for a contingent food pellet reward on a continuous schedule of reinforcement. During training, rats were food-restricted such that no rat dropped below 90% of its starting body weight. Only those rats that acquired at least 80 pellets during the last session of training were selected and prepared for surgery. Intrajugular surgeries were performed according to a previously described procedure (De La Peña et al., 2012). After surgery, each rat was individually housed in a transparent plastic cage. Rats were allowed to recover for 5 days after surgery. During the first 4 days following surgery, rats received an antibiotic treatment (gentamicin, 1 mg/kg i.p.). After a recovery period of 5 days, SA testing commenced and was performed for 2 h/day. Rats (n=6–8 per group) were placed under a fixed-ratio (FR)1 schedule for 7 days. During the experiment, a press of the left lever (active lever) resulted in an injection of 0.1 mL of PYT HCl, PIT HCl, DBT HCl, 2-MT HCl (0.03, 0.1, or 0.3 mg/kg/infusion), METH (0.1 mg/kg/infusion), or veh. METH was used as a reference drug because this drug has been evaluated in rats self-administrating METH (Mcfadden et al., 2012). Simultaneously, the house light was switched off and the stimulus light was illuminated and remained lit for 20 s after the end of the infusion (time-out period). Lever presses during the time-out period were recorded but did not have any corresponding effects. Similarly, presses of the right lever (inactive lever) were not reinforced. Catheter patency was assessed by injecting 0.1 ml of thiopental sodium (10 mg/kg) one day before and on the last day of the SA testing period. Rats (n=6) that did not lose muscle tone within 3–5 s were excluded from the experiments.
Open field test
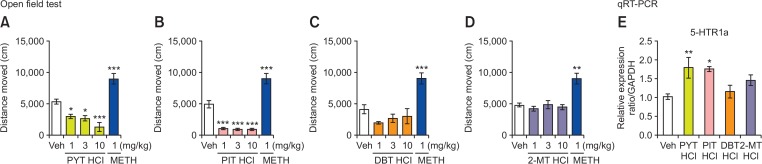
The OFT was performed according to a previous study (Botanas et al., 2017). Mouse locomotor activity was assessed in a square black Plexiglas container with white surface in an open field arena (42×42×42 cm3). The apparatus was illuminated by a 100 W lamp placed 2 m above the center of the floor of the whole apparatus. For the first day, mice (n=10 mice per group) were allowed to habituate to the apparatus for 30 min. On the second day, their locomotor activity was recorded and used as a baseline. Thereafter, mice were treated with PYT HCl, PIT HCl, DBT HCl, 2-MT HCl (1, 3, 10 mg/kg), METH (1 mg/kg), or vehicle. We used METH as positive control because this drug has been shown to increase locomotor activity (Kitanaka et al., 2003). Locomotor activity [distance moved (cm)] was immediately measured by a computer-based video-tracking system (Ethovision) for 30 min.
Head-twitch response
The HTR is a distinct twitching behavior of the head of rodents. The HTR procedure followed that described by our previous study (Abiero et al., 2019). It is easily monitored and usually cannot be mistaken for other head movements such as head-shakes or head-jerks. A camera mounted above the observation cage began recording the behavior and continued to do so for 30 min; the amount of HTRs were later scored after 2 min of habituation by two trained observers (blinded to the grouping).
To assess the acute and repeated effects, mice (n=6 per group) were treated i.p. with vehicle, PYT HCl, PIT HCl, DBT HCl and 2-MT HCl (3 mg/kg) once a day for 7 days and challenged with the same drug and dose following 7 days of drug abstinence. A dose of 3mg/kg was selected for the present study because in a previous study it had submaximal effects and reliably increased the number of head twitches (Abiero et al., 2019). To determine the involvement of 5-HTR2a, another cohorts of mice (n=6 per group) were injected with KS (0.1 mg/kg) 10 min before PYT HCl, PIT HCl, DBT HCl, and 2-MT HCl (3 mg/kg) or veh administration and were observed for the HTR.
Tissue collection, RNA preparation, and quantitative real-time PCR
Mice (n=6 per group) treated with PYT HCl, PIT HCl, DBT HCl, and 2-MT HCl (3 mg/kg) or veh for 1-day, 7-day and challenge day were sacrificed by cervical dislocation and decapitation 30 min after the last drug dose. Brains were rapidly and carefully removed as described previously (Spijker, 2011) and placed in ice-cold saline to prevent damage to the brain. The PFC was dissected out and immediately frozen at −70°C until further use. The PFC was used because this brain region has been implicated in motor activity and in the induction of the HTR (Fuster, 1991; Willins and Meltzer, 1997; Erberk and Rezaki, 2007). Total RNA was isolated using Trizol (Invitrogen, Carlsbad, CA, USA) according to the manufacturer’s instructions. The RNA was further purified using the Hybrid-RTM kit (Geneall Biotechnology, Seoul, Korea). The concentration of total RNA was determined using a Colibri Microvolume Spectrometer (Titertek-Berthold, Pforzheim, Germany).
A quantitative real-time polymerase chain reaction (qRT-PCR) was performed to determine the levels of 5-HTR1a and 5-HTR2a mRNA in the PFC. The method was similar to that used in our previous study (Botanas et al., 2017). In brief, 1 μg of total RNA was reverse transcribed into cDNA using AccuPower® CycleScript RT PreMix (Bioneer, Seoul, Korea) according to the manufacturer’s instructions. The cDNA was amplified using a set of custom sequence-specific primers (Cosmogenetech, Seoul, Korea) and detected using SYBR Green (SolGent, Inc., Daejon, Korea). The following primer sequences were used: 5-HTR1a, forward 5′-GACAGGCGGCAACGATACT-3′, reverse 5′-CCAAGGAGCCGATGAGATAGTT-3′; 5-HTR2a, forward 5′-TAATGCAATTAGGTGACGACTCG-3′, reverse 5′-GCAGGAGAGGTTGGTTCTGTTT-3′; Glyceraldehyde 3-phosphate dehydrogenase (GAPDH), forward 5′-AGGTCGGTGTGAACGGATTTG-3′, reverse 5′-TGTAGACCATGTAGTTGAGGTCA-3′. The qRT-PCR reactions were performed in triplicates. Values were normalized to the relative amounts of GAPDH mRNA. Results are shown as a relative expression level calculated using the 2−ΔΔCT method (Vanguilder et al., 2008).
Data analysis
The results from CPP (Fig. 2), open field tests (Fig. 4A–4D) and qRT-PCR for 5-HTR1a (Fig. 4E) experiments were analyzed by one-way analysis of variance (ANOVA), followed by Dunnett’s test to compare the effects of PYT HCl, PIT HCl, DBT HCl, 2-MT HCl, and METH to the veh. Data from the active lever responses and the number of infusions (Fig. 3) during the 2-h/d, 7-day SA test, the HTR (Fig. 5A) and qRT-PCR for 5-HTR2a (Fig. 5C) experiments were analyzed using a two-way repeated measures analysis of variance (ANOVA) with treatment as the between-subject factor and SA days/treatment days as the within-subject factor. Bonferroni’s test was utilized to further compare the effects of PYT HCl, PIT HCl, DBT HCl, 2-MT HCl, and METH to the veh and each SA day/treatment day. The mean infusion during stable days (days 4–7) was also presented and analyzed with one-way ANOVA for PYT HCl, PIT HCl, DBT HCl, 2-MT HCl, and the unpaired, two tailed t-test for METH. A one-way ANOVA was used to analyze the effects of KS pretreatment on the HTR (Fig. 5B), followed by Bonferroni’s test to determine the differences between the group. The criterion for statistical significance was considered to be p<0.05. Values are expressed as the as mean ± standard error of the mean (SEM). All analyses were performed with GraphPad Prism software (GraphPad Software, San Diego, CA, USA).
Fig. 2.
The effects of PYT HCl, PIT HCl, DBT HCl, 2-MT HCl, and METH on the CPP test in mice. Each bar represents the mean ± SEM of the CPP score, calculated as the difference in the time spent in the drug-paired or saline-paired side during the postminus the pre-conditioning phases. n=7–8 animals per group. ***p<0.001 significantly different from the veh group (Dunnett’s posttest).
Fig. 4.
(A–D) The effects of PYT HCl, PIT HCl, DBT HCl, 2-MT HCl, and METH on the locomotor activity (n=10 animals per group). (E) mRNA expression levels of 5-HTR1a in the mouse prefrontal cortex (n=6 animals per group). Mice were treated with vehicle, PYT HCl, PIT HCl, DBT HCl, 2-MT HCl, and METH. Values are (mean ± SEM). *p<0.05, **p<0.01, ***p<0.001 significantly different from the veh group (Dunnett’s posttest).
Fig. 3.
The effects of PYT HCl, PIT HCl, DBT HCl, 2-MT HCl, and METH on the self-administration in rats. Active lever responses made and number of infusions obtained during the 2-h/d, 7-day SA test, under a FR1 schedule; values are mean ± SEM. n=6-8 animals per group. *p<0.05, **p<0.01, ***p<0.001 relative to veh group (Bonferroni’s posttest).
Fig. 5.
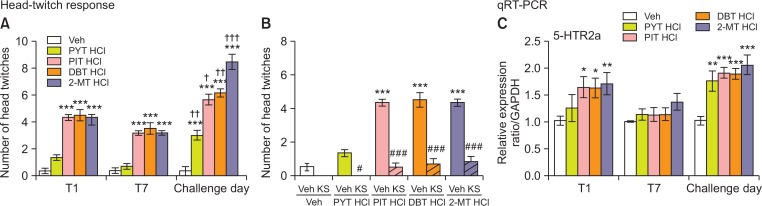
(A, C) Effects of PYT HCl, PIT HCl, DBT HCl, and 2-MT HCl treatment in the head-twitch response (HTR) and the mRNA expression levels of 5-HTR2a in the mouse prefrontal cortex during 7 days of treatment and on the day of challenge. (B) Another cohort of mice were used to examine the effect of ketanserin pretreatment (0.1 mg/kg) 10 min prior to treatment with PYT HCl, PIT HCl, DBT HCl, or 2-MT HCl during the HTR assay. Mice were treated with vehicle, PYT HCl (3 mg/kg), PIT HCl (3 mg/kg), DBT HCl (3 mg/kg), and 2-MT HCl (3 mg/kg) repeatedly for 7 days, and then challenged with the same drug and dose following a 7-day withdrawal period. HTRs and 5-HTR2a mRNA expression levels were assessed on the 1st and 7th day of treatment and on the challenge day. Values are mean ± S.E.M. n=5–6 animals per group. *p<0.05, **p<0.01, ***p<0.001 significantly different from the veh, †p<0.05, ††p<0.01, †††p<0.001 compared to acute treatment; #p<0.05, ###p<0.001 compared to PYT HCl, PIT HCl, DBT HCl, or 2-MT HCl pretreated with veh (Bonferroni’s posttest).
RESULTS
Conditioned place preference
Fig. 2 displays the CPP scores of mice treated with the test drugs (i.e., PYT HCl, PIT HCl, DBT HCl, and 2-MT HCl), METH, and veh. A one-way ANOVA revealed that METH significantly increased the CPP score [F (13,105)=16.12, p<0.001], but none of the four test drugs had an effect.
Self-administration test
Fig. 3 shows the number of active lever responses and infusion of veh, PYT HCl, PIT HCl, DBT HCl-and 2-MT HCl-treated rats during the 2-h/day, 7-day SA sessions under the FR1 schedule. Rats self-administering PYT HCl displayed a significant difference between treatments [F (3,168)=3.829, p<0.05], SA days [F (6,168)=30.10, p<0.001], and interaction between them [F (18,168)=3.918, p<0.001]. A significant difference in the number of infusions in SA days [F (6,168)=19.78, p<0.001] was also observed. However, post-hoc analyses showed that there was an increase in lever pressing only during the first (p<0.001) and second day (p<0.05) of SA with exposure to 0.03 mg/kg/infusion. Bonferroni post-tests revealed an increase in the number of infusions during the first day of SA with exposure to 0.1 mg/kg/infusion (p<0.05) and 0.03 mg/kg/infusion (p<0.001). In a similar manner, a significant variation in SA days [F (6,138)=7.910, p<0.001] but no effect on treatments [F (3,138)=0.3904, p>0.05] and interaction between them [F (18,138)=1.635, p>0.05] was observed in rats self-administering PIT HCl. A significant difference in the number of infusions in SA days [F (6,138)=6.021, p<0.001] and interaction between them [F (18,138)=1.687, p<0.05] but not with treatments [F (3,138)=1.332, p>0.05] was also observed. Bonferroni post-tests revealed that there was an increase in lever pressing (p<0.01) and the number of infusions (p<0.05) only on the first day of SA with exposure to 0.03 mg/kg/infusion. SA of DBT HCl led to a significant difference in SA days [F (6,144)=11.45, p<0.001] but not with the treatments [F (3,144)=1.118, p>0.05] and interaction between them [F (18,144)=1.465, p>0.05]. There was also a significant variation in the number of infusion in SA days [F (6,144)=9.281, p<0.001] and interaction between them [F (18,144)=2.008, p<0.05]. Post-hoc tests revealed that DBT HCl increased the number of lever presses only during the first day of SA when administered at dosages of 0.3 mg/kg/infusion (p<0.01) and 0.1 mg/kg/infusion (p<0.05) and increased the number of infusions when administered at dosages of 0.3 mg/kg/infusion (p<0.001) and 0.03 mg/kg/infusion (p<0.05). A repeated measures two-way ANOVA revealed a significant difference in treatment [F (3,162)=3.141, p<0.05] and SA days [F (6,162)=14.06, p<0.001] but no effect on interaction between them [F (18,162)=1.111, p>0.05] when 2-MT HCl was compared to vehicle. Similarly, a significant effect of SA days [F (6,162)=12.28, p<0.001] was also observed on the number of infusions. Bonferroni post-tests revealed an increase in lever pressing during the first day of SA with exposure to 0.1 mg/kg/infusion (p<0.01). There was also an increase in the number of infusions during the first (p<0.05) and seventh day (p<0.05) of the SA test of exposure to 0.1 mg/kg/infusion. One-way ANOVA of mean infusions from days 4–7 also revealed no differences (p>0.05) in rats self-administering PYT HCl, PIT HCl, DBT HCl, and 2-MT HCl when compared to the vehicle group. In contrast, METH-treated rats exhibited a significant increase in active lever responses [F (1,78)=40.33, p<0.001] and number of infusions [F (1,78)=146.3, p<0.001] in treatments, and post-hoc tests revealed that there was an increase in the lever pressing and number of infusions across all days (p<0.001) of the 7-day test. Moreover, unpaired t-test revealed that during the stable SA days (days 4–7), there was a significant increase in infusions (p<0.001).
Open field test
Fig. 4A–4D illustrate the locomotor activity of the mice treated with PYT HCl, PIT HCl, DBT HCl, 2-MT HCl (1, 3, 10 mg/kg), METH (1 mg/kg), or veh. It was revealed that PYT and PIT dose-dependently decreased the locomotor activity of the mice. One-way ANOVA showed a significant locomotor alteration [PYT HCl [F (4,48)=25.90, p<0.001]] and [PIT HCl [F (4,49)=57.93, p<0.001]]. Post-hoc analyses further revealed that mice treated with 1 (p<0.05), 3 (p<0.05), and 10 (p<0.001) mg/kg PYT HCl and PIT HCl (p<0.001) displayed a significant decreased locomotor activity of the mice compared with the vehicle group. In contrast, METH-treated mice displayed increased locomotor activity (p<0.001). Fig. 4E demonstrates the effects of acute PYT HCl, PIT HCl, DBT HCl, 2-MT HCl, or veh treatment on the levels of 5-HT1a mRNA in the PFC of the mice. A one-way ANOVA showed a significant effect of the treatments on the expression levels of 5-HT1a [F (4,29)=4.469, p<0.01]. It was further revealed that PYT HCl (p<0.01) and PIT HCl (p<0.05) significantly increased 5-HT1a mRNA levels.
Tryptamine-derived hallucinogens induce head-twitch response and are blocked by pretreatment with ketanserin that is modulated by the 5-HT2a receptor
Fig. 5A demonstrates the acute and repeated effects of mice treated with PYT HCl, PIT HCl, DBT HCl, 2-MT HCl, or veh in the HTR assay. A two-way repeated measures ANOVA revealed a significant difference between treatments [F (4,25)=83.90, p<0.001], treatment days [F (2,25)=126.5, p<0.001], and interaction between the two factors [F (8,25)=14.90, p<0.001]. Post hoc analysis showed that mice treated with PIT HCl (p<0.001), DBT HCl (p<0.001) and 2-MT HCl (p<0.001) displayed a significant increase in the number of the HTR on the first, seventh, and challenge days of treatment and PYT HCl (p<0.001) on the challenge day. Consequently, a drug challenge treatment with PYT HCl (p<0.01), PIT HCl (p<0.05), DBT HCl (p<0.01) and 2-MT HCl (p<0.001) led to increased head-twitch behavior (p<0.001) when compared to acute treatment. Fig. 5B shows the effects of KS pretreatment on the PYT HCl, PIT HCl, DBT HCl-and 2-MT HCl-induced HTR in mice. A one-way ANOVA revealed a significant difference between treatments [F (9,59)=57.72, p<0.001]. Similar to the results above, PIT HCl (p<0.001), DBT HCl (p<0.001), and 2-MT HCl (p<0.001) without KS pretreatment led to an increased number of HTRs in mice. Pretreatment with KS significantly decreased the number of HTRs in mice treated with tryptamine-derived hallucinogens (p<0.05, p<0.001). Fig. 5C reveals the effects of PYT HCl, PIT HCl, DBT HCl, 2-MT HCl, or veh treatment on the levels of 5-HTR2a mRNA in the PFC. Significant differences were seen between treatments [F (4,22)=14.17, p<0.001] and treatment days [F (2,22)=15.53, p<0.001] but there was no significant interaction between treatment and treatment days [F (8,22)=1.219, p>0.05] in 5-HTR2a mRNA levels. Further comparisons revealed that acute with PIT HCl (p<0.05), DBT HCl (p<0.05), and 2-MT (p<0.01) increased levels of 5-HTR2a mRNA compared to veh and challenge treatment with PYT HCl (p<0.01), PIT HCl (p<0.001), DBT HCl (p<0.001), and 2-MT HCl (p<0.001) on the challenge day. Repeated PYT HCl, PIT HCl, DBT HCl, and 2-MT HCl treatment led to reduced 5-HTR2a mRNA levels when compared to acute treatment.
DISCUSSION
In the present study, we found that the novel synthetic tryptamine analogs PYT HCl, PIT HCl, DBT HCl, and 2-MT HCl did not induce CPP in mice and were not reliably self-administered by rats, suggesting that these drugs have no or minimal rewarding and reinforcing effects. This result is similar to other tryptamines that fail to produce CPP and SA in animals (Fantegrossi et al., 2008; Abiero et al., 2019). Previous studies have shown that several tryptamines and hallucinogens lack the affinity for dopamine receptors or dopamine transporters, which may render them unable to affect brain dopaminergic neurotransmission that is associated with drug addiction and reinforcing properties (Nichols, 2004). These tryptamines also affect the serotonin system by increasing the release and inhibiting the reuptake of serotonin in the brain: an effect attributed to their decreased reinforcing efficacy, resulting in the difficulty to initiate reliable SA (Rickli et al., 2015). Considering that PYT HCl, PIT HCl, DBT HCl, and 2-MT HCL are synthetic tryptamines, the aforementioned effects of the tryptamines might have been the same effects of the four novel tryptamines in the present study. Additional experiments are needed to examine the effects of PYT HCl, PIT HCl, DBT HCl, and 2-MT HCL in the dopamine and serotonin system. Furthermore, tryptamines have been reported to be difficult to elicit CPP and SA response in animals, despite the fact that they are being abused by humans (Nichols, 2004; Fantegrossi et al., 2008). However, the two-lever drug discrimination studies show that tryptamines can be a substitute for abused hallucinogens (Winter, 2009; Gatch et al., 2011), suggesting that this paradigm is sensitive to the rewarding stimuli of tryptamines. Thus, it would also be worthwhile to assess the abuse potential of PYT HCl, PIT HCl, DBT HCl, and 2-MT HCL in other behavioral paradigms, such as drug discrimination test, in the future. Nevertheless, our results suggest that these novel synthetic tryptamine analogs have a low abuse potential.
Consistent with the results of other tryptamines, PYT HCl and PIT HCl dose-dependently decreased the locomotor activity of the mice (Krebs-Thomson et al., 1998). It has been suggested that tryptamines decrease the locomotor activity by stimulating the 5-HTR1a in the brain (Krebs-Thomson et al., 2006). For instance, pretreatment with 5-HTR1a antagonist WAY-100635 blocked the reduction of locomotor activity in mice with 5-MeO-DMT, psilocin, LSD, and DMT (Krebs-Thomson et al., 1998, 2006; Halberstadt and Geyer, 2011). Furthermore, 5-HTR1a −/− knockout mice did not exhibit decreased locomotor activity following 5-MeO-DMT (Van Den Buuse et al., 2011). Here, we have shown that PYT HCl and PIT HCl increased the mRNA levels of 5-HTR1a in the PFC. This result suggests that 5-HTR1a is involved in the PYT HCl-and PIT HCl-induced reduction of locomotor activity in mice. However, further studies are needed to determine why DBT HCl and 2-MT HCl did not alter the locomotor activity given their structural similarities with PYT HCl and PIT HCl.
Similar to other tryptamines, acute treatment with PYT HCl, PIT HCl, DBT HCl, and 2-MT HCl induced the HTR in mice (Fig. 5A) (Fantegrossi et al., 2006, 2010; Canal and Morgan, 2012; Abiero et al., 2019). This result indicates that novel tryptamines can induce hallucinogenic effects. As mentioned above, the HTR is mediated by 5-HTR2a activation in the PFC (Nichols, 2016). In line with this, our results show that KS pretreatment blocked the HTR induced by PYT HCl, PIT HCl, DBT HCl, and 2-MT HCl. Furthermore, these compounds increased the 5-HTR2a mRNA levels in the PFC following acute treatment. These findings suggest that the induced HTR by the novel tryptamines was mediated through 5-HTR2a activation. Although repeated treatment with the novel tryptamines induced the HTR, this is inconsistent with findings that other tryptamines lead to decreased HTR after repeated administration (Pranzatelli and Pluchino, 1991; Smith et al., 2014). Nevertheless, our result is in agreement with previous studies showing that repeated treatment of DMT did not lead to a reduced number of HTRs (Carbonaro and Gatch, 2016). On the contrary, qRT-PCR results show that repeated treatment decreased 5-HTR2a mRNA levels. Repeated administration of 1-(2, 5-dimethoxy-4-iodophenyl)-2-aminopropane (DOI), a selective 5-HT 2A/2C agonist, inducing HTR did not result in changes in the affinity for 5-HTR2a (Anji et al., 2000). Therefore, it is possible that the induced HTR after repeated treatment with the novel tryptamines is most likely due to the unaffected affinity for the 5-HT2a receptors. Interestingly, during challenge day, the novel tryptamines elicited higher number of HTRs and an increasing trend in 5-HTR2a mRNA levels as compared to acute treatment. A theory on classical monoamine receptor adaptation suggests that chronic deprivation of receptor stimulation normally results in receptor up-regulation, which may result in an enhanced behavioral response (Darmani et al., 1990a). Thus, it is likely that the increased HTR during the drug challenge corresponds with the increasing trend in 5-HTR2a mRNA levels. Taken together, this study suggests that PYT HCl, PIT HCl, DBT HCl, and 2-MT HCl have hallucinogenic properties, and highlights the parallel dynamics of the HTR induced by the novel tryptamines and 5-HTR2a mRNA levels in the PFC.
In conclusion, PYT HCl, PIT HCl, DBT HCl, and 2-MT HCl do not induce rewarding and reinforcing effects; therefore these drugs are likely to have a low potential for abuse. Furthermore, these novel tryptamines induce hallucinogenic effects, as evidenced in the HTR assay, which is probably mediated by the activation of 5-HTR2a in the PFC. However, additional studies (e.g. structure-activity relationship) are necessary to clarify the differential effects of each analog in locomotor activity and HTR assays. Nonetheless, the present study provides valuable information that may be useful in predicting the abuse potential and psychopharmacological effects of potential synthetic tryptamines.
Acknowledgments
This work was supported by the Ministry of Food and Drug Safety (MFDS) of Korea (14182MFDS979, 19182MFDS410). The authors have no conflicts of interest to declare.
REFERENCES
- Abiero A, Botanas CJ, Sayson LV, Custodio RJ, de la Peña JB, Kim M, Lee HJ, Seo JW, Ryu IS, Chang CM, Yang JS, Lee YS, Jang CG, Kim HJ, Cheong JH. 5-Methoxy-α-methyltryptamine (5-MeO-AMT), a tryptamine derivative, induces head-twitch responses in mice through the activation of serotonin receptor 2a in the prefrontal cortex. Behav. Brain Res. 2019;359:828–835. doi: 10.1016/j.bbr.2018.07.020. [DOI] [PubMed] [Google Scholar]
- Anji A, Kumari M, Hanley NS, Bryan GL, Hensler JG. Regulation of 5-HT2A receptor mRNA levels and binding sites in rat frontal cortex by the agonist DOI and the antagonist mianserin. Neuropharmacology. 2000;39:1996–2005. doi: 10.1016/S0028-3908(00)00026-5. [DOI] [PubMed] [Google Scholar]
- Araújo AM, Carvalho F, de Lourdes Bastos M, De Pinho PG, Carvalho M. The hallucinogenic world of tryptamines: an updated review. Arch. Toxicol. 2015;89:1151–1173. doi: 10.1007/s00204-015-1513-x. [DOI] [PubMed] [Google Scholar]
- Berry JN, Neugebauer NM, Bardo MT. Reinstatement of methamphetamine conditioned place preference in nicotine-sensitized rats. Behav. Brain Res. 2012;235:158–165. doi: 10.1016/j.bbr.2012.07.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Botanas CJ, de la Peña JB, Custodio RJ, dela Peña IJ, Kim M, Woo T, Kim HJ, Kim HI, Cho MC, Lee YS, Cheong JH. Methoxetamine produces rapid and sustained antidepressant effects probably via glutamatergic and serotonergic mechanisms. Neuropharmacology. 2017;126:121–127. doi: 10.1016/j.neuropharm.2017.08.038. [DOI] [PubMed] [Google Scholar]
- Canal CE, Morgan D. Head-twitch response in rodents induced by the hallucinogen 2, 5-dimethoxy-4-iodoamphetamine: a comprehensive history, a re-evaluation of mechanisms, and its utility as a model. Drug Test. Anal. 2012;4:556–576. doi: 10.1002/dta.1333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carbonaro TM, Gatch MB. Neuropharmacology of N, N-dimethyltryptamine. Brain Res. Bull. 2016;126:74–88. doi: 10.1016/j.brainresbull.2016.04.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corne S, Pickering R. A possible correlation between drug-induced hallucinations in man and a behavioural response in mice. Psychopharmacologia. 1967;11:65–78. doi: 10.1007/BF00401509. [DOI] [PubMed] [Google Scholar]
- Corne S, Pickering R, Warner B. A method for assessing the effects of drugs on the central actions of 5-hydroxytryptamine. Br. J. Pharmacol. Chemother. 1963;20:106–120. doi: 10.1111/j.1476-5381.1963.tb01302.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Creehan KM, Vandewater SA, Taffe MA. Intravenous self-administration of mephedrone, methylone and MDMA in female rats. Neuropharmacology. 2015;92:90–97. doi: 10.1016/j.neuropharm.2015.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darmani NA, Martin BR, Glennon RA. Withdrawal from chronic treatment with (+/−)-DOI causes super-sensitivity to 5-HT2 receptor-induced head-twitch behaviour in mice. Eur. J. Pharmacol. 1990a;186:115–118. doi: 10.1016/0014-2999(90)94066-7. [DOI] [PubMed] [Google Scholar]
- Darmani NA, Martin BR, Pandey U, Glennon RA. Do functional relationships exist between 5-HT1A and 5-HT2 receptors? Pharmacol. Biochem. Behav. 1990b;36:901–906. doi: 10.1016/0091-3057(90)90098-3. [DOI] [PubMed] [Google Scholar]
- de la Peña JBI, Lee HC, de la Peña IC, Woo TS, Yoon SY, Lee HL, Han JS, Lee JI, Cho YJ, Shin CY, Cheong JH. Rewarding and reinforcing effects of the NMDA receptor antagonist-benzodiazepine combination, zoletil®: Difference between acute and repeated exposure. Behav. Brain Res. 2012;233:434–442. doi: 10.1016/j.bbr.2012.05.038. [DOI] [PubMed] [Google Scholar]
- Drug Enforcement Administration (DEA), Department of Justice Schedules of controlled substances: temporary placement of alpha-methyltryptamine and 5-methoxy-N, N-diisopropyltryptamine into Schedule I. Final rule. Fed. Regist. 2003;68:16427. [PubMed] [Google Scholar]
- Erberk NO, Rezaki M. Prefrontal cortex: implications for memory functions and dementia. Turk Psikiyatri Derg. 2007;18:262–269. [PubMed] [Google Scholar]
- Fantegrossi W, Harrington A, Kiessel C, Eckler J, Rabin R, Winter J, Coop A, Rice K, Woods J. Hallucinogen-like actions of 5-methoxy-N, N-diisopropyltryptamine in mice and rats. Pharmacol. Biochem. Behav. 2006;83:122–129. doi: 10.1016/j.pbb.2005.12.015. [DOI] [PubMed] [Google Scholar]
- Fantegrossi W, Simoneau J, Cohen M, Zimmerman S, Henson C, Rice K, Woods J. Interaction of 5-HT2A and 5-HT2C receptors in R (−)-2, 5-dimethoxy-4-iodoamphetamine-elicited head twitch behavior in mice. J. Pharmacol. Exp. Ther. 2010;335:728–734. doi: 10.1124/jpet.110.172247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fantegrossi WE, Murnane KS, Reissig CJ. The behavioral pharmacology of hallucinogens. Biochem. Pharmacol. 2008;75:17–33. doi: 10.1016/j.bcp.2007.07.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuster JM. Progress in brain research. Vol. 87. Elsevier; 1991. The prefrontal cortex and its relation to behavior; pp. 201–211. [DOI] [PubMed] [Google Scholar]
- Gatch MB, Forster MJ, Janowsky A, Eshleman AJ. Abuse liability profile of three substituted tryptamines. J. Pharmacol. Exp. Ther. 2011;338:280–289. doi: 10.1124/jpet.111.179705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibbons S. ‘Legal highs’-novel and emerging psychoactive drugs: a chemical overview for the toxicologist. Clin. Toxicol. 2012;50:15–24. doi: 10.3109/15563650.2011.645952. [DOI] [PubMed] [Google Scholar]
- Gribble GW, Pelcman B. Total syntheses of the marine sponge pigments fascaplysin and homofascaplysin B and C. J. Org. Chem. 1992;57:3636–3642. doi: 10.1021/jo00039a024. [DOI] [Google Scholar]
- Halberstadt AL, Geyer MA. Multiple receptors contribute to the behavioral effects of indoleamine hallucinogens. Neuropharmacology. 2011;61:364–381. doi: 10.1016/j.neuropharm.2011.01.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halberstadt AL, Koedood L, Powell SB, Geyer MA. Differential contributions of serotonin receptors to the behavioral effects of indoleamine hallucinogens in mice. J. Psychopharmacol. (Oxf.) 2011;25:1548–1561. doi: 10.1177/0269881110388326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill SL, Thomas SH. Clinical toxicology of newer recreational drugs. Clin. Toxicol. 2011;49:705–719. doi: 10.3109/15563650.2011.615318. [DOI] [PubMed] [Google Scholar]
- Kitanaka J, Kitanaka N, Takemura M. Chronic methamphetamine administration reduces histamine-stimulated phosphoinositide hydrolysis in mouse frontal cortex. Biochem. Biophys. Res. Commun. 2003;300:932–937. doi: 10.1016/S0006-291X(02)02948-0. [DOI] [PubMed] [Google Scholar]
- Krebs-Thomson K, Paulus MP, Geyer MA. Effects of hallucinogens on locomotor and investigatory activity and patterns: influence of 5-HT2A and 5-HT2C receptors. Neuropsychopharmacology. 1998;18:339–351. doi: 10.1016/S0893-133X(97)00164-4. [DOI] [PubMed] [Google Scholar]
- Krebs-Thomson K, Ruiz EM, Masten V, Buell M, Geyer MA. The roles of 5-HT 1A and 5-HT 2 receptors in the effects of 5-MeO-DMT on locomotor activity and prepulse inhibition in rats. Psychopharmacology. 2006;189:319–329. doi: 10.1007/s00213-006-0566-1. [DOI] [PubMed] [Google Scholar]
- McFadden LM, Hadlock GC, Allen SC, Vieira-Brock PL, Stout KA, Ellis JD, Hoonakker AJ, Andrenyak DM, Nielsen SM, Wilkins DG, Hanson GR, Fleckenstein AE. Methamphetamine self-administration causes persistent striatal dopaminergic alterations and mitigates the deficits caused by a subsequent methamphetamine exposure. J. Pharmacol. Exp. Ther. 2012;340:295–303. doi: 10.1124/jpet.111.188433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagai F, Nonaka R, Kamimura KSH. The effects of non-medically used psychoactive drugs on monoamine neurotransmission in rat brain. Eur. J. Pharmacol. 2007;559:132–137. doi: 10.1016/j.ejphar.2006.11.075. [DOI] [PubMed] [Google Scholar]
- Nichols DE. Hallucinogens. Pharmacol. Ther. 2004;101:131–181. doi: 10.1016/j.pharmthera.2003.11.002. [DOI] [PubMed] [Google Scholar]
- Nichols DE. Psychedelics. Pharmacol. Rev. 2016;68:264–355. doi: 10.1124/pr.115.011478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peden NR, Macaulay K, Bissett AF, Crooks J, Pelosi A. Clinical toxicology of ‘magic mushroom’ ingestion. Postgrad. Med. J. 1981;57:543–545. doi: 10.1136/pgmj.57.671.543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pranzatelli MR, Pluchino RS. The relation of central 5-HT1A and 5-HT2 receptors: Low dose agonist-induced selective tolerance in the rat. Pharmacol. Biochem. Behav. 1991;39:407–413. doi: 10.1016/0091-3057(91)90199-C. [DOI] [PubMed] [Google Scholar]
- Ray TS. Psychedelics and the human receptorome. PLoS ONE. 2010;5:e9019. doi: 10.1371/journal.pone.0009019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rickli A, Kopf S, Hoener MC, Liechti ME. Pharmacological profile of novel psychoactive benzofurans. Br. J. Pharmacol. 2015;172:3412–3425. doi: 10.1111/bph.13128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith DA, Bailey JM, Williams D, Fantegrossi WE. Tolerance and cross-tolerance to head twitch behavior elicited by phenethylamine-and tryptamine-derived hallucinogens in mice. J. Pharmacol. Exp. Ther. 2014;351:485–491. doi: 10.1124/jpet.114.219337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spijker S. Neuroproteomics. Springer; 2011. Dissection of rodent brain regions; pp. 13–26. [DOI] [Google Scholar]
- Tittarelli R, Mannocchi G, Pantano F, Saverio Romolo F. Recreational use, analysis and toxicity of tryptamines. Curr. Neuropharmacol. 2015;13:26–46. doi: 10.2174/1570159X13666141210222409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vallejos G, Fierro A, Rezende MC, Sepúlveda-Boza S, Reyes-Parada M. Heteroarylisopropylamines as MAO inhibitors. Bioorg. Med. Chem. 2005;13:4450–4457. doi: 10.1016/j.bmc.2005.04.045. [DOI] [PubMed] [Google Scholar]
- van den Buuse M, Ruimschotel E, Martin S, Risbrough VB, Halberstadt AL. Enhanced effects of amphetamine but reduced effects of the hallucinogen, 5-MeO-DMT, on locomotor activity in 5-HT1A receptor knockout mice: implications for schizophrenia. Neuropharmacology. 2011;61:209–216. doi: 10.1016/j.neuropharm.2011.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- VanGuilder HD, Vrana KE, Freeman WM. Twenty-five years of quantitative PCR for gene expression analysis. Biotechniques. 2008;44:619–626. doi: 10.2144/000112776. [DOI] [PubMed] [Google Scholar]
- Willins DL, Meltzer HY. Direct injection of 5-HT2A receptor agonists into the medial prefrontal cortex produces a head-twitch response in rats. J. Pharmacol. Exp. Ther. 1997;282:699–706. [PubMed] [Google Scholar]
- Winter JC. Hallucinogens as discriminative stimuli in animals: LSD, phenethylamines, and tryptamines. Psychopharmacology. 2009;203:251–263. doi: 10.1007/s00213-008-1356-8. [DOI] [PubMed] [Google Scholar]