Abstract
Objective
The gamma-glutamyl cycle catalyzed by gamma-glutamyl transferase (GGT) plays an important role in glutathione (GSH) homeostasis in the cell. In cells continuously exposed to the drug, the main phase of the enzymatic detoxification is the conjugation of the drug with GSH catalyzed by glutathione-S-transferase (GST). Conjugation of drugs with GSH is the first step in the development of chemotherapeutic drug resistance. In this study, we aimed to investigate the relationship between GGT and GSH in molecular subgroups of breast cancer patients.
Materials and Methods
Serum GGT activity and GSH levels for patients diagnosed with breast cancer (n=58) and healthy controls (n=8) were measured by a spectrophotometric method and a colorimetric kit, respectively.
Results
GGT activity was significantly higher in the total patient group and in the molecular subgroups than those in the control groups (p<0.05). Serum GSH levels were higher in the patient groups compared to controls without reaching statistical significance (p>0.05). GGT activity was positively correlated with GSH levels in the total patients and healthy controls (p<0.001 and p<0.05, respectively). There was also a positive correlation between GGT activity and GSH levels in Luminal A, HER2-positive (Human epidermal growth factor receptor 2), and Triple-negative groups (p<0.05).
Conclusion
This is the first study showing the relationship between GGT and GSH in molecular subgroups of breast cancer. An increase in GGT activity may affect intracellular GSH synthesis. Therefore, having a correlation between GGT and GSH in some molecular subgroups may affect the course of treatment in these patients.
Keywords: Gamma-glutamyltransferase, glutathione, molecular subgroups of breast cancer
Introduction
Gamma-glutamyl transferase (GGT) (GGT, EC 2.3.2.2) is an enzyme known as (5-L-glutamyl) -peptide: amino acid 5-glutamyl transferase in systematic nomenclature. GGT is located on the outer surface of plasma membranes of cells which has ecto-enzyme activity. The enzyme is a dimeric glycoprotein composed of a heavy chain and a light subunit bound by a non-covalent bond, processed from a single chain precursor with an autocatalytic cleavage in prokaryotes and eukaryotes (1, 2). GGT is located in the plasma membrane of almost all cells, but mainly involved in epithelial tissues with secretory or absorbing functions (1). Although the enzyme is shown in many organs, the highest GGT activity is present in the kidney, then in the duodenum, small intestine and gallbladder, respectively (3). GGT is present in the biliary pole of hepatocytes and cholangiocytes in adult liver and thus secreted into bile. It is known that the main source of plasma GGT is the liver (1).
Glutathione (GSH) (GSH, L-glutamyl-L-cysteinylglycine) is a tripeptide which has a thiol group and it is present in 1–10 mM concentration in all mammalian tissues (4). It is the most abundant antioxidant molecule in cells and is involved in various critical cellular functions such as detoxification of xenobiotics and/or their metabolites, cell proliferation, apoptosis, and modulation of fibrogenesis (4). GSH is also an important determinant of sulfur assimilation, protection of cells against oxidative stress and storage and transport of nitric oxide and cysteine. The gamma-glutamyl cycle catalyzed by GGT uses GSH as a continuous source of cysteine for cells (5). GSH is synthesized in the cytosol and then transferred out of the cell. The extracellular GSH metabolism is initiated by GGT, which is the first enzyme of the GSH destruction pathway, and is then finished with membrane dipeptidases (6). The γ-glutamyl moiety released by the breakdown of GSH by GGT is transferred to other amino acids and the resulting γ-glutamyl amino acid is reintroduced into the cell (6). This final compound is metabolized to form 5-oxoproline, which is then converted to glutamate which can be used in the formation of GSH again and amino acid (7). On the other hand, cysteinylglycine, which occurs after the removal of the gamma-glutamyl moiety of GSH, is also degraded by dipeptidases to form glycine and cysteine which will be transported back into the cell (7). Most of the cysteine taken by the cell is used to synthesize GSH again, and the remaining amount is introduced into newly synthesized proteins or is degraded into the sulphate and taurine (8).
One of the first studies on GGT activity was published in 1956 by Ball et al. (9). Despite a period of over 60 years, studies on GGT have not been concluded. Following the disclosure of the human genome, detection of the presence of other GGT genes with possibly overlapping activity has made the subject more complex but has aroused interest in it. GGT expression is often significantly increased in human cancers. It has been suggested that gamma-GGT can be used as an indicator of cancer risk, as well as its use as a marker of diabetes, cardiovascular and chronic kidney diseases (10). There are several hypotheses for the role of GGT in cancer. One of them is the increased GSH catabolism initiated by GGT. As described above, the extracellular degradation of GSH by GGT provides gamma-glutamyl amino acid and also cysteinylglycine, which is a highly reactive metabolite (11). Cysteinylglycine allows the reduction of Fe3+ to Fe2+, resulting in the production of reactive oxygen species. It, as a pro-oxidant, has been shown to induce low density lipoprotein (LDL) oxidation (12), lipid peroxidation as well as oxidative damage to DNA bases.
Living organisms are constantly exposed to xenobiotics or drugs. The main phase of enzymatic detoxification is the conjugation of activated xenobiotics/drugs with GSH catalyzed by GST (13). It has been reported that some compounds, once converted to glutathione-S-conjugates, enter the mercapturic acid pathway and generate highly reactive and toxic end products for the cell (14). The cytotoxicity of these GSH conjugates is mainly dependent on GST and GGT, which are enzymes that initiate the mercapturic acid synthesis pathway (15). High GST or GGT activity in cancer cells causes accumulation of GSH-drug conjugates and increases drug resistance (14).
The aims of this study are to investigate GGT activity and GSH levels in breast cancer and to evaluate the relationship between them in breast cancer according to the molecular subgroups.
Materials and Methods
Fifty-eight patients who applied to applied to the Istanbul University, Institute of Oncology, Clinical Oncology Department, Oncology Surgical Unit and and were diagnosed with breast cancer and had operation due to their illness, were included in the study. The patients were informed for participation in the study with approval prior to the operation date and informed consent forms from the patients were obtained. Serum samples were taken from 58 patients before the operation. Eight healthy women who applied to Surgical Oncology Unit for macromastia and for breast reduction surgery and no any breast cancer history in their family, between 18 to 70 years of age, without any known chronic illnesses (e.g. hypertension, diabetes mellitus, coronary artery disease, chronic liver disease, hepatitis, hyperlipidemia), any neoplastic and hormone related diseases, and history of regular alcohol consumption were included as the control group. Table 1 gives the main characteristics and clinic-pathological findings of the patients and the controls. Serum samples were stored at −80°C until use. The protocol for this research was approved by The Clinical Research Ethics Committee of Istanbul Faculty of Medicine.
Table 1.
Demographic characteristics and laboratory tests of the patient group
| Control (n=8) | Patient (n=58) | |
|---|---|---|
| Age, average (SD) | 36.3 (9.6) | 53.1 (12.0) |
| Menopause Status | ||
| Premenopausal, n (%) | 6 (75) | 33 (56.9) |
| Postmenopausal, n (%) | 2 (25) | 25 (43.1) |
| Cancer Stage, n (%) | ||
| I | - | 5 (8.6) |
| II | - | 23 (39.7) |
| III | - | 30 (51.7) |
| Tumor Location, n (%) | ||
| Right | - | 31 (53.5) |
| Left | - | 26 (44.8) |
| Right + Left | - | 1 (1.7) |
| Molecular Subtype, n (%) | ||
| Luminal A | - | 16 (27.6) |
| Luminal B / HER-2 (−) | - | 8 (13.8) |
| Luminal B-HER-2 (+) | - | 9 (15.5) |
| HER2 (+) | - | 9 (15.5) |
| Triple negative | - | 16 (27.6) |
| Laboratory tests | ||
| Estrogen Receptor, mean (SD) | - | 43.5 (43.3) |
| Progesterone Receptor, mean (SD) | - | 21.5 (32.8) |
| Ki-67, mean (SD) | - | 40.8 (26.2) |
SD: standard deviation
Histopathological analysis and staging
All cases underwent standard histopathological evaluation, including macroscopic and microscopic analysis. Immunohistochemical staining for ER (estrogen receptor), PR (progesterone receptor), HER-2 and Ki-67 were performed on sections of formalin-fixed paraffin-embedded tissue from the primary tumours. Histopathological analyses were performed in the accredited laboratory of Department of Pathology of Istanbul Medical Faculty.
For persistence of ER and PR receptors were included all results with +, ++ or +++ on immunohistochemical examination. For persistence of HER-2 receptors were included all patients with +++ result on immunohistochemical analysis. In cases where ICT determined HER-2 neu positive status ++ patients underwent FISH analyses for defining the HER2-neu gene amplification status. Staging criteria for breast cancer were determined by using criteria from American Join Committee (AJC) and TNM classification according to UICC (International Union for Cancer Control). According to the classification system for breast cancer subtypes, breast cancer is divided in Luminal A, Luminal B with HER2 negative, Luminal B with HER2 positive, HER2 enriched and basal-like (triple negative) (Table 2).
Table 2.
Parameters Used in the Classification of Breast Cancer Patients
| Parameter | |
|---|---|
| Luminal A | ER(+)/PR(+)/Ki-67<25% |
| Luminal B/HER-2 (−) | ER(+)/PR(+)/Ki-67≥25% |
| Luminal B/HER-2 (+) | ER(+)/PR(+)/HER-2(+) |
| HER2-positive | ER(−)/PR(−)/HER-2(+) |
| Triple-negative | ER(−)/PR(−)/HER-2(−) |
ER: estrogen receptor; PR: progesterone receptor
Measurement of serum GGT activity
To measure serum GGT activity, kinetic method based on the measurement of transpeptidase activity was used. This method, developed by Szasz (16), was modified in our study to measure with the microplate. GGT activity was measured at 0.05 mM 2-amino-2-methyl-1.3-propanediol (Sigma-Aldrich, Germany) buffer pH 8.6 in the presence of MgCl2.6H2O (Sigma-Aldrich, Germany), Gly-Gly (Sigma-Aldrich, Germany) and L-gamma-glutamyl-p-nitroanilide (PubChem, Bethesda, MD, USA) as GGT substrate. The reaction was monitored by following the increase in absorbance at 405 nm linked to the release of p-nitroanilide (17). All data are expressed as mean (standard deviation, SS).
Total glutathione analysis
Total serum glutathione (tGSH) analysis was performed using a colorimetric kit (Glutathione (GSH) Assay Kit; Oxford Biomedical Research, MI, USA). In the 96-well microplate, both the standards and the samples were analyzed in accordance with the kit procedure. Measurements were carried out in absorbance (A) at 400 nm. All data are expressed as mean (standard deviation, SS).
Statistical analysis
The homogeneity of the data was evaluated with the Kolmogorov-Smirnov test. Since the data were not normally distributed, the results were compared using nonparametric tests. The Mann-Whitney U test was used to compare differences between patient and healthy controls. Spearman-correlation test was used to examine the relationship between the parameters for the non-normally distributed data. P values of less than 0.05 were regarded as statistically significant. Statistical analyzes were performed using the Statistical Package for Social Sciences for Windows software version 22 (IBM Corp.; Armonk, NY, USA).
Results
To determine whether the data from serum GGT enzyme activity and GSH analysis were distributed normally, a Kolmogorov-Smirnov test was used. According to test results, GGT enzyme activity and GSH data did not show a normal distribution (p<0.001).
Serum GGT enzyme activity and GSH levels in the total patient group and breast cancer molecular subgroup average values are given in Table 3. GGT activity was statistically significantly higher in the total patient group and in the molecular subgroups than those in the control group (p<0.05). Serum GSH levels were higher in the patient groups compared to controls, but not statistically significant (p>0.05). When GGT activity and GSH levels were compared between molecular subgroups of breast cancer, no statistically significant difference was observed (p>0.05).
Table 3.
Mean values and comparison of serum GGT enzyme activity and GSH levels of patient groups according to total and molecular subtypes
| n | Serum GGT Activity (U/L) | Serum GSH (μmol/L) | |
|---|---|---|---|
| Controls | 8 | 18.8 (4.4) | 5.8 (1.4) |
| Total patients | 58 | 26.2 (10.3)* | 7.8 (5.5) |
| Luminal A | 16 | 25.3 (8.1)** | 6.3 (3.7) |
| Luminal B / HER-2 (−) | 8 | 26.9 (9.8)** | 10.1 (7.8) |
| Luminal B / HER2 (+) | 9 | 25.1 (6.4) | 7.6 (4.2) |
| HER2 (+) | 9 | 32.9 (16.3)** | 9.2 (7.1) |
| Triple (−) | 16 | 27.0 (10.0)*** | 8.1 (6.3) |
p<0.01 compared with the control group
p<0.05 compared to the control group
p<0.02compared to the control group
GGT: gamma-glutamyl transferase; GSH: glutathione
When the relationship between GGT enzyme activity and GSH levels in total patient and control groups were examined, a statistically significant correlation was observed (p<0.001 and p<0.05, respectively) (Figure 1). In addition, Luminal A, HER2-positive, and Triple-negative patients showed a statistically significant correlation between GGT activity and GSH levels (p<0.05). No statistically significant correlation was observed in Luminal B and Luminal B-HER2-positive patients (p>0.05) (Table 4).
Figure 1.
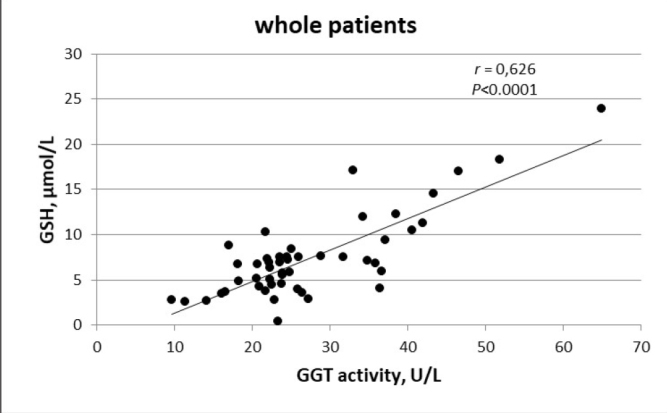
The relationship between GGT activity and GSH levels in controls and patients
Table 4.
The relationship between GGT activity and GSH levels in molecular sub-groups
| n | GGT – GSH r (p) | |
|---|---|---|
| Luminal A | 16 | 0.800 (0.003) |
| Luminal B/HER-2 negative | 8 | 0.714 (0.071) |
| Luminal B-Her2-positive | 9 | 0.100 (0.798) |
| Her2-positive | 9 | 0.800 (0.010) |
| Triple-negative | 16 | 0.552 (0.041) |
GGT: gamma-glutamyl transferase; GSH: glutathione
Discussion and Conclusion
Various hypotheses have been suggested for the role of GGT in carcinogenesis, in the literature. One of them is the increased GSH catabolism initiated by increased GGT activity. Extracellular GSH degradation by GGT provides cysteine, a rate-limiting amino acid for GSH synthesis in the cell. Therefore, GGT plays an important role in GSH and cysteine homeostasis (18–21). GSH protects cells against carcinogens and regulates neoplastic transformation and viability of cells. Because of its reducing properties, GSH can inactivate some carcinogens, protect DNA against free radicals that are damaging, protect the integrity of different tissues, and prevent lipid peroxidation (22). On the other hand, cysteinylglycine, a product of the extracellular degradation of GSH by GGT, is a highly reactive carcinogenic metabolite. The second hypothesis for the role of GGT in cancer is its activity in the synthesis and metabolism of leukotrienes. It is believed that the relationship between chronic inflammation and cancer is due in part to the infiltration of the tumor microenvironment through inflammatory cells from which a number of proinflammatory mediators such as prostagladin and leukotriene are released (23–24). In addition, GGT promotes free iron release from transferrin, which provides iron to malignant cells (25).
In our study, GGT activity was higher in the patients in all molecular-subgroups than those in the controls. Also, patients in the drug-resistant HER2-positive breast cancer group had slightly higher GGT activity than patients in the other sub-groups. Recently, in a study in which Shackshaft et al. (26) examined serum GGT activity in breast cancer subgroups, serum GGT activity was found to be slightly higher in breast cancer patients compared to the control group. They also found significant associations between serum GGT activity and development of ER+, ER− and PR+ breast cancers compared to controls and inverse associations between GGT levels and PR− breast cancers compared to PR+ (26). In a study by Staudigl et al. (27), no relationship was found between GGT enzyme activity and hormone receptor and HER2-status. Fentiman et al. (28) reported a positive correlation between increased GGT activity and breast cancer incidence in premenopausal women. On the other hand, Van Hemelrijck (29) explained that increased GGT levels were an independent risk-factor for breast cancer.
Expression of GGT involved in the mercapturic acid pathway has been reported to be induced in cancer cells, especially drug-resistant cancer cells (15). Since overproduction of GGT results in increased intracellular GSH synthesis, it plays an important role in the development of resistance to certain chemotherapeutics, such as alkylating agents (30). In our study, GSH levels were found to be higher in both total patient group and molecular subgroups in comparison with the control group without reaching statistical significance. However, there were significant positive correlations between GGT activity and GSH levels both in the whole patient group and in the Luminal A, Her2-positive, and triple-negative subgroups. This result supports the relationship between the increase in GGT activity and the increase in GSH levels. Although there are not many studies examining GGT and GSH in breast cancer at the same time, Mishra et al. (31) showed significant increases in GSH levels in breast cancer patients with/without metastasis when compared to healthy controls and increases in GGT levels in breast cancer patients with metastasis when compared to non-metastatic patients. However, they did not examine the correlation between the two and therefore could not explain the relationship between high GSH levels and GGT.
In the breast cancer, the main treatment in hormone-positive patients is with tamoxifen or aromatase inhibitors (32). HER2-positive breast cancer cells respond to monoclonal antibodies and kinase inhibitors that block HER2 receptor, such as trastuzumab and lapatinib (33, 34). Since three receptors that are important for the development and proliferation of tumor cells in triple-negative breast cancer (TNBC) are not expressed (ER−, PR−, HER2−), standard hormone therapy and/or targeted treatment agents for these receptors cannot be used. Therefore, patients with TNBC are usually treated with chemotherapeutic agents that are cytotoxic. The efficacy of various chemotherapy agents such as anthracyclines, taxanes, ixabepilone, and platinum derivatives has been shown in different studies in the treatment of TNBC (35). However, different response rates are observed in patients. For example, while only 30% of patients with TNBC respond to chemotherapy, the remaining 70% of patients does not respond to chemotherapy or show resistance (36). Therefore, prevention of drug resistance in these patients is important for a positive treatment process. As suggested by our study’s results and other studies, if increased GGT activity causes the accumulation of GSH-drug conjugates, we may consider that chemotherapeutic drug resistance may develop, and the treatment process may be affected in patients with high GGT activity and GSH levels. However, new studies are needed on the role of GGT activity and GSH in the development of drug resistance.
Footnotes
Ethics Committee Approval: Ethics committee approval was received for this study from the Ethics Committee of Istanbul Faculty of Medicine (23.03.2016).
Informed Consent: Written informed consent was obtained from patients who participated in this study.
Peer-review: Externally peer-reviewed.
Author Contributions: Concept - S.Y.A.; Design - S.Y.A., E.M.S.; Supervision - S.Y.A., E.M.S., D.G.; Resources - S.Y.A.; Materials - H.K., S.D.; Data Collection and/or Processing - S.Y.A., E.M.S., H.K., S.D.; Analysis and/or Interpretation - S.Y.A., E.M.S., D.G., H.K., S.D.; Literature Search - S.Y.A., E.M.S., D.G., H.K., S.D.; Writing Manuscript - S.Y.A., E.M.S., D.G., H.K., S.D.; Critical Review - S.Y.A., E.M.S., D.G., H.K., S.D.
Conflict of Interest: The authors declared that this study has received no financial support.
Financial Disclosure: This study was supported by Gazi University Research Fund (Project No: 02/2017-02).
References
- 1.Fornaciari I, Fierabracci V, Corti A, Aziz Elawadi H, Lorenzini E, Emdin M, Paolicchi A, Franzini M. Gamma-Glutamyltransferase Fractions in Human Plasma and Bile: Characteristic and Biogenesis. PLoS One. 2014;9:e88532. doi: 10.1371/journal.pone.0088532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Heisterkamp N, Groffen J, Warburton D, Sneddon TP. The human gamma-glutamyltransferase gene family. Hum Genet. 2008;123:321–332. doi: 10.1007/s00439-008-0487-7. [DOI] [PubMed] [Google Scholar]
- 3.Owen AD, Schapira AH, Jenner P, Marsden CD. Oxidative stress and Parkinson’s disease. Ann N Y Acad Sci. 1996;786:217–223. doi: 10.1111/j.1749-6632.1996.tb39064.x. [DOI] [PubMed] [Google Scholar]
- 4.Kadoğlu D, Akçay T. Glutation Metabolism and Clinical Importance. Turkiye Klinikleri Journal of Medical Sciences. 1995;15:214–218. [Google Scholar]
- 5.Wickham S, West MB, Cook PF, Hanigan MH. Gamma-glutamyl compounds: Substrate specificity of gamma-glutamyl transpeptidase enzymes. Anal Biochem. 2011;414:208–214. doi: 10.1016/j.ab.2011.03.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ribas V, García-Ruiz C, Fernández-Checa JC. Glutathione and mitochondria. Front Pharmacol. 2014;5:151. doi: 10.3389/fphar.2014.00151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lu SC. Regulation of glutathione synthesis. Mol Aspects Med. 2009;30:42–59. doi: 10.1016/j.mam.2008.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wang Y, Li J, Matye D, Zhang Y, Dennis K, Ding WX, Li T. Bile acids regulate cysteine catabolism and glutathione regeneration to modulate hepatic sensitivity to oxidative injury. JCI Insight. 2018;3:99676. doi: 10.1172/jci.insight.99676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ball EG, Cooper O, Revel JP. The quantitative measurement of gamma glutamyl transpeptidase activity. J Biol Chem. 1956;221:895–908. [PubMed] [Google Scholar]
- 10.Whitfield JB. Gamma glutamyl transferase. Crit Rev Clin Lab Sci. 2001;38:263–355. doi: 10.1080/20014091084227. [DOI] [PubMed] [Google Scholar]
- 11.Estrela JM, Ortega A, Obrador E. Glutathione in cancer biology and therapy. Crit Rev Clin Lab Sci. 2006;43:143–181. doi: 10.1080/10408360500523878. [DOI] [PubMed] [Google Scholar]
- 12.Hanigan MH. Gamma-Glutamyl transpeptidase, a glutathionase: its expression and function in carcinogenesis. Chem Biol Interact. 1998;111–112:333–42. doi: 10.1016/S0009-2797(97)00170-1. [DOI] [PubMed] [Google Scholar]
- 13.Banneau G, Guedj M, MacGrogan G, de Mascarel I, Velasco V, Schiappa R, Bonadona V, David A, Dugast C, Gilbert-Dussardier B, Ingster O, Vabres P, Caux F, de Reynies A, Iggo R, Sevenet N, Bonnet F, Longy M. Molecular apocrine differentiation is a common feature of breast cancer in patients with germline PTEN mutations. Breast Cancer Res. 2010;12:R63. doi: 10.1186/bcr2626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Longley DB, Johnston PG. Molecular mechanisms of drug resistance. J Pathol. 2005;205:275–292. doi: 10.1002/path.1706. [DOI] [PubMed] [Google Scholar]
- 15.Ramsay EE, Dilda PJ. Glutathione S-conjugates as prodrugs to target drug-resistant tumors. Front Pharmacol. 2014;5:181. doi: 10.3389/fphar.2014.00181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Szasz G. A kinetic photometric method for serum gamma-glutamyl transpeptidase. Clin Chem. 1969;15:124–136. [PubMed] [Google Scholar]
- 17.Burtis CA, Ashwood ER. Tietz Textbook of Clinical Chemistry. 2nd Edition. WB Philadelphia (USA): Saunder Co; 1964. p. 2326. [Google Scholar]
- 18.Corti A, Franzini M, Paolicchi A, Pompella A. Gamma-glutamyltransferase of cancer cells at the crossroads of tumor progression, drug resistance and drug targeting. Anticancer Res. 2010;30:1169–1181. [PubMed] [Google Scholar]
- 19.Pompella A, Corti A, Paolicchi A, Giommarelli C, Zunino F. Gamma-glutamyltransferase, redox regulation and cancer drug resistance. Curr Opin Pharmacol. 2007;7:360–366. doi: 10.1016/j.coph.2007.04.004. [DOI] [PubMed] [Google Scholar]
- 20.Roomi MW, Gaal K, Yuan QX, French BA, Fu P, Bardag-Gorce F, French SW. Preneoplastic liver cell foci expansion induced by thioacetamide toxicity in drug-primed mice. Exp Mol Pathol. 2006;81:8–14. doi: 10.1016/j.yexmp.2006.02.006. [DOI] [PubMed] [Google Scholar]
- 21.Whitfield JB. Gamma glutamyl transferase. Crit Rev Clin Lab Sci. 2001;38:263–355. doi: 10.1080/20014091084227. [DOI] [PubMed] [Google Scholar]
- 22.Traverso N, Ricciarelli R, Nitti M, Marengo B, Furfaro AL, Pronzato MA, Marinari UM, Domenicotti C. Role of Glutathione in Cancer Progression and Chemoresistance. Oxid Med Cell Longev. 2013;2013 doi: 10.1155/2013/972913. 972913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Coussens LM, Werb Z. Inflammation and cancer. Nature. 2002;420:860–867. doi: 10.1038/nature01322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wang D, Dubois RN. Eicosanoids and cancer. Nat Rev Cancer. 2010;10:181–193. doi: 10.1038/nrc2809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Dominici S, Pieri L, Comporti M, Pompella A. Possible role of membrane gamma-glutamyltransferase activity in the facilitation of transferrin-dependent and -independent iron uptake by cancer cells. Cancer Cell Int. 2003;3:7. doi: 10.1186/1475-2867-3-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Shackshaft L, Van Hemelrijck M, Garmo H, Malmström H, Lambe M, Hammar N, Walldius G, Jungner I, Wulaningsih W. Circulating gamma-glutamyl transferase and development of specific breast cancer subtypes: findings from the Apolipoprotein Mortality Risk (AMORIS) cohort. Breast Cancer Res. 2017;19:22. doi: 10.1186/s13058-017-0816-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Staudigl C, Concin N, Grimm C, Pfeiler G, Nehoda R, Singer CF, Polterauer S. Prognostic Relevance of Pretherapeutic Gamma-Glutamyltransferase in Patients with Primary Metastatic Breast Cancer. PLoS One. 2015;10:e0125317. doi: 10.1371/journal.pone.0125317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fentiman IS, Allen DS. Gamma-Glutamyl transferase and breast cancer risk. Br J Cancer. 2010;103:90–93. doi: 10.1038/sj.bjc.6605719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Van Hemelrijck M, Jassem W, Walldius G, Fentiman IS, Hammar N, Lambe M, Garmo H, Jungner I, Holmberg L. Gamma-glutamyltransferase and risk of cancer in a cohort of 545,460 persons - the Swedish AMORIS study. Eur J Cancer. 2011;47:2033–2041. doi: 10.1016/j.ejca.2011.03.010. [DOI] [PubMed] [Google Scholar]
- 30.Bansal A, Simon MC. Glutathione metabolism in cancer progression and treatment resistance. J Cell Biol. 2018;217:2291–2298. doi: 10.1083/jcb.201804161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mishra S, Sharma DC, Sharma P. Studies of biochemical parameters in breast cancer with and without metastasis. Indian J Clin Biochem. 2004;19:71–75. doi: 10.1007/BF02872394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Romond EH, Perez EA, Bryant J, Suman VJ, Geyer CE, Jr, Davidson NE, Tan-Chiu E, Martino S, Paik S, Kaufman PA, Swain SM, Pisansky TM, Fehrenbacher L, Kutteh LA, Vogel VG, Visscher DW, Yothers G, Jenkins RB, Brown AM, Dakhil SR, Mamounas EP, Lingle WL, Klein PM, Ingle JN, Wolmark N. Trastuzumab plus adjuvant chemotherapy for operable HER2-positive breast cancer. N Engl J Med. 2005;353:1673–1684. doi: 10.1056/NEJMoa052122. [DOI] [PubMed] [Google Scholar]
- 33.Geyer CE, Forster J, Lindquist D, Chan S, Romieu CG, Pienkowski T, Jagiello-Gruszfeld A, Crown J, Chan A, Kaufman B, Skarlos D, Campone M, Davidson N, Berger M, Oliva C, Rubin SD, Stein S, Cameron D. Lapatinib plus capecitabine for HER2-positive advanced breast cancer. N Engl J Med. 2006;355:2733–2743. doi: 10.1056/NEJMoa064320. [DOI] [PubMed] [Google Scholar]
- 34.Piccart-Gebhart MJ, Procter M, Leyland-Jones B, Goldhirsch A, Untch M, Smith I, Gianni L, Baselga J, Bell R, Jackisch C, Cameron D, Dowsett M, Barrios CH, Steger G, Huang CS, Andersson M, Inbar M, Lichinitser M, Láng I, Nitz U, Iwata H, Thomssen C, Lohrisch C, Suter TM, Rüschoff J, Suto T, Greatorex V, Ward C, Straehle C, McFadden E, Dolci MS, Gelber RD Herceptin Adjuvant (HERA) Trial Study Team. Trastuzumab after adjuvant chemotherapy in HER2-positive breast cancer. N Engl J Med. 2005;353:1659–1672. doi: 10.1056/NEJMoa052306. [DOI] [PubMed] [Google Scholar]
- 35.Andreopoulou E, Sparano JA. Chemotherapy in Patients with Anthracycline- and Taxane-Pretreated Metastatic Breast Cancer: An Overview. Curr Breast Cancer Rep. 2013;5:42–50. doi: 10.1007/s12609-012-0097-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rivera E, Gomez H. Chemotherapy resistance in metastatic breast cancer: the evolving role of ixabepilone. Breast Cancer Res. 2010;12(Suppl 2):S2. doi: 10.1186/bcr2573. [DOI] [PMC free article] [PubMed] [Google Scholar]


