Abstract
Pure ductal carcinoma in situ of male breast (DCIS) is extremely rare. Only a few cases have been reported until now. Its treatment is not well established. Prognosis is as good as in women. In this study, we reported 3 cases of pure ductal carcinoma in situ in the male breast. The mean age of DCIS patients was 58.3 years. The main symptom was a breast mass. The median size of the tumor was 25 mm. Two patients had an axillary lymph node. The left side was reached in 2 cases. All of the patients underwent mastectomy. The histopathological assessment showed papillary, cribriform, and comedocarcinoma in situ. There was no evidence of invasive carcinoma. In one case, the DCIS was associated with Paget’s disease of the nipple. One patient received hormonotherapy. The time of follow-up ranged between 6 and 117 months. One patient developed an invasive recurrence.
Keywords: Male breast cancer, ductal carcinoma in situ, treatment
Introduction
Pure Ductal carcinoma in situ (DCIS) of the man is extremely rare. The incidence is approximately 1% of all malignancies in men and 5% to 7% of male breast cancer (1). It is usually associated with invasive carcinoma. We reported three cases on DCIS in men. The aim of our study is to further emphasize the importance of this disease for men and to evaluate the management of this rare tumor.
Case Presentations
Case 1
A 58-year old man consulted for a left breast mass that has been evolving for 3 months. There was no remarkable personal history or family history of breast’s disease. He had a remarkable history of smoking with 38 packages per year. On physical examination, we found a mobile, well-defined mass, measuring 20 mm × 20 mm without axillary lymph node. The right breast was unremarkable. Mammography and ultrasound showed a circumscribed nodule without calcifications in the left breast. This was considered as ACR 3 of the classification of the American College of Radiology (ACR) (Figure 1). We performed a core needle biopsy. The histological findings showed a DCIS. The patient underwent a mastectomy with sentinel node. Macroscopically, the tumor was greyish to white and measured 17 mm in its greater axis. The definitive histopathological assessments showed DCIS with papillary and cribriform patterns (Figure 2). The nuclear grade was intermediate, and there was no necrosis. Cells were polarized. The margins were free, with a clearance of 15 mm. No invasive cancer was present. The nuclear grade was I of Van Nuys. Van Nuys Prognostic Index score (VNPI) was 6 (Table 1). Sentinel lymph node sampling brought back three lymph nodes which were all negative. The immunohistochemical examination of estrogen (ER) and progesterone (PR) receptors were negative for both. The patient was noted to be doing well until now, and he is regularly followed up, with a total duration of follow-up of 10 years.
Figure 1.

A retro-areolar, well-defined mass of the left breast
Figure 2.

a–d. Papillary patterns of DCIS (a), cribriform patterns of DCIS (b), comedocarcinoma on DCIS (c), nuclear grade 2 of Van Nuys (d)
Table 1.
Scoring system according to the new Silverstein classification of VNPI
| VNPI scoring system | 1 | 2 | 3 |
|---|---|---|---|
| Tumor size (diameter in mm) | less or equal to 15 | 16–40 | greater or equal to 41 |
| Margin width (in mm) | less or equal to 10 | 1–9 | <1 |
| Pathologic Classification | non-high grade, (nuclear grades 1 and 2) no necrosis | non-high grade, (nuclear grades 1 and 2)with necrosis | high grade (nuclear grade 3) with or without necrosis |
| Age (in years) | 61 or older | 40–60 | 39 or younger |
| Overall VNPI score | 4–6 | 7–9 | 10–12 |
VNPI: Van Nuys Prognostic Index score; Mm: millimeters
A written informed consent was obtained from the patient.
Case 2
An 85-year old man was referred for a retro-areolar mass of the left breast and nipple retraction for more than 6 months. He had a medical history of hypothyroidism and he didn’t have a remarkable family history of breast disease. On physical examination, there was a mobile retro-areolar mass, measuring 25 mm × 20 mm in greater diameter with inflammatory skin changes (Figure 3). We also found an ipsilateral axillary lymph node. The mammogram and ultrasound revealed a suspicious retro-areolar masse which considered as ACR4. The core biopsy with histological assessment showed patterns of DCIS. A mastectomy with axillary lymph node dissection was performed. In histological findings, macroscopically the tumor measured 27 mm in its greatest diameter. The architectural patterns were cribriform and papillary, with Paget’s disease of the nipple (Figure 2). We didn’t identify necrosis. The nuclear grade was intermediate, and cells were polarized. The margins were free, with a clearance of 10 mm. The nuclear grade was I of Van Nuys. VNPI score was 5. All lymph nodes were negative. The ER and the PR were positive. Due to inflammatory skin signs in the first clinical presentation, the patient received Tamoxifen 20 mg per day for five years (TEVA Sante, MACORS, Paris, France). He is still being followed up without recurrence, with a total follow-up duration of 4 years.
Figure 3.
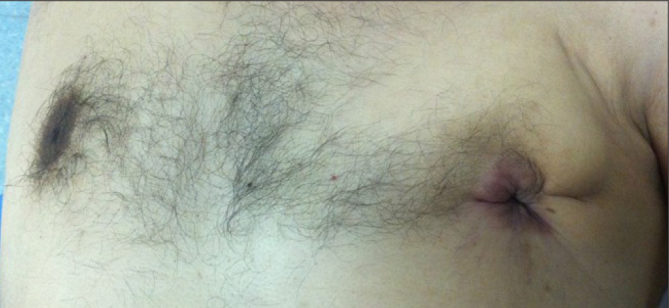
Clinical appearance of a retro-areolar lesion with nipple retraction and skin involvement
A written informed consent was obtained from the patient.
Case 3
A 35-year-old man had a 3 weeks history of a painful lump in the periareolar region of the right breast. There was no remarkable personal history or family history of breast’s disease. The patient was neither smoking nor alcoholic. On physical examination, we noted an elastic gynecomastia of the right breast without any palpable mass (Figure 4). There were no palpable lymph nodes. Mammography and ultrasound showed homogenous gynecomastia without nodules. A subcutaneous mastectomy was performed. Histological examination revealed a DCIS measured 10 mm in its greatest diameter, with papillary and comedocarcinoma patterns, and the nuclear grade was intermediate (Figure 2). We identified the necrosis (Figure 5). Cells were rarely polarized. The ER and the PR were negative. The margins were free with a clearance of 5 mm. No invasive cancer was seen. The nuclear grade was II of Van Nuys. VNPI score was 8. The patient was immediately lost from view postoperatively. He consulted two years later for a retro-areolar mass. A core biopsy was done and the histological findings showed a recurrence of DCIS. A mastectomy with sentinel node was performed. The final histological exam showed a poorly limited mass that measured 30 mm in its greatest diameter. We noted the presence of necrosis. The ER and the PR were negative. The margins were free with a clearance of 10 mm. The sentinel lymph nodes were free of disease. The nuclear grade was II of Van Nuys. VNPI score was 8. The recurrence was confirmed, and we didn’t recommend additional treatment for the patient. He was lost from view again. He consulted 6 months later for peri-cicatricial mass. The biopsy showed invasive ductal carcinoma. The chest X-ray, ultrasound of the abdomen, and bone scintigraphy were performed and there was no evidence of distant metastases. A large excision was done followed by radiotherapy. The patient is still followed up with no evidence of recurrence with a total duration of regular follow-up of 5 years.
Figure 4.

Gynecomastia combining a tumor of the right breast
Figure 5.

Microscopic section showing necrosis
A written informed consent was obtained from the patient.
Discussion and Conclusion
Male DCIS is a rare entity, the incidence of male DCIS is 7% of all male breast cancer (1). This low incidence is thought to be partly due to the lack of breast screening in male patients.
There is no clinical particularity of the DCIS in man. The median age of presentation is 65 years (range 25–94 years) but it is usually diagnosed at an advanced age (1, 2) as we reported in the second case. The most frequent clinical symptoms are subareolar mass in 58% followed by nipple discharge in 35% and rarely associated with gynecomastia in 19%, as we reported in the third case (2).
The radiological evaluation of the male breast is not standardized, the mammography is done first and is followed by ultrasound (1, 3).
The comparison with female breast shows that the calcifications are less frequently seen on the mammography as in our case series; we didn’t found microcalcification (4). Ultrasonography of male DCIS typically reveals a cystic lesion (4).
Therefore, the DCIS of the male is expected to have a good prognosis with simple mastectomy only and no axillary sentinel node biopsy or chemotherapy is needed as in female patients. Meanwhile, some authors actually recommend the use of sentinel lymph node systematically (1, 4). Radiotherapy may be recommended for a male patient with DCIS treated by lumpectomy or patients with involved margins for reducing the local recurrence.
The European Organisation for Research and Treatment of Cancer (EORTC) cohort concluded to be DCIS is the most commonly observed precursor lesion in male breast cancer, which can explain the aggressive behavior that we reported in the third case (5).
Histological examination showed that the papillary lesion was the most frequently observed histological subtype at 74% and that the cribriform patterns were less common with a rate of 27%. The low-grade is the most frequent one (57%), and the intermediate grade is less common (43%) as we reported in our case series, where all the patients expressed a nuclear grade 2. On the contrary, high-grade DCIS is considered to be a very rare lesion in pure DCIS (6, 7). The grading system for DCIS is very varied; we used Van Nuys grading, which took into account nuclear features, and the presence or absence of necrosis.
Silverstein established the VNPI to attempt to identify the aggressiveness of DCIS in terms of local recurrence following breast-conserving surgeries (Table 1) (8). Initially, the index evaluated three factors frequently connected with the aggressiveness of DCIS. These included: total tumor size, classification of pathological ‘ nuclear grade ‘ (including presence or lack of necrosis), and margin clearance. Later, Silverstein added patient age to the stratification scoring (9). He reviewed the record of 706 patients with pure DCIS, who were treated with breast preservation. In patients with VNPI scores of 4, 5 or 6, regardless of whether radiation therapy was used, there was no statistical difference in the 12-year local recurrence-free survival (p=not significant). However, patients with VNPI scores of 7, 8, or 9 received a statistically significant average of 12% to 15% local recurrence-free survival benefit when treated with radiation therapy (p=0.03) (9).
In our series, all the patients had a surgical resection consisting of mastectomy with sentinel lymph node in two cases and a lymph node dissection in one case due to the presence of ipsilateral palpable lymph node. We didn’t indicate radiotherapy as an adjuvant treatment due to the free margins in the histological exam and the absence of invasive underlying carcinoma. However, as we reported in the third case, even with radical treatment and free margins, the patient had a local recurrence with invasive behavior. In this case, the patient was under 40 years old and we observed necrosis in the histological assessment. The tumor measured 3 cm in its greatest diameter and the VNPI score was 8 according to the new score established by Silverstein (9). This statement leads to the consideration of radiotherapy for some patients even when mastectomy was done. Due to the rarity of this entity in men, further investigations have to be conducted to confirm this hypothesis.
The hormone receptor expression rate in DCIS remains undefined due to the rarity of this entity. In a recent cohort of EORTC, including 1483 patients, the authors found that ER, PR, and androgen receptors (AR) were mostly positive in invasive breast male cancer, and the tumor was frequently Luminal B-like/human epidermal growth factor receptor 2 negative (HER2). In our series, we found positive ER and PR in only one patient (10).
Hormonotherapy is not often used; however, some previous studies demonstrated a benefit of the use of Tamoxifen (3, 6). In our series, only one patient had hormonotherapy and was aged 85, we can conclude that hormonotherapy in DCIS was efficient.
The prognosis is uncertain because of the small number of populations in the literature. We consider the third case unusual in the sense that a DCIS in a male patient presented with gynecomastia, which was radically treated, but the patient exhibited an invasive local recurrence. Meanwhile, the prognosis is usually excellent. Some studies showed worse prognosis for male DCIS than female (11). We recommend regular follow up with clinical examination and regular ultrasound and mammography.
In conclusion, there are few studies about DCIS in men, so there are no clear guidelines for its management. Breast cancer should be considered for any male patients presenting with mass breast, gynecomastia or nipple discharge, which would lead to earlier detection and better overall prognosis. We recommend a mastectomy with sentinel lymph node as treatment standard. We can administer hormonotherapy when the ER and PR are positive. The place of radiotherapy remains uncertain and further investigations are needed to select the right candidate for it. The prognosis is excellent, but we are still waiting for future studies to understand the biology of this disease.
Footnotes
Informed Consent: Written informed consent was obtained from patients who participated in this study.
Peer-review: Externally peer-reviewed.
Author Contributions: Concept - C.R; Design - B.M.; Supervision - R.K.; Materials - A.O., K.S.; Data Collection and/or Processing - K.S.; Analysis and/or Interpretation - B.M., J.O.; Literature Search - J.O.; Writing Manuscript - S.S.; Critical Review - C.R., R.K.
Conflict of Interest: The authors have no conflicts of interest to declare.
Financial Disclosure: The authors declared that this study has received no financial support.
References
- 1.Chern J, Liao L, Baraldi R, Tinney E, Hendershott K, Germaine P. Case Report: Ductal Carcinoma in Situ in the Male Breast. Case Rep Radiol. 2012 doi: 10.1155/2012/532527. 532527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Brents M, Hancock J. Ductal Carcinoma In situ of the Male Breast. Breast Care. 2016;11:288290. doi: 10.1159/000447768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cutuli B, Dilhuydy JM, De Lafontan B, Berlie J, Lacroze M, Lesaunier F, Graic Y, Tortochaux J, Resbeut M, Lesimple T, Gamelin E, Campana F, Reme-Saumon M, Moncho-Bernier V, Cuilliere JC, Marchal C, De Gislain G, N’Guyen TD, Teissier E, Velten M. Ductal carcinoma in situ of the male breast. Analysis of 31 cases. Eur J Cancer. 1997;33:3538. doi: 10.1016/S0959-8049(96)00436-4. [DOI] [PubMed] [Google Scholar]
- 4.Coroneos CJ, Hamm C. Ductal carcinoma in situ in a 25-year-old man presenting with apparent unilateral gynecomastia. Curr Oncol. 2010;17:133137. doi: 10.3747/co.v17i4.525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Doebar SC, Slaets L, Cardoso F, Giordano SH, Bartlett JM, Tryfonidis K, Dijkstra NH, Schröder CP, van Asperen CJ, Linderholm B, Benstead K, Dinjens WN, van Marion R, van Diest PJ, Martens JW, van Deurzen CH. Male breast cancer precursor lesions: analysis of the EORTC 10085/TBCRC/BIG/NABCG International Male Breast Cancer Program. Mod Pathol. 2017;30:509–518. doi: 10.1038/modpathol.2016.229. [DOI] [PubMed] [Google Scholar]
- 6.Isley LM, Leddy RJ, Rumboldt T, Bernard JM. Asymptomatic Incidental Ductal Carcinoma in situ in a Male Breast Presenting with Contralateral Gynecomastia. J Clin Imaging Sci. 2012;2:9. doi: 10.4103/2156-7514.94021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Deutsch M, Rosenstein MM. Ductal carcinoma in situ (DCIS) of the male breast treated by lumpectomy and breast irradiation. Clin Oncol (R Coll Radiol) 1998;10:204–205. doi: 10.1016/S0936-6555(98)80073-3. [DOI] [PubMed] [Google Scholar]
- 8.Silverstein MJ, Lagios MD, Craig PH, Waisman JR, Lewinsky BS, Colburn WJ, Poller DN. A prognostic index for ductal carcinoma in situ of the breast. Cancer. 1996;77:2267–2274. doi: 10.1002/(SICI)1097-0142(19960601)77:11<2267::AID-CNCR13>3.0.CO;2-V. [DOI] [PubMed] [Google Scholar]
- 9.Silverstein MJ. The University of Southern California/Van Nuys prognostic index for ductal carcinoma in situ of the breast. Am J Surg. 2003;186:337–343. doi: 10.1016/S0002-9610(03)00265-4. [DOI] [PubMed] [Google Scholar]
- 10.Cardoso F, Bartlett JMS, Slaets L, van Deurzen CHM, van Leeuwen-Stok E, Porter P, Linderholm B, Hedenfalk I, Schröder C7, Martens J, Bayani J, van Asperen C, Murray M, Hudis C, Middleton L, Vermeij J, Punie K, Fraser J, Nowaczyk M, Rubio IT, Aebi S, Kelly C, Ruddy KJ, Winer E, Nilsson C, Dal Lago L, Korde L, Benstead K, Bogler O, Goulioti T, Peric A, Litière S, Aalders KC, Poncet C, Tryfonidis K, Giordano SH. Characterization of male breast cancer: results of the EORTC 10085/TBCRC/BIG/NABCG International Male Breast Cancer Program. Ann Onco. 2018;29:405–417. doi: 10.1093/annonc/mdx651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fentiman I. Male breast cancer: a review. Ecancermedicalscience. 2009;3:140. doi: 10.3332/ecancer.2009.140. [DOI] [PMC free article] [PubMed] [Google Scholar]


