Abstract
Objective
We compared the breast cancer patients with invasive lobular carcinoma (ILC), invasive ductal carcinoma (IDC) and mixed invasive ductal and lobular carcinoma (IDLC) in terms of clinicopathological and treatment features, metastatic patterns and long-term survival.
Materials and Methods
In a 10 years patient cohort, 3412 patients with unilateral breast carcinoma were enrolled in the study. Tumors were classified histologically according to criteria described by World Health Organization classification.
Results
The highest rate of T3 tumors were found in IDLC patients, the lowest in IDC patients, and the difference between groups was significant only in comparison of IDC vs IDLC. Axillary positivity rate was highest in IDLC, lowest in ILC; differences were significant in comparisons of IDLC vs ILC and IDLC vs IDC. There was no significant difference between the patient groups in terms of surgical treatment, mastectomy and breast conserving surgery. Rate of bone metastasis was highest in IDLC, lowest in IDC, with significant difference between IDLC and IDC. Locoregional recurrence-free survival (LRFS) rate was 90.9% in ILC patients, 92.5% in IDC patients, 92.9% in IDLC patients, with no significant difference between the groups; in multivariate Cox analysis, histological type had no prognostic significance (p=0.599). Distant metastasis-free survival (DMFS) rate was 66.2% in ILC patients, 66.7% in IDC patients, 57.1% in IDLC patients; in multivariate Cox analysis, histological type had no prognostic significance (p=0.392).
Conclusion
Although these results suggest that IDLC may have a worse prognosis than IDC and ILC, in multivariate analysis LRFS and DMFS were not significantly different among the histological type groups.
Keywords: Breast cancer, Invasive lobular carcinoma, Invasive ductal carcinoma, mixed invasive ductal and lobular carcinoma, survival
Introduction
Invasive breast cancer is a histologically heterogeneous disease; among numerous histological types, invasive ductal carcinoma (IDC) is the most common, present in 70%–75% of the cases (1, 2), followed by invasive lobular carcinoma (ILC), present in 5%–15% of the cases (1–3). Mixed invasive ductal and lobular carcinoma (IDLC), which has characteristics of both invasive ductal and lobular carcinoma, is present in approximately 5% of the cases (2). Lately, the prevalence of the lobular breast tumors has been on the rise, particularly in postmenopausal women; this increase has been linked with evidence suggesting that frequent use of hormone replacement therapy in recent years has increased the risk of ILC and IDLC development more than that of IDC (4–6). Clinicopathological characteristics and survival outcomes of ILC and IDC have been compared in numerous studies with conflicting results. On the other hand, few studies have compared IDLC with ILC and IDC.
In this study, we compared ILC, IDC and IDLC in terms of clinicopathological and treatment features, metastatic patterns and long-term survival retrospectively in a 10 years patient cohort.
Materials and Methods
Ethical standards
The research protocol of this clinical study was approved by the Ethics Committee of the University of Health Sciences, Istanbul Okmeydanı Training and Research Hospital (the date/protocol number: 04.24.2019/1236). The study was conducted according to the principles of the Helsinki Declaration and its later amendments or comparable ethical standards. In addition, all patients were routinely informed about the procedures and their written informed consent was obtained.
Patients
We reviewed the file records of women who underwent surgery for breast carcinoma between January 1993 and December 2002 who were then followed up in Istanbul Okmeydanı Training and Research Hospital. Inclusion criteria for the patients were a histological diagnosis of unilateral breast ILC, IDC and IDLC; tumors were classified histologically according to criteria described by World Health Organization classification; no previous or concomitant malignant disease; known pathological tumor size (patients with T4 tumor were not included), for multifocal/multicentric (MFMC) tumors, largest dimension of the largest tumor was accepted as the tumor size; at least one lymph node removed by axillary dissection; no metastasis in ipsilateral internal mammary or supraclavicular lymph nodes and distant sites at the time of diagnosis; microscopically tumor-free surgical margins; completion of adjuvant therapy planned according to standard therapy protocols (patients received neoadjuvant therapy were not included); and a follow-up period at least five years in patients without disease recurrence. A total of 3412 patients (including 668 patients who underwent surgery at the study hospital) who met these criteria were enrolled in the current study.
Removal of at least six nonmetastatic lymph nodes is required to describe the axillary lymph node status as “negative” according to TNM classification (7). Thus, within the node-negative patient group, 132 patients (nine patients with ILC, 118 patients with IDC, five patients with IDLC) with one to five lymph node(s) removed by axillary dissection were not included in the analyses of axillary status assessment and survival.
Follow-up data were obtained from file records and, in some patients, through telephone calls. The endpoint of the study was first disease recurrence. Locoregional recurrence was defined as the recurrence involving the chest wall or tumor excision site in the breast (local) or/and ipsilateral axillary, supraclavicular and internal mammary lymph nodes (regional). Locoregional recurrence-free survival (LRFS) and distant metastasis-free survival (DMFS) times were defined as the time interval between tumor excision and detection of first locoregional recurrence or distant metastasis, respectively, or the date of last follow-up. In 147 patients who developed a second malignancy (excluding three patients with basal cell carcinoma), the diagnosis date of second malignancy was considered as the last follow-up date. In 66 patients whose death was unrelated to cancer, the date of death was considered as the last follow-up date.
Statistical analysis
The chi-squared and Fisher’s exact tests were used to evaluate the differences between proportions and Student’s t-test was used to evaluate the continuous data for comparisons of the clinicopathological and treatment features, metastatic pattern and metachronous contralateral breast cancer development of the patient groups. Kaplan-Meier method was used for calculation and plotting of the LRFS and DMFS curves of the patient groups, and log-rank test was used for the comparison of the survival curves. The relative importance of the prognostic features was investigated using the Cox proportional hazards model; prognostic parameters present in all patients were included in the Cox analysis. All comparisons were two-tailed, and p value less than 0.05 was considered to be statistically significant. All statistical analyses were performed using Statistical Package for Social Sciences version 17.0 (SPSS Inc., Chicago, IL, USA).
Results
Clinicopathological features
Among 3621 patients, including 209 patients with invasive carcinoma of other histological types (mucinous, medullary, papillary, metaplastic and other) who were in the patient cohort during the same period but not included in this study, 272 (7.5%) had ILC, 2981 (82.3%) had IDC, and 159 (4.4%) had IDLC. Clinicopathological features of 3412 patients are shown in Table 1. Patients with ILC had the highest mean age, while patients with IDLC had the lowest; significant age difference was found for comparisons of ILC vs IDC and ILC vs IDLC. Considering age status according to the cutoff of 35 years, there was no significant difference among the histological types in the rates of the patients below 35 years and 35 and above 35 years. The rate of postmenopausal patients was highest in ILC group and lowest in IDLC group; no significant difference was detected in comparison of ILC vs IDC, while the rate of postmenopausal women in IDLC group was significantly lower than those in the ILC and IDC groups. Mean tumor size was largest in IDLC patients, smallest in IDC patients; the difference was borderline significant for comparison of ILC vs IDC (p=0.051), significant for IDC vs IDLC, not significant for ILC vs IDLC. According to TNM classification, the highest rate of T1 tumors were found in IDC patients, lowest in IDLC patients; the highest rate of T3 tumors were found in IDLC patients, the lowest in IDC patients, and the difference between groups was significant only in comparison of IDC vs IDLC. The rate of MFMC tumors was highest in IDLC patients, and the difference was statistically significant compared with both ILC and IDC; there was no significant difference between ILC and IDC.
Table 1.
Clinicopathological features of the patients
| ILC | IDC | IDLC | p | ||||||
|---|---|---|---|---|---|---|---|---|---|
|
|
|
|
|
||||||
| Feature | n | % | n | % | n | % | ILC vs IDC | ILC vs IDLC | IDC vs IDLC |
| Age, years | 0.023 | 0.008 | 0.119 | ||||||
| Mean (SD) | 50.9 (11.2) | 49.3 (11.0) | 47.9 (11.3) | ||||||
| Median | 49.5 | 48.0 | 47.0 | ||||||
| Range | 24.0–84.0 | 20.0–86.0 | 22.0–80.0 | ||||||
| Age, years | 0.377 | 0.305 | 0.602 | ||||||
| <35 | 17 | 6.3 | 237 | 8.0 | 15 | 9.4 | |||
| ≥35 | 255 | 93.7 | 2744 | 92.0 | 144 | 90.6 | |||
| Menopausal status | 0.188 | 0.006 | 0.021 | ||||||
| Premenopausal | 129 | 47.4 | 1538 | 51.6 | 97 | 61.0 | |||
| Postmenopausal | 143 | 52.6 | 1443 | 48.4 | 62 | 39.0 | |||
| Tumor size, cm | 0.051 | 0.132 | 0.002 | ||||||
| Mean (SD) | 3.3 (2.0) | 3.1 (1.8) | 3.6 (2.1) | ||||||
| Median | 3.0 | 2.9 | 3.0 | ||||||
| Range | 0.3–13.0 | 0.2–15.0 | 0.7–11.0 | ||||||
| Tumor size, TNM | 0.204 | 0.367 | 0.008 | ||||||
| T1 | 86 | 31.6 | 966 | 32.4 | 42 | 26.4 | |||
| T2 | 143 | 52.6 | 1654 | 55.5 | 85 | 53.5 | |||
| T3 | 43 | 15.8 | 361 | 12.1 | 32 | 20.1 | |||
| MFMC tumors | 0.790 | 0.001 | <0.001 | ||||||
| Yes | 17 | 6.3 | 205 | 6.9 | 26 | 16.4 | |||
| No | 255 | 93.7 | 2776 | 93.1 | 133 | 83.6 | |||
| Vascular invasion | 0.047 | 0.521 | 0.292 | ||||||
| Negative | 65 | 52.4 | 751 | 43.3 | 56 | 48.3 | |||
| Positive | 59 | 47.6 | 985 | 56.7 | 60 | 51.7 | |||
| Unknown | 148 | 1245 | 43 | ||||||
| Perineural invasion | 0.371 | 0.076 | 0.001 | ||||||
| Negative | 52 | 66.7 | 759 | 72.1 | 40 | 52.6 | |||
| Positive | 26 | 33.3 | 294 | 27.9 | 36 | 47.4 | |||
| Unknown | 194 | 1928 | 83 | ||||||
| Estrogen receptor | 0.288 | 0.149 | 0.008 | ||||||
| Negative | 45 | 34.9 | 631 | 39.6 | 24 | 25.8 | |||
| Positive | 84 | 65.1 | 961 | 60.4 | 69 | 74.2 | |||
| Unknown | 143 | 1389 | 66 | ||||||
| Progesterone receptor | 0.122 | 0.047 | <0.001 | ||||||
| Negative | 42 | 33.9 | 636 | 41.0 | 18 | 20.5 | |||
| Positive | 82 | 66.1 | 917 | 59.0 | 70 | 79.5 | |||
| Unknown | 148 | 1428 | 71 | ||||||
| Axillary lymph node status | 0.391 | 0.029 | 0.046 | ||||||
| Negative | 103 | 39.2 | 1045 | 36.5 | 44 | 28.6 | |||
| Positive | 160 | 60.8 | 1818 | 63.5 | 110 | 71.4 | |||
ILC: invasive lobular carcinoma; IDC: invasive ductal carcinoma; IDLC: mixed invasive ductal and lobular carcinoma; SD: standard deviation; MFMC: Multifocal or Multicentric
During the examination period of this study patients, vascular invasion, perineural invasion, estrogen receptor (ER) and progesterone receptor (PR) evaluations were not performed routinely in our hospital and in our country. Even though the number of patients having these evaluations was not high, we analyzed the available data. Vascular invasion rate was lowest in ILC, highest in IDC; the difference was significant for comparison of ILC vs IDC, while other group comparisons showed no significant difference. Perineural invasion rate was highest in IDLC, lowest in IDC; the difference was significant in comparison of IDLC vs IDC, while IDLC vs ILC difference was close to the level of significance (p=0.076). ER positivity rate was highest in IDLC, lowest in IDC; the difference between groups was significant only in comparison of IDLC vs IDC. PR positivity rate was highest in IDLC, lowest in IDC; this rate was significantly higher in IDLC compared with ILC and IDC. In the evaluation of axillary lymph node status, axillary positivity rate was highest in IDLC, lowest in ILC; differences were significant in comparisons of IDLC vs ILC and IDLC vs IDC, but not significant for ILC vs IDC.
Treatment features
Surgery and adjuvant treatment features of patients are presented in Table 2. There was no significant difference among histological type groups in terms of surgery, adjuvant hormonal therapy, and radiotherapy. Adjuvant chemotherapy application was highest rate in IDLC patients, lowest rate in ILC patients; the difference was significant in comparison of ILC vs IDC and ILC vs IDLC, but not significant for IDC vs IDLC.
Table 2.
Treatment features of the patients
| ILC | IDC | IDLC | p | ||||||
|---|---|---|---|---|---|---|---|---|---|
|
|
|
|
|
||||||
| Feature | n | % | n | % | n | % | ILC vs IDC | ILC vs IDLC | IDC vs IDLC |
| Surgery | 0.998 | 0.904 | 0.829 | ||||||
| Mastectomy | 244 | 89.7 | 2674 | 89.7 | 144 | 90.6 | |||
| Breast-conserving | 28 | 10.3 | 307 | 10.3 | 15 | 9.4 | |||
| Chemotherapy | 0.009 | 0.010 | 0.200 | ||||||
| Yes | 202 | 74.3 | 2409 | 80.8 | 135 | 84.9 | |||
| No | 70 | 25.7 | 572 | 19.2 | 24 | 15.1 | |||
| Hormonal therapy | 0.161 | 0.742 | 0.484 | ||||||
| Yes | 199 | 73.2 | 2059 | 69.1 | 114 | 71.7 | |||
| No | 73 | 26.8 | 922 | 30.9 | 45 | 28.3 | |||
| Radiotherapy | 0.502 | 0.086 | 0.116 | ||||||
| Yes | 184 | 67.6 | 2075 | 69.6 | 120 | 75.5 | |||
| No | 88 | 32.4 | 906 | 30.4 | 39 | 24.5 | |||
ILC: invasive lobular carcinoma; IDC: invasive ductal carcinoma; IDLC: mixed invasive ductal and lobular carcinoma
Metastasis sites and metachronous contralateral breast carcinoma
Table 3 presents data regarding the location of metastases (in one site or more sites concomitantly) and the development of metachronous contralateral breast carcinoma for the whole series encompassing 3412 patients. There was no significant difference among the histological type groups in terms of metastasis to unilateral axillary lymph nodes. Distant metastasis sites were not significantly different among the groups except for the bone. Development rate of bone metastasis was highest in IDLC patients, lowest in IDC patients; the difference was significant in comparison of IDC vs IDLC, but not significant for ILC vs IDC and ILC vs IDLC. In addition to metastasis sites reported in Table 3, metastases developed in cecum, pancreas, urinary bladder, thyroid, pericardium, retroperitoneal soft tissue in one patient each and in the eye in two patients. Due to their low numbers, these locations were not considered in the statistical analysis. The rates of metachronous contralateral breast carcinoma were not significantly different among the three histological type groups.
Table 3.
Metastatis sites and metachronous contralateral breast carcinoma in patient groups according to histological types
| ILC | IDC | IDLC | p | ||||||
|---|---|---|---|---|---|---|---|---|---|
|
|
|
|
|
||||||
| Metastasis sites | n | % | n | % | n | % | ILC vs IDC | ILC vs IDLC | IDC vs IDLC |
| Axillary lymph nodes | 0.929 | 0.410 | 0.325 | ||||||
| Yes | 4 | 1.5 | 52 | 1.7 | 5 | 3.1 | |||
| No | 268 | 98.5 | 2929 | 98.3 | 154 | 96.9 | |||
| Bone | 0.162 | 0.085 | 0.001 | ||||||
| Yes | 54 | 19.9 | 493 | 16.5 | 43 | 27.0 | |||
| No | 218 | 80.1 | 2488 | 83.5 | 116 | 73.0 | |||
| Lung | 0.429 | 1.000 | 0.700 | ||||||
| Yes | 25 | 9.2 | 320 | 10.7 | 15 | 9.4 | |||
| No | 247 | 90.8 | 2661 | 89.3 | 144 | 90.6 | |||
| Pleura | 0.961 | 0.591 | 0.306 | ||||||
| Yes | 4 | 1.5 | 51 | 1.7 | 5 | 3.1 | |||
| No | 268 | 98.5 | 2930 | 98.3 | 154 | 96.9 | |||
| Liver | 0.779 | 0.519 | 0.574 | ||||||
| Yes | 18 | 6.6 | 217 | 7.3 | 14 | 8.8 | |||
| No | 254 | 93.4 | 2764 | 92.7 | 145 | 91.2 | |||
| Central nervous system | 0.071 | 1.000 | 0.235 | ||||||
| Yes | 3 | 1.1 | 98 | 3.3 | 2 | 1.3 | |||
| No | 269 | 98.9 | 2883 | 96.7 | 157 | 98.7 | |||
| Gynecologic | 1.000 | 1.000 | 1.000 | ||||||
| Yes | 1 | 0.4 | 6 | 0.2 | 0 | 0.0 | |||
| No | 271 | 99.6 | 2975 | 99.8 | 159 | 100.0 | |||
| Distant lymph nodes | 0.146 | 1.000 | 0.265 | ||||||
| Yes | 10 | 3.7 | 63 | 2.1 | 6 | 3.8 | |||
| No | 262 | 96.3 | 2918 | 97.9 | 153 | 96.2 | |||
| Skin-subcutaneous | 0.545 | 1.000 | 1.000 | ||||||
| Yes | 1 | 0.4 | 8 | 0.3 | 0 | 0.0 | |||
| No | 271 | 99.6 | 2973 | 99.7 | 159 | 100.0 | |||
| Adrenal | 1.000 | - | 1.000 | ||||||
| Yes | 0 | 0.0 | 5 | 0.2 | 0 | 0.0 | |||
| No | 272 | 100.0 | 2976 | 99.8 | 159 | 100.0 | |||
| Peritoneum | 0.354 | 1.000 | 0.229 | ||||||
| Yes | 1 | 0.4 | 4 | 0.1 | 1 | 0.6 | |||
| No | 271 | 99.6 | 2977 | 99.9 | 158 | 99.4 | |||
| Contralateral breast carcinoma | 0.496 | 0.252 | 0.405 | ||||||
| Yes | 3 | 1.1 | 56 | 1.9 | 5 | 3.1 | |||
| No | 269 | 98.9 | 2925 | 98.1 | 154 | 96.9 | |||
ILC: invasive lobular carcinoma; IDC: invasive ductal carcinoma; IDLC: mixed invasive ductal and lobular carcinoma
Survival
Survival analyses were conducted on 3280 patients, excluding 132 node-negative patients who had 1–5 lymph node(s) removed by axillary dissection. Until the end of the study on November 2017, 251 patients developed locoregional recurrence, 1107 patients developed distant metastasis, and 57 patients developed concomitant locoregional recurrence and distant metastasis. In patients without disease recurrence, the median follow-up time was 148 months (range:60–297 months).
LRFS rate was 90.9% in ILC patients, 92.5% in IDC patients, 92.9% in IDLC patients, with no significant difference between the groups (Figure 1); in multivariate Cox analysis, histological type had no prognostic significance (p=0.599) (Table 4). DMFS rate was 66.2% in ILC patients, 66.7% in IDC patients, 57.1% in IDLC patients, with no significant difference between the ILC patients and IDC patients (log-rank x2=0.040, p=0.842); DMFS of IDLC patients was significantly worse than IDC patients (log-rank x2=5.867, p=0.015); it was also worse than that of ILC patients, but the difference was outside the limit of significance (log-rank x2=3.065, p=0.080) (Figure 2); in multivariate Cox analysis, histological type had no prognostic significance (p=0.392) (Table 5).
Figure 1.
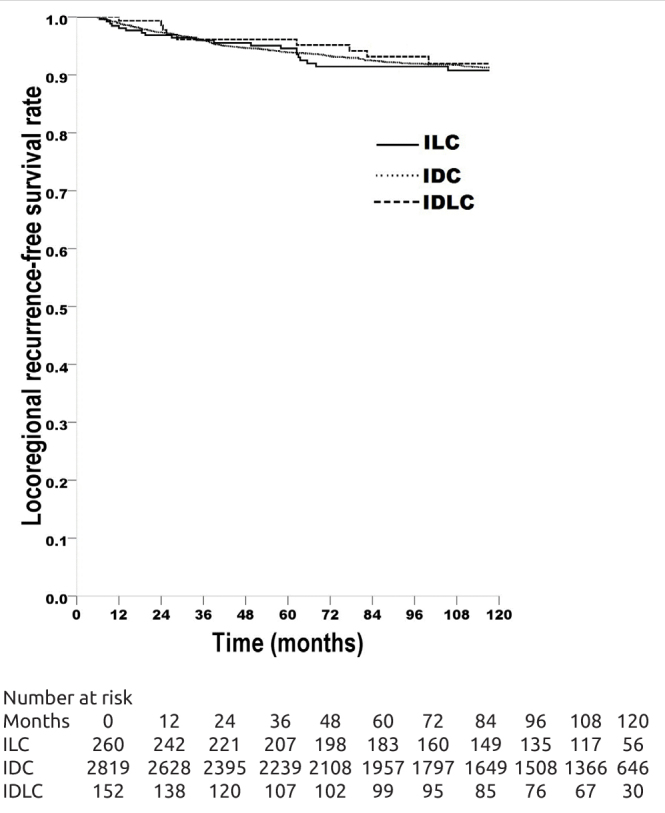
Locoregional recurrence-free survival (LRFS) rates of the breast carcinoma patients with invasive lobular carcinoma (ILC, 263 patients, LRFS rate 90.9%), with invasive ductal carcinoma (IDC, 2863 patients, LRFS rate 92.5%), with mixed invasive ductal and lobular carcinoma (IDLC, 154 patients, LRFS rate 92.9%). ILC vs IDC, log-rank x2=0.842, p=0.359; ILC vs IDLC, log-rank x2=0.295, p=0.587; IDC vs IDLC, log-rank x2=0.000, p=0.993.
Table 4.
Cox proportional hazards model analysis of the clinicopathological and treatment features in terms of locoregional recurrence-free survival
| Feature | Relative risk | 95% CI | p |
|---|---|---|---|
| Age, years | 0.009 | ||
| <35 | 1.00 | ||
| ≥35 | 0.57 | 0.38–0.87 | |
| Menopausal status | 0.103 | ||
| Premenopausal | 1.00 | ||
| Postmenopausal | 1.26 | 0.95–1.67 | |
| Tumor size | <0.001 | ||
| T1 | 1.00 | ||
| T2 | 1.72 | 1.27–2.33 | |
| T3 | 2.18 | 1.40–3.41 | |
| Multifocality/multicentricity | 0.031 | ||
| Yes | 1.00 | ||
| No | 0.62 | 0.40–0.96 | |
| Histological type | 0.599 | ||
| ILC | 1.00 | ||
| IDC | 0.80 | 0.52–1.23 | |
| IDLC | 0.80 | 0.39–1.63 | |
| Axillary lymph node status | 0.018 | ||
| Negative | 1.00 | ||
| Positive | 1.51 | 1.07–2.12 | |
| Surgery | 0.001 | ||
| Mastectomy | 1.00 | ||
| Breast-conserving | 1.92 | 1.29–2.85 | |
| Chemotherapy | 0.863 | ||
| Yes | 1.00 | ||
| No | 1.03 | 0.70–1.52 | |
| Hormonal therapy | <0.001 | ||
| Yes | 1.00 | ||
| No | 1.82 | 1.39–2.38 | |
| Radiotherapy | 0.001 | ||
| Yes | 1.00 | ||
| No | 1.87 | 1.31–2.66 |
CI: confidence interval; ILC: invasive lobular carcinoma; IDC: invasive ductal carcinoma; IDLC: mixed invasive ductal and lobular carcinoma
Figure 2.
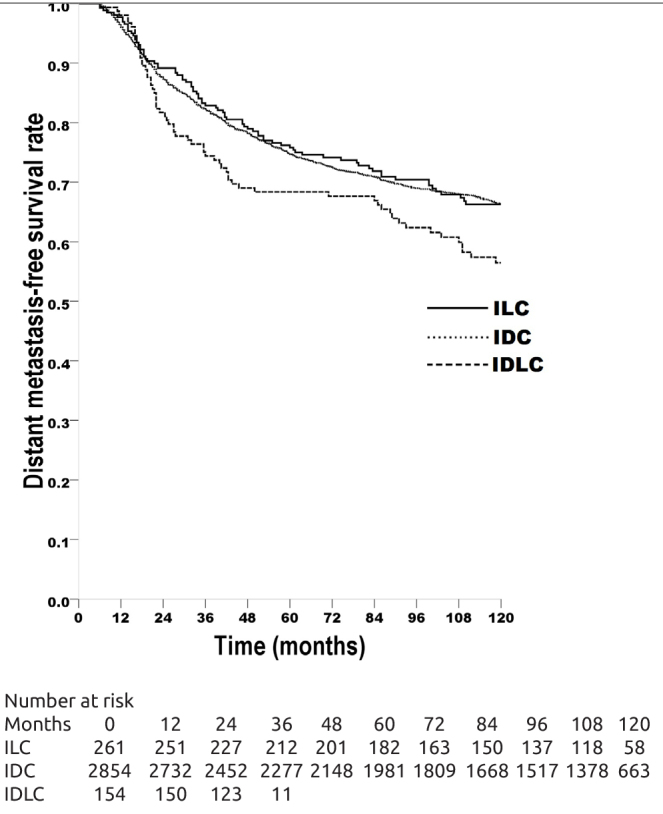
Distant metastasis-free survival (DMFS) rates of the breast carcinoma patients with invasive lobular carcinoma (ILC, 263 patients, DMFS rate 66.2%), with invasive ductal carcinoma (IDC, 2863 patients, DMFS rate 66.7%), with mixed invasive ductal and lobular carcinoma (IDLC, 154 patients, DMFS rate 57.1%). ILC vs IDC, log-rank x2=0.040, p=0.842; ILC vs IDLC, log-rank x2=3.065, p=0.080; IDC vs IDLC, log-rank x2=5.867, p=0.015.
Table 5.
Cox proportional hazards model analysis of the clinicopathological and treatment features in terms of distant metastasis-free survival
| Feature | Relative risk | 95% CI | p |
|---|---|---|---|
| Age, years | <0.001 | ||
| <35 | 1.00 | ||
| ≥35 | 0.68 | 0.56–0.84 | |
| Menopausal status | 0.263 | ||
| Premenopausal | 1.00 | ||
| Postmenopausal | 1.08 | 0.94–1.23 | |
| Tumor size | <0.001 | ||
| T1 | 1.00 | ||
| T2 | 1.55 | 1.33–1.82 | |
| T3 | 2.49 | 2.05–3.03 | |
| Multifocality/multicentricity | 0.001 | ||
| Yes | 1.00 | ||
| No | 0.72 | 0.59–0.88 | |
| Histological type | 0.392 | ||
| ILC | 1.00 | ||
| IDC | 0.96 | 0.77–1.19 | |
| IDLC | 1.14 | 0.83–1.57 | |
| Axillary lymph node status | <0.001 | ||
| Negative | 1.00 | ||
| Positive | 2.41 | 1.99–2.93 | |
| Surgery | 0.661 | ||
| Mastectomy | 1.00 | ||
| Breast-conserving | 0.95 | 0.76–1.19 | |
| Chemotherapy | 0.301 | ||
| Yes | 1.00 | ||
| No | 0.89 | 0.71–1.11 | |
| Hormonal therapy | <0.001 | ||
| Yes | 1.00 | ||
| No | 1.36 | 1.20–1.55 | |
| Radiotherapy | 0.068 | ||
| Yes | 1.00 | ||
| No | 0.83 | 0.67–1.01 |
CI: confidence interval; ILC: invasive lobular carcinoma; IDC: invasive ductal carcinoma; IDLC: mixed invasive ductal and lobular carcinoma
Discussion and Conclusion
In our study, comparison of clinicopathological features of patients with ILC, IDC and IDLC revealed highest mean age in ILC, lowest mean age in IDLC, with significant difference in comparisons of ILC vs IDC and ILC vs IDLC. When patients were analyzed in two groups according to 35-year cutoff, no significant difference was found among the histological types. In some studies, significantly advanced age was found in ILC compared with IDC (8–13). In other studies, no significant age difference was found between ILC and IDC (14–18). In one study, the rate of patients below the age of 50 years was significantly lower in IDLC compared with ILC, while no significant difference was seen between IDLC and IDC (19). In a study comparing IDLC with ILC and IDC, the rate of women over the age of 50 years was significantly higher in ILC (20). In our series, the rate of postmenopausal women was highest in ILC, lowest in IDLC, with significant difference for IDLC vs ILC and IDLC vs IDC comparisons. In a study, menopausal status was not significantly different between ILC and IDC (1). In a study, the rate of postmenopausal patients was significantly lower in IDLC than in ILC, while no significant difference was found between IDLC and IDC (19). In a study comparing ILC, IDC and IDLC, there was no significant difference between the histological groups in terms of menopausal status (20).
In our study, both mean tumor size and the rate of T3 tumors were highest in IDLC, lowest in IDC, with significant difference between IDLC and IDC. In some studies comparing ILC and IDC, no significant difference was found between the two histological types in terms of tumor size (9, 14, 15, 17, 18, 21, 22); in other studies, tumor size was significantly larger in ILC compared with IDC (1, 8, 10–13, 23); in one study the rate of T1 tumors was significantly lower in ILC compared with IDC (16). In a study comparing IDLC with IDC and ILC, the rate of T3 tumors was significantly higher in IDLC than in IDC, while no significant difference was found between IDLC and ILC (24); in another study, mean tumor size was largest in IDLC, smallest in IDC, with significant difference in histological group comparisons (there were no pairwise comparisons) (20); in another study no significant difference was found in IDLC compared with ILC and IDC in terms of tumor size (19). In our series, the rate of MFMC tumors was highest in IDLC, lowest in ILC, with significant difference found in comparisons of IDLC vs ILC and IDLC vs IDC and no significant difference found for ILC vs IDC. In some studies comparing ILC and IDC, the rate of MFMC tumors was found to be significantly higher in ILC compared with IDC (12, 13, 17, 18). In one study, no significant difference was found between the two histological types in terms of MFMC tumor rate (15). In a study investigating IDLC, ILC and IDC, MFMC tumor rate was found to be significantly higher in ILC (20).
In our series, within the subset of patients with vascular invasion, perineural invasion, ER and PR status evaluations, vascular invasion positivity rate was highest in IDC, lowest in ILC, with significant difference in comparison of ILC vs IDC. In studies comparing ILC and IDC, vascular invasion was significantly lower in ILC vs IDC (1, 9, 23). In our series, the rates of perineural invasion and ER positivity were highest in IDLC, lowest in IDC, with significant difference between IDLC and IDC for both. Similarly, PR positivity rate was highest in IDLC, lowest in IDC, with significant difference detected in comparisons of IDLC vs ILC and IDLC vs IDC. In some studies comparing ILC and IDC, ER and PR positivity were found at significantly higher rates in ILC than in IDC (8, 10–13, 16, 18, 21–23) while some studies found no significant difference between these two histological types in terms of ER status (9,15). In one of the studies comparing IDLC with IDC and ILC, ER positivity rate was significantly higher in IDLC than in IDC, with no significant difference between IDLC and ILC, and PR positivity rate was significantly higher in IDLC than in IDC and ILC (24); in another study, no difference was found between the three histological types in terms of ER and PR positivity (20); in a different study, ER and PR positivity was significantly higher in IDLC and ILC compared with IDC (25).
In our series, the rate of axillary lymph node positivity was highest in IDLC, lowest in ILC, with pairwise comparisons of IDLC vs ILC and IDLC vs IDC significant, while ILC vs IDC was not significant. In some studies comparing ILC and IDC, axillary lymph node positivity rate was not significantly different between ILC and IDC (1, 8–10, 15, 16, 18, 21–23). In other studies, it was significantly lower in ILC compared with IDC (14, 17); in some other studies it was significantly higher in ILC compared with IDC (11, 13). In one study comparing IDLC with IDC and ILC, axillary lymph node positivity rate was significantly higher in IDLC than in IDC, with no significant difference between IDLC and ILC (24); in another study, there was no significant difference between the three histological types in terms of axillary lymph node positivity (20).
In our study, there was no significant difference between the patient groups according to histological type in terms of surgical treatment, mastectomy and breast-conserving surgery. Among studies comparing ILC and IDC, some had no difference in mastectomy and breast-conserving surgery rates (15, 16, 18, 22); while some found significantly more mastectomy performed in patients with ILC than breast-conserving surgery (1, 8, 10–12), more frequent application of mastectomy in ILC patients may be related to more frequent presence or higher likelihood of multicentric tumors in this histological type. In a study comparing IDLC with IDC and ILC, surgical treatment was not significantly different between the histological groups (20).
In our study, rates of metastasis to various locations did not vary significantly between the histological types, except for bone metastasis. Rate of bone metastasis was highest in IDLC, lowest in IDC, with significant difference between IDLC and IDC. In a study comparing these three histological types, no significant difference was found regarding the metastatic sites (20). Among studies comparing metastatic sites of ILC and IDC, some found no difference between the two histological types (16, 18, 21), while some found significantly higher rates of bone metastasis in ILC (1, 12); some studies found significantly more frequent lung metastases in IDC (1, 10, 12, 22, 26), while one study found it to be significantly higher in ILC (27). Some studies reported the rare occurrence of peritoneum-retroperitoneum metastases, as also seen in our series, and more frequently in ILC than in IDC (26, 27).
In this series, there was no significant difference between the three histological type groups in terms of metachronous contralateral breast cancer occurrence. Some studies found higher rates of metachronous contralateral breast cancer in ILC compared with IDC (10, 14, 16, 28), while others found no significant difference between the two histological types (1, 17, 21, 29, 30).
In our study, LRFS was not statistically significant among the patient groups with three histological types in univariate and multivariate analyses. In univariate analysis, DMFS rate was highest in IDC, lowest in IDLC, with the difference close to the level of significance for IDLC vs ILC and significant for IDLC vs IDC; however, there was no significant difference among the histological groups in multivariate analysis. In various studies comparing survival in ILC and IDC, no significant survival difference was found between the two groups (9, 10, 12, 14–18, 21–23, 26, 31, 32); in some studies, survival was found to be significantly better in ILC compared with IDC (8, 11). In two studies comparing IDLC with ILC and IDC, survival was not significantly different between the groups (19, 20); in another study, IDLC had significantly worse survival compared with IDC, while no significant survival difference was found between IDLC and ILC (24).
In our study two important prognostic factors according to TNM classification, namely tumor size and axillary lymph node status, were not significantly different between ILC and IDC, while IDLC had significantly larger tumor size and higher rates of axillary lymph node positivity than IDC; compared with ILC, IDLC had significantly higher lymph node positivity rate, but no significant difference in terms of tumor size. Although these results suggest that IDLC may have a worse prognosis than IDC and ILC, in multivariate analysis LRFS and DMFS were not significantly different among the histological type groups. In our series, rates of metastasis to various locations did not vary significantly between the histological types, except for bone metastasis. Rate of bone metastasis was highest in IDLC, lowest in IDC, with significant difference between IDLC and IDC. Since the risk of developing metachronous contralateral breast carcinoma was similar in all three histological type groups, it is reasonable to use a similar approach for all histological types in the evaluation of contralateral breast in post-treatment follow-up of these patients. Retrospective nature of our study is a limitation. Future evaluations of prognostic characteristics of histological types should involve prospective controlled studies and include current, new prognostic characteristics in addition to the clinicopathological characteristics that were available within the period of the present study.
Acknowledgements
We would like to thank Archive staff for their cooperation.
Footnotes
Ethics Committee Approval: Ethics committee approval was received for this study from the Ethics Committee of Health Sciences Istanbul Okmeydanı Training and Research Hospital (04.24.2019/1236).
Informed Consent: Written informed consent was obtained from patients who participated in this study.
Peer-review: Externally peer-reviewed.
Author Contributions: Concept - N.D., S.H.; Design - N.D., S.H.; Supervision - N.D., S.H.; Resources - N.D., S.H.; Materials - S.H., A.A.; Data Collection and/or Processing - A.A., P.Ö.N.; Analysis and/or Interpretation - N.D., S.H.; Literature Search - N.D., S.H.; Writing Manuscript - N.D., S.H.; Critical Review - N.D., S.H.
Conflict of Interest: The authors have no conflicts of interest to declare.
Financial Disclosure: The authors declared that this study has received no financial support.
References
- 1.Pestalozzi BC, Zahrieh D, Mallon E, Gusterson BA, Price KN, Gelber RD, Holmberg SB, Lindtner J, Snyder R, Thürlimann B, Murray E, Viale G, Castiglione-Gertsch M, Coates AS, Goldhirsch A. Distinct clinical and prognostic features of infiltrating lobular carcinoma of the breast: combined results of 15 International Breast Cancer Study Group clinical trials. J Clin Oncol. 2008;26:3006–3014. doi: 10.1200/JCO.2007.14.9336. [DOI] [PubMed] [Google Scholar]
- 2.Corben AD. Pathology of invasive breast disease. Surg Clin North Am. 2013;93:363–392. doi: 10.1016/j.suc.2013.01.003. [DOI] [PubMed] [Google Scholar]
- 3.Mamtani A, King TA. Lobular breast cancer: different disease, different algorithms? Surg Oncol Clin N Am. 2018;27:81–94. doi: 10.1016/j.soc.2017.07.005. [DOI] [PubMed] [Google Scholar]
- 4.Chen C-L, Weiss NS, Newcomb P, Barlow W, White E. Hormone replacement therapy in relation to breast cancer. JAMA. 2002;287:734–741. doi: 10.1001/jama.287.6.734. [DOI] [PubMed] [Google Scholar]
- 5.Li CI, Anderson BO, Daling JR, Moe RE. Trends in incidence rates of invasive lobular and ductal breast carcinoma. JAMA. 2003;289:1421–1424. doi: 10.1001/jama.289.11.1421. [DOI] [PubMed] [Google Scholar]
- 6.Biglia N, Mariani L, Sgro L, Mininanni P, Moggio G, Sismondi P. Increased incidence of lobular breast cancer in women treated with hormone replacement therapy: implications for diagnosis, surgical and medical treatment. Endocr Relat Cancer. 2007;14:549–567. doi: 10.1677/ERC-06-0060. [DOI] [PubMed] [Google Scholar]
- 7.Edge SB, Byrd DR, Compton CC, Fritz AG, Greene FL, Trotti A. AJCC Cancer Staging Handbook. 7th edn. New York: Springer-Verlag; 2010. [Google Scholar]
- 8.Silverstein MJ, Lewinsky BS, Waisman JR, Gierson ED, Colburn WJ, Senofsky GM, Gamagami P. Infiltrating lobular carcinoma: is it different from infiltrating duct carcinoma? Cancer. 1994;73:1673–1677. doi: 10.1002/1097-0142(19940315)73:6<1673::aid-cncr2820730620>3.0.co;2-b. [DOI] [PubMed] [Google Scholar]
- 9.Mersin H, Yıldırım E, Gülben K, Berberoğlu U. Is invasive lobular carcinoma different from invasive ductal carcinoma? Eur J Surg Oncol. 2003;29:390–395. doi: 10.1053/ejso.2002.1423. [DOI] [PubMed] [Google Scholar]
- 10.Arpino G, Bardou VJ, Clark GM, Elledge RM. Infiltrating lobular carcinoma of the breast: tumor characteristics and clinical outcome. Breast Cancer Res. 2004;6:R149–R156. doi: 10.1186/bcr767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wasif N, Maggard MA, Ko CY, Giuliano AE. Invasive lobular vs. ductal breast cancer: a stage-matched comparison of outcomes. Ann Surg Oncol. 2010;17:1862–1869. doi: 10.1245/s10434-010-0953-z. [DOI] [PubMed] [Google Scholar]
- 12.Kwast AB, Groothuis-Oudshoorn KC, Grandjean I, Ho VK, Voogd AC, Menke-Pluymers MB, van der Sangen MJ, Tjan-Heijnen VC, Kiemeney LA, Siesling S. Histological type is not an independent prognostic factor for the risk pattern of breast cancer recurrences. Breast Cancer Res Treat. 2012;135:271–280. doi: 10.1007/s10549-012-2160-z. [DOI] [PubMed] [Google Scholar]
- 13.Brouckaert O, Laenen A, Smeets A, Christiaens MR, Vergote I, Wildiers H, Moerman P, Floris G, Neven P. Prognostic implications of lobular breast cancer histology: new insights from a single hospital cross-sectional study and SEER data. Breast. 2014;23:371–377. doi: 10.1016/j.breast.2014.01.007. [DOI] [PubMed] [Google Scholar]
- 14.Toikkanen S, Pylkkänen L, Joensuu H. Invasive lobular carcinoma of the breast has better short- and long-term survival than invasive ductal carcinoma. Br J Cancer. 1997;76:1234–1240. doi: 10.1038/bjc.1997.540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jayasinghe UW, Bilous AM, Boyages J. Is survival from infiltrating lobular carcinoma of the breast different from that of infiltrating ductal carcinoma? Breast J. 2007;13:479–485. doi: 10.1111/j.1524-4741.2007.00468.x. [DOI] [PubMed] [Google Scholar]
- 16.Cao AY, Huang L, Wu J, Lu JS, Liu GY, Shen ZZ, Shao ZM, Di GH. Tumor characteristics and the clinical outcome of invasive lobular carcinoma compared to infiltrating ductal carcinoma in a Chinese population. World J Surg Oncol. 2012;10:152. doi: 10.1186/1477-7819-10-152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fortunato L, Mascaro A, Poccia I, Andrich R, Amini M, Costarelli L, Cortese G, Farina M, Vitelli C. Lobular breast cancer: same survival and local control compared with ductal cancer, but should both be treated the same way? Analysis of an institutional database over a 10-year period. Ann Surg Oncol. 2012;19:1107–1114. doi: 10.1245/s10434-011-1907-9. [DOI] [PubMed] [Google Scholar]
- 18.Biglia N, Maggiorotto F, Liberale V, Bounous VE, Sgro LG, Pecchio S, D’Alonzo M, Ponzone R. Clinical-pathologic features, long term-outcome and surgical treatment in a large series of patients with invasive lobular carcinoma (ILC) and invasive ductal carcinoma (IDC) Eur J Surg Oncol. 2013;39:455–460. doi: 10.1016/j.ejso.2013.02.007. [DOI] [PubMed] [Google Scholar]
- 19.Rakha EA, Gill MS, El-Sayed ME, Khan MM, Hodi Z, Blamey RW, Evans AJ, Lee AH, Ellis IO. The biological and clinical characteristics of breast carcinoma with mixed ductal and lobular morphology. Breast Cancer Res Treat. 2009;114:243–250. doi: 10.1007/s10549-008-0007-4. [DOI] [PubMed] [Google Scholar]
- 20.Zengel B, Yararbas U, Duran A, Uslu A, Elıyatkın N, Demırkıran MA, Cengiz F, Şimşek C, Postacı H, Vardar E, Durusoy R. Comparison of the clinicopathological features of invasive ductal, invasive lobular, and mixed (invasive ductal + invasive lobular) carcinoma of the breast. Breast Cancer. 2015;22:374–381. doi: 10.1007/s12282-013-0489-8. [DOI] [PubMed] [Google Scholar]
- 21.Korhonen T, Huhtala H, Holli K. A comparison of the biological and clinical features of invasive lobular and ductal carcinomas of the breast. Breast Cancer Res Treat. 2004;85:23–29. doi: 10.1023/B:BREA.0000021038.97593.8b. [DOI] [PubMed] [Google Scholar]
- 22.Korhonen T, Kuukasjärvi T, Huhtala H, Alarmo E-L, Holli K, Kallioniemi A, Pylkkänen L. The impact of lobular and ductal breast cancer histology on the metastatic behavior and long term survival of breast cancer patients. Breast. 2013;22:1119–1124. doi: 10.1016/j.breast.2013.06.001. [DOI] [PubMed] [Google Scholar]
- 23.Molland JG, Donnellan M, Janu NC, Carmalt HL, Kennedy CW, Gillet DJ. Infiltrating lobular carcinoma – a comparison of diagnosis, management and outcome with infiltrating duct carcinoma. Breast. 2004;13:389–396. doi: 10.1016/j.breast.2004.03.004. [DOI] [PubMed] [Google Scholar]
- 24.Arps DP, Healy P, Zhao L, Kleer CG, Pang JC. Invasive ductal carcinoma with lobular features: a comparison study to invasive ductal and invasive lobular carcinomas of the breast. Breast Cancer Res Treat. 2013;138:719–726. doi: 10.1007/s10549-013-2493-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bharat A, Gao F, Margenthaler JA. Tumor characteristics and patient outcomes are similar between invasive lobular and mixed invasive ductal/lobular breast cancers but differ from pure invasive ductal breast cancers. Am J Surg. 2009;198:516–519. doi: 10.1016/j.amjsurg.2009.06.005. [DOI] [PubMed] [Google Scholar]
- 26.Inoue M, Nakagomi H, Nakada H, Furuya K, Ikegame K, Watanabe H, Omata M, Oyama T. Specific sites of metastases in invasive lobular carcinoma: a retrospective cohort study of metastatic breast cancer. Breast Cancer. 2017;24:667–672. doi: 10.1007/s12282-017-0753-4. doi: 10.1007/s12282-017-0753-4. [DOI] [PubMed] [Google Scholar]
- 27.Borst MJ, Ingold JA. Metastatic patterns of invasive lobular versus invasive ductal carcinoma of the breast. Surgery. 1993;114:637–642. [PubMed] [Google Scholar]
- 28.Moran MS, Yang Q, Haffty BG. The Yale University experience of early-stage invasive lobular carcinoma (ILC) and invasive ductal carcinoma (IDC) treated with breast conservation treatment (BCT): analysis of clinical-pathologic features, long-term outcomes, and molecular expression of COX-2, Bcl-2, and p53 as a function of histology. Breast J. 2009;15:571–578. doi: 10.1111/j.1524-4741.2009.00833.x. [DOI] [PubMed] [Google Scholar]
- 29.Chung MA, Cole B, Wanebo HJ, Bland KI, Chang HR. Optimal surgical treatment of invasive lobular carcinoma of the breast. Ann Surg Oncol. 1997;4:545–550. doi: 10.1007/BF02305534. [DOI] [PubMed] [Google Scholar]
- 30.Santiago RJ, Harris EER, Qin L, Hwang W-T, Solin LJ. Similar long-term results of breast-conservation treatment for stage I and II invasive lobular carcinoma compared with invasive ductal carcinoma of the breast: the University of Pennsylvania experience. Cancer. 2005;103:2447–2454. doi: 10.1002/cncr.21071. [DOI] [PubMed] [Google Scholar]
- 31.Mhuircheartaigh JN, Curran C, Hennessy E, Kerin MJ. Prospective matched-pair comparison of outcome after treatment for lobular and ductal breast carcinoma. Br J Surg. 2008;95:827–833. doi: 10.1002/bjs.6042. [DOI] [PubMed] [Google Scholar]
- 32.Viale G, Rotmensz N, Maisonneuve P, Orvieto E, Maiorano E, Galimberti V, Luini A, Colleoni M, Goldhirsch A, Coates AS. Lack of prognostic significance of “classic” lobular breast carcinoma: a matched, single institution series. Breast Cancer Res Treat. 2009;117:211–214. doi: 10.1007/s10549-008-0112-4. [DOI] [PubMed] [Google Scholar]


