Abstract
Objective
Phyllodes tumors are biphasic tumors consisting of epithelial and stromal components that account for less than 1% of all breast tumors. According to the World Health Organization (WHO) phyllodes tumors are classified into three categories as benign, borderline and malignant. It has been reported that these tumors are usually benign and both the stromal component and the epithelial component may progress to malignancy. In this descriptive study, it was aimed to present the cases of phyllodes tumor and to evaluate the clinicopathological features of these tumors in the light of the literature.
Materials and Methods
In our study, 55 cases of phyllodes tumor diagnosed between 2005–2018 in the Department of Medical Pathology were retrospectively studied. A total of 55 cases were included in the study.
Results
All cases were female with a mean age of 39.7+15.2 years. Fifty-seven tumors diagnosed in 55 cases were classed as benign in 20 cases (35.1%), borderline in 14 cases (24.6%) and malignant phyllodes tumors in 23 cases (40.3%). Ductal carcinoma in situ (solid and cribriform type) were detected in one case with malignant phyllodes tumor, whereas invasive ductal carcinoma was detected in one case. Bilateral ductal carcinoma in situ was present in the patient with invasive ductal carcinoma.
Conclusion
These tumors which rapidly grow into large masses can be clinically and pathologically confused with benign lesions, macroscopic and microscopic evaluation of concomitant in situ-invasive carcinomas should be considered. Phyllodes tumors have an important role in breast surgery and pathology.
Keywords: Fibroepithelial lesion, phyllodes tumor, breast
Introduction
Phyllodes tumor of the breast is a rare biphasic tumor accounting for less than 1% of all primary breast tumors (1). This tumor was first described in 1774 as a giant type of fibroadenoma and was first named as “cystosarcoma phyllodes” by Johannes Muller in 1838. World Health Organization (WHO) adapted similar terminology in 1982 and uses the term “phyllodes tumor” in the classification (2, 3).
World Health Organization classifies phyllodes tumors in three groups as benign, borderline and malignant, based on histopathological features such as tumor margins, stromal cellularity, stromal cell atypia, mitotic activity, stromal overgrowth and the presence of malignant heterologous elements (4). The incidence of benign phyllodes tumor is 35–64%, whereas the incidence of malignant phyllodes tumor as 25% (5).
The development of lobular carcinoma in situ, ductal carcinoma in situ, invasive lobular carcinoma, invasive ductal carcinoma, infiltrative carcinoma and squamous cell carcinoma have been reported in patients with phyllodes tumor (6–8).
In this descriptive study, it was aimed to present cases of phyllodes tumors and evaluate clinicopathological features of these tumors in light of the literature.
Material and Methods
55 cases of phyllodes tumor diagnosed between 2005–2018 in the Department of Surgical Pathology were retrospectively analyzed. Hematoxylin-eosin and immunohistochemically stained slides were re-evaluated.
The inclusion criteria in the study were cases diagnosed as phyllodes tumor, cases with available clinical data and suitability of blocks and slides for re-evaluation. Cases without an available clinical data, with insufficient tissue and slide quality for evaluation and cases without available blocks and slides were excluded from the study.
Phyllodes tumors are classified into three groups as benign, borderline and malignant phyllodes tumors according to WHO classification based on histopathological features such as stromal cellularity, stromal cell atypia, tumor margins, mitotic activity, stromal overgrowth and the presence of malignant heterologous elements. Tumors with well-circumscribed, mildly increased stromal cellularity, with or without minimal atypia, a mitotic activity generally <5 per 10 high-power fields, no marked stromal overgrowth and no heterologous elements are classified as benign phyllodes tumor. Tumors with focal infiltrative borders, moderate stromal cellularity, mild or moderate atypia, mitotic activity between 5–9 per 10 high-power fields, marked focal stromal overgrowth and no malignant heterologous elements are classified as borderline phyllodes tumor. Tumors with infiltrative borders, marked stromal cellularity and atypical stromal cells, high mitotic count (≥10 per 10 high-power fields), stromal overgrowth and heterologous elements are evaluated as malignant phyllodes tumor.
The immunohistochemically stained slides were re-evaluated using Ki-67 (RM SL6 Monoclonal Clone, 1/250 dilution Cell Marque) antibody in Leica Bond-Max Automatic Immunohistochemistry Staining Device (Leica Microsystems, Berlin, Germany) on the sections taken from the formalin-fixed paraffin-embedded blocks at a thickness of 4 micrometers. Ki-67 proliferative index was counted with Olympos CX31 binocular microscope in 1000 cells in areas where the proliferative activity is the highest.
Demographic information such as gender, age, tumor localization and tumor size; clinical information such as clinical presentation, radiological imaging, choice of treatment, follow-up period, recurrence and metastasis were obtained from the patient files in the electronic hospital database.
The conformity of continuous variables to normal distribution was analyzed using Shapiro-Wilk test. Variables were expressed as median (minimum: maximum) and mean ± standard deviation values. The Mann-Whitney U or Kruskal-Wallis tests were used to compare the continuous variables among the study groups according to the test of normality. When Kruskal-Wallis test was found to be significant, paired comparisons between groups were performed using the Dunn-Bonferroni approach. For statistical analysis, SPSS Statistical software (IBM Corp. Released 2012. IBM SPSS Statistics for Windows, Version 21.0. Armonk, NY: IBM Corp.) was used and p<0.05 was considered statistically significant.
The study was approved by the Uludağ University Clinical Research Ethics Committee with the decision no. 2018-1/28 on 25 September 2018. Informed consent was not received due to the retrospective nature of the study.
Results
A total of 55 cases diagnosed with phyllodes tumors between 2005–2018 were detected. The general characteristics of patients is summarized in Table-1. All cases were female with a mean age of 39.8 + 15.3 years (Age range: 15–75).
Table 1.
Clinicopathological findings of cases (n=55)
| Variable | No. of cases | |
|---|---|---|
| Gender | Female | 55 |
| Male | 0 | |
| Age | ≤30 | 18 |
| 31–49 | 23 | |
| >50 | 14 | |
| Tumor site | Left | 19 |
| Right | 33 | |
| Bilateral | 2 | |
| Initial diagnosis | Fibroadenoma | 26 |
| Malignancy | 17 | |
| Phyllodes Tumor | 10 | |
| Diagnosis | Benign | 20 |
| Borderline | 14 | |
| Malignant | 23 | |
| Initial treatment | Excision | |
| Mastectomy and | 41 | |
| sentinel lymph | 7 | |
| node dissection | ||
| Mastectomy | 4 | |
| Follow-up (months) | 34 (Range: 2–142) |
Fifty-seven tumors diagnosed in 55 cases were classified as benign in 20 cases (35.1%), borderline in 14 cases (24.6%) and malignant phyllodes tumor in 23 cases (40.3%). Tumors were located in the right breast in 33 cases (61.1%), left breast in 19 cases (35.2%) and bilateral in 2 cases (3.7%). One of the cases was consulted from an external center, tumor localization was not specified and no clinical information was available. One other case had two foci in the same breast while all the other cases had a single focus of tumor.
24 tumors were located in the upper outer quadrant, 4 in the lower outer quadrant, 7 in the lower inner quadrant, 9 in the upper inner quadrant, 5 in the subareolar region and 1 case had extensive tumor occupying all quadrants. The tumor localization of 7 cases could not be determined.
The mean diameter of benign phyllodes tumors was 3 cm (Range: 0.9–9), borderline phyllodes tumors diameter was 4.5 cm (Range: 1.2–12) and malignant phyllodes tumor was 3.7 cm (Range: 1.5–12). The tumor diameter in a total of 6 cases (1 benign, 3 borderline and 2 malignant) could not be detected.
Ki-67 proliferative index was counted as 52/1000 cells in benign phyllodes tumor, 110/1000 cells in borderline phyllodes tumor and 200/1000 cells in malignant phyllodes tumor.
Forty-four patients were admitted to the clinic with complaints of a palpable breast mass (Figure 1), 5 with pain and 1 with discharge. One of the cases had an incidental tumor and information of clinical presentation was not available in 6 cases.
Figure 1.
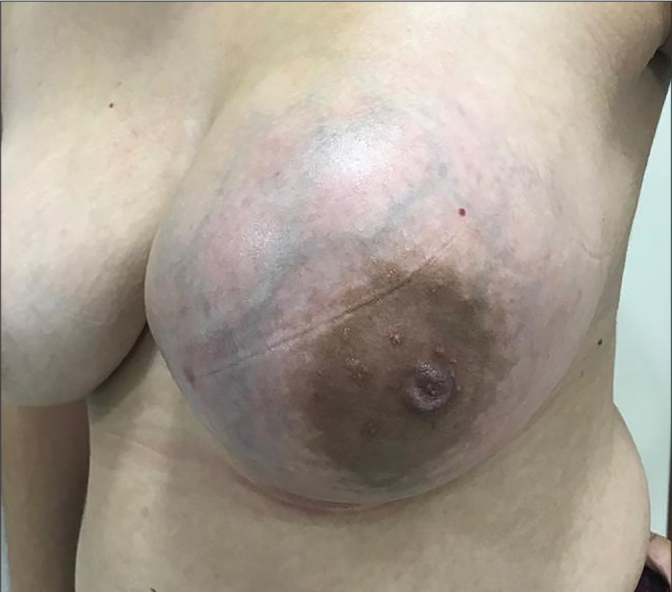
The patient admitted to the clinic with complaints of a palpable breast mass with borderline phyllodes tumor
Ultrasonographic examination of 51 patients revealed a well-defined and hypoechoic solid mass lesion. Eight patients with available mammographic images had macrolobulated well-circumscribed lesions. Dynamic contrast-enhanced magnetic resonance imaging was performed in 17 patients. Fast, heterogenous contrast-enhanced lesion was observed in the early stage of dynamic imaging after the contrast agent was given (Figure 2).
Figure 2.
a–d. Benign phyllodes tumor mammography imaging (a). Borderline phyllodes tumor mammography imaging (b). Malign phyllodes tumor mammography imaging (c). Malign phyllodes tumor magnetic resonance imaging (d)
The physical examination and radiological findings suggested the diagnosis of fibroadenoma in 26 cases, malignancy in 17 cases and phyllodes tumor in 10 cases. The clinical data of 7 cases were not available.
Of the 39 patients who underwent core biopsy, 30 were diagnosed with fibroepithelial lesion, 8 with phyllodes tumor and 1 with fibrocystic changes. Core biopsy results of 16 patients were not available.
Surgical excision was planned in patients with the diagnosis of fibroepithelial lesion on core biopsy, patients who had clinical and radiological findings suggestive of malignancy, patients with fast growing lesions and with increased mitotic activity and high ki-67 proliferative index. Patients who were scheduled for radical mastectomy with a tumor of 5 cm diameters or more (considering the technical failure of sentinel lymph node dissection in case of detection of malignancy postoperatively) and who had a recurrent tumor with chest wall and axilla involvement, underwent surgical excision with sentinel lymph node dissection.
Forty-one patients were treated with wide local excision (lumpectomy), 7 underwent mastectomy and sentinel lymph node dissection, and 4 underwent mastectomy only. The surgical procedure of three cases were not available.
Gross examination of all the resection materials showed a solid multinodular mass with relatively smooth margins, greyish cut surface. Some of them had areas of cystic degeneration.
Histopathological examination revealed tumors composed of epithelial and myoepithelial cell layers with an intracanalicular growth pattern, branching cleft-like spaces and a stroma with increased cellularity around the cleft-like spaces (Figure 3). Benign phyllodes tumors were well-circumscribed. Cytological atypia or heterologous elements were not observed. Average mitosis was counted as 2.7 in 10 consecutive high-power fields. In malignant phyllodes tumors nuclear pleomorphism, prominent nucleoli and cytological atypia was observed. Lesions showed infiltrative borders and stroma was highly cellular. Average mitosis was counted as 13.2 in 10 consecutive high-power fields. Ductal carcinoma in situ (solid and cribriform type) was detected in one case of malignant phyllodes tumor and invasive ductal carcinoma was detected in another. Bilateral ductal carcinoma in situ (solid, micropapillary, mucinous type) was present in the case with invasive ductal carcinoma. In addition, in another case of malignant phyllodes tumor, invasive ductal carcinoma was detected concomitantly in the contralateral breast. Histopathological examination of sentinel lymph node biopsy showed no evidence of metastasis.
Figure 3.
a–f. Benign Phyllodes Tumor: Typical leaf-like pattern, slight increase in cellularity of the stromal component and low ki-67 proliferative index (H&E, immunohistochemistry stain ×40) (a, b). Borderline Phyllodes Tumor: Mildly increased cellularity and high ki-67 proliferative index (H&E, immunohistochemistry stain ×40) (c, d). Malign Phyllodes Tumor: Stromal overgrowth, marked cellular atypia and brisk mitotic activity and ki-67 proliferative index (H&E, immunohistochemistry stain ×100) (e, f)
Five patients (8.9%) developed recurrence after the treatment. Three of the recurrent cases were initially diagnosed as borderline and 2 as malignant phyllodes tumor. One of the cases with borderline phyllodes tumor recurred as malignant phyllodes tumor after 33 months of initial diagnosis. In 4 out of 5 cases, the initial diagnosis was made in an external center and surgical margin information could not be obtained. In 1 case, the surgical margins of the resection material were positive and extended resection material revealed tumor at a distance of 0.2 cm in the closest surgical margin. Lung metastases were detected in 2 cases of malignant phyllodes tumor and one of them recurred two times. The patient who had lung metastasis 27 months after the first diagnosis, was given 5 cycles of chemotherapy and died after 36 months of initial diagnosis. The other case was a patient who had lung metastasis 2 months postoperatively. The patient started receiving chemotherapy but died after 1 month.
Adjuvant chemotherapy (doxorubicin, cyclophosphamide) or hormone therapy (tamoxifen, letrozole) along with radiotherapy (Mean dose: 30 Gray) was given to 1 case of borderline and 5 cases of malignant phyllodes tumor. Chemotherapy or hormone therapy was given to 2 cases of borderline and 4 cases of malignant phyllodes tumor, and radiotherapy alone was given to 7 cases of malignant phyllodes tumor.
The mean follow-up period of 55 cases was 34 months (Range: 2–142). Follow-up data was not available for eight cases.
Discussion and Conclusion
Phyllodes tumor is a biphasic tumor consisting of mesenchymal and epithelial elements, constituting less than 1% of all breast tumors with an incidence of 2.1/1000000 (1, 9). They are usually diagnosed in females in 4th or 5th decades of life and are very rare in men with few cases reported in the literature (5, 10). In our series, all of the cases were female and the mean age of diagnosis was 39.8 ± 15.3 years.
Although hyperestrogenism and breast trauma are thought to play a role in the development of phyllodes tumor, its etiology has not been yet fully elucidated (11). Cases of phyllodes tumor in pregnancy have been reported (12). In our series, 2 cases were diagnosed during pregnancy. Recurrence was observed in one of these cases following surgery.
Patients usually present with complaints of a breast mass. Complaint of a rapidly growing painless mass is a significant finding for phyllodes tumor (13). Tumors are usually unifocal. The most commonly involved site is upper outer quadrant. Multifocality and bilaterality have been reported in the literature (14, 15). In our series, the most commonly involved site was also the upper outer quadrant consistent with the literature. Two of the cases were bilateral and 1 was multifocal.
Phyllodes tumors does not have a pathognomonic radiological finding that distinguishes fibroadenoma from benign, borderline, and malignant phyllodes tumor. In recent studies, findings on contrast-enhanced MRI including tumor size over 3 cm, poorly demarcated and microlobulated architecture, heterogeneous appearance in echogenicity, hypervascularity and presence of internal cystic spaces have been reported to support phyllodes tumor in the differential diagnosis of two tumors with similar mammographical findings (16). In dynamic gadolinium-enhanced MRI, fast contrast-bearing tumors in dynamic imaging, well-circumscribed and high signal density in fat-saturated T2-weighted images with internal septation support benign phyllodes tumor (17).
Grossly, phyllodes tumors appear as well-defined, firm and multinodular masses. The cut surface is grayish white and has a homogeneous appearance. Myxoid areas, cystic spaces, areas of hemorrhage and necrosis can be seen (18). In a study including 145 benign, 33 borderline and 15 malignant phyllodes tumors Kim et al. (19) found the mean tumor diameter to be 4 cm. The mean tumor diameter was reported as 3.7 cm in patients with benign phyllodes tumor, whereas 4.2 cm in borderline phyllodes tumor and 6.2 cm in malignant phyllodes tumor. In our series, the mean tumor diameter was 3.2 cm in benign phyllodes tumors, 5.06 cm in borderline phyllodes tumors and 4.6 cm in malignant phyllodes tumors.
Phyllodes tumor is histopathologically characterized by leaf-like phyllodes structures lined by double layered epithelium, an internal epithelium with a myoepithelium outside, that has cleft-like cystic spaces with hypercellular stroma and intracanalicular growth pattern. Pseudoangiomatous stromal hyperplasia, cartilaginous, osseous, lipomatous metaplasia or stromal giant cells can be seen. Squamous and apocrine metaplasia of the epithelium is uncommon. Rarely ductal and lobular carcinoma in situ and invasive carcinoma may develop from the epithelium of phyllodes tumor (20). Rodrigues et al. (9) reported a total of 11 cases with malignant epithelial transformation in a series of 183 cases. 6 of the cases developed ductal carcinoma in situ, 4 cases lobular carcinoma in situ, and 1 case developed invasive ductal carcinoma. In our series, ductal carcinoma in situ was detected in one case of malignant phyllodes tumor, whereas invasive ductal carcinoma was detected in another case. Bilateral ductal carcinoma in situ (solid, micropapillary, mucinous) was also present in the patient with invasive ductal carcinoma.
Phyllodes tumors are classified as benign, borderline and malignant according to WHO classification (21). Tan et al. (22) reported 72.7% benign, 18.4% borderline and 8.9% malignant phyllodes tumor in their series consisting of 605 cases. In our series, 34.5% benign, 24.1% borderline and 39.7% malignant phyllodes tumor were detected. We determined that the rate of malignant phyllodes tumor is not compatible with the data available in the literature because our center is the only tertiary health institution in its region.
The differential diagnosis of phyllodes tumor includes cellular fibroadenoma, spindle cell carcinoma, primary and metastatic breast sarcomas. Fibroadenoma should not be interpreted as phyllodes tumor based on only the histopathological findings of increased cellularity and mitotic activity which are more frequently detected especially in the pediatric group (23). Phyllodes tumor is difficult to differentiate in core biopsy materials and should be reported as ‘fibroepithelial lesion with increased stromal cellularity’ and the excision of the mass should be recommended (24). In the fibroepithelial lesion series consisting of 54 patients aged between 10 and 18 years conducted by Ross et al., juvenile fibroadenoma was detected in 23 cases. In cases of juvenile fibroadenoma, 1–7 mitosis was observed and increased stromal cellularity were present in 61% (25).
Primary or metastatic breast sarcomas are extremely rare but should be considered in the differential diagnosis of phyllodes tumors. When a sarcomatous tumor is encountered in the breast, the tumor should be examined with plenty sections and the presence of a benign epithelial component should be investigated (4).
In the treatment of phyllodes tumors, surgical excision is the main treatment and a wide local excision of the tumor with adequate margins of at least 1 cm is necessary. Radiotherapy, chemotherapy and hormone therapy are controversial in the treatment of phyllodes tumors (26–28). In their series, Chaney et al. (29) recommended adjuvant radiotherapy in malignant phyllodes tumor cases which had high risk of local recurrence. Surgical margin positivity, presence of tumor less than 0.5 cm of the surgical margin, detection of recurrent tumor or tumor diameter over 10 cm were considered high risk for local recurrence. There appears to be no consensus regarding the dosage that should be used in treatment.
Although there is no routine chemotherapy protocol for treatment of phyllodes tumors, it is suggested that these tumors should be treated like a sarcoma rather than a carcinoma when giving treatment. Especially patients with malignant phyllodes tumors larger than 5 cm and with high risk of recurrence are candidate for chemotherapy. Doxorubicin and dacarbazine were used as single agents but it is reported that treatment response is better in combined treatments with cisplatin or iphosphamide (30, 31).
Studies suggest that axillary lymph node sampling is not necessary in cases of phyllodes tumors. In their series consisting 48 cases of malignant phyllodes tumors, Kapiris et al. (32) did not detect metastasis in 21 axillary lymph node samples. In a series of 106 phyllodes tumor cases Ben Hassouna et al. (33) identified one patient with lymph node metastasis out of 20 cases that had undergone axillary lymph node biopsy. In our series, no metastasis was found in 7 patients who underwent axillary lymph node dissection.
In patients with phyllodes tumor, varying rates of recurrence can be observed during follow-up. Recurrence of benign, borderline and malignant phyllodes tumors have been reported as 17%, 25% and 27%, respectively (22). The epithelial expression of E-cadherin which affects the Wnt signaling pathway in phyllodes tumors, is thought to be correlated with recurrence rates (26). Recurrent malignant phyllodes tumors might have a more aggressive biological behavior than the initial tumor. Borderline and malignant phyllodes tumors might also metastasize to distant organs. It has been reported that the tumor usually spreads by hematogenous route and metastasis is found most frequently in lungs and bone but can be detected in any localization. Histopathologically, stromal component is frequently found rather than the epithelial component in a focus of metastatic phyllodes tumor (34, 35). In 2012, Tan et al. (36) reported 12 cases with distant metastases in their series of 605 cases. All the cases with liver, lung, pleural, soft tissue and vertebrae metastases were diagnosed as malignant phyllodes tumor and no metastasis was detected in borderline and benign phyllodes tumors. The role of surgery and radiotherapy in the treatment of metastatic disease is controversial and reports are found that chemotherapy might be useful (37). In our series, 5 cases developed recurrence and lung metastasis was observed in 2 cases.
In conclusion, phyllodes tumors are rare tumors showing epithelial and mesenchymal components. The grading of phyllodes tumors is crucial due to diverse potential for recurrence and metastasis. The treatment of choice for phyllodes tumors is surgical excision. The role of radiotherapy and chemotherapy in preventing possible recurrences and metastases is controversial and clinical and radiological follow-up of the patients is recommended.
Acknowledgements
Authors would like to thank patients who participated this study.
Footnotes
Ethics Committee Approval: Ethics committee approval was received for this study from the Uludağ University Clinical Research Ethics Committee.
Informed Consent: Informed consent was not received due to the retrospective nature of the study.
Peer-review: Externally peer-reviewed.
Author Contributions: Concept - S.H., M.Ö., Ş.T.; Design - S.H., M.Ö., Ş.T.; Supervision - Ş.T.; Resources - S.H., M.Ö., Ş.T.; Materials - Ş.T., M.Ş.G.; Data Collection and/or Processing - S.H., M.Ö.; Analysis and/or Interpretation - Ş.T., S.H., M.Ö.; Literature Search - S.H., M.Ö.; Writing Manuscript - S.H., M.Ö., Ş.T.; Critical Review - Ş.T., M.Ş.G.
Conflict of Interest: The authors have no conflicts of interest to declare.
Financial Disclosure: The authors declared that this study has received no financial support.
References
- 1.Lakhani SR, Elis IO, Schnitt SJ, Tan PH, Vijver MJ. World Health Organization Classification of Tumours of the Breast: Fibroepithelial tumors. 4th ed. Lyon, France: IARC Press; 2012. pp. 142–47. [Google Scholar]
- 2.Atalay C, Kınaş V, Çelebioğlu S. Analysis of patients with phyllodes tumor of the breast. Ulus Cerrahi Derg. 2014;30:129–132. doi: 10.5152/UCD.2014.2719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mishra SP, Tiwary SK, Mishra M, Khanna AK. Phyllodes tumor of breast: a review article. ISRN Surg. 2013;2013 doi: 10.1155/2013/361469. 361469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Schnitt SJ, Collins LC. Bıopsy Interpretation Series Biopsy Interpretation of the Breast: Phyllodes Tumor. 2th ed. Philadelphia: 2013. pp. 186–199. [Google Scholar]
- 5.Spitaleri G, Toesca A, Botteri E, Bottiglieri L, Rotmensz N, Boselli S, Sangalli C, Catania C, Toffalorio F, Noberasco C, Delmonte A, Luini A, Veronesi P, Colleoni M, Viale G, Zurrida S, Goldhirsch A, Veronesi U, De Pas T. Breast phyllodes tumor: a review of literature and a single center retrospective series analysis. Crit Rev Oncol Hematol. 2013;88:427–436. doi: 10.1016/j.critrevonc.2013.06.005. [DOI] [PubMed] [Google Scholar]
- 6.Wu DI, Zhang H, Guo L, Yan XU, Fan Z. Invasive ductal carcinoma within borderline phyllodes tumor with lymph node metastases: A case report and review of the literature. Oncol Lett. 2016;11:2502–2506. doi: 10.3892/ol.2016.4238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Abdul Aziz M, Sullivan F, Kerin MJ, Callagy G. Malignant phyllodes tumour with liposarcomatous differentiation, invasive tubular carcinoma, and ductal and lobular carcinoma in situ: case report and review of the literature. Patholog Res Int. 2010;2010:501274. doi: 10.4061/2010/501274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Shin YD, Lee SK, Kim KS, Park MJ, Kim JH, Yim HS, Choi YJ. Collision tumor with inflammatory breast carcinoma and malignant phyllodes tumor: a case report and literature review. World J Surg Oncol. 2014;12:5. doi: 10.1186/1477-7819-12-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rodrigues MF, Truong PT, McKevitt EC, Weir LM, Knowling MA, Wai ES. Phyllodes tumors of the breast: The British Columbia Cancer Agency experience. Cancer Radiother. 2018;22:112–119. doi: 10.1016/j.canrad.2017.08.112. [DOI] [PubMed] [Google Scholar]
- 10.Chougule A, Bal A, Rastogi P, Das A. Recurrent phyllodes tumor in the male breast in a background of gynaecomastia. Breast Dis. 2015;35:139–142. doi: 10.3233/bd-140393. [DOI] [PubMed] [Google Scholar]
- 11.Sbeih MA, Engdahl R, Landa M, Ojutiku O, Morrison N, Depaz H. A giant phyllodes tumor causing ulceration and severe breast disfigurement: case report and review of giant phyllodes. J Surg Case Rep. 2015;2015 doi: 10.1093/jscr/rjv162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gentile LF, Gaillard WF, Wallace J, Spiguel LRP, Alizadeh L, Lentz A, Shaw C. A cesa of a giant borderline phyllodes tumor early in pregnancy treated with mastectomy and immediate breast reconstruction. Breast J. 2016;22:683–687. doi: 10.1111/tbj.12663=. [DOI] [PubMed] [Google Scholar]
- 13.Zhao H, Cheng X, Sun S, Yang W, Kong F, Zeng F. Synchronous bilateral primary breast malignant phyllodes tumor and carcinoma of the breast with Paget’s disease: a case report and review of the literature. Int J Clin Exp Med. 2015;8:17839–17841. [PMC free article] [PubMed] [Google Scholar]
- 14.Karczmarek-Borowska B, Bukala A, Syrek-Kaplita K, Ksiazek M, Filipowska J, Gradalska-Lampart M. A Rare Case of Breast Malignant Phyllodes Tumor with Metastases to the Kidney: Case Report. Medicine (Baltimore) 2015;94:e1312. doi: 10.1097/MD.0000000000001312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mallory MA, Chikarmane SA, Raza S, Lester S, Caterson SA, Golshan M. Bilateral synchronous benign phyllodes tumors. Am Surg. 2015;81:E192–194. [PMC free article] [PubMed] [Google Scholar]
- 16.Duman L, Gezer NS, Balcı P, Altay C, Başara I, Durak MG, Sevinç AI. Differentiation between Phyllodes Tumors and Fibroadenomas Based on Mammographic Sonographic and MRI Features. Breast Care (Basel) 2016;11:123–127. doi: 10.1159/000444377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Balaji R, Ramachandran KN. Magnetic Resonance Imaging of a Benign Phyllodes Tumor of the Breast. Breast Care (Basel) 2009;4:189–191. doi: 10.1159/000220604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Venter AC, Roşca E, Daina LG, Muţiu G, Pirte AN, Rahotă D. Phyllodes tumor: diagnostic imaging and histopathology findings. Rom J Morphol Embryol. 2015;56:1397–1402. [PubMed] [Google Scholar]
- 19.Kim S, Kim JY, Kim do H, Jung WH, Koo JS. Analysis of phyllodes tumor recurrence according to the histologic grade. Breast Cancer Res Treat. 2013;141:353–363. doi: 10.1007/s10549-013-2684-x. [DOI] [PubMed] [Google Scholar]
- 20.Hoda SA, Brogl E, Koerner FC, Rosens PP. Rosens Breast Patholgoy: Fibroepithelial neoplasms. 4rd edition. Philadelphia: Lippincott Williams&Wilkins; 2014. pp. 232–70. [Google Scholar]
- 21.Zhang Y, Kleer CG. Phyllodes Tumor of the Breast: Histopathologic Features, Differential Diagnosis, and Molecular/Genetic Updates. Arch Pathol Lab Med. 2016;140:665–671. doi: 10.5858/arpa.2016-0042-RA. [DOI] [PubMed] [Google Scholar]
- 22.Tan PH, Thike AA, Tan WJ, Thu MM, Busmanis I, Li H, Chay WY, Tan MH Phyllodes Tumour Network Singapore. Predicting clinical behaviour of breast phyllodes tumours: a nomogram based on histological criteria and surgical margins. J Clin Pathol. 2012;65:69–76. doi: 10.1136/jclinpath-2011-200368. [DOI] [PubMed] [Google Scholar]
- 23.Tay TK, Chang KT, Thike AA, Tan PH. Paediatric fibroepithelial lesions revisited: pathological insights. J Clin Pathol. 2015;68:633–641. doi: 10.1136/jclinpath-2015-202956. [DOI] [PubMed] [Google Scholar]
- 24.Wiratkapun C, Piyapan P, Lertsithichai P, Larbcharoensub N. Fibroadenoma versus phyllodes tumor: distinguishing factors in patients diagnosed with fibroepithelial lesions after a core needle biopsy. Diagn Interv Radiol. 2014;20:27–33. doi: 10.5152/dir.2013.13133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ross DS, Giri DD, Akram MM, Catalano J, Van Zee KJ, Brogi E. Fibroepithelial lesions in the breast of adolescent females: a clinicopathological profile of 35 cases. Mod Pathol. 2012;25(Suppl 2):64a. doi: 10.1111/tbj.12706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tan BY, Acs G, Apple SK, Badve S, Bleiweiss IJ, Brogi E, Calvo JP, Dabbs DJ, Ellis IO, Eusebi V, Farshid G, Fox SB, Ichihara S, Lakhani SR, Rakha EA, Reis-Filho JS, Richardson AL, Sahin A, Schmitt FC, Schnitt SJ, Siziopikou KP, Soares FA, Tse GM, Vincent-Salomon A, Tan PH. Phyllodes tumours of the breast: a consensus review. Histopathology. 2016;68:5–21. doi: 10.1111/his.12876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Morales-Vásquez F, Gonzalez-Angulo AM, Broglio K, Lopez-Basave HN, Gallardo D, Hortobagyi GN, De La Garza JG. Adjuvant chemotherapy with doxorubicin and dacarbazine has no effect in recurrence-free survival of malignant phyllodes tumors of the breast. Breast J. 2007;13:551–556. doi: 10.1111/j.1524-4741.2007.00510.x. [DOI] [PubMed] [Google Scholar]
- 28.Prakash S, Raj P. A Very Large Malignant Phyllodes Tumor with Skin Ulceration and Nipple Areola Complex Involvement-Still a Reality!!! Indian J Surg. 2013;75:39–42. doi: 10.1007/s12262-012-0499-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chaney AW, Pollack A, McNeese MD, Zagars GK. Adjuvant radiotherapy for phyllodes tumor of breast. Radiat Oncol Investig. 1998;6:264–267. doi: 10.1002/(SICI)1520-6823(1998)6:6<264::AID-ROI3>3.0.CO;2-J. [DOI] [PubMed] [Google Scholar]
- 30.Roberts N, Runk DM. Aggressive malignant phyllodes tumor. Int J Surg Case Rep. 2015;8:161–165. doi: 10.1016/j.ijscr.2014.12.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mouna B, Rhiziane B, Boutayeb S, Errihani H. The Efficacy of Chemotherapy against Metastatic Malignant Phyllodes Tumors of the Breast. J Clinic Case Reports. 2012;2:6. doi: 10.4172/2165-7920.1000122. [DOI] [Google Scholar]
- 32.Kapiris I, Nasiri N, A’Hern R, Healy V, Gui GPH. Outcome and predictive factors of local recurrence and distant metastases following primary surgical treatment of high-grade malignant phyllodes tumours of the breast. Eur J Surg Oncol. 2001;27:723–730. doi: 10.1053/ejso.2001.1207. [DOI] [PubMed] [Google Scholar]
- 33.Ben Hassouna J, Damak T, Gamoudi A, Chargui R, Khomsi F, Mahjoub S, Slimene M, Ben Dhiab T, Hechiche M, Boussen H, Rahal K. Phyllodes tumors of the breast: a case series of 106 patients. Am J Surg. 2006;192:141–147. doi: 10.1016/j.amjsurg.2006.04.007. [DOI] [PubMed] [Google Scholar]
- 34.Prakash S, Raj P. A Very Large Malignant Phyllodes Tumor with Skin Ulceration and Nipple Areola Complex Involvement-Still a Reality!!! Indian J Surg. 2013;75:39–42. doi: 10.1007/s12262-012-0499-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Koh VCY, Thike AA, Tan PH. Distant metastases in phyllodes tumours of the breast: an overview. Applied Cancer Research. 2017;37:15. doi: 10.1186/s41241-017-0028-6. [DOI] [Google Scholar]
- 36.Tan P, Thike A, Tan W, Thu M, Busmanis I, Li H, Chay WY, Tan MH. Predicting clinical behaviour of breast phyllodes tumours: a nomogram based on histological criteria and surgical margins. J Clin Pathol. 2012;65:69–76. doi: 10.1136/jclinpath-2011-200368. [DOI] [PubMed] [Google Scholar]
- 37.Mituś JW, Blecharz P, Walasek T, Reinfuss M, Jakubowicz J, Kulpa J. Treatment of Patients with Distant Metastases from Phyllodes Tumor of the Breast. World J Surg. 2016;40:323–328. doi: 10.1007/s00268-015-3262-7. [DOI] [PMC free article] [PubMed] [Google Scholar]




