Abstract
PURPOSE
Radiotherapy (RT) is an essential component of cancer treatment. There is a lack of RT services in sub-Saharan Africa as well as limited knowledge regarding clinical practices. The purpose of this study was to identify and describe the patterns for RT treatment in Ethiopia.
METHODS AND MATERIALS
We performed a retrospective analysis of 1,823 patients treated with cobalt RT at a large referral hospital in Addis Ababa, Ethiopia, from May 2015 through January 2018. Paper charts were reviewed for patient and treatment characteristics. Descriptive statistics were computed using SPSS (IBM, Armonk, NY).
RESULTS
Among patients treated for cancer, 98% (n = 1,784) were adults, 78% (n = 1,426) were female, 5% (n = 85) were HIV positive, 30% (n = 555) were from Addis Ababa, and the median age was 48 years (interquartile range [IQR], 38-58 years). Cervical cancer was the most frequent cancer treated (47%, n = 851), followed by breast cancer (15%, n = 274) and head and neck cancer (10%, n = 184). Seventy-three percent of patients (n = 1,339) presented at a late stage, and 62% (n = 1,138) received palliative RT. The wait times were the shortest for patients receiving palliative treatment (median, 0 days; IQR, 0-15 days; n = 1,138), whereas wait times were longer for patients receiving curative treatment (median, 150 days; IQR, 60-210 days; n = 685). Three percent of patients (n = 56) had documented grade 3 or 4 acute toxicity; of these, 59% (n = 33) were patients with head and neck cancer.
CONCLUSION
Cervical cancer accounted for half of patients treated; thus, a majority of patients were adult females. Most patients had advanced-stage cancer, and goals of care were palliative. Wait times were long for patients with curative-intent cancer as a result of low capacity for RT services.
INTRODUCTION
Radiotherapy (RT) plays an important role in oncology and can be used as the principle treatment method or combined with other modalities to provide cure in up to 40% of patients with a variety of cancers.1 RT is also a highly effective treatment for palliation of cancer symptoms.2 In low- and middle-income countries (LMICs), it is estimated that up to 70% of patients would benefit from RT at some point in their illness as a result of its utility in alleviating symptoms of late-stage disease.3 Moreover, RT is also a cost-effective modality in LMICs where personnel costs are low.4 Despite the clear necessity of RT in LMICs, there is stark inequality in access to care. Nearly 60% of new cancer diagnoses occur in LMICs, whereas <40% of RT equipment is found in these regions.5,6 In sub-Saharan Africa (excluding southern Africa), the disparity is striking, with fewer than one RT machine per 1 million population, 10 times lower than that of North America.6 In fact, more than half the countries in Africa lack a single RT center.7 Therefore, it is estimated that 700 additional RT machines are needed to increase capacity to an acceptable level in Africa.8 This need will only continue to grow because cancer incidence is projected to double in lower resourced regions of Africa by 2040.5
CONTEXT
Key Objective There is a paucity of high-quality epidemiological data on radiotherapy utilization patterns in sub-Saharan Africa. This study seeks to describe current patterns of cobalt radiotherapy in Ethiopia and understand unmet needs.
Knowledge Generated Nearly 2,000 patients were included from the only radiotherapy treating facility in Ethiopia. Cervical cancer accounted for half of patients treated. Most radiotherapy was palliative and wait times for curative patients were long due to low capacity for radiotherapy services.
Relevance This study provides important epidemiology-based cancer research highlighting the need for increased radiotherapy capacity and providing pivotal information on areas of priority for cancer control planning strategies.
Ethiopia is a low-income country in East Africa with one of the highest populations in sub-Saharan Africa. With the global health burden shifting from communicable to noncommunicable diseases, cancer is now a leading cause of death in Ethiopian adults.9,10 At present, Ethiopia has one functioning cobalt teletherapy machine, serving more than 100 million inhabitants. Tikur Anbessa Specialized Hospital (TASH) in Addis Ababa, the only oncologic referral center in Ethiopia, opened in 1998 and treats more than 1,700 RT patients a year.11 In the 2016 to 2020 Ethiopian National Cancer Control Plan, inadequate RT equipment relative to population need was recognized.12 In response, the government has prioritized expanding RT services to 5 regional teaching hospitals across the country and has committed to purchasing linear accelerators rather than increasing the number of cobalt machines.
Cancer diagnoses are dramatically increasing in Africa, and it is evident that RT provides an invaluable tool for cure and palliation. Because this has been recognized by governments and investors, there is a broad consideration to increase availability of RT across the continent. However, few data are available to understand the current use and dynamics of RT treatment in Africa, with most studies in the region based on small or cancer-specific cohorts. Epidemiology-based cancer research is pivotal for cancer control planning in all settings and is particularly critical where resources are limited to prioritize funding toward evidence-based policies that focus on highest need.13 The primary aim of this study was to provide a broad descriptive analysis of the current patterns of cobalt RT in Ethiopia in a large cohort of patients with cancer to inform cancer control policy and provide a baseline for assessing the effectiveness of future RT interventions.
METHODS AND MATERIALS
Study Design and Participants
This retrospective, cross-sectional study included adult and pediatric patients treated with cobalt RT for malignant diagnoses between May 2015 and January 2018 at TASH in Addis Ababa, Ethiopia. It is estimated that 4,250 patients were treated during the study period, and a random sample of 1,826 charts (43%) were evaluated. Inclusion of all patients treated during the study period was not considered feasible due to difficulty locating paper charts from incomplete registration data in an overcrowded file room. Therefore, charts were randomly identified by hospital administrative staff in the RT department.
General demographic information (age, sex, and region), tumor characteristics (histology and stage), and data regarding specific cancer therapy were extracted from the patients’ paper files. Charts of individuals treated with RT for a benign condition were excluded.
Institutional Characteristics
TASH had one functional cobalt teletherapy unit (Theratron Equinox, Best Theratronics, Ottawa, Ontario, Canada). Brachytherapy capacity was added in 2016. There were 6 radiation oncologists and 28 residents by the end of the study. There were 4 medical physicists, 5 radiation therapists, and 26 oncology nurses on staff.
Diagnosis, Staging of Cancer, and HIV Status
Histology and diagnoses of cancer (International Classification of Diseases–Oncology) were taken from the charts and grouped into categories modeled after the International Agency for Research on Cancer.5 Advanced imaging (computed tomography [CT] and magnetic resonance imaging [MRI]) was available at TASH; however, the bulk of staging was performed by x-ray and ultrasound. For example, staging for advanced breast and cervical cancers is performed using chest x-ray and abdominal ultrasound. CT staging was reserved for head and neck, thoracic, and select GI cancers and sarcomas. MRI was used in CNS disease, rectal cancer, and select sarcomas.
Staging was classified by International Federation of Gynecology and Obstetrics (FIGO) classification for gynecologic malignancies, Ann Arbor staging for lymphomas, and American Joint Committee on Cancer (AJCC) seventh edition staging for all other cancers except for CNS malignancies and sarcomas, which were not staged but rather classified as operable or inoperable. Multiple myeloma was not staged because of a lack of molecular profiling. The stage at time of pathologic diagnosis was not collected; therefore, stage was determined at the planning visit just before initiating RT. Stage was then classified as early stage, late stage, locoregional recurrence, distant recurrence, not reported, or not applicable. Early stage was defined as AJCC/Ann Arbor/FIGO stage I or II or operable for CNS malignancies and sarcoma. Late stage was classified as AJCC/Ann Arbor/FIGO stage III or IV or inoperable for CNS malignancies and sarcoma. Presence or absence of distant metastasis was also recorded. The baseline symptoms were recorded (if present), and self-reported HIV status was documented as unknown, negative, positive on highly active antiretroviral therapy (HAART), or positive not documented to be on HAART.
Cancer Treatment
Patients with cancer were referred from all regions of Ethiopia and nearby countries (ie, Eritrea and Djibouti) for RT. Typically patients were referred in a stepwise approach, starting at the primary level of care (health center, health post, or primary hospital); then patients were referred to the secondary level (general hospital) before being referred to TASH. At the initial appointment, if RT was indicated, the patients were either given an appointment to return for treatment planning or put on a call list because of high patient volume. Patients with acute symptoms or impending complications from their disease were given priority, followed by patients younger than 25 years old. Treatment aim was assigned at the second visit when treatment was planned and classified as curative (radical, adjuvant, or neoadjuvant) or palliative.
Most treatment planning was performed by anatomic landmarks, even after June 2018 when a planning CT was obtained and reserved for selected complicated planning (ie, approximately 1 of 6 patients with head and neck cancer) predominately for training purposes. Waiting time was calculated as the time between the date of the initial visit and the start of RT (in days). Body site(s) treated, total dose (grays), number of fractions, and dates of external-beam treatment were documented, along with use of brachytherapy when indicated. Any documented acute toxicities (type and grade) were recorded (assessed at end of treatment and the recommended 4-week follow-up, although follow-up after that is often sporadic and toxicity not routinely documented). It was reported whether treatment was completed as planned, interrupted temporarily but completed, or discontinued. Whether patients received chemotherapy and, if so, the timing (before, during, or after RT) were reported. Concurrent chemotherapy was not commonly used given long RT wait times.
Statistical Analysis
Descriptive statistical analysis was performed using IBM SPSS Statistics version 24 (IBM, Armonk, NY).
Ethical and Quality Considerations
Ethical approval was obtained from the Addis Ababa University Clinical Oncology Department Ethical Review Board. The data were deidentified and managed in a secured Microsoft Excel 2018 v16.18 spreadsheet (Microsoft, Redmond, WA). The study was conducted without individual informed consent because the study relied on retrospective data collected as part of routine patient care.
RESULTS
Cancer Distribution and HIV Status
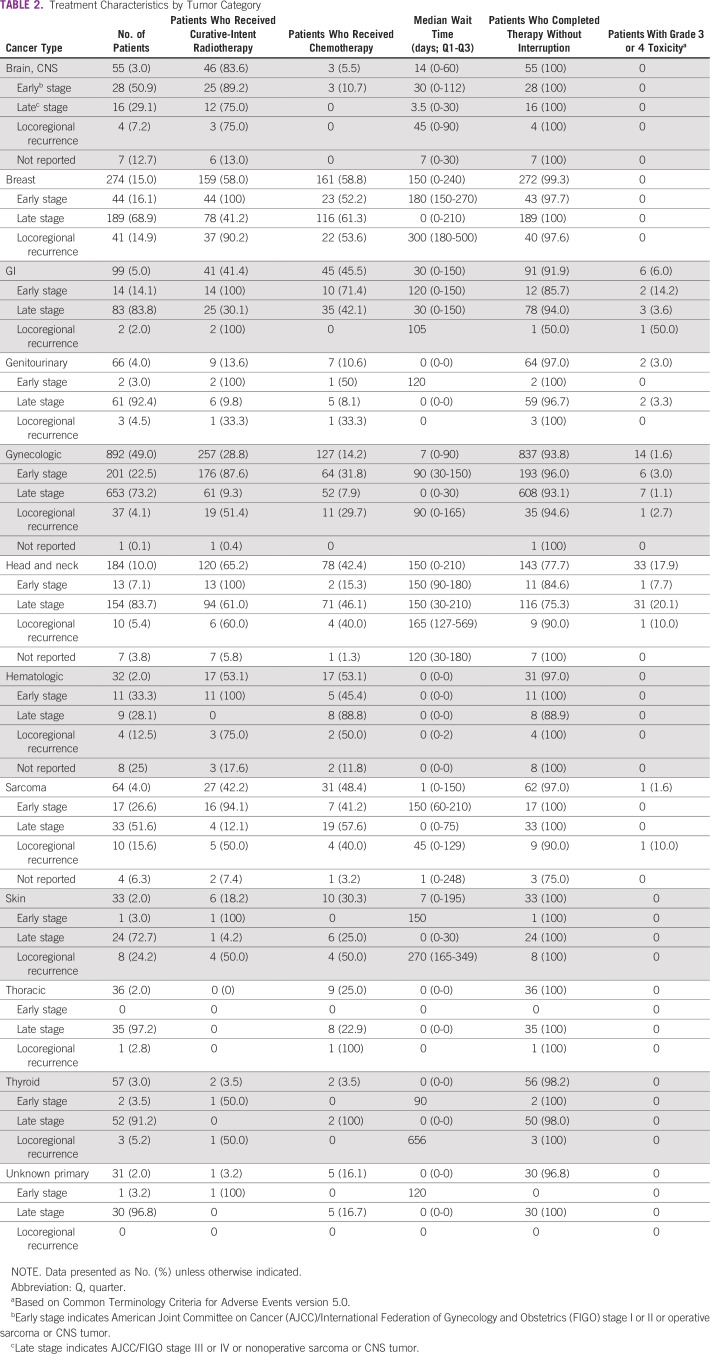
Of 1,826 patient charts evaluated, 3 were excluded (unclassified diagnoses), resulting in 1,823 patients evaluable and included in this analysis. Patient and general treatment characteristics are listed in Table 1. Stage at the initiation of treatment was as follows: 13% not staged (n = 239), 1% stage I (n = 16), 15% stage II (n = 266), 22% stage III (n = 407), and 50% stage IV (n = 895). Thus, three quarters of patients with cancer (n = 1,339) presented at late stage (stage III-IV and inoperable). Five percent of the total cohort (n = 85) reported HIV positivity, and the majority were patients with gynecologic cancer (n = 60, 71% of all HIV-positive patients). Of these gynecologic patients with HIV, 70% (n = 42) had stage III or IV disease. Nearly all HIV-positive patients (n = 83, 98%) reported being treated with HAART.
TABLE 1.
Patient and General Treatment Characteristics (N = 1,823)
Treatment Characteristics
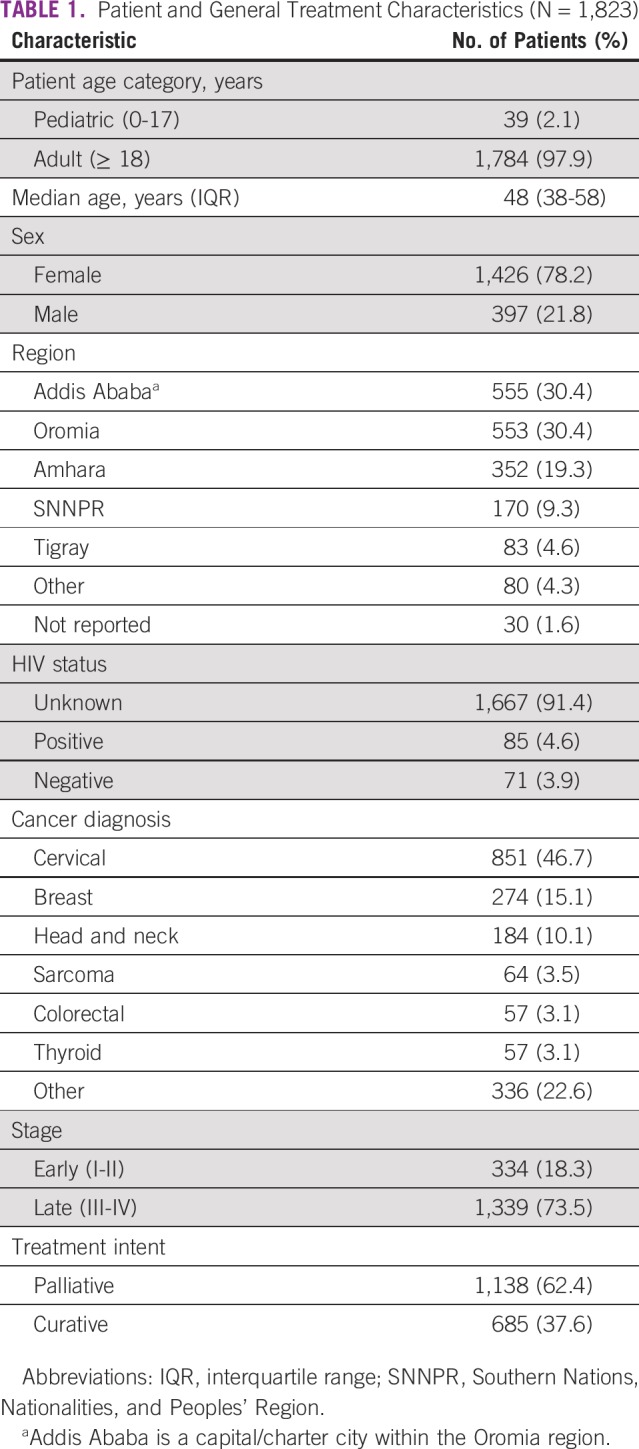
Primary indications for palliative RT included pain (n = 368, 32%), pain and bleeding (n = 261, 23%), bleeding (n = 149, 13%), obstructive symptoms (n = 37, 3%), discharge (n = 32, 2.8%), neurologic symptoms (n = 25, 2.2%), other (n = 3), and not reported (n = 263, 23%). The cancer categories with highest percentage of curative intent included brain and CNS (84%), head and neck (65%), breast (58%), and hematologic malignancies (53%; Table 2). The tumors least often treated with curative intent were thoracic (lung and thymic, 0%), unknown primary (3%), thyroid (4%), genitourinary (14%), and skin (18%). As expected, patients who presented at an early stage were more likely to be treated with curative intent. Twenty-seven percent of patients (n = 494) received chemotherapy at some point in their treatment; of these, the predominate cancer types were breast cancer (59%), hematologic malignancies (53%), sarcoma (48%), GI malignancies (45%), and head and neck cancers (42%).
TABLE 2.
Treatment Characteristics by Tumor Category
Treatment Completion and Toxicity
Ninety-four percent of patients (n = 1,710) completed therapy without interruption, and 3% (n = 56) had documented grade 3 or 4 acute toxicity. Lowest rates of completion (without interruption) were in patients with head and neck cancer (78%, n = 143), who also had the highest rates of grade 3 or 4 acute toxicity (17.9%, n = 33).
Dosing Regimens
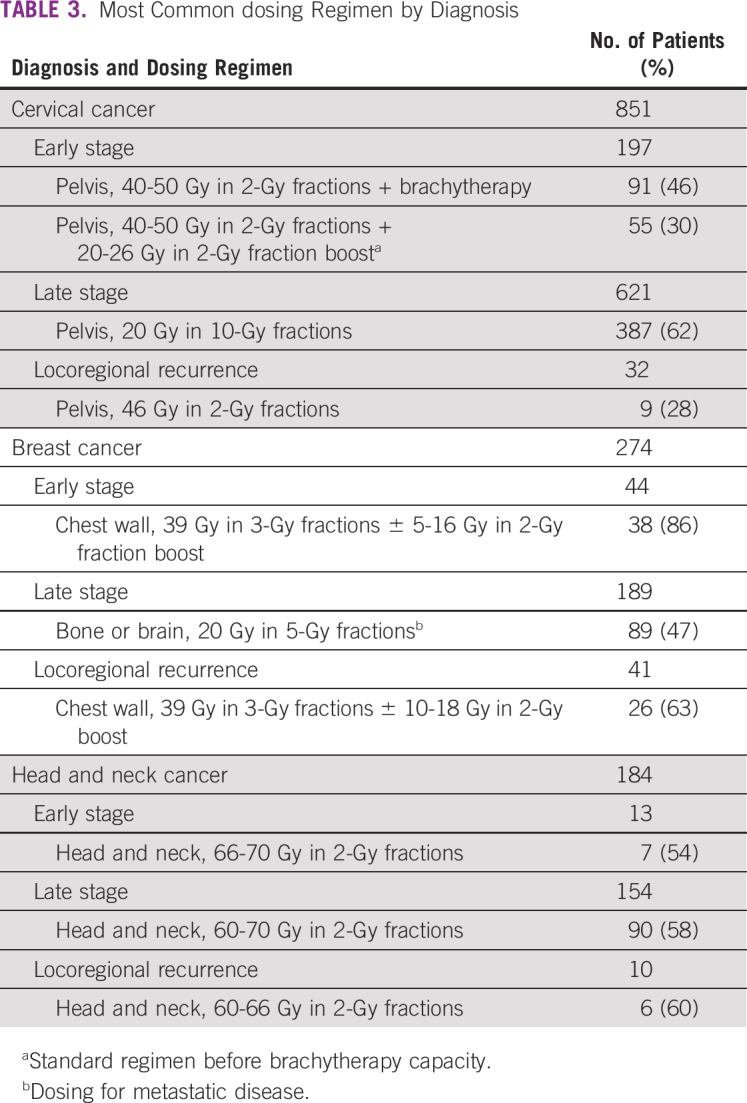
The most frequent RT regimen was 20 Gy in 2 fractions for advanced-stage cervical cancer and 20 Gy in 5 fractions for many other advanced-stage cancers (n = 672, 37%), suggestive of palliative treatment (Table 3).
TABLE 3.
Most Common dosing Regimen by Diagnosis
Wait Times
The wait times were the shortest for patients who received short palliative treatment (median, 0 days; interquartile range [IQR], 0-15 days; n = 1,138), whereas wait times were long for patients who received curative treatment (median, 150 days; IQR, 60-210 days; n = 685).
DISCUSSION
This cross-sectional study on the patterns of RT in Ethiopia is, to our knowledge, the largest descriptive analysis of RT use for all cancers in Ethiopia and, more broadly, the African continent. In our cohort, women disproportionally carried the cancer burden as a result of high rates of breast and cervical cancer, consistent with published data from the Addis Ababa population-based cancer registry that breast and cervical cancer account for nearly a third of all cancers, followed by colorectal cancer, non-Hodgkin lymphoma, leukemia, prostate cancer, and thyroid cancer.11,14,15 According to the International Agency for Research on Cancer, cervical cancer is the fourth most common cancer in women worldwide, but it is the most frequent cancer in women in many countries in sub-Saharan Africa where incidence is the highest in the world. This is a stark disparity because 85% of women diagnosed with cervical cancer (a largely avoidable disease) live in LMICs with limited access to preventative services and treatment.16-18
The prevalence of HIV in our cohort was 3-fold higher than that of the general Ethiopian population (4.6% v 1.5%, respectively), which has also been observed in Uganda.19,20 A majority of patients in the study were female. Females have a higher prevalence of HIV than males; in addition, HIV is an independent risk factor for cervical cancer, which is preferentially treated by RT. A study by Kantelhardt et al21 reported a 9% prevalence of HIV in Ethiopian patients with cervical cancer, and our study found a similar rate in Ethiopian patients with gynecologic cancers. These HIV rates in patients with cervical cancer are lower compared with other countries in sub-Saharan Africa as a result of the overall lower prevalence of HIV in Ethiopia.22,23
Nearly three-quarters of all patients with cancer in this study presented at late stage, which is consistent with findings from other countries in sub-Saharan Africa. This is at least partially a result of the lack of national screening programs in most African regions.24-26 In addition, a great majority of cancer care in Ethiopia is centralized to the tertiary level of care. Even palliative care services are centralized and not initiated until a patient is seen at TASH, a system that is critically over capacity. Our study not only emphasizes the need for high-impact, low-cost prevention and screening strategies in Ethiopia and the African continent at large, with the aim of diagnosing cancer at early stages when RT and other treatment modalities can be used with curative intent, but also emphasizes the need to decentralize palliative care and train primary- and secondary-level providers to provide palliative care at the community level.
In most cases of palliative RT, patients returned to their communities and were not seen in follow-up; therefore, documented toxicity was overall low. Patients with head and neck cancer had the highest rates of treatment interruption and the highest rates of grade 3 or 4 toxicity, which is similar to the literature.27 To our knowledge, there are no other published reported rates of acute RT toxicity in head and neck cancer in sub-Saharan Africa. Moelle et al28 reported grade 3 early toxicity in 5% of patients with cervical cancer who received curative RT in the same center in Ethiopia, which was a considerably higher rate than in this study. This is at least partially a result of the addition of brachytherapy during our study period, which alleviates the need for an external-beam boost, causing higher toxicity to the surrounding tissue.
One of the most striking findings of this study was the long waiting times of patients with potential for cure. Waiting times are influenced by the priority given to the large burden of patients who require palliation of debilitating symptoms such as pain, obstruction, or bleeding and risk reduction of emergent conditions such as pathologic fracture and cord compression. In addition, priority is given to younger patients. These reasons, coupled with limited treatment availability, played a major role in the observed wait times. This situation leads to a long wait for many individuals with potentially curative disease, risking their conversion to the palliative category as the disease progresses. Kantelhardt et al21 showed comparable rates of late-stage cervical cancer and wait times in Ethiopia but also reported that the proportion of patients with late-stage cervical cancer (FIGO stage IIIb and greater) increased from 44% to 69% during the wait time. In our study, stage was recorded immediately before starting RT; therefore, the rates of conversion during the wait time are unknown; however, it is possible that a 20% conversion also occurred during the waiting times. A correlation has been made between long wait time and worse outcomes in head and neck cancer; a Taiwanese study showed worse outcomes after 40-day delay, and a larger Dutch study showed worse outcome after 90 days.29,30 The median wait time for radical and adjuvant head and neck cancer was 5 months. This finding is of importance because it calls for better planning and stratification of treatment in an attempt to increase cure rates for early-stage tumors. It is likely that as more RT equipment is available in the region, waiting times will decrease and better curative-intent stratification will occur.
In regard to RT dosing at TASH, conventional fractionation is preferred for all curative diagnoses except for breast cancer, for which the hypofractionation schedule is used based on the START-A trial to maximize limited RT resources.31 Since brachytherapy was obtained in 2016, all curative patients are receiving brachytherapy unless probe insertion is not possible. Furthermore, because of limited capacity, concurrent chemoradiation is not yet feasible for cervical cancer.
There are multiple limitations to this study. First, the retrospective nature narrows the qualitative descriptors of the data to those previously recorded in the patient file. Second, the data collection was completed after approximately half of the estimated cohort was enrolled as a result of feasibility because data analysis at 1,823 patients was unchanged from data analysis after 500 patients, indicating a random sample. Third, cancer staging was performed by the treating physician through standardized methods agreed upon throughout the department. However, the eighth and latest edition of the AJCC staging system was not used because of lack of molecular diagnostics. For example, estrogen receptor–, progesterone receptor–, and human epidermal growth factor receptor–status is not routinely determined in patients with breast cancer. However, this is a limitation applicable to many resource-limited areas and speaks of the need to create differential guidelines in these settings. Finally, the availability of documented follow-up data were rare; thus, late toxicities and patient outcomes were not available. Information about acute toxicity was also not routinely recorded and likely significantly underreported, highlighting the need for future prospective studies that can more accurately describe toxicity and outcomes. Because more RT equipment needs to be purchased, it is imperative for stakeholders to have accurate outcome information from the African context about different equipment choices to help inform decisions.
To our knowledge, this study is the largest of its kind, describing the basic epidemiology and findings of more than 1,800 patients treated with cobalt RT at a large referral hospital in sub-Saharan Africa. Most patients were treated for gynecologic cancer and presented at advanced stage. The goal of treatment was palliative in a majority of patients, and limited radiation capacity led to long waiting times. The need for increased RT capacity is critical, and future prospective studies are needed on outcomes for RT modalities in sub-Saharan Africa.
Footnotes
Supported by Varian Medical Systems.
AUTHOR CONTRIBUTIONS
Conception and design: Tara Rick, Biruk Habtamu, Wondemagegnhu Tigeneh, Aynalem Abreha., Surbhi Grover, Mathewos Assefa, Luca Incrocci
Administrative support: Mathewos Assefa
Provision of study materials or patients: Aynalem Abreha
Collection and assembly of data: Tara Rick, Biruk Habtamu, Luca Incrocci
Data analysis and interpretation: Tara Rick, Yvette van Norden, Surbhi Grover, Luca Incrocci
Manuscript writing: All authors
Final approval of manuscript: All authors
Accountable for all aspects of the work: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated unless otherwise noted. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or ascopubs.org/jgo/site/misc/authors.html.
Open Payments is a public database containing information reported by companies about payments made to US-licensed physicians (Open Payments).
Tara Rick
Research Funding: Varian Medical Systems (Inst)
Travel, Accommodations, Expenses: Varian Medical Systems
Luca Incrocci
Research Funding: Varian Medical Systems (Inst), Janssen Cilag (Inst)
No other potential conflicts of interest were reported.
REFERENCES
- 1.Price P, Sikora K. Treatment of Cancer. (ed 5). London, United Kingdom: Arnold Hodder; 2008. [Google Scholar]
- 2.Barton MB, Frommer M, Shafiq J. Role of radiotherapy in cancer control in low-income and middle-income countries. Lancet Oncol. 2006;7:584–595. doi: 10.1016/S1470-2045(06)70759-8. [DOI] [PubMed] [Google Scholar]
- 3.International Atomic Energy Agency https://www.iaea.org/publications/8419/planning-national-radiotherapy-services-a-practical-tool IAEA Human Health Series, No. 14: Planning national radiotherapy services: A practical tool.
- 4.Van Der Giessen PH, Alert J, Badri C, et al. Multinational assessment of some operational costs of teletherapy. Radiother Oncol. 2004;71:347–355. doi: 10.1016/j.radonc.2004.02.021. [DOI] [PubMed] [Google Scholar]
- 5.Ferlay J, Ervik M, Lam F, et al. https://gco.iarc.fr/today Global Cancer Observatory: Cancer Today. Lyon, France, International Agency for Research on Cancer, 2018.
- 6.International Atomic Energy Agency https://dirac.iaea.org DIRAC: Directory of Radiotherapy Centres.
- 7.Zubizarreta EH, Fidarova E, Healy B, et al. Need for radiotherapy in low and middle income countries: The silent crisis continues. Clin Oncol (R Coll Radiol) 2015;27:107–114. doi: 10.1016/j.clon.2014.10.006. [DOI] [PubMed] [Google Scholar]
- 8.Abdel-Wahab M, Bourque JM, Pynda Y, et al. Status of radiotherapy resources in Africa: An International Atomic Energy Agency analysis. Lancet Oncol. 2013;14:e168–e175. doi: 10.1016/S1470-2045(12)70532-6. [DOI] [PubMed] [Google Scholar]
- 9.Misganaw A, Mariam DH, Araya T. The double mortality burden among adults in Addis Ababa, Ethiopia, 2006-2009. Prev Chronic Dis. 2012;9:E84. doi: 10.5888/pcd9.110142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Weldearegawi B, Melaku YA, Spigt M, et al. Applying the InterVA-4 model to determine causes of death in rural Ethiopia. Glob Health Action. 2014;7:25550. doi: 10.3402/gha.v7.25550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Tigeneh W, Molla A, Abreha A, et al: Pattern of cancer in Tikur Anbessa Specialized Hospital Oncology Center in Ethiopia from 1998 to 2010. Int J Cancer Res Mol Mech 10.16966/ 2381-3318.103. [Google Scholar]
- 12.Ethiopian Federal Ministry of Health https://www.iccp-portal.org/sites/default/files/plans/NCCP%20Ethiopia%20Final%20261015.pdf National Cancer Control Plan 2016-2020. Addis Ababa, Ethiopia, Federal Ministry of Health, 2015.
- 13.Adewole I, Martin DN, Williams MJ, et al. Building capacity for sustainable research programmes for cancer in Africa. Nat Rev Clin Oncol. 2014;11:251–259. doi: 10.1038/nrclinonc.2014.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Memirie ST, Habtemariam MK, Asefa M, et al. doi: 10.1200/JGO.17.00175. Estimates of cancer incidence in Ethiopia in 2015 using population-based registry data. J Glob Oncol 10.1200/JGO.17.00175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Timotewos G, Solomon A, Mathewos A, et al. First data from a population based cancer registry in Ethiopia. Cancer Epidemiol. 2018;53:93–98. doi: 10.1016/j.canep.2018.01.008. [DOI] [PubMed] [Google Scholar]
- 16.Ferlay J, Soerjomataram I, Dikshit R, et al. Cancer incidence and mortality worldwide: Sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer. 2015;136:E359–E386. doi: 10.1002/ijc.29210. [DOI] [PubMed] [Google Scholar]
- 17.Ginsburg O, Bray F, Coleman MP, et al. The global burden of women’s cancers: A grand challenge in global health. Lancet. 2017;389:847–860. doi: 10.1016/S0140-6736(16)31392-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Arbyn M, Castellsagué X, de Sanjosé S, et al. Worldwide burden of cervical cancer in 2008. Ann Oncol. 2011;22:2675–2686. doi: 10.1093/annonc/mdr015. [DOI] [PubMed] [Google Scholar]
- 19.Central Statistical Agency and ICF International https://dhsprogram.com/pubs/pdf/fr255/fr255.pdf Ethiopia Demographic and Health Survey 2011.
- 20.Bender Ignacio R, Ghadrshenas M, Low D, et al. HIV status and associated clinical characteristics among adult patients with cancer at the Uganda Cancer Institute. J Glob Oncol. doi: 10.1200/JGO.17.00112. 10.1200/JGO.17.00112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kantelhardt EJ, Moelle U, Begoihn M, et al. Cervical cancer in Ethiopia: Survival of 1,059 patients who received oncologic therapy. Oncologist. 2014;19:727–734. doi: 10.1634/theoncologist.2013-0326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Grover S, Bvochora-Nsingo M, Yeager A, et al. Impact of human immunodeficiency virus infection on survival and acute toxicities from chemoradiation therapy for cervical cancer patients in a limited-resource setting. Int J Radiat Oncol Biol Phys. 2018;101:201–210. doi: 10.1016/j.ijrobp.2018.01.067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Simonds HM, Neugut AI, Jacobson JS. doi: 10.1097/IGC.0000000000000441. HIV status and acute hematologic toxicity among patients with cervix cancer undergoing radical chemoradiation. Int J Gynecol Cancer 25:884-890, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jedy-Agba E, McCormack V, Adebamowo C, et al. Stage at diagnosis of breast cancer in sub-Saharan Africa: A systematic review and meta-analysis. Lancet Glob Health. 2016;4:e923–e935. doi: 10.1016/S2214-109X(16)30259-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Asombang AW, Madsen R, Simuyandi M, et al. Descriptive analysis of colorectal cancer in Zambia, Southern Africa using the National Cancer Disease Hospital Database. Pan Afr Med J. 2018;30:248. doi: 10.11604/pamj.2018.30.248.12464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gilyoma JM, Rambau PF, Masalu N, et al. Head and neck cancers: A clinico-pathological profile and management challenges in a resource-limited setting. BMC Res Notes. 2015;8:772. doi: 10.1186/s13104-015-1773-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Trotti A, Bellm LA, Epstein JB, et al. Mucositis incidence, severity and associated outcomes in patients with head and neck cancer receiving radiotherapy with or without chemotherapy: A systematic literature review. Radiother Oncol. 2003;66:253–262. doi: 10.1016/s0167-8140(02)00404-8. [DOI] [PubMed] [Google Scholar]
- 28.Moelle U, Mathewos A, Aynalem A, et al. Cervical cancer in Ethiopia: The effect of adherence to radiotherapy on survival. Oncologist. 2018;23:1024–1032. doi: 10.1634/theoncologist.2017-0271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Fortin A, Bairati I, Albert M, et al. Effect of treatment delay on outcome of patients with early-stage head-and-neck carcinoma receiving radical radiotherapy. Int J Radiat Oncol Biol Phys. 2002;52:929–936. doi: 10.1016/s0360-3016(01)02606-2. [DOI] [PubMed] [Google Scholar]
- 30.van Harten MC, de Ridder M, Hamming-Vrieze O, et al. The association of treatment delay and prognosis in head and neck squamous cell carcinoma (HNSCC) patients in a Dutch comprehensive cancer center. Oral Oncol. 2014;50:282–290. doi: 10.1016/j.oraloncology.2013.12.018. [DOI] [PubMed] [Google Scholar]
- 31.Haviland JS, Owen JR, Dewar JA, et al. The UK Standardisation of Breast Radiotherapy (START) trials of radiotherapy hypofractionation for treatment of early breast cancer: 10-year follow-up results of two randomised controlled trials. Lancet Oncol. 2013;14:1086–1094. doi: 10.1016/S1470-2045(13)70386-3. [DOI] [PubMed] [Google Scholar]