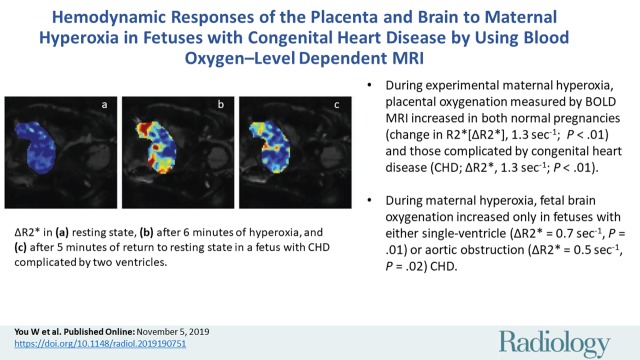
Abstract
Background
Impaired brain development in fetuses with congenital heart disease (CHD) may result from inadequate cerebral oxygen supply in utero.
Purpose
To test whether fetal cerebral oxygenation can be increased by maternal oxygen administration, effects of maternal hyperoxia on blood oxygenation of the placenta and fetal brain were examined by using blood oxygenation level–dependent (BOLD) functional MRI.
Materials and Methods
In this prospective study, BOLD MRI was performed in 86 fetuses (56 healthy fetuses and 30 fetuses diagnosed with CHD) between 22 and 39 weeks gestational age (GA) from May 2015 to December 2017, with the following study design: phase I, 2-minute resting state at baseline (room air); phase II, 6-minute maternal hyperoxia with 100% oxygen; and phase III, 5.6-minute return to resting state. After motion correction, the signals were averaged over the placenta and fetal brain and converted to the change in R2* (ΔR2*). Fetuses with CHD were categorized into those with a single ventricle (SV) or two ventricles (TVs) and those with aortic obstruction (AO) or non-AO. Data were analyzed by using generalized linear mixed models controlling for GA and sex.
Results
Placental ΔR2* increased during maternal hyperoxia in healthy fetuses and fetuses with CHD, but it was higher in SV CHD (mean ΔR2*, 1.3 sec−1 ± 0.1 [standard error; P < .01], 1.9 sec−1 ± 0.2 [P < .01], and 1.0 sec−1 ± 0.3 [P < .01], respectively, for control fetuses, fetuses with SV CHD, and fetuses with TV CHD). Placental ΔR2* during maternal hyperoxia changed with GA in healthy control fetuses and fetuses with SV or AO CHD (ΔR2* per week, 0.1 sec−1 ± 0 [P < .01], 0.2 sec–1 ± 0 [P = .01], and 0.2 sec−1 ± 0 [P = .01], respectively), but not in fetuses with CHD and TV or non-AO. Fetal brain ΔR2* was constant across all phases in healthy control fetuses and fetuses with TV CHD but increased during maternal hyperoxia in fetuses with SV or AO CHD (mean ΔR2*, 0.7 sec−1 ± 0.2 [P = .01] and 0.5 sec−1 ± 0.2 [P = .02], respectively).
Conclusion
Six minutes of maternal hyperoxia increased placental oxygenation in healthy fetuses and fetuses with congenital heart disease, and it selectively increased cerebral blood oxygenation in fetuses with single ventricle or aortic obstruction.
© RSNA, 2019
Summary
Short-term maternal hyperoxia increased placental oxygenation at blood oxygen level−dependent MRI in both normal pregnancies and those complicated by congenital heart disease and increased fetal cerebral blood oxygenation in fetuses with either single-ventricle physiologic structure or aortic obstruction.
Key Results
■ During experimental maternal hyperoxia, placental oxygenation measured by blood oxygenation level–dependent MRI increased in both normal pregnancies (change in R2*[ΔR2*], 1.3 sec−1; P < .01) and those complicated by congenital heart disease (CHD; ΔR2*, 1.3 sec−1; P < .01).
■ During maternal hyperoxia, fetal brain oxygenation increased only in fetuses with either single-ventricle (ΔR2* = 0.7 sec−1, P = .01) or aortic obstruction (ΔR2* = 0.5 sec−1, P = .02) CHD.
Introduction
Impaired fetal brain development during the third trimester has been increasingly reported as a contributing factor to brain injury and neurodevelopmental disabilities in survivors of complex congenital heart disease (CHD) (1,2). Available evidence suggests that alterations in fetal oxygen delivery may contribute to aberrant brain growth in this high-risk fetal population and raises the possibility of maternal hyperoxygenation as a potential fetal therapy for certain types of CHD (3–5). The role of maternal oxygen administration, or maternal hyperoxia (HO), to improve fetal outcomes has been studied primarily in pregnancies complicated by fetal growth restriction, presumably to support declining placental function. Most of these studies focused on the redistribution of placental and cerebral blood flow during HO as measured at Doppler US, however, the potential impact of this therapy remains unclear (6–9). Recent advances in fetal MRI allow for more sophisticated and quantitative analyses of fetal hemodynamics and oxygenation, including phase-contrast MRI, T2 mapping, and blood oxygenation level–dependent (BOLD) functional MRI (10,11).
The fetal response to maternal HO has been reported in the healthy fetus by using BOLD functional MRI, which demonstrated negligible changes in fetal brain oxygenation even in the setting of increased placental oxygenation (8). In fetuses with CHD, T2* mapping has been studied to estimate fetal cerebral tissue oxygenation. These studies have revealed significant decreases in blood oxygenation and oxygen consumption of the fetal brain in CHD compared with healthy control fetuses (12), which was associated with impaired brain growth (4). In light of these findings, it has been proposed that administration of oxygen to a woman carrying a fetus with CHD may improve fetal cerebral oxygenation by increasing blood oxygen content. To our knowledge, only one study (13) has examined the effects of maternal HO in fetuses with hypoplastic left-heart syndrome by using Doppler US. However, to our knowledge, the direct effects of maternal HO on placental and fetal cerebral blood oxygenation in CHD have not been studied.
In our study, we sought to investigate the effects of maternal HO on placental and cerebral oxygenation in fetuses with CHD by using BOLD functional MRI. We hypothesized that the fetus with CHD will have a significant increase in cerebral oxygenation in response to maternal HO compared with healthy control fetuses.
Materials and Methods
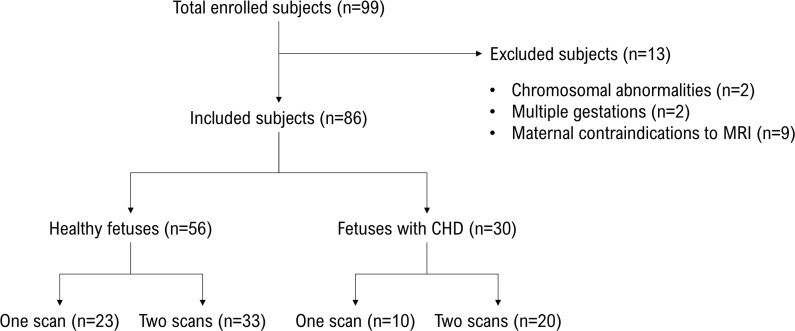
This prospective study was approved by the institutional review board at Children’s National Hospital (Washington, DC), and written informed consent was obtained from all study participants. BOLD functional MRI signals were acquired from enrolled pregnant women between May 2015 and December 2017. Healthy control participants were eligible if there were no maternal comorbidities and all pregnancy screening evaluations were normal. CHD was diagnosed at fetal echocardiogram according to established guidelines (14). Mothers with a fetus with CHD that was expected to undergo cardiac surgery with cardiopulmonary bypass within the first 30 days of life were eligible for enrollment. Exclusion criteria included dysmorphic fetal features diagnosed at antenatal US, chromosomal abnormalities diagnosed by chorionic villous sampling or amniocentesis, multiple gestations, congenital infections, and maternal contraindications to MRI (Fig 1). Fetuses with CHD were classified as either single-ventricle (SV) or two-ventricle (TV) CHD for analysis of CHD subtypes. Fetuses with CHD were further classified as either CHD with aortic obstruction (AO) or CHD without AO (non-AO).
Figure 1:
Flowchart for the selection of study subjects from the enrolled pregnant women in the Children’s National Hospital. CHD = congenital heart disease.
Pregnant women underwent fetal MRI with a 1.5-T MRI system (Discovery MR450; GE Healthcare, Waukesha, Wis). We performed gradient-echo planar imaging sequences by using an eight-channel receive-only surface coil placed on the maternal abdomen in either supine or left-lateral positions. No sedation or exogenous contrast agent was used. A total of 408 volumes were acquired for each examination on the coronal acquisition plane with the following parameters: repetition time msec/echo time msec, 2000/60; field of view, 420 × 420 mm; 128 × 128 matrix; 17–18 slices; slice thickness, 7–12 mm; slice gap, 2 mm; and flip angle, 90°. Additional fast spin-echo T2-weighted imaging was performed with the following parameters: 1095/163; field of view, 320 × 320 mm; slice thickness, 2 mm; and flip angle, 90°; the images served as an anatomical reference to manually identify the regions of interest for the fetal brain and placenta.
The maternal HO design consisted of three consecutive phases: phase I, a 2-minute resting state at baseline normoxia (room air with 21% oxygen); phase II, a 6-minute maternal HO with 100% oxygen inhaled through a facial oxygen nonrebreather mask at 15 L/min (CareFusion, Yorba Linda, Calif); and phase III, return to resting state (RR) at normoxic conditions for 5.6 minutes. The examination was paused for 5 seconds between each phase.
The degradation of BOLD signals because of severe fetal motion was corrected by using the design-optimized motion correction pipeline (15,16). In this pipeline, after excluding the first five volumes in each phase, the inhomogeneous distribution of signal intensities produced by MRI bias field was corrected by using the four-dimensional nonparametric bias estimator (17).
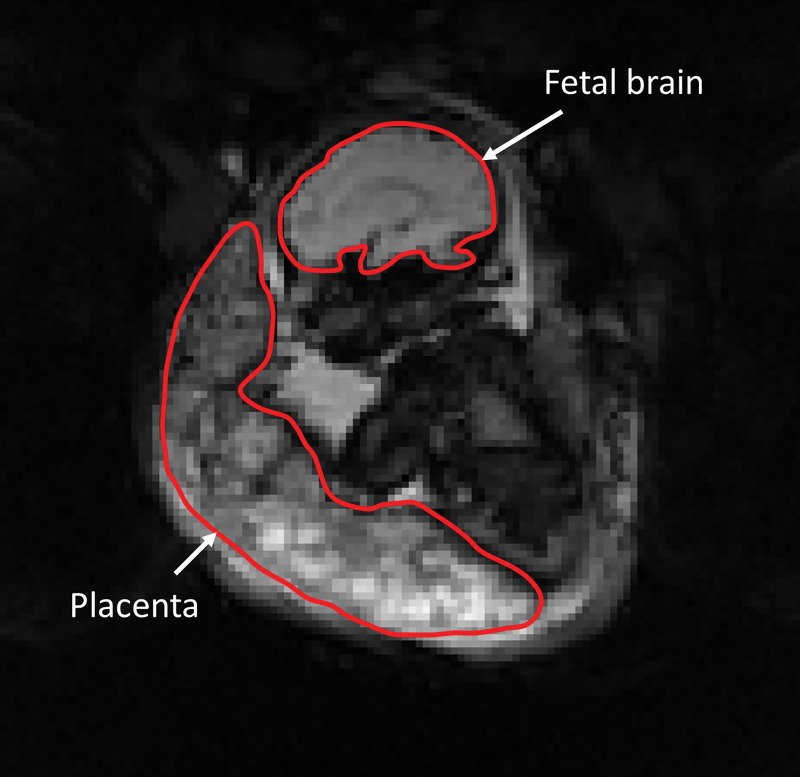
A volume with minimum variation from other volumes was automatically selected as a template for image registration. The region-of-interest masks of the fetal brain and placenta were manually mapped on the template volume by using ITK-SNAP (18), and their anatomic coherence was verified by using the corresponding T2-weighted images from MRI. An example of the region-of-interest masks is shown in Figure 2.
Figure 2:
Coronal image from functional MRI from a normal pregnancy of a single female fetus at 35 gestational weeks. The red lines show the regions of interest for the placenta and fetal brain acquired during maternal hyperoxia.
The between-slice motion artifact was corrected by using a straightforward algorithm that decomposes each volume into two subvolumes of odd and even slices and aligns the two interleaved subvolumes (19). The between-volume motion artifact was corrected separately in the fetal brain and placenta by using the region-of-interest masks. Both rigid-body and nonrigid-body image registrations were applied to the fetal brain by using the advanced neuroimaging tools (20), whereas nonrigid body image registration was applied to the placenta by using the Image Registration Toolkit (21). Volumes that remained misaligned after motion correction were automatically eliminated through volume outlier rejection and were recovered by using data imputation (15,16).
The BOLD signals were averaged as the median value of voxel intensities over each region of interest. The change in BOLD signal, S, from the baseline signal, S0, was converted to the change in R2* (ΔR2*) according to the equation ΔR2* = –log (S/S0)/TE, where TE is echo time, by using software (Matlab 2018a; MathWorks, Natick, Mass) (22). The region-of-interest–averaged ΔR2* of the fetal brain and placenta were separated into 13 phases that were abbreviated to RS (ie, resting state), HO1–HO6 (ie, each minute of phase II), and RR1–RR6 (ie, each minute of phase III). The ΔR2* phase averages were computed as the mean of ΔR2* during each phase.
A one-sample t test was used to evaluate whether ΔR2* was significantly different from zero at each phase by subject group. To account for within-patient clustering, complex survey analysis techniques were employed by treating repeated patient measures as individual clusters; standard errors were adjusted by using Taylor series estimation (23). Subsequently, the generalized linear mixed models accounting for repeat patient sampling were used to assess differences in ΔR2* in groups with CHD compared with healthy control participants. Gestational age (GA) and sex were controlled for all models. Groups by time interaction terms were included in generalized linear mixed model to evaluate whether associations across GA differed by group; GA was considered to be a continuous variable. Model parameters were estimated by using restricted maximum likelihood with robust standard errors. Fixed-effect R2 estimates for goodness of fit were calculated by using an estimation method for linear mixed models (24). All analyses were performed by using statistical software (SAS 9.4; SAS Institute, Cary, NC). A formal power analysis was performed on the original study whereas no power analysis was performed on this secondary study. A P value less than .05 was considered to indicate statistical significance.
Results
Study Population
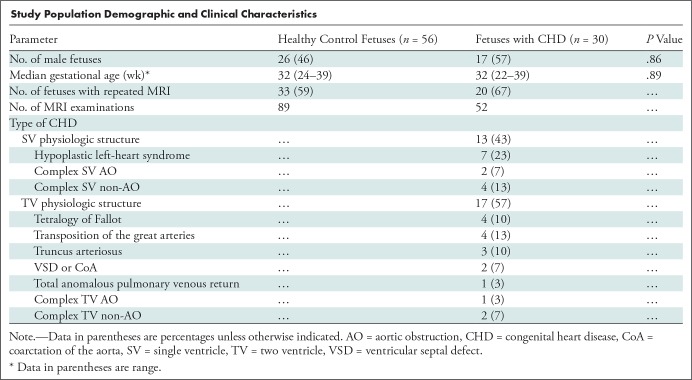
We enrolled a total of 86 pregnant women (56 with healthy fetuses and 30 with fetuses with CHD) at a mean GA of 31 5/7 weeks (ranging from 22 1/7 to 39 4/7 weeks). A subset of 53 pregnant women (33 healthy fetuses and 20 fetuses with CHD) underwent two MRI examinations after study enrollment: the first MRI examination was performed before 30 weeks GA and the second MRI examination was performed after 30 weeks GA, for a total 141 MRI examinations (89 examinations for healthy fetuses and 52 examinations for fetuses with CHD). A flowchart demonstrating the selection of study subjects from the enrolled pregnant women is shown in Figure 1. No adverse events were noted in the fetuses or mothers at or immediately after MRI. The quality of functional MRI data was assessed after data preprocessing; 12 of 141 examinations were excluded from the placental analysis because of severe MRI artifacts and failure of motion correction; and 57 examinations were excluded from the analysis of the fetal brain because of significant MRI artifact, failure of motion correction, and partial data acquisition of the fetal brain. The demographic and clinical properties of the remaining study participants are summarized in the Table.
Study Population Demographic and Clinical Characteristics
Placental ΔR2*
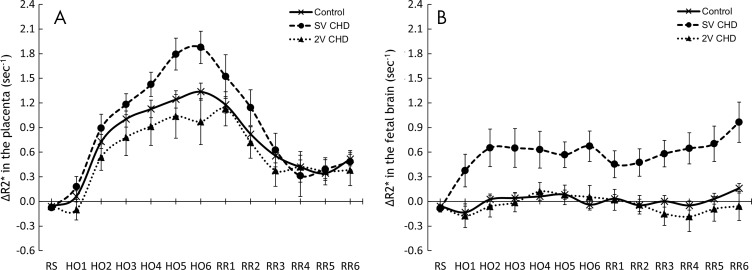
The ΔR2* values of the placenta and fetal brain are summarized in Tables E1 and E2 (online). Figure 3 shows the temporal trends of placental and fetal brain ΔR2* over maternal HO and RR. Figures 4 and 5 show the local variation of placental and fetal brain ΔR2*. The placental ΔR2* increased during HO in both healthy fetuses and fetuses with CHD (mean ΔR2* at HO6, 1.3 sec–1 ± 0.1 [standard error; P < .01], 1.9 sec−1 ± 0.2 [P < .01], and 1.0 sec–1 ± 0.3 [P < .01], respectively, in healthy control fetuses, fetuses with SV CHD, and fetuses with TV CHD). Whereas placental ΔR2* was higher in fetuses with SV CHD compared with healthy control fetuses for the last 2 minutes of HO (P = .01), the values were similar in fetuses with TV CHD (HO6, P = .10) (Table E1 [online]).
Figure 3:
Graphs show the changes in R2* of the, A, placenta and, B, fetal brain at resting state (RS) during maternal hyperoxia (HO) and during return to resting state (RR) in normal pregnancies and those complicated by congenital heart disease (CHD) with single ventricle (SV) or two ventricles (2V). Data for ΔR2* are expressed as means and standard errors. HO1–HO6 = each minute of HO, RR1–RR6 = each minute of RR.
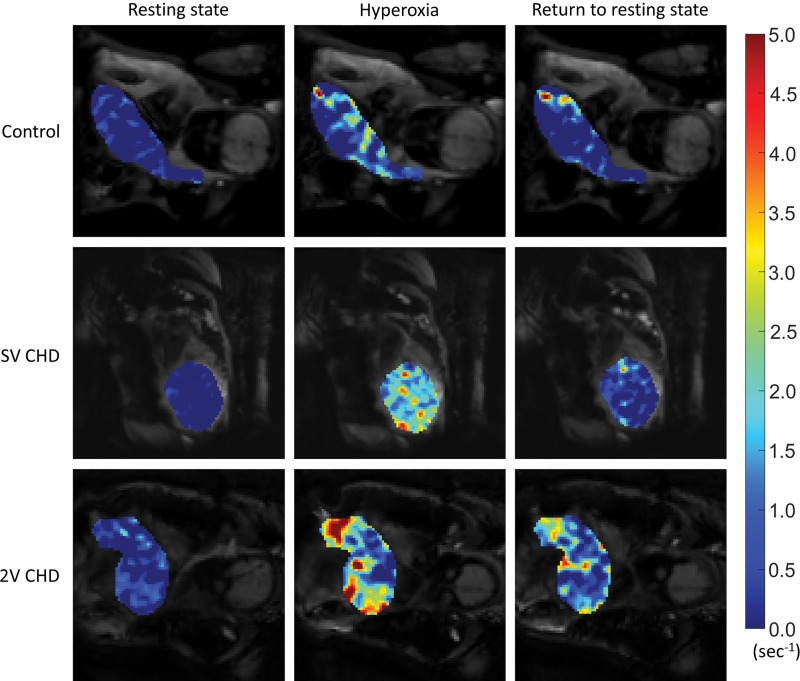
Figure 4:
Comparison of voxel-wise placental change in R2* (ΔR2*) in resting state (left), after 6 minutes of hyperoxia (middle), and after 5 minutes of return to resting state (right) between healthy pregnancies and pregnancies complicated by congenital heart disease (CHD) with single ventricle (SV) or two ventricles (2V). The color map indicates the magnitude of ΔR2*.
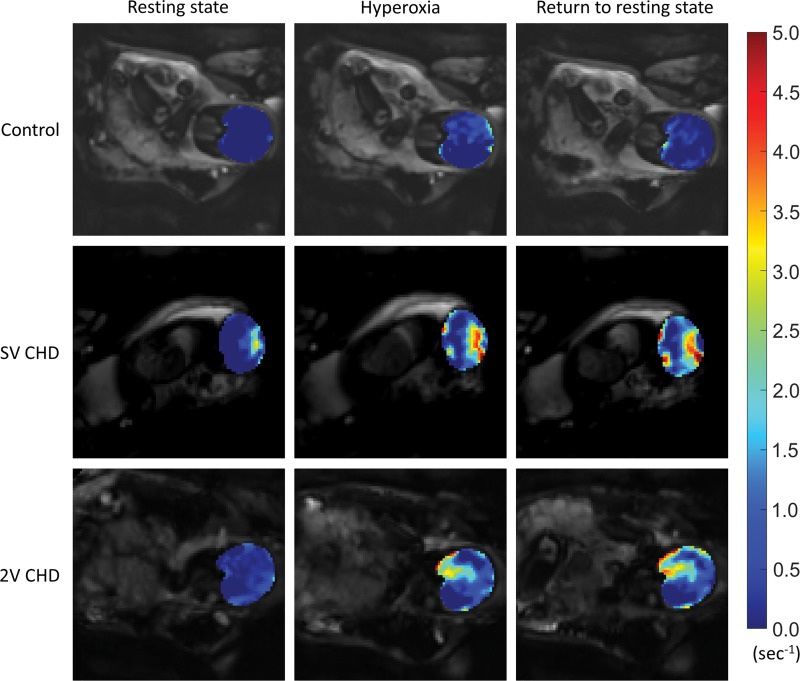
Figure 5:
Comparison of voxel-wise change in R2* (ΔR2*) in fetal brain in resting state (left), after 6 minutes of hyperoxia (middle), and after 5 minutes of return to resting state (right) between healthy pregnancies and pregnancies complicated by congenital heart disease (CHD) with single ventricle (SV) or two ventricles (2V). The color map indicates the magnitude of ΔR2*.
The placental ΔR2* in fetuses with AO CHD were not different in any phases compared with fetuses with non-AO CHD (mean ΔR2* at HO6, 1.4 sec−1 ± 0.3 and 1.2 sec–1 ± 0.3, respectively, in fetuses with AO CHD and non-AO CHD; P = .87) (Table E2, Fig E1 [online]).
Fetal Brain ΔR2*
The fetal brain ΔR2* did not increase during maternal HO for healthy fetuses and fetuses with TV CHD (mean ΔR2* at HO6, 0 sec−1 ± 0.1 [P = .55] and 0.1 sec−1 ± 0.1 [P = .70], respectively). However, fetal brain ΔR2* was higher in fetuses with SV CHD during maternal HO (mean ΔR2* at HO6, 0.7 ± 0.2 sec−1; P = .01). Higher ΔR2* persisted through phase III (mean ΔR2* at RR6, 1.0 sec−1 ± 0.2; P = .01). The fetal brain ΔR2* was higher in fetuses with SV CHD compared with healthy fetuses throughout all periods in phases II and III (in HO6 and RR6, P < .01). Similar to fetuses with SV CHD, the fetal brain ΔR2* values for the fetuses with AO CHD increased during HO (ΔR2* at HO6, 0.5 sec−1 ± 0.2; P = .02), and the group difference between healthy control fetuses and fetuses with AO CHD was statistically significant during HO4 to RR2 phases (P < .05 for all; Table E2 [online], Fig E1 [online]).
Relationship between ΔR2* and GA
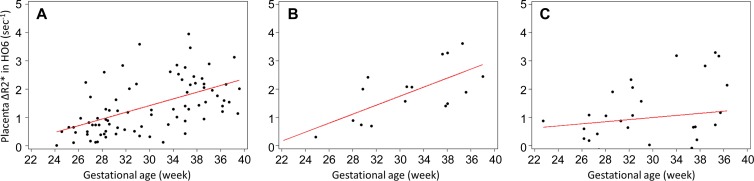
The effects of GA on placental and fetal brain ΔR2* in healthy fetuses and fetuses with CHD are summarized in Table E3 (online). The placental ΔR2* between phases HO3 and RR1 increased with advancing GA for healthy control fetuses and fetuses with SV CHD (P < .05 for all), although the increase of placental ΔR2* over GA persisted through the RR4 phase in healthy control fetuses. Figure 6 shows the relationship between placental ΔR2* during terminal HO with advancing GA, with a positive association between regional placental oxygenation and GA for both healthy control fetuses and fetuses with SV CHD (GA, β = .1 sec–1 per week ± .0 [P < .01] and .2 sec–1 per week ± .0 [P = .01], respectively; fetuses with TV CHD, β = .0 sec−1 per week ± .1 [P = .57]). The fetuses with AO CHD also showed an increase in the placental ΔR2* over GA between HO3 and RR1 phases (Fig E2 [online]) (GA at HO6, AO CHD vs non-AO CHD: β = .2 sec−1 per week ± .0 [P = .01] and .1 sec−1 per week ± .1, respectively [P = .40]).
Figure 6:
Scatterplots show comparison of placental change in R2* (ΔR2*; after 6 minutes of maternal hyperoxia [HO6] phase) versus gestational age between, A, normal pregnancies and those complicated by congenital heart disease with, B, single ventricle or, C, two ventricles. Data points and linear regression lines are plotted.
The fetal brain ΔR2* at terminal HO did not show any longitudinal change over advancing GA in both healthy fetuses and fetuses with CHD. However, there was a relationship between the fetal brain ΔR2* at early HO (HO1 to HO3 phases) and GA for SV CHD only (Table E3 [online]) (GA at HO1 phase, β = .1 sec−1 per week ± .0; P = .03).
Discussion
In our study, we reported the effects of short-term maternal hyperoxia (HO) on the placenta and fetal brain-blood oxygenation in pregnancies complicated by congenital heart disease (CHD) by using blood oxygenation level–dependent (BOLD) MRI to assess whether maternal HO can be used to improve fetal oxygenation in CHD. We demonstrated that placental change in R2* (ΔR2*) during HO was higher in the fetuses with single-ventricle (SV) CHD compared with healthy control fetuses (ΔR2*, 1.9 sec−1 vs 1.3 sec−1, respectively; P = .01), and that fetal brain ΔR2* increased during HO in the fetuses with either SV or aortic obstruction (AO) CHD (ΔR2*, 0.7 sec−1 and 0.5 sec−1, respectively; P < .01).
The increase in placental ΔR2* during maternal HO in both healthy pregnancies and pregnancies that involve CHD may reflect the anatomic nature of placental vasculature. It has been shown that whereas the concentration of oxyhemoglobin in the maternal blood entering the placenta remains relatively unchanged with HO, maternal arterial partial pressure of oxygen, or PaO2, increases. This allows for more oxygen to be diffused into the fetal compartment with less saturated blood, thus increasing the oxygen saturation in the umbilical vein (10,25,26).
Interestingly, the increase in placental ΔR2* was higher in fetuses with SV CHD compared with healthy control fetuses. A decrease in oxygen saturation of the ascending aorta in SV CHD had been previously demonstrated (4). If SV CHD results in the most deoxygenated blood return to the placenta, this may explain in part why our study found the most statistically significant increase in placental oxygenation in this cohort. Moreover, the unique response of the placental ΔR2* in SV CHD also might be reflective of distinct placental disease. Pathologic examination of the placenta in CHD has revealed high rates of hemorrhage and infarction (27). Placental injury may cause larger hyperoxic increase in blood oxygenation in isolated regions. Indeed, placental disease in CHD may also be a contributing factor to growth restriction, as decreased fetal growth has been reported in CHD (3,28).
Placental ΔR2* during maternal HO significantly increased over GA in healthy fetuses. This is supported by a previous study that demonstrated reduced T2* values of the placenta in the mature pregnancy, suggesting that less placental oxygenation at baseline later in gestation may lead to larger hyperoxic increase in ΔR2* (29). However, we did not detect a significant association with placental ΔR2* and GA in fetuses with TV or non-AO CHD. This may potentially reflect independent placental pathologic mechanisms that limit oxygen transfer in this group, though further investigation is warranted (30).
In healthy fetuses, cerebral blood oxygenation remained constant across maternal oxygenation states. This is consistent with previous studies (8,31) of MRI in the human fetus that reported negligible hyperoxic changes in fetal cerebral BOLD (related to oxygen saturation) and change in R1 (related to the change in partial oxygen pressure). There are conflicting studies that reported increasing cerebral oxygenation during maternal HO (32,33). These may be in part because of differences in HO design, imaging modality, and maternal condition like labor as a potential fetal stressor (31,34).
The insignificant hyperoxic response in fetal cerebral oxygenation differs from previous studies (8,35) that demonstrated increased oxygenation in the fetal liver and kidney during maternal HO. Sørensen et al (8) suggested this may be reflective of cerebral autoregulatory mechanisms through which the brain can maintain stable cerebral blood flow across a variety of intrauterine conditions to secure sufficient delivery of oxygen and nutrients. During fetal hypoxia, the cerebral vascular resistance is changed to regulate cerebral blood flow and maintain cerebral oxygen delivery, whereas the opposite may be true during fetal HO (3,8,31,36).
A significant and persistent increase in fetal cerebral oxygenation during maternal HO was found in SV and AO CHD. Of note, Sun et al (4) recently described lower oxygen delivery in fetuses with SV versus TV CHD. We hypothesize that the lower baseline cerebral oxygenation in SV CHD may result in greater increases in cerebral oxygen delivery in response to maternal HO in this population. Ongoing research is needed to address these intriguing preliminary findings.
Different than the placenta, the fetal brain response to HO did not show statistically significant longitudinal changes over GA for healthy control fetuses and fetuses with CHD. This provides further evidence that cerebrovascular autoregulation continues to mature with advancing gestation, maintaining constant oxygen delivery despite maternal HO (37). However, in the fetus with SV CHD, the increase in cerebral oxygenation during the early phases of maternal HO was positively associated with GA. Whereas the underlying mechanism is unclear, this may represent progressive hypoxia of the fetal cerebral circulation that would be exaggerated in late gestation with the rising metabolic demands to support brain development.
There were important study limitations that deserve mention. Our experiments were designed to observe short-term transient changes in blood oxygenation responding to maternal HO of 6 minutes, whereas other studies (8,35,38) used longer durations of HO (10–20 minutes). Therefore, the ΔR2* may not reflect a fully saturated condition of the placenta or fetal brain. In addition, the optimal length of time to reach the return to baseline is unknown. Future work should consider variable paradigms to best capture true oxygen saturation and recovery rates for both the placenta and fetal brain. As well, our CHD cohort had variable underlying diagnoses with unique pathologic structure of the fetal circulation, although the functional categorization into SV and TV and AO and non-AO CHD did reveal unique differences in hyperoxic oxygenation of the placenta and fetal brain. Finally, the data from many fetuses had to be excluded from analysis because of the technical reasons we described (eg, motion artifacts and related image degradation). Technical optimization and motion compensation tools need to be further developed and optimized.
Our study provided evidence for increased cerebral oxygenation in fetuses with single-ventricle and aortic obstruction congenital heart disease (CHD) in response to a brief period of maternal hyperoxia. Although the underlying mechanism needs further investigation, our data suggested that maternal oxygen therapy may improve oxygen delivery to the brain in selected fetuses with specific CHD subtypes. Future investigations to better understand cerebral oxygenation of the fetus can build on the blood oxygenation level−dependent studies presented here, combined with techniques to measure blood flow, such as phase-contrast imaging. Investigating changes to visceral perfusion and oxygenation in CHD will also be important to better understand the fetal ability to compensate for altered cardiovascular function. Whether chronic maternal hyperoxia can provide a sustained improvement in cerebral oxygenation and improve neurodevelopmental outcomes is, to our knowledge, currently unknown and beyond the scope of our current study. The safety and feasibility of maternal hyperoxia in pregnant women with fetal CHD remains to be determined (5,39–41).
APPENDIX
SUPPLEMENTAL FIGURES
Acknowledgments
Acknowledgments
We thank the research coordinators, patients, and their families who took part in this study.
C.L. supported by the National Institutes of Health (R01 HL116585-01); M.J. supported by the National Institutes of Health (1U54HD090257-01); study supported by the National Institutes of Health Intellectual and Developmental Disabilities Research Centers (5U54HD090257-04).
Disclosures of Conflicts of Interest: W.Y. disclosed no relevant relationships. N.N.A. disclosed no relevant relationships. K.K. disclosed no relevant relationships. M.T.D. disclosed no relevant relationships. M.J. disclosed no relevant relationships. C.L. disclosed no relevant relationships.
Abbreviations:
- ΔR2*
- change in R2*
- AO
- aortic obstruction
- BOLD
- blood oxygenation level dependent
- CHD
- congenital heart disease
- GA
- gestational age
- HO
- hyperoxia
- RR
- return to resting state
- SV
- single ventricle
- TV
- two ventricle
References
- 1.Limperopoulos C, Tworetzky W, McElhinney DB, et al. Brain volume and metabolism in fetuses with congenital heart disease: evaluation with quantitative magnetic resonance imaging and spectroscopy. Circulation 2010;121(1):26–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Miller SP, McQuillen PS, Hamrick S, et al. Abnormal brain development in newborns with congenital heart disease. N Engl J Med 2007;357(19):1928–1938. [DOI] [PubMed] [Google Scholar]
- 3.Donofrio MT, Bremer YA, Schieken RM, et al. Autoregulation of cerebral blood flow in fetuses with congenital heart disease: the brain sparing effect. Pediatr Cardiol 2003;24(5):436–443. [DOI] [PubMed] [Google Scholar]
- 4.Sun L, Macgowan CK, Sled JG, et al. Reduced fetal cerebral oxygen consumption is associated with smaller brain size in fetuses with congenital heart disease. Circulation 2015;131(15):1313–1323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Co-Vu J, Lopez-Colon D, Vyas HV, Weiner N, DeGroff C. Maternal hyperoxygenation: A potential therapy for congenital heart disease in the fetuses? A systematic review of the current literature. Echocardiography 2017;34(12):1822–1833. [DOI] [PubMed] [Google Scholar]
- 6.Nicolaides KH, Campbell S, Bradley RJ, Bilardo CM, Soothill PW, Gibb D. Maternal oxygen therapy for intrauterine growth retardation. Lancet 1987;1(8539):942–945. [DOI] [PubMed] [Google Scholar]
- 7.Battaglia C, Artini PG, D’Ambrogio G, Galli PA, Segre A, Genazzani AR. Maternal hyperoxygenation in the treatment of intrauterine growth retardation. Am J Obstet Gynecol 1992;167(2):430–435. [DOI] [PubMed] [Google Scholar]
- 8.Sørensen A, Peters D, Simonsen C, et al. Changes in human fetal oxygenation during maternal hyperoxia as estimated by BOLD MRI. Prenat Diagn 2013;33(2):141–145. [DOI] [PubMed] [Google Scholar]
- 9.Simchen MJ, Tesler J, Azami T, et al. Effects of maternal hyperoxia with and without normocapnia in uteroplacental and fetal Doppler studies. Ultrasound Obstet Gynecol 2005;26(5):495–499. [DOI] [PubMed] [Google Scholar]
- 10.Huen I, Morris DM, Wright C, et al. R1 and R2 * changes in the human placenta in response to maternal oxygen challenge. Magn Reson Med 2013;70(5):1427–1433. [DOI] [PubMed] [Google Scholar]
- 11.Ogawa S, Lee TM, Kay AR, Tank DW. Brain magnetic resonance imaging with contrast dependent on blood oxygenation. Proc Natl Acad Sci U S A 1990;87(24):9868–9872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lauridsen MH, Uldbjerg N, Henriksen TB, et al. Cerebral Oxygenation Measurements by Magnetic Resonance Imaging in Fetuses With and Without Heart Defects. Circ Cardiovasc Imaging 2017;10(11):e006459. [DOI] [PubMed] [Google Scholar]
- 13.Szwast A, Tian Z, McCann M, Donaghue D, Rychik J. Vasoreactive response to maternal hyperoxygenation in the fetus with hypoplastic left heart syndrome. Circ Cardiovasc Imaging 2010;3(2):172–178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Donofrio MT, Moon-Grady AJ, Hornberger LK, et al. Diagnosis and treatment of fetal cardiac disease: a scientific statement from the American Heart Association. Circulation 2014;129(21):2183–2242 [Published correction appears in Circulation 2014;129(21):e512.] 10.1161/01.cir.0000437597.44550.5d. [DOI] [PubMed] [Google Scholar]
- 15.You W, Evangelou IE, Zun Z, Andescavage N, Limperopoulos C. Robust preprocessing for stimulus-based functional MRI of the moving fetus. J Med Imaging (Bellingham) 2016;3(2):026001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.You W, Serag A, Evangelou IE, Andescavage N, Limperopoulos C. Robust motion correction and outlier rejection of in vivo functional MR images of the fetal brain and placenta during maternal hyperoxia. In: Gimi B, Molthen RC, eds. Proceedings of SPIE: medical imaging 2015—biomedical applications in molecular, structural, and functional imaging. Vol 9417. Bellingham, Wash: International Society for Optics and Photonics, 2015; 94170O. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tustison NJ, Avants BB, Cook PA, et al. N4ITK: improved N3 bias correction. IEEE Trans Med Imaging 2010;29(6):1310–1320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yushkevich PA, Piven J, Hazlett HC, et al. User-guided 3D active contour segmentation of anatomical structures: significantly improved efficiency and reliability. Neuroimage 2006;31(3):1116–1128. [DOI] [PubMed] [Google Scholar]
- 19.Turk EA, Luo J, Gagoski B, et al. Spatiotemporal alignment of in utero BOLD-MRI series. J Magn Reson Imaging 2017;46(2):403–412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Avants BB, Tustison NJ, Song G, Cook PA, Klein A, Gee JC. A reproducible evaluation of ANTs similarity metric performance in brain image registration. Neuroimage 2011;54(3):2033–2044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rueckert D, Sonoda LII, Hayes C, Hill DLGL, Leach MOO, Hawkes DJJ. Nonrigid registration using free-form deformations: application to breast MR images. IEEE Trans Med Imaging 1999;18(8):712–721. [DOI] [PubMed] [Google Scholar]
- 22.Zhou J, van Zijl PCM. Perfusion imaging using FAIR with a short predelay. Magn Reson Med 1999;41(6):1099–1107. [DOI] [PubMed] [Google Scholar]
- 23.Woodruff RS. A Simple Method for Approximating the Variance of a Complicated Estimate. J Am Stat Assoc 1971;66(334):411–414. [Google Scholar]
- 24.Jaeger BC, Edwards LJ, Das K, Sen PK. An R2 statistic for fixed effects in the generalized linear mixed model. J Appl Stat 2017;44(6):1086–1105. [Google Scholar]
- 25.Sørensen A, Peters D, Fründ E, Lingman G, Christiansen O, Uldbjerg N. Changes in human placental oxygenation during maternal hyperoxia estimated by blood oxygen level-dependent magnetic resonance imaging (BOLD MRI). Ultrasound Obstet Gynecol 2013;42(3):310–314. [DOI] [PubMed] [Google Scholar]
- 26.Ingram E, Morris D, Naish J, Myers J, Johnstone E. MR Imaging Measurements of Altered Placental Oxygenation in Pregnancies Complicated by Fetal Growth Restriction. Radiology 2017;285(3):953–960. [DOI] [PubMed] [Google Scholar]
- 27.Rychik J, Goff D, McKay E, et al. Characterization of the Placenta in the Newborn with Congenital Heart Disease: Distinctions Based on Type of Cardiac Malformation. Pediatr Cardiol 2018;39(6):1165–1171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wallenstein MB, Harper LM, Odibo AO, et al. Fetal congenital heart disease and intrauterine growth restriction: a retrospective cohort study. J Matern Fetal Neonatal Med 2012;25(6):662–665. [DOI] [PubMed] [Google Scholar]
- 29.Sinding M, Sørensen A, Peters D, et al. OP22.09: Placental T2 * in normal pregnancy and in two cases of fetal growth restriction. Ultrasound Obstet Gynecol 2014;44(S1):165–166. [Google Scholar]
- 30.Burton GJ, Jauniaux E. Development of the human placenta and fetal heart: Synergic or independent? Front Physiol 2018;9:373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Huen I, Morris DM, Wright C, Sibley CP, Naish JH, Johnstone ED. Absence of PO2 change in fetal brain despite PO2 increase in placenta in response to maternal oxygen challenge. BJOG 2014;121(13):1588–1594. [DOI] [PubMed] [Google Scholar]
- 32.Aldrich CJ, Wyatt JS, Spencer JAD, Reynolds EOR, Delpy DT. The effect of maternal oxygen administration on human fetal cerebral oxygenation measured during labour by near infrared spectroscopy. Br J Obstet Gynaecol 1994;101(6):509–513. [DOI] [PubMed] [Google Scholar]
- 33.Tomimatsu T, Peña JP, Longo LD. Fetal cerebral oxygenation: the role of maternal hyperoxia with supplemental CO2 in sheep. Am J Obstet Gynecol 2007;196(4):359.e1–359.e5. [DOI] [PubMed] [Google Scholar]
- 34.Sørensen A, Pedersen M, Tietze A, Ottosen L, Duus L, Uldbjerg N. BOLD MRI in sheep fetuses: a non-invasive method for measuring changes in tissue oxygenation. Ultrasound Obstet Gynecol 2009;34(6):687–692. [DOI] [PubMed] [Google Scholar]
- 35.Luo J, Abaci Turk E, Bibbo C, et al. In Vivo Quantification of Placental Insufficiency by BOLD MRI: A Human Study. Sci Rep 2017;7(1):3713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Baschat AA. Fetal responses to placental insufficiency: an update. BJOG 2004;111(10):1031–1041. [DOI] [PubMed] [Google Scholar]
- 37.Rhee CJ, Fraser CD, 3rd, Kibler K, et al. The ontogeny of cerebrovascular pressure autoregulation in premature infants. J Perinatol 2014;34(12):926–931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tomimatsu T, Pereyra Peňa J, Hatran DP, Longo LD. Maternal oxygen administration and fetal cerebral oxygenation: studies on near-term fetal lambs at both low and high altitude. Am J Obstet Gynecol 2006;195(2):535–541. [DOI] [PubMed] [Google Scholar]
- 39.Edwards LA, Lara DA, Sanz Cortes M, et al. Chronic Maternal Hyperoxygenation and Effect on Cerebral and Placental Vasoregulation and Neurodevelopment in Fetuses with Left Heart Hypoplasia. Fetal Diagn Ther 2019;46(1):45–57. [DOI] [PubMed] [Google Scholar]
- 40.Say L, Gülmezoglu AM, Hofmeyr GJ. Maternal oxygen administration for suspected impaired fetal growth. Cochrane Database Syst Rev 2003;(1):CD000137. [DOI] [PubMed] [Google Scholar]
- 41.Porayette P, Madathil S, Sun L, et al. MRI reveals hemodynamic changes with acute maternal hyperoxygenation in human fetuses with and without congenital heart disease. Prenat Diagn 2016;36(3):274–281. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.