Abstract
PURPOSE
Escalated BEACOPP (bleomycin, etoposide, doxorubicin, cyclophosphamide, vincristine, procarbazine, and prednisone) improves overall survival (OS) in patients with Hodgkin lymphoma (HL) relative to ABVD (doxorubicin, bleomycin, vinblastine, and dacarbazine) therapy. However, the associated higher cost and toxicity discourage clinicians from prescribing it. Identifying high-risk patients and administering escalated BEACOPP remains an effective strategy. We assessed the significance of interim positron emission tomography (iPET) scan after 2 cycles (iPET2) in identifying this high-risk subset.
PATIENTS AND METHODS
This cohort study used secondary data from 12 tertiary care centers in South India gathered over 10 years (2008-2018). OS, event-free survival (EFS), determinants of EFS, and complete response (CR) in iPET2 were assessed.
RESULTS
The study included 409 patients with HL (mean age, 34.5 years; male/female ratio, 1.4:1). The median duration of follow-up was 2.8 years. Of 409 patients, 63% underwent PET-based staging and 37% underwent computerized tomography (CT) staging. Stage IV (28.9%) and bone involvement (9.2%) were seen more often with PET than with CT staging (9.2% and 2%, respectively). Among 171 patients with iPET2 results, 24% did not achieve CR, and no factors were significantly associated. The 5-year EFS and OS rates of the entire cohort were 78% and 97%, respectively. The 5-year EFS and OS rates of patients with CR on iPET2 were 90% and 99%, respectively, whereas these were 65% and 100%, respectively, for patients not achieving CR. On univariable analysis, sex, stage, and iPET2 response significantly predicted inferior EFS. On multivariate analysis, only iPET2 response significantly predicted EFS (P < .000).
CONCLUSION
Our study supports the use of PET for staging and iPET2 for response assessment. Nonachievement of CR on iPET2 indicates unfavorable outcome, and such patients may benefit from more intensive treatment.
INTRODUCTION
Hodgkin lymphoma (HL) accounts for approximately 10% of all lymphomas, with excellent long-term cure rates.1 The ABVD (doxorubicin, bleomycin, vinblastine, and dacarbazine) combination yields a complete response (CR) rate of 75% and an overall survival (OS) rate of 73% and has been considered a standard regimen for several decades.2,3 However, ABVD fails to result in remission in 20% to 30% of patients with HL, who then require salvage chemotherapy and transplantation. Intensive regimens like dose-escalated BEACOPP (bleomycin, etoposide, doxorubicin, cyclophosphamide, vincristine, procarbazine, and prednisone; [EB]) have shown better cure rates versus ABVD but are rarely used because of increased treatment-related mortality and morbidity.4-6 Therefore, it is important to identify patients with high risk of unfavorable outcome and treat them with intense regimens such as EB is important.
International Prognostic Score (IPS7) and modified scoring system (IPS3) are used for prognostication, but they do not demarcate risk groups sufficiently to justify deviation from standard ABVD therapy.7-10 More recently, an interim positron emission tomography (iPET)/computed tomography (CT) scan performed after 2 cycles of ABVD therapy (iPET2) was reported to be a better prognosticator than IPS.11,12
CONTEXT
Key Objective
Although dose-escalated BEACOPP (bleomycin, etoposide, doxorubicin, cyclophosphamide, vincristine, procarbazine, and prednisone; EB) improves outcome in Hodgkin lymphoma (HL), it is not widely used because of cost and toxicity. Identifying high-risk patients and treating them with EB should be the norm. We evaluated the efficacy of positron emission tomography (PET) scanning in identifying this high-risk subset in a real-world setting.
Knowledge Generated
Four of every 6 patients were staged with PET, and we noticed more stage IV disease and skeletal involvement with PET than with computed tomography–based staging. The 5-year event-free and overall survival rates of the entire cohort were 78% and 97%, respectively. Patients with positive interim PET after 2 cycles of first-line therapy (iPET2) had a 5-time increased risk of unfavorable outcome than iPET2-negative patients. International Prognostic Score–based risk showed no correlation with survival.
Relevance
PET for patients with HL for both staging and response assessment at 2 months is recommended. Not achieving complete remission on iPET2 indicates poor prognosis, and such patients may benefit from treatment intensification.
In India, younger age, male preponderance, and increased frequency of mixed cellularity are the major features of the disease.13-21 This study was conducted to provide real-world evidence of the role of iPET2 in the management of HL and analyze various factors predicting its outcome.
PATIENTS AND METHODS
Study Design
This was a cohort study involving secondary data.
Setting
Study sites.
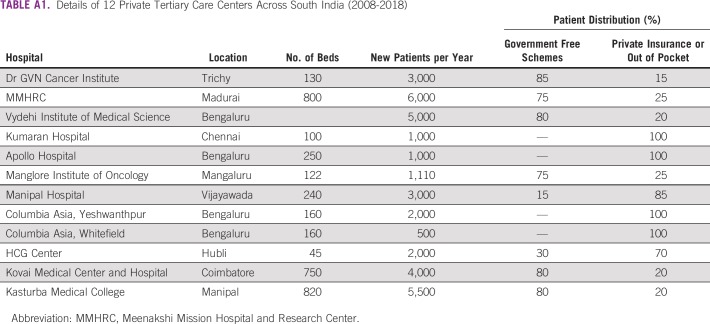
The study was conducted in 12 private tertiary care centers across 3 states in South India (Tamil Nadu, Andhra Pradesh, and Karnataka; Appendix Table A1). Patients either paid out of pocket or were covered under the state-funded or private health insurance schemes.
Routine diagnosis and management of HL.
All patients required histopathologic diagnosis with excisional nodal, core needle, or bone marrow biopsy. Morphologic evaluation and classification of the patients were performed using the revised European-American lymphoma classification.22
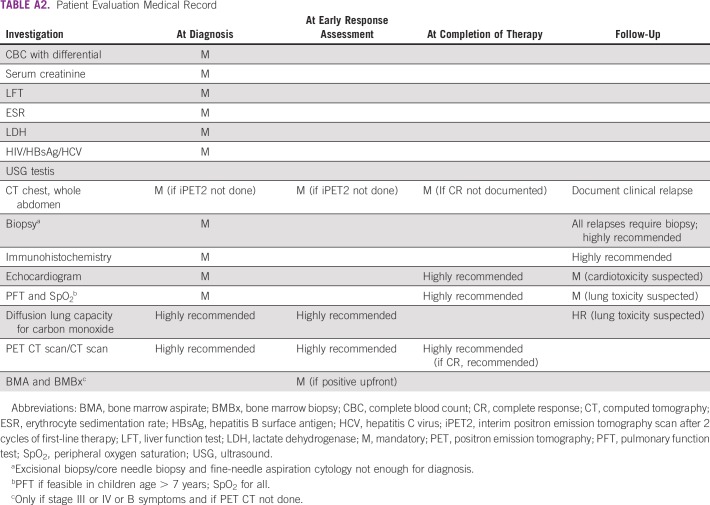
The workup included documentation of presenting complaints, including B symptoms (unexplained fever, > 10% weight loss, and/or drenching night sweats), physical examination, and investigation reports (Appendix; Appendix Table A2). Stage was assigned based on the Ann Arbor staging system with Cotswolds modifications and determined using clinical examination and CT and/or PET scan.23 Early-stage prognostic grouping included stages I, IIA, IX, and IIX, and advanced-stage grouping included stages IB, IIB, III, and IV. Treatment strategy was in accordance with National Comprehensive Cancer Network guidelines.24
All patients received chemotherapy in a daycare facility as outpatients. ABVD was delivered as per the original schedule,2 EB according to the HD-9 study,25 and BEACOPP-14 as per the RATHL study.26 Interim response assessment was performed using PET or CT imaging after 2 or 4 cycles based on clinician opinion and institutional policy. iPET2 was performed 2 to 3 days before the third cycle of chemotherapy. Repeat PET or CT imaging was performed at the end of treatment. PET images were interpreted by an experienced nuclear medicine physician at the particular center, and no second review or central review was performed in our study. CT and PET scan reporting was based on the Lugano recommendation for response assessment, and PET images were scored according to the 5-point Deauville score.11,12
CR based on PET imaging was defined as a Deauville score of 1, 2, and 3 on interim scans and a score of 1 or 2 at the end-of-treatment scan. On the basis of CT scan findings, CR was defined as complete disappearance of all clinical and radiologic evidence of disease; partial response (PR) was defined as a 50% reduction in tumor area (the product of the 2 longest diameters) but less than that in a CR. Appearance of a new lesion or a 50% increase in an existing lesion was considered progressive disease (PD). All other responses were considered stable disease.11,12 Patients who did not achieve CR after 2 cycles of ABVD, had bulky disease at presentation, had early-stage disease, and had residual disease at the end of treatment were considered for consolidation radiotherapy (RT). After completing treatment, patients were observed as per the institution policy.
HL electronic database.
A list of all newly registered patients with HL was prepared from patient case records, outpatient department files, and investigation reports. This was captured in an electronic database using online Google forms in all 12 tertiary care centers. A check for missing data was conducted every month, and the database was updated after referring to the paper-based records.
Study Population
The study population included all patients diagnosed with and initiating treatment for HL between January 1, 2008, and October 31, 2018. They were observed on record until December 31, 2018 (date of censoring). Patients with nodular lymphocyte–predominant HL were excluded from the study.
Data Variables and Sources
Variables extracted from the electronic database included name of treating center, patient ID, age, stage, sex, B symptoms, site of lymphadenopathy, albumin level, erythrocyte sedimentation rate, IPS, histology, extranodal sites, WBC count, absolute lymphocyte count, mediastinal involvement, interim and end-of-treatment response, treatment modifications with iPET2, and outcome (alive and in remission, relapse, death, or lost to follow-up). Date of diagnosis, treatment initiation of ABVD, iPET, and outcome or censoring (whichever was earlier) were also collected.
An event (unfavorable outcome) was defined as relapse after previous CR, progression after documenting PR or PD on CT scan assessments during treatment, or death resulting from any cause or treatment failure, whichever occurred first. Not achieving CR on the last date of follow-up was considered treatment failure. If a patient had a relapse followed by loss to follow-up or death, the outcome was documented as relapse. Loss to follow-up was defined as missing 2 scheduled visits to the center and not responding to telephonic reminders. The date of last visit to the center was considered the date of loss to follow-up. Patients who were lost to follow-up were censored and not considered for analysis after the date of loss to follow-up. The primary end point was event-free survival (EFS), which was calculated from the date of diagnosis to the date of occurrence of the event. The secondary end point was OS, defined as time from diagnosis until death resulting from any cause or censored at the date of last information of the patient being alive.
Data Analysis
Data were analyzed using STATA (version 12.1; STATA, College Station, TX). Incidence rates of events per 100 person-years of follow-up were calculated. The 2- and 5-year EFS and 2- and 5-year OS rates were calculated. Crude and adjusted hazard ratios were calculated using Cox proportional hazards regression to determine the risk factors for events. Among those undergoing iPET2, factors associated with not attaining CR were assessed using log binomial regression.
The regression models included age, sex, and variables with a crude P value < .2 (stage, iPET2 response, B symptoms). Bone, spleen, extralymphatic involvement, and site of lymphadenopathy were used to determine stage. Therefore, we considered stage over these variables in the regression model. Prognostic grouping was based on stage and B symptoms, which were therefore included instead of prognostic grouping.27
Ethics
Ethics approval was obtained from the Dr GVN Ethics Committee of the Dr GVN Cancer Institute (Tiruchirappalli, India; dated July 30, 2018) and the Ethics Advisory Group (EAG) of the International Union Against Tuberculosis and Lung Disease (Paris, France; EAG No. 29/18; dated June 11, 2018). Because the study involved review of patient records (secondary data), a waiver for informed consent was sought and approved by the ethics committees. Administrative approval was obtained from collaborative institutions before study initiation.
RESULTS
Baseline Characteristics
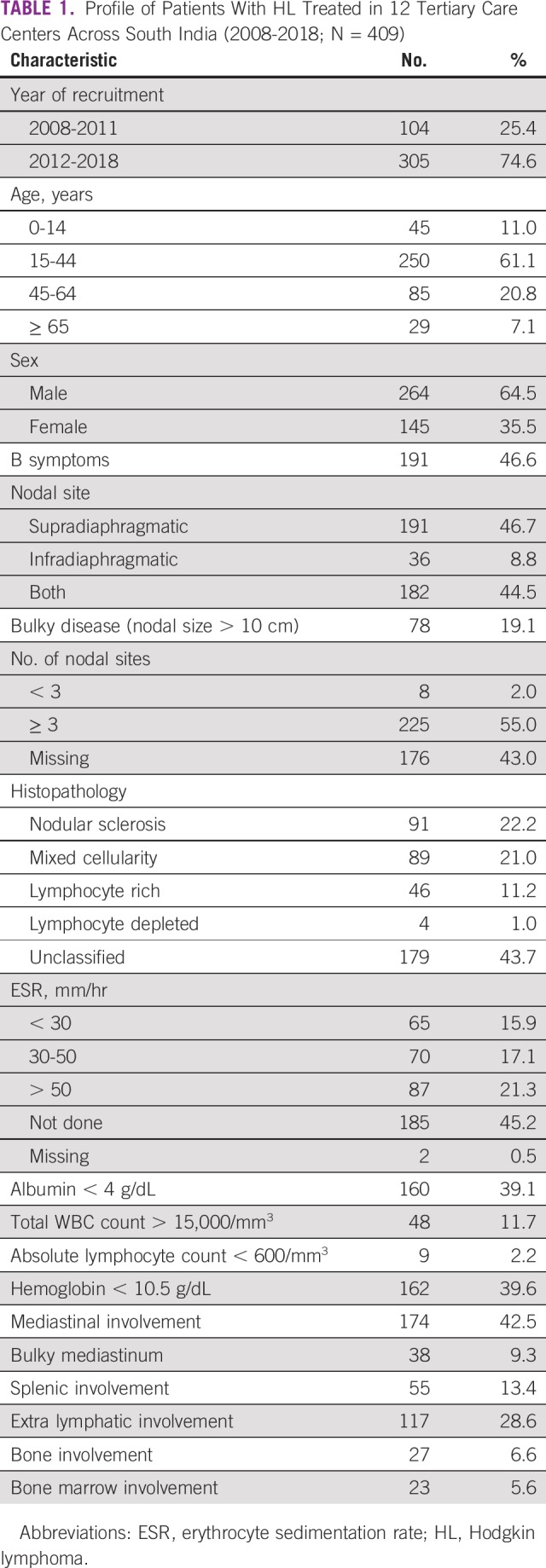
The baseline characteristics and staging profiles of 409 patients are summarized in Table 1. The mean age was 34.5 years (standard deviation, 17.8 years), and the male/female ratio was 1.4:1. B symptoms were seen in 198 (48.4%) and bulky disease in 78 patients (19.1%). The most common histopathologic subtype was nodular sclerosis (n = 91; 22.2%). Of 409 patients with HL, 102 (24.9%) received treatment under a state government–supported health insurance scheme, and the rest (75.1%) paid either out of pocket or through private health insurance schemes.
TABLE 1.
Profile of Patients With HL Treated in 12 Tertiary Care Centers Across South India (2008-2018; N = 409)
Prognostic Grouping and Treatment Profile
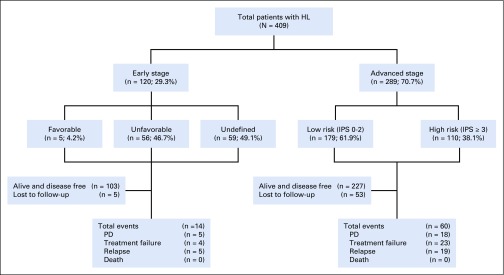
Stage, prognostic grouping, risk stratification, and treatment profile details are summarized in Tables 2 and 3 and Figure 1. Of 409 patients with HL, 259 (63.3%) underwent PET-based and 150 (36.7%) underwent CT-based initial staging evaluation. Stage IV (28.9%) and bone involvement (9.2%) were more often seen with PET-based staging than with CT-based staging (9.2% and 2%, respectively; data not shown). Per prognostic grouping, 120 patients (29.3%) had early-stage and 289 (70.6%) had advanced-stage disease.
TABLE 2.
Staging, Prognostic Grouping, and Risk Stratification of Patients With HL Treated in 12 Tertiary Care Centers Across South India (2008-2018; N = 409)
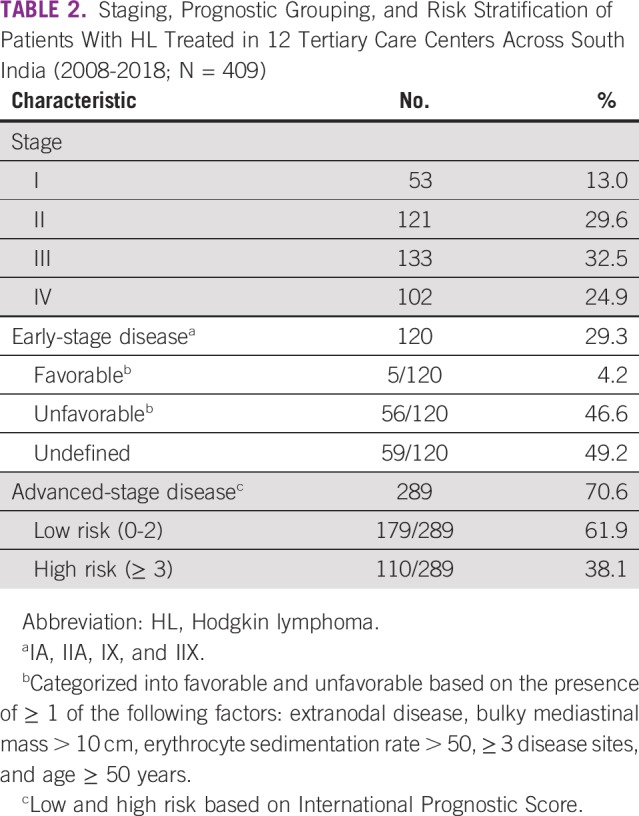
TABLE 3.
Treatment Profile of Patients With HL Treated in 12 Tertiary Care Centers Across South India (2008-2018)
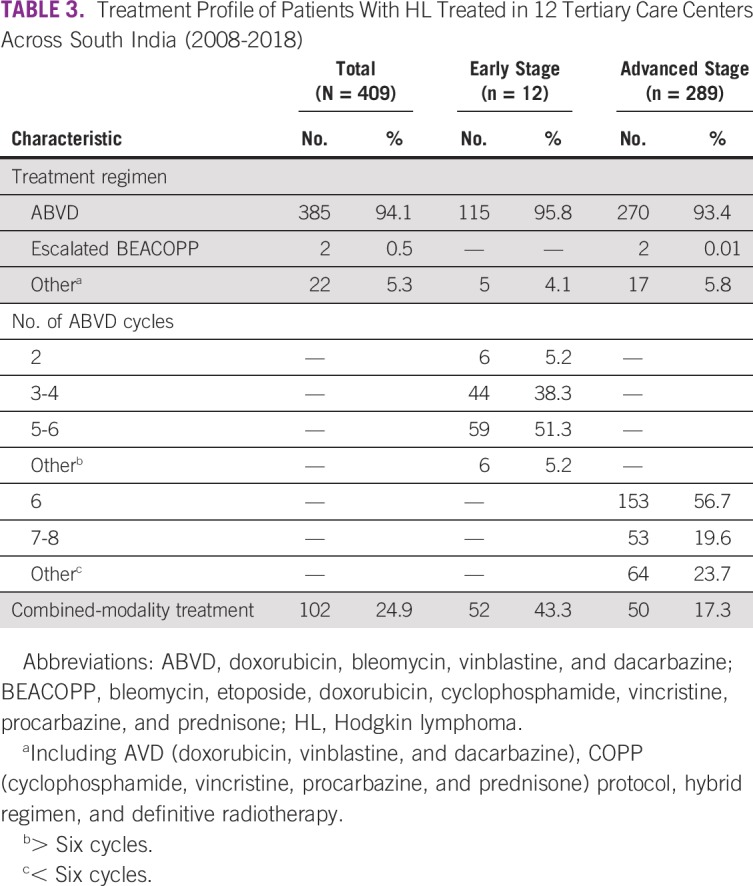
FIG 1.
Flowchart depicting the outcome of all patients with Hodgkin lymphoma (HL) treated in 12 tertiary care centers across South India (2008-2018; N = 409). IPS, International Prognostic Score; PD, progressive disease.
A median of 6 cycles of chemotherapy was administered to patients with both early- (range, 2-8 cycles) and advanced-stage disease (range, 4-8 cycles). Combined-modality treatment was administered to 52 patients (43.3%) with early-stage disease and 50 (17.3%) with advanced-stage disease. Of the 52 patients with early-stage disease, 5 received 20 Gy of involved-site RT (ISRT) along with 2 ABVD cycles, and the remaining patients received 30 Gy of ISRT as a partial combined-modality treatment. The patients with advanced HL received 30 to 36 Gy of ISRT; the indications were bulky disease (n = 28), iPET2-positive sites (slow responders; n = 5), residual disease at the end of treatment (n = 14), and consolidation treatment of an extranodal site (n = 3).
Response Assessment
The results of interim and end-of-treatment response assessments after the end of first-line therapy are summarized in Table 4. Interim response assessment was performed for 408 patients: 280 were PET based and 128 were CT based. Among patients with an interim PET scan, 171 underwent their scans after 2 cycles and 109 after 4 cycles. One patient missed the interim assessment and directly underwent the end-of-treatment assessment. CR rates at interim and end-of-treatment assessments were 72.9% and 75.3% with PET and 39% and 73.5% with CT imaging, respectively. On follow-up of patients achieving CR with interim PET scan (n = 204), 186 (91.1%) were alive and disease free, 12 (5.8%) had a relapse, 2 had PD, and 4 were receiving treatment. Among patients achieving CR on interim CT scan (n = 50), 44 (90%) were alive and disease free, 5 (10%) had a relapse, and 1 was receiving treatment. Of the 93 patients not achieving CR (based on either PET or CT) at the end of first-line therapy, 66 achieved CR at a later date with further salvage therapy.
TABLE 4.
Response Assessment of All Patients With HL Treated in 12 Tertiary Care Centers Across South India (2008-2018)
iPET2 Response Assessment and Treatment Modification
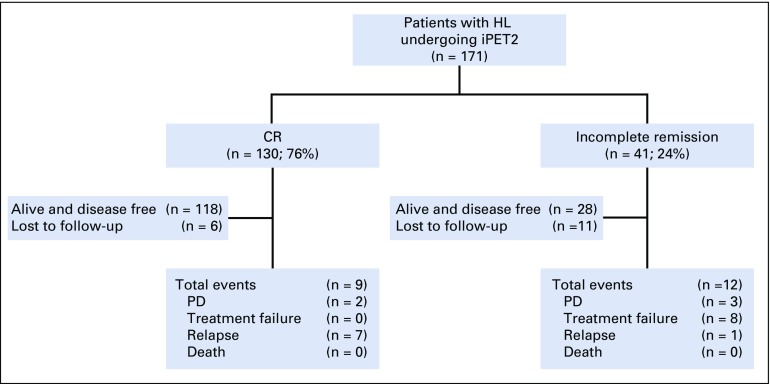
Among the 409 patients, 171 (41.8%) underwent iPET2. Figure 2 shows the outcome of all patients who underwent the iPET2 scan. Of these 171 patients, 130 (76%) achieved CR and 41 (24%) did not. None of the baseline factors significantly predicted iPET2 response (data not shown). On follow-up of patients achieving CR (n = 130) on iPET2, 118 (90.7%) were alive and disease free, 7 (5.3%) had a relapse, 2 had PD, and 3 were receiving treatment. Among patients not achieving CR (n = 41), 28 (68.2%) were alive and disease free, 8 (19.5%) experienced treatment failure, 3 (7.3%) had PD, 1 had a relapse, and 1 was receiving treatment.
FIG 2.
Flowchart depicting the outcome of patients with Hodgkin lymphoma (HL) undergoing interim positron emission tomography scan after 2 cycles of first-line therapy (iPET2) treated in 12 tertiary care centers across South India (2008-2018; n = 171). CR, complete response; PD, progressive disease.
Among patients not achieving CR (n = 41), dose escalation was administered to 4 patients, of whom 3 received BEACOPP-14 and 1 received EB. Of these 4 patients, 3 achieved CR and 1 had PR status at the end of treatment; on the last follow-up, 2 continued to remain in CR, 1 experienced a relapse, and 1 had PD. Of 130 patients with CR on iPET2, dose de-escalation to AVD was performed for 8 patients (6.1%), of whom 5 achieved CR, 1 experienced a relapse, and 2 were receiving treatment.
Toxicity
The most common acute toxicity was grade IV febrile neutropenia, seen in 22 patients (5.7%) receiving ABVD, 3 patients receiving EB (100%), 3 receiving BEACOPP-14 (100%), and 1 receiving COPP (100%). Bleomycin toxicity was seen in 21 patients (5.1%), of whom 19 required hospitalization and 1 died. Four patients with HL developed doxorubicin-related cardiotoxicity with no documented death. Dose modification because of treatment toxicity was required in 28 patients (7.2%) receiving ABVD, 3 receiving EB (100%), 1 receiving BEACOPP-14 (33.3%), and 1 receiving COPP (100%).
Survival
Of the total 1,154 person-years of follow-up, 74 events were documented, translating to an incidence rate of 6.4 (95% CI, 5.1 to 8.1) per 100 person-years of follow-up. Of the 74 events, 27 were treatment failure, 24 were relapse, and 23 were PD. Seven deaths were documented, of which 4 resulted from PD, 2 from relapse, and 1 from relapse with bleomycin toxicity.
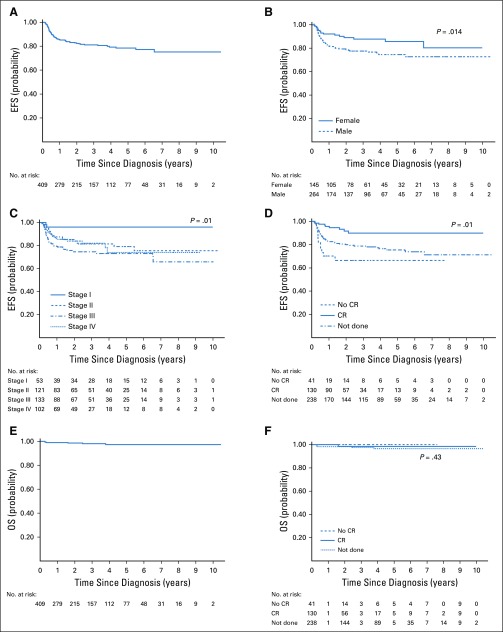
The median duration of follow-up was 2.8 years (interquartile range, 0.75-4.25 years), and the 2- and 5-year EFS rates for the entire cohort were 82% (95% CI, 78% to 86%) and 78% (95% CI, 73% to 83%), respectively. The 2- and 5-year OS rates for the entire cohort were 98% (95% CI, 96% to 99%) and 97% (95% CI, 94% to 99%), respectively. Both 2- and 5-year EFS rates of patients with CR on iPET2 were 90% (95% CI, 84% to 95%), whereas these were 65% (95% CI, 46% to 79%) for patients not achieving CR. The OS rate of patients with CR on iPET2 was 99% (95% CI, 91% to 100%) at 2 and 5 years, and no death was reported among patients not achieving CR.
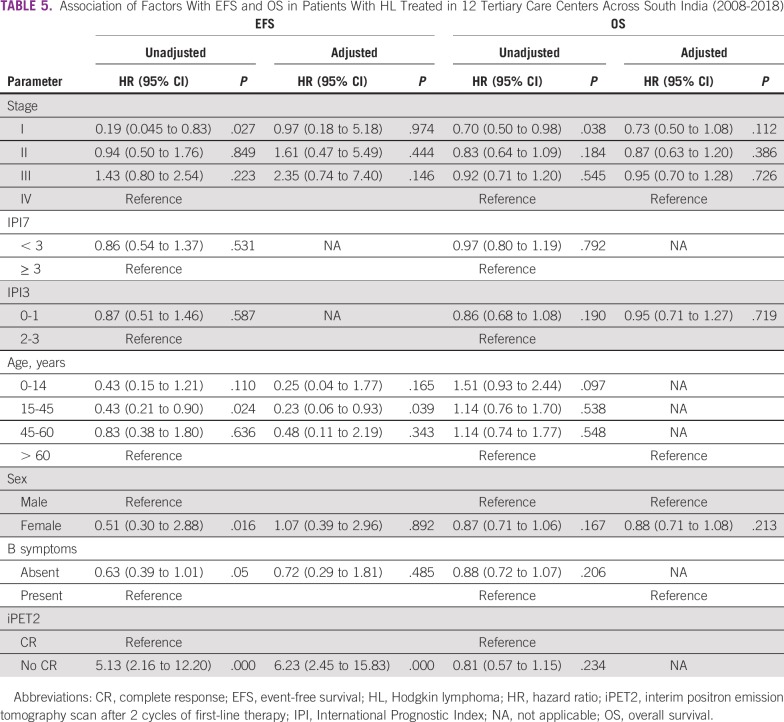
Table 5 summarizes the unadjusted and adjusted associations of factors with EFS and OS. The survival curves stratified by the associated factors are shown in Figure 3. On univariable analysis, sex, stage, and iPET2 response significantly predicted inferior EFS. On multivariable analysis, only response on iPET2 scan significantly predicted EFS (P < .000).
TABLE 5.
Association of Factors With EFS and OS in Patients With HL Treated in 12 Tertiary Care Centers Across South India (2008-2018)
FIG 3.
(A-D) Event-free survival (EFS) and (E-F) overall survival (OS) of patients with Hodgkin lymphoma treated in 12 tertiary care centers across South India (2008-2018), (A, E) overall and (B-D, F) stratified by factors significantly predicting survival (N = 409): (B) sex, (C) stage, and (D, E) interim positron emission tomography scan after 2 cycles of first-line therapy (iPET2). CR, complete response.
DISCUSSION
This study is the largest multicenter study to our knowledge from India that provides insights into the demographic profile, management, and outcome of patients with HL and the role of iPET2 as a predictor of EFS. A high proportion of patients had advanced-stage disease at presentation, and approximately three-fourths had EFS for 5 years after diagnosis. The iPET2 scan results were a strong predictor of survival. One key limitation was that only two-fifths of the patients underwent iPET2 scans.
Prior studies from India presented similar results with regard to age distribution, male/female ratio, and proportion of patients with advanced-stage disease on presentation.13-21 The possible reasons for advanced-stage presentations are delay in diagnosis, which can be explained by late referral from primary care physicians, high incidence of tuberculosis leading to misdiagnosis, lack of financial assistance, and poor access to health care facilities. The high proportion of men may be because of women not seeking appropriate treatment.
By histopathologic subtype, both nodular sclerosis and mixed cellularity had nearly equal distribution, with no clear dominant pattern. Most studies from developed nations indicate nodular sclerosis is the most common subtype.3,27 In India, some studies showed mixed cellularity as the most common HL subtype, whereas others reported nodular sclerosis.28-31
A combined-modality approach was used in 43% patients with early-stage and 17% with advanced-stage HL. Despite recent studies showing superior efficacy of the combined-modality approach over chemotherapy alone in early-stage HL, 67% of our early-stage patients did not receive RT.32-35 This may be because of concern among practitioners regarding the long-term effects of RT. Omission of RT did not affect outcome in our study, because most of our patients with early-stage disease received 6 ABVD cycles instead of the standard 4 cycles. Similar findings on additional chemotherapy cycles negating the benefit of RT have been reported.36,37
Four of every 6 patients were evaluated with PET, and we observed a higher incidence of stage IV disease and skeletal involvement in PET-based staging than in CT-based staging. Similar findings have been reported in other studies, where incorporation of PET resulted in stage changes in approximately 20% of patients, and half of these patients may require aggressive management.38-40
Two-fifths of patients underwent iPET2, of whom 24% did not achieve CR. Similar results with iPET2 scans have been reported in the literature, and an Indian study of patients with advanced HL reported 16% not achieving CR with iPET2.37,40,41 In our study, the reasons for not intensifying treatment in most patients despite not achieving CR on iPET2 could have been high cost, clinician choice, lack of supporting health care facilities, noncoverage under state insurance schemes, and patient preference. Similarly, de-escalation after CR on iPET2 was seen in few patients. Because of the lack of evidence supporting de-escalation, most clinicians preferred removing bleomycin only for patients at high risk of bleomycin toxicity, and all patients treated before 2016 had received bleomycin irrespective of iPET2 results.26 In our study, the CR rate at the end of treatment was 75.3%, 5-year EFS rate was 78%, and 5-year OS rate was 97%. The better EFS rate compared with CR rate at the end of treatment can be explained by the practice of continuing first-line treatment with salvage chemotherapy followed by autologous transplantation in patients not achieving CR at the end of 6 ABVD cycles. Our 5-year EFS rate is comparable to those in other studies.42-45 Male sex, stage III disease, and iPET2 positivity predicted poor EFS. IPS-based risk classification (IPS7 and IPS3) into high- and low-risk groups did not show a significant difference in survival. Given similar results from recent studies, the significance of IPS as a predictor of outcome and its ability to risk stratify HL in the current timeframe is questionable.7,8
According to our results, we recommend performing PET for all patients with HL for both staging and response assessment at 2 months. We recommend using PET results after 2 cycles as a predictor of survival. Not achieving CR on iPET2 indicates poor prognosis, and such patients may benefit from more intensive treatment programs. Similarly, de-escalation could be considered in case of CR. The real-world effectiveness of this strategy needs to be assessed in future studies, especially in developing countries like India, where the cost and toxicity of EB keep clinicians from using it as first-line therapy.
ACKNOWLEDGMENT
This research was conducted through the Structured Operational Research and Training Initiative (SORT IT), a global partnership led by the Special Program for Research and Training in Tropical Diseases at the World Health Organization (WHO/TDR). The training model is based on a course developed jointly by the International Union Against Tuberculosis and Lung Disease (The Union) and Medécins sans Frontières (MSF/Doctors Without Borders). The specific SORT IT program which resulted in this publication was jointly developed and implemented by: Fenivi Research Solutions Private Limited, Chennai, India; The Union South-East Asia Office, New Delhi, India; and the Center for Operational Research, The Union, Paris, France. Mentorship and the coordination/facilitation of this particular SORT IT program was provided through Fenivi Research Solutions Private Limited, Chennai, India; The Union South-East Asia Office, New Delhi, India; the Center for Operational Research, The Union, Paris, France; Jawaharlal Institute of Postgraduate Medical Education and Research (JIPMER), Puducherry, India; All India Institute of Medical Sciences (AIIMS), Nagpur, India and Velammal Medical College Hospital and Research Institute, Madurai, India. The specific SORT IT program which resulted in this publication was based on the data shared by the members of Collaborative Medical Oncology Group (CMOG), India. J. Joy, H.M. Malini, and K. Jenisha provided support in data extraction/analysis.
APPENDIX
Details of clinical workup on diagnosis of Hodgkin lymphoma:
1. Physical examination should be careful and complete
a. Each involved site and lymph node regions are measured and noted
b. The size of liver/spleen in centimeters below costal margin
c. Baseline pubertal status
2. Mandatory laboratory investigations
a. Complete blood counts and differential leukocyte counts
b. Lactate dehydrogenase, liver function tests, and serum creatinine
c. Computed tomography (CT) chest and whole abdomen are mandatory
d. Adequate bilateral bone marrow biopsy should be performed in patients with stage III or IV disease or B symptoms and if upfront positron emission tomography (PET)/CT is not done
e. HIV, hepatitis B surface antigen, and hepatitis C virus status should be determined
Optional investigations:
1. Upfront PET/CT scan is highly recommended
2. Bone scan: indicated in case of bone pain and elevated alkaline phosphatase; not needed if PET scan has been done
3. Any node with longest transverse diameter > 1.5 cm at the time of diagnosis should be considered compatible with lymphomatous involvement in the absence of a compelling alternative etiology, such as infection. This includes supraclavicular, infraclavicular, epitrochlear, brachial, preauricular, and popliteal nodes. Cervical, axillary, inguinal, and mesenteric lymph nodes may reach a diameter of 2 cm before being considered involved with lymphoma if reactive hyperplasia is considered possible. If there is doubt about a particular site of disease noted by CT or physical examination, and PET scan is feasible for the patient, PET avidity can be used to determine whether a site is involved. If involvement of a site will change the patient’s risk group or significantly alter the radiation therapy field, biopsy should be considered to document or exclude involvement.
4. Any focal mass lesion of a visceral organ (eg, liver, spleen, kidney) is considered lymphomatous in the absence of reasonable alternative explanation (eg, cyst, hemangioma, abscess), unless too small to characterize. Lesions too small to characterize are indeterminate unless follow-up studies allow characterization or tissue sampling is performed. Hepatosplenomegaly is not considered involvement of the organs with lymphoma but can instead result from cytokine production.
5. A measurable lesion by CT is a lesion that can be accurately measured in 2 orthogonal dimensions. Nonmeasurable assessable lesions include permeative bone lesions, malignant ascites, malignant effusions, lymphangitic spread, and lesions too small to accurately measure in 2 dimensions by CT. Measurable lesions up to a maximum of 6 lesions in total, representative of all involved organs, will be measured as target lesions at baseline and observed for response. Target lesions will be selected on the basis of size (eg, largest lesions) and suitability for accurate repeated measurements by imaging or clinical examination. Size is to be recorded using metric notation. Lesion size is expressed as the product of the perpendicular diameters (PPDs) and serves as a surrogate measurement of area with dimensions of square centimeters. The PPD is obtained by multiplying the longest diameter of the lesion by the maximal diameter perpendicular to the longest diameter. The sum of the PPDs is obtained by adding the PPDs of all measurable lesions.
6. All nonmeasurable assessable lesions should be recorded and noted at follow-up.
TABLE A1.
Details of 12 Private Tertiary Care Centers Across South India (2008-2018)
TABLE A2.
Patient Evaluation Medical Record
Footnotes
The content of this article does not necessarily reflect the views of the organizations with which the authors are affiliated.
AUTHOR CONTRIBUTIONS
Conception and design: Arun Seshachalam, Shashidhar V. Karpurmath, Krishnakumar Rathnam, Krishna Reddy Golamari, Basawantrao Malipatil, Hemant Deepak Shewade
Administrative support: Arun Seshachalam, Neelesh Reddy, Krishna Reddy Golamari
Provision of study material or patients: Arun Seshachalam, Shashidhar V. Karpurmath, Krishnakumar Rathnam, S.G. Raman, Krishna Prasad, Channappa Patil, Satish Kumar Anumula, Sirigeri Prabhakar Roopa, Krishna Reddy Golamari, Bharath Rangarajan, Karthik S. Udupa, Manjunath Nandennavar
Collection and assembly of data: Arun Seshachalam, Shashidhar V. Karpurmath, Krishnakumar Rathnam, S.G. Raman, M. Janarthinakani, Krishna Prasad, Channappa Patil, Parameswaran Anoop, Neelesh Reddy, Satish Kumar Anumula, Sirigeri Prabhakar Roopa, Krishna Reddy Golamari, Madhav Danthala, Prasad Gunari, Basawantrao Malipatil, Bharath Rangarajan, Karthik S. Udupa, Manjunath Nandennavar
Data analysis and interpretation: Arun Seshachalam, Shashidhar V. Karpurmath, Channappa Patil, Krishna Reddy Golamari, Madhav Danthala, Prasad Gunari, Basawantrao Malipatil, K. Niraimathi, Hemant Deepak Shewade
Manuscript writing: All authors
Final approval of manuscript: All authors
Accountable for all aspects of the work: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated unless otherwise noted. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or ascopubs.org/jgo/site/misc/authors.html.
Open Payments is a public database containing information reported by companies about payments made to US-licensed physicians (Open Payments).
Krishna Prasad
Stock and Other Ownership Interests: NATCO, Merck
Honoraria: AstraZeneca, Dr Reddy’s, Cipla, Mylan
Bharath Rangarajan
Employment: Kovai Medical Center and Hospitals
Stock and Other Ownership Interests: Heartcare Associates
Hemant Deepak Shewade
Employment: SRL Diagnostics (I)
No other potential conflicts of interest were reported.
REFERENCES
- 1.Siegel RL, Miller KD, Jemal A. Cancer Statistics, 2017. CA Cancer J Clin. 2017;67:7–30. doi: 10.3322/caac.21387. [DOI] [PubMed] [Google Scholar]
- 2.Bonadonna G, Zucali R, Monfardini S, et al. Combination chemotherapy of Hodgkin’s disease with adriamycin, bleomycin, vinblastine, and imidazole carboxamide versus MOPP. Cancer. 1975;36:252–259. doi: 10.1002/1097-0142(197507)36:1<252::aid-cncr2820360128>3.0.co;2-7. [DOI] [PubMed] [Google Scholar]
- 3.Canellos GP, Anderson JR, Propert KJ, et al. Chemotherapy of advanced Hodgkin’s disease with MOPP, ABVD, or MOPP alternating with ABVD. N Engl J Med. 1992;327:1478–1484. doi: 10.1056/NEJM199211193272102. [DOI] [PubMed] [Google Scholar]
- 4.Federico M, Luminari S, Iannitto E, et al. ABVD compared with BEACOPP compared with CEC for the initial treatment of patients with advanced Hodgkin’s lymphoma: Results from the HD2000 Gruppo Italiano per lo Studio dei Linfomi Trial. J Clin Oncol. 2009;27:805–811. doi: 10.1200/JCO.2008.17.0910. [DOI] [PubMed] [Google Scholar]
- 5.Viviani S, Zinzani PL, Rambaldi A, et al. ABVD versus BEACOPP for Hodgkin’s lymphoma when high-dose salvage is planned. N Engl J Med. 2011;365:203–212. doi: 10.1056/NEJMoa1100340. [DOI] [PubMed] [Google Scholar]
- 6.Engert A, Haverkamp H, Kobe C, et al. Reduced-intensity chemotherapy and PET-guided radiotherapy in patients with advanced stage Hodgkin’s lymphoma (HD15 trial): A randomised, open-label, phase 3 non-inferiority trial. Lancet. 2012;379:1791–1799. doi: 10.1016/S0140-6736(11)61940-5. [DOI] [PubMed] [Google Scholar]
- 7. Tartas NE, Zerga M, Santos MI, et al: International Prognostic Score (IPS) is not useful in stages I-II Hodgkin’s lymphoma (HL): An experience of the Buenos Aires Leukemia Group (BALG). Blood 108, 2006 (abstr 4659) [Google Scholar]
- 8.Moccia AA, Donaldson J, Chhanabhai M, et al. International Prognostic Score in advanced-stage Hodgkin’s lymphoma: Altered utility in the modern era. J Clin Oncol. 2012;30:3383–3388. doi: 10.1200/JCO.2011.41.0910. [DOI] [PubMed] [Google Scholar]
- 9.Diefenbach CS, Li H, Hong F, et al. Evaluation of the International Prognostic Score (IPS-7) and a Simpler Prognostic Score (IPS-3) for advanced Hodgkin lymphoma in the modern era. Br J Haematol. 2015;171:530–538. doi: 10.1111/bjh.13634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ganesan P, Dhanushkodi M, Ganesan TS, et al. Prognostic utility of the IPS 3 score for predicting outcomes in advanced Hodgkin lymphoma. Clin Lymphoma Myeloma Leuk. 2019;19:116–122. doi: 10.1016/j.clml.2018.11.009. [DOI] [PubMed] [Google Scholar]
- 11.Barrington SF, Qian W, Somer EJ, et al. Concordance between four European centres of PET reporting criteria designed for use in multicentre trials in Hodgkin lymphoma. Eur J Nucl Med Mol Imaging. 2010;37:1824–1833. doi: 10.1007/s00259-010-1490-5. [DOI] [PubMed] [Google Scholar]
- 12.Meignan M, Gallamini A, Meignan M, et al. Report on the First International Workshop on interim-PET scan in lymphoma. Leuk Lymphoma. 2009;50:1257–1260. doi: 10.1080/10428190903040048. [DOI] [PubMed] [Google Scholar]
- 13.Talvalkar GV, Sampat MB, Gangadharan P. Hodgkin’s disease in Western India: Review of 1082 cases. Cancer. 1982;50:353–359. doi: 10.1002/1097-0142(19820715)50:2<353::aid-cncr2820500232>3.0.co;2-#. [DOI] [PubMed] [Google Scholar]
- 14.Shanta V, Sastri DV, Sagar TG, et al. A review of Hodgkin’s disease at the Cancer Institute, Madras. Clin Oncol. 1982;8:5–15. [PubMed] [Google Scholar]
- 15.Sagar TG, Chandra A, Raman SG. Childhood Hodgkin disease treated with COPP/ABV hybrid chemotherapy: A progress report. Med Pediatr Oncol. 2003;40:66–69. doi: 10.1002/mpo.10017. [DOI] [PubMed] [Google Scholar]
- 16.Jain S, Kapoor G, Bajpai R. ABVD-based therapy for Hodgkin lymphoma in children and adolescents: Lessons learnt in a tertiary care oncology center in a developing country. Pediatr Blood Cancer. 2016;63:1024–1030. doi: 10.1002/pbc.25935. [DOI] [PubMed] [Google Scholar]
- 17.Trehan A, Singla S, Marwaha RK, et al. Hodgkin lymphoma in children: Experience in a tertiary care centre in India. J Pediatr Hematol Oncol. 2013;35:174–179. doi: 10.1097/MPH.0b013e318271f587. [DOI] [PubMed] [Google Scholar]
- 18.Dinand V, Arya LS. Epidemiology of childhood Hodgkins disease: Is it different in developing countries? Indian Pediatr. 2006;43:141–147. [PubMed] [Google Scholar]
- 19.Arya LS, Dinand V, Thavaraj V, et al. Hodgkin’s disease in Indian children: Outcome with chemotherapy alone. Pediatr Blood Cancer. 2006;46:26–34. doi: 10.1002/pbc.20157. [DOI] [PubMed] [Google Scholar]
- 20.Chandra J, Naithani R, Singh V, et al. Developing anticancer chemotherapy services in a developing country: Hodgkin lymphoma experience. Pediatr Blood Cancer. 2008;51:485–488. doi: 10.1002/pbc.21609. [DOI] [PubMed] [Google Scholar]
- 21.Laskar S, Gupta T, Vimal S, et al. Consolidation radiation after complete remission in Hodgkin’s disease following six cycles of doxorubicin, bleomycin, vinblastine, and dacarbazine chemotherapy: Is there a need? J Clin Oncol. 2004;22:62–68. doi: 10.1200/JCO.2004.01.021. [DOI] [PubMed] [Google Scholar]
- 22. Swerdlow SH, Campo E, Harris NL, et al (eds): WHO Classification of Tumours of Haematopoietic and Lymphoid Tissues (ed 4). Lyon, France, IARC Press, 2018.
- 23.Olweny CL. Cotswolds modification of the Ann Arbor staging system for Hodgkin’s disease. J Clin Oncol. 1990;8:1598. [PubMed] [Google Scholar]
- 24.Hoppe RT, Advani RH, Ai WZ, et al. Hodgkin Lymphoma Version 1.2017, NCCN clinical practice guidelines in oncology. J Natl Compr Canc Netw. 2017;15:608–638. doi: 10.6004/jnccn.2017.0064. [DOI] [PubMed] [Google Scholar]
- 25.Engert A, Diehl V, Franklin J, et al. Escalated-dose BEACOPP in the treatment of patients with advanced-stage Hodgkin’s lymphoma: 10 years of follow-up of the GHSG HD9 study. J Clin Oncol. 2009;27:4548–4554. doi: 10.1200/JCO.2008.19.8820. [DOI] [PubMed] [Google Scholar]
- 26.Johnson P, Federico M, Kirkwood A, et al. Adapted treatment guided by interim PET-CT scan in advanced Hodgkin’s lymphoma. N Engl J Med. 2016;374:2419–2429. doi: 10.1056/NEJMoa1510093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bursac Z, Gauss CH, Williams DK, et al. Purposeful selection of variables in logistic regression. Source Code Biol Med. 2008;3:17. doi: 10.1186/1751-0473-3-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ganesan P, Kumar L, Raina V, et al. Hodgkin’s lymphoma: Long-term outcome—An experience from a tertiary care cancer center in North India. Ann Hematol. 2011;90:1153–1160. doi: 10.1007/s00277-011-1262-8. [DOI] [PubMed] [Google Scholar]
- 29.Dinand V, Dawar R, Arya LS, et al. Hodgkin’s lymphoma in Indian children: Prevalence and significance of Epstein-Barr virus detection in Hodgkin’s and Reed-Sternberg cells. Eur J Cancer. 2007;43:161–168. doi: 10.1016/j.ejca.2006.08.036. [DOI] [PubMed] [Google Scholar]
- 30.Dinshaw KA, Advani SH, Gopal R, et al. Management of Hodgkin’s disease in western India. Cancer. 1984;54:1276–1282. doi: 10.1002/1097-0142(19841001)54:7<1276::aid-cncr2820540708>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- 31.Vashisht S, Aikat BK. Hodgkin’s disease (retrospective study of 119 cases) Indian J Cancer. 1973;10:263–279. [PubMed] [Google Scholar]
- 32.Raemaekers JMM, André MPE, Federico M, et al. Omitting radiotherapy in early positron emission tomography-negative stage I/II Hodgkin lymphoma is associated with an increased risk of early relapse: Clinical results of the preplanned interim analysis of the randomized EORTC/LYSA/FIL H10 trial. J Clin Oncol. 2014;32:1188–1194. doi: 10.1200/JCO.2013.51.9298. [DOI] [PubMed] [Google Scholar]
- 33.André MPE, Girinsky T, Federico M, et al. Early positron emission tomography response-adapted treatment in stage I and II Hodgkin lymphoma: Final results of the randomized EORTC/LYSA/FIL H10 trial. J Clin Oncol. 2017;35:1786–1794. doi: 10.1200/JCO.2016.68.6394. [DOI] [PubMed] [Google Scholar]
- 34.Koshy M, Rich SE, Mahmood U, et al. Declining use of radiotherapy in stage I and II Hodgkin’s disease and its effect on survival and secondary malignancies. Int J Radiat Oncol Biol Phys. 2012;82:619–625. doi: 10.1016/j.ijrobp.2010.10.069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Parikh RR, Grossbard ML, Harrison LB, et al. Early-stage classic Hodgkin lymphoma: The utilization of radiation therapy and its impact on overall survival. Int J Radiat Oncol Biol Phys. 2015;93:684–693. doi: 10.1016/j.ijrobp.2015.06.039. [DOI] [PubMed] [Google Scholar]
- 36.Radhakrishnan V, Dhanushkodi M, Ganesan TS, et al. Pediatric Hodgkin lymphoma treated at cancer institute, Chennai, India: Long-term outcome. J Glob Oncol. 2016;3:545–554. doi: 10.1200/JGO.2016.005314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Dann EJ, Bairey O, Bar-Shalom R, et al. Modification of initial therapy in early and advanced Hodgkin lymphoma, based on interim PET/CT is beneficial: A prospective multicentre trial of 355 patients. Br J Haematol. 2017;178:709–718. doi: 10.1111/bjh.14734. [DOI] [PubMed] [Google Scholar]
- 38.Barrington SF, Mikhaeel NG. When should FDG-PET be used in the modern management of lymphoma? Br J Haematol. 2014;164:315–328. doi: 10.1111/bjh.12601. [DOI] [PubMed] [Google Scholar]
- 39.Naumann R, Beuthien-Baumann B, Reiss A, et al. Substantial impact of FDG PET imaging on the therapy decision in patients with early-stage Hodgkin’s lymphoma. Br J Cancer. 2004;90:620–625. doi: 10.1038/sj.bjc.6601561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Barrington SF, Kirkwood AA, Franceschetto A, et al. PET-CT for staging and early response: Results from the Response-Adapted Therapy in Advanced Hodgkin Lymphoma study. Blood. 2016;127:1531–1538. doi: 10.1182/blood-2015-11-679407. [DOI] [PubMed] [Google Scholar]
- 41.Ganesan P, Rajendranath R, Kannan K, et al. Phase II study of interim PET-CT-guided response-adapted therapy in advanced Hodgkin’s lymphoma. Ann Oncol. 2015;26:1170–1174. doi: 10.1093/annonc/mdv077. [DOI] [PubMed] [Google Scholar]
- 42.Jain H, Sengar M, Nair R, et al. Treatment results in advanced stage Hodgkin’s lymphoma: A retrospective study. J Postgrad Med. 2015;61:88–91. doi: 10.4103/0022-3859.150446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Duggan DB, Petroni GR, Johnson JL, et al. Randomized comparison of ABVD and MOPP/ABV hybrid for the treatment of advanced Hodgkin’s disease: Report of an intergroup trial. J Clin Oncol. 2003;21:607–614. doi: 10.1200/JCO.2003.12.086. [DOI] [PubMed] [Google Scholar]
- 44.Gobbi PG, Levis A, Chisesi T, et al. ABVD versus modified Stanford V versus MOPPEBVCAD with optional and limited radiotherapy in intermediate- and advanced-stage Hodgkin’s lymphoma: Final results of a multicenter randomized trial by the Intergruppo Italiano Linfomi. J Clin Oncol. 2005;23:9198–9207. doi: 10.1200/JCO.2005.02.907. [DOI] [PubMed] [Google Scholar]
- 45.Johnson PWM, Radford JA, Cullen MH, et al. Comparison of ABVD and alternating or hybrid multidrug regimens for the treatment of advanced Hodgkin’s lymphoma: Results of the United Kingdom Lymphoma Group LY09 trial (ISRCTN97144519) J Clin Oncol. 2005;23:9208–9218. doi: 10.1200/JCO.2005.03.2151. [DOI] [PubMed] [Google Scholar]