Abstract
Introduction
Given FDA’s authority to implement a cigarette nicotine reduction policy, possible outcomes of this regulation must be examined, especially among those who may be most affected, such as those with comorbid psychiatric disorders.
Methods
In this secondary analysis of a multisite, randomized, clinical laboratory study, we used analyses of variance to examine the effects of nicotine dose (0.4, 2.4, 5.2, and 15.8 mg/g of tobacco), depressive and anxiety diagnoses (depression only, anxiety only, both, or neither), and depressive and anxiety symptom severity on cigarette choice, smoke exposure, craving, and withdrawal across three vulnerable populations: socioeconomically disadvantaged women of reproductive age, opioid-dependent individuals, and those with affective disorders (n = 169).
Results
Diagnosis and symptom severity largely had no effects on smoking choice, total puff volume, or CO boost. Significant main effects on craving and withdrawal were observed, with higher scores in those with both anxiety and depression diagnoses compared with depression alone or no diagnosis, and in those with more severe depressive symptoms (p’s < .001). These factors did not interact with nicotine dose. Cigarettes with <15.8 mg/g nicotine were less reinforcing, decreased total puff volume, and produced significant but lower magnitude and shorter duration reductions in craving and withdrawal than higher doses (p’s < .01).
Conclusions
Reducing nicotine dose reduced measures of cigarette addiction potential, with little evidence of moderation by either psychiatric diagnosis or symptom severity, providing evidence that those with comorbid psychiatric disorders would respond to a nicotine reduction policy similarly to other smokers.
Implications
Thus far, controlled studies in healthy populations of smokers have demonstrated that use of very low nicotine content cigarettes reduces cigarette use and dependence without resulting in compensatory smoking. These analyses extend those findings to a vulnerable population of interest, those with comorbid psychiatric disorders. Cigarettes with very low nicotine content were less reinforcing, decreased total puff volume, and produced significant but lower magnitude and shorter duration reductions in craving and withdrawal than higher doses. These nicotine dose effects did not interact with psychiatric diagnosis or mood symptom severity suggesting that smokers in this vulnerable population would respond to a nicotine reduction strategy similarly to other smokers.
Introduction
The 2009 Family Smoking Prevention and Tobacco Control Act (FSPTCA) granted the FDA regulatory authority over cigarettes and other tobacco products.1 Accordingly, the FDA has proposed several product standards to address cigarette smoking, including setting a maximal nicotine level in cigarettes.2 As this product standard is considered, it is necessary to examine the potential public health impact of very low nicotine content (VLNC) cigarettes. Thus far, controlled studies in healthy populations of smokers have demonstrated that use of VLNC cigarettes reduces cigarette use and dependence without resulting in mood disruption or compensatory smoking.3,4
However, concerns persist about how this regulation may affect smokers with psychiatric comorbidities. Affective disorders (ADs), which include major depressive disorder and anxiety disorders, are the most common mental health conditions (MHCs) in the United States; in 2015, 7% of US adults reported past-year major depressive disorder and 18% reported a past-year anxiety disorder.5 Although smoking rates in the general US population have declined over the past 50 years, there has been little to no decline among those with MHCs and smoking prevalence among this group is elevated compared with the general population (40% vs. 15.5%).6 In addition to an increased prevalence of smoking, cigarette dependence appears to be higher in smokers with elevated depression symptoms and those with anxiety disorders.7–9 Accordingly, smokers with ADs have greater difficulty quitting smoking and greater likelihood of relapse compared with those without ADs.10–14 It should also be noted that ADs frequently co-occur (eg, depression and anxiety) and as the presence of multiple MHCs increases, so does the likelihood of nicotine dependence in this population.15
It is hypothesized that reducing the nicotine content of cigarettes to a minimally addictive level could improve long-term health outcomes in smokers with MHCs.16,17 Reductions in nicotine content should reduce dependence, potentially both preventing experimental smokers from becoming regular smokers and making it easier for established smokers to quit.2 Investigations of VLNC cigarettes in smokers with MHCs and other populations especially vulnerable to tobacco use have been promising, generally resulting in reduced reinforcing effects and relief from craving and withdrawal, with no evidence of compensatory smoking.18–23 For a specific example, Tidey and colleagues examined depressive symptom severity as a moderator of response to VLNC cigarettes and found that those above and below a clinical cut-off for depression had similar reductions in smoking with VLNC cigarettes.23
However, little research has been reported examining the impact of VLNC in smokers with MHCs, and this issue needs to be thoroughly examined. No studies have examined whether those with more severe anxiety symptomology or multiple affective disorders are at increased risk for unintended negative consequences of a nicotine reduction policy. Compensatory smoking, differences in sensitivity to nicotine, or an inability for VLNC cigarettes to ameliorate withdrawal in smokers with more severe psychiatric burden could have public health implications. The aim of the current study was to examine the effect of type and number of diagnoses, as well as severity of depressive and anxious symptomology, on acute response to cigarettes varying in nicotine content by examining effects on cigarette choice (relative reinforcing effects), compensatory smoking, craving, and withdrawal. It was hypothesized that the effects of nicotine dose would be minimally affected by symptom severity or diagnosis.
Methods
Study Sample
Data for this secondary analysis were drawn from participants in a multisite study (University of Vermont, Brown University, Johns Hopkins University School of Medicine) which included 169 adult daily smokers (56 with affective disorders as an exemplar of smokers with mental illness, 60 with opioid dependence as an exemplar of smokers with other substance use disorders, and 53 socioeconomically disadvantaged women of reproductive age as an exemplar of smokers with socioeconomic disadvantage). Study inclusion and exclusion criteria have been reported previously.18,19 Briefly, all participants smoked ≥5 cigarettes per day (CPD) for at least 1 year, had limited use of other tobacco products, and were not currently interested in quitting smoking. All participants completed screening and intake visits where detailed sociodemographic and smoking characteristics were collected. As part of these measures, participants completed the Fagerström Test for Nicotine Dependence (FTND) and the heaviness of smoking index (HSI) scores were calculated by summing scores from items 1 and 4 of the FTND.24,25 Participants also completed the Mini-International Neuropsychiatric interview26 (MINI version 6.0, which assesses DSM-IV disorders) and were determined to have either a depressive disorder (major depression, dysthymia), an anxiety disorder (Panic Disorder, Social Phobia, Specific Phobia, Obsessive Compulsive Disorder, Post-Traumatic Stress Disorder, General Anxiety Disorder), both, or neither. Additionally, participants self-reported depressive and anxious symptomology by completing the Beck Depression Inventory-II (BDI) and the Overall Anxiety Severity and Impairment Scale (OASIS).27,28 Study procedures were approved by the University of Vermont IRB and all participants provided written informed consent.
Research Cigarettes
The study used SPECTRUM research cigarettes (22nd Century Group, Clarence, NY). Participants whose usual brand was mentholated were assigned to menthol research cigarettes. Four nicotine doses were investigated. The average nicotine content of doses across menthol and nonmenthol products was 15.8, 5.2, 2.4, and 0.4 mg of nicotine per gram of tobacco (mg/g) with the 15.8 mg/g nicotine dose serving as a control for commercial cigarettes. All cigarettes were labeled with arbitrary letter codes and administered under double-blind conditions. The dose and letter code combinations were determined randomly.
Procedure
Procedures for this study have been described previously.18,19 Briefly, participants completed fourteen 2- to 4-h sessions in a within-subjects design organized into three phases. The present study focuses on Phases 1 and 2. Prior to each session, participants abstained from smoking for a minimum of 6–8 h, operationalized as breath carbon monoxide (CO) at ≤50% of study-intake CO level. Sessions were conducted a minimum of 48 h apart with order of exposure to nicotine doses randomized across participants.
In Phase 1, participants were oriented to the study protocol (Session 1) and then sampled each of the four research cigarette doses across separate sessions (Sessions 2–5). Cigarettes were smoked ad lib using a Clinical Research Support System (CReSS) device to record smoking topography.29 The primary topography measure was total cigarette puff volume, i.e., the sum of the volumes of all puffs taken within each cigarette, which is a sensitive summary measure of exposure.30 Prior to smoking and every 15 min for 60 min following smoking, participants also completed the Minnesota Tobacco Withdrawal Scale (MTWS),31 the Questionnaire of Smoking Urges-brief (QSU-B),32 and breath CO levels were collected to calculate CO boost (postsmoking CO level minus pre-smoking CO level) as a measure of smoke intake.
In Phase 2 (Sessions 6–11), at each session, participants were presented with pairs of the research cigarettes concurrently and were able to take puffs from either cigarette.33,34 Each of the six possible cigarette dose-pair combinations was tested once in separate sessions. In these 3-h sessions, a participant sat alone in a ventilated room. When they wished to smoke, they used a computer mouse to click on one of two icons on a computer screen representing the two cigarettes available that session. After ten clicks on the icon, they could take two puffs of the associated cigarette.33 Participants were free to choose either option as often as they wished, or to abstain if they did not wish to smoke.
Statistical Approach
This secondary analysis examined effects of diagnosis and symptom severity on responses to cigarettes varying in nicotine content using a combined sample of smokers with affective disorders, opioid use disorders, and socioeconomic disadvantage.18,19 Minimal differences across samples on response to nicotine dose were seen in the parent study.19 Collapsing across subsamples maximized statistical power to detect interactions between dose and psychiatric factors, with the larger sample having greater variability in diagnoses and severity of symptoms.
Participants were categorized three ways: by number/type of diagnosis (none, depression only, anxiety only, both depression and anxiety), by severity of depressive symptoms (BDI < 20 vs. ≥ 20), and by severity of anxious symptoms (OASIS < 8 vs. ≥ 8). These cut-points were based on prior validation studies.35,36 Outcome variables of interest were examined in relation to these categories. Demographic variables were compared between diagnosis and symptom severity categories using Chi-Square Tests of Independence for categorical variables and Kruskal–Wallis tests for continuous variables.
Effects on smoking choices were examined using the proportion of choices participants made for the higher-level nicotine content cigarette during Phase 2 concurrent choice testing. Effects on craving and withdrawal were examined using MNWS total score, the “Desire to Smoke” item on the MNWS, and QSU scores. QSU was examined by factor with Factor 1 characterized by desire and intent to smoke due to positive reinforcing effects of smoking and Factor 2 with craving to smoke related to relief from nicotine withdrawal or negative affect.37 Potential compensatory smoking was examined using total puff volume and CO boost.
Mixed model repeated measures analyses of variance were used to test for the effects of diagnosis, nicotine dose, and their interactions on all study outcomes, with dose as the within-participant factor. To examine effects of symptom severity on these outcomes, this analysis approach was repeated with BDI symptom severity (high/low) and OASIS symptom severity (high/low) substituted for diagnosis. Analyses also included fixed effects of sex, session, and population as covariates, and a random effect to account for the three study sites. Multivariable models were used, with independent variables/predictors included in the same model, with the base model being primary predictor/dose/interaction and covariates added. For analyses of MNWS scores, QSU factor scores, and CO boost, time was added to the models as another within-participant factor, along with time-by-diagnosis/severity and time-by-dose interactions. When not significant, interaction effects were dropped from the models. Statistical significance level alpha was set a priori at 0.05. Significant main or interaction effects were followed by post hoc testing, with p-values subject to Bonferroni correction. All statistical analyses were conducted using SAS version 9.4, SAS Institute, Inc., Cary, NC.
Results
Participants
Participant characteristics are provided in Table 1. The mean age of the participants was 36 and the sample was 71% female. 73% of the sample was non-Latino White and about half had at least some college education. Average number of cigarettes per day was 16 and average age started smoking regularly was 16 with an average HSI of 3. Few differences in demographics were seen between diagnosis groups or by symptom severity. However, there were gender differences by severity of both depressive and anxiety symptoms, with men more likely to be in the groups with more severe symptoms. Educational attainment differed by anxiety symptom severity with higher severity being associated with higher educational attainment. Additionally, there were race/ethnicity differences by both diagnosis and by anxiety symptom severity with non-Latino Whites being more likely to have both an anxiety and a depression diagnosis and more likely to have more severe anxiety symptoms. Participants met CO reduction goals prior to experimental sessions. Baseline CO levels were 22.4 ppm on average and average CO prior to the experimental sessions was 7.4 ppm.
Table 1.
Sample characteristics
| All (n = 169) | Diagnosis groups | p | BDI groups | p | OASIS groups | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| None (n = 54) | Anxiety (n = 17) | Depression (n = 33) | Both (n = 65) | BDI >= 20 (n = 44) | BDI < 20 (n = 125) | OASIS >= 8 (n = 60) | OASIS < 8 (n = 108) | p | ||||
| Age (M ± SD) | 35.56 ± 11.38 | 36.2 ± 11.35 | 32.71 ± 11.3 | 33.48 ± 11.51 | 36.83 ± 11.36 | 0.25 | 33.7 ± 11.53 | 36.22 ± 11.31 | 0.13 | 33.6 ± 10.89 | 36.73 ± 11.57 | 0.07 |
| Gender (% Female) | 120 (71.01) | 44 (81.48) | 13 (76.47) | 24 (72.73) | 39 (60) | 0.07 | 24 (54.55) | 96 (76.8) | 0.01 | 33 (55) | 86 (79.63) | 0.001 |
| Race/Ethnicity | 0.02 | 0.35 | 0.05 | |||||||||
| Non-Latino White | 123 (72.78) | 36 (66.67) | 10 (58.82) | 24 (72.73) | 53 (81.54) | 34 (77.27) | 89 (71.2) | 48 (80) | 74 (68.52) | |||
| Non-Latino Black | 23 (13.61) | 14 (25.93) | 1 (5.88) | 5 (15.15) | 3 (4.62) | 3 (6.82) | 20 (16) | 2 (3.33) | 21 (19.44) | |||
| Latino | 6 (3.55) | 1 (1.85) | 1 (5.88) | 2 (6.06) | 2 (3.08) | 2 (4.55) | 4 (3.2) | 3 (5) | 3 (2.78) | |||
| Non-Latino Other or >1 race | 15 (8.88) | 3 (5.56) | 4 (23.53) | 2 (6.06) | 6 (9.23) | 4 (9.09) | 11 (8.8) | 6 (10) | 9 (8.33) | |||
| Non-Latino Asian | 1 (0.59) | 0 (0) | 0 (0) | 0 (0) | 1 (1.54) | 1 (2.27) | 0 (0) | 1 (1.67) | 0 (0) | |||
| Non-Latino Hawaiian | 1 (0.59) | 0 (0) | 1 (5.88) | 0 (0) | 0 (0) | 0 (0) | 1 (0.8) | 0 (0) | 1 (0.93) | |||
| Education | 0.98 | 0.08 | 0.02 | |||||||||
| 8th Grade or Less | 4 (2.37) | 1 (1.85) | 1 (5.88) | 1 (3.03) | 1 (1.54) | 1 (2.27) | 3 (2.4) | 1 (1.67) | 3 (2.78) | |||
| Some High School | 23 (13.61) | 8 (14.81) | 3 (17.65) | 5 (15.15) | 7 (10.77) | 6 (13.64) | 17 (13.6) | 5 (8.33) | 18 (16.67) | |||
| High School Graduate/ Equivalent | 58 (34.32) | 20 (37.04) | 5 (29.41) | 10 (30.3) | 23 (35.38) | 12 (27.27) | 46 (36.8) | 18 (30) | 40 (37.04) | |||
| Some College | 64 (37.87) | 20 (37.04) | 6 (35.29) | 12 (36.36) | 26 (40) | 15 (34.09) | 49 (39.2) | 22 (36.67) | 41 (37.96) | |||
| 2-Year Associate’s Degree | 10 (5.92) | 2 (3.7) | 1 (5.88) | 3 (9.09) | 4 (6.15) | 3 (6.82) | 7 (5.6) | 6 (10) | 4 (3.7) | |||
| College Graduate/4-Year Degree | 6 (3.55) | 2 (3.7) | 1 (5.88) | 2 (6.06) | 1 (1.54) | 4 (9.09) | 2 (1.6) | 4 (6.67) | 2 (1.85) | |||
| Graduate or Professional Degree | 4 (2.37) | 1 (1.85) | 0 (0) | 0 (0) | 3 (4.62) | 3 (6.82) | 1 (0.8) | 4 (6.67) | 0 (0) | |||
| Marital Status | 0.41 | 0.12 | 0.27 | |||||||||
| Married | 27 (15.98) | 10 (18.52) | 3 (17.65) | 3 (9.09) | 11 (16.92) | 3 (6.82) | 24 (19.2) | 7 (11.67) | 20 (18.52) | |||
| Never Married | 103 (60.95) | 34 (62.96) | 11 (64.71) | 23 (69.7) | 35 (53.85) | 32 (72.73) | 71 (56.8) | 40 (66.67) | 62 (57.41) | |||
| Divorced or Separated | 35 (20.71) | 10 (18.52) | 2 (11.76) | 5 (15.15) | 18 (27.69) | 9 (20.45) | 26 (20.8) | 13 (21.67) | 22 (20.37) | |||
| Widowed | 4 (2.37) | 0 (0) | 1 (5.88) | 2 (6.06) | 1 (1.54) | 0 (0) | 4 (3.2) | 0 (0) | 4 (3.7) | |||
| Cigarettes Per Day (M ± SD) | 15.79 ± 7.46 | 15.09 ± 6.47 | 15 ± 8.28 | 14.15 ± 4.93 | 17.42 ± 8.82 | 0.33 | 16.7 ± 7.88 | 15.47 ± 7.32 | 0.39 | 16.35 ± 7.74 | 15.54 ± 7.34 | 0.53 |
| Primary Menthol Smoker | 61 (36.09) | 23 (42.59) | 6 (35.29) | 10 (30.3) | 22 (33.85) | 0.66 | 14 (31.82) | 47 (37.6) | 0.49 | 19 (31.67) | 41 (37.96) | 0.41 |
| Breath CO (ppm) (M ± SD) | 22.37 ± 11.96 | 22.02 ± 10.45 | 21.29 ± 10.43 | 20.48 ± 11.55 | 23.91 ± 13.66 | 0.49 | 23.02 ± 11.65 | 22.14 ± 12.1 | 0.50 | 24.73 ± 14.83 | 21.19 ± 9.86 | 0.21 |
| Age Started Smoking Regularly (M years ± SD) | 16.26 ± 4.27 | 16.31 ± 3.67 | 18.29 ± 6.73 | 15.91 ± 5.2 | 15.86 ± 3.23 | 0.40 | 16.57 ± 2.9 | 16.15 ± 4.66 | 0.13 | 16.43 ± 3.31 | 16.17 ± 4.74 | 0.12 |
| Heaviness of Smoking Index (M ± SD) | 2.99 ± 1.27 | 3.09 ± 1.20 | 2.29 ± 1.53 | 2.67 ± 1.14 | 3.26 ± 1.25 | 0.04 | 2.98 ± 1.32 | 3.00 ± 1.26 | 0.75 | 3.02 ± 1.36 | 2.98 ± 1.24 | 0.98 |
Cigarette Choice
There was a significant main effect of dose pair on proportion of higher dose choices (F[5,835] = 6.12, p < .001, Cohen’s d = 0.44) driven largely by significantly higher proportions of choices for the highest dose in tests comparing the 0.4 versus 15.8 and 2.4 versus 15.8 dose pairs (Supplementary Table 1). There were no main effects of diagnosis or symptom severity on proportion of higher dose choices. Furthermore, there was no interaction between dose pair and either diagnosis or symptom severity, indicating that neither variable significantly moderated preference for the higher doses. Participants chose the higher nicotine dose at significantly greater than chance levels across each of the six dose pairs independent of the psychiatric variables examined.
Craving
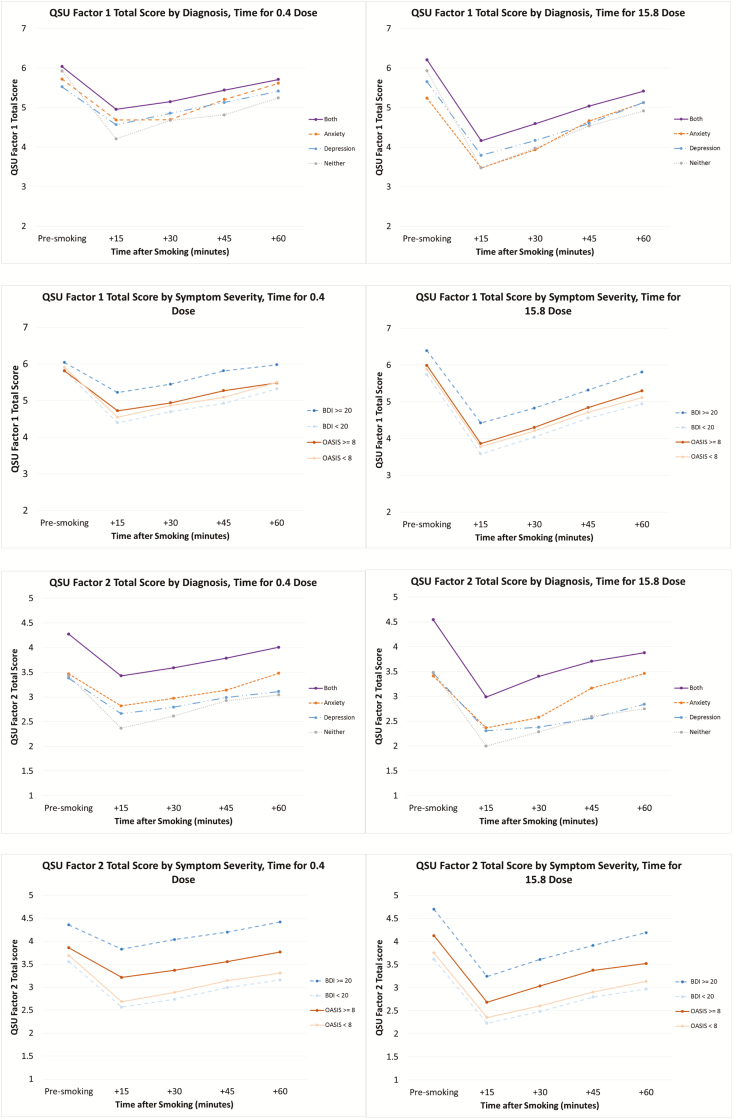
There was a main effect of depression symptom severity on both QSU Factor 1 and Factor 2 scores, as well as on the MNWS “Desire to Smoke” item with higher scores among those with elevated BDI scores (Factor 1: F[1,164] = 5.50, p = .02, Cohen’s d = 0.20; Factor 2: F[1,163] = 11.26, p = .001, Cohen’s d = 0.25; Desire to Smoke: F[1,164] = 5.26, p = .02, Cohen’s d = 0.28; Figure 1). There was also a main effect of diagnosis on QSU Factor 2 (F[3,161] = 3.75, p = .01, Cohen’s d = 0.36). Those with both disorders had higher scores than those with depression alone (p = .03) or neither diagnosis (p = .02). There were no main effects of anxiety symptom severity on QSU scores. As previously reported, there were significant main effects of nicotine dose and time on MNWS “desire to smoke” and both factors of the QSU; all cigarettes reduced craving relative to baseline, but the magnitude of effects was greater and effects were of longer duration with the higher-nicotine content cigarettes, as shown by significant dose*time interactions (QSU Factor 1: F[12,2014] = 9.04, p < .001; QSU Factor 2: F[12,2014] = 5.22, p < .001; Desire to Smoke: F[12,2014] = 5.98, p < .001). There were no significant interactions between psychiatric symptomology/diagnoses and time or nicotine dose.
Figure 1.
Craving by dose, time, and diagnosis/symptom severity.
Withdrawal
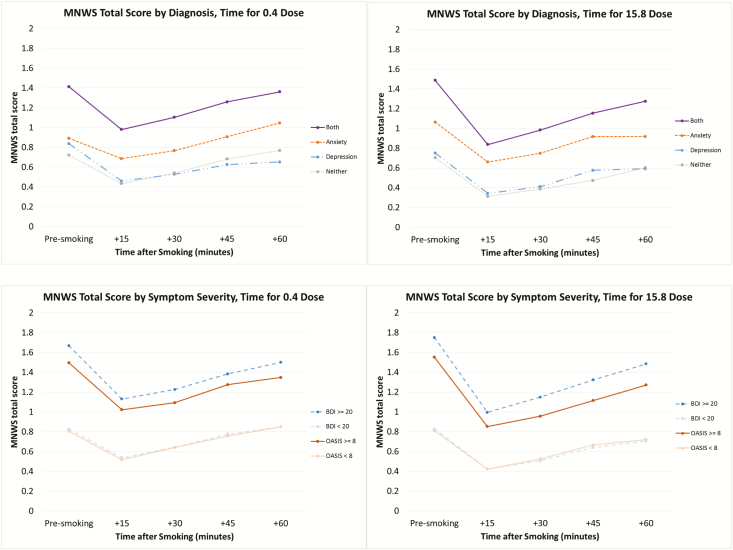
There were significant main effects on withdrawal by diagnosis (F[3,158] = 7.23, p < .001, Cohen’s d = 0.88), depression symptom severity (F[1,164] = 20.29, p < .001, Cohen’s d = 0.88), and anxiety symptom severity (F[1,148] = 10.16, p = .002, Cohen’s d = 0.66; Figure 2). Higher withdrawal levels were present in those with both an anxiety and depression diagnosis when compared with those with depression alone (p = .001) or with no diagnosis (p = .001). Higher withdrawal levels were seen in those with more severe depressive or anxious symptoms compared with less severe symptoms (p’s ≤ .002). Consistent interactions were found between both diagnoses and symptom severity by time. In those groups with higher withdrawal scores, return to baseline withdrawal was less complete than in those with lower baseline levels of withdrawal (F’s = 1.95–10.29, p’s < .05). As previously reported, there were significant main effects of nicotine dose and time on withdrawal; all cigarettes reduced withdrawal relative to baseline, but the magnitude of effects was greater and effects were of longer duration with the higher-nicotine content cigarettes, as shown by a significant dose*time interaction (F[12,2014] = 2.63, p = .002). However, there were no significant interactions between psychiatric variables and dose for withdrawal.
Figure 2.
Withdrawal by dose, time, and diagnosis/symptom severity.
Smoke Exposure Measures
There were no significant main effects of diagnosis or symptom severity on either CO boost or total puff volume (Table 2). Reducing the nicotine content of cigarettes did not affect CO boost, but significantly reduced total puff volume (F[3,489] = 4.67, p = .003, Cohen’s d = 0.28). There were no significant interactions between dose with diagnosis or symptom severity, indicating that neither variable moderated effects of nicotine dose on measures of smoke exposure.
Table 2.
Measures of compensatory smoking by diagnosis, BDI level, OASIS level, and nicotine dose
| Total Puff Volume (mL) | p | CO Boost* (ppm) | p | |
|---|---|---|---|---|
| Mean ± SEM | Mean ± SEM | |||
| Diagnosis level | ||||
| Neither | 653.66 ± 61.01 | 0.14 | 5.37 ± 0.32 | 0.47 |
| Anxiety | 512.09 ± 85.64 | 5.67 ± 0.52 | ||
| Depression | 522.14 ± 69.35 | 6.03 ± 0.38 | ||
| Both | 617.55 ± 58.89 | 5.92 ± 0.29 | ||
| BDI level | ||||
| < 20 | 622.31 ± 55.14 | 0.33 | 5.89 ± 0.22 | 0.25 |
| >= 20 | 557.91 ± 72.56 | 5.36 ± 0.38 | ||
| OASIS level | ||||
| < 8 | 601.26 ± 60.41 | 0.86 | 5.73 ± 0.25 | 0.88 |
| >= 8 | 613.71 ± 71.46 | 5.79 ± 0.34 | ||
| Nicotine dose | ||||
| 0.4 mg/g | 535.77 ± 55.38a | 0.003 | 5.68 ± 0.21 | 0.73 |
| 2.4 mg/g | 557.02 ± 55.43b | 5.73 ± 0.24 | ||
| 5.2 mg/g | 560.44 ± 55.39c | 5.67 ± 0.22 | ||
| 15.8 mg/g | 652.21 ± 55.49a,b,c | 5.68 ± 0.21 |
Note: Boldface indicates statistical significance (p = .05).
Results having matching letters indicate significant pairwise differences between levels.
*CO Boost 15 min following smoking.
Conclusions
Our results show no evidence that response to cigarettes differing in nicotine content is moderated by psychiatric diagnosis or symptom severity. When considering effects of nicotine content on cigarette choice (ie, relative reinforcing effects), we saw no evidence of effect of psychiatric variables as a main effect or in interactions. Higher dose cigarettes were chosen more often, independent of the psychiatric factors examined. Similarly, a previous study, while not testing choice directly, found that ratings of cigarette liking were driven by nicotine content and were not influenced by depression history or current anxiety symptomology.38 These results suggest that reducing the nicotine content of cigarettes to a level that does not support smoking in the general population may also be effective at reducing smoking in people with ADs, including those with elevated symptoms of anxiety and depression.
Levels of craving and withdrawal were affected by the psychiatric variables examined, although those effects did not interact with nicotine dose. Craving was higher across nicotine doses among those with higher BDI or OASIS scores or among those with both a depression and an anxiety diagnosis. Of interest, the largest effects of diagnosis and symptom severity appeared to be in QSU Factor 2, which represents anticipation of relief from negative affect.37 The elevated QSU Factor 2 scores among those with elevated depressive symptoms are consistent with prior literature demonstrating increased smoking urges among those with higher depressive symptoms and findings that those with increased depressive symptoms may be disproportionately prone to smoke to relieve the negative affect symptoms caused by withdrawal.39,40
Effects on withdrawal showed a similar pattern. MNWS scores were higher among those with higher BDI or OASIS scores or those with both a depression and an anxiety diagnosis relative to participants lower in depression and anxiety. Elevated MNWS scores in smokers with affective disorders are typically observed in studies of smokers with mental health conditions20,23,41 and are likely due, at least in part, to the overlap between MNWS items and mood/psychiatric symptoms.42 Once again, the largest effects appeared to be driven by depressive symptom severity and having dual anxiety/depression diagnoses.
The overall pattern of effects of diagnosis and symptom severity on craving and withdrawal symptoms indicates that, for smokers with depression in this study, symptom severity was a more significant risk factor for craving and withdrawal symptoms than diagnosis. These findings are consistent with prior reports where it has been suggested that current depressive symptomology is a better predictor of smoking-related outcomes than diagnosis.43 For smokers with anxiety, diagnosis and symptom severity were similar in terms of how they affected craving and withdrawal scores. Interestingly, although a depression diagnosis did not affect QSU Factor 2 or MNWS scores on its own, it increased the effects of anxiety diagnosis on these scores. However, the influences of diagnosis and symptom severity were restricted to main effects, and there was no evidence that those with more severe symptoms or multiple diagnoses would respond differently to lower nicotine content cigarettes.
In regard to potential compensatory smoking, we found no evidence of effect of psychiatric variables either as a main effect or in interactions with dose on our measures of smoke exposure (CO boost and total puff volume). These findings are consistent with other reports in smokers with serious mental illness,22 and those with elevated depressive symptoms23 which also did not find effects of psychiatric variables on measures of smoke exposure. The only variable that affected smoke exposure was nicotine content, with lower nicotine content cigarettes resulting in smaller total puff volumes, the opposite of what would be predicted if compensatory smoking were occurring.18,19 A recent study examining the pharmacokinetic profile of Spectrum cigarettes found that, as expected, boosts in plasma nicotine concentration were reflective of the nicotine content of the cigarettes, also suggesting that compensatory smoking is not occurring with these reduced nicotine content cigarettes.44
Strengths of the study include the large sample, the inclusion of several nicotine doses, the use of well-validated measures to assess psychiatric diagnosis and symptom severity, and the testing of cigarette effects under monitored and controlled conditions. Potential limitations of the present study are that (1) this is a secondary analysis of data collected to test other hypotheses and thus may be underpowered to detect effects of diagnosis and symptom severity; (2) the parent study recruited fairly psychiatrically stable participants, so results may not generalize to more severely impaired smokers; and (3) only acute exposure to the cigarettes was examined, leaving unanswered whether results will generalize to effects of extended exposure. These limitations notwithstanding, the present study helps address the important regulatory question of whether psychiatric diagnosis type, number, or symptom severity will moderate response to a policy reducing the nicotine content of cigarettes. In particular, to the best of our knowledge, this is the first study to examine responses to VLNC cigarettes among smokers with anxiety disorders or to examine the effects of comorbid depression and anxiety disorders on responses to reduced-nicotine cigarettes. This research provides evidence that those with comorbid psychiatric disorders would respond to a nicotine reduction policy similarly to other smokers, and underscores that the potential benefits of reducing the nicotine content of cigarettes to decrease the addiction potential of smoking should extend to those with more severe symptomology and multiple diagnoses.
Funding
The research presented in this paper was supported by Tobacco Centers of Regulatory Science award U54DA036114 from the National Institute on Drug Abuse (NIDA) and Food and Drug Administration (FDA). Author support during the preparation of this paper was also provided by Center of Biomedical Research Excellence award P20GM103644 from the National Institute of General Medical Sciences (NIGMS), NIDA Institutional Training Grant T32DA007242, and NIDA/FDA grant U54DA031659. The content is solely the responsibility of the authors and does not necessarily represent the official views of NIDA, NIGMS, or the FDA.
Declaration of Interests
Dr. Hughes has received consulting and speaking fees from several companies that develop or market pharmacological and behavioral treatments for smoking cessation or harm reduction and from several nonprofit organizations that promote tobacco control. He has recently received consulting fees from Swedish Match, Altria and Philip Morris International to assist their efforts to develop less-risky tobacco products. All other authors have no disclosures to declare.
Supplementary Material
References
- 1. Family Smoking Prevention and Tobacco Control Act. https://www. govtrack.us/congress/bills/111/hr1256/text. Accessed September 5, 2019.
- 2. US Food and Drug Administration. Tobacco Product Standard for Nicotine Level of Combusted Cigarettes. A Proposed Rule by the Food and Drug Administration https://www.federalregister.gov/documents/2018/03/16/2018–05345/tobaccoproduct-standard-for-nicotine-level-of-combusted-cigarettes. Accessed March 4, 2019.
- 3. Donny EC, Denlinger RL, Tidey JW, et al. Randomized trial of reduced-nicotine standards for cigarettes. N Engl J Med. 2015;373(14):1340–1349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Hatsukami DK, Luo X, Jensen JA, et al. Effect of immediate vs gradual reduction in nicotine content of cigarettes on biomarkers of smoke exposure: A randomized clinical trial. JAMA. 2018;320(9):880–891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Center for Behavioral Health Statistics and Quality. Behavioral Health Trends in the United States: Results from the 2014 National Survey on Drug Use and Health 2015. http://www.samhsa.gov/data/. Accessed September 5, 2019.
- 6. Smith PH, Mazure CM, McKee SA. Smoking and mental illness in the U.S. population. Tob Control. 2014;23(e2):e147–e153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Fergusson DM, Goodwin RD, Horwood LJ. Major depression and cigarette smoking: Results of a 21-year longitudinal study. Psychol Med. 2003;33(8):1357–1367. [DOI] [PubMed] [Google Scholar]
- 8. Johnson JG, Cohen P, Pine DS, Klein DF, Kasen S, Brook JS. Association between cigarette smoking and anxiety disorders during adolescence and early adulthood. JAMA. 2000;284(18):2348–2351. [DOI] [PubMed] [Google Scholar]
- 9. Moylan S, Jacka FN, Pasco JA, Berk M. Cigarette smoking, nicotine dependence and anxiety disorders: A systematic review of population-based, epidemiological studies. BMC Med. 2012;10:123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Prochaska JJ, Rossi JS, Redding CA, et al. Depressed smokers and stage of change: Implications for treatment interventions. Drug Alcohol Depend. 2004;76(2):143–151. [DOI] [PubMed] [Google Scholar]
- 11. Young-Wolff KC, Fromont SC, Delucchi K, Hall SE, Hall SM, Prochaska JJ. PTSD symptomatology and readiness to quit smoking among women with serious mental illness. Addict Behav. 2014;39(8):1231–1234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Cooper J, Borland R, McKee SA, Yong HH, Dugué PA. Depression motivates quit attempts but predicts relapse: Differential findings for gender from the International Tobacco Control Study. Addiction. 2016;111(8):1438–1447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Hitsman B, Papandonatos GD, McChargue DE, et al. Past major depression and smoking cessation outcome: A systematic review and meta-analysis update. Addiction. 2013;108(2):294–306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Weinberger AH, Kashan RS, Shpigel DM, et al. Depression and cigarette smoking behavior: A critical review of population-based studies. Am J Drug Alcohol Abuse. 2017;43(4):416–431. [DOI] [PubMed] [Google Scholar]
- 15. Swendsen J, Conway KP, Degenhardt L, et al. Mental disorders as risk factors for substance use, abuse and dependence: Results from the 10-year follow-up of the National Comorbidity Survey. Addiction. 2010;105(6):1117–1128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Gaalema DE, Miller ME, Tidey JW. Predicted impact of nicotine reduction on smokers with affective disorders. Tob Regul Sci. 2015;1(2):154–165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Tidey JW, Davis DR, Miller ME, Pericot-Valverde I, Denlinger-Apte RL, Gaalema DE. Modeling nicotine regulation: A review of studies in smokers with mental health conditions. Prev Med. 2018;117:30–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Higgins ST, Heil SH, Sigmon SC, et al. Response to varying the nicotine content of cigarettes in vulnerable populations: An initial experimental examination of acute effects. Psychopharmacology (Berl). 2017;234(1):89–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Higgins ST, Heil SH, Sigmon SC, et al. Addiction potential of cigarettes with reduced nicotine content in populations with psychiatric disorders and other vulnerabilities to tobacco addiction. JAMA Psychiatry. 2017;74(10):1056–1064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Tidey JW, Colby SM, Xavier EM. Effects of smoking abstinence on cigarette craving, nicotine withdrawal, and nicotine reinforcement in smokers with and without schizophrenia. Nicotine Tob Res. 2014;16(3):326–334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Tidey JW, Rohsenow DJ, Kaplan GB, Swift RM, Ahnallen CG. Separate and combined effects of very low nicotine cigarettes and nicotine replacement in smokers with schizophrenia and controls. Nicotine Tob Res. 2013;15(1):121–129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Tidey JW, Cassidy RN, Miller ME. Smoking topography characteristics of very low nicotine content cigarettes, with and without nicotine replacement, in smokers with schizophrenia and controls. Nicotine Tob Res. 2016;18(9):1807–1812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Tidey JW, Pacek LR, Koopmeiners JS, et al. Effects of 6-week use of reduced-nicotine content cigarettes in smokers with and without elevated depressive symptoms. Nicotine Tob Res. 2017;19(1):59–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Heatherton TF, Kozlowski LT, Frecker RC, Fagerström KO. The Fagerström Test for nicotine dependence: A revision of the Fagerström Tolerance Questionnaire. Br J Addict. 1991;86(9):1119–1127. [DOI] [PubMed] [Google Scholar]
- 25. Borland R, Yong HH, O’Connor RJ, Hyland A, Thompson ME. The reliability and predictive validity of the heaviness of smoking index and its two components: Findings from the international tobacco control four country study. Nicotine Tob Res. 2010;12 (Suppl):S45–S50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Sheehan DV, Lecrubier Y, Sheehan KH, et al. The Mini-International Neuropsychiatric Interview (M.I.N.I): The development and validation of a structured diagnostic psychiatric interview for DSM-IV and ICD-10. J Clin Psychiatry. 1998;59 (Suppl 20):22–33;quiz 34–57. [PubMed] [Google Scholar]
- 27. Beck AT, Steer RA, Garbin MG. Psychometric properties of the Beck Depression Inventory: Twenty-five years of evaluation. Clin Psychol Rev. 1988;8(1):77–100. [Google Scholar]
- 28. Norman SB, Cissell SH, Means-Christensen AJ, Stein MB. Development and validation of an Overall Anxiety Severity And Impairment Scale (OASIS). Depress Anxiety. 2006;23(4):245–249. [DOI] [PubMed] [Google Scholar]
- 29. Lee EM, Malson JL, Waters AJ, Moolchan ET, Pickworth WB. Smoking topography: Reliability and validity in dependent smokers. Nicotine Tob Res. 2003;5(5):673–679. [DOI] [PubMed] [Google Scholar]
- 30. Scherer G. Smoking behaviour and compensation: A review of the literature. Psychopharmacology (Berl). 1999;145(1):1–20. [DOI] [PubMed] [Google Scholar]
- 31. Hughes JR, Hatsukami D. Signs and symptoms of tobacco withdrawal. Arch Gen Psychiatry. 1986;43(3):289–294. [DOI] [PubMed] [Google Scholar]
- 32. Cox LS, Tiffany ST, Christen AG. Evaluation of the brief questionnaire of smoking urges (QSU-brief) in laboratory and clinical settings. Nicotine Tob Res. 2001;3(1):7–16. [DOI] [PubMed] [Google Scholar]
- 33. Lussier JP, Higgins ST, Badger GJ. Influence of the duration of abstinence on the relative reinforcing effects of cigarette smoking. Psychopharmacology (Berl). 2005;181(3):486–495. [DOI] [PubMed] [Google Scholar]
- 34. Johnson MW, Bickel WK, Kirshenbaum AP. Substitutes for tobacco smoking: A behavioral economic analysis of nicotine gum, denicotinized cigarettes, and nicotine-containing cigarettes. Drug Alcohol Depend. 2004;74(3):253–264. [DOI] [PubMed] [Google Scholar]
- 35. Beck AT, Steer RA, Brown GK.. BDI-II Manual. San Antonio, TX: The Psychological Corporation; 1996. [Google Scholar]
- 36. Campbell-Sills L, Norman SB, Craske MG, et al. Validation of a brief measure of anxiety-related severity and impairment: The Overall Anxiety Severity and Impairment Scale (OASIS). J Affect Disord. 2009;112(1–3):92–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Tiffany ST, Drobes DJ. The development and initial validation of a questionnaire on smoking urges. Br J Addict. 1991;86(11):1467–1476. [DOI] [PubMed] [Google Scholar]
- 38. Perkins KA, Karelitz JL, Giedgowd GE, Conklin CA, Sayette MA. Differences in negative mood-induced smoking reinforcement due to distress tolerance, anxiety sensitivity, and depression history. Psychopharmacology (Berl). 2010;210(1):25–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Reid HH, Ledgerwood DM. Depressive symptoms affect changes in nicotine withdrawal and smoking urges throughout smoking cessation treatment: Preliminary results. Addict Res Theory. 2016;24(1):48–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Leventhal AM, Ameringer KJ, Osborn E, Zvolensky MJ, Langdon KJ. Anxiety and depressive symptoms and affective patterns of tobacco withdrawal. Drug Alcohol Depend. 2013;133(2):324–329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Piper ME, Cook JW, Schlam TR, Jorenby DE, Baker TB. Anxiety diagnoses in smokers seeking cessation treatment: Relations with tobacco dependence, withdrawal, outcome and response to treatment. Addiction. 2011;106(2):418–427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Tonkin SS, Williams TF, Simms LJ, et al. Withdrawal symptom, treatment mechanism, and/or side effect? Developing an explicit measurement model for smoking cessation research. Nicotine Tob Res. 2018. doi: 10.1093/ntr/nty262 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Cook J, Spring B, McChargue D, Doran N. Effects of anhedonia on days to relapse among smokers with a history of depression: A brief report. Nicotine Tob Res. 2010;12(9):978–982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Kamens HM, Silva CP, Nye RT, et al. Pharmacokinetic profile of Spectrum reduced nicotine cigarettes. Nicotine Tob Res. ntz045. doi: 10.1093/ntr/ntz045 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.