Abstract
Introduction
Young adults (aged 18–24 years) have a higher smoking prevalence than younger and older age groups and young adulthood is an important developmental period during which long-term behavior patterns like cigarette smoking are established. The aim of the current study was to examine how young adult smokers with additional vulnerabilities to smoking respond to reduced nicotine content cigarettes.
Methods
This is a secondary analysis of a double-blind, within-subject experiment conducted with 169 cigarette smokers recruited from populations with comorbid psychiatric conditions or socioeconomic disadvantage assessing acute effects of research cigarettes varying in nicotine content (0.4, 2.4, 5.2, 15.8 mg/g). Participants were dichotomized by chronological age (18–24 vs. ≥25 years). Across 14 laboratory sessions effects of nicotine content were examined on measures of relative reinforcing efficacy (Cigarette Purchase Task [CPT] and Concurrent Choice testing), subjective effects, craving/withdrawal, and smoking topography. Repeated measures analysis of variances were used to examine potential moderating effects of age.
Results
Young adults exhibited lower demand for reduced nicotine content cigarettes than older adults across three of five CPT indices (ps < .05). No differences by age were observed on other measures of reinforcing efficacy, subjective effects, craving/withdrawal, or smoking topography where effects generally decreased as an orderly function of decreasing nicotine content (ps <.05).
Conclusion
Overall, these findings suggest that reducing the nicotine content of cigarettes would decrease the addiction potential of cigarette smoking in young adult smokers as much or perhaps more than older adult smokers from populations at increased vulnerability to smoking, addiction, and smoking-related health consequences.
Implications
Reducing the nicotine content in cigarettes to lower addiction potential of smoking has been proposed as a means to improve overall population health. It is imperative to examine how young adults may respond to a nicotine reduction policy. We saw minimal evidence that age moderates acute response and where there was evidence it was in the direction of reduced nicotine content cigarettes having less addictive potential among young versus older adults (eg, steeper decreases in demand for very low nicotine content cigarettes among young versus older adults). Overall, a nicotine reduction policy has the potential to reduce smoking across age groups.
Introduction
Cigarette smoking contributes to approximately half a million deaths in the United States per year,1 with nicotine well established as the constituent in cigarette smoke that promotes repeated use and eventual dependence.1,2 Reducing the nicotine content in cigarettes to very low levels is hypothesized to have the potential to reduce the reinforcing value of cigarettes to a level that may help current smokers quit and reduce the likelihood that those experimenting with cigarettes would go on to develop dependence.3 The US Food and Drug Administration is considering a product standard to reduce the maximal nicotine content in cigarettes in order to lower the addiction potential of cigarette smoking, as well as reduce initiation, facilitate smoking cessation, and improve overall population health.4 Initial research investigating nicotine reduction in cigarettes has proven promising. Studies examining reduced nicotine content (RNC) cigarettes in relatively healthy smokers5–7 and smokers with additional vulnerabilities to cigarette smoking and dependence8–12 have demonstrated that cigarettes with very low nicotine content (VLNC; ie, ≤ 2.4 mg of nicotine per gram of tobacco [mg/g]) may lower the addiction potential of smoking without causing untoward withdrawal, craving, or compensatory smoking.
As RNC cigarettes are investigated with the aim of informing tobacco regulation, it is imperative to examine how different subsets of the smoking population may be affected by a nicotine reduction policy. One group of interest is young adults who have a higher prevalence of smoking compared to younger and older age groups.13 In addition, smoking initiation is increasingly occurring in young adulthood, rather than adolescence.14,15 This group is also in a relatively early stage of their smoking history where many quit smoking while others transition to a long-term pattern of chronic smoking and related health consequences.16–18 Young adults not only have a relatively different smoking profile compared to older, more established smokers, but are explicitly targeted in tobacco marketing19,20 with manufacturers recognizing this age group as being in transition between experimental and established smoking and using marketing strategies to promote the latter.21
To the best of our knowledge, there is only one prior study in the literature examining age as a moderator of response to RNC cigarettes among young adults.22 That study was a secondary analysis of a randomized trial of 6 weeks of exposure to cigarettes varying in nicotine content in smokers sampled from the general population. After 2 weeks of exposure, young adults (aged 18–24 years) reported fewer positive subjective effects from VLNC cigarettes and smoked fewer cigarettes per day compared to older adults (≥ 25), although by 6 weeks the older adults had similarly decreased their positive ratings and use of the VLNC cigarettes.22 These initial differences observed between the two age groups suggest a potential greater sensitivity to the effects of nicotine reduction among younger compared to older adult smokers, perhaps due to shorter smoking histories and weaker conditioned reinforcing effects that help to maintain smoking.
As we examine the potential impacts of a nicotine reduction policy, it is important to investigate effects in those with increased vulnerability to smoking, such as those with comorbid psychiatric conditions or socioeconomic disadvantage, groups in which smoking prevalence has remained relatively stable or even increased as overall rates declined in the general population.23,24 These vulnerable populations often have higher rates of smoking, addiction severity, and less success in quitting.23–25 As we examine vulnerable populations, it is also important to examine how age or other sociodemographic risk factors for smoking may moderate response to nicotine reduction in these groups, which have come to represent a relatively large proportion of the overall current smoking population. Evidence suggests that risk factors for smoking (eg, age, psychiatric comorbidities, socioeconomic disadvantage) have an independent and summative effect on overall smoking risk.26
The primary aim of the current study was to provide a comprehensive examination of whether age (18–24 vs. ≥ 25 years) moderates response to RNC cigarettes within populations with comorbid psychiatric conditions or socioeconomic disadvantage. Items of interest are those especially relevant to the addiction potential of smoking including relative reinforcing effects, positive subjective effects, and reductions in withdrawal and craving, as well as measures related to toxin exposure including compensatory smoking. In addition, this study allows for comparisons with findings from the prior study22 mentioned earlier examining potential moderating effects of age on response to RNC cigarettes in a population of relatively healthy smokers.
Methods
Study Sample
Participants in this multisite study were 169 adults aged 18–70 years who were daily smokers (≥ 5 cigarettes/day) from three populations with comorbid psychiatric conditions or socioeconomic disadvantage (56 with affective disorder, 60 maintained on medication for opioid dependence, 53 socioeconomically disadvantaged women of reproductive age). All participants provided written informed consent and study inclusion and exclusion criteria have been reported previously.9 Briefly, all participants reported being daily smokers for at least the past year with limited current use of other nicotine and tobacco products (<10 days in the past month), and no current illicit drug use other than marijuana. For this secondary analysis, participants across all three populations were combined and categorized by age (18–24 or 25–70 years). These age brackets were selected to examine study participants in early versus later adulthood27 and to be able to compare results to the prior study on this topic.22
Research Cigarettes
The study used Spectrum research cigarettes manufactured by 22nd Century Group (Clarence, NY) and obtained from the National Institute on Drug Abuse. Four nicotine doses were investigated with nicotine content averaged across menthol and non-menthol products (assignment of a menthol or non-menthol product was based on a participant’s usual brand): 15.8, 5.2, 2.4, and 0.4 mg/g). The 15.8 mg/g dose served as a control for nicotine levels typical of commercial cigarettes. All cigarettes were tested under double-blind conditions.
Procedure
These procedures have been described previously.9 Briefly, in a within-subjects design, participants completed fourteen 2–4 hour experimental sessions following overnight smoking abstinence operationalized as a breath carbon monoxide (CO) reading less than one-half of baseline levels. Sessions were organized into three phases.
Phase 1 (Sessions 2–5)
Following a baseline session (Session 1) in which participants were oriented to the research protocol using their usual brand cigarette, participants completed four sessions in which each of the four research cigarettes was sampled once. Research cigarettes were administered in randomized order across sessions. In each session, participants smoked one of the four research cigarettes ad-lib using a plastic cigarette holder connected to a device that recorded smoking topography (Clinical Research Support System): puff volume, duration, velocity, interpuff interval, and number of puffs.28
Immediately following smoking the research cigarettes, participants completed two questionnaires; the modified cigarette evaluation questionnaire (mCEQ), a 12-item questionnaire with five subscales (Smoking Satisfaction, Craving Reduction, Psychological Reward, Enjoyment of Respiratory Tract Sensations, and Aversion) evaluating aspects of the subjective effects of cigarette smoking,29 and the Cigarette Purchase Task (CPT), a measure of the relative reinforcing effects of smoking that assesses cigarette demand under constraint.30 The CPT simulates consumer demand under constraint by having participants estimate the number of cigarettes they would anticipate smoking in a 24-hour period across a wide range of cigarette prices; those estimates are used to model (1) participant cigarette smoking rate when unconstrained by cost (Intensity), (2) maximal amount of money one is willing to spend on daily smoking (Maximum Expenditure), (3) the price at which smoking rate begins decreasing proportionate to increasing price (Maximum Price), (4) the price at which one would quit smoking rather than incur the cost (Breakpoint), and (5) overall sensitivity of demand to price (Elasticity). Greater Intensity, Maximum Expenditure, Maximum Price, and Breakpoint and lower Elasticity represent greater demand for cigarettes.
Measures of smoking craving and withdrawal—Minnesota Tobacco Withdrawal Scale (MTWS)31 and Questionnaire of Smoking Urges-brief scale (QSU-brief)32—were collected at each session: once prior to smoking and every 15 minutes for 1 hour following smoking. Expired breath CO was also collected prior to and every 15 minutes for 1 hour following smoking the research cigarettes.
Phase 2 (Sessions 6–11)
After the four research cigarettes had been sampled in Phase 1, a direct test of the relative reinforcing efficacy of the research cigarettes was conducted in Phase 2 (Sessions 6–11) by allowing participants to choose which cigarette they preferred to smoke in a series of two-cigarette concurrent test sessions.33,34 Each of the six possible dose–pair combinations was tested once in separate sessions.
In these 3-hour sessions, participants sat alone in a comfortable, well-ventilated room. When they wished to smoke they used a computer mouse to click on one of two icons on a computer screen representing the two cigarettes available during that test session. After ten clicks on an icon, participants could take two standardized, controlled puffs from the selected cigarette. Participants were free to smoke as much as they wanted or to abstain completely during test sessions.
Phase 3 (Sessions 12–14)
Phase 3 used the same arrangement as Phase 2, but compared only the least and most preferred cigarettes in Phase 2, which were the 0.4 and 15.8 mg/g cigarettes, respectively. This phase was designed to assess whether product preference could be reliably shifted, increasing the response cost for the preferred product. The low dose cigarette remained available for 10 computer mouse clicks on the corresponding icon in Phase 3 sessions while access to the highest dose was available under a progressive-ratio schedule where availability started at a cost of 10 computer mouse clicks and then increased each time it was chosen to 160, 320, 640, 1280, 2400, 3600, 4800, 6000, 7200, 8400.35 This same arrangement was tested across three sessions to assess the stability of effects.
Statistical Methods
All outcomes across Phase 1 were examined using repeated-measures analysis of variance, with nicotine dose and time across session (where applicable) as the within-participant factors and age as a fixed effect with two levels (18–24 vs. 25–70 years old). Outcome measures were mCEQ subscales, CPT indices, smoking topography indices, MTWS, MTWS item no. 4 rating desire to smoke, QSU-Brief subscales, and change in CO levels (ie, CO boost) across sessions. To measure CO boost, pre-smoking CO values were subtracted from post-smoking CO values. Analyses also included fixed effects for (1) session, (2) the three primary study vulnerable populations who were studied in independent parallel experiments using common research protocols, (3) sequence of presentation of cigarettes, and (4) study site. The Fagerström Test for Nicotine Dependence scores and cigarettes-per-day (CPD) were included as covariates in all analyses as they differed significantly between age groups (p < .05). Time-by-dose interactions were included to test whether measures collected before and after smoking differed by dose; age-by-dose interactions were included to test for differential effects of age by dose; when not significant, interaction effects were dropped from models.
For CPT, four of the five indices (Intensity, Maximum Expenditure, Maximum Price, and Breakpoint) were calculated from observed values and log transformed to correct for skewness. To quantify participant-level CPT demand Elasticity, a demand curve was fit to individual consumption at each price for each dose.36 Elasticity values greater than 1.00 were winsorized to 1.00 prior to statistical analysis (22 of 845 cases). We reviewed CPT results and found systematic patterns in 92.7% of demand curves; no data were excluded from analyses. In cases where participants reported zero consumption across all prices (54 of 845 cases), curve fitting was not possible; so elasticity was not analyzed and other demand indices were quantified as zero.30
Differences in preference among all possible dose pairs in Phase 2 Concurrent Choice testing were similarly examined using repeated-measures analysis of variance, with each pairwise combination as the within-participant factor and age, population, site, and session as fixed effects. Differences among participants in preference for the highest- versus lowest-dose cigarettes in Phase 3 were examined using analysis of variance, with session as the repeating factor; age as the between-subjects factor; and site, session, and population as the fixed effects.
Across all analyses, statistical significance was defined as p < .05 (two-tailed). Significant main or interaction effects were followed by post hoc testing to determine mean differences by dose, age, or interaction effects using Bonferroni corrections, dividing the critical value (p < .05) by the number of comparisons to derive a more conservative Type I error rate. All analyses were conducted using SAS 9.4 (SAS Institute, Cary, NC).
Analyses were also conducted with age treated as a continuous variable. Doing so did not alter results in any meaningful manner from those where age was treated as a categorical variable (ie, 18–24 vs. 25–70). Thus only results from the categorical analyses are reported below. Reporting results from the categorical analyses also facilitates comparisons to the prior study22 on this topic which also treated age as a categorical variable using the same age brackets.
Results
Participant Characteristics
Demographic and smoking characteristics are displayed in Table 1. Approximately 17% of participants were between 18–24 years. Age groups differed by vulnerable population, dependence severity, and CPD, with younger adults being less likely to be opioid maintained, having lower nicotine dependence severity, and smoking fewer CPD (ps < .05).
Table 1.
Baseline Characteristics by Age Group Category
| 18–24 | ≥25 | p valuea | |
|---|---|---|---|
| N | 28 | 141 | |
| Gender no. (% female) | 22 (78.6) | 98 (69.5) | .37 |
| Population no. (%) | <.0001 | ||
| Affective disorders | 13 (46.3) | 43 (30.5) | |
| Opioid maintained | 1 (3.6) | 59 (41.8) | |
| Disadvantaged women | 14 (50) | 39 (27.7) | |
| Race no. (%) | |||
| Asian | 0 (0) | 1 (0.7) | .34 |
| Black | 3 (10.7) | 20 (14.2) | |
| Hawaiian/Pacific Islander | 0 (0) | 1 (0.7) | |
| Latino/Hispanic | 3 (10.7) | 3 (2.1) | |
| More than one race | 3 (10.7) | 12 (8.5) | |
| White | 19 (67.9) | 104 (73.8) | |
| Menthol status no. (% menthol) | 9 (32.) | 50 (35.5) | .39 |
| Cigarettes per day | 13.1 (6.7) | 16.3 (7.5) | .04 |
| FTND, mean (SD) | 4.3 (1.9) | 5.1 (2.8) | .04 |
| Age at first cigarette | 16.4 (2.5) | 16.2 (4.5) | .90 |
| Breath carbon monoxide | 18.4 (6.7) | 23.2 (12.6) | .05 |
a p values represent t-tests of differences across groups or chi-squared tests for differences across categorical variables. Bold text indicates significant effects at below 0.05. FTND = Fagerström Test for Nicotine Dependence.
Phase 1 Testing
Cigarette Purchase Task
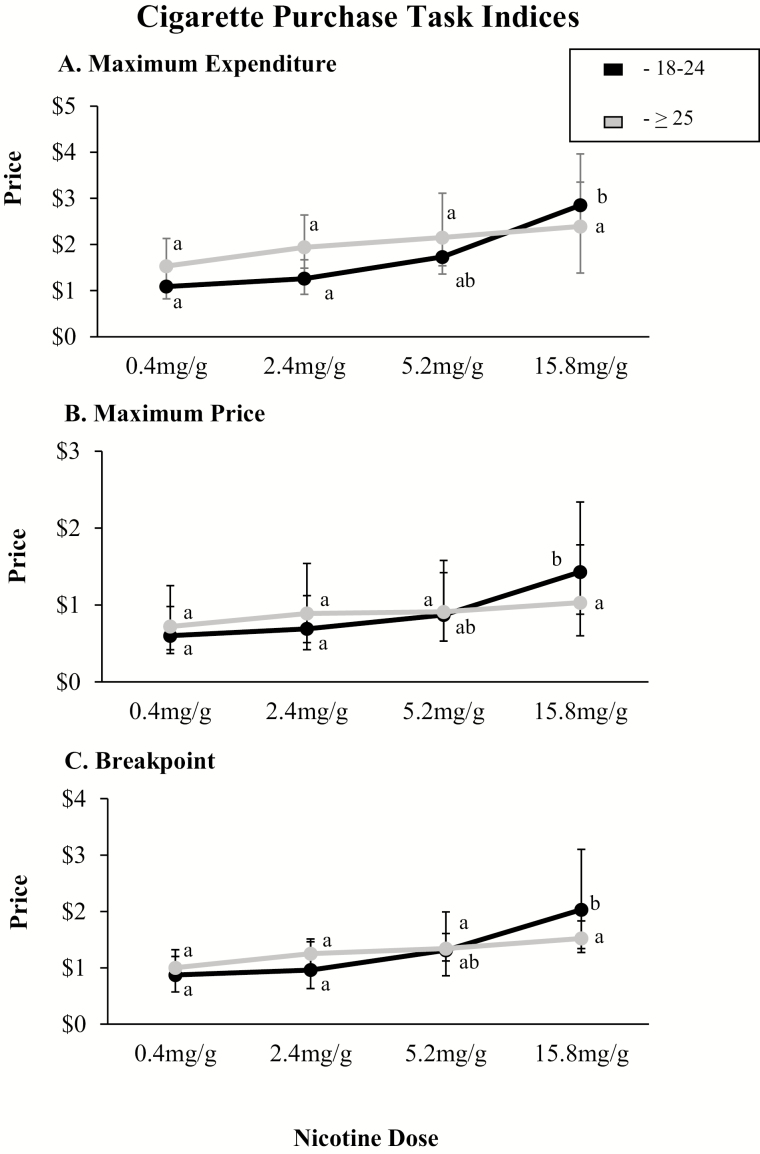
In simulated testing of the relative reinforcing effects of the different cigarette doses using the CPT, demand increased as a function of dose across each of the five indices of demand (Intensity: F3,501 = 5.65, p = .0008; Maximum Expenditure: F3,501 = 13.78, p < .0001; Maximum Price:F3,501 = 13.19, p < .0001; Breakpoint: F3,501 = 15.60, p < .0001; Elasticity: F3,456 = 7.47, p <.0001). There was no main effect of age on any of the CPT indices, but there were significant interactions of dose by age on Maximum Expenditure (F3,498 = 2.78, p = .04), Maximum Price (F3,498 = 3.87, p = .01), and Breakpoint (F3,498 = 3.01, p = .03) with dose–effect curves for these indices generally being steeper among the younger compared to older smokers. Post hoc testing demonstrated a pattern wherein Maximum Expenditure, Maximum Price, and Breakpoint for the 0.4 and 2.4 mg/g cigarettes were significantly lower than the 15.8 mg/g cigarette in the younger age group but not the older age group. The 5.2 mg/g cigarette was not significantly different than the 0.4, 2.4, or 15.8 mg/g cigarette in 18–24 or the ≥ 25 age groups (Figure 1).
Figure 1.
Demand Indices from the Cigarette Purchase Task simulating demand for cigarette smoking at escalating price. Error bars represent 95% confidence intervals (CIs). Data points not sharing a letter differ significantly by dose within age group (p < .05). Panel A represents mean values by age group (18–24 vs. ≥ 25 years old) at each dose for maximum expenditure per cigarette. Panel B represents mean values by age group (18–24 vs. ≥ 25 years old) at each dose for price per cigarette at which smoking rate begins decreasing proportionate to increasing price (Maximum Price). Panel C represents mean values by age group (18–24 vs. ≥ 25 years old) at each dose for Breakpoint, or price per cigarette at which smoking rate decreases to 0. Analyses included session order, project, CPD, and FTND as covariates. FTND = Fagerström Test for Nicotine Dependence.
Modified Cigarette Evaluation Scale
Ratings on each of the five mCEQ subscales varied as a graded function of nicotine content, with subjective value increasing as nicotine content for the cigarettes increased (Smoking Satisfaction: F3,498 = 27.15, p < .0001; Craving Reduction: F3,498 = 16.13, p < .0001; Psychological Reward: F3,498 = 13.71, p < .0001; Enjoyment of Respiratory Tract Sensations: F3,498 = 19.25, p < .0001; Aversion: F3,498 = 5.04, p < .01) (Table 2). No significant effects of age nor interactions of dose by age were noted on any of the mCEQ subscales.
Table 2.
Modified Cigarette Evaluation Questionnaire Subscale Scores by Age and Cigarette Nicotine Dose
| Smoking satisfaction | Psychological reward | Aversion | Enjoyment of respiratory tract sensations | Craving reduction | |
|---|---|---|---|---|---|
| Age, mean ± SEM | |||||
| 18–24 | 3.58 ± 0.24 | 2.83 ± 0.24 | 1.74 ± 0.21 | 3.28 ± 0.26 | 3.48 ± 0.38 |
| ≥25 | 3.89 ± 0.11 | 3.05 ± 0.10 | 1.53 ± 0.14 | 3.49 ± 0.11 | 3.82 ± 0.26 |
| Nicotine dose, mean ± SEM | |||||
| 0.4 mg/g | 3.09 ± 0.14a | 2.61 ± 0.14a | 1.59 ± 0.16a | 2.85 ± 0.17a | 3.13 ± 0.30a |
| 2.4 mg/g | 3.52 ± 0.14b | 2.78 ± 0.14ab | 1.59 ± 0.16a | 3.10 ± 0.17a | 3.44 ± 0.30ab |
| 5.2 mg/g | 3.78 ± 0.14b | 3.00 ± 0.14b | 1.59 ± 0.16a | 3.50 ± 0.17b | 3.73 ± 0.30b |
| 15.8 mg/g | 4.54 ± 0.14c | 3.38 ± 0.14c | 1.82 ± 0.16b | 4.08 ± 0.17c | 4.30 ± 0.30c |
Tabled values represent least square means (± SEM). Post hoc mean comparisons were only conducted when significant main effects or interactions were observed. Upper panel shows mean ratings for each modified Cigarette Evaluation Questionnaire subscale/item averaged across participants and nicotine dose for smokers categorized by age. There were no significant effects of age nor interactions of age and dose. Lower panel shows mean ratings by nicotine dose averaged across all participants. There were significant main effects of dose across all subscales. Data points not sharing a superscript letter differ significantly within each subscale or dose in post-hoc testing with Bonferroni corrections. All analyses included session order, project, CPD, and FTND as covariates. FTND = Fagerström Test for Nicotine Dependence.
Craving and Withdrawal
There was a significant interaction of dose by time on MTWS total scores, with all doses decreasing withdrawal scores immediately following smoking. This dose effect dissipated over the 1-hour interval time period, but the duration of effects persisted longer at the 15.8 mg/g dose (F12,2014 = 2.64, p = .002) (Supplementary Table 1). There was no significant effect of age nor interactions involving age, dose, or time noted on withdrawal (ps >.05).
Like the measure of withdrawal, significant interactions of nicotine dose by time were observed on craving as measured using the “Desire to Smoke” item (ie, Item no. 4) on the MTWS and the two factors of the QSU (ie, Factor 1 and Factor 2) (Supplementary Tables 1 and 2). All doses decreased craving ratings to some degree with effects dissipating across time (Desire to Smoke: F12,2014 = 5.86, p < .0001; QSU Factor 1: F12, 2014 = 8.92, p < .0001; QSU Factor 2: F12,2014 = 5.22, p < .0001), but magnitude and duration of effects were greatest at the 15.8 mg/g content cigarette. No effects of age nor interactions involving age were noted for craving (ps > .05).
Smoking Topography and CO Boost
The only significant effects of dose on measures of smoking topography were increases in total puff volume (F3,487 = 4.57, p = .004) and number of puffs per cigarette (F3,484 = 13.32, p < .001) at the higher compared to lower doses. There were no significant effects of age nor age by dose interactions on puff topography measures (ps > .05) (Supplementary Table 3). The only significant effect on CO boost was for time (F3,504 = 104.46, p < .0001), with levels decreasing across doses as time since smoking increased (ps > .05) (data not shown).
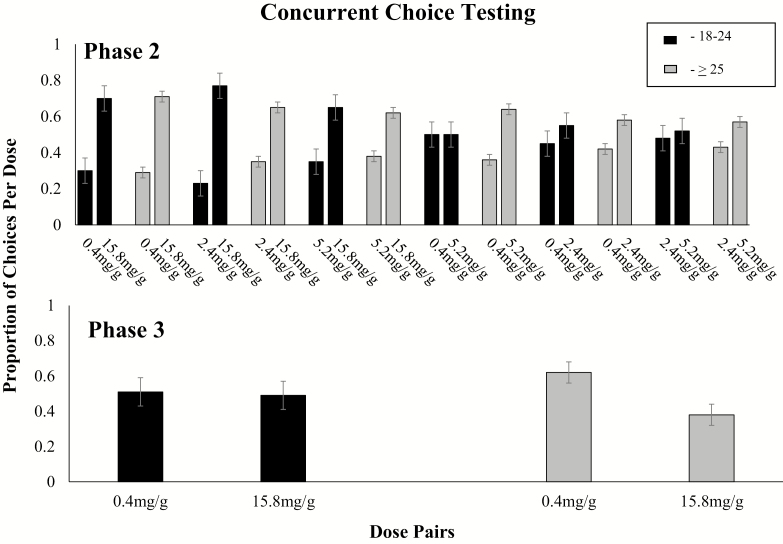
Phase 2 Testing
No significant differences by age were observed in direct testing of the relative reinforcing effects of the different dose pairs using the concurrent choice arrangement (Figure 2, upper panel). When response cost was equal across the dose pairs, there was a main effect of dose on cigarette choice (F5,830 = 5.98, p < .0001), with the higher nicotine content cigarette in each pair being chosen significantly more often than the lower nicotine content. There was no significant effect of age nor dose by age interactions on preference for higher over lower doses within each dose pair (ps > .05).
Figure 2.
Upper panel: mean proportion of choices allocated to all possible 2-dose comparisons across the four nicotine dose cigarettes (0.4, 2.4, 5.2, 15.8 mg/g tobacco) across Phase 2 choice sessions within each age group (18–24 vs. ≥ 25 years old). Lower panel: mean proportion of choices across Phase 3 sessions within each age group (18–24 vs. ≥ 25 years old). Data points represent mean proportion of choices allocated to the different nicotine dose cigarettes. Error bars represent ± SEM. No significant differences observed by age were noted. Analyses included session order, project, CPD, and FTND as covariates. FTND = Fagerström Test for Nicotine Dependence.
Phase 3 Testing
When the 0.4 and 15.8 mg/g cigarettes were retested in the concurrent choice arrangement with the higher dose cigarette now on a progressive-ratio schedule, preference shifted to the low dose cigarette (t1,158 = 3.41, p <.001) (Figure 1, bottom panel). There was no significant effect of age on this relationship (F1,160 = 2.85, p = .09).
Discussion
The aim of the current study was to provide a comprehensive examination of potential moderating effects of age on acute response to RNC cigarettes in a population with comorbid psychiatric conditions or socioeconomic disadvantage. More specifically, we were interested in whether young adults with these comorbid conditions might respond to nicotine reduction in ways that could preclude them from benefitting from an RNC policy. Overall, we saw minimal evidence that age moderates response to reduced nicotine cigarettes in these vulnerable populations. Moreover, where we did see evidence of moderation in these vulnerable populations it was in a direction where young adults could potentially benefit more from an RNC policy. That is, young adults exhibited significant decreases in demand for the VLNC compared to the higher dose cigarettes across three of the five CPT indices, whereas the older adults did not. This may suggest that there is a greater difference in reinforcing efficacy between the high and low nicotine content cigarettes among young compared to older adults. Overall, this pattern suggests that a policy that established a low maximal nicotine standard in cigarettes should benefit vulnerable adult smokers independent of age, but may be even more impactful among younger adults.
In the prior investigation of age as a potential moderator of response to extended exposure to RNC cigarettes,22 young adults also exhibited evidence of greater sensitivity to nicotine content differences than older adults. As described earlier, young adults exhibited significant decreases in smoking rates in the first two weeks of exposure to the VLNC cigarettes while comparable reductions only emerged among older adults after six weeks of exposure. Whether these findings suggesting a greater sensitivity to dose reductions among younger smokers are a result of a shorter smoking history and thus less sensitivity to conditioned reinforcing effects or a greater sensitivity to the direct effects of nicotine among young adults is unclear. Importantly, any greater sensitivity to nicotine content reductions among young adults was not associated with evidence of compensatory smoking in the prior or present study. In the present study, for example, we saw no evidence of changes in puff topography or CO boost in the direction of young adults attempting to obtain greater nicotine exposure from the RNC cigarettes. Taken together, these findings suggest that young adults from both the more vulnerable and general populations of smokers may behave similarly in the face of a nicotine reduction policy.
Finally, we saw no evidence that age moderated effects of RNC cigarettes on craving or withdrawal. Effects on these measures were dose dependent, but each of the cigarettes examined produced significant amelioration of craving and withdrawal suggesting that an RNC policy is not likely to cause problems of untoward craving or withdrawal in young adults or older smokers despite the presence of comorbid conditions, effects consistent with prior findings in these populations.10–12,22
Limitations of the present study include being a secondary analysis of a study not designed to examine age effects.9 As a result, only a relatively small proportion of the total sample of 169 participants (16.6% or 28 participants) were in the younger age category. We cannot rule out the possibility that a larger sample of young adults and associated greater statistical power may have revealed a greater influence of age on the outcomes examined. As noted earlier, however, treating age as a continuous rather than a categorical variable did not alter the results in any meaningful way. Moreover, our findings are consistent with those in the only prior study on this topic.22 Another limitation is that study inclusion criteria required participants to be daily smokers and to smoke five or more CPD. Considering that a sizeable proportion of young adult smokers do not smoke daily37,38 the findings from the present and prior study on this topic may not generalize to young adult lighter smokers. These limitations notwithstanding, this investigation in a vulnerable group of smokers and the prior findings in a relatively healthier population of smokers22 suggest that a nicotine reduction policy is as likely or perhaps even more likely to reduce the addiction potential of smoking in young as older adult daily smokers.
Funding
This project was supported by Tobacco Centers of Regulatory Science award U54DA036114 from the National Institute on Drug Abuse (NIDA) and Food and Drug Administration (FDA), Center of Biomedical Research Excellence award P20GM103644 from the National Institute of General Medical Sciences (NIGMS), and NIDA Institutional Training Grant T32DA007242. The content is solely the responsibility of the authors and does not necessarily represent the official views of NIDA, FDA, or NIGMS.
Declaration of Interests
None declared.
Supplementary Material
References
- 1. The Health Consequences of Smoking—50 Years of Progress: A Report of the Surgeon General. Atlanta, GA: U.S. Department of Health and Human Services, Centers for Disease Control and Prevention, National Center for Chronic Disease Prevention and Health Promotion, Office on Smoking and Health; 2014. https://www.ncbi.nlm.nih.gov/books/NBK179276/pdf/Bookshelf_NBK179276.pdf [Google Scholar]
- 2. The Health Consequences of Smoking: Nicotine Addiction: A Report of the Surgeon General. Washington, DC: US. Department of Health and Human Services (DHHS Publication No. CDC 90-8416), U.S. Government Printing Office. 1988;https://profiles.nlm.nih.gov/ps/access/nnbbzd.pdf. [Google Scholar]
- 3. Benowitz NL, Henningfield JE. Establishing a nicotine threshold for addiction. The implications for tobacco regulation. N Engl J Med. 1994;331(2):123–125. [DOI] [PubMed] [Google Scholar]
- 4. Tobacco Product Standard for Nicotine Level of Combusted Cigarettes: A Proposed Rule by the Food and Drug Administration on 3/16/2018. Washington, DC: Food and Drug Administration, HHS; 3/16/2018. https://www.federalregister.gov/documents/2018/03/16/2018-05345/tobacco-product-standard-for-nicotine-level-of-combusted-cigarettes [Google Scholar]
- 5. Donny EC, Denlinger RL, Tidey JW, et al. Randomized trial of reduced-nicotine standards for cigarettes. N Engl J Med. 2015;373(14):1340–1349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Hatsukami DK, Luo X, Jensen JA, et al. Effect of immediate vs gradual reduction in nicotine content of cigarettes on biomarkers of smoke exposure: a randomized clinical trial. JAMA. 2018;320(9):880–891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Hatsukami DK, Heishman SJ, Vogel RI, et al. Dose-response effects of spectrum research cigarettes. Nicotine Tob Res. 2013;15(6):1113–1121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Higgins ST, Heil SH, Sigmon SC, et al. Response to varying the nicotine content of cigarettes in vulnerable populations: an initial experimental examination of acute effects. Psychopharmacology (Berl). 2017;234(1):89–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Higgins ST, Heil SH, Sigmon SC, et al. Addiction potential of cigarettes with reduced nicotine content in populations with psychiatric disorders and other vulnerabilities to tobacco addiction. JAMA Psychiatry. 2017;74(10):1056–1064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Tidey JW, Rohsenow DJ, Kaplan GB, Swift RM, Ahnallen CG. Separate and combined effects of very low nicotine cigarettes and nicotine replacement in smokers with schizophrenia and controls. Nicotine Tob Res. 2013;15(1):121–129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Tidey JW, Cassidy RN, Miller ME. Smoking topography characteristics of very low nicotine content cigarettes, with and without nicotine replacement, in smokers with schizophrenia and controls. Nicotine Tob Res. 2016;18(9):1807–1812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Tidey JW, Pacek LR, Koopmeiners JS, et al. Effects of 6-week use of reduced-nicotine content cigarettes in smokers with and without elevated depressive symptoms. Nicotine Tob Res. 2017;19(1):59–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. SAMHSA. Results from the 2012 National Survey on Drug Use and Health: Summary of National Findings. Rockville, MD: Substance Abuse and Mental Health Services Administration, U.S. Department of Health and Human Services; 2013. https://www.samhsa.gov/data/sites/default/files/NSDUHmhfr2012/NSDUHmhfr2012.pdf [Google Scholar]
- 14. Thompson AB, Mowery PD, Tebes JK, McKee SA. Time trends in smoking onset by sex and race/ethnicity among adolescents and young adults: findings from the 2006-2013 national survey on drug use and health. Nicotine Tob Res. 2018;20(3):312–320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Perry CL, Pérez A, Bluestein M, et al. Youth or young adults: which group is at highest risk for tobacco use onset? J Adolesc Health. 2018;63(4):413–420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Richardson A, Williams V, Rath J, Villanti AC, Vallone D. The next generation of users: prevalence and longitudinal patterns of tobacco use among US young adults. Am J Public Health. 2014;104(8):1429–1436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Hertel AW, Mermelstein RJ. Smoker identity development among adolescents who smoke. Psychol Addict Behav. 2016;30(4):475–483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Chen P, Jacobson KC. Developmental trajectories of substance use from early adolescence to young adulthood: gender and racial/ethnic differences. J Adolesc Health. 2012;50(2):154–163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Soneji S, Ambrose BK, Lee W, Sargent J, Tanski S. Direct-to-consumer tobacco marketing and its association with tobacco use among adolescents and young adults. J Adolesc Health. 2014;55(2):209–215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Biener L, Albers AB. Young adults: vulnerable new targets of tobacco marketing. Am J Public Health. 2004;94(2):326–330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Ling PM, Glantz SA. Why and how the tobacco industry sells cigarettes to young adults: evidence from industry documents. Am J Public Health. 2002;92(6):908–916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Cassidy RN, Tidey JW, Cao Q, et al. Age moderates smokers’ subjective response to very-low nicotine content cigarettes: evidence from a randomized controlled trial. Nicotine Tob Res. 2019;21(7):962–969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Chilcoat HD. An overview of the emergence of disparities in smoking prevalence, cessation, and adverse consequences among women. Drug Alcohol Depend. 2009;104(suppl 1):S17–S23. [DOI] [PubMed] [Google Scholar]
- 24. Higgins ST, Chilcoat HD. Women and smoking: an interdisciplinary examination of socioeconomic influences. Drug Alcohol Depend. 2009;104(suppl 1):S1–S5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Lasser K, Boyd JW, Woolhandler S, Himmelstein DU, McCormick D, Bor DH. Smoking and mental illness: a population-based prevalence study. JAMA. 2000;284(20):2606–2610. [DOI] [PubMed] [Google Scholar]
- 26. Higgins ST, Kurti AN, Redner R, et al. Co-occurring risk factors for current cigarette smoking in a U.S. nationally representative sample. Prev Med. 2016;92:110–117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Arnett JJ. Emerging adulthood. A theory of development from the late teens through the twenties. Am Psychol. 2000;55(5):469–480. [PubMed] [Google Scholar]
- 28. Lee EM, Malson JL, Waters AJ, Moolchan ET, Pickworth WB. Smoking topography: reliability and validity in dependent smokers. Nicotine Tob Res. 2003;5(5):673–679. [DOI] [PubMed] [Google Scholar]
- 29. Cappelleri JC, Bushmakin AG, Baker CL, Merikle E, Olufade AO, Gilbert DG. Confirmatory factor analyses and reliability of the modified cigarette evaluation questionnaire. Addict Behav. 2007;32(5):912–923. [DOI] [PubMed] [Google Scholar]
- 30. Jacobs EA, Bickel WK. Modeling drug consumption in the clinic using simulation procedures: demand for heroin and cigarettes in opioid-dependent outpatients. Exp Clin Psychopharmacol. 1999;7(4):412–426. [DOI] [PubMed] [Google Scholar]
- 31. Hughes JR, Hatsukami D. Signs and symptoms of tobacco withdrawal. Arch Gen Psychiatry. 1986;43(3):289–294. [DOI] [PubMed] [Google Scholar]
- 32. Cox LS, Tiffany ST, Christen AG. Evaluation of the brief questionnaire of smoking urges (QSU-brief) in laboratory and clinical settings. Nicotine Tob Res. 2001;3(1):7–16. [DOI] [PubMed] [Google Scholar]
- 33. Lussier JP, Higgins ST, Badger GJ. Influence of the duration of abstinence on the relative reinforcing effects of cigarette smoking. Psychopharmacology (Berl). 2005;181(3):486–495. [DOI] [PubMed] [Google Scholar]
- 34. Johnson MW, Bickel WK, Kirshenbaum AP. Substitutes for tobacco smoking: a behavioral economic analysis of nicotine gum, denicotinized cigarettes, and nicotine-containing cigarettes. Drug Alcohol Depend. 2004;74(3):253–264. [DOI] [PubMed] [Google Scholar]
- 35. Sigmon SC, Tidey JW, Badger GJ, Higgins ST. Acute effects of D-amphetamine on progressive-ratio performance maintained by cigarette smoking and money. Psychopharmacology (Berl). 2003;167(4):393–402. [DOI] [PubMed] [Google Scholar]
- 36. Hursh SR, Silberberg A. Economic demand and essential value. Psychol Rev. 2008;115(1):186–198. [DOI] [PubMed] [Google Scholar]
- 37. Wortley PM, Husten CG, Trosclair A, Chrismon J, Pederson LL. Nondaily smokers: a descriptive analysis. Nicotine Tob Res. 2003;5(5):755–759. [DOI] [PubMed] [Google Scholar]
- 38. Moran S, Wechsler H, Rigotti NA. Social smoking among US college students. Pediatrics. 2004;114(4):1028–1034. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.