Abstract
Introduction
The US Food and Drug Administration is considering implementing a reduced-nicotine standard for cigarettes. Given the high rate of smoking among people with serious mental illness (SMI), it is important to examine the responses of these smokers to very low nicotine content (VLNC) cigarettes.
Methods
This trial compared the effects of VLNC (0.4 mg nicotine/g tobacco) and normal nicotine content cigarettes (15.8 mg/g) over a 6-week period in non-treatment-seeking smokers with schizophrenia, schizoaffective disorder, or bipolar disorder (n = 58). Linear regression was used to examine the effects of cigarette condition on cigarettes per day, subjective responses, nicotine and tobacco toxicant exposure, craving, withdrawal symptoms, and psychiatric symptoms.
Results
At week 6, participants in the VLNC condition smoked fewer cigarettes per day, had lower breath carbon monoxide levels, lower craving scores, and rated their study cigarettes lower in satisfaction, reward, enjoyment, and craving reduction than those in the normal nicotine content condition (ps < .05). Week 6 psychiatric and extrapyramidal symptoms did not differ by condition, except for scores on a measure of parkinsonism, which were lower in the VLNC condition (p < .05). There were no differences across conditions on total nicotine exposure, 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanol, withdrawal symptoms, or responses to abstinence.
Conclusions
These results suggest that a reduced-nicotine standard for cigarettes would reduce smoking among smokers with SMI. However, the lack of effect on total nicotine exposure indicates VLNC noncompliance, suggesting that smokers with SMI may respond to a reduced-nicotine standard by substituting alternative forms of nicotine.
Implications
Results from this trial suggest that a reduced-nicotine standard for cigarettes would reduce smoking rates and smoke exposure in smokers with SMI, without increasing psychiatric symptoms. However, noncompliance with VLNC cigarettes was observed, suggesting that these smokers might respond to a reduced-nicotine standard by substituting alternative forms of nicotine.
Introduction
People with serious mental illness (SMI; ie, those with schizophrenia spectrum or bipolar disorder) are two to three times more likely to be current smokers than the general US population,1 and about half of all deaths in people with SMI are because of tobacco-related disease.2 Factors that appear to contribute to low cessation rates among people with SMI include high severity of nicotine dependence, high levels of craving and negative affect during abstinence, and inadequate access to effective cessation treatments.3 Pharmacological and behavioral cessation treatments are effective in people with SMI,4 but quit rates are low among those who do not have access to these treatments.5
The US Food and Drug Administration is considering whether to mandate a reduction in the nicotine content of cigarettes to a minimally addictive level, which may reduce the likelihood that new smokers will become dependent on tobacco and increase the likelihood that established smokers will quit.6,7 In randomized clinical trials conducted under double-blind conditions, smokers from the general US population who are switched to very low nicotine content (VLNC) cigarettes (ie, cigarettes with ≤ 2.4 mg nicotine/g tobacco) smoke fewer cigarettes and have lower levels of nicotine and tobacco toxicant exposure than those who are switched to normal nicotine content (NNC) research cigarettes.8–11 Although some noncompliant use of conventional cigarettes is common among participants assigned to VLNC cigarettes,12 significant reductions in nicotine and tobacco toxicant exposure are observed despite this noncompliance.
By decreasing smoking rates and cigarette dependence, a reduced-nicotine standard for cigarettes has the potential to increase cessation among smokers with SMI. However, smokers with SMI might also respond to a reduced-nicotine standard for cigarettes with high levels of craving and negative mood and might increase their smoking rates in an attempt to cope with these symptoms. Studies that have assessed responses to VLNC cigarettes in smokers with mental health conditions include a laboratory study that assessed acute responses to cigarettes varying in nicotine content among smokers with opioid use disorders, affective disorders, or socioeconomic disadvantages,13 and one that compared the effects of acute VLNC and NNC cigarette use in smokers with schizophrenia versus smokers without psychiatric disorders.14 Both studies reported that VLNC cigarette use reduced behavioral and subjective measures of cigarette abuse liability without increasing smoke intake.13–15 In addition, a secondary analysis of a large clinical trial9 compared responses to 6-week VLNC or NNC cigarette use among smokers who had depressive symptoms above or below a cutoff associated with clinical depression.16 In both groups of participants, those assigned to VLNC cigarettes smoked fewer cigarettes per day (CPD), had lower levels of nicotine exposure, and had lower cigarette dependence and craving scores at week 6 than those assigned to NNC cigarettes; furthermore, those with elevated depressive symptoms at baseline who were assigned to VLNC cigarettes had lower depressive symptoms at week 6 than those who had been assigned to NNC cigarettes.16 These studies provide support for the idea that the potential benefits of a reduced-nicotine standard for cigarettes that have been observed among smokers without psychiatric disorders would extend to smokers with mental health conditions. However, to date, there have been no reports of the effects of extended VLNC cigarette use in smokers with SMI.
The aim of this study was to examine the effects of 6-week VLNC cigarette use on smoking and related subjective and physiological measures in smokers with SMI. We hypothesized that participants randomized to the VLNC condition would have lower measures of cigarette consumption, cigarette craving, and nicotine dependence compared to those in the NNC condition at week 6. We compared effects of VLNC versus NNC cigarette use on measures of mood, psychiatric symptoms, and responses to cigarette abstinence, without testing specific hypotheses.
Methods
Participants
Participants were recruited from the Providence, Rhode Island US area using community-based advertisements. Participants were required to be 18–70 years of age, to meet diagnostic criteria for schizophrenia, schizoaffective disorder, or bipolar disorder based on the Structured Clinical Interview for DSM-IV (SCID),17 to smoke at least 10 CPD, and to have breath carbon monoxide (CO) levels at least 8 ppm or urinary cotinine levels at least 100 ng/ml. Potential participants were excluded if they were pregnant or breast feeding, had a positive breath alcohol level or positive toxicology screen for illicit drugs other than cannabis, reported binge alcohol drinking (> 4/5 drinks within 2 h for women/men) on more than 9 of the past 30 days, had significant unstable medical conditions as determined by the study’s licensed medical practitioner, reported changes in psychiatric symptoms or medications within the past 4 weeks, displayed severe disorientation or uncooperativeness during the screening, reported past-month suicidal ideation or past-year suicide attempt, were seeking treatment for smoking, had made a serious quit attempt within the past 30 days, intended to quit within the next 30 days, exclusively used roll-your-own cigarettes, or had used tobacco products other than machine-made cigarettes on more than 9 of the past 30 days. Data were collected from November 2014 to September 2017. The study initially included only smokers with schizophrenia or schizoaffective disorder and was amended in July 2016 to include those with bipolar disorder given challenges with recruitment (no changes were made to stratification procedures). All procedures were approved by the Brown University Institutional Review Board.
Procedure
Participants completed a brief phone screen, followed by an in-person consenting and screening session. At the end of that session, those eligible to participate completed baseline assessments (study timeline shown in Supplementary Figure 1). During the following week, participants continued to smoke their usual brand cigarettes. One week later, participants completed a second baseline session, at the end of which they were randomized to either the 15.8 mg nicotine/g tobacco (NNC) or the 0.4 mg nicotine/g tobacco (VLNC) condition. Cigarette tar yields were 8–10 mg and participants received menthol or non-menthol cigarettes according to their preference. Study cigarettes (Spectrum cigarettes, 22nd Century Group, Inc) were provided by the National Institute on Drug Abuse and have been described in detail elsewhere.9 Randomization was conducted by the research center’s Biostatistics Core at the University of Minnesota and was stratified by gender and age group (18–30, 31–50, 51–70 years). Cigarettes were labeled with only a number code when they arrived at the study site, and all investigators, staff, and participants were blind to condition assignment.
Participants visited the laboratory weekly throughout the 6-week intervention period. At each visit, participants were provided with free study cigarettes and were instructed to use only those cigarettes if they smoked. Study cigarette compliance monitoring and counseling were provided weekly. Throughout the baseline and intervention periods, participants used an interactive voice response system (InterVision Media) to report the number of study and non-study cigarettes smoked on the previous day (reported separately). Other measures are described later. The total possible compensation for completing study procedures was $684.
In addition, participants could receive a $200 lottery-based bonus payment based on session attendance, biologically verified compliance with study cigarettes (VLNC condition only), and honesty about compliance (ie, self-reported compliance status was consistent with biochemically verified compliance status; VLNC condition only). As study cigarette compliance in the NNC condition could not be determined biochemically, participants in the NNC condition were yoked with a participant in the VLNC condition to ensure equal compensation across groups. The incentive system included a semi-bogus pipeline such that urine samples were collected weekly from all participants, and participants were told that these samples would be used to determine whether they had been compliant; in reality, samples were tested monthly and only samples from the VLNC condition were tested. To maintain the blind among staff, samples from all participants were sent to the Biomarkers Core at the University of Minnesota for analysis. Study staff were informed who had won the drawing but were not given any other information.
From post-randomization week 6 to week 7, participants were asked to abstain from smoking for as long as they could, and could receive daily financial incentives for meeting a biological criterion of abstinence during the following week (CO < 50% of the previous day’s CO or ≤ 7 ppm). Incentives for abstinence decreased daily ($80 on day 1, decreasing by 50% each day), based on an analog model of smoking lapse.18 To complete these CO tests, participants were provided with a study cell phone and CO monitor (Bedfont piCO+) and received training on how to text videos of themselves providing breath CO samples twice per day (8 am–12 pm and 4 pm –8 pm) to study staff. During laboratory visits on the first and last days of the abstinence week, participants provided CO samples and completed measures of craving and withdrawal symptoms. Thirty days after the second abstinence session, participants were recontacted by telephone and were asked if they had made a quit attempt since the last visit.
Measures
Behavioral and Physiological Measures
The primary outcome measure was total number of cigarettes smoked per day (study plus non-study) at week 6 as measured by interactive voice response. Cigarette and other tobacco use were also collected using Timeline Follow-back interviews19 at each weekly session as a secondary measure. Breath CO levels (Bedfont Scientific, Ltd) were collected weekly during the baseline and intervention weeks, and twice daily during the abstinence week as described previously. First-void urine samples were collected at baseline, week 2, and week 6 for assessment of total nicotine equivalents (TNE), a measure of nicotine exposure, which is the sum of nicotine, cotinine, trans 3’-hydroxycotinine, and their glucuronides.20 Samples collected at baseline and week 6 were also assessed for 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanol (NNAL), a measure of exposure to the tobacco-specific nitrosamine 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone (NNK). TNE and total NNAL (NNAL and its glucuronides) levels were assayed by the Biomarkers Core at the University of Minnesota using liquid chromatography with tandem mass spectrometry.
Questionnaires and Psychiatric Symptom Assessments
The following measures were collected at baseline, week 2 and week 6 except where noted. All measures were administered by trained and experienced raters. Cigarette dependence was measured using the Fagerström Test for Cigarette Dependence.21 Craving for the product currently being used (usual brand at baseline; randomized condition at week 6 and abstinence assessment) was measured weekly using the 10-item Questionnaire on Smoking Urges–brief scale (QSU).22 Nicotine withdrawal symptoms were measured weekly using the 8-item Minnesota Nicotine Withdrawal Scale (MNWS).23 Subjective responses to the cigarettes were measured weekly using the modified Cigarette Evaluation Scale (CES),24 which includes five subscales: Satisfaction (satisfaction, taste, enjoyment), Psychological Reward (calm, feel more awake, less irritable, help to concentrate, reduce hunger items), Craving Reduction (single item), Enjoyment of Respiratory Tract Sensations (single item) and Aversion (dizziness, nausea items). Positive and negative mood states were measured using the Positive and Negative Affect Schedule (PANAS).25 Psychiatric symptoms were measured using the Positive and Negative Syndrome Scale (PANSS),26 the Scale for Assessment of Negative Symptoms (SANS),27 the Center for Epidemiologic Studies—Depression Scale (CES-D),28 and the Calgary Depression Scale for Schizophrenia.29 The Brief Psychiatric Rating Scale (BPRS)30 was used to measure psychiatric symptom severity at visits in which the PANSS and SANS were not administered (weeks 1, 3, 4 and 5). Extrapyramidal symptoms related to antipsychotic medication use were measured using the Abnormal Involuntary Movements Scale (AIMS),31 which assesses tardive dyskinesia, the Simpson-Angus Scale,32 which measures parkinsonism, and the Barnes Akathisia Rating Scale (BARS),33 which measures motor restlessness.
Statistical Analyses
Participant characteristics at baseline were compared using two-sample t-tests with equal variances or Fisher’s exact tests. Linear regression analyses were used to examine the effects of cigarette condition on outcome measures collected at post-randomization week 6, first controlling only for baseline levels of each variable, and then also adjusting for the stratification variables, gender, and age. Most outcomes are expressed in the tables as mean differences (95% confidence interval [CI]) between the cigarette conditions, with negative values indicating that those in the VLNC condition reported a reduction in this measure relative to those in the NNC condition. Associations between nicotine content and the biomarkers TNE and total NNAL are summarized using ratios of geometric means (95% CI) from the VLNC condition relative to geometric means from the NNC condition.
During the abstinence period, days to lapse was estimated using the Kaplan–Meier method and analyzed by the Cox proportional hazard model. The total number of days abstinent was analyzed using simple linear regression (unadjusted). Linear regression was used to examine the effects of cigarette condition on QSU and MNWS scores collected during the first day of abstinence, controlling for levels of these variables at the week 6 session and the stratification variables, gender, and age. Data from the last day of the abstinence week were not analyzed and are not included in this report. Likelihood of having made at least one quit attempt during the 30-day follow-up period was compared across conditions using a Fisher’s exact test.
All analyses were conducted using the intent-to-treat principle. Tests were considered significant at α = 0.05, two-tailed. As this research focused on examining the potential unintended negative consequences of nicotine reduction in smokers with SMI, we considered it more important to avoid type II error than type I error. Therefore, we specified a priori that we would not include corrections for the multiple statistical tests.
Results
A total of 400 people responded to the study advertisements, of whom 126 completed the in-person consenting/screening session, 61 were eligible for the study, and 58 were randomized. The primary reasons for ineligibility at the phone or in-person screening stage were not meeting diagnostic criteria for schizophrenia, schizoaffective disorder, or bipolar disorder (n = 120), intending to quit within 30 days (n = 67), a psychiatric medication change within the past 4 weeks (n = 55) or smoking fewer than 10 CPD (n = 47). Baseline characteristics of participants randomized to the VLNC and NNC conditions are shown in Table 1. Overall, participants were 43.2 ± 10.2 (M ± SD) years old, 41% female, 59% white, and 19% African American. At enrollment, participants smoked 19.2 ± 8.3 CPD, and had FTND scores of 6.7 ± 1.5, indicating high levels of nicotine dependence. Participants were clinically stable with low-to-moderate psychiatric symptom levels. No significant differences in these measures were observed at baseline across groups. Twenty-six participants in the VLNC condition and 25 in the NNC condition completed interactive voice response through week 6 (87% and 89%, respectively; p = 1.0), and 24 participants in the VLNC condition and 25 in the NNC condition completed the week 6 in-person session (80% and 89%, respectively; p = .473).
Table 1.
Baseline Characteristics of Participants Randomized to Each Cigarette Conditiona
| VLNC (0.4 mg/g) (n = 30) | NNC (15. 8 mg/g) (n = 28) | p-value | |
|---|---|---|---|
| Age (years) | 43.4 (9.6) | 43.1 (11.0) | .924 |
| Gender (female) no. (%) | 12 (40.0) | 12 (42.9) | 1.00 |
| Race no. (%) | .412b | ||
| White | 15 (50.0)† | 19 (67.9) | |
| Black | 6 (20.0) | 5 (17.9) | |
| Multiracial | 6 (20.0) | 4 (14.3) | |
| Hispanic ethnicity no. (%) | 3 (10.0) | 0 (0) | .237 |
| Diagnosis no. (%) | .534 | ||
| Schizophrenia/schizoaffective | 22 (73.3) | 23 (82.1) | |
| Bipolar | 8 (26.7) | 5 (17.9) | |
| Psychiatric medication use (%) | |||
| Antipsychotics | 23 (76.7) | 20 (71.4) | .767 |
| Antidepressants | 12 (40.0) | 15 (53.6) | .430 |
| Antianxiety/hypnotics | 12 (40.0) | 8 (28.6) | .416 |
| Mood stabilizers | 10 (33.3) | 8 (28.6) | .780 |
| Menthol use no. (%) | 23 (76.7) | 18 (64.3) | .390 |
| Cigarettes per day | 20.1 (8.8) | 18.2 (7.7) | .374 |
| Carbon monoxide level (ppm) | 20.4 (13.0) | 21.1 (11.9) | .835 |
| TNE (nmol/mg creatinine) | 79.9 (62.8, 101.8) | 78.3 (61.7, 99.2)†† | .900 |
| NNAL (pmol/mg creatinine) | 1.60 (1.13, 2.25) | 1.74 (1.29, 2.33) †† | .709 |
| FTCD | 6.8 (1.5) | 6.5 (1.5) | .438 |
| QSU Total (Usual Brand) | 39.0 (17.1) | 44.0 (17.8) | .284 |
| MNWS | 13.6 (4.9) | 13.3 (6.7) | .842 |
| CES Satisfaction | 12.6 (4.4) | 12.9 (4.1) | .794 |
| CES Psychological Reward | 14.8 (6.4) | 16.2 (9.2) | .524 |
| CES Enjoyment of Sensations | 3.2 (1.9) | 3.0 (2.2) | .705 |
| CES Craving Reduction | 4.1 (1.6) | 4.1 (2.1) | .901 |
| CES Aversion | 1.1 (1.7) | 1.9 (2.4) | .195 |
| PANAS Positive Affect | 32.5 (7.5) | 32.2 (6.2) | .904 |
| PANAS Negative Affect | 19.9 (8.4) | 19.4 (7.5) | .784 |
| PANSS Positive symptoms | 10.1 (2.7) | 10.8 (3.0) | .366 |
| PANSS Negative symptoms | 14.0 (4.5) | 13.6 (4.7) | .768 |
| PANSS General symptoms | 24.3 (4.7) | 23.6 (6.0) | .629 |
| SANS | 17.8 (12.2) | 18.8 (13.2) | .769 |
| Brief Psychiatric Rating Scale | 31.0 (6.8) | 30.6 (6.5) | .837 |
| Calgary Depression Scale | 4.2 (3.1) | 3.8 (2.7) | .531 |
| CES-D | 15.6 (11.6) | 18.8 (9.9) | .257 |
| AIMS | 0.7 (1.0) | 1.0 (1.2) | .268 |
| Barnes Akathisia Scale | 0.5 (1.3) | 0.8 (1.3) | .466 |
| Simpson-Angus Scale | 1.5 (1.5) | 1.7 (1.7) | .619 |
TNE, total nicotine equivalents; FTCD, Fagerström Test of Cigarette Dependence; QSU, Questionnaire on Smoking Urges; MNWS, Minnesota Nicotine Withdrawal Scale; CES, Cigarette Evaluation Scales; PANAS, Positive and Negative Affect Schedule; PANSS, Positive and Negative Syndrome Scale; SANS, Scale for Assessment of Negative Symptoms; CES-D, Center for Epidemiologic Studies Depression Scale; AIMS, Abnormal Involuntary Movements Scale
aUnless otherwise indicated, values represent mean (standard deviation) or geometric mean (95% confidence interval) for creatinine-corrected TNE and NNAL.
b p-value is based on the comparison between whites and non-whites.
†Three participants did not report race.
††One participant was missing creatinine at baseline.
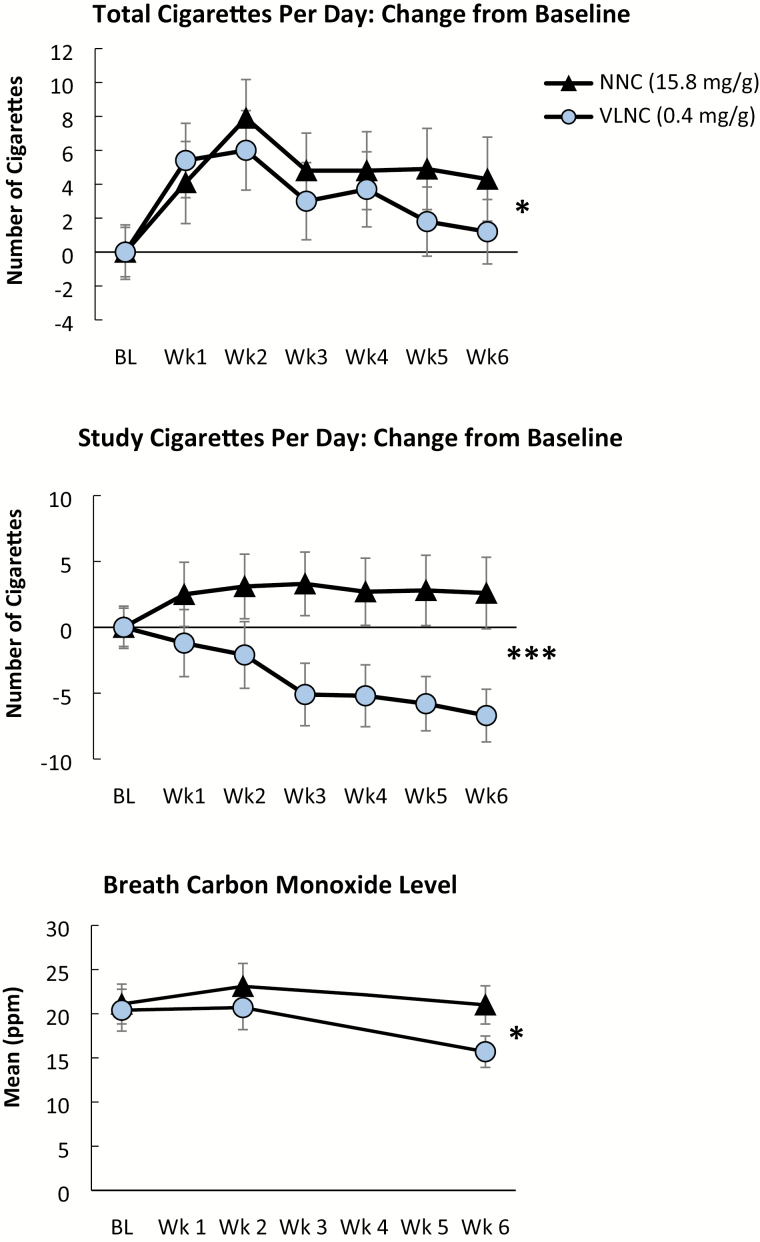
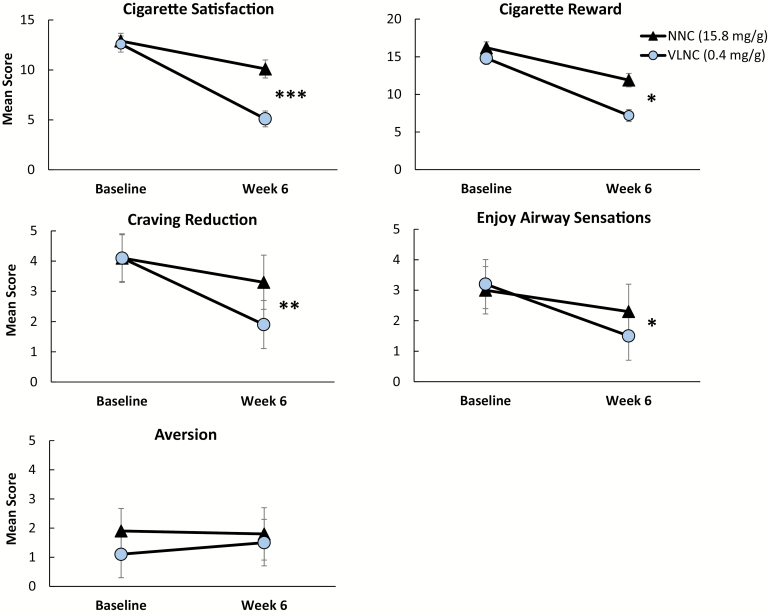
Effects of cigarette condition on outcome measures are provided in Table 2, and effects of condition on total CPD, study CPD, and breath CO levels are shown in Figure 1. The adjusted regression results show that at week 6, participants assigned to the VLNC condition smoked fewer total CPD (p < .05) and fewer study CPD (p < .001), had lower CO levels (p < .05), and had lower QSU scores (p < .05) compared to those in the NNC condition. The VLNC cigarettes were rated significantly lower than NNC cigarettes on the CES Satisfaction, Psychological Reward, Enjoyment of Respiratory Sensations and Craving Reduction subscales (ps < .05), although groups did not differ on the Aversion subscale (Figure 2). No significant differences between conditions were observed on week 6 TNE or NNAL levels, or on Fagerström Test for Cigarette Dependence, MNWS, or PANAS scores. No significant differences between conditions were observed on most measures of psychiatric symptoms, with the exception of the Simpson-Angus Scale, on which scores were significantly lower at week 6 in the VLNC group relative to the NNC group (p < .05).
Table 2.
Results of Linear Regression Analyses Testing for Effects of Cigarette Condition (0.4 mg/g Nicotine–15.8 mg/g Nicotine) at Week 6 Unadjusted (Baseline Only) and Adjusted for Gender, Age, and Baseline Levels of Each Variable
| Unadjusted linear regression | Adjusted linear regression | |||
|---|---|---|---|---|
| Mean difference (95% CI) | p-value | Mean difference (95% CI) | p-value | |
| Total cigarettes per day | –4.01 (–8.25 to 0.22) | .063 | –4.23 (–8.40 to –0.06) | .047 |
| Study cigarettes per day | –9.57 (–15.43 to –3.72) | .002 | –9.96 (–15.59 to –4.34) | <.001 |
| Non-study cigarettes per day | 5.56 (1.60 to 9.52) | .007 | 5.73 (1.81 to 9.65) | .005 |
| Carbon monoxide level (ppm) | –4.93 (–9.49 to –0.37) | .035 | –5.29 (–9.80 to –0.79) | .022 |
| TNE (nmol/mg creatinine) | 0.86 (0.43 to 1.72) | .658 | 0.86 (0.42 to 1.75) | .678 |
| NNAL (nmol/mg creatinine) | 1.19 (0.73 to 1.96) | .470 | 1.16 (0.70 to 1.92) | .545 |
| FTCD | –0.49 (–1.39 to 0.41) | .282 | –0.51 (–1.40 to 0.39) | .262 |
| QSU total (study brand) | –9.47 (–17.88 to –1.07) | .028 | –9.76 (–18.42 to –1.09) | .028 |
| MNWS | 0.36 (–2.62 to 3.33) | .810 | 0.56 (–2.42 to 3.54) | .707 |
| CES Satisfaction | –4.96 (–7.42 to –2.51) | <.001 | –5.03 (–7.52 to –2.54) | <.001 |
| CES Psychological Reward | –4.19 (–7.67 to –0.72) | .019 | –3.98 (–7.52 to –0.44) | .028 |
| CES Enjoyment of Sensations | –0.97 (–1.89 to –0.05) | .039 | –0.95 (–1.88 to –0.02) | .046 |
| CES Craving Reduction | –1.42 (–2.41 to –0.43) | .006 | –1.40 (–2.41 to –0.38) | .008 |
| CES Aversion | 0.04 (–1.24 to 1.32) | .954 | 0.06 (–1.24 to 1.37) | .923 |
| PANAS Positive Affect | 0.40 (–3.71 to 4.51) | .845 | 0.14 (–3.93 to 4.21) | .945 |
| PANAS Negative Affect | –1.16 (–5.17 to 2.85) | .563 | –1.34 (–5.37 to 2.69) | .507 |
| PANSS Positive Symptoms | 0.05 (–1.46 to 1.57) | .945 | –0.01 (–1.53 to 1.50) | .985 |
| PANSS Negative Symptoms | –0.10 (–2.11 to 1.91) | .920 | –0.03 (–2.03 to 1.98) | .979 |
| PANSS General Symptoms | –0.27 (–2.72 to 2.18) | .826 | –0.27 (–2.72 to 2.18) | .826 |
| SANS | –1.19 (–6.47 to 4.09) | .651 | –0.99 (–6.27 to 4.28) | .706 |
| Brief Psychiatric Rating Scale | –0.15 (–2.70 to 2.40) | .905 | 0.09 (–2.55 to 2.74) | .945 |
| Calgary Depression Scale | 0.22 (–1.23 to 1.67) | .759 | 0.21 (–1.24 to 1.66) | .768 |
| CES-D | 2.02 (–2.54 to 6.59) | .377 | 1.88 (–2.71 to 6.46) | .413 |
| AIMS | 0.18 (–0.41 to 0.77) | .537 | 0.21 (–0.38 to 0.80) | .473 |
| Barnes Akathisia Scale | 0.15 (–0.24 to 0.54) | .435 | 0.17 (–0.22 to 0.56) | .387 |
| Simpson-Angus Scale | –0.77 (–1.37 to –0.17) | .013 | –0.77 (–1.39 to –0.15) | .016 |
TNE, total nicotine equivalents; FTCD, Fagerström Test of Cigarette Dependence; QSU, Questionnaire on Smoking Urges; MNWS, Minnesota Nicotine Withdrawal Scale; CES, Cigarette Evaluation Scales; PANAS, Positive and Negative Affect Schedule; PANSS, Positive and Negative Syndrome Scale; SANS, Scale for Assessment of Negative Symptoms; CES-D, Center for Epidemiologic Studies Depression Scale; AIMS, Abnormal Involuntary Movements Scale
Figure 1.
Effects of the 0.4 mg/g (very low nicotine content) cigarette condition (circles) and the 15.8 mg/g (normal nicotine content) cigarette condition (triangles) on change from baseline in total number of cigarettes smoked per day (top), change in baseline in study cigarettes only (middle), and breath carbon monoxide (CO) levels across study weeks. Symbols represent M ± SEM. Significant main effects of cigarette type on week 6 outcomes are indicated with asterisks (*p < .05, ***p < .001).
Figure 2.
Effects of the 0.4 mg/g (very low nicotine content) cigarette condition (circles) and the 15.8 mg/g (normal nicotine content) cigarette condition (triangles) on Cigarette Evaluation Scale subscale scores at week 6 relative to levels at baseline with usual brand cigarettes. Symbols represent M ± SEM. Significant main effects of cigarette type on week 6 outcomes are indicated with asterisks (*p < .05, **p < .01, ***p < .001).
Of those who attended the week 6 visit, 14 participants in the VLNC condition and 11 in the NNC condition were CO-confirmed as abstinent on the first morning of the abstinence period (58% and 44%, respectively; p = .396). The median (95% CI) time to first lapse was 2 (1, 6) days in the VLNC condition and 1.5 (1, 3) days in the NNC condition (p = .631), and those assigned to VLNC cigarettes were abstinent for 3.1 ± 2.8 days compared to 1.9 ± 2.8 days for the NNC condition (p = .163). QSU scores on the first day of the abstinence period did not statistically differ by condition for either Factor 1 (mean difference [95% CI] = 3.67 [–1.48 to 8.83], p = .157) or Factor 2 (mean difference [95% CI] = 2.86 [–1.45 to 7.17], p = .186). Results were similar when only those abstinent on the first day of the abstinence week were included in the analysis (mean difference [95% CI] = 2.55 [–3.27 to 8.36], p = .372 for Factor 1; 1.33 [–2.63 to 5.28], p = .492 for Factor 2). Likewise, MNWS scores on the first day of the abstinence week did not significantly differ across conditions when all participants were included (mean difference [95% CI] = 0.18 [–4.0 to 4.35], p = .931) or when analyses included only abstinent participants (mean difference [95% CI] = 0.91 [–5.02 to 6.84], p = .752). Likelihood of having made at least one quit attempt during the 30-day follow-up period did not differ across groups (47% in the VLNC condition, 39% in the NNC condition; p = .733).
Discussion
Clinical trials of smokers sampled from the general population have supported the hypothesis that reducing the nicotine content of cigarettes to a minimally addictive level may be an effective regulatory approach to reducing the public health burden of tobacco in the United States.8–11 The results of the current trial indicate that the potential benefits of a reduced-nicotine standard for cigarettes may extend to smokers with SMI, in that these smokers also reduce their smoking rates and CO intake when they are switched from their usual brand to VLNC cigarettes. The effect of VLNC cigarettes on smoking rate in this trial was slightly smaller than that observed in a parallel trial conducted in adult smokers without SMI (ie, the difference between the VLNC and NNC conditions on total CPD at week 6 was 4.2 CPD in the current trial and 6.2 CPD in the previous trial).9 However, the null finding on TNE indicates that substantial noncompliance with study cigarettes occurred among those assigned to VLNC cigarettes. This null finding was not attributable to use of noncombusted nicotine products (eg, nicotine patches, electronic cigarettes), which was minimal in this trial. This finding suggests that if a reduced-nicotine standard for cigarettes were used, many smokers with SMI who are not interested in quitting may respond to this regulation by seeking out alternative sources of nicotine. Despite this noncompliance, the significant effect of cigarette condition on reduction in CO level indicates that total smoke exposure was reduced in the VLNC condition relative to the NNC condition.
To our knowledge, this is the first study to investigate the effects of extended VLNC cigarette use among people with SMI. Previous studies of the acute effects of VLNC cigarettes in people with SMI and other mental health conditions found that VLNC cigarettes were lower in abuse liability and reversed the effects of abstinence on craving and withdrawal, with no evidence of compensatory smoking.13–15 Furthermore, these and other studies of smokers with mental health conditions who smoked VLNC cigarettes in the laboratory have reported either no effect or reductions in psychiatric symptoms,13–15,34 although these studies may have been too short for changes in psychiatric symptoms to emerge. A secondary analysis that compared the effects of VLNC or NNC cigarette use over a 6-week period among smokers with elevated depressive symptoms found that those assigned to VLNC cigarettes smoked fewer cigarettes and had lower CO levels and depressive symptoms at week 6 than those who had been assigned to NNC cigarettes.16 In this study, mood, psychiatric, and extrapyramidal symptoms were closely monitored because (1) smokers with SMI report smoking to reduce negative affect35 and (2) smoking induces the metabolism of some antipsychotic medications.36 However, there was no indication that participants in the VLNC condition experienced increases in mood, psychiatric, or extrapyramidal symptoms, and in fact scores on a measure of parkinsonism were decreased in the VLNC condition. Similarly, increases in psychiatric or extrapyramidal symptoms have not been noted in smoking treatment studies of smokers with SMI.37 In summary, results from the current study are consistent with previous reports that VLNC cigarette use reduces behavioral, physiological, and subjective measures of cigarette abuse liability in smokers with mental health conditions, without increasing smoke exposure or psychiatric symptoms.
The results of this study must be considered in light of its limitations. The primary limitation was noncompliance with study cigarettes in the VLNC condition, as demonstrated by a larger decrease in study cigarette use compared to the decrease in total cigarette use. Noncompliance with VLNC cigarettes has been reported in studies of smokers sampled from the general population, but reductions in TNE and NNAL were found despite this noncompliance.9,12 In the current trial, the high level of VLNC cigarette noncompliance may be because of the participants’ high levels of nicotine dependence, or possibly another characteristic associated with their psychiatric diagnosis. We attempted to limit noncompliance by (1) providing free study cigarettes, thus increasing the relative cost of usual-brand cigarettes, (2) providing weekly study cigarette compliance monitoring and counseling, as in previous studies,9,10 and (3) providing financial incentives for meeting a biomarker criterion indicative of study cigarette compliance. However, biomarker analysis was conducted off-site, which introduced a delay between the desired behavior (compliance) and the incentive, and delays to reinforcement are known to reduce the effectiveness of contingent incentives.38 Furthermore, participants could receive incentives for attendance or honest reporting even if they were not compliant, which also may have reduced the effect of the compliance incentive. Alternative methods of ensuring VLNC compliance include sequestering participants in controlled environments,39 but such studies have not been conducted in smokers with SMI. Given this noncompliance, the effects of VLNC cigarette use on withdrawal and psychiatric symptoms reported herein must be considered tentative. In addition, this study excluded people with unstable medical or psychiatric symptoms, those using illicit drugs other than cannabis, binge alcohol drinkers, those smoking fewer than 10 CPD, and those intending to quit smoking within the next 30 days. These exclusions may reduce the extent to which our findings generalize to the broader population of smokers with SMI.
Notwithstanding these limitations, the high rate of noncompliance with VLNC cigarettes in this study is an important observation because it suggests that if a reduced-nicotine standard for cigarettes were implemented, smokers with SMI may be likely to seek out alternative sources of nicotine. Providing adjunctive nicotine replacement may increase VLNC compliance, thereby enhancing the effectiveness of this approach. A laboratory study that investigated the acute effects of VLNC cigarettes combined with either 42 mg transdermal nicotine replacement or placebo patches found that both were equally efficacious at reducing smoke intake, but participants were only observed for a single session under each condition.14,15 Furthermore, VLNC cigarettes combined with 42 mg transdermal nicotine replacement reversed cognitive performance decrements observed when VLNC cigarettes were combined with placebo patches.40 These findings, along with results from the current trial, suggest that longer investigations of the effects of VLNC cigarettes combined with nicotine replacement, including sources of noncombusted nicotine that have greater appeal for smokers with SMI (such as electronic cigarettes),41,42 are warranted.
Funding
This research was supported by grant U54DA031659 from the National Institute on Drug Abuse (NIDA) and the Food and Drug Administration Center for Tobacco Products (FDA). Additional author support during the preparation of this paper was provided by National Institutes of Health (NIH) grants U54DA036114, K01CA189300, and R36DA045183. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH or FDA.
Declaration of Interests
None declared.
Supplementary Material
Acknowledgments
We thank Mollie Miller; Netesha Reid; Emily Xavier; Ashley Marzullo; Kimberly Duguay; Tonya Lane; the Center for Evaluation of Nicotine in Tobacco (CENIC) Administrative, Biomarkers, and Biostatistics Cores; and the study participants for their essential contributions to this research.
References
- 1. McClave AK, McKnight-Eily LR, Davis SP, Dube SR. Smoking characteristics of adults with selected lifetime mental illnesses: results from the 2007 National Health Interview Survey. Am J Public Health. 2010;100(12):2464–2472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Callaghan RC, Veldhuizen S, Jeysingh T, et al. . Patterns of tobacco-related mortality among individuals diagnosed with schizophrenia, bipolar disorder, or depression. J Psychiatr Res. 2014;48(1):102–110. [DOI] [PubMed] [Google Scholar]
- 3. Tidey JW, Miller ME. Smoking cessation and reduction in people with chronic mental illness. BMJ. 2015;351(8028):h4065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Evins AE, Benowitz NL, West R, et al. . Neuropsychiatric safety and efficacy of varenicline, bupropion, and nicotine patch in smokers with psychotic, anxiety, and mood disorders in the EAGLES Trial. J Clin Psychopharmacol. 2019;39(2):108–116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Prochaska JJ, Das S, Young-Wolff KC. Smoking, mental illness, and public health. Annu Rev Public Health. 2017;38:165–185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Food and Drug Administration. Statement of FDA Commission Scott Gottlieb, M.D., on pivotal public health step to dramatically reduce smoking rates by lowering nicotine in combustible cigarettes to minimally or non-addictive levels. Accessed August 13, 2019.
- 7. Benowitz NL, Henningfield JE. Reducing the nicotine content to make cigarettes less addictive. Tob Control. 2013;22(suppl 1):i14–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Benowitz NL, Dains KM, Hall SM, et al. . Smoking behavior and exposure to tobacco toxicants during 6 months of smoking progressively reduced nicotine content cigarettes. Cancer Epidemiol Biomarkers Prev. 2012;21(5):761–769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Donny EC, Denlinger RL, Tidey JW, et al. . Randomized trial of reduced-nicotine standards for cigarettes. N Engl J Med. 2015;373(14):1340–1349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Hatsukami DK, Luo X, Jensen JA, et al. . Effect of immediate vs gradual reduction in nicotine content of cigarettes on biomarkers of smoke exposure: a randomized clinical trial. JAMA. 2018;320(9):880–891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Shiffman S, Kurland BF, Scholl SM, Mao JM. Nondaily smokers’ changes in cigarette consumption with very low-nicotine-content cigarettes: a randomized double-blind clinical trial. JAMA Psychiatry. 2018;75(10):995–1002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Nardone N, Donny EC, Hatsukami DK, et al. . Estimations and predictors of non-compliance in switchers to reduced nicotine content cigarettes. Addiction. 2016;111(12):2208–2216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Higgins ST, Heil SH, Sigmon SC, et al. . Addiction potential of cigarettes with reduced nicotine content in populations with psychiatric disorders and other vulnerabilities to tobacco addiction. JAMA Psychiatry. 2017;74(10):1056–1064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Tidey JW, Rohsenow DJ, Kaplan GB, Swift RM, Ahnallen CG. Separate and combined effects of very low nicotine cigarettes and nicotine replacement in smokers with schizophrenia and controls. Nicotine Tob Res. 2013;15(1):121–129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Tidey JW, Cassidy RN, Miller ME. Smoking topography characteristics of very low nicotine content cigarettes, with and without nicotine replacement, in smokers with schizophrenia and controls. Nicotine Tob Res. 2016;18(9):1807–1812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Tidey JW, Pacek LR, Koopmeiners JS, et al. . Effects of 6-week use of reduced-nicotine content cigarettes in smokers with and without elevated depressive symptoms. Nicotine Tob Res. 2017;19(1):59–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. First MB, Spitzer RL, Gibbon M, Williams JB.. Structured Clinical Interview for DSM-IV Axis-I Disorders—Patient Edition (SCID -I / P, Version 2.0). New York, NY: Biometric Research Department; 1994. [Google Scholar]
- 18. Juliano LM, Donny EC, Houtsmuller EJ, Stitzer ML. Experimental evidence for a causal relationship between smoking lapse and relapse. J Abnorm Psychol. 2006;115(1):166–173. [DOI] [PubMed] [Google Scholar]
- 19. Sobell LC, Sobell MB. Timeline follow-back: a technique for assessing self-reported ethanol consumption. In: Allen J, Litten RZ, eds. Measuring Alcohol Consumption: Psychosocial and Biological Methods. Totowa, NJ: Humana Press; 1992: 41–72. [Google Scholar]
- 20. Scherer G, Engl J, Urban M, Gilch G, Janket D, Riedel K. Relationship between machine-derived smoke yields and biomarkers in cigarette smokers in Germany. Regul Toxicol Pharmacol. 2007;47(2):171–183. [DOI] [PubMed] [Google Scholar]
- 21. Heatherton TF, Kozlowski LT, Frecker RC, Fagerström KO. The Fagerström Test for Nicotine Dependence: a revision of the Fagerström Tolerance Questionnaire. Br J Addict. 1991;86(9):1119–1127. [DOI] [PubMed] [Google Scholar]
- 22. Cox LS, Tiffany ST, Christen AG. Evaluation of the brief questionnaire of smoking urges (QSU-brief) in laboratory and clinical settings. Nicotine Tob Res. 2001;3(1):7–16. [DOI] [PubMed] [Google Scholar]
- 23. Hughes JR, Hatsukami D. Signs and symptoms of tobacco withdrawal. Arch Gen Psychiatry. 1986;43(3):289–294. [DOI] [PubMed] [Google Scholar]
- 24. Cappelleri JC, Bushmakin AG, Baker CL, Merikle E, Olufade AO, Gilbert DG. Confirmatory factor analyses and reliability of the modified cigarette evaluation questionnaire. Addict Behav. 2007;32(5):912–923. [DOI] [PubMed] [Google Scholar]
- 25. Watson D, Clark LA, Tellegen A. Development and validation of brief measures of positive and negative affect: the PANAS scales. J Pers Soc Psychol. 1988;54(6):1063–1070. [DOI] [PubMed] [Google Scholar]
- 26. Kay SR, Fiszbein A, Opler LA. The positive and negative syndrome scale (PANSS) for schizophrenia. Schizophr Bull. 1987;13(2):261–276. [DOI] [PubMed] [Google Scholar]
- 27. Andreasen N. The Scale for the Assessment of Negative Symptoms (SANS): Conceptual and Theoretical Foundations. Br J Psychiatry. 1989; 155( S7):49–52. doi:10.1192/S0007125000291496 [PubMed] [Google Scholar]
- 28. Radloff LS. 1977. The CES-D Scale: a self-report depression scale for research in the general population. Appl Psychol Meas. 1977;1(3):385–401. [Google Scholar]
- 29. Addington D, Addington J, Maticka-Tyndale E. Assessing depression in schizophrenia: the calgary depression scale. Br J Psychiatry Suppl. 1993;22:39–44. [PubMed] [Google Scholar]
- 30. Overall JE, Gorham DR. The Brief Psychiatric Rating Scale. Psychol Rep. 1962;10(3):799–812. [Google Scholar]
- 31. National Institute of Mental Health (NIMH). Abnormal Involuntary Movement Scale (AIMS). ECDEU Assessment Manual for Psychopharmacology, 1976. National Institute of Mental Health, Psychopharmacology Research Branch, Division of Basic Brain and Behavioral Sciences, Rockville, MD. [Google Scholar]
- 32. Simpson GM, Angus JW. A rating scale for extrapyramidal side effects. Acta Psychiatr Scand Suppl. 1970;45(S212):11–19. [DOI] [PubMed] [Google Scholar]
- 33. Barnes TR. A rating scale for drug-induced akathisia. Br J Psychiatry. 1989;154:672–676. [DOI] [PubMed] [Google Scholar]
- 34. Smith RC, Singh A, Infante M, Khandat A, Kloos A. Effects of cigarette smoking and nicotine nasal spray on psychiatric symptoms and cognition in schizophrenia. Neuropsychopharmacology. 2002;27(3):479–497. [DOI] [PubMed] [Google Scholar]
- 35. Tidey JW, Rohsenow DJ. Smoking expectancies and intention to quit in smokers with schizophrenia, schizoaffective disorder and non-psychiatric controls. Schizophr Res. 2009;115(2–3):310–316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Zevin S, Benowitz NL. Drug interactions with tobacco smoking. An update. Clin Pharmacokinet. 1999;36(6):425–438. [DOI] [PubMed] [Google Scholar]
- 37. Evins AE, Cather C, Laffer A. Treatment of tobacco use disorders in smokers with serious mental illness: toward clinical best practices. Harv Rev Psychiatry. 2015;23(2):90–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Roll JM, Reilly MP, Johanson CE. The influence of exchange delays on cigarette versus money choice: a laboratory analog of voucher-based reinforcement therapy. Exp Clin Psychopharmacol. 2000;8(3):366–370. [DOI] [PubMed] [Google Scholar]
- 39. Denlinger RL, Smith TT, Murphy SE, et al. . Nicotine and anatabine exposure from very low nicotine content cigarettes. Tob Regul Sci. 2016;2(2):186–203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. AhnAllen CG, Bidwell LC, Tidey JW. Cognitive effects of very low nicotine content cigarettes, with and without nicotine replacement, in smokers with schizophrenia and controls. Nicotine Tob Res. 2015;17(5):510–514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. O’Brien B, Knight-West O, Walker N, Parag V, Bullen C. E-cigarettes versus NRT for smoking reduction or cessation in people with mental illness: secondary analysis of data from the ASCEND trial. Tob Induc Dis. 2015;13(1):5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Meurk C, Ford P, Sharma R, Fitzgerald L, Gartner C. Views and preferences for nicotine products as an alternative to smoking: a focus group study of people living with mental disorders. Int J Environ Res Public Health. 2016;13(11):1166. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.