Abstract
Introduction
Minimal research exists on adolescent smokers’ perceptions of very low-nicotine-content (VLNC) cigarettes. As approximately half of adolescent smokers prefer menthol cigarettes, it is important to consider the influence of menthol preference on VLNC cigarette perceptions and to what extent menthol preference may affect VLNC smoking behavior. This study examined the effects of cigarette nicotine content and menthol preference or menthol smoking on health risk perceptions, subjective ratings, and carbon monoxide (CO) boost in adolescent smokers.
Methods
Across two counterbalanced sessions, adolescent smokers sampled VLNC and normal nicotine content (NNC) research cigarettes following overnight abstinence. Cigarettes were mentholated or non-mentholated consistent with participants’ usual brand. In each session, participants smoked the research cigarette and then completed the Perceived Health Risk Scale and Cigarette Evaluation Scale. Breath CO readings were obtained pre- and post-smoking. Mixed-factor ANOVA tests compared outcomes with cigarette type (VLNC vs. NNC) as the within-subjects factor and menthol preference as the between-subjects factor.
Results
Participants (N = 50) were M = 17.7 years old, smoked M = 8.2 cigarettes/day, and 56% typically smoked menthol cigarettes. Participants reported lower risk of developing lung cancer, other cancers, emphysema, bronchitis, and heart disease (ps ≤ .05) when smoking VLNC cigarettes relative to NNC cigarettes. Perceived risk of addiction and stroke did not differ by nicotine content. Menthol preference or menthol smoking did not moderate risk perceptions, subjective ratings, or CO boost.
Conclusions
Adolescents may incorrectly perceive that VLNC cigarettes are less harmful products. Health communication campaigns could help to correct VLNC misperceptions and potentially minimize unintended consequences of a nicotine reduction policy.
Introduction
Each day in the United States, more than 3000 adolescents smoke cigarettes for the first time.1 With nearly 90% of smokers starting by the age of 18, tobacco control policies aimed at minimizing adolescent smoking are critical to reduce the public health burden of tobacco.2 One promising approach for reducing adolescent smoking is for the US Food and Drug Administration (FDA) to implement a low-nicotine product standard for cigarettes.3 Because nicotine is the primary reinforcing constituent in cigarettes responsible for establishing and maintaining smoking behavior,4 reducing the nicotine content in cigarettes to a minimally addictive level could reduce the number of adolescents who transition from cigarette experimenters to dependent adult smokers and help current adolescent smokers quit.
The current literature indicates that a low-nicotine product standard for cigarettes could have a major positive public health impact in the United States. A policy simulation concluded that if the FDA were to implement a nicotine reduction policy for cigarettes, it would prevent the uptake of smoking in an estimated 16 million people by 2060.5 During clinical trials, when adult smokers use very low-nicotine-content (VLNC) cigarettes, they experience reductions in urinary nicotine levels and toxicant exposure,6–12 reductions in the number of cigarettes smoked per day,6,9,11–13 and increases in cessation outcomes.6,9,13–15 Although encouraging, these trials enrolled adult smokers, so little is known about the potential impact of a nicotine reduction policy on adolescent smokers.
In general, many smokers misunderstand the health risks of nicotine in cigarettes and in other nicotine-containing products. For example, previous studies of nicotine replacement therapies report that some smokers incorrectly believe that using nicotine replacement therapies is as harmful as smoking cigarettes, whereas other studies report that smokers incorrectly think that the nicotine in cigarettes is primarily responsible for the development of tobacco-related diseases.16–19 Therefore, a potential unintended consequence of a nicotine reduction policy is that adolescent smokers may misperceive VLNC cigarettes as being less harmful than conventional cigarettes because of the reduced nicotine levels. Such a misunderstanding has recently been reported by adult smokers when they were asked to rate the harmfulness of cigarettes they were told had most of the nicotine removed because of government regulation.20 VLNC cigarettes are only harm reduction products to the extent that they have lower risk of addiction and may reduce tobacco consumption; otherwise, they are still combusted tobacco products containing similar levels of some carcinogens as conventional cigarettes.21 However, smokers may perceive them as safer products either because information about lower nicotine levels may lead to misperceptions or because cigarette risk perceptions are partially explained by the sensory experiences of nicotine (eg, throat hit, taste). For example, in a previous study, smokers who sampled research cigarettes varying in nicotine content under double-blind conditions rated cigarettes with low nicotine as less harmful for several tobacco-related diseases compared to cigarettes with moderate nicotine levels.22 Two additional studies of adult smokers who sampled VLNC cigarettes under both blinded and un-blinded conditions reported lower health risk ratings by participants after smoking VLNC cigarettes.23,24 Inaccurate risk perceptions of VLNC cigarettes by adolescents are particularly concerning because adolescent smokers already underestimate the potential harms of smoking.25,26 If current adolescent smokers believe that VLNC cigarettes are less harmful, then they might respond to a low-nicotine standard with reductions in quit attempts, as has been previously reported by adult smokers,20 or possibly even increase their cigarette use.
An important consideration regarding VLNC cigarette use among adolescent smokers is the strong preference for menthol cigarettes in this population. Adolescent and young adult smokers report using menthol cigarettes at significantly higher rates than older adult smokers.27–29 Adolescent smoking initiation with menthol cigarettes is also associated with increased odds of progression to becoming an established smoker and with nicotine dependence.30–34 In addition, menthol flavoring contributes to the appeal of cigarettes such that one study found nearly 30% of adolescents rated menthol cigarettes as having more appealing sensory effects (ie, “refreshing taste”) and approximately 20% perceived menthol cigarettes as less harmful, less addictive, or containing less nicotine compared to non-menthol cigarettes.35 As such, adolescents who use menthol cigarettes may be more susceptible to misperceptions regarding the harms of smoking VLNC cigarettes.
Another concern regarding the potential interaction between cigarette nicotine content and menthol preference or menthol smoking is compensatory smoking, which occurs when smokers attempt to titrate their nicotine exposure by increasing the number of cigarettes smoked per day or by changing their smoking intensity (eg, deeper inhalation; more puffs per cigarette). Because nicotine contributes to the sensory experience of smoking (ie, “throat hit”), reducing the nicotine level in cigarettes could make it easier for smokers, especially adolescents, to inhale smoke deeper into their lungs. In addition, because menthol flavoring masks the harshness of tobacco,36,37 menthol cigarettes could exacerbate any effects of nicotine reduction on compensation. If compensatory smoking of VLNC cigarettes were prolonged, it would be a major negative consequence of a nicotine reduction policy. The literature indicates that adult smokers do not engage in compensatory smoking behavior when using VLNC cigarettes for extended periods.9,12 Regarding compensation among adolescent smokers, the evidence is mixed, albeit limited. One laboratory study found short-term compensation of VLNC cigarettes as measured by smoking topography indices.38 However, another study did not find evidence of compensation as measured by acute carbon monoxide exposure after smoking VLNC cigarettes.39 Importantly, neither adolescent study of VLNC cigarettes explored the impact of menthol preference or menthol smoking on compensation. Therefore, it is important to determine if adolescents using menthol VLNC cigarettes engage in compensatory smoking behavior to a greater extent than those using non-menthol VLNC cigarettes.
The aims of the current study were (1) to measure the separate and combined effects of cigarette nicotine content and menthol preference or menthol smoking on adolescent smokers’ health risk perceptions and (2) to assess the combined effects of cigarette nicotine content and menthol preference or menthol smoking on subjective cigarette ratings and carbon monoxide boost. The data analyzed in this study were obtained from a multi-day, laboratory assessment measuring the acute effects of smoking research cigarettes varying in nicotine content among adolescent smokers.39 On the basis of previous studies of VLNC cigarette health risk perceptions among adult smokers,22–24 we hypothesized that adolescents would rate VLNC research cigarettes as less harmful compared to the NNC research cigarettes for all tobacco-related diseases included in the Perceived Health Risk Scale. Because menthol cigarettes are perceived as less harmful and more appealing by some adolescents,35 we hypothesized that cigarette flavor (ie, menthol vs. non-menthol) would moderate the relationship between nicotine content and risk perceptions such that participants smoking menthol VLNC cigarettes would rate their cigarettes as even less harmful than participants smoking non-menthol VLNC cigarettes. Because menthol flavoring masks the harshness or bitterness of tobacco,36,37 we hypothesized that menthol smokers will have greater CO boosts (ie, increased smoke inhalation) after smoking VLNC cigarettes compared to non-menthol smokers.
In prior studies, participants rated VLNC cigarettes less favorably on the Cigarette Evaluation Scale (ie, lower ratings on measures of liking and satisfaction) compared to NNC cigarettes.39–41 One previous laboratory study of Spectrum research cigarettes, similar to those used in the current study, found that adult smokers rated menthol VLNC cigarettes less favorably than non-menthol VLNC cigarettes.22 Although the direction of this difference may seem surprising, commercial brands of menthol cigarettes range in menthol content.42 It is possible that the menthol content of the Spectrum research cigarettes may have differed from that of the participants’ usual brands. Therefore, we hypothesized that participants smoking menthol VLNC cigarettes will report less favorable subjective ratings compared to participants smoking non-menthol VLNC cigarettes.
Methods
Study Design
Between February 2015 and June 2016, adolescent smokers aged 15–19 were recruited from Providence, Rhode Island, and the surrounding area via advertisements including flyers, brochures, Facebook/Instagram, Craigslist, and public transit. Research staff also hosted informational booths at local community events and high school cafeterias to provide study information to interested adolescents. Initial study eligibility was assessed via a telephone screening questionnaire. To be eligible, participants had to smoke at least one cigarette per day for the past 6 months, smoke cigarettes 28 of the last 30 days, not be interested in quitting smoking or seeking cessation treatment, and have an expired breath CO more than 6 ppm or a urinary cotinine level more than 100 ng/mL. Participants could not be pregnant, breastfeeding, or currently experiencing suicidal ideation or have had a lifetime suicide attempt. Individuals meeting eligibility criteria completed a baseline assessment visit and four experimental sessions. Participants under 18 years of age received informed consent documents in the mail for parent/guardian review and signature and provided written informed assent at the baseline visit. Participants age 18 and 19 provided written informed consent during the baseline visit.
At the baseline visit, participants provided demographic and smoking history information, as described in the primary manuscript.39 Before each subsequent experimental session, participants were asked to stop smoking by 10:00 PM the previous night. In order for the experimental sessions to begin, participants’ CO readings had to be reduced by 50% relative to their baseline reading, or less than or equal to 6 ppm, consistent with a previous VLNC clinical trial.9 If CO readings were above the abstinence cutoff, then participants were rescheduled for later in the day or on a different day to meet the abstinence criteria. Experimental sessions were conducted on separate days with an allowable session window of at least 2 but no more than 7 days between visits.
Those meeting the abstinence criteria smoked one research cigarette per session (described later) using a CReSS handheld smoking topography device (Borgwaldt, KC). CO readings were collected again immediately after smoking. Participants then completed the Perceived Health Risk Scale (PHRS)43 and Cigarette Evaluation Scale (CES).44 The PHRS asked participants to rate their perceived likelihood of developing the following tobacco-related diseases: lung cancer, other cancers, emphysema, bronchitis, heart disease, stroke, and risk of addiction, using 1–10 point Likert scales (“very low risk” to “very high risk”). The CES measured subjective cigarette effects on 1–7 Likert scales (“not at all” to “extremely”). Five validated subscales are obtained from the CES: Satisfaction (mean of ratings on the satisfaction, taste and enjoyment items), Psychological Reward (mean of ratings on the calm, feel more awake, less irritable, help to concentrate and reduce hunger items), Craving Reduction (single item), Enjoyment of Respiratory Tract Sensations (single item) and Aversion (mean of ratings on the dizziness and nausea items).44 We calculated CO boost by subtracting the pre-smoking CO level from the post-smoking CO level. The Brown University Institutional Review Board approved all study procedures.
Study Cigarettes
Spectrum research cigarettes used during the study’s four experimental sessions were supplied by the National Institute on Drug Abuse (NOT-DA-14-004) and had the following nicotine contents: 0.4, 1.3, 5.2, and 15.8 mg nicotine/g tobacco. For the current analysis, we only included the 0.4 and 15.8 mg nicotine/g tobacco research cigarettes. We selected the 0.4 mg nicotine/g tobacco cigarette because it falls within the range of nicotine contents (0.3–0.5 mg nicotine/g tobacco) of greatest interest to the FDA as it considers a low-nicotine product standard for cigarettes,45 and the 15.8 mg nicotine/g tobacco cigarette because it is similar in nicotine content to commercial cigarette brands.21,46 Participants received mentholated or non-mentholated research cigarettes consistent with their usual brand cigarette flavor preference. The order of the research cigarettes administered during the sessions was counterbalanced across participants. Participants and research staff were blind to the nicotine content of the research cigarette used during each session.
Statistical Analyses
Mixed-factor ANOVA tests were used to compare the effects of the within-subjects factor cigarette type (15.8 mg/g [NNC] or 0.4 mg/g [VLNC] cigarette) and the between-subjects factor menthol preference (menthol or non-menthol) on an overall disease risk score (average across all diseases except risk of addiction),24 PHRS individual disease risk scores, CES subscale scores, and CO boosts. Gender was explored as an additional between-subjects factor. The significance threshold was set at the p value of less than .05 level. Effects sizes (partial eta squared, ƞ p2) are provided when a p value of less than .05 is found in conjunction with an ƞ p2 less than or equal to 0.05 indicating small, 0.06–0.13 medium, and more than or equal to 0.14 large effect sizes.47 Analyses were conducted using SPSS statistical software, version 25 (IBM).
Results
Fifty-five participants were eligible after completing the baseline session. Four participants withdrew before completing the first experimental session, and one participant was dismissed from the study because of protocol non-adherence. In total, 50 participants completed the study. Participants completing the study were, on average, 17.7 years old (SD = 1.1), smoked 8.3 cigarettes per day (SD = 4.6) and 50% were female.39 Menthol smokers (n = 28) were younger, smoked more cigarettes per day, and had higher dependence scores compared to non-menthol smokers (n = 22). Table 1 reports baseline demographics and smoking characteristics by menthol smoking status.
Table 1.
Baseline Demographic and Smoking Characteristics by Menthol Smoking Status
| Characteristics | Menthol smokers (N = 28) | Non-menthol smokers (N = 22) | p Value |
|---|---|---|---|
| Age, mean (SD), years | 17.4 (1.3) | 18.1 (0.6) | .02 |
| Female, no. (%) | 15 (53.6) | 10 (45.5) | .57 |
| Race, no. (%) | .28 | ||
| Non-White | 11 (39.3) | 12 (54.5) | |
| White | 17 (60.7) | 10 (45.5) | |
| Cigarettes per day, mean (SD) | 9.4 (4.2) | 6.9 (4.7) | .06 |
| Modified Fagerstrom Tolerance Questionnaire (mFTQ), mean (SD) | 4.9 (1.4) | 3.3 (1.4) | <.01 |
| Weekly income in US dollars (total from parents, work, and other sources), mean (SD) | 119.46 (125.37) | 118.86 (91.90) | .99 |
Baseline demographics for all participants by menthol preference or menthol smoking. p-values are from chi-square tests for categorical variables and independent t-tests assuming equal variances for continuous variables.
We found a significant main effect of nicotine content for the overall perceived risk score [F(1, 46) = 7.5, p < .01; ƞ p2= 0.14] with lower risk perceptions for the VLNC cigarette compared to the NNC cigarette. When analyzing individual disease risk scores, we found significant main effects of cigarette nicotine content for perceived risk of developing lung cancer [F(1, 46) = 6.2, p = .02; ƞ p2 = 0.12], other cancers [F(1, 46) = 11.8, p < .01; ƞ p2 = 0.20], emphysema [F(1,46) = 6.7, p = .01; ƞ p2 = 0.13], and bronchitis [F(1,46) = 5.5, p = .02; ƞ p2 = 0.11] with lower risk perceptions for the VLNC cigarette than the NNC cigarette (Table 2). A trend was observed for reporting lower perceived risk of developing heart disease [F(1, 46) = 4.0, p = .05; ƞ p2 = 0.08] after smoking the VLNC cigarettes. Perceived risk of addiction and stroke did not differ by cigarette nicotine content. Menthol smokers did not differ significantly from non-menthol smokers on perceived risk for disease or addiction, and smoking a menthol versus a non-menthol cigarette did not moderate the effects of cigarette nicotine content on risk perceptions. In addition, there were no differences in perceived risk ratings by gender.
Table 2.
Perceived Health Risk Scale Mean Scores
| Disease risk | Normal nicotine content Mean (SE) | Very low nicotine content Mean (SE) | Nicotine content p value | Menthol p value | Interaction p value | ||
|---|---|---|---|---|---|---|---|
| Menthol | Non-menthol | Menthol | Non-menthol | ||||
| Lung cancer | 5.7 (0.5) | 6.3 (0.6) | 5.0 (0.5) | 5.7 (0.6) | .02 | .41 | .96 |
| Other cancers | 5.9 (0.5) | 5.9 (0.6) | 5.2 (0.5) | 5.0 (0.6) | <.01 | .86 | .70 |
| Emphysema | 5.4 (0.5) | 6.2 (0.6) | 4.7 (0.5) | 5.6 (0.6) | .01 | .22 | .91 |
| Bronchitis | 5.7 (0.5) | 6.6 (0.6) | 5.3 (0.5) | 5.8 (0.6) | .02 | .35 | .33 |
| Heart disease | 5.5 (0.6) | 5.7 (0.6) | 5.2 (0.5) | 5.0 (0.5) | .05 | .99 | .39 |
| Stroke | 5.1 (0.6) | 5.4 (0.6) | 4.8 (0.5) | 4.3 (0.6) | .08 | .86 | .70 |
| Addiction | 5.9 (0.6) | 6.6 (0.6) | 6.1 (0.6) | 6.4 (0.7) | .93 | .53 | .60 |
Mean scores for PHRS by cigarette type and menthol preference or menthol smoking.
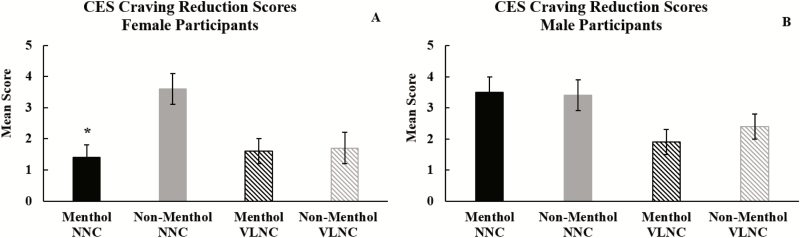
VLNC cigarettes significantly reduced CES scores as previously reported.39 Menthol Spectrum cigarettes were rated more highly than non-menthol Spectrums on the Psychological Reward subscale but had lower ratings on the Satisfaction, Craving Reduction, and Enjoyment in the Respiratory Tract subscales. A significant main effect of menthol emerged for Craving Reduction such that menthol smokers reported less craving reduction relative to non-menthol smokers [F(1, 46) = 4.7, p = .04; ƞ p2 = 0.09], but there was not a significant menthol × nicotine content interaction (Table 3). However, a three-way interaction was observed for Craving Reduction [F(1, 46) = 4.2, p < .05; ƞ p2 = 0.08] such that female menthol smokers reported less craving reduction from the NNC cigarettes compared to female non-menthol smokers and male menthol and non-menthol smokers (Figure 1). No other significant main effects of menthol and no significant interactions between nicotine content and menthol on CES scores emerged.
Table 3.
Cigarette Evaluation Scale—Subscales Mean Scores
| Subscale | Normal nicotine content Mean (SE) | Very low nicotine content Mean (SE) | Nicotine content p value | Menthol p value | Interaction p value | ||
|---|---|---|---|---|---|---|---|
| Menthol | Non-menthol | Menthol | Non-menthol | ||||
| Satisfaction | 3.2 (0.3) | 3.3 (0.4) | 2.3 (0.3) | 2.7 (0.3) | <.01 | .56 | .48 |
| Psychological reward | 2.6 (0.3) | 2.1 (0.3) | 2.3 (0.2) | 2.0 (0.3) | .34 | .20 | .64 |
| Enjoyment in respiratory tract | 3.0 (0.3) | 3.2 (0.4) | 2.5 (0.3) | 2.7 (0.4) | .07 | .61 | .86 |
| Craving reduction | 2.4 (0.3) | 3.5 (0.4) | 1.8 (0.3) | 2.1 (0.3) | <.01 | .04 | .23 |
| Aversion | 1.1 (0.2) | 1.0 (0.3) | 1.0 (0.2) | 0.4 (0.3) | .12 | .27 | .30 |
Mean scores for CES Subscales by cigarette type and menthol preference or menthol smoking. There are no significant interactions by cigarette type and menthol preference or menthol smoking.
Figure 1.
Mean scores for the Cigarette Evaluation Scale (CES) Craving Reduction subscale by cigarette nicotine content and cigarette flavor. (A) Female participants’ mean scores and (B) male participants’ mean scores. Black bars represent menthol smokers and gray bars represent non-menthol smokers. Solid bars represent normal nicotine content (NNC) cigarettes whereas lined bars represent very low-nicotine-content (VLNC) cigarettes. *Significant three-way interaction. Female menthol smokers reported significantly lower craving reduction scores when smoking the NNC cigarette relative to female non-menthol NNC smokers and male smokers (p < .05).
There were no significant effects of cigarette nicotine content on CO boost as previously reported.39 Menthol preference or menthol smoking did not significantly affect CO boost, and there was no interaction between nicotine content and menthol preference or menthol smoking on CO boost. CO boosts for the NNC cigarettes were 3.8 ppm (SE = 0.6) for menthol and 4.3 ppm (SE = 0.6) for non-menthol cigarettes, and CO boosts for the VLNC cigarettes were 3.6 (SE = 0.5) for menthol and 4.2 (SE = 0.6) for non-menthol cigarettes.
Discussion
To our knowledge, this is the first study to report the effects of cigarette nicotine content and menthol preference or menthol smoking on cigarette risk perceptions among adolescent smokers who have sampled VLNC cigarettes. Overall, risk perception scores were significantly lower when participants smoked the VLNC cigarettes compared to the NNC cigarettes. Specifically, adolescent smokers rated their risk for developing lung cancer and other cancers as significantly lower when smoking VLNC cigarettes compared to NNC cigarettes. In addition, participants reported reductions in perceived risk of developing respiratory diseases and tended to rate their risk of developing cardiovascular diseases as lower when smoking VLNC cigarettes. Surprisingly, however, there were no differences across cigarette condition in perceived risk of addiction.
In general, these results bolster the findings from the primary manuscript associated with this study in which adolescent smokers were able to discern differences in subjective experiences when smoking VLNC cigarettes relative to smoking NNC cigarettes, despite being blind to the nicotine content in the research cigarettes. Adolescent smokers reported dose-dependent reductions in cigarette craving and satisfaction ratings with lower scores being associated with lower nicotine doses.39 This study indicates that the sensory properties of the cigarettes also influenced perceived risk ratings. These findings are particularly concerning because when a nicotine reduction policy is in place, smokers will not only experience different respiratory sensations but they will also likely be aware that their cigarettes have less nicotine; and such information has been shown to reduce risk perceptions in adults on its own.20 Recent surveys indicate that nearly half or more of smokers, especially young adults, incorrectly believe that nicotine is the constituent in cigarettes primarily responsible for the development of lung cancer and other tobacco-related diseases.19,20,48 Thus, if the FDA mandates a low-nicotine product standard for cigarettes, it will be crucial to correct this misperception so that adolescent smokers do not mistakenly believe that smoking VLNC cigarettes will reduce their risk for developing tobacco-related cancers. This misperception, coupled with the reduced sensory experience from VLNC cigarettes, could lead to potential unintended consequences such as reductions in cessation attempts by current adolescent smokers or increases in cigarette experimentation among novice users. More research is needed to determine if VLNC cigarette risk misperceptions as well as general misunderstandings regarding the role of nicotine in developing tobacco-related diseases affect adolescent smoking behavior in the real world.
Alternatively, if a nicotine reduction policy is enacted, adolescent smokers could also switch to other nicotine or tobacco products that would be higher in nicotine content relative to VLNC cigarettes. These alternative products could be reduced harm products, such as e-cigarettes or vaping devices, or they could have similar levels of harm, such as little cigars or cigarillos. Therefore, future studies interested in VLNC cigarette risk perceptions should also consider assessing perceived health risks of alternative tobacco and nicotine products to achieve a more complete understanding of adolescent risk perceptions. We currently have such a study ongoing to assess the potential effects of a nicotine reduction policy on alternative product use in adolescent polytobacco users (NCT#03860077). Public health officials developing health communication messages for a nicotine reduction policy could potentially use this information to ensure that adolescent smokers understand the relative risks of switching to alternative products versus quitting smoking completely.
Because nearly 30% of cigarettes sold in the United States are menthol flavored,27 it is important to examine how a nicotine reduction policy may affect menthol cigarette smokers, especially adolescents who favor menthol cigarettes to a greater extent than adults. Under acute laboratory conditions, adolescents smoking menthol VLNC cigarettes did not have higher smoke exposure compared to those smoking non-menthol VLNC cigarettes, which is encouraging. However, more research is needed to determine whether compensation occurs when adolescents use VLNC cigarettes in the real world for extended periods and what impact, if any, menthol preference or menthol smoking has on VLNC cigarette smoking behavior. Such a trial is underway and will likely provide crucial information on VLNC cigarette use among adolescent menthol smokers.49
Menthol preference or menthol smoking did not affect VLNC cigarette risk perceptions or subjective cigarette ratings, which is consistent with the adult literature.24,50 However, we found a significant three-way interaction (menthol × nicotine content × gender) for Craving Reduction, suggesting that adolescent female smokers using menthol cigarettes were less sensitive to the effects of cigarette nicotine content on craving reduction compared to female smokers using non-menthol cigarettes and male smokers. This finding aligns with previous studies of adult smokers sampling Spectrum research cigarettes or Quest brand cigarettes reporting that female smokers were less sensitive to the effects of the nicotine content in cigarettes.51,52 Researchers should continue to explore the interaction between cigarette nicotine content and menthol preference or menthol smoking among adolescent female smokers to fully understand how a nicotine reduction policy may affect this population.
There are several limitations to this study. First, menthol preference or menthol smoking was not randomly assigned. Participants selected whether they smoked menthol or non-menthol research cigarettes during the study, which resulted in significant baseline differences between those smoking menthol and non-menthol cigarettes. Future research could test the effects of menthol versus non-menthol cigarettes, independent of menthol preference, to isolate the effects of menthol flavoring and eliminate the potential confounding that exists within this study. Second, the sample size was modest, and the study was underpowered to test for potential interactions between cigarette nicotine content and menthol preference or menthol smoking on study outcomes as well as to detect differences by gender. Future studies with larger sample sizes should be conducted to further examine adolescent VLNC cigarette risk perceptions and the impact of menthol preference or menthol smoking and gender on perceptions of harm. Third, the study only enrolled adolescent daily smokers. Therefore, the results may not generalize to adolescent non-daily smokers or early experimenters, which are a larger proportion of adolescent smokers.53 Fourth, adolescents in general may be less familiar with the diseases on the PHRS (eg, emphysema) or of their relationship to cigarettes (eg, smoking is associated with increased risk of stroke) compared to adults. In addition, the PHRS does not assess the short-term cosmetic health consequences of cigarette smoking, such as facial skin wrinkles or yellow teeth, which may resonate more with adolescent smokers rather than the long-term serious health consequences of smoking.54 Future studies could address these limitations by measuring adolescent health literacy, providing brief descriptions of the tobacco-related diseases included in the PHRS, and including adolescent-focused questions assessing the short-term consequences of cigarette smoking. Finally, participants were blind to the nicotine content of the research cigarettes, which does not necessarily represent a real-world policy scenario. If the FDA implements a nicotine reduction policy for cigarettes, smokers would likely be aware of the reduced nicotine content in their cigarettes. However, the study results can still inform public health officials when creating nuanced messages about how differences in the sensory experiences of smoking VLNC cigarettes relative to conventional cigarettes should not lead smokers to believe they are safer cigarettes. To date, no studies have been conducted in adolescent or adult smokers assessing the impact of corrective statements regarding the health risks of VLNC cigarettes on smoking behavior or motivation to quit. Therefore, additional studies providing participants with explicit knowledge of the nicotine content in the research cigarettes may be informative for understanding adolescent VLNC cigarette risk perceptions, with the caveat that such information may increase the possibility that adolescents will perceive lower disease risk with VLNC cigarettes.
Implications for Tobacco Regulation
Consistent with adult smokers, likely based on their sensory experience while smoking VLNC cigarettes, adolescent smokers have inaccurate perceptions that VLNC cigarettes are lower in risk for developing tobacco-related diseases when they are introduced to VLNC cigarettes under double-blind conditions. As the FDA moves forward with implementing a low-nicotine product standard for cigarettes, health communication experts must educate the public about the purpose of a nicotine reduction policy (eg, to reduce adolescent smoking acquisition; to increase successful quit attempts) to maximize the potential benefits as well as make corrective statements clarifying that VLNC cigarettes have similar health risks as conventional cigarettes to minimize potential unintended consequences for adolescent and adult smokers.
Funding
The study was funded by National Cancer Institute grant K01 CA189300 (PI: Cassidy). Author support during data analyses and manuscript preparation was provided by National Institute on Drug Abuse and Food and Drug Administration (FDA) Center for Tobacco Products grants R36 DA045183, U54 DA031659, U54 DA036114, and by NIDA T32 DA016184. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health or the FDA.
Declaration of Interests
The authors have no conflicts of interest to report.
Acknowledgments
The authors wish to acknowledge Graham DiGuiseppi, Tim Souza, and Suzanne Sales for their work on this project. Ms. Denlinger-Apte would like to thank Drs. Kate Carey and Christopher Kahler for their feedback during manuscript development.
References
- 1. U.S. Department of Health and Human Services. The Health Consequences of Smoking: 50 Years of Progress. A Report of the Surgeon General. Atlanta, GA: U.S. Department of Health and Human Services, Centers for Disease Control and Prevention, National Center for Chronic Disease Prevention and Health Promotion, Office on Smoking and Health; 2014. [Google Scholar]
- 2. U.S. Department of Health and Human Services. Preventing Tobacco Use Among Youth and Young Adults: A Report of the Surgeon General. Atlanta, GA: U.S. Department of Health and Human Services, Centers for Disease Control and Prevention, National Center for Chronic Disease Prevention and Health Promotion, Office on Smoking and Health; 2012. [Google Scholar]
- 3. Gottlieb S, Zeller M. A nicotine-focused framework for public health. N Engl J Med. 2017;377(12):1111–1114. [DOI] [PubMed] [Google Scholar]
- 4. Benowitz NL. Pharmacology of nicotine: addiction, smoking-induced disease, and therapeutics. Annu Rev Pharmacol Toxicol. 2009;49:57–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Apelberg BJ, Feirman SP, Salazar E, et al. . Potential public health effects of reducing nicotine levels in cigarettes in the United States. N Engl J Med. 2018;378(18):1725–1733. [DOI] [PubMed] [Google Scholar]
- 6. Hatsukami DK, Kotlyar M, Hertsgaard LA, et al.. Reduced nicotine content cigarettes: effects on toxicant exposure, dependence and cessation. Addiction. 2010;105(2):343–355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Benowitz NL, Dains KM, Hall SM, et al.. Smoking behavior and exposure to tobacco toxicants during 6 months of smoking progressively reduced nicotine content cigarettes. Cancer Epidemiol Biomarkers Prev. 2012;21(5):761–769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Hammond D, O’Connor RJ. Reduced nicotine cigarettes: smoking behavior and biomarkers of exposure among smokers not intending to quit. Cancer Epidemiol Biomarkers Prev. 2014;23(10):2032–2040. [DOI] [PubMed] [Google Scholar]
- 9. Donny EC, Denlinger RL, Tidey JW, et al.. Randomized trial of reduced-nicotine standards for cigarettes. N Engl J Med. 2015;373(14):1340–1349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Mercincavage M, Souprountchouk V, Tang KZ, et al.. A randomized controlled trial of progressively reduced nicotine content cigarettes on smoking behaviors, biomarkers of exposure, and subjective ratings. Cancer Epidemiol Biomarkers Prev. 2016;25(7):1125–1133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Shiffman S, Kurland BF, Scholl SM, Mao JM. Nondaily smokers’ changes in cigarette consumption with very low-nicotine-content cigarettes: a randomized double-blind clinical trial. JAMA Psychiatry. 2018;75(10):995–1002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Hatsukami DK, Luo X, Jensen JA, et al. . Effect of immediate vs gradual reduction in nicotine content of cigarettes on biomarkers of smoke exposure: a randomized clinical trial. JAMA. 2018;320(9):880–891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Hatsukami DK, Hertsgaard LA, Vogel RI, et al.. Reduced nicotine content cigarettes and nicotine patch. Cancer Epidemiol Biomarkers Prev. 2013;22(6):1015–1024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Walker N, Howe C, Bullen C, et al.. The combined effect of very low nicotine content cigarettes, used as an adjunct to usual quitline care (nicotine replacement therapy and behavioural support), on smoking cessation: a randomized controlled trial. Addiction. 2012;107(10):1857–1867. [DOI] [PubMed] [Google Scholar]
- 15. McRobbie H, Przulj D, Smith KM, Cornwall D. Complementing the standard multicomponent treatment for smokers with denicotinized cigarettes: a randomized trial. Nicotine Tob Res. 2016;18(5):1134–1141. [DOI] [PubMed] [Google Scholar]
- 16. Bobak A, Shiffman S, Gitchell JG, Bery J, Ferguson SG. Perceived safety of nicotine and the use of nicotine replacement products among current smokers in Great Britain: Results from two national surveys. J Smok Cessat. 2010;5(2):115–122. [Google Scholar]
- 17. Bansal MA, Cummings KM, Hyland A, Giovino GA. Stop-smoking medications: who uses them, who misuses them, and who is misinformed about them? Nicotine Tob Res. 2004;6 (suppl 3):S303–S310. [DOI] [PubMed] [Google Scholar]
- 18. Wilson S, Partos T, McNeill A, Brose LS. Harm perceptions of e-cigarettes and other nicotine products in a UK sample. Addiction. 2019;114(5):879–888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. O’Brien EK, Nguyen AB, Persoskie A, Hoffman AC. U.S. adults’ addiction and harm beliefs about nicotine and low nicotine cigarettes. Prev Med. 2017;96:94–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Byron MJ, Jeong M, Abrams DB, Brewer NT. Public misperception that very low nicotine cigarettes are less carcinogenic. Tob Control. 2018;27(6):712–714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Richter P, Steven PR, Bravo R, et al.. Characterization of SPECTRUM variable nicotine research cigarettes. Tob Regul Sci. 2016;2(2):94–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Hatsukami DK, Heishman SJ, Vogel RI, et al.. Dose-response effects of spectrum research cigarettes. Nicotine Tob Res. 2013;15(6):1113–1121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Denlinger-Apte RL, Joel DL, Strasser AA, Donny EC. Low nicotine content descriptors reduce perceived health risks and positive cigarette ratings in participants using very low nicotine content cigarettes. Nicotine Tob Res. 2017;19(10):1149–1154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Pacek LR, Joseph McClernon F, Denlinger-Apte RL, et al.. Perceived nicotine content of reduced nicotine content cigarettes is a correlate of perceived health risks. Tob Control. 2018;27(4):420–426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Song AV, Morrell HE, Cornell JL, et al.. Perceptions of smoking-related risks and benefits as predictors of adolescent smoking initiation. Am J Public Health. 2009;99(3):487–492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Halpern-Felsher BL, Biehl M, Kropp RY, Rubinstein ML. Perceived risks and benefits of smoking: differences among adolescents with different smoking experiences and intentions. Prev Med. 2004;39(3):559–567. [DOI] [PubMed] [Google Scholar]
- 27. Giovino GA, Villanti AC, Mowery PD, et al.. Differential trends in cigarette smoking in the USA: is menthol slowing progress? Tob Control. 2015;24(1):28–37. [DOI] [PubMed] [Google Scholar]
- 28. Villanti AC, Johnson AL, Ambrose BK, et al.. Flavored tobacco product use in youth and adults: findings from the first wave of the PATH study (2013-2014). Am J Prev Med. 2017;53(2):139–151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Villanti AC, Mowery PD, Delnevo CD, Niaura RS, Abrams DB, Giovino GA. Changes in the prevalence and correlates of menthol cigarette use in the USA, 2004–2014. Tob Control. 2016;25 (suppl 2):ii14–ii20. [DOI] [PubMed] [Google Scholar]
- 30. Nonnemaker J, Hersey J, Homsi G, Busey A, Allen J, Vallone D. Initiation with menthol cigarettes and youth smoking uptake. Addiction. 2013;108(1):171–178. [DOI] [PubMed] [Google Scholar]
- 31. Hersey JC, Ng SW, Nonnemaker JM, et al.. Are menthol cigarettes a starter product for youth? Nicotine Tob Res. 2006;8(3):403–413. [DOI] [PubMed] [Google Scholar]
- 32. Hersey JC, Nonnemaker JM, Homsi G. Menthol cigarettes contribute to the appeal and addiction potential of smoking for youth. Nicotine Tob Res. 2010;12 (suppl 2):S136–S146. [DOI] [PubMed] [Google Scholar]
- 33. Collins CC, Moolchan ET. Shorter time to first cigarette of the day in menthol adolescent cigarette smokers. Addict Behav. 2006;31(8):1460–1464. [DOI] [PubMed] [Google Scholar]
- 34. Wackowski O, Delnevo CD. Menthol cigarettes and indicators of tobacco dependence among adolescents. Addict Behav. 2007;32(9):1964–1969. [DOI] [PubMed] [Google Scholar]
- 35. Brennan E, Gibson L, Momjian A, Hornik RC. Are young people’s beliefs about menthol cigarettes associated with smoking-related intentions and behaviors? Nicotine Tob Res. 2015;17(1):81–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Kreslake JM, Wayne GF, Connolly GN. The menthol smoker: tobacco industry research on consumer sensory perception of menthol cigarettes and its role in smoking behavior. Nicotine Tob Res. 2008;10(4):705–715. [DOI] [PubMed] [Google Scholar]
- 37. Yerger VB, McCandless PM. Menthol sensory qualities and smoking topography: a review of tobacco industry documents. Tob Control. 2011;20 (suppl 2):ii37–ii43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Kassel JD, Greenstein JE, Evatt DP, et al.. Smoking topography in response to denicotinized and high-yield nicotine cigarettes in adolescent smokers. J Adolesc Health. 2007;40(1):54–60. [DOI] [PubMed] [Google Scholar]
- 39. Cassidy RN, Colby SM, Tidey JW, et al.. Adolescent smokers’ response to reducing the nicotine content of cigarettes: acute effects on withdrawal symptoms and subjective evaluations. Drug Alcohol Depend. 2018;188:153–160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Higgins ST, Heil SH, Sigmon SC, et al.. Addiction potential of cigarettes with reduced nicotine content in populations with psychiatric disorders and other vulnerabilities to tobacco addiction. JAMA Psychiatry. 2017;74(10):1056–1064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Cassidy RN, Tidey JW, Cao Q, et al.. Age moderates smokers’ subjective response to very-low nicotine content cigarettes: evidence from a randomized controlled trial. Nicotine Tob Res. 2019;21(7):962–969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Ai J, Taylor KM, Lisko JG, Tran H, Watson CH, Holman MR. Menthol content in US marketed cigarettes. Nicotine Tob Res. 2016;18(7):1575–1580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Hatsukami DK, Vogel RI, Severson HH, Jensen JA, O’Connor RJ. Perceived health risks of snus and medicinal nicotine products. Nicotine Tob Res. 2016;18(5):794–800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Cappelleri JC, Bushmakin AG, Baker CL, Merikle E, Olufade AO, Gilbert DG. Confirmatory factor analyses and reliability of the modified cigarette evaluation questionnaire. Addict Behav. 2007;32(5):912–923. [DOI] [PubMed] [Google Scholar]
- 45. Food and Drug Administration. Tobacco Product Standard for Nicotine Level of Combusted Cigarettes. Vol. 83 FR 11818. Washington, D.C.: Federal Register; 2018:11818–11843. [Google Scholar]
- 46. Federal Trade Commission. Tar, Nicotine, and Carbon Monoxide Report - 1998. Washington, D.C.: Federal Trade Commission; 2000. [Google Scholar]
- 47. Cohen J. Statistical Power Analysis for the Behavioral Sciences. 2nd ed New Jersey: Lawrence Erlbaum Associates, Publishers; 1988. [Google Scholar]
- 48. Mumford EA, Pearson JL, Villanti AC, Evans WD. Nicotine and e-cigarette beliefs and policy support among US smokers and nonsmokers. Tob Regul Sci. 2017;3(3):293–305. [Google Scholar]
- 49. Cassidy RN, Colby SM, Tidey JW, Cioe PA, Krishnan-Sarin S, Hatsukami DK. Modeling the effects a nicotine reduction policy in adolescent smokers: Protocol of an ongoing clinical trial. Poster presented at: NIH-FDA Tobacco Regulatory Science Annual Meeting 2018; Bethesda, MD. [Google Scholar]
- 50. Davis DR, Miller ME, Streck JM, et al. . Response to reduced nicotine content in vulnerable populations: effect of menthol status. Tob Regul Sci. 2019;5(2):135–142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Perkins KA, Karelitz JL. Sex differences in acute relief of abstinence-induced withdrawal and negative affect due to nicotine content in cigarettes. Nicotine Tob Res. 2015;17(4):443–448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Perkins KA, Karelitz JL, Kunkle N. Sex differences in subjective responses to moderate versus very low nicotine content cigarettes. Nicotine Tob Res. 2018;20(10):1258–1264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Youth Risk Behavior Survey. Centers for Disease Control and Prevention https://www.cdc.gov/healthyyouth/data/yrbs/index.htm. Accessed May 14, 2018.
- 54. Brubach AL. The case and context for “The Real Cost” campaign. Am J Prev Med. 2019;56(2S1):S5–S8. [DOI] [PubMed] [Google Scholar]