Abstract
Background
Cardiac amyloidosis (CA) is a secondary form of cardiomyopathy where abnormal accumulation of amyloid protein in the myocardial interstitium causes cardiac hypertrophy and myocardial fibrosis. If primary CA advances to heart failure, most patients do not survive for very long after the diagnosis.
Case summary
A 40-year-old man was admitted to our hospital for dyspnoea, progressive anaemia, and decreased appetite. He has diagnosed with amyloid light-chain (AL) amyloidosis. Although BD treatment (bortezomib + dexamethasone) and medical treatment were started, there was no sign of improvement. Then, high-dose chemotherapy followed by autologous peripheral blood stem cell transplantation (auto-PBSCT) was initiated. Pretreatment echocardiography revealed typical findings of CA, such as ventricular wall thickening, valvular thickening, diastolic dysfunction, and pericardial effusion. Global longitudinal strain (GLS) was significantly reduced, and bull's-eye mapping showed typical apical sparing. After auto-PBSCT, GLS gradually improved and was almost normal after 2 years. Other echocardiographic parameters, functional status, and laboratory data also showed that there was significant regression of CA.
Discussion
Although the prognosis in primary CA is extremely poor, we achieved long-term survival in a patient with effective high-dose chemotherapy and auto-PBSCT. Global longitudinal strain may be a useful marker of prognosis, regression, and recovery.
Keywords: Cardiac amyloidosis, Echocardiography, Speckle tracking, Global longitudinal strain, Improvement, Case report
Learning points
Global longitudinal strain (GLS) may be a useful marker of regression and recovery after auto peripheral blood stem cell transplantation in patients with cardiac amyloidosis (CA).
Serial measurements of GLS may identify cardiac function improvement without demonstrable changes in standard echocardiographic parameters among light-chain CA patients.
Introduction
Cardiac amyloidosis (CA) is part of a systemic disease characterized by the deposition of amyloid in multiple tissues. Cardiac amyloidosis results in characteristic macroscopic structural and functional changes on transthoracic echocardiography (TTE).1 Global longitudinal strain (GLS) is significantly reduced, and bull’s-eye mapping typically shows apical sparing.2 Cardiac amyloidosis is usually accompanied by refractory heart failure and conduction abnormalities; hence, most patients do not survive for very long after the diagnosis.3 However, recent reports have demonstrated that select patients who achieve haematologic remission after high-dose chemotherapy and autologous peripheral blood stem cell transplantation (auto-PBSCT) may regress these cardiac features.4,5 We report a patient with light-chain (AL)-CA who showed significant improvement in the anatomic features associated with CA, including GLS.
Timeline
| Events | |
|---|---|
| 2 months prior to autologous peripheral blood stem cell transplantation (auto-PBSCT) | Patient admitted to our hospital for dyspnoea, progressive anaemia, and decreased appetite |
| Transthoracic echocardiography led us to strongly suspect cardiac amyloidosis (CA) with heart failure | |
| 1 month prior to auto-PBSCT | Diagnosed with light-chain CA |
| BD treatment (bortezomib + dexamethasone) and medical treatment was started | |
| February 2014 | High-dose chemotherapy (melphalan, 140 mg/m2) with auto-PBSCT was started |
| Brain natriuretic peptide (BNP) = 1194 pg/mL, global longitudinal strain (GLS) = −6.2%, and left ventricular (LV) wall = 14 mm | |
| 4 months after auto-PBSCT | Symptoms were getting relieved and discharged hospital |
| BNP levels declined to the 700s pg/mL | |
| 6 months after auto-PBSCT | BNP levels declined to the 200s pg/mL |
| GLS showed only a slight change (−8.3%) | |
| 11 months after auto-PBSCT | BNP levels declined to the 80s pg/mL |
| GLS improved to a value of −12.2% | |
| LV wall showed only a slight change (12 mm) | |
| 22 months after auto-PBSCT | BNP levels normalized. |
| GLS improved to a value of −16.2% | |
| LV wall improved to 10 mm | |
| 60 months after auto-PBSCT | Patient has been no relapse and continues to visit the outpatient clinic. |
Case presentation
A 40-year-old man presented to a local clinic complaining of dyspnoea, progressive anaemia, and decreased appetite. N-terminal pro-brain natriuretic peptide (NT-proBNP) was markedly elevated to 12 994 pg/mL, and he was admitted to our hospital. At the first visit, his blood pressure was 84/46 mmHg and pulse rate was 65 b.p.m. with a regular rhythm. Precordial examination revealed a third heart sound. Chest examination revealed bilateral diminished air entry over both lung bases. He displayed oedema of the lower extremities. A blood test indicated markedly elevated levels of BNP (1490 pg/mL). Complete blood count showed anaemia with haemoglobin of 10.9 g/dL.
Diagnostic assessment
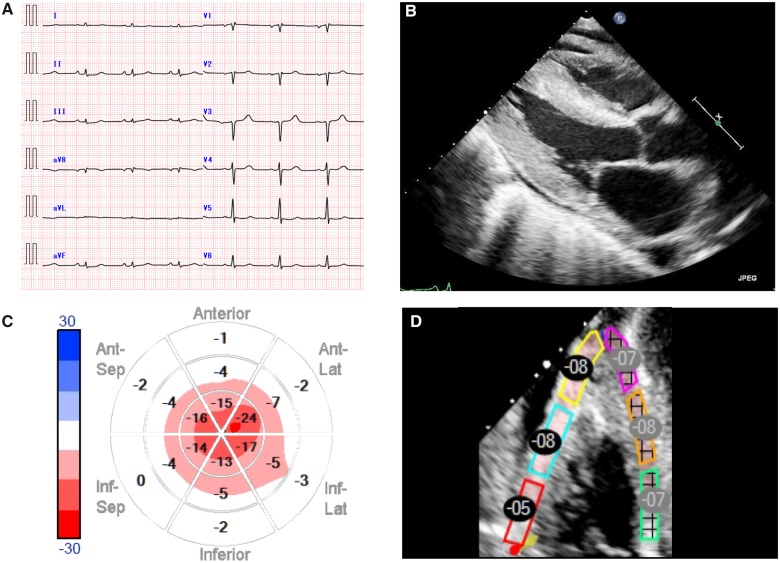
Electrocardiography displayed a low-amplitude R-wave on the limb leads and a QS pattern on leads V1–V3 (Figure 1A). Transthoracic echocardiography revealed moderately increased left ventricular (LV) wall thickness (septal wall, 14 mm; posterior wall, 14 mm) with a small pericardial effusion (Figure 1B). Thickening of the right ventricular (RV) wall was also shown. Left ventricular systolic function was preserved, with an ejection fraction (EF) of 63%, as measured by the modified Simpson method. Longitudinal myocardial systolic strain based on two-dimensional speckle-tracking echocardiography showed far more significant LV dysfunction. Global longitudinal strain was markedly reduced to −6.2%, and bull’s eye mapping revealed the characteristic apical sparing pattern (Figure 1C). The regional values of the RV free wall longitudinal strain (RV free wall LS) were reduced to −7.0% (Figure 1D). Left atrial (LA) strain was also reduced to −14%. The mitral inflow profile showed a restricted pattern such that the patient's peak early filling (E) and late diastolic filling (A) velocity ratio was 3.2. Furthermore, the septal and lateral tissue Doppler e‘ values were low, with an E/e’ ratio >15. Pulmonary artery systolic pressure could not be estimated due to absence of tricuspid regurgitation. Inferior vena cava (IVC) was dilated and showed decreased respiratory variability. These findings led us to strongly suspect CA with heart failure. Cardiac magnetic resonance imaging (MRI) showed diffuse subendocardial mild enhancement of the biventricular myocardium. Subsequently, the patient was diagnosed with primary systemic AL amyloidosis by cardiac muscle, duodenum, and bone marrow biopsy (Supplementary material online, Figure S1).
Figure 1.
Imaging examinations before treatment. (A) Electrocardiogram. (B) Parasternal echocardiographic view demonstrated biventricular hypertrophy and a small pericardial effusion. (C) Bull’s-eye mapping. Regional values of the left ventricle longitudinal strain decreased markedly in the base even though the apex remained unchanged, indicating an apical sparing pattern. (D) Regional values of the right ventricle free wall longitudinal strain. These changes are considered to exhibit high sensitivity and specificity for the diagnosis of cardiac amyloidosis.
Interventions and outcomes
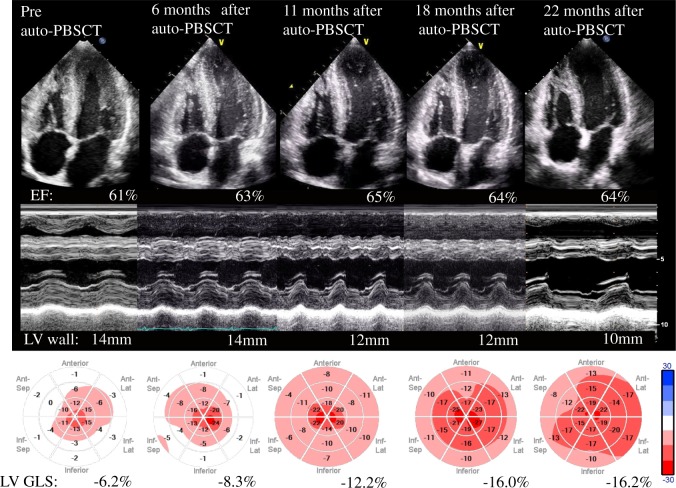
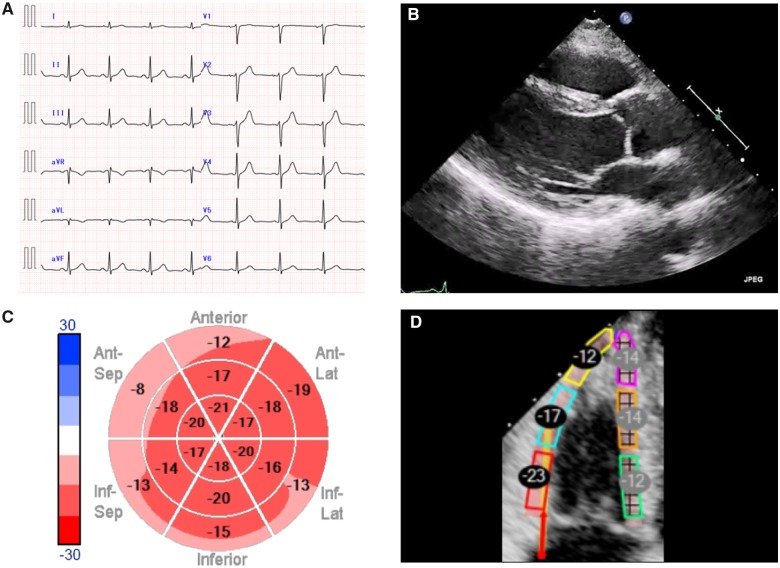
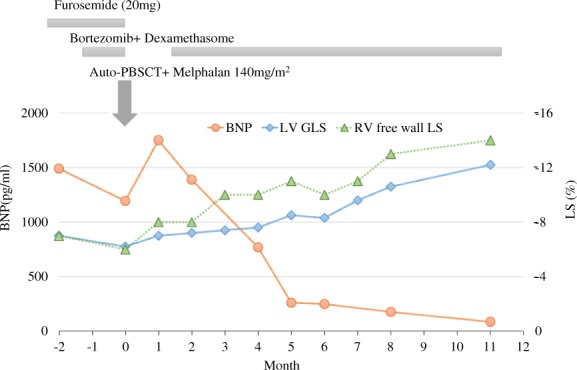
Although BD treatment (bortezomib + dexamethasone) and medical treatment were started, the patient’s condition gradually worsened. Then, high-dose chemotherapy (melphalan, 140 mg/m2) with auto-PBSCT was started. The clinical course of auto-PBSCT is shown in Figure 2. Brain natriuretic peptide levels declined to the 200 s pg/mL at 6 months after auto-PBSCT and continued to decrease. Left ventricular diastolic function measurements corresponded to progressive improvement in the BNP level. Doppler demonstrated a relaxation abnormality pattern with an E/A <0.8, and an IVC diameter <15 mm at 6 months after auto-PBSCT. These improvements were accompanied by an improvement in biventricular LS. While GLS showed a slight change at 6 months after auto-PBSCT was started, it significantly improved thereafter. Twenty-two months after auto-PBSCT, GLS improved to a value of −16.2%, and bull’s-eye mapping showed a more normal pattern. Right ventricular free wall LS and LA strain were also improved to −17% and −27%, respectively. Left ventricular EF did not change significantly from pretreatment levels. The anatomic changes in ventricular wall thickness occurred at a slower rate than the changes in GLS. Left ventricular wall thickness improved to 10 mm at 22 months after auto-PBSCT (Figure 3, Supplementary material online, Videos S1 and S2). Interestingly, the subendocardial enhancement disappeared on cardiac MRI after auto-PBSCT. Currently, the patient has had no relapse over 60 months (Figure 4).
Figure 2.
Transthoracic echocardiography. Left ventricular cardiac apex (upper), M mode view (middle), and speckle tracking (lower). Although the left ventricular ejection fraction remained unchanged at 60–65%, global longitudinal strain gradually improved. Gradual alleviation of left ventricular wall hypertrophy was noted, ∼1 year after therapy.
Figure 3.
Clinical course of the patient on high-dose chemotherapy with auto-PBSCT. After autologous peripheral blood stem cell transplantation, the brain natriuretic peptide spiked to 1700 pg/mL, but declined to ∼200 pg/mL after 6 months. These improvements were accompanied by a reduction in the global longitudinal strain levels.
Figure 4.
Imaging examinations 60 months after treatment. (A) Electrocardiogram. Electrocardiography demonstrated a R-wave improvement on the limb leads. (B) Parasternal echocardiographic. (C) Bull’s-eye mapping. (D) Regional values of the right ventricle free wall longitudinal strain.
Discussion
Amyloid light-chain-CA is an infiltrative cardiomyopathy beginning with subclinical myocardial deposition of fibrils, LV and RV wall thickening, and impairment of diastolic function.6 Cardiac involvement occurs in ∼50% of amyloidosis cases. As the volume of infiltration increases, patients develop clinical heart failure with preserved EF. Prognosis is poor, with survival reported as 48 months, dropping to 5–8 months with cardiac involvement.4
Our patient had a rapid reduction in BNP and has maintained a good condition over a period of 5 years. This improvement may have been due to shifting the treatment to high-dose melphalan and auto-PBSCT. Plasma levels of the natriuretic peptides (BNP and NT-proBNP) tend to be high in CA independent of the severity of heart failure and have prognostic value as an index of response to therapy.7 In contrast, a recent study showed patients who achieve haematologic remission after auto-PBSCT have significant regression of the classical echocardiographic changes in CA.8 However, several studies have reported that serial assessments of wall thickness and EF do indeed improve in association with a maintained complete haematologic response, and these changes typically require many years (three or more) to observe; they are rarely, if ever, observed in the short term.9
Speckle-tracking echocardiography has been widely reported to be useful in the diagnosis and prognosis of CA. Global longitudinal strain and an apical sparing pattern have been widely reported to discriminate between AL-CA and other wall thickening processes, and predict survival associated with heart failure development in a cohort of AL amyloidosis patients (cardiac and non-cardiac).2,10,11 Furthermore, several studies have reported that GLS may unmask early cardiac recovery following chemotherapy in patients with CA.12 Fitzgerald et al.5 reported a case of an AL-CA patient who showed complete remission with normalization of GLS ∼2 years after PBSCT. In this previous case, the anatomic changes (regression in wall thickness) occurred at a slower rate than the changes in GLS. These findings are consistent with our case. Improvement in GLS may be related to a reduction in the amount of myocardial amyloid deposition. Ternacle et al.13 reported that areas with low strain showed a tendency for higher levels of myocardial amyloid deposition by histopathology of myocardial biopsies. A further analysis is needed to determine whether or not our findings can be generalized to other cases.
Conclusion
Our report provides detailed echocardiography follow-up of a patient throughout the clinical course of CA.
Lead author biography

Yukina Hirata is the sonographer at Tokushima University Hospital. She majored in cardiovascular Medicine and received her PhD degree from Graduate School of Medical Sciences, Tokushima University, Japan in March 2016. Her main research theme is examine the clinical utility of epicardial adipose tissue using echocardiography. Recently, she is interested in artificial intelligence and supervised by Dr Kenya Kusunose.
Supplementary material
Supplementary material is available at European Heart Journal - Case Reports online.
Supplementary Material
Acknowledgements
The authors are deeply grateful to staffs and Drs Hisanori Uehara at Department of Pathology of Tokushima University Hospital for data acquisitions of Pathological specimen and to all staffs at Ultrasound Examination Center, Tokushima University Hospital for acquisitions of echocardiographic parameters. Finally, we are gratefully acknowledge to Masataka Sata at Department of Cardiovascular Medicine of Tokushima University Hospital for advice with this paper.
Funding
This work was supported by JSPS Kakenhi Grants [17K13037 to Y.H., 15K19381/17K09506 to K.K.].
Slide sets: A fully edited slide set detailing this case and suitable for local presentation is available online as Supplementary data.
Consent: The author/s confirm that written consent for submission and publication of this case report including image(s) and associated text has been obtained from the patient in line with COPE guidance.
Conflict of interest: none declared.
References
- 1. Falk RH, Comenzo RL, Skinner M.. The systemic amyloidosis. N Engl J Med 1997;337:898–909. [DOI] [PubMed] [Google Scholar]
- 2. Phelan D, Collier P, Thavendiranathan P, Popović ZB, Hanna M, Plana JC, Marwick TH, Thomas JD.. Relative apical sparing of longitudinal strain using two-dimensional speckle-tracking echocardiography is both sensitive and specific for the diagnosis of cardiac amyloidosis. Heart 2012;98:1442–1448. [DOI] [PubMed] [Google Scholar]
- 3. Dubrey SW, Cha K, Anderson J, Chamarthi B, Reisinger J, Skinner M, Falk RH. The clinical features of immunoglobulin light-chain (AL) amyloidosis with heart involvement. QJM 1998;91:141–157. [DOI] [PubMed] [Google Scholar]
- 4. Fitzgerald BT, Bashford J, Scalia GM.. The return of the normal heart: resolution of cardiac amyloidosis after chemotherapy and bone marrow transplantation. Heart Lung Circ 2013;22:655–660. [DOI] [PubMed] [Google Scholar]
- 5. Fitzgerald BT, Bashford J, Scalia GM.. Regression of the anatomic cardiac features of amyloid light chain cardiac amyloidosis accompanied by normalization of global longitudinal strain. CASE (Phila) 2017;1:46–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Falk RH. Cardiac amyloidosis: a treatable disease, often overlooked. Circulation 2011;124:1079–1085. [DOI] [PubMed] [Google Scholar]
- 7. Merlini G, Lousada I, Ando Y, Dispenzieri A, Gertz MA, Grogan M, Maurer MS, Sanchorawala V, Wechalekar A, Palladini G, Comenzo RL.. Rationale, application and clinical qualification for NT-proBNP as a surrogate end point in pivotal clinical trials in patients with al amyloidosis. Leukemia 2016;30:1979–1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Salinaro F, Meier-Ewert HK, Miller EJ, Pandey S, Sanchorawala V, Berk JL, Seldin DC, Ruberg FL.. Longitudinal systolic strain, cardiac function improvement, and survival following treatment of light-chain (AL) cardiac amyloidosis. Eur Heart J Cardiovasc Imaging 2017;18:1057–1064. [DOI] [PubMed] [Google Scholar]
- 9. Meier-Ewert HK, Sanchorawala V, Berk J, Finn KT, Skinner M, Seldin DC, Ruberg FL. Regression of cardiac wall thickness following chemotherapy and stem cell transplantation for light chain (AL) amyloidosis. Amyloid 2011;18 Suppl 1:130–131. [DOI] [PubMed] [Google Scholar]
- 10. Buss SJ, Emami M, Mereles D, Korosoglou G, Kristen AV, Voss A, Schellberg D, Zugck C, Galuschky C, Giannitsis E, Hegenbart U, Ho AD, Katus HA, Schonland SO, Hardt SE.. Longitudinal left ventricular function for prediction of survival in systemic light-chain amyloidosis: incremental value compared with clinical and biochemical markers. J Am Coll Cardiol 2012;60:1067–1076. [DOI] [PubMed] [Google Scholar]
- 11. Liu D, Hu K, Niemann M, Herrmann S, Cikes M, Störk S, Gaudron PD, Knop S, Ertl G, Bijnens B, Weidemann F.. Effect of combined systolic and diastolic functional parameter assessment for differentiation of cardiac amyloidosis from other causes of concentric left ventricular hypertrophy. Circ Cardiovasc Imaging 2013;6:1066–1072. [DOI] [PubMed] [Google Scholar]
- 12. Tuzovic M, Kobayashi Y, Wheeler M, Barrett C, Liedtke M, Lafayette R, Schrier S, Haddad F, Witteles R.. Functional cardiac recovery and hematologic response to chemotherapy in patients with light-chain amyloidosis (from the Stanford University Amyloidosis Registry). Am J Cardiol 2017;120:1381–1386. [DOI] [PubMed] [Google Scholar]
- 13. Ternacle J, Bodez D, Guellich A, Audureau E, Rappeneau S, Lim P, Radu C, Guendouz S, Couetil J-P, Benhaiem N, Hittinger L, Dubois-Randé J-L, Plante-Bordeneuve V, Mohty D, Deux J-F, Damy T.. Causes and consequences of longitudinal LV dysfunction assessed by 2D strain echocardiography in cardiac amyloidosis. JACC Cardiovasc Imaging 2016;9:126–138. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.