Photosynthetic triose phosphate utilization limitation is discussed, highlighting misleading points in physiology and focusing on regulation.
Keywords: Gas exchange, phosphate metabolism, photosynthesis modeling, regulation of photosynthesis, sink strength, TPU limitation, triose phosphate utilization
Abstract
During photosynthesis, plants fix CO2 from the atmosphere onto ribulose-bisphosphate, producing 3-phosphoglycerate, which is reduced to triose phosphates (TPs). The TPs are then converted into the end products of photosynthesis. When a plant is photosynthesizing very quickly, it may not be possible to commit photosynthate to end products as fast as it is produced, causing a decrease in available phosphate and limiting the rate of photosynthesis to the rate of triose phosphate utilization (TPU). The occurrence of an observable TPU limitation is highly variable based on species and especially growth conditions, with TPU capacity seemingly regulated to be in slight excess of typical photosynthetic rates the plant might experience. The physiological effects of TPU limitation are discussed with an emphasis on interactions between the Calvin–Benson cycle and the light reactions. Methods for detecting TPU-limited data from gas exchange data are detailed and the impact on modeling of some physiological effects are shown. Special consideration is given to common misconceptions about TPU.
Introduction
Triose phosphate utilization (TPU) is one of the three canonical biochemical limitations of photosynthesis in gas exchange analysis of C3 plants. It reflects a steady-state condition in which assimilation of carbon is limited by the ability to regenerate phosphate through production of end products of photosynthesis. Phosphate is required by ATP synthase to produce ATP, of which three are needed to fix a single carbon. Although all three ATPs are used for phosphorylation of carbon chains, two are immediately released when the 3-phosphoglyceric acid (PGA) kinase reaction is followed by glyceraldehyde-3-phosphate (GAP) dehydrogenase. Regeneration of ribulose 1,5-bisphosphate (RuBP) releases two phosphates per three fixed carbons, one from fructose-1,6-bisphosphatase (FBPase) and one from sedoheptulose bisphosphatase (SBPase). One phosphate per three carbons remains on the triose phosphates (TPs) GAP and dihydroxyacetone phosphate (DHAP), which are used for synthesis of starch and sucrose. The capacity for end product synthesis relative to carbon fixation can determine the concentration of inorganic phosphate. If the capacity for TPU is high relative to carbon fixation, the concentration of phosphate will be high. A high concentration of phosphate will inhibit starch synthesis and, less so, sucrose synthesis, changing the partitioning of carbon among the end products. A high concentration of phosphate could also make ATP synthesis easier and so interfere with the acidification of the stromal lumen, which is necessary to induce energy-dependent quenching (qE) in PSII. If TP use is too quick relative to carbon fixation, it may deplete Calvin–Benson cycle intermediates and lead to difficulty in regenerating RuBP. On the other hand, if the capacity for TPU is low relative to carbon fixation, the phosphate concentration decreases, leading to reduced conductivity of protons through thylakoid ATP synthase that ultimately slows photosynthesis (Kanazawa and Kramer, 2002; Takizawa et al., 2008; Kiirats et al., 2009). One minute after becoming TPU limited, the ATP/ADP ratio can fall from 2.3 to 1.2 although after 18 min other regulatory processes can allow it to recover to 1.6 (Sharkey et al., 1986b).
The decline in ATP is a form of feedback limitation and is potentially quite dangerous to the plant. Feedback conditions are known to cause photodamage due to the inability to move energy downstream (Pammenter et al., 1993; Takizawa et al., 2008; Kiirats et al., 2009). To avoid photodamage, instead of maintaining phosphate-restricted feedback, a series of regulatory steps are engaged to slow photosynthetic electron transport and carbon fixation by Rubisco. While the capacity is determined by phosphate balance, the steady-state rate is set by regulatory effects that serve to ameliorate feedback conditions. This includes reduction in the PSII quantum yield (ΦPSII) (Sharkey et al., 1988; Kiirats et al., 2009) and reduced activation state of Rubisco (Sharkey et al., 1986a; Socias et al., 1993; Viil et al., 2004; Cen and Sage, 2005). In this review, we discuss the effect of end product synthesis on the overall rate and regulation of photosynthesis.
How are triose phosphates used?
The maximal photosynthetic rate under TPU limitation is primarily, but not exclusively, determined by the rate of conversion of TPs into starch and sucrose. The synthesis of sugar alcohols in some plant species (Escobar-Gutiérrez and Gaudillère, 1997; Loescher et al., 2000) has the same effect as sucrose synthesis. The limitation on assimilation is based on the release of phosphate from Calvin–Benson cycle intermediates that leave the cycle, and the most immediate release is from the activity of FBPase in the chloroplast for starch synthesis or in the cytosol for sucrose synthesis. Sucrose synthesis begins with the translocation of TPs through the triose phosphate/phosphate translocator (TPT) (Riesmeier et al., 1993). This removes carbon from the Calvin–Benson cycle and returns phosphate from the cytosol to the chloroplast. Each sucrose molecule requires the combination of two hexose molecules, for a total of four triose phosphates. Net phosphate release from organic phosphates during sucrose synthesis occurs at FBPase (2), UDP-glucose pyrophosphorylase (1), and sucrose-phosphate phosphatase (1). Sucrose synthesis is typically measured at between 25% and 50% of total carbon assimilation (Sharkey et al., 1985; Escobar-Gutiérrez and Gaudillère, 1997; Szecowka et al., 2013; Abadie et al., 2018), with some studies demonstrating up to 75% (Stitt et al., 1983). It is likely that the species and environmental conditions have an effect on partitioning of carbon into sucrose.
In starch synthesis, phosphate release occurs at stromal FBPase and ADP-glucose pyrophosphorylase. The flux to starch varies considerably with the growth conditions of the plant; for example, Arabidopsis growing in an 18 h photoperiod committed only 24% of fixed carbon to starch but in a 6 h photoperiod committed 51% (Sulpice et al., 2014). Other studies show that between 30% and 60% of fixed carbon goes to starch (Sharkey et al., 1985; Escobar-Gutiérrez and Gaudillère, 1997; Szecowka et al., 2013; Abadie et al., 2018), but the amount of carbon partitioned to starch can vary greatly among plant species (Huber, 1981). A small amount of phosphate is added to starch in photosynthesizing leaves by glucan-water dikinase and phosphoglucan-water dikinase, but the amount is very low, 0.1–0.9% of glucose moieties (McPherson and Jane, 1999; Ritte et al., 2002; Kötting et al., 2005), and so is not relevant for understanding gas exchange properties of photosynthesis.
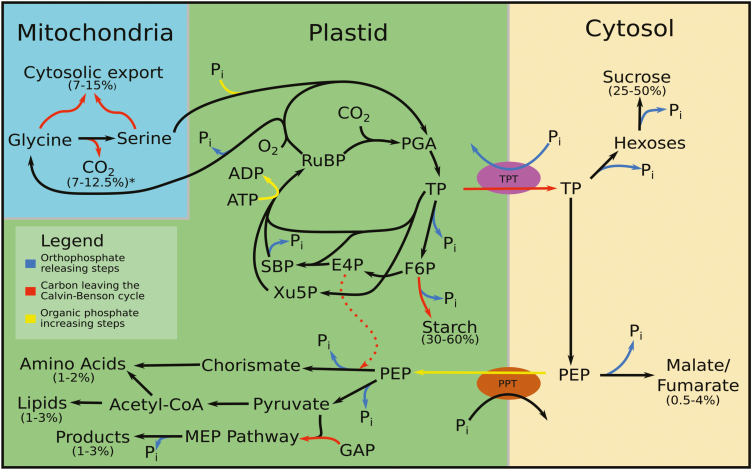
There are a number of other routes by which carbon is exported from the Calvin–Benson cycle (Fig. 1). Any carbon metabolism pathway that begins with a phosphorylated Calvin–Benson cycle intermediate and ends with a non-phosphorylated molecule will contribute to TPU. The shikimate pathway to aromatic amino acid synthesis begins with the export of GAP from the chloroplast to make phosphoenolpyruvate (PEP). PEP is reimported into the chloroplast through the phosphoenolpyruvate/phosphate translocator (PPT) and combines with erythrose 4-phosphate (E4P) and ends with chorismate, accounting for 1–2% of fixed carbon (Escobar-Gutiérrez and Gaudillère, 1997; Abadie et al., 2018). Fatty acids and branched chain amino acids are synthesized from acetyl-CoA from pyruvate and account for 1–3% of fixed carbon (Bao et al., 2000). It has been shown that oil biosynthesis can be increased as a carbon sink, and this would contribute to a higher capacity for TPU (Sanjaya et al., 2011). The methyl-erythrtitol 4-phosphate (MEP) pathway begins with GAP and pyruvate to produce isoprenoids consuming up to 3% of fixed carbon (Rasulov et al., 2014). Pyruvate is made from TP exported from the chloroplast and dephosphorylated by pyruvate kinase, freeing phosphate in the cytosol, or by beta elimination of phosphate during the Rubisco reaction (Andrews and Kane, 1991) freeing phosphate in the stroma.
Fig. 1.
A depiction of the major phosphate and carbon exits from the Calvin–Benson cycle. Rates: sucrose, 25–50%; starch, 30–60%; photorespiratory amino acids, 7–15%; shikimate pathway, 1–2%; lipids 1–3%; methylerythritol pathway, 1–3%; PEP carboxylation, 0.5–4%; CO2 release from photorespiration*, 7–12.5% of fixed carbon lost and does not contribute to TPU capacity. Abbreviations: E4P, erythrose 4-phosphate; F6P, fructose 6-phosphate; GAP, glyceraldehyde 3-phosphate; PEP, phosphoenolpyruvate; PGA, 3-phosphoglyceric acid; SBP, sedoheptulose bisphosphate; TP, triose phosphates; Xu5P, xylulose 5-phosphate.
Amino acid intermediates in the photorespiratory pathway can be exported from the leaf or used in the cytosol as carbon skeletons, for transamination, or for protein construction. It is estimated that an average of 30% to a high of 70% of photorespiratory glycolate carbon is exported from the Calvin–Benson cycle as modeled from gas exchange measurements (Busch et al., 2018). If the ratio of oxygenation to carboxylation (ϕ) is assumed to be 0.25, this represents carbon export from the Calvin–Benson cycle equivalent to 7–15% of fixed carbon. In addition, CO2 lost from conversion of glycine to serine will allow for increased rates of carboxylation, though it does not increase the maximum assimilation rate. If we assume ϕ is 0.25 and no glycine export, this would represent 12.5% of fixed carbon lost, but under TPU-limited conditions excess carboxylation capacity allows fixation of the same amount of CO2. This is part of the reason photosynthesis becomes insensitive to CO2 even though the rate of photorespiration varies with CO2.
Plants are capable of carboxylating PEP and releasing the phosphate on PEP. The resulting oxaloacetate can be transaminated to aspartate or reduced to malate for use in anapleurotic reactions or storage in the vacuole (sometimes as fumarate). PEP carboxylation contributes to TPU as PEP may come from TPs exported from the chloroplast, and the carboxylation consumes atmospheric carbon which would be measured in gas exchange. Gauthier et al. (2010) found that amino acids made from α-ketoglutarate are quickly labeled by [15N]ammonium nitrate but not 13CO2 fed to photosynthesizing leaves, indicating that the carbon for these amino acids comes from pre-existing pools and so does not contribute to TPU. Szecowka et al. (2013) showed that no more than 2.6% of label goes through PEP to organic acids or amino acids, including non-carboxylation reactions. Ma et al. (2014), using extensive in silico modeling combined with MS measurements, found that PEP carboxylation represented 0.5–4% of fixed carbon, depending on how much PEP carbon is assumed to be directly from the Calvin–Benson cycle and the overall rate of photosynthesis. Another study found that the rate of PEP carboxylation varied with the rate of photosynthesis, increasing significantly in its proportion at low assimilation from 2% to 25% of fixed carbon (Abadie and Tcherkez, 2019). In Arabidopsis, a significant amount of carbon is stored in the vacuole as fumarate; it is not known how much of this carbon is recent (and therefore contributes to TPU) and how much is pre-existing carbon (Chia et al., 2000; Pracharoenwattana et al., 2010; Zell et al., 2010; Ma et al., 2014). This is also true of sunflower (Abadie et al., 2018).
In summary, TPU is primarily starch and sucrose synthesis (~80%). The next most important ‘use’ of TPs may be in removal of glycine or serine from the photorespiratory cycle, potentially reaching 15% but probably usually well below 10%. Many other metabolic pathways account for the remainder, but none of these is likely to exceed 5% of the rate of carbon fixation and so they usually do not have a significant impact on TPU limitation behavior.
TPU and gas exchange
TPU is typically assessed from gas exchange data obtained using infrared gas analyzers to measure rates of CO2 uptake. Because of the usefulness of fluorescence parameters in analyzing gas exchange data, gas exchange measurements are frequently combined with chlorophyll fluorescence analysis. Measuring the stomatal conductance to gas exchange by transpiration allows the calculation of the partial pressure of CO2 inside the leaf (Ci) (Sharkey et al., 1982). Diffusion resistance within the mesophyll will further reduce the effective partial pressure of CO2, resulting in the partial pressure of CO2 at the site of carboxylation (Cc). TPU-limited photosynthesis is mostly insensitive to CO2, so resistance to diffusion of CO2 has little or no effect on TPU-limited photosynthesis.
Plots of carbon assimilation (A) as a function of Ci (or better Cc when mesophyll conductance can be estimated) can be interpreted using Rubisco kinetics to predict what biochemical process is limiting assimilation. At low Cc, assimilation is typically limited by binding affinity of Rubisco for CO2 (and the inhibition by oxygen), known as the Rubisco limitation (often abbreviated as C limitation). At intermediate Cc or when given insufficient light, assimilation is typically limited by the rate of regeneration of ribulose 1,5-bisphosphate (RuBP), frequently referred to as J limitation. TPU limitation, sometimes called P limitation, only happens when the plant has a greater capacity to fix carbon than it has to remove carbon from the Calvin–Benson cycle in end product synthesis. In many plants, this can be seen at high Cc and saturating light. The requirement for a high photosynthetic rate may be why TPU limitation is so hard to detect in plants with low inherent photosynthetic rates such as Arabidopsis (Yang et al., 2016).
Lack of, or reverse, sensitivity of A to oxygen partial pressure changes and CO2 partial pressure increases is the primary gas exchange behavior of TPU limitation (Sharkey, 1985a). Insensitivity had been reported for many years (Ludwig and Canvin, 1971; Jolliffe and Tregunna, 1973; von Caemmerer and Farquhar, 1981). Critically, Harris et al. (1983) found insensitivity following feeding with mannose, which sequesters phosphate. Later it was shown that oxygen insensitivity was correlated with CO2 insensitivity (Sharkey, 1985a). Leegood and Furbank (1986) found that oxygen-insensitive photosynthesis in leaf discs was induced by a combination of low temperature and high CO2 partial pressure. Feeding of phosphate restored normal oxygen sensitivity and also increased CO2 assimilation rate, showing that phosphate metabolism was involved in both oxygen sensitivity and the limitation of assimilation. From this and other considerations, Sharkey (1985a) concluded “(a)s the rate of CO2 assimilation increases, starch and sucrose synthesis must increase as well. If not, triose-P and PGA will build up and phosphate will decline. These changes in pool size will stimulate starch and sucrose synthesis. However, there is a limit to how far the phosphate pool can fall before it begins to limit photophosphorylation. Once this limit is reached, CO2 will be assimilated at the rate at which starch and sucrose synthesis can metabolize triose-P, regardless of whether oxygenation occurs or not”.
When photosynthesis is limited either by Rubisco or by RuBP regeneration, increasing CO2 or decreasing O2 should increase A. When A is Rubisco limited, A will increase because of (i) the affinity of Rubisco for CO2 and the effects of O2 on CO2 affinity and (ii) the reduced CO2 release in photorespiration. When A is limited by RuBP regeneration, A will increase because of (i) the reduced CO2 release in photorespiration (as above) and (ii) the diversion of RuBP from oxygenation to carboxylation when photorespiration is suppressed. TPU-limited photosynthesis does not exhibit this increase or exhibits a reduced stimulation when photorespiration is suppressed (Badger et al., 1984; Sharkey, 1985a). The insensitivity of A while TPU is limited happens because the controlling factor is the ability of the leaf to make end products and this is not affected by CO2, O2, or the rate of photorespiration. Increasing photorespiration by increasing O2 or decreasing CO2 partial pressures will be compensated by increased RuBP regeneration and carboxylation but, because these capacities are in excess in a TPU-limited state, this will not affect A. Use of oxygen or CO2 insensitivity to determine photosynthetic limitations in A/Ci curves is discussed in greater detail in Busch and Sage (2017).
It is not possible to determine whether C4 plants suffer TPU limitation. The carbon pump of C4 metabolism makes it difficult to see the gas exchange behaviors that characterize TPU limitation. C4 plants at high photosynthetic rates are interpreted to be limited by CO2-saturated Rubisco activity, and at lower rates by PEP carboxylase activity (Collatz et al., 1992). Even if Rubisco is not saturated with CO2, oxygen-dependent changes in the rate of photorespiratory CO2 release change the CO2 concentration in the bundle sheaths, making the C4 photosynthesis rate independent of the photorespiration rate (von Caemmerer, 2000). Thus, the CO2 and O2 dependence that results from the variation in the ratio of carboxylation to oxygenation is not observed in C4 photosynthesis and, because this is the gas exchange characteristic that is used to diagnose TPU limitation, it is not possible to tell if C4 plants have a TPU-limited state.
Reverse sensitivity to CO2 and O2 partial pressures
While the TPU limitation offered understanding of insensitivity to increasing O2 and CO2 partial pressures, it did not immediately explain reverse sensitivity. It has long been known that oxygen inhibits photorespiration due to competitive binding to Rubisco and photorespiratory CO2 release (Warburg, 1919; Ludwig and Canvin, 1971; McVetty and Canvin, 1981). It was therefore unexpected to find that reducing oxygen or increasing CO2 partial pressures could sometimes reduce the rate of CO2 assimilation. As photorespiration releases CO2, it is counterintuitive that altering the gas composition to favor carboxylation would result in decreased carbon assimilation. Yet data dating back decades show that once at high CO2, increasing CO2 can cause a decrease in net assimilation (Jolliffe and Tregunna, 1973; Canvin, 1978; von Caemmerer and Farquhar, 1981), and increasing O2 can cause an increase in net assimilation (Viil et al., 1977).
Photorespiration was one key to understanding the reverse oxygen sensitivity under TPU-limiting conditions. Phosphoglycolate is dephosphorylated by phosphoglycolate phosphatase before export through PLGG1 or BASS6 (South et al., 2017). Photorespiratory metabolism of two glycolate molecules leads to re-import of carbon as glycerate, which is phosphorylated to phosphoglyceric acid. The extra phosphate released can be used to make ATP that phosphorylates ribulose 5-phosphate to produce RuBP that will be used to accept a CO2, balancing the photorespiratory loss of one carbon. However, the two amino acid intermediates in the photorespiratory pathway can be used in the cytosol, resulting in net carbon export from the Calvin–Benson cycle. This carbon is effectively lost from RuBP and not directly from CO2 fixed from the atmosphere. Photorespiratory carbon that never returns to the chloroplast was parameterized as α, the fraction of glycolate carbon that leaves the photorespiratory cycle as amino acids (Harley and Sharkey, 1991). The α parameter was later refined to αG and αS, the fraction of glycolate carbon that leaves as glycine and serine, respectively (Busch et al., 2018). When glycine is exported instead of serine, no CO2 is released. As these amino acids come from phosphorylated plastidic metabolites, and permanently leave the Calvin–Benson cycle, they contribute to TPU capacity. Adjusting the gas composition to decrease ϕ reduces the export of glycine and serine, and therefore reduces TPU capacity, reducing the maximum photosynthetic rate. This can explain the reverse sensitivity of A to CO2 and O2.
Starch synthesis is also affected by oxygen partial pressure and can contribute to severe reverse sensitivity. Beans photosynthesizing quickly then transferred to low oxygen were found to have reduced rates of starch synthesis but a minimal change in the rate of sucrose synthesis. A concurrent reduction in the ratio of glucose-6-phosphate to fructose-6-phosphate indicates inhibition of phosphoglucose isomerase (Dietz, 1985; Vassey and Sharkey, 1989). The precise mechanism of this inhibition is unclear.
Modeling
TPU models have seen some recent changes to account for our enhanced understanding of the possible role of photorespiration in nitrogen metabolism. Original models that account for TPU relied on simple stoichiometry (Sharkey, 1985b):
| (1) |
where Wp is the rate of carboxylation when limited by phosphate metabolism. Under this model, photosynthetic carboxylation would equal the rate of carbon export from the Calvin–Benson cycle for starch and sucrose synthesis (numerator) adjusted by the amount of carbon released during photorespiration (denominator). Under TPU limitation, A is given by
| (2) |
When Equation 1 is plugged into Equation 2, the (1–0.5ϕ) term cancels out and so A is independent of the rate of photorespiration. This is because Rubisco is not limiting so the amount of CO2 released during photorespiration can be compensated by increased Rubisco activity.
However, this model did not account for reverse sensitivity of assimilation to oxygen or CO2 frequently observed. The model also describes all carbon export as TPU, which is not directly true. Any carbon that leaves the Calvin–Benson cycle and is dephosphorylated will contribute to the maximum TPU capacity. While all carbon in the Calvin–Benson cycle derives from TP, some of the end products are made from Calvin–Benson cycle intermediates other than TPs. Despite this, the simple model has some advantages. It requires no estimation of RL, mesophyll conductance (gm), or Γ*. These three parameters are currently impossible to measure directly, and there is some debate about our ability to fit them accurately and the constancy of these parameters.
A recent model for TPU incorporates parameters for glycine or serine exit from the photorespiratory cycle. The glycine and serine need not accumulate and could have a range of metabolic fates, as long as the carbon does not re-enter the Calvin–Benson cycle. From Busch et al. (2018):
| (3) |
The denominator in the equation has three terms to account for carbon that exits photorespiration as glycine (αG) or serine (αS). As one carbon out of four is lost as CO2 in the formation of serine, αS cannot be greater than 0.75. If αG and αS are zero, Equations 1 and 3 are identical. Unlike the simple model of Equation 1, Equation 3 requires knowledge of the relative rate of photorespiration, and therefore relies on fitting for Γ*. There is little signal to differentiate αS and αG by gas exchange, which can make fitting these two parameters challenging. For conversion of Equation 3 to assimilation as would be measured by gas exchange, Wp must be adjusted for respiratory carbon loss:
| (4) |
where Γ*αG is the Rubisco–Cc compensation point given the reduced rate of photorespiratory CO2 release due to export of glycine. Γ*αG/Cc is equivalent to 0.5ϕ if αG=0.
Current modeling software is available with varying numbers of parameters to fit. Sharkey (2016) presented an Excel tool which allows picking of points from A/Ci curves, with options to fit RL, gm, αG, and αS. Bellasio et al. (2016) provide a highly detailed Excel tool that uses combined gas exchange and fluorescence to fit RL, gm, Jmax, Vcmax, Γ*, and Rubisco specificity for CO2 versus oxygen (Sc/o), but not α; much of the basis of this fitting is also discussed by Yin et al. (2009). Dubois et al. (2007) provide an SAS program which allows fitting of RL, gm, Jmax, Vcmax, Γ*, Sc/o, and α. Moualeu-Ngangue et al. (2017) propose to improve the Dubois fitting by reducing the number of assumptions made, though they do not fit α. Gu et al. (2010) provide a website for fully automated leaf data analysis called LeafWeb which does not require selecting limitations point-wise or specific software. It should be noted that no current model attempts to incorporate other carbon sinks, and TPU is treated as a single variable.
Temperature sensitivity
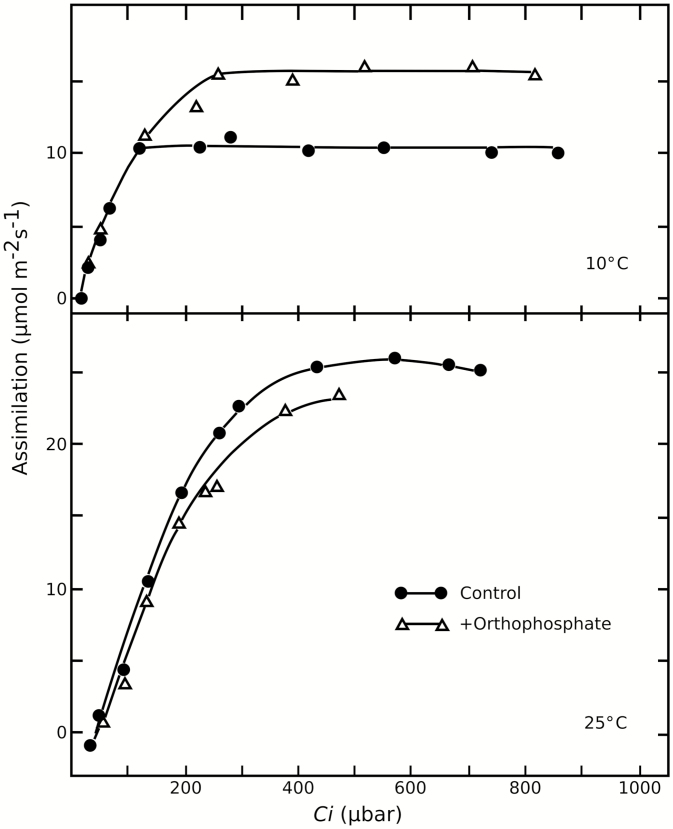
Photosynthesis under TPU limitation is highly temperature sensitive. Though the other photosynthetic limitations demonstrate temperature sensitivity (Cen and Sage, 2005; Sage and Kubien, 2007; Sharkey and Bernacchi, 2012; Busch and Sage, 2017), TPU-limiting conditions are the most temperature sensitive (Sharkey and Bernacchi, 2012; Yang et al., 2016) perhaps because of the strong temperature sensitivity of sucrose-phosphate synthase (Stitt and Grosse, 1988; Leegood and Edwards, 1996) or altered sensitivity of cytosolic FBPase to 2,6-fructose bisphosphate (Stitt and Grosse, 1988). Other enzymes implicated in TPU limitation are also temperature sensitive, such as nitrate reductase (Leegood and Edwards, 1996; Busch et al., 2018). Because of the different ways by which temperature affects the three limitations, the conditions in which they appear change with temperature. At temperatures lower than growth conditions, the plant is significantly more likely to become TPU limited (Stitt, 1986; Sage and Sharkey, 1987; Labate and Leegood, 1988). Labate and Leegood (1988) demonstrated a temperature-sensitive increase in photosynthesis from phosphate feeding. Leaf discs floated on a solution containing phosphate at 25 °C saw a marginal reduction in assimilation. However, discs fed phosphate at 10 °C experienced significant photosynthetic gains, indicating that reduced temperatures result in greater limitation of photosynthesis by TPU (Fig. 2).
Fig. 2.
Rate of CO2 assimilation of barley versus Ci at 10 °C (top) and 25 °C (bottom) with and without the addition of phosphate. A temperature-dependent increase in photosynthetic assimilation is observed upon addition of phosphate. Adapted by permission from Springer: Springer Planta. Limitation of photosynthesis by changes in temperature, Labate, CA, Leegood, RC, Copyright 1988.
Acclimation of TPU
The capacity for TPU is not immutable. Plants grown under poor conditions are highly adaptive, and those grown under low temperature tend to have greatly elevated TPU capacity (Guy et al., 1992; Holaday et al., 1992; Sage and Kubien, 2007). This acclimation largely comes from increased expression of sucrose biosynthesis enzymes (Guy et al., 1992; Holaday et al., 1992; Strand et al., 1999; Hurry et al., 2000), and it has been proposed that this acclimation is signaled by low phosphate levels (Hurry et al., 2000). This increased capacity offsets the decreased activity of starch synthase and sucrose-phosphate synthase at low temperature and makes it less likely that the plant will be TPU limited (Cornic and Louason, 1980; Sage and Sharkey, 1987). Plants transferred to an elevated CO2 environment developed increased phosphate regeneration capacity, demonstrating acclimation (Sharkey et al., 1988; Sage et al., 1989).
Plants experiencing water stress reduce their TPU capacity, possibly reflecting the reduced internal CO2 partial pressure that results from stomatal closure (von Caemmerer and Farquhar, 1984; Vassey and Sharkey, 1989; Cornic et al., 1992). Transgenic plants overexpressing alternative oxidase cope better with water stress (Dahal et al., 2014, 2015) and experience reduced negative effects on assimilation from TPU capacity. The reduced occurrence of TPU limitation in plants overexpressing the alternative oxidase was correlated with higher amounts of chloroplast ATP synthase, which might allow ATP synthesis at lower phosphate concentration. This adaptability shows that TPU will influence the metabolic investments of the plant; it will enhance the ability to handle high TP production, but only when it is required for the current output of photosynthesis.
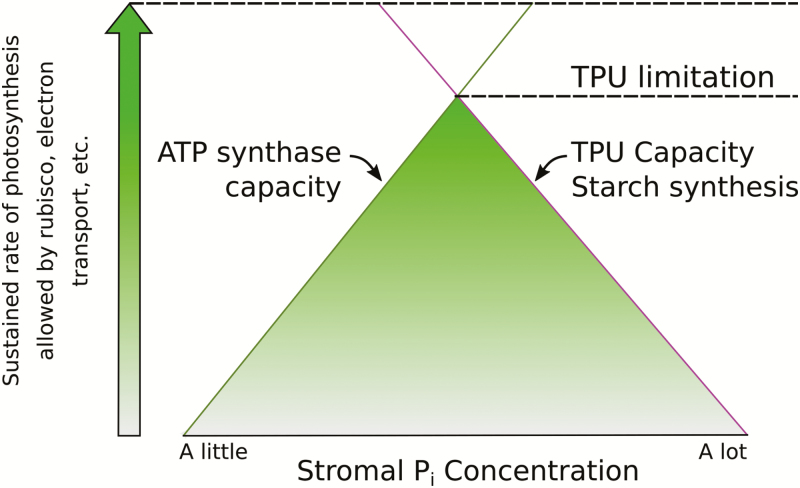
The adaptability of TPU is important for fulfilling the role of stromal phosphate in balancing starch synthesis and ATP synthesis (Fig. 3). Starch synthesis is highly sensitive to phosphate due to inhibition of ADP-glucose pyrophosphorylase (Preiss, 1982), and ATP synthase is kinetically (Takizawa et al., 2007) and thermodynamically sensitive to phosphate. This relationship can help explain the very low partitioning of carbon into starch at a low photosynthetic rate (Escobar-Gutiérrez and Gaudillère, 1997), which is exacerbated by reduced levels of PGA which would otherwise stimulate starch production (Heldt et al., 1977). If sucrose synthesis is in excess, the balance of starch versus sucrose synthesis during the day could become unfavorable for growth, and the extra phosphate could even collapse the Calvin–Benson cycle by driving export of too much TP out of the chloroplast. This has been reported in isolated chloroplasts (Leegood and Walker, 1983) but not in intact leaves. High phosphate outside of chloroplasts has also been shown to result in starch breakdown in the light (Stitt and Heldt, 1981). The highest rate of photosynthesis will be achieved with a fine balance of phosphate usage and phosphate release. In an environment where expected photosynthetic rates are lower, the plant will benefit from reduced TPU capacity. This allows phosphate to fall, correcting several issues with starch and sucrose metabolism and reducing the risk of overconsumption of TPs. When expected photosynthetic rates are higher, the plant will benefit from increased TPU capacity allowing better recycling of phosphate and improved ATP synthase throughput, and alleviating the potential for photodamage due to feedback conditions.
Fig. 3.
As the photosynthetic rate increases, the gap between the phosphate concentration required by the ATP synthase and the phosphate concentration to inhibit starch synthesis narrows. The shapes of the responses are represented by straight lines only for simplicity. When TPU limits the photosynthetic rate, any increase in phosphate required for higher ATP synthase activity would inhibit starch synthesis, restricting phosphate release.
Effects on the light reactions
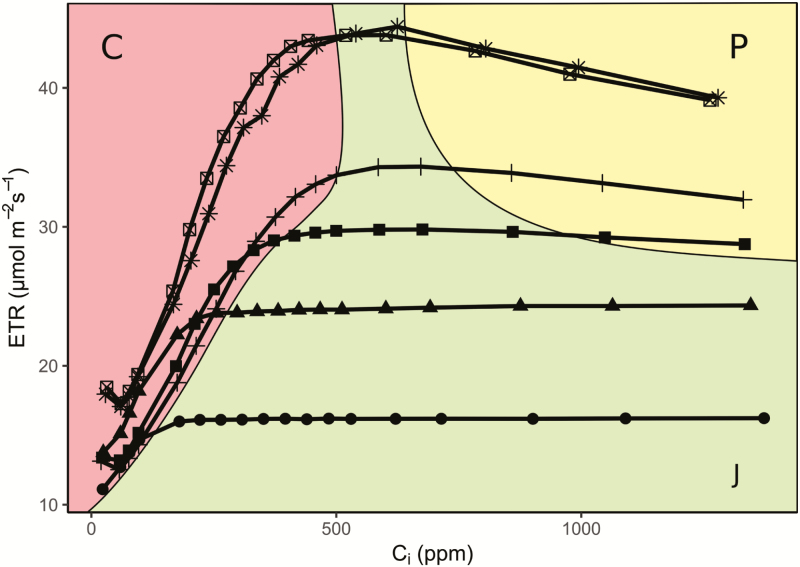
Elevating CO2 partial pressure when photosynthesis is limited by TPU will cause a decrease in ΦPSII. Rubisco binds CO2 and O2 competitively, meaning that an increase in CO2 partial pressure reduces the rate of use of RuBP for oxygenation. This does not lead to an increase in assimilation when TPU is controlling. Rather, it reduces the rate of carboxylation because less carbon is lost through photorespiration, resulting in reduced total Rubisco activity. Both carboxylation and oxygenation require ATP and NADPH, which come from electron transport. Therefore, increasing CO2 partial pressures over TPU-limited leaves results in an overall reduction in electron transport requirements (Stitt, 1986; Sharkey et al., 1988; Stitt and Grosse, 1988). Regulatory processes lead to reduced ΦPSII, a phenomenon which can be useful in discriminating TPU limitation using combined gas exchange and fluorescence data (Fig. 4).
Fig. 4.
The decline in electron transport rate is diagnostic of TPU limitation. Combined gas exchange and fluorescence data in A/Ci curves of Nicotiana benthamiana at varying light intensity and 35 °C. At low CO2, plants are limited by Rubisco activity (C limitation, red), characterized by a sharp upwards slope of both A and ETR with increasing CO2. When light is insufficient, plants will be limited by the rate of RuBP regeneration (J limitation, green), characterized by a flat slope of the ETR with increasing CO2. Only when the plant has ample CO2 and electron transport will TPU limitation (P, yellow) be seen, characterized by a decline in ETR with increasing CO2. ETR is calculated from fluorescence-derived ΦPSII. Light intensity (µmol m−2 s−1):  , 250;
, 250;  , 400;
, 400;  , 550;
, 550;  , 750;
, 750;  , 1000;
, 1000;  1500.
1500.
There are effects on the kinetics of the light reactions that happen concurrently with reduction of electron transport rate. Proton conductivity across the thylakoid membrane goes down under TPU limitation (Takizawa et al., 2008; Kiirats et al., 2009; Yang et al., 2016). It is proposed that this kinetic change occurs because of a reduced pool of available phosphate in the stroma, which reduces the rate of ATP synthase. The Km of chloroplast ATP synthase for phosphate has been measured at 0.2–1 mM (Selman-Reimer et al., 1981; Grotjohann and Gräber, 2002). Stromal phosphate concentration during feedback conditions is estimated to be between 0 mM and 1.7 mM depending on how much phosphate is assumed to be free (Sharkey and Vanderveer, 1989), so it is reasonable to suggest that the phosphate concentration may drop below the Km of ATP synthase. Co-occurring with a decrease in ATP synthase conductivity is an increase in proton-motive force (PMF). The energy needed to make ATP will depend on the concentration of phosphate.
| (5) |
As the effective [Pi] declines, ∆GATP will increase, requiring a greater PMF for ATP synthesis. Increased PMF leads to controls on electron transport through qE, reducing energy arrival at P680 or reduction in the rate of electron flow at the cytochrome b6f complex, leading to reduced electron transport rates (ETRs) (Kramer and Crofts, 1996; Owens, 1996). While phosphate seems to play a role in linking the light reactions and the Calvin–Benson cycle, it is less clear what other molecular mechanisms may be important. It is likely that we do not yet know some important regulatory components that control ETR when TPU limits the rate of photosynthesis.
TPU and sink strength
TPU limitation is a form of very short-term sink/source disequilibrium, separate from long-term sinks such as fruit or root growth, though the two could be related. TPU is concerned with the ability to dephosphorylate and remove carbon quickly from the Calvin–Benson cycle. The half-life of Calvin–Benson cycle intermediates tends to be very short, with many <1 s; some larger pools such as glucose 6-phosphate and UDP-glucose have a half-life of <1 min (Stitt et al., 1980; Arrivault et al., 2009). Pool lifetimes this short mean that TPU limitation can build up and diminish very rapidly. Over a longer time frame, a greater sink can be important in freeing up short-term sinks. It has been reported that defruited wheat experiences significant down-regulation of photosynthesis (King et al., 1967), though not all plants experience this effect (Farquhar and von Caemmerer, 1982). Build up of sucrose in source leaves could result in reduced TPU capacity due to reduced sucrose-phosphate synthase activity, as shown in some experiments (Huber, 1981; Paul and Foyer, 2001), or increased invertase activity (Mengin et al., 2017). In some experiments using conditions consistent with TPU limitation, starch builds up and causes a decline in photosynthetic rate (Sasek et al., 1985; Peet et al., 1986; Ramonell et al., 2001). The source of this decline is still to be conclusively determined. A long-term sink which can absorb carbon will allow the plant to recover (Sasek et al., 1985; Arp, 1991).
TPU and plant nutrition
TPU limitation is often incorrectly interpreted as a nutritional deficiency. It is true that plants transferred to media without any phosphate experience significant reduction in photosynthetic capacity (Brooks, 1986; Foyer and Spencer, 1986). However, less dramatic differences in phosphate nutrition result in relatively small changes in photosynthetic rate. This is due to the vacuole buffering the phosphate concentration in the rest of the cell on a time scale of hours (Rebeille et al., 1983; Woodrow et al., 1984). Under increased or decreased phosphate nutrition, large changes in vacuolar phosphate concentration are seen, but only relatively small changes are seen in plastidic phosphate concentration (Rebeille et al., 1983; Foyer and Spencer, 1986). Plants grown with different phosphate nutrition are therefore not significantly more or less likely to experience TPU limitation. Most phosphate in photosynthesizing cells will be used in nucleic acids and phospholipids (Dissanayaka et al., 2018), and growth is more sensitive to phosphate nutrition than is photosynthetic rate (Mo et al., 2018). Ellsworth et al. (2015) showed that Australian plants growing in the wild with varying phosphate availability were adapted to their environment, and TPU limitation was more likely at high phosphate nutrition. Furthermore, TPU limitation can only be seen when the plant is photosynthesizing very quickly, which usually cannot occur if the plant is nutritionally deprived. Plants with reduced nitrogen were not capable of photosynthesizing quickly enough to reach TPU limitation (Sage et al., 1990).
Oscillations
Oscillations in carbon assimilation rate are a common side effect of TPU limitation (Ogawa, 1982; Sivak and Walker, 1986, 1987). They are typically seen after a perturbation in the environment of a plant that results in high photosynthetic rates, such as sharp increases in illumination or CO2. Oscillations then continue without further input for a variable amount of time. Oscillations include tandem changes in carbon assimilation and fluorescence parameters, indicating simultaneous changes in both the light reactions and the Calvin–Benson cycle (Ogawa, 1982; Walker et al., 1983; Peterson et al., 1988; Stitt and Grosse, 1988). The amplitude of oscillations can increase with conditions that further exacerbate TPU limitation, such as low temperature or low O2 (Peterson et al., 1988; Stitt and Grosse, 1988). Oscillations showed a significant impact on organic phosphates and their relevant ratios, notably large initial spikes in PGA, and reduction in RuBP and ATP pools (Sharkey et al., 1986b; Sage et al., 1988; Stitt and Grosse, 1988; Laisk et al., 1991).
A few models have been produced to explain oscillations. The most significant theory is that there is a delay in activation of sucrose synthesis after a photosynthetic increase that causes oscillations (Laisk and Walker, 1986). The delay may also originate from cytosolic FBPase inhibition by fructose-2,6-bisphosphate (Stitt et al., 1984; Laisk and Eichelmann, 1989; Laisk et al., 1989) or post-translational regulation (Huber and Huber, 1996). An additional interpretation of these oscillations has been proposed originating from the light reactions, with damping caused by a slow leak of protons across the thylakoid membrane (Kocks and Ross, 1995).
Environmental impact
The changing climate, resulting in large measure from increasing CO2, has the potential to affect the frequency and severity of TPU limitations to photosynthesis. Since this syndrome occurs when carbon fixation and light capture have a greater capacity than end product synthesis, increasing CO2 should increase the occurrence of TPU limitation. However, because TPU is stimulated by increasing temperature, there could be a reduction in the occurrence of TPU limitation in the future. It is hard to predict which effect will dominate, and whether TPU limitation will be observed more or less frequently based on climate change predictions. However, beyond the short-term effects of temperature and CO2, it is important to consider how the plant responds when it is TPU limited. Generally, plants growing in elevated CO2 show less propensity for TPU limitation because they have reduced capacity for other processes in photosynthesis (Sage et al., 1989). This suggests that plants cannot or do not make full use of the greater potential for photosynthesis. We hypothesize that understanding TPU will help in predicting acclimation responses of plants to increasing atmospheric CO2. How plants might acclimate could depend on such things as stochasticity of their environment and the typical day/night change in temperature. If night (and dawn) temperature rises more than day temperature, this could affect optimal TPU capacity.
It is often found that TPU limitation occurs whenever photosynthesis is stimulated to be ~20% higher than was occurring in the plant under natural conditions (Yang et al., 2016). Increasing CO2, decreasing oxygen, or lowering the temperature usually allows TPU limitation to be observed. In a large study of published A/Ci curves, Wullschleger (1993) found 23 cases (out of 109) where investigators reported TPU limitations. It is likely that the phenomenon is observed but not recognized much more often. For example, a curve presented in Wullschleger et al. (Fig. 1B, taken from Ireland et al., 1988), shows evidence of TPU limitation but this was not one of the 26 instances of TPU limitation cited. It is common for the TPU limitation to be ignored even when it is evident in data.
Since the components of photosynthesis must all work in concert and in strict stoichiometry, it is not surprising that there might be a relationship between Vcmax and TPU capacity. This has been invoked in global models of photosynthesis, although many models do not include TPU. Lombardozzi et al. (2018) used several estimates of the ratio of Vcmax and TPU capacity, and concluded that current global models may overestimate how much CO2 will be fixed by plants in the future because TPU limitations, or adjustments to avoid TPU limitation, will reduce photosynthetic capacity. It is important to realize that even though plants growing in elevated CO2 do not show TPU limitation, TPU may still be setting an upper bound and that plants adjust other capacities to keep below the upper bound of TPU because TPU can cause damage.
Conclusions
TPU is a metabolic condition that incorporates numerous signals to reflect the state of photosynthesis across the whole cell. Most metabolites in the chloroplast are phosphorylated, and so phosphate can reflect the metabolic state of the chloroplast. Phosphate is linked throughout the cytosol, where sucrose synthesis takes place, and thus phosphate represents the photosynthetic state across all chloroplasts. Phosphate concentrations are carefully regulated, and TPU limitation is very unlikely to be found at ambient conditions. A low phosphate level naturally signals to the other processes that photosynthesis is very fast, kinetically controls the ATP synthase, and leads to downstream effects on photosynthesis by accumulation of PMF and engaging qE. The reduction in phosphate signals the plant to build up starch by relieving phosphate inhibition of ADP-glucose pyrophosphorylase (Preiss, 1982). Plants which are photosynthesizing slowly can reduce their TPU capacity, which will lower their phosphate regeneration, helping to produce starch and prevent cycle collapse from overexport of TPs; conversely, increasing TPU capacity in plants which are photosynthesizing quickly will raise their phosphate regeneration and help produce ATP. In this way, TPU sets the span on expected photosynthesis. We believe that the gas exchange behavior in TPU conditions reflects several important regulatory features. Yet, the role of TPU as regulation is relatively unexplored. Experimental determination of the molecular mechanisms that underpin this system, and ecological studies to examine the broader effects of TPU are exciting future directions in this field.
A number of misconceptions cloud the field in regards to TPU. Even the term ‘TPU’ can now be seen not to be wholly accurate. It largely describes phosphate metabolism, but not all effects on carbon metabolism related to phosphate can be accurately described as TPU. At steady state, there are other sources of phosphate release that contribute to the assimilation cap. Amino acid release from photorespiration, methylerythritol 4-phosphate and shikimate pathways, and other carbon sinks for Calvin–Benson cycle intermediates will all contribute to the maximal assimilation rate when photosynthesis is TPU limited. An alternative view is that all Calvin–Benson cycle exports are downstream of TP, and thus constitute a form of TPU. The specific terminology and nuance are less important than the total understanding, which is that TPU limitation is the result of insufficient capacity for carbon export from the Calvin–Benson cycle. Other carbon metabolism pathways in the chloroplast that do not immediately originate in the Calvin–Benson cycle, while important for the overall physiology of the plant, will not be discernible in gas exchange measurements.
Maintaining TPU limitation is unhealthy for the plant due to risk of oxidative stress from photosystem oxidation (Pammenter et al., 1993). Electron transport regulation as assessed by chlorophyll fluorescence quenching analysis and deactivation of Rubisco lead to an overall slowing of photosynthesis lower than TPU, eventually reaching a steady state with the assimilation rate based on the rate of TPU. Excess assimilation when already low on phosphate would further deprive ATP synthase of phosphate it needs. Contrary to what one might expect given the term ‘TPU limitation’, TPs do not necessarily need to build up, though phosphate levels should be low (Sharkey and Vanderveer, 1989). This is why plants can be drained of phosphate via mannose or deoxyglucose feeding and be TPU limited (Herold and Lewis, 1977; Herold, 1980; Sivak and Walker, 1986). It is the relationship between the need for phosphate for ATP synthase and the phosphate sensitivity of starch and sucrose synthesis that results in TPU (Herold, 1980).
Acknowledgements
This research was funded by the US Department of Energy Grant DE-FG02-91ER2002 (to TDS and AMM). AMM is partially supported by a fellowship from Michigan State University under the Training Program in Plant Biotechnology for Health and Sustainability (T32-GM110523). Partial salary support for TDS came from Michigan AgBioResearch. We thank the UIUC Plant Journal Club for comments on a bioRxiv preprint of this manuscript.
Glossary
Abbreviations
- A
net assimilation of carbon, or, when negative, net respiration of carbon
- Cc
partial pressure of CO2 at the site of carboxylation
- Ci
partial pressure of CO2 inside the leaf
- DHAP
dihydroxyacetone phosphate
- E4P
erythrose 4-phosphate
- ETR
photosynthetic electron transport rate
- FBPase
fructose-1,6-bisphosphatase
- GAP
glyceraldehyde 3-phosphate
- gm
mesophyll conductance to CO2
- Jmax
maximum rate of electron transport under infinite light
- MEP
methylerythritol 4-phosphate
- PEP
phosphoenolpyruvate
- PGA
phosphoglyceric acid
- PMF
proton-motive force
- PPT
phosphoenolpyruvate/phosphate translocator
- qE
energy-dependent quenching
- R
rate of RuBP consumption
- RL
rate of respiration in the light
- RuBP
ribulose 1,5-bisphosphate
- Sc/o
specificity of Rubisco for CO2 versus oxygen
- TP
triose phosphate
- TPT
triose phosphate/phosphate translocator
- TPU
triose phosphate utilization
- Vcmax
maximum velocity of carboxylation
- W
rate of carboxylation
- α
the fraction of glycolate carbon which leaves the Calvin–Benson cycle as amino acids
- αG
the fraction of glycolate carbon which leaves the Calvin–Benson cycle as glycine
- αS
the fraction of glycolate carbon which leaves the Calvin–Benson cycle as serine
- Γ*
The Rubisco CO2 compensation point ignoring the effect of RL
- ϕ
the ratio of oxygenations to carboxylations
- ΦPSII
quantum yield of PSII
References
- Abadie C, Bathellier C, Tcherkez G. 2018. Carbon allocation to major metabolites in illuminated leaves is not just proportional to photosynthesis when gaseous conditions (CO2 and O2) vary. New Phytologist 218, 94–106. [DOI] [PubMed] [Google Scholar]
- Abadie C, Tcherkez G. 2019. In vivo phosphoenolpyruvate carboxylase activity is controlled by CO2 and O2 mole fraction and represents a major flux at high photorespiration rates. New Phytologist 221, 1843–1852. [DOI] [PubMed] [Google Scholar]
- Andrews TJ, Kane HJ. 1991. Pyruvate is a by-product of catalysis by ribulosebisphosphate carboxylase/oxygenase. Journal of Biological Chemistry 266, 9447–9452. [PubMed] [Google Scholar]
- Arp WJ. 1991. Effects of souce–sink relations on photosynthetic acclimation to elevated CO2. Plant, Cell & Environment 14, 869–875. [Google Scholar]
- Arrivault S, Guenther M, Ivakov A, Feil R, Vosloh D, van Dongen JT, Sulpice R, Stitt M. 2009. Use of reverse-phase liquid chromatography, linked to tandem mass spectrometry, to profile the Calvin cycle and other metabolic intermediates in Arabidopsis rosettes at different carbon dioxide concentrations. The Plant Journal 59, 826–839. [DOI] [PubMed] [Google Scholar]
- Badger MR, Sharkey TD, von Caemmerer S. 1984. The relationship between steady-state gas exchange of bean leaves and the levels of carbon-reduction-cycle intermediates. Planta 160, 305–313. [DOI] [PubMed] [Google Scholar]
- Bao X, Focke M, Pollard M, Ohlrogge J. 2000. Understanding in vivo carbon precursor supply for fatty acid synthesis in leaf tissue. The Plant Journal 22, 39–50. [DOI] [PubMed] [Google Scholar]
- Bellasio C, Beerling DJ, Griffiths H. 2016. An Excel tool for deriving key photosynthetic parameters from combined gas exchange and chlorophyll fluorescence: theory and practice. Plant, Cell & Environment 39, 1180–1197. [DOI] [PubMed] [Google Scholar]
- Brooks A. 1986. Effects of phosphorus nutrition on ribulose-1,5-bisphosphate carboxylase activation, photosynthetic quantum yield and amounts of some Calvin-cycle metabolites in spinach leaves. Australian Journal of Plant Physiology 13, 221–237. [Google Scholar]
- Busch FA, Sage RF. 2017. The sensitivity of photosynthesis to O2 and CO2 concentration identifies strong Rubisco control above the thermal optimum. New Phytologist 213, 1036–1051. [DOI] [PubMed] [Google Scholar]
- Busch FA, Sage RF, Farquhar GD. 2018. Plants increase CO2 uptake by assimilating nitrogen via the photorespiratory pathway. Nature Plants 4, 46–54. [DOI] [PubMed] [Google Scholar]
- Canvin DT. 1978. Photorespiration and the effect of oxygen on photosynthesis. In: Siegelman HW, Hind G, eds. Photosynthetic carbon assimilation. Boston, MA: Springer US, 61–76. [Google Scholar]
- Cen YP, Sage RF. 2005. The regulation of Rubisco activity in response to variation in temperature and atmospheric CO2 partial pressure in sweet potato. Plant Physiology 139, 979–990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chia DW, Yoder TJ, Reiter WD, Gibson SI. 2000. Fumaric acid: an overlooked form of fixed carbon in Arabidopsis and other plant species. Planta 211, 743–751. [DOI] [PubMed] [Google Scholar]
- Collatz G, Ribas-Carbo M, Berry J. 1992. Coupled photosynthesis–stomatal conductance model for leaves of C4 plants. Australian Journal of Plant Physiology 19, 519. [Google Scholar]
- Cornic G, Ghashghaie J, Genty B, Briantais J-M. 1992. Leaf photosynthesis is resistant to a mild drought stress. Photosynthetica 27, 295–309. [Google Scholar]
- Cornic G, Louason G. 1980. The effects of O2 on net photosynthesis at low temperature (5°C). Plant, Cell & Environment 3, 149–157. [Google Scholar]
- Dahal K, Martyn GD, Vanlerberghe GC. 2015. Improved photosynthetic performance during severe drought in Nicotiana tabacum overexpressing a nonenergy conserving respiratory electron sink. New Phytologist 208, 382–395. [DOI] [PubMed] [Google Scholar]
- Dahal K, Wang J, Martyn GD, Rahimy F, Vanlerberghe GC. 2014. Mitochondrial alternative oxidase maintains respiration and preserves photosynthetic capacity during moderate drought in Nicotiana tabacum. Plant Physiology 166, 1560–1574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dietz K-J. 1985. A possible rate-limiting function of chloroplast hexosemonophosphate isomerase in starch synthesis of leaves. Biochimica et Biophysica Acta 839, 240–248. [Google Scholar]
- Dissanayaka DMSB, Plaxton WC, Lambers H, Siebers M, Marambe B, Wasaki J. 2018. Molecular mechanisms underpinning phosphorus-use efficiency in rice. Plant, Cell & Environment 41, 1483–1496. [DOI] [PubMed] [Google Scholar]
- Dubois JJ, Fiscus EL, Booker FL, Flowers MD, Reid CD. 2007. Optimizing the statistical estimation of the parameters of the Farquhar–von Caemmerer–Berry model of photosynthesis. New Phytologist 176, 402–414. [DOI] [PubMed] [Google Scholar]
- Ellsworth DS, Crous KY, Lambers H, Cooke J. 2015. Phosphorus recycling in photorespiration maintains high photosynthetic capacity in woody species. Plant, Cell & Environment 38, 1142–1156. [DOI] [PubMed] [Google Scholar]
- Escobar-Gutiérrez AJ, Gaudillère JP. 1997. Carbon partitioning in source leaves of peach, a sorbitol-synthesizing species, is modified by photosynthetic rate. Physiologia Plantarum 100, 353–360. [Google Scholar]
- Farquhar GD, von Caemmerer S. 1982. Modelling of photosynthetic response to environmental conditions. In: Lange OL, Nobel PS, Osmond CB, Ziegler H, eds. Physiological plant ecology II. Berlin Heidelberg New York: Springer-Verlag, 549–582. [Google Scholar]
- Foyer C, Spencer C. 1986. The relationship between phosphate status and photosynthesis in leaves: effects on intracellular orthophosphate distribution, photosynthesis and assimilate partitioning. Planta 167, 369–375. [DOI] [PubMed] [Google Scholar]
- Gauthier PP, Bligny R, Gout E, Mahé A, Nogués S, Hodges M, Tcherkez GG. 2010. In folio isotopic tracing demonstrates that nitrogen assimilation into glutamate is mostly independent from current CO2 assimilation in illuminated leaves of Brassica napus. New Phytologist 185, 988–999. [DOI] [PubMed] [Google Scholar]
- Grotjohann I, Gräber P. 2002. The H+-ATPase from chloroplasts: effect of different reconstitution procedures on ATP synthesis activity and on phosphate dependence of ATP synthesis. Biochimica et Biophysica Acta 1556, 208–216. [DOI] [PubMed] [Google Scholar]
- Gu L, Pallardy SG, Tu K, Law BE, Wullschleger SD. 2010. Reliable estimation of biochemical parameters from C3 leaf photosynthesis–intercellular carbon dioxide response curves. Plant, Cell & Environment 33, 1852–1874. [DOI] [PubMed] [Google Scholar]
- Guy CL, Huber JL, Huber SC. 1992. Sucrose phosphate synthase and sucrose accumulation at low temperature. Plant Physiology 100, 502–508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harley PC, Sharkey TD. 1991. An improved model of C3 photosynthesis at high CO2: reversed O2 sensitivity explained by lack of glycerate reentry into the chloroplast. Photosynthesis Research 27, 169–178. [DOI] [PubMed] [Google Scholar]
- Harris GC, Cheesbrough JK, Walker DA. 1983. Effects of mannose on photosynthetic gas exchange in spinach leaf discs. Plant Physiology 71, 108–111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heldt HW, Chon CJ, Maronde D. 1977. Role of orthophosphate and other factors in the regulation of starch formation in leaves and isolated chloroplasts. Plant Physiology 59, 1146–1155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herold A. 1980. Regulation of photosynthesis by sink activity—the missing link. New Phytologist 86, 131–144. [Google Scholar]
- Herold A, Lewis DH. 1977. Mannose and green plants: occurrence, physiology and metabolism, and use as a tool to study the role of orthophosphate. New Phytologist 79, 1–40. [Google Scholar]
- Holaday AS, Martindale W, Alred R, Brooks AL, Leegood RC. 1992. Changes in activities of enzymes of carbon metabolism in leaves during exposure of plants to low temperature. Plant Physiology 98, 1105–1114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huber SC. 1981. Interspecific variation in activity and regulation of leaf sucrose phosphate synthase. Zeitschrift für Pflanzenphysiologie 102, 443–450. [Google Scholar]
- Huber SC, Huber JL. 1996. Role and regulation of sucrose-phosphate synthase in higher plants. Annual Review of Plant Physiology and Plant Molecular Biology 47, 431–444. [DOI] [PubMed] [Google Scholar]
- Hurry V, Strand A, Furbank R, Stitt M. 2000. The role of inorganic phosphate in the development of freezing tolerance and the acclimatization of photosynthesis to low temperature is revealed by the pho mutants of Arabidopsis thaliana. The Plant Journal 24, 383–396. [DOI] [PubMed] [Google Scholar]
- Ireland CR, Telfer A, Covello PS, Baker NR, Barber J. 1988. Studies on the limitations to photosynthesis in leaves of the atrazine-resistant mutant of Senecio vulgaris L. Planta 173, 459–467. [DOI] [PubMed] [Google Scholar]
- Jolliffe PA, Tregunna EB. 1973. Environmental regulation of the oxygen effect on apparent photosynthesis in wheat. Canadian Journal of Botany 51, 841–853. [Google Scholar]
- Kanazawa A, Kramer DM. 2002. In vivo modulation of nonphotochemical exciton quenching (NPQ) by regulation of the chloroplast ATP synthase. Proceedings of the National Academy of Sciences, USA 99, 12789–12794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiirats O, Cruz JA, Edwards GE, Kramer DM. 2009. Feedback limitation of photosynthesis at high CO2 acts by modulating the activity of the chloroplast ATP synthase. Functional Plant Biology 36, 893–901. [DOI] [PubMed] [Google Scholar]
- King RW, Wardlaw IF, Evans LT. 1967. Effect of assimilate utilization on photosynthetic rate in wheat. Planta 77, 261–276. [DOI] [PubMed] [Google Scholar]
- Kocks P, Ross J. 1995. Kinetic model for (damped) oscillations of transthylakoid pH in plants. Journal of Physical Chemistry 99, 16490–16497. [Google Scholar]
- Kötting O, Pusch K, Tiessen A, Geigenberger P, Steup M, Ritte G. 2005. Identification of a novel enzyme required for starch metabolism in Arabidopsis leaves. The phosphoglucan, water dikinase. Plant Physiology 137, 242–252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kramer DM, Crofts AR. 1996. Control and measurement of photosynthetic electron transport in vivo. In: Baker NR, Govindjee, eds. Photosynthesis and the environment. Dordrecht: Kluwer Academic, 25–66. [Google Scholar]
- Labate CA, Leegood RC. 1988. Limitation of photosynthesis by changes in temperature: factors affecting the response of carbon-dioxide assimilation to temperature in barley leaves. Planta 173, 519–527. [DOI] [PubMed] [Google Scholar]
- Laisk A, Eichelmann H. 1989. Towards understanding oscillations: a mathematical model of the biochemistry of photosynthesis. Philosophical Transactions of the Royal Society B: Biological Sciences 323, 369–384. [Google Scholar]
- Laisk A, Eichelmann H, Oja V, Eatherall A, Walker DA. 1989. A mathematical model of the carbon metabolism in photosynthesis. Difficulties in explaining oscillations by fructose 2,6-bisphosphate regulation. Proceedings of the Royal Society B: Biological Sciences 237, 389–415. [Google Scholar]
- Laisk A, Siebke K, Gerst U, Eichelmann H, Oja V, Heber U. 1991. Oscillations in photosynthesis are initiated and supported by imbalances in the supply of ATP and NADPH to the Calvin cycle. Planta 185, 554–562. [DOI] [PubMed] [Google Scholar]
- Laisk A, Walker DA. 1986. Control of phosphate turnover as a rate-limiting factor and possible cause of oscillations in photosynthesis: a mathematical model. Proceedings of the Royal Society B: Biological Sciences 227, 281–302. [Google Scholar]
- Leegood RC, Edwards GE. 1996. Carbon metabolism and photorespiration: temperature dependence in relation to other environmental factors. In: Baker NR, ed. Photosynthesis and the environment. Dordrecht: Springer Netherlands, 191–221. [Google Scholar]
- Leegood RC, Furbank RT. 1986. Stimulation of photosynthesis by 2% oxygen at low temperatures is restored by phosphate. Planta 168, 84–93. [DOI] [PubMed] [Google Scholar]
- Leegood RC, Walker DA. 1983. The role of transmembrane solute flux in regulation of CO2 fixation in chloroplasts. Biochemical Society Transactions 11, 74–76. [DOI] [PubMed] [Google Scholar]
- Loescher WH, Everard JD, Leegood RC, Sharkey TD, Von Caemmerer S. 2000. Regulation of sugar alcohol biosynthesis. In: Leegood RC, Sharkey TD, von Caemmerer S, eds. Photosynthesis: physiology and metabolism. Advances in photosynthesis. Dordrecht: Kluwer Academic, 275–299. [Google Scholar]
- Lombardozzi DL, Smith NG, Cheng SJ, Dukes JS, Sharkey TD, Rogers A, Fisher R, Bonan GB. 2018. Triose phosphate limitation in photosynthesis models reduces leaf photosynthesis and global terrestrial carbon storage. Environmental Research Letters 13, 074025. [Google Scholar]
- Ludwig LJ, Canvin DT. 1971. The rate of photorespiration during photosynthesis and the relationship of the substrate of light respiration to the products of photosynthesis in sunflower leaves. Plant Physiology 48, 712–719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma F, Jazmin LJ, Young JD, Allen DK. 2014. Isotopically nonstationary 13C flux analysis of changes in Arabidopsis thaliana leaf metabolism due to high light acclimation. Proceedings of the National Academy of Sciences, USA 111, 16967–16972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McPherson AE, Jane J. 1999. Comparison of waxy potato with other root and tuber starches. Carbohydrate Polymers 40, 57–70. [Google Scholar]
- McVetty PBE, Canvin DT. 1981. Inhibition of photosynthesis by low oxygen concentrations. Canadian Journal of Botany 59, 721–725. [Google Scholar]
- Mengin V, Pyl ET, Alexandre Moraes T, Sulpice R, Krohn N, Encke B, Stitt M. 2017. Photosynthate partitioning to starch in Arabidopsis thaliana is insensitive to light intensity but sensitive to photoperiod due to a restriction on growth in the light in short photoperiods. Plant, Cell & Environment 40, 2608–2627. [DOI] [PubMed] [Google Scholar]
- Mo Q, Li Z, Sayer EJ, Lambers H, Li Y, Zou B, Tang J, Heskel M, Ding Y, Wang F. 2018. Foliar phosphorus fractions reveal how tropical plants maintain photosynthetic rates despite low soil phosphorus availability. Functional Ecology. [Google Scholar]
- Moualeu-Ngangue DP, Chen TW, Stützel H. 2017. A new method to estimate photosynthetic parameters through net assimilation rate–intercellular space CO2 concentration (A–Ci) curve and chlorophyll fluorescence measurements. New Phytologist 213, 1543–1554. [DOI] [PubMed] [Google Scholar]
- Ogawa T. 1982. Simple oscillations in photosynthesis of higher plants. Biochimica et Biophysica Acta 681, 103–109. [Google Scholar]
- Owens TG. 1996. Processing of excitation energy by antenna pigments. In: Govindjee Baker NR, eds. Photosynthesis and the environment. Dordrecht: Kluwer Academic, 1–23. [Google Scholar]
- Pammenter NW, Loreto F, Sharkey TD. 1993. End product feedback effects on photosynthetic electron transport. Photosynthesis Research 35, 5–14. [DOI] [PubMed] [Google Scholar]
- Paul MJ, Foyer CH. 2001. Sink regulation of photosynthesis. Journal of Experimental Botany 52, 1383–1400. [DOI] [PubMed] [Google Scholar]
- Peet MM, Huber SC, Patterson DT. 1986. Acclimation to high CO2 in monoecious cucumbers: II. Carbon exchange rates, enzyme activities, and starch and nutrient concentrations. Plant Physiology 80, 63–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peterson RB, Sivak MN, Walker DA. 1988. Carbon dioxide-induced oscillations in fluorescence and photosynthesis: role of thylakoid membrane energization in regulation of photosystem II activity. Plant Physiology 88, 1125–1130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pracharoenwattana I, Zhou W, Keech O, Francisco PB, Udomchalothorn T, Tschoep H, Stitt M, Gibon Y, Smith SM. 2010. Arabidopsis has a cytosolic fumarase required for the massive allocation of photosynthate into fumaric acid and for rapid plant growth on high nitrogen. The Plant Journal 62, 785–795. [DOI] [PubMed] [Google Scholar]
- Preiss J. 1982. Regulation of the biosynthesis and degradation of starch. Annual Review of Plant Physiology and Plant Molecular Biology 33, 431–454. [Google Scholar]
- Ramonell KM, Kuang A, Porterfield DM, Crispi ML, Xiao Y, McClure G, Musgrave ME. 2001. Influence of atmospheric oxygen on leaf structure and starch deposition in Arabidopsis thaliana. Plant, Cell & Environment 24, 419–428. [DOI] [PubMed] [Google Scholar]
- Rasulov B, Bichele I, Laisk A, Niinemets Ü. 2014. Competition between isoprene emission and pigment synthesis during leaf development in aspen. Plant, Cell & Environment 37, 724–741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rebeille F, Bligny R, Martin JB, Douce R. 1983. Relationship between the cytoplasm and the vacuole phosphate pool in Acer pseudoplatanus cells. Archives of Biochemistry and Biophysics 225, 143–148. [DOI] [PubMed] [Google Scholar]
- Riesmeier JW, Flügge UI, Schulz B, Heineke D, Heldt HW, Willmitzer L, Frommer WB. 1993. Antisense repression of the chloroplast triose phosphate translocator affects carbon partitioning in transgenic potato plants. Proceedings of the National Academy of Sciences, USA 90, 6160–6164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ritte G, Lloyd JR, Eckermann N, Rottmann A, Kossmann J, Steup M. 2002. The starch-related R1 protein is an α-glucan, water dikinase. Proceedings of the National Academy of Sciences, USA 99, 7166–7171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sage RF, Kubien DS. 2007. The temperature response of C3 and C4 photosynthesis. Plant, Cell & Environment 30, 1086–1106. [DOI] [PubMed] [Google Scholar]
- Sage RF, Sharkey TD. 1987. The effect of temperature on the occurrence of O2 and CO2 insensitive photosynthesis in field grown plants. Plant Physiology 84, 658–664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sage RF, Sharkey TD, Pearcy RW. 1990. The effect of leaf nitrogen and temperature on the CO2 response of photosynthesis in the C3 dicot Chenopodium album L. Australian Journal of Plant Physiology 17, 135–148. [Google Scholar]
- Sage RF, Sharkey TD, Seemann JR. 1988. The in-vivo response of the ribulose-1,5-bisphosphate carboxylase activation state and the pool sizes of photosynthetic metabolites to elevated CO2 in Phaseolus vulgaris L. Planta 174, 407–416. [DOI] [PubMed] [Google Scholar]
- Sage RF, Sharkey TD, Seemann JR. 1989. Acclimation of photosynthesis to elevated CO2 in five C3 species. Plant Physiology 89, 590–596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanjaya Durrett TP, Weise SE, Benning C. 2011. Increasing the energy density of vegetative tissues by diverting carbon from starch to oil biosynthesis in transgenic Arabidopsis. Plant Biotechnology Journal 9, 874–883. [DOI] [PubMed] [Google Scholar]
- Sasek TW, Delucia EH, Strain BR. 1985. Reversibility of photosynthetic inhibition in cotton after long-term exposure to elevated CO2 concentrations. Plant Physiology 78, 619–622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selman-Reimer S, Merchant S, Selman BR. 1981. Isolation, purification, and characterization of coupling factor 1 from Chlamydomonas reinhardi. Biochemistry 20, 5476–5482. [DOI] [PubMed] [Google Scholar]
- Sharkey TD. 1985a O2-insensitive photosynthesis in C3 plants. Plant Physiology 78, 71–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharkey TD. 1985b Photosynthesis in intact leaves of C3 plants. The Botanical Review 5, 53–105. [Google Scholar]
- Sharkey TD. 2016. What gas exchange data can tell us about photosynthesis. Plant, Cell & Environment 39, 1161–1163. [DOI] [PubMed] [Google Scholar]
- Sharkey TD, Bernacchi CJ. 2012. Photosynthetic responses to high temperature. In: Flexas J, Loreto F, Medrano H, eds. Terrestrial photosynthesis in a changing environment: a molecular, physiological, and ecological approach. Cambridge: Cambridge University Press, 294–302. [Google Scholar]
- Sharkey TD, Berry JA, Raschke K. 1985. Starch and sucrose synthesis in Phaseolus vulgaris as affected by light, CO2, and abscisic acid. Plant Physiology 77, 617–620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharkey TD, Berry JA, Sage RF. 1988. Regulation of photosynthetic electron-transport in Phaseolus vulgaris L., as determined by room-temperature chlorophyll a fluorescence. Planta 176, 415–424. [DOI] [PubMed] [Google Scholar]
- Sharkey TD, Imai K, Farquhar GD, Cowan IR. 1982. A direct confirmation of the standard method of estimating intercellular partial pressure of CO2. Plant Physiology 69, 657–659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharkey TD, Seemann JR, Berry JA. 1986a Regulation of ribulose-1,5-bisphosphate carboxylase activity in response to changing partial pressure of O2 and light in Phaseolus vulgaris. Plant Physiology 81, 788–791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharkey TD, Stitt M, Heineke D, Gerhardt R, Raschke K, Heldt HW. 1986b Limitation of photosynthesis by carbon metabolism: II. O2-insensitive CO2 uptake results from limitation of triose phosphate utilization. Plant Physiology 81, 1123–1129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharkey TD, Vanderveer PJ. 1989. Stromal phosphate concentration is low during feedback limited photosynthesis. Plant Physiology 91, 679–684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sivak MN, Walker DA. 1986. Photosynthesis in vivo can be limited by phosphate supply. New Phytologist 102, 499–512. [Google Scholar]
- Sivak MN, Walker DA. 1987. Oscillations and other symptoms of limitation of in vivo photosynthesis by inadequate phosphate supply to the chloroplast. Plant Physiology and Biochemistry 25, 635–648. [Google Scholar]
- Socias FX, Medrano H, Sharkey TD. 1993. Feedback limitation of photosynthesis of Phaseolus vulgaris L. grown in elevated CO2. Plant, Cell & Environment 16, 81–86. [Google Scholar]
- South PF, Walker BJ, Cavanagh AP, Rolland V, Badger M, Ort DR. 2017. Bile acid sodium symporter BASS6 can transport glycolate and is involved in photorespiratory metabolism in Arabidopsis thaliana. The Plant Cell 29, 808–823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stitt M. 1986. Limitation of photosynthesis by carbon metabolism: I. Evidence for excess electron transport capacity in leaves carrying out photosynthesis in saturating light and CO2. Plant physiology 81, 1115–1122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stitt M, Grosse H. 1988. Interactions between sucrose synthesis and CO2 fixation IV. Temperature-dependent adjustment of the relation between sucrose synthesis and CO2 fixation. Journal of Plant Physiology 133, 392–400. [Google Scholar]
- Stitt M, Heldt HW. 1981. Simultaneous synthesis and degradation of starch in spinach chloroplasts in the light. Biochimica et Biophysica Acta 638, 1–11. [Google Scholar]
- Stitt M, Kürzel B, Heldt HW. 1984. Control of photosynthetic sucrose synthesis by fructose 2,6-bisphosphate: II. Partitioning between sucrose and starch. Plant Physiology 75, 554–560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stitt M, Wirtz W, Heldt HW. 1980. Metabolite levels during induction in the chloroplast and extrachloroplast compartments of spinach protoplasts. Biochimica et Biophysica Acta 593, 85–102. [DOI] [PubMed] [Google Scholar]
- Stitt M, Wirtz W, Heldt HW. 1983. Regulation of sucrose synthesis by cytoplasmic fructosebisphosphatase and sucrose phosphate synthase during photosynthesis in varying light and carbon dioxide. Plant Physiology 72, 767–774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strand Å, Hurry V, Henkes S, Huner N, Gustafsson P, Gardeström P, Stitt M. 1999. Acclimation of Arabidopsis leaves developing at low temperatures. Increasing cytoplasmic volume accompanies increased activities of enzymes in the Calvin cycle and in the sucrose-biosynthesis pathway. Plant Physiology 119, 1387–1398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sulpice R, Flis A, Ivakov AA, Apelt F, Krohn N, Encke B, Abel C, Feil R, Lunn JE, Stitt M. 2014. Arabidopsis coordinates the diurnal regulation of carbon allocation and growth across a wide range of photoperiods. Molecular Plant 7, 137–155. [DOI] [PubMed] [Google Scholar]
- Szecowka M, Heise R, Tohge T, et al. 2013. Metabolic fluxes in an illuminated Arabidopsis rosette. The Plant Cell 25, 694–714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takizawa K, Cruz JA, Kanazawa A, Kramer DM. 2007. The thylakoid proton motive force in vivo. Quantitative, non-invasive probes, energetics, and regulatory consequences of light-induced pmf. Biochimica et Biophysica Acta 1767, 1233–1244. [DOI] [PubMed] [Google Scholar]
- Takizawa K, Kanazawa A, Kramer DM. 2008. Depletion of stromal Pi induces high ‘energy-dependent’ antenna exciton quenching (qE) by decreasing proton conductivity at CFO-CF1 ATP synthase. Plant, Cell & Environment 31, 235–243. [DOI] [PubMed] [Google Scholar]
- Vassey TL, Sharkey TD. 1989. Mild water stress of Phaseolus vulgaris plants leads to reduced starch synthesis and extractable sucrose phosphate synthase activity. Plant Physiology 89, 1066–1070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Viil J, Ivanova H, Pärnik T, Pärsim E. 2004. Regulation of ribulose-1,5-bisphosphate carboxylase/oxygenase in vivo: control by high CO2 concentration. Photosynthetica 42, 283–290. [Google Scholar]
- Viil J, Laisk A, Oja V, Pärnik T. 1977. Enhancement of photosynthesis caused by oxygen under saturating irradiance and high CO2 concentrations. Photosynthetica 11, 251–259. [Google Scholar]
- von Caemmerer S. 2000. Biochemical models of leaf photosynthesis. Collingwood, Victoria, Australia: CSIRO Publishing. [Google Scholar]
- von Caemmerer S, Farquhar GD. 1981. Some relationships between the biochemistry of photosynthesis and the gas exchange of leaves. Planta 153, 376–387. [DOI] [PubMed] [Google Scholar]
- von Caemmerer S, Farquhar GD. 1984. Effects of partial defoliation, changes of irradiance during growth, short-term water stress and growth at enhanced p(CO2) on the photosynthetic capacity of leaves of Phaseolus vulgaris L. Planta 160, 320–329. [DOI] [PubMed] [Google Scholar]
- Walker DA, Sivak MN, Prinsley RT, Cheesbrough JK. 1983. Simultaneous measurement of oscillations in oxygen evolution and chlorophyll a fluorescence in leaf pieces. Plant Physiology 73, 542–549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warburg VO. 1919. Über die Geschwindigkeit der photocliemischen Kohlen- saürezersetzung in lebenden Zellen. Biochemische Zeitschrift 100, 230–270. [Google Scholar]
- Woodrow IE, Raymond Ellis J, Jellings A, Foyer CH. 1984. Compartmentation and fluxes of inorganic phosphate in photosynthetic cells. Planta 161, 525–530. [DOI] [PubMed] [Google Scholar]
- Wullschleger SD. 1993. Biochemical limitations to carbon assimilation in C3 plants—a retrospective analysis of the A/Ci curves from 109 species. Journal of Experimental Botany 44, 907–920. [Google Scholar]
- Yang JT, Preiser AL, Li Z, Weise SE, Sharkey TD. 2016. Triose phosphate use limitation of photosynthesis: short-term and long-term effects. Planta 243, 687–698. [DOI] [PubMed] [Google Scholar]
- Yin X, Struik PC, Romero P, Harbinson J, Evers JB, Van Der Putten PEL, Vos J. 2009. Using combined measurements of gas exchange and chlorophyll fluorescence to estimate parameters of a biochemical C photosynthesis model: a critical appraisal and a new integrated approach applied to leaves in a wheat (Triticum aestivum) canopy. Plant, Cell & Environment 32, 448–464. [DOI] [PubMed] [Google Scholar]
- Zell MB, Fahnenstich H, Maier A, et al. 2010. Analysis of Arabidopsis with highly reduced levels of malate and fumarate sheds light on the role of these organic acids as storage carbon molecules. Plant Physiology 152, 1251–1262. [DOI] [PMC free article] [PubMed] [Google Scholar]