Abstract
AbstractSphingosine 1-phosphate (S1P), a sphingolipid mediator, regulates various cellular functions via high-affinity G protein-coupled receptors, S1P1-5. The S1P-S1P receptor signaling system plays important roles in lymphocyte trafficking and maintenance of vascular integrity, thus contributing to the regulation of complex inflammatory processes. S1P is enriched in blood and lymph while maintained low in intracellular or interstitial fluids, creating a steep S1P gradient that is utilized to facilitate efficient egress of lymphocytes from lymphoid organs. Blockage of the S1P-S1P receptor signaling system results in a marked decrease in circulating lymphocytes because of a failure of lymphocyte egress from lymphoid organs. This provides a basis of immunomodulatory drugs targeting S1P1 receptor such as FTY720, an immunosuppressive drug approved in 2010 as the first oral treatment for relapsing–remitting multiple sclerosis. The S1P-S1P receptor signaling system also plays important roles in maintenance of vascular integrity since it suppresses sprouting angiogenesis and regulates vascular permeability. Dysfunction of the S1P-S1P receptor signaling system results in various vascular defects, such as exaggerated angiogenesis in developing retina and augmented inflammation due to increased permeability. Endothelial-specific deletion of S1P1 receptor in mice fed high-fat diet leads to increased formation of atherosclerotic lesions. This review highlights the importance of the S1P-S1P receptor signaling system in inflammatory processes. We also describe our recent findings regarding a specific S1P chaperone, apolipoprotein M, that anchors to high-density lipoprotein and contributes to shaping the endothelial-protective and anti-inflammatory properties of high-density lipoprotein.
Keywords: G-protein-coupled receptor, lipid mediator, lysophospholipid, sphingolipid
Introduction
Sphingosine 1-phosphate (S1P) is a pleiotropic lipid mediator involved in various cellular functions such as proliferation, migration, rearrangement of cytoskeleton, adhesion and inflammation in many types of cells, most notably in the immune and vascular systems. Regulation of immune cell trafficking and vascular integrity are two important functions of the S1P-S1P receptor signaling system that are highly relevant to the regulation of inflammatory processes.
S1P activity is regulated in multiple steps. We begin this review by overviewing the regulatory mechanisms of the S1P-S1P receptor signaling system: S1P metabolism, S1P transport and S1P receptors. We then detail the functions of the S1P-S1P receptor signaling system in the regulation of lymphocyte trafficking and vascular integrity. We also describe our recent findings regarding a specific S1P chaperone, apolipoprotein M (ApoM), its role in vascular inflammation and therapeutic approaches.
Overview of the S1P-S1P receptor signaling system
S1P is a lipid mediator that belongs to a class of lipids called sphingolipids. Almost all cells metabolize sphingolipids, which are building blocks of cellular membranes as well as cellular signaling mediators (1). S1P activity as an intercellular signaling mediator is regulated by multiple steps: S1P metabolism, S1P transport and S1P receptors (Fig. 1). In this section, we briefly summarize the regulatory mechanisms of the S1P-S1P receptor signaling system.
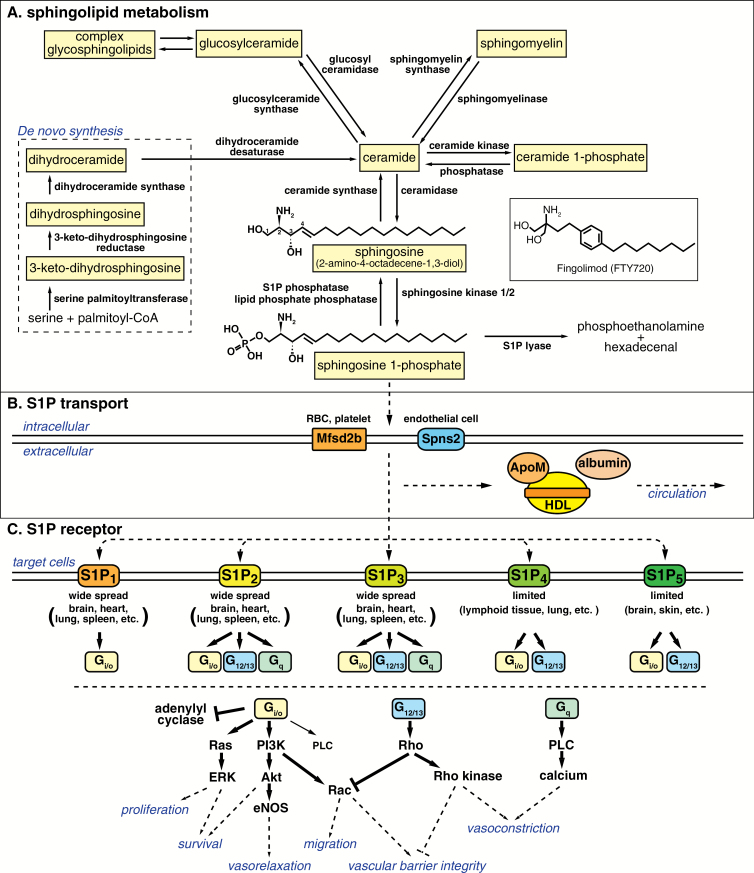
Fig. 1.
Overview of the S1P-S1P receptor signaling system. (A) De novo sphingolipid metabolism starts at the endoplasmic reticulum where serine palmitoyltransferase condensates serine and palmitoyl-CoA to produce ceramide. Ceramide, generated either from the de novo pathway or from degradation of complex sphingolipids such as sphingomyelin and glycosphingolipids, is deacylated by ceramidase to sphingosine, followed by phosphorylation by sphingosine kinase 1/2 to produce S1P. S1P is dephosphorylated to sphingosine by S1P phosphatase or by lipid phosphate phosphatases, or is irreversibly degraded to phosphoethanolamine and hexadecenal by S1P lyase. (B) S1P produced intracellularly is transported by specific transporters such as Mfsd2b [red blood cell (RBC) and platelet] and Spns2 (endothelial cells) and carried by albumin and HDL-associated specific S1P chaperone protein, ApoM. (C) S1P activates S1P receptors, S1P1-5, which transmit diverse intracellular signals depending on the coupled Gα subunits of heterotrimeric G protein and the expression pattern of each receptors in a given cell type. Modified from Obinata and Hla (75) with permission.
S1P metabolism
De novo synthesis of sphingolipids starts with condensation of serine and palmitoyl-CoA to produce 3-keto-dihydrosphingosine by the action of serine palmitoyltransferase, followed by several enzymatic reactions to produce ceramide (Fig. 1A) (1, 2). Ceramide is further metabolized to generate complex sphingolipids such as sphingomyelin and glycosphingolipids, major phospholipids in cellular membranes. On the other hand, ceramide can be hydrolyzed by ceramidase to sphingosine, from which S1P is produced by the action of sphingosine kinase 1 and 2, rate-limiting enzymes for S1P production. Usually, intracellular concentrations of S1P are maintained low by the action of either S1P phosphatase or S1P lyase. S1P lyase irreversibly degrades S1P to phosphoethanolamine and hexadecenal, which serves as the final degradation step of sphingolipid species (3).
Among all sphingolipids, S1P is the most well-characterized intercellular signaling molecule. Structurally, S1P is classified as a lysophospholipid, with a polar head group and a hydrophobic acyl tail. This amphipathic character allows lysophospholipids to leave the plasma membrane and function as a diffusible intercellular mediator. While S1P is maintained low in intracellular or interstitial fluids, it is enriched in blood and lymph in the sub-micromolar range, creating a steep S1P gradient (4). This vascular S1P gradient is utilized to regulate trafficking of immune cells such as lymphocytes and hematopoietic progenitor cells as discussed later in this review.
S1P transport
Major sources of circulating S1P are erythrocytes and endothelial cells (Fig. 1B). Erythrocytes lack the S1P degrading enzymes (5) and express the specific S1P transporter Mfsd2b (6). Mfsd2b deficiency results in a 50% decrease of the plasma S1P. Endothelial cells express another specific S1P transporter Spns2 and contribute to maintenance of the circulating S1P concentration as well (7, 8). Spns2 deficiency results in a 40 and 80% decrease of the plasma and lymph S1P concentrations, respectively, suggesting that lymph S1P is mainly maintained by lymphatic endothelial cells (9, 10). S1P secretion from endothelial cells is stimulated by fluid shear stress (7). Platelets also lack the S1P degrading enzymes and express the S1P transporter Mfsd2b (6), but the contribution of platelets to the circulating S1P concentration is negligible since mice that lack platelets did not show changes in the plasma S1P concentration (7, 11). Platelets probably contribute to local exaggerated synthesis of S1P during thrombotic episodes (12). A family of lipid phosphate phosphatases, which are localized on the surface of endothelial cells and have broad substrate specificity to phosphate-containing lipid species, contributes to the degradation of extracellular S1P (13).
Because of its hydrophobic nature, S1P is poorly water soluble and requires carrier proteins for efficient transport and circulation. In plasma, around 65% of S1P is carried by high-density lipoprotein (HDL) and the remainder by albumin (14). We have identified HDL-bound ApoM as a specific S1P chaperone (15), which is defined as a S1P carrier protein that facilitate S1P receptor activation to evoke specific biological responses. ApoM is contained only in 5% of plasma HDL particles, but all of the HDL-bound S1P is found in ApoM-containing HDL particles (15). In ApoM-deficient mice, the plasma S1P concentration decreases by 60%, and the remaining S1P is found in the albumin fraction (15). On the other hand, albumin-deficient mice show only a 15% decrease in the plasma S1P concentrations (H. Obinata, unpublished observation), suggesting the importance of ApoM as a physiologically relevant S1P chaperone.
S1P receptors
S1P exerts various physiological effects mostly via specific, high-affinity G protein-coupled receptors, while some reports have also indicated potential intracellular S1P roles (16, 17). So far, five subtypes of S1P receptors have been identified and are named S1P1-5 (18). As illustrated in Fig. 1(C), S1P1-3 are widely distributed with highest expression levels in the cardiovascular and immune systems. S1P4 and S1P5 show limited expression in lymphatic and nervous systems, respectively, and the expression levels of S1P4 and S1P5 are relatively low compared to S1P1-3. S1P1 exclusively couples with the Gi/o alpha subunit of heterotrimeric G proteins, whereas S1P2 and S1P3 couple with Gi/o, Gq and G12/13, and S1P4 and S1P5 with Gi/o and G12/13. These differential but overlapping expression patterns and intracellular signaling pathways of each receptor probably form the molecular basis for the diverse S1P functions.
S1P and lymphocyte trafficking
S1P-S1P1 signaling plays a pivotal role in the regulation of lymphocyte egress from thymus and secondary lymphoid organs (19). S1P receptors are the targets of Fingolimod (FTY720), which is an immunomodulatory drug and was approved by the US Food and Drug Administration in 2010 as the first oral treatment for relapsing–remitting multiple sclerosis (20, 21). In this section, we summarize the functions of the S1P-S1P receptor signaling system in lymphocyte trafficking and the mechanisms of action of Fingolimod.
S1P and lymphocyte trafficking
Mutant mice that specifically lack S1P1 in hematopoietic cells show no clear defect in lymphocyte maturation, but show profound lymphopenia (lack of circulating lymphocytes) due to trapping in thymus and peripheral lymphoid organs (22). When S1P1-deficient mature lymphocytes are adoptively transferred intravenously into wild-type mice, they enter but cannot exit from secondary lymphoid organs (22). These observations clearly show that S1P1 is essential for the lymphocyte egress from thymus and peripheral lymphoid organs but is otherwise not needed for entry into secondary lymphoid organs.
As mentioned above, S1P concentrations are low in intracellular and interstitial fluids, whereas S1P is enriched within blood and lymph in the sub-micromolar range, creating a steep S1P gradient. Lymphocytes utilize this S1P gradient between lymphoid organs and the circulation as a cue for the egress process. Disruption of the S1P gradient either by inhibition of sphingosine kinase 1/2 (responsible for S1P production) (11) or by inhibition of S1P lyase (responsible for S1P degradation) (23) results in lymphopenia due to the defect in the lymphocyte egress.
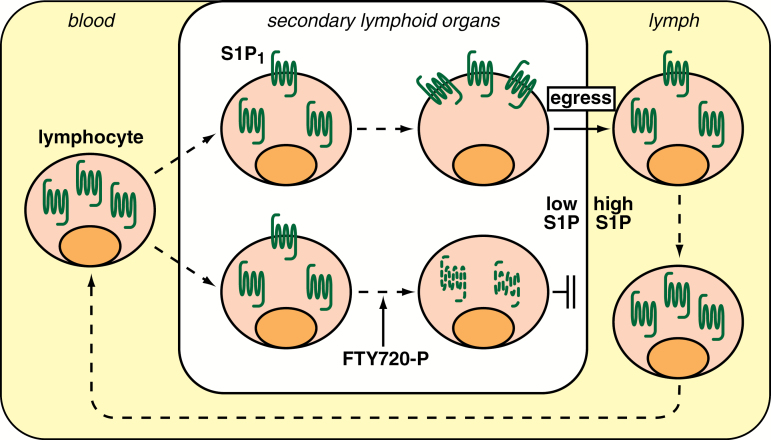
S1P1 on thymocytes gets internalized almost completely after incubation with as little as 1 nM S1P for 20 min ex vivo (23), indicating that S1P1 on lymphocytes is very sensitive to S1P exposure. The internalization/desensitization of S1P1 is important for circulating lymphocytes to enter lymphoid organs against the S1P gradient. Lymphocytes that lack GRK2 (a critical regulator of internalization step) display a reduced ability to enter lymphoid organs, which can be restored by S1P1 deficiency. Although S1P1 is undetectable on the surfaces of circulating T cells, the receptor is readily detectable on the surfaces of T cells in lymphoid organs (23). The current model for the regulation of lymphocyte egress by S1P-S1P1 signaling is illustrated in Fig. 2. Lymphocytes in circulation have their S1P1 mostly internalized due to the high S1P concentration. When lymphocytes enter lymphoid organs where the S1P concentration is low, they gradually recover the surface expression of S1P1 and regain the ability to migrate out from lymphoid organs toward the high S1P in blood or lymph. The same S1P gradient-dependent strategy is also utilized for splenic B-cell shuttling (24) and regulation of trafficking of the other immune cells, including dendritic cells (25), natural killer cells (26, 27) and hematopoietic stem cells (28).
Fig. 2.
S1P and lymphocyte trafficking. Lymphocytes in circulation have their S1P1 mostly internalized due to the high S1P concentration. Upon entrance in secondary lymphoid organs where the S1P concentration is low, they gradually recover the surface expression of S1P1 and regain ability to egress from the lymphoid organs toward S1P in circulation. FTY720 is phosphorylated by sphingosine kinase 2 and induces the internalization and subsequent degradation of S1P1 and thereby inhibits the lymphocyte egress. Reproduced from Obinata and Hla (75) with permission.
S1P and Fingolimod
Fingolimod (FTY720) is an immunomodulatory drug targeting S1P receptors and approved as an oral treatment for relapsing–remitting multiple sclerosis (Gilenya®) (20, 21). FTY720 was synthesized as a potent immunosuppressor with low cytotoxicity using myriocin as a lead compound (29). Myriocin is one of the active ingredients of Isaria sinclairii, a fungus with medicinal properties used in Eastern traditional medicine (30). Different from immunomodulatory drugs targeting calcineurin, FTY720 does not affect proliferation and activation of lymphocytes, but it induces a marked decrease of circulating lymphocytes (31, 32). Structural similarity between FTY720 and sphingosine (Fig. 1A) led to the elucidation that S1P signaling plays a critical role in lymphocyte trafficking as mentioned above, and that the phosphorylated form of FTY720 modulates S1P signaling.
When administered in vivo, FTY720 gets phosphorylated to FTY720-P by sphingosine kinase 2 (33–35), and FTY720-P acts as an acute potent agonist on all of the S1P receptors except S1P2 in the nanomolar range (36, 37). Agonist stimulation induces the desensitization of S1P1 through phosphorylation of its C-terminal tail, followed by internalization via clathrin-coated endocytosis (38, 39). In the case of S1P stimulation, most of the internalized S1P1 receptors recycle back to the plasma membrane. In contrast, FTY720-P induces sustained internalization followed by WWP2 (ubiquitin E3 ligase)-dependent polyubiquitinylation and consequent degradation of S1P1, leading to the massive decrease of S1P1 expression level (39). As a result of this functional antagonism of S1P1, FTY720 inhibits the lymphocyte egress from lymphoid organs, which is a basis of FTY720 as a potent immunosuppressor (Fig. 2). An S1P1 mutant that lacks C-terminal phosphorylation sites shows delayed internalization and defective desensitization after agonist stimulation, and the mice with this S1P1 mutant exhibit significant resistance against FTY720-induced lymphopenia (40), supporting the idea that FTY720 exerts its effects through modulating the cell surface residence and activity of S1P1.
While FTY720 is an effective oral treatment for relapsing–remitting multiple sclerosis, its use is associated with several serious adverse effects such as bradycardia and macular edema. Bradycardia is associated with the agonistic effects of FTY720-P on S1P1-dependent activation of the G protein-coupled inwardly rectifying potassium (GIRK) channel in cardiomyocytes in humans, while S1P3 is involved in the GIRK channel activation in rodents (41). Macular edema is likely due to impaired vascular barrier function by functional antagonism of endothelial S1P1 (42). Development of competitive S1P1 antagonists that specifically target hematopoietic S1P1 but not endothelial S1P1 would be beneficial to avoid these adverse effects of FTY720.
S1P and vascular integrity
Another important function of the S1P-S1P receptor signaling system is the regulation of vascular integrity. In this section, we describe S1P function in vascular development as a potent suppressor of sprouting angiogenesis. We also describe the role of S1P in vascular inflammation as a regulator of vascular permeability including our recent findings.
S1P in angiogenesis
Genetic deletion of S1P1 results in embryonic lethality between E12.5 and E14.5 as a result of excessive vascular leak and hemorrhage (43). Endothelial-specific deletion of S1P1 shows the same phenotype as the global deletion (44). Single deletion of either S1P2 or S1P3 does not lead to the embryonic lethality, but simultaneous deletion of S1P2 and S1P3 results in about 50% embryonic death around E13.5 due to the hemorrhage (45–48). Triple null mice lacking all of the S1P1-3 show the severest bleeding, leading to embryonic death between E10.5 and E11.5 (48), which is earlier than S1P1 single deletion. These results indicate that S1P1 plays a pivotal role in vascular maturation, with S1P2 and S1P3 redundant and/or supportive roles. Double knockout of sphingosine kinase 1/2, the rate-limiting enzymes for S1P production, also leads to the embryonic lethality with the same phenotypes as S1P1-deleted mice (49), demonstrating the importance of the S1P-S1P receptor signaling system in vascular development.
In S1P1-deleted mice, no clear defect is observed in vasculogenesis or early angiogenesis. However, vascular maturation is incomplete due to defective coverage by vascular smooth muscle cells/pericytes in the dorsal aorta (43). This is partly explained by the report showing that S1P1 regulates N-cadherin-mediated cell adhesion between endothelial and mural cells (50). Close observation of the dorsal aorta revealed a massive endothelial hyperplasia with abnormal microvasculature around the aorta (51), which is considered as another cause hampering proper coverage by pericytes.
We engineered inducible and endothelial-specific S1P1-deficient/-overexpressing mice, and observed that the density of the vascular networks changed according to the S1P1 expression levels in the postnatal angiogenesis in retina; namely, S1P1-deficient mice showed a denser vascular network while S1P1-overexpressing mice had a sparser network (52). In S1P1-deficient mice, endothelial cells in the leading front of the vascularization showed hyper-sprouting phenotypes with an increased number of filopodia-containing tip cells, branch points and dilated vessels, which results in the formation of the denser network. In sprouting angiogenesis, angiogenic growth factors like vascular endothelial growth factor are produced in the region of hypoxia, and act on the blood vessels to loosen adherens junctions and induce the sprouting of endothelial cells (53). On the other hand, S1P1 strengthens the adherens junctions between endothelial cells (51, 52). Probably, S1P1 signaling acts as a vascular-intrinsic stabilization mechanism, protecting developing blood vessels against aberrant angiogenic responses (Fig. 3).
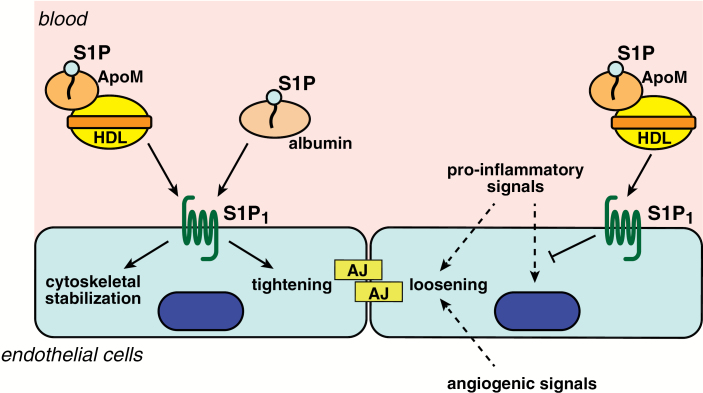
Fig. 3.
S1P and vascular integrity. S1P, either carried by albumin or HDL-ApoM, acts on S1P1 and induces cytoskeletal stabilization and tightening of adherens junctions (AJs) in endothelial cells, while angiogenic signals such as vascular endothelial growth factor from hypoxic tissues or pro-inflammatory signals such as histamine and leukotrienes induce loosening of AJs. S1P carried by HDL-ApoM also suppresses pro-inflammatory endothelial responses induced by cytokines. These anti-angiogenic and anti-inflammatory actions by the S1P-S1P receptor signaling system contribute to the maintenance of vascular integrity.
S1P in vascular inflammation
Regulation of endothelial adherens junctions by S1P signaling also plays critical roles in the maintenance of the vascular barrier integrity. A dysfunctional endothelial barrier leads to increased vascular permeability, which is one of the central features of inflammation, tumor metastasis and atherosclerosis. Pro-inflammatory mediators such as histamine and leukotrienes induce loosening of adherens junctions between endothelial cells to allow exudation of plasma proteins such as antibodies and complement, and migration of leukocytes and lymphocytes to the site of the inflammation. On the other hand, S1P signaling strengthens the adherens junctions to limit exaggerated inflammation (Fig. 3). Mutant mice engineered to selectively lack plasma S1P by an inducible sphingosine kinase 1/2 double-deletion system display increased vascular leak and impaired survival after anaphylactic challenges by platelet-activating factor or histamine (54). Similarly, pharmacological antagonism of S1P1 or ApoM deficiency results in increased vascular permeability and augmented inflammation (15, 55). Brain endothelial S1P1 signaling also maintains the blood–brain barrier by regulating the proper localization of tight junction proteins (56).
Important events to maintain vascular integrity are rearrangements of cytoskeleton and assembly of adherens junctions in endothelial cells (Fig. 3). S1P induces reorganization of the actin cytoskeleton and accumulation of VE-cadherin and α-, β- and γ-catenin to the sites of cell–cell contact in conjunction with adherens junction assembly (57). Activation of small G proteins Rac and Rho downstream of the S1P1 and S1P3 signaling pathways are involved in the processes (Fig. 1C) (57, 58).
Although the precise molecular mechanisms remain to be clarified, S1P1 signaling is involved also in the regulation of the inflammatory status of vascular endothelial cells. When S1P1 deletion was induced in an endothelial-specific manner, up-regulations of the pro-inflammatory endothelial markers such as VCAM-1 and ICAM-1 were observed in the descending aorta (59), where expression of these pro-inflammatory molecules are usually suppressed compared to the inflammation-susceptible areas under the disturbed blood flow such as larger curvature and bifurcation points of the aorta. S1P1 shows disturbed intracellular localization in the inflammation-susceptible areas, while S1P1 are accumulated in the adherens junctions in the endothelial cells under the laminar flow (52, 59). These observations indicate that proper S1P1 localization and signaling are important to maintain vascular homeostasis, and that the impairment of S1P1 signaling because of receptor internalization predisposes endothelial cells to an inflammatory phenotype. In accordance with this notion, endothelial S1P1-deficient mice that were fed a high-fat diet show increased formation of atherosclerotic lesions in the descending aorta (59).
Chaperone-dependent S1P functions
As mentioned in the first section, ~65% of the S1P in circulation is carried by HDL-anchored ApoM and the remainder by albumin (14). Identification of ApoM as a specific S1P chaperone revealed the importance of ApoM-bound S1P in mediating anti-inflammatory effects of HDL on endothelial cells. In this section, we describe functional differences between ApoM- and albumin-bound S1P, the contribution of ApoM-S1P to the HDL functions and a novel therapeutic strategy utilizing ApoM-S1P.
Functional differences between ApoM- and albumin-bound S1P
In vitro studies showed that both ApoM- and albumin-bound S1P can activate S1P1-3 to evoke intracellular signaling events such as ERK1/2 and Akt phosphorylation (Fig. 1C) (15, 59, 60). However, suppression of cAMP production downstream of the Gαi-related pathway is only observed by albumin-bound S1P in S1P1-expressing cells (Fig. 1C), and S1P1-internalization upon S1P stimulation is induced more efficiently by albumin-S1P than by ApoM-S1P (59). In endothelial culture systems, ApoM-S1P increases trans-endothelial electric resistance (an indicator of the barrier function) in a sustained manner over a few hours, while albumin-S1P induces a transient increase that goes back to the base-line level after around 30 min (60, 61). In addition, only ApoM-S1P suppresses cytokine-induced inflammatory responses in endothelial cells (Fig. 3) (59). These results in endothelial cells suggest that ApoM is a more physiologically relevant carrier protein for S1P than albumin in terms of the maintenance of vascular integrity. In lymphocytes, ApoM-S1P regulates lymphopoiesis in bone marrow (62).
The molecular basis for the functional differences between ApoM- and albumin-bound S1P remains to be elucidated. One possibility is that ApoM-S1P acts on the receptors in a sustained release manner, because ApoM has higher affinity to S1P than albumin and protects S1P from degradation by lipid phosphate phosphatases (7, 63, 64). Another possibility is that ApoM or some other molecules on HDL interact with S1P receptors or other receptor-associated molecules to form a signaling complex that allows context-dependent signaling via S1P receptors. While albumin serves as a promiscuous binding protein for various hydrophobic molecules, ApoM probably plays pivotal roles as a specific S1P chaperone in the S1P transport and the receptor activation to evoke specific biological responses.
Anti-inflammatory functions of ApoM-S1P contained in HDL
Multiple epidemiological studies have established the inverse-correlation between HDL-cholesterol levels and cardiovascular disease. This is mainly attributed to the ‘reverse-cholesterol transport’ by HDL that extracts excessive cholesterol from peripheral tissues and carries it back to the liver. In addition, HDL has various athero-protective functions such as anti-oxidative, anti-thrombotic and anti-inflammatory properties (65). ApoM-S1P on HDL serves as one of the main components that exert the anti-inflammatory effects. Cytokine-induced inflammatory responses in endothelial cells were suppressed by ApoM-S1P-containing HDL (Fig. 3) but not by ApoM-deficient HDL prepared from ApoM knockout mice (59). HDL particles prepared from patients with coronary artery disease show a reduced S1P content and some functional defects such as impaired NO-dependent vasodilation and suppression of cytokine-induced inflammation of vascular smooth muscle cells (66–69). A diminished HDL-S1P content is also observed in acute myocardial infarction (66), type II diabetes (70, 71) and chronic kidney disease (72). It remains to be elucidated how the decrease in HDL-S1P content is induced in these pathological conditions.
Novel therapeutic strategy utilizing ApoM-S1P
So far, clinical trials that raised the HDL-cholesterol levels have not been successful in reducing the risk of cardiovascular diseases. HDL function, rather than the HDL-cholesterol level, is likely more important for the beneficial properties of HDL (73). Since ApoM-S1P has been proven to be one of the major components of HDL that carry anti-inflammatory effects (59), therapeutic intervention that aims to increase the ApoM-S1P concentration could be promising. Along the lines of this concept, we engineered ApoM-Fc, in which the C-terminal end of ApoM is fused with the Fc region of IgG to avoid rapid clearance of free ApoM by kidney (74). S1P bound to ApoM-Fc maintained the ability to activate S1P receptors and promoted endothelial function in a sustained manner in vitro (60). When injected intraperitoneally in mice, ApoM-Fc showed a plasma half-life of about 90 h and 100 µg ApoM-Fc administration increased the plasma S1P concentration by 30% after 24 h of the injection (60). On the other hand, ApoM-Fc-TM (triple mutant ApoM that lacks the ability of S1P binding) showed a comparable plasma half-life but did not increase the plasma S1P concentration. ApoM-Fc administration reduced blood pressure in hypertensive mice, attenuated myocardial damage after ischemia/reperfusion injury and reduced brain infarct volume in the experimental model of stroke, which were not observed by ApoM-Fc-TM administration (60). Reduction of the blood pressure and attenuation of the ischemic injury are probably due to the increased NO production and the improved vascular barrier function, respectively, via the strengthened S1P1 signaling by ApoM-Fc in endothelial cells. In contrast, ApoM-Fc did not modulate circulating lymphocyte numbers, suggesting that it specifically activated endothelial S1P receptors. This study suggests that selective and sustained targeting of endothelial S1P receptors by ApoM-Fc could be a beneficial therapeutic strategy in vascular diseases.
Conclusions
In this short review, we summarized the importance of the S1P-S1P receptor signaling system in the regulation of inflammatory processes. The steep S1P gradient between lymphoid organs and circulation, and fine tuning of the intracellular S1P1 localization enable efficient egress of lymphocytes from lymphoid organs. Blockage of the S1P-S1P receptor signaling system results in lymphopenia due to accumulation of lymphocytes in lymphoid organs, which provides a basis for immunomodulatory drugs targeting S1P1. The S1P-S1P receptor signaling system also plays important roles in the maintenance of vascular integrity as a potent suppressor of sprouting angiogenesis as well as a regulator of vascular permeability and inflammation. Especially, ApoM-bound S1P on HDL contributes to shaping the anti-inflammatory properties of HDL. While blockage of the S1P-S1P receptor signaling system in lymphocytes is beneficial for the suppression of inflammatory responses, it could have adverse effects in terms of the maintenance of vascular integrity. Our recent study (60) indicated that therapeutic intervention that aims to increase the ApoM-S1P concentration could be beneficial for treating vascular diseases without affecting lymphocyte trafficking. Further studies in preclinical models as well as clinical trials will warrant the development of immunomodulatory drugs based on the anti-inflammatory property of ApoM-bound S1P.
Funding
H.O. is supported by the Japan Society for the Promotion of Science (JSPS) KAKENHI Grant Number JP16K98576 and a grant from ONO Medical Research Foundation. T.H. is supported by National Institutes of Health (NIH) grant R35-HL135821 and a Fondation Leducq Trans-Atlantic networkgrant (SphingoNet).
Conflicts of interest statement: H.O. declares no conflicts of interest. T.H. acknowledges grant support from ONO Pharmaceuticals, has filed patents/patent applications of ApoM, ApoM-Fc and HDL containing ApoM, and has consulted for Astellas, Steptoe and Johnson, Gerson Lehrman Group Council, Janssen Research & Development, LLC.
References
- 1. Gault C. R., Obeid L. M. and Hannun Y. A. 2010. An overview of sphingolipid metabolism: from synthesis to breakdown. Adv. Exp. Med. Biol. 688:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Yanagida K. and Hla T. 2017. Vascular and immunobiology of the circulatory sphingosine 1-phosphate gradient. Annu. Rev. Physiol. 79:67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Nakahara K., Ohkuni A., Kitamura T. et al. 2012. The Sjögren-Larsson syndrome gene encodes a hexadecenal dehydrogenase of the sphingosine 1-phosphate degradation pathway. Mol. Cell 46:461. [DOI] [PubMed] [Google Scholar]
- 4. Hla T., Venkataraman K. and Michaud J. 2008. The vascular S1P gradient-cellular sources and biological significance. Biochim. Biophys. Acta 1781:477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Ito K., Anada Y., Tani M. et al. 2007. Lack of sphingosine 1-phosphate-degrading enzymes in erythrocytes. Biochem. Biophys. Res. Commun. 357:212. [DOI] [PubMed] [Google Scholar]
- 6. Vu T. M., Ishizu A. N., Foo J. C. et al. 2017. Mfsd2b is essential for the sphingosine-1-phosphate export in erythrocytes and platelets. Nature 550:524. [DOI] [PubMed] [Google Scholar]
- 7. Venkataraman K., Lee Y. M., Michaud J. et al. 2008. Vascular endothelium as a contributor of plasma sphingosine 1-phosphate. Circ. Res. 102:669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Kawahara A., Nishi T., Hisano Y., Fukui H., Yamaguchi A. and Mochizuki N. 2009. The sphingolipid transporter spns2 functions in migration of zebrafish myocardial precursors. Science 323:524. [DOI] [PubMed] [Google Scholar]
- 9. Hisano Y., Kobayashi N., Yamaguchi A. and Nishi T. 2012. Mouse SPNS2 functions as a sphingosine-1-phosphate transporter in vascular endothelial cells. PLoS ONE 7:e38941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Mendoza A., Bréart B., Ramos-Perez W. D. et al. 2012. The transporter Spns2 is required for secretion of lymph but not plasma sphingosine-1-phosphate. Cell Rep. 2:1104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Pappu R., Schwab S. R., Cornelissen I. et al. 2007. Promotion of lymphocyte egress into blood and lymph by distinct sources of sphingosine-1-phosphate. Science 316:295. [DOI] [PubMed] [Google Scholar]
- 12. Igarashi Y. and Yatomi Y. 1998. Sphingosine 1-phosphate is a blood constituent released from activated platelets, possibly playing a variety of physiological and pathophysiological roles. Acta Biochim. Pol. 45:299. [PubMed] [Google Scholar]
- 13. Pyne S., Long J. S., Ktistakis N. T. and Pyne N. J. 2005. Lipid phosphate phosphatases and lipid phosphate signalling. Biochem. Soc. Trans. 33(Pt 6):1370. [DOI] [PubMed] [Google Scholar]
- 14. Murata N., Sato K., Kon J. et al. 2000. Interaction of sphingosine 1-phosphate with plasma components, including lipoproteins, regulates the lipid receptor-mediated actions. Biochem. J. 352 Pt 3:809. [PMC free article] [PubMed] [Google Scholar]
- 15. Christoffersen C., Obinata H., Kumaraswamy S. B. et al. 2011. Endothelium-protective sphingosine-1-phosphate provided by HDL-associated apolipoprotein M. Proc. Natl Acad. Sci. USA 108:9613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Hait N. C., Allegood J., Maceyka M. et al. 2009. Regulation of histone acetylation in the nucleus by sphingosine-1-phosphate. Science 325:1254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Alvarez S. E., Harikumar K. B., Hait N. C. et al. 2010. Sphingosine-1-phosphate is a missing cofactor for the E3 ubiquitin ligase TRAF2. Nature 465:1084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Chun J., Hla T., Lynch K. R., Spiegel S. and Moolenaar W. H. 2010. International Union of Basic and Clinical Pharmacology. LXXVIII. Lysophospholipid receptor nomenclature. Pharmacol. Rev. 62:579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Schwab S. R. and Cyster J. G. 2007. Finding a way out: lymphocyte egress from lymphoid organs. Nat. Immunol. 8:1295. [DOI] [PubMed] [Google Scholar]
- 20. Cohen J. A., Barkhof F., Comi G. et al. ; TRANSFORMS Study Group. 2010. Oral fingolimod or intramuscular interferon for relapsing multiple sclerosis. N. Engl. J. Med. 362:402. [DOI] [PubMed] [Google Scholar]
- 21. Kappos L., Radue E. W., O’Connor P. et al. ; FREEDOMS Study Group. 2010. A placebo-controlled trial of oral fingolimod in relapsing multiple sclerosis. N. Engl. J. Med. 362:387. [DOI] [PubMed] [Google Scholar]
- 22. Matloubian M., Lo C. G., Cinamon G. et al. 2004. Lymphocyte egress from thymus and peripheral lymphoid organs is dependent on S1P receptor 1. Nature 427:355. [DOI] [PubMed] [Google Scholar]
- 23. Schwab S. R., Pereira J. P., Matloubian M., Xu Y., Huang Y. and Cyster J. G. 2005. Lymphocyte sequestration through S1P lyase inhibition and disruption of S1P gradients. Science 309:1735. [DOI] [PubMed] [Google Scholar]
- 24. Green J. A., Suzuki K., Cho B. et al. 2011. The sphingosine 1-phosphate receptor S1P₂ maintains the homeostasis of germinal center B cells and promotes niche confinement. Nat. Immunol. 12:672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Czeloth N., Bernhardt G., Hofmann F., Genth H. and Förster R. 2005. Sphingosine-1-phosphate mediates migration of mature dendritic cells. J. Immunol. 175:2960. [DOI] [PubMed] [Google Scholar]
- 26. Walzer T., Chiossone L., Chaix J. et al. 2007. Natural killer cell trafficking in vivo requires a dedicated sphingosine 1-phosphate receptor. Nat. Immunol. 8:1337. [DOI] [PubMed] [Google Scholar]
- 27. Jenne C. N., Enders A., Rivera R. et al. 2009. T-bet-dependent S1P5 expression in NK cells promotes egress from lymph nodes and bone marrow. J. Exp. Med. 206:2469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Massberg S., Schaerli P., Knezevic-Maramica I. et al. 2007. Immunosurveillance by hematopoietic progenitor cells trafficking through blood, lymph, and peripheral tissues. Cell 131:994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Adachi K., Kohara T., Nakao N. et al. 1995. Design, synthesis, and structure-activity relationships of 2-substituted-2-amino-1,3-propanediols: discovery of a novel immunosuppressant, FTY720. Bioorg. Med. Chem. Lett. 5:853. [Google Scholar]
- 30. Fujita T., Inoue K., Yamamoto S. et al. 1994. Fungal metabolites. Part 11. A potent immunosuppressive activity found in Isaria sinclairii metabolite. J. Antibiot. (Tokyo) 47:208. [DOI] [PubMed] [Google Scholar]
- 31. Chiba K., Yanagawa Y., Masubuchi Y. et al. 1998. FTY720, a novel immunosuppressant, induces sequestration of circulating mature lymphocytes by acceleration of lymphocyte homing in rats. I. FTY720 selectively decreases the number of circulating mature lymphocytes by acceleration of lymphocyte homing. J. Immunol. 160:5037. [PubMed] [Google Scholar]
- 32. Yanagawa Y., Sugahara K., Kataoka H., Kawaguchi T., Masubuchi Y. and Chiba K. 1998. FTY720, a novel immunosuppressant, induces sequestration of circulating mature lymphocytes by acceleration of lymphocyte homing in rats. II. FTY720 prolongs skin allograft survival by decreasing T cell infiltration into grafts but not cytokine production in vivo. J. Immunol. 160:5493. [PubMed] [Google Scholar]
- 33. Sanchez T., Estrada-Hernandez T., Paik J. H. et al. 2003. Phosphorylation and action of the immunomodulator FTY720 inhibits vascular endothelial cell growth factor-induced vascular permeability. J. Biol. Chem. 278:47281. [DOI] [PubMed] [Google Scholar]
- 34. Kharel Y., Lee S., Snyder A. H. et al. 2005. Sphingosine kinase 2 is required for modulation of lymphocyte traffic by FTY720. J. Biol. Chem. 280:36865. [DOI] [PubMed] [Google Scholar]
- 35. Zemann B., Kinzel B., Müller M. et al. 2006. Sphingosine kinase type 2 is essential for lymphopenia induced by the immunomodulatory drug FTY720. Blood 107:1454. [DOI] [PubMed] [Google Scholar]
- 36. Brinkmann V., Davis M. D., Heise C. E. et al. 2002. The immune modulator FTY720 targets sphingosine 1-phosphate receptors. J. Biol. Chem. 277:21453. [DOI] [PubMed] [Google Scholar]
- 37. Mandala S., Hajdu R., Bergstrom J. et al. 2002. Alteration of lymphocyte trafficking by sphingosine-1-phosphate receptor agonists. Science 296:346. [DOI] [PubMed] [Google Scholar]
- 38. Liu C. H., Thangada S., Lee M. J., Van Brocklyn J. R., Spiegel S. and Hla T. 1999. Ligand-induced trafficking of the sphingosine-1-phosphate receptor EDG-1. Mol. Biol. Cell 10:1179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Oo M. L., Thangada S., Wu M. T. et al. 2007. Immunosuppressive and anti-angiogenic sphingosine 1-phosphate receptor-1 agonists induce ubiquitinylation and proteasomal degradation of the receptor. J. Biol. Chem. 282:9082. [DOI] [PubMed] [Google Scholar]
- 40. Thangada S., Khanna K. M., Blaho V. A. et al. 2010. Cell-surface residence of sphingosine 1-phosphate receptor 1 on lymphocytes determines lymphocyte egress kinetics. J. Exp. Med. 207:1475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Camm J., Hla T., Bakshi R. and Brinkmann V. 2014. Cardiac and vascular effects of fingolimod: mechanistic basis and clinical implications. Am. Heart J. 168:632. [DOI] [PubMed] [Google Scholar]
- 42. Jain N. and Bhatti M. T. 2012. Fingolimod-associated macular edema: incidence, detection, and management. Neurology 78:672. [DOI] [PubMed] [Google Scholar]
- 43. Liu Y., Wada R., Yamashita T. et al. 2000. Edg-1, the G protein-coupled receptor for sphingosine-1-phosphate, is essential for vascular maturation. J. Clin. Invest. 106:951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Allende M. L., Yamashita T. and Proia R. L. 2003. G-protein-coupled receptor S1P1 acts within endothelial cells to regulate vascular maturation. Blood 102:3665. [DOI] [PubMed] [Google Scholar]
- 45. Ishii I., Friedman B., Ye X. et al. 2001. Selective loss of sphingosine 1-phosphate signaling with no obvious phenotypic abnormality in mice lacking its G protein-coupled receptor, LP(B3)/EDG-3. J. Biol. Chem. 276:33697. [DOI] [PubMed] [Google Scholar]
- 46. Ishii I., Ye X., Friedman B. et al. 2002. Marked perinatal lethality and cellular signaling deficits in mice null for the two sphingosine 1-phosphate (S1P) receptors, S1P(2)/LP(B2)/EDG-5 and S1P(3)/LP(B3)/EDG-3. J. Biol. Chem. 277:25152. [DOI] [PubMed] [Google Scholar]
- 47. Kono M., Belyantseva I. A., Skoura A. et al. 2007. Deafness and stria vascularis defects in S1P2 receptor-null mice. J. Biol. Chem. 282:10690. [DOI] [PubMed] [Google Scholar]
- 48. Kono M., Mi Y., Liu Y. et al. 2004. The sphingosine-1-phosphate receptors S1P1, S1P2, and S1P3 function coordinately during embryonic angiogenesis. J. Biol. Chem. 279:29367. [DOI] [PubMed] [Google Scholar]
- 49. Mizugishi K., Yamashita T., Olivera A., Miller G. F., Spiegel S. and Proia R. L. 2005. Essential role for sphingosine kinases in neural and vascular development. Mol. Cell. Biol. 25:11113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Paik J. H., Skoura A., Chae S. S. et al. 2004. Sphingosine 1-phosphate receptor regulation of N-cadherin mediates vascular stabilization. Genes Dev. 18:2392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Gaengel K., Niaudet C., Hagikura K. et al. 2012. The sphingosine-1-phosphate receptor S1PR1 restricts sprouting angiogenesis by regulating the interplay between VE-cadherin and VEGFR2. Dev. Cell 23:587. [DOI] [PubMed] [Google Scholar]
- 52. Jung B., Obinata H., Galvani S. et al. 2012. Flow-regulated endothelial S1P receptor-1 signaling sustains vascular development. Dev. Cell 23:600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Eilken H. M. and Adams R. H. 2010. Dynamics of endothelial cell behavior in sprouting angiogenesis. Curr. Opin. Cell Biol. 22:617. [DOI] [PubMed] [Google Scholar]
- 54. Camerer E., Regard J. B., Cornelissen I. et al. 2009. Sphingosine-1-phosphate in the plasma compartment regulates basal and inflammation-induced vascular leak in mice. J. Clin. Invest. 119:1871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Christensen P. M., Liu C. H., Swendeman S. L. et al. 2016. Impaired endothelial barrier function in apolipoprotein M-deficient mice is dependent on sphingosine-1-phosphate receptor 1. FASEB J. 30:2351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Yanagida K., Liu C. H., Faraco G. et al. 2017. Size-selective opening of the blood–brain barrier by targeting endothelial sphingosine 1-phosphate receptor 1. Proc. Natl Acad. Sci. USA 114:4531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Lee M. J., Thangada S., Claffey K. P. et al. 1999. Vascular endothelial cell adherens junction assembly and morphogenesis induced by sphingosine-1-phosphate. Cell 99:301. [DOI] [PubMed] [Google Scholar]
- 58. Garcia J. G., Liu F., Verin A. D. et al. 2001. Sphingosine 1-phosphate promotes endothelial cell barrier integrity by Edg-dependent cytoskeletal rearrangement. J. Clin. Invest. 108:689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Galvani S., Sanson M., Blaho V. A. et al. 2015. HDL-bound sphingosine 1-phosphate acts as a biased agonist for the endothelial cell receptor S1P1 to limit vascular inflammation. Sci. Signal. 8:ra79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Swendeman S. L., Xiong Y., Cantalupo A. et al. 2017. An engineered S1P chaperone attenuates hypertension and ischemic injury. Sci. Signal. 10:eaal2722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Wilkerson B. A., Grass G. D., Wing S. B., Argraves W. S. and Argraves K. M. 2012. Sphingosine 1-phosphate (S1P) carrier-dependent regulation of endothelial barrier: high density lipoprotein (HDL)-S1P prolongs endothelial barrier enhancement as compared with albumin-S1P via effects on levels, trafficking, and signaling of S1P1. J. Biol. Chem. 287:44645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Blaho V. A., Galvani S., Engelbrecht E. et al. 2015. HDL-bound sphingosine-1-phosphate restrains lymphopoiesis and neuroinflammation. Nature 523:342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Kimura T., Sato K., Kuwabara A. et al. 2001. Sphingosine 1-phosphate may be a major component of plasma lipoproteins responsible for the cytoprotective actions in human umbilical vein endothelial cells. J. Biol. Chem. 276:31780. [DOI] [PubMed] [Google Scholar]
- 64. Zhang H., Pluhackova K., Jiang Z. and Böckmann R. A. 2016. Binding characteristics of sphingosine-1-phosphate to ApoM hints to assisted release mechanism via the ApoM calyx-opening. Sci. Rep. 6:30655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Brewer H. B., Jr 2011. Clinical review: the evolving role of HDL in the treatment of high-risk patients with cardiovascular disease. J. Clin. Endocrinol. Metab. 96:1246. [DOI] [PubMed] [Google Scholar]
- 66. Sattler K. J., Elbasan S., Keul P. et al. 2010. Sphingosine 1-phosphate levels in plasma and HDL are altered in coronary artery disease. Basic Res. Cardiol. 105:821. [DOI] [PubMed] [Google Scholar]
- 67. Argraves K. M., Sethi A. A., Gazzolo P. J. et al. 2011. S1P, dihydro-S1P and C24:1-ceramide levels in the HDL-containing fraction of serum inversely correlate with occurrence of ischemic heart disease. Lipids Health Dis. 10:70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Sattler K., Gräler M., Keul P. et al. 2015. Defects of high-density lipoproteins in coronary artery disease caused by low sphingosine-1-phosphate content: correction by sphingosine-1-phosphate-loading. J. Am. Coll. Cardiol. 66:1470. [DOI] [PubMed] [Google Scholar]
- 69. Keul P., Polzin A., Kaiser K. et al. 2019. Potent anti-inflammatory properties of HDL in vascular smooth muscle cells mediated by HDL-S1P and their impairment in coronary artery disease due to lower HDL-S1P: a new aspect of HDL dysfunction and its therapy. FASEB J. 33:1482. [DOI] [PubMed] [Google Scholar]
- 70. Tong X., Peng H., Liu D. et al. 2013. High-density lipoprotein of patients with type 2 diabetes mellitus upregulates cyclooxygenase-2 expression and prostacyclin I-2 release in endothelial cells: relationship with HDL-associated sphingosine-1-phosphate. Cardiovasc. Diabetol. 12:27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Tong X., Lv P., Mathew A. V. et al. 2014. The compensatory enrichment of sphingosine -1- phosphate harbored on glycated high-density lipoprotein restores endothelial protective function in type 2 diabetes mellitus. Cardiovasc. Diabetol. 13:82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Prüfer N., Kleuser B. and van der Giet M. 2015. The role of serum amyloid A and sphingosine-1-phosphate on high-density lipoprotein functionality. Biol. Chem. 396:573. [DOI] [PubMed] [Google Scholar]
- 73. Rader D. J. and Tall A. R. 2012. Bench to bedside: is it time to revise the HDL cholesterol hypothesis? Nat. Med. 18:1344. [DOI] [PubMed] [Google Scholar]
- 74. Christoffersen C., Ahnström J., Axler O., Christensen E. I., Dahlbäck B. and Nielsen L. B. 2008. The signal peptide anchors apolipoprotein M in plasma lipoproteins and prevents rapid clearance of apolipoprotein M from plasma. J. Biol. Chem. 283:18765. [DOI] [PubMed] [Google Scholar]
- 75. Obinata H. and Hla T. 2012. Sphingosine 1-phosphate in coagulation and inflammation. Semin. Immunopathol. 34:73. [DOI] [PMC free article] [PubMed] [Google Scholar]