Abstract
Background and Aims
Inflammation of the pouch after ileal pouch-anal anastomosis (IPAA) can significantly impact quality of life and be difficult to treat. We assessed the effectiveness and safety of vedolizumab in Crohn’s disease (CD) of the pouch and chronic antibiotic-dependent or antibiotic-refractory pouchitis.
Methods
This was a retrospective, multicenter cohort study at 5 academic referral centers in the United States. Adult patients with endoscopic inflammation of the pouch who received vedolizumab were included. The primary outcome was clinical response at any time point. Secondary outcomes included clinical remission, endoscopic response, and remission. Univariate analysis and multivariate analysis were performed for the effect of the following variables on clinical response: fistula, onset of pouchitis less than 1 year after IPAA, younger than 35 years old, gender, previous tumor necrosis factor inhibitor-alpha use, and BMI >30.
Results
Eighty-three patients were treated with vedolizumab for inflammation of the pouch between January 2014 and October 2017. Median follow-up was 1.3 years (interquartile range 0.7–2.1). The proportion of patients that achieved at least a clinical response was 71.1%, with 19.3% achieving clinical remission. Of the 74 patients with a follow-up pouchoscopy, the proportion of patients with endoscopic response and mucosal healing was 54.1% and 17.6%, respectively. Patients who developed pouchitis symptoms less than 1 year after undergoing IPAA were less likely to respond to vedolizumab, even after controlling for other risk factors.
Conclusions
Vedolizumab is safe and effective in the management of CD of the pouch and chronic pouchitis. Further studies are needed to compare vedolizumab with other biologic therapies for pouchitis and CD of the pouch.
Keywords: pouchitis, vedolizumab, Crohn’s disease
In a multicenter cohort study of 83 patients treated with vedolizumab for pouchitis and Crohn’s of the pouch, 71% achieved clinical response, 19.3% clinical remission, whereas endoscopic response and mucosal healing were observed in 54.1% and 17.6%, respectively.
INTRODUCTION
Surgical therapy for medically refractory ulcerative colitis (UC) or UC-associated dysplasia with a total proctocolectomy and ileal pouch-anal anastomosis (IPAA) is often curative.1 Many patients, however, develop inflammation of the pouch leading to symptoms such as pain, urgency, and increased stool frequency, with estimated incidences ranging from 46%–82%.1–3 The first line treatment for pouchitis is antibiotics, but some patients may fail to respond, termed “chronic antibiotic-refractory pouchitis” (CARP) or develop symptoms after antibiotics are stopped, termed “chronic antibiotic-dependent pouchitis” (CADP). Some of these patients have de novo Crohn’s disease (CD) in this location or have been misdiagnosed as UC before colectomy.4 Chronic antibiotic-dependent pouchitis or chronic antibiotic-refractory pouchitis occurs in up to 19% of patients.5 There is no consensus criteria for CD of the pouch, but up to 15% of patients may be diagnosed with CD after IPAA.6 Crohn’s disease of the pouch can be characterized either by the histologic findings of granulomas or by fistulizing disease, intestinal strictures especially in the region of the afferent limb, or extensive and/or serpiginous ulcerations of the afferent limb causing symptoms of obstruction, diarrhea, bleeding, infection, and pain.7
Chronic inflammatory disorders of the pouch can be difficult to treat. Up to 63% of patients with CD require pouch excision, leaving these patients with a permanent diverting ileostomy.4, 8 The optimal treatment for pouchitis that is refractory or dependent to antibiotics is unknown.4 The immunomodulators and biologics used to treat Crohn’s disease have been used with varying success.9–12 Vedolizumab is a gut-specific monoclonal antibody against alpha 4-beta 7 integrin, approved in 2014 for the treatment of CD and UC. Vedolizumab is successful in inducing remission in 14.5% of patients with CD, and of those that achieve a response, 39% are still in remission at 1 year.13 Similarly positive results have been observed in clinical practice.14 There have been only a few studies examining the use of vedolizumab for refractory pouchitis, with the largest study of 20 patients demonstrating antibiotic-free management of pouchitis in ~90% of the study patients.15–21
We assembled a multicenter cohort of patients with CD of the pouch and chronic antibiotic-dependent pouchitis (CADP) and chronic antibiotic refractory pouchitis (CARP) treated with vedolizumab to further examine the clinical effectiveness and safety of vedolizumab in a real-world setting for this difficult-to-treat condition.
METHODS
Patient Population
We performed a retrospective, multicenter cohort study evaluating the efficacy of vedolizumab as a treatment for CD of the pouch and chronic pouchitis (CADP or CARP) at 5 academic referral centers in the US with expertise in inflammatory bowel diseases (IBD). These were the Medical College of Wisconsin (Milwaukee, WI), Cedars-Sinai Medical Center (Los Angeles, CA), the Mayo Clinic (Rochester, MN), the University of North Carolina at Chapel Hill (Chapel Hill, NC), and Washington University in Saint Louis (St. Louis, MO).
Adult IBD patients (18 years or older) with a history of total proctocolectomy with IPAA with endoscopically visualized inflammation of the pouch who received vedolizumab with at least 3 months of follow-up were eligible for inclusion. Exclusion criteria included the use of vedolizumab before colectomy and swithcing to vedolizumab for reasons other than active disease (eg, if a patient was in remission on natalizumab but was switched due to John Cunningham virus positivity). Patients who had undergone pouch excision or had a diverting ileostomy before initiating vedolizumab therapy were not eligible for the study. All patients received standard induction therapy with 300 mg of vedolizumab at weeks 0, 2, and 6, followed by maintenance doses every 4 to 8 weeks.
Study data were collected and managed using REDCap electronic data capture tools hosted at Washington University in St. Louis.22 REDCap (Research Electronic Data Capture) is a secure, web-based application designed to support data capture for research studies, providing 1) an intuitive interface for validated data entry, 2) audit trails for tracking data manipulation and export procedures, 3) automated export procedures for seamless data downloads to common statistical packages, and 4) procedures for importing data from external sources. A number of clinical and endoscopic variables were collected from the electronic medical record using prespecified definitions and criteria for coding. Endoscopy and imaging reports were reviewed for location of inflammation (pouch, cuff, afferent limb, proximal small bowel), fistula connections if present, and location of strictures if present (ileoanal anastomosis, afferent limb). For the purposes of analysis with time-dependent variables, if only the year was known, the date was recorded as January 1 of that year. If the year and month were recorded, the day was assumed to be the first of the month.
As there is no standard definition of Crohn’s disease of the pouch,4 we used the following characteristics as features suggestive of CD: inflammation/stricture of afferent limb/proximal small bowel, fistula involving perianal region or small bowel, fistula greater than 6 months after surgery, or granulomas. A stricture at the ileoanal anastomosis was felt to be likely related to surgery rather than CD, so it was not classified as suggestive of CD. All other patients were defined as having chronic pouchitis, which included CADP or CARP.
Outcomes
The primary outcome was clinical response at any time point, and secondary outcomes included clinical remission, endoscopic response, and endoscopic remission. Clinical response at any time was determined through chart review as defined in a prior study by our group as a decrease in the number of bowel movements, abdominal pain, or fistula drainage.23 Clinical remission was similarly defined as in the prior study as a complete return to normal function with reported normal, nonbloody bowel movements without pain, urgency, or increased nocturnal bowel movements, and the absence of a fistula (if a fistula was previously present). Normal bowel frequency referred to bowel frequency after IPAA before the onset of pouchitis-like symptoms, but after IPAA. The reports from endoscopy performed for assessment of response after initiation of vedolizumab were reviewed in cases where a pre- and post-treatment endoscopic images were available. Endoscopic response and remission or mucosal healing were defined as any improvement in mucosal inflammation (defined as ulcer or erosion) and achievement of completely normal mucosa, respectively. Clinical and endoscopic response/remission were recorded at 3 and 6 months and if a patient ever responded. Other secondary outcomes recorded included the need for subsequent antibiotics or steroids, endoscopic dilation, fistula intervention or pouch excision, and discontinuation of vedolizumab. Safety outcomes included serious infection while on vedolizumab and hypersensitivity reaction to vedolizumab. If vedolizumab was discontinued, the reason for discontinuation was recorded.
Statistical Analysis
Continuous variables were reported using means and standard deviations and compared using Student t tests or Wilcoxon Rank Sum testing where appropriate. Univariate analysis was performed using χ2 or the Fisher exact test (where appropriate) for the effect of the following variables on clinical response: fistula (yes/no), stricture (yes/no), features of CD of the pouch (yes/no), onset of pouchitis symptoms (less than/equal to 1 year vs greater than 1 year after IPAA), symptom duration (less than/equal to 5 years vs greater than 5 years), age (less than/equal to 35 years), sex, previous tumor necrosis factor-alpha inhibitor (anti-TNF) use, and BMI >30. Multivariate logistic regression was performed using the following variables: onset of pouchitis symptoms less than 1 year after IPAA, previous anti-TNF use and BMI >30. Statistical analysis was performed using SAS version 9.4 (Cary, NC, USA). The institutional review board at each institution approved the study.
RESULTS
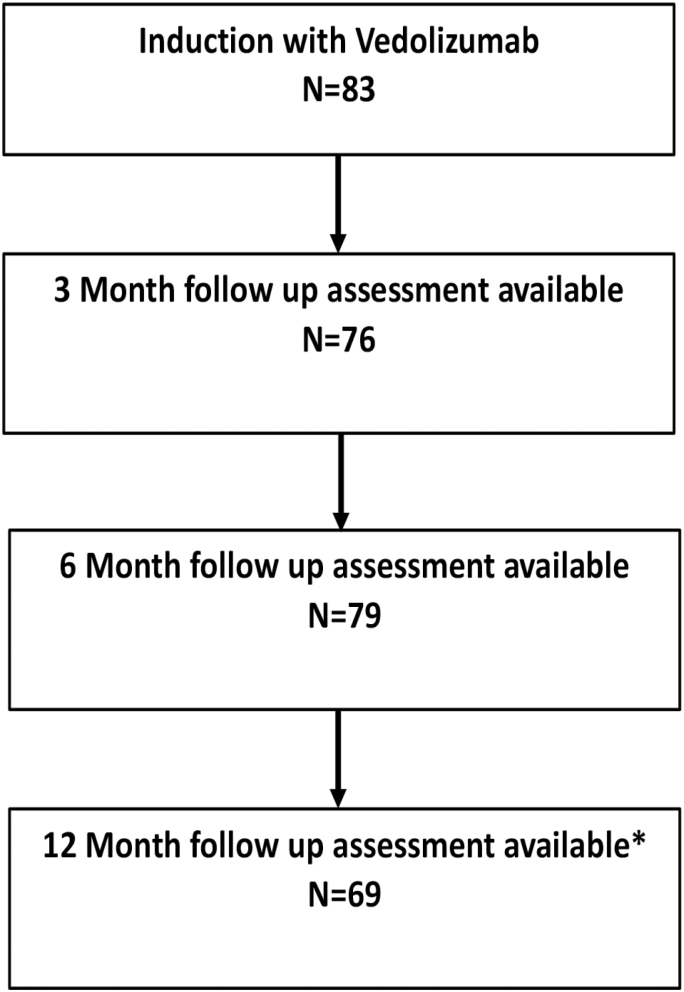
Eighty-three patients were treated with vedolizumab for Crohn’s disease of the pouch or chronic pouchitis at the 5 IBD centers between January 2014 and October 2017 (Table 1, Fig. 1). The majority of patients had UC (81.9%) and had IPAA for medically refractory disease (95.2%). The median duration of IBD before IPAA was 4.9 years (interquartile range [IQR], 2.0–9.9), whereas the median duration for development of pouchitis after IPAA was 1.3 years (IQR, 0.7–5.7). Over half of patients (51.2%) had extraintestinal manifestations of IBD at some point during their illness.
TABLE 1.
Demographic and Baseline Clinical Characteristics of Patients Treated With Vedolizumab
| N = 83 | |
|---|---|
| Female Gender, n (%) | 45 (54.2) |
| Smoker, former or current, n (%) | 19 (22.9) |
| Age at initiation of vedolizumab in years, median (IQR) | 42.1 (31.1–53.4) |
| Extra-intestinal manifestations, n (%) | 43 (51.2) |
| Years from IBD diagnosis to IPAA in years, median (IQR) | 4.9 (2.0–9.9) |
| Years from IPAA to pouchitis in years, median (IQR) | 1.3 (0.7–5.7) |
| Obese (BMI >30), n (%) | 19 (22.9) |
| Pre-operative diagnosis | |
| UC, n (%) | 68 (81.9) |
| CD, n (%) | 9 (10.8) |
| Indeterminate colitis, n (%) | 6 (7.2) |
| Indication for IPAA | |
| Medically refractory disease, n (%) | 79 (95.2) |
| Dysplasia, n (%) | 2 (2.4) |
| Othera, n (%) | 2 (2.4) |
| Infectious complication after IPAA | |
| Presacral abscess, n (%) | 11 (13.3) |
| SSI of ostomy, n (%) | 4 (4.8) |
| Perianal abscess, n (%) | 6 (7.2) |
| Family history of IBD | 26 (31.3) |
| Opioid use | 16 (19.3) |
| Ever had fistula, n (%) | 35 (42.2) |
| Fistula at time of starting vedolizumab | 18 (21.7) |
| Fistula type | |
| Perianal | 17 (20.5) |
| Vaginal | 9 (10.8) |
| Ileal | 3 (3.6) |
| Otherc | 6 (7.2) |
| Ileoanal stricture | 26 (30.3) |
| CD of the pouch b , n (%) | 54 (65.1) |
| Afferent limb stricture | 13 (15.7) |
| Fistula | 31 (37.3) |
| Duration from pouchitis diagnosis to vedolizumab initiation in years, median (IQR) | 3.95 (1.3–8.6) |
| Previous treatments for pouchitis | |
| Topical ASA, n (%) | 18 (21.7) |
| Oral ASA, n (%) | 12 (14.5) |
| Azathioprine or 6MP, n (%) | 29 (34.9) |
| Methotrexate, n (%) | 24 (28.9) |
| Anti-TNF monotherapy, n (%) | 43 (51.8) |
| Anti-TNF + immunomodulator, n (%) | 14 (16.9) |
| Topical steroids, n (%) | 25 (30.1) |
| Systemic corticosteroids, n (%) | 34 (40.9) |
| Antibiotics, n (%) | 72 (86.8) |
ASA, aminosalicylate; SSI, surgical site infection
aOne patient developed pneumatosis coli after receiving the second infliximab induction dose; another patient had a colonic perforation.
bInflammation or stricture of afferent limb or more proximal small bowel, fistula involving perineum or small bowel, fistula involving the pouch >6 months after surgery, granulomas
cIncludes 2 perirectal, 3 perineal, 1 pouch labial
FIGURE 1.
Flow diagram of patient follow-up. *3 Three patients were kept on vedolizumab and had a response charted after 1 year and remained on therapy. One patient did not have a clinical assessment until after one year and did respond and remains on therapy.
Patients had pouchitis symptoms for a median of 3.95 years before starting vedolizumab (IQR 1.3–8.6). Fifty-four patients (65.1%) had CD of the pouch, and 29 (34.9) had chronic pouchitis. Of the 54 patients with CD of the pouch, 13 (15.7%) had a stricture in the afferent limb, and 31 (37.3%) had fistulizing disease. An additional 4 patients (4.8%) had a fistula that was not felt to be related to CD. Eighteen patients (21.7%) had a fistula when vedolizumab was started. Twenty-six patients (30.3%) had a stricture at the ileoanal anastomosis. When evaluating prior therapy for pouch-related disorders after IPAA, 29 (34.9%) had been treated with a thiopurine, 24 (28.9%) had been treated with methotrexate, and 57 (68.7%) had received at least 1 anti-TNF therapy. Of these, 43 (51.8%) had anti-TNF monotherapy, and 14 (16.9%) had combination therapy (anti-TNF + immunomodulator).
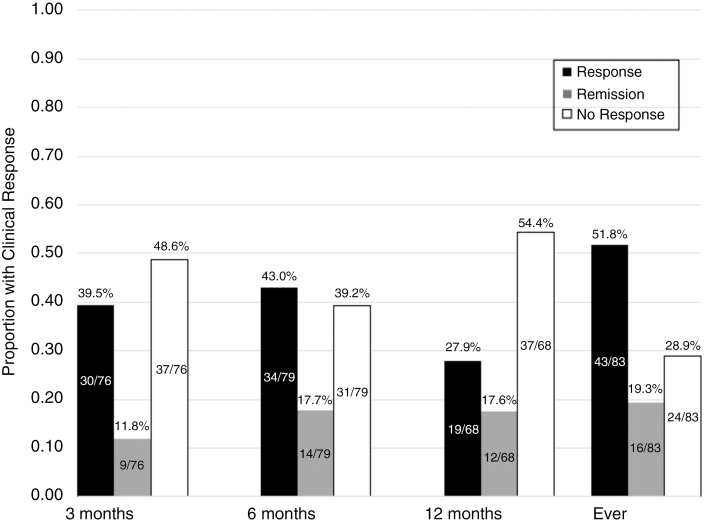
The median duration of follow-up while on vedolizumab was 1.3 years (IQR 0.7–2.1) (Table 2). The proportion of patients that achieved at least a clinical response was 71.1%, with 19.3% of patients achieving remission (Fig. 2). At 3 months, 51% had a clinical response, and 12% were in remission, and at 6 months, these values were 60.7% and 17.7%, respectively. At 12 months, 45.6% of patients were responding, and 17.6% were in remission. Among those with prior anti-TNF exposure, either before or after colectomy, 69.3% achieved a clinical response compared with 87.5% among those naïve to anti-TNFs (Supplementary Figure S1). In the subgroup of patients with CD of the pouch, 72.2% had a clinical response (Supplementary Figure S3).
TABLE 2.
Outcomes After Initiation of Vedolizumab for Pouchitis
| Duration of follow-up in years, median (IQR) | 1.3 (0.7–2.1) |
| Time until clinical response in years, median (IQR) | 0.3 (0.2–0.5) |
| Endoscopic Response, n (%) | |
| 3 months | 11/23 (47.8) |
| 6 months | 22/40 (55.0) |
| Ever | 40/74 (54.1) |
| Endoscopic Remission, n (%) | |
| 3 months | 3/23 (13.0) |
| 6 months | 6/40 (15.0) |
| Ever | 13/74 (17.6) |
| Corticosteroid-free response | 40/83 (48.2) |
| Corticosteroid-free remission | 14/83 (16.9) |
| Antibiotic and steroid free response | 21/83 (25.3) |
| Need for subsequent procedures | |
| Exam under anesthesia, n (%) | 12 (14.5) |
| Stricture dilation, n (%) | 16 (19.3) |
| Seton placement, n (%) | 7 (8.4) |
| Fistulotomy, n (%) | 2 (2.4) |
| Abscess drainage, n (%) | 4 (4.8) |
| Diverting ileostomy, n (%) | 4 (4.8) |
| Pouch excision, n (%) | 3 (3.6) |
| Corticosteroid prescription, n (%) | 28 (33.7) |
| Antibiotic prescription, n (%) | 50 (60.2) |
| Episodic, n (%) | 29 (34.9) |
| Continuous, n (%) | 22 (25.3) |
| Serious infection, n (%) a | 3 (3.6) |
| Relapse after initial response, n (%) | 16 (27.1) |
| Discontinuation of vedolizumab, n (%) b | 30 (36.1) |
aClostridium difficile infection, norovirus infection, intra-abdominal abscess requiring percutaneous drainage
bSee text for reasons for discontinuation.
FIGURE 2.
Proportion of patients experiencing clinical response and remission to vedolizumab for pouchitis
Of the 74 patients that had a follow-up endoscopy, the proportion of patients achieving endoscopic response and mucosal healing was 54.1% and 17.6%, respectively. At 3 months, 47.8% were in endoscopic response, and 13% demonstrated mucosal healing; at 6 months, these numbers were 55% and 15%, respectively. Comparing those with anti-TNF exposure, 51.5% had an endoscopic response compared with 83.3% in those who had never received an anti-TNF (Supplementary Figure S2). In the subgroup of patients with CD of the pouch, 56.9% had an endoscopic response (Supplementary Figure S4). Of those with inflammation of the cuff, 75.0% had an endoscopic response. Of those with CD and inflammation of the pouch, 65.0% had an endoscopic response. Of those with inflammation of the afferent limb, 70.0% achieved an endoscopic response.
Although 86% of the patients were on antibiotics before initiating vedolizumab, a majority of patients (60.2%) continued to require antibiotics at some point during follow-up, including 61.1% of patients who responded to vedolizumab. Of the patients that achieved remission, 56.3% required antibiotics at some point during follow-up. Corticosteroids were needed in 34.6% of patients. Forty patients (48.2%) achieved a corticosteroid-free response, and 14 (16.9%) achieved corticosteroid-free remission. Thirty patients (36.1%) required subsequent procedures, with the most common being exam under anesthesia (14.5%), stricture dilation (19.3%), and seton placement (8.4%). Of the 16 patients who required dilation, 10 of them required dilation of the ileoanal anastomosis, which was likely a surgical complication rather than CD that might respond to vedolizumab. Four patients had a diverting ileostomy (4.8%), and 3 patients (3.6%) had their pouch excised.
Factors Associated With Response
In univariate analysis, comparing those with clinical response (ever) to vedolizumab, patients who developed pouchitis symptoms less than 1 year after undergoing IPAA were less likely to respond than those that developed symptoms more than 1 year after IPAA (Table 3). This was still significant after adjustment for prior anti-TNF use and BMI (Table 4). Age, gender, BMI, presence of a fistula or stricture, features of CD, and previous anti-TNF use were not predictive of clinical response to vedolizumab. None of the variables were predictive of clinical or endoscopic response or remission to vedolizumab at 3 or 6 months. Furthermore, no differences were found on univariate analysis in the subgroups of those naïve to an anti-TNF and those with prior anti-TNF exposure or those with CD of the pouch.
TABLE 3.
Comparison Between Patients With and Without Clinical Response to Vedolizumab
| Variable | Clinical Response |
No Clinical Response | P |
|---|---|---|---|
| (N = 59) | (N = 24) | ||
| Female (n, %) | 34 (57.6) | 11 (45.8) | 0.33 |
| Fistula (n, %) | 25 (42.4) | 11 (45.8) | 0.77 |
| Stricture (n, %) | 28 (47.5) | 11 (45.8) | 0.89 |
| Previous Anti-TNF (n, %) | 52 (88.1) | 23 (95.8) | 0.43 |
| CD features (n, %) | 39 (66.1) | 15 (62.5) | 0.76 |
| Pouchitis ≤1 year after IPAA (n, %) a | 16 (28.1) | 13 (59.1) | 0.01 |
| Obese (n, %) | 16 (27.1) | 3 (12.5) | 0.15 |
| Age <35 years (n, %) | 40 (67.8) | 15 (62.5) | 0.64 |
| Symptoms >5 years (n, %) | 24 (42.1) | 13 (59.1) | 0.18 |
| Smoker (n, %) | 13 (22.0) | 6 (25.0) | 0.77 |
a2 patients were missing a vedolizumab initiation date and were excluded from this analysis.
TABLE 4.
Logistic Regression Model for Factors Predictive of Clinical Response (ever) to Vedolizumab
| Variable | Odds Ratio (CI) | P |
|---|---|---|
| Previous Anti-TNF exposure | 0.5 (0.05–4.3) | 0.50 |
| Pouchitis ≤1 year after IPAA | 0.3 (0.1–0.9) | 0.03 |
| Obesity | 3.1 (0.6–15.5) | 0.17 |
CI, confidence interval
Adverse Effects and Discontinuation
Thirty patients (36.1%) discontinued vedolizumab based on an assessment by the treating clinician. Three patients developed infusion reactions, with vedolizumab being discontinued in 2 of the cases. One patient had to stop vedolizumab for financial reasons. Three patients (3.61%) developed serious infections while on vedolizumab: Clostridium difficile infection, norovirus, and intra-abdominal abscess requiring percutaneous drainage (Table 2). Vedolizumab was discontinued in the patient that developed an intra-abdominal abscess due to this infection and ongoing disease activity, but the medication was continued in the other 2 patients with Clostridium difficile and norovirus infections. The remaining patients had their treatment discontinued due to failure to achieve durable remission per the discretion of the treating clinician.
DISCUSSION
In this multicenter retrospective study across 5 academic IBD centers in the United States, vedolizumab improved clinical symptoms in more than half of the patients with chronic pouch inflammation including CD of the pouch at 3 and 6 months of therapy. Additionally, over half of patients had endoscopic improvement at 3 and 6 months of therapy. Rapid onset of pouch inflammation within a year of IPAA construction was predictive of nonresponse to vedolizumab. Few patients experienced adverse effects while on vedolizumab.
Prior experience with the use of vedolizumab in patients with inflammation of the IPAA has been limited to case reports and small case series (Table 5).15, 16, 18–21, 24 In the largest published study of 20 patients, Bar et al found that 65% had clinical improvement and 64% had an endoscopic response.21 Shelton at el examined vedolizumab for refractory IBD patients, of whom 9 had pouchitis or CD of the pouch.16 The rate of clinical response was 75%, and 1 patient achieved clinical remission at 14 weeks.16 Khan et al found that 66.7% of patients with CD of the pouch had a clinical response.24 Philpott et al reported clinical and endoscopic response in 4 patients treated with vedolizumab, though 1 still required budesonide.15 Our data on clinical and endoscopic response are consistent with the data from Bar et al, while providing additional data specific to anti-TNF exposure and the subset of patients diagnosed with CD of the pouch. Our data is also consistent with real-world clinical response (32%) and clinical remission (18%) at 6 months of therapy in the Victory consortium for patients with CD.25 The clinical response rate in the major clinical trial for vedolizumab in CD was 25.7% after 6 weeks, with 43.5% maintaining a response after 1 year.13
TABLE 5.
Review of the Literature
| Reference | No. Patients | Prior anti-TNF exposure (n, %) | Clinical response (n, %) | Endoscopic response (n, %) |
|---|---|---|---|---|
| Bar et al. 18 | 20 | 11 (55) | 13 (65) | 9 (64.3) |
| Khan et al. 21 | 12 | 8 (66.7) | 8 (66.7) | 10 (83.3) |
| Philpott et al. 12 | 4 | 4 (100) | 4 (100) | 4 (100) |
| Shelton et al. 13 | 8a | Not reported | 6 (75) | Not reported |
| Coletta et al. 16 | 1 | 1 (100) | 1 (100) | 1 (100) |
| Bethge et al. 17 | 1 | 1 (100) | 1 (100) | 1 (100) |
| Schmid et al. 15 | 1 | 0 (0) | 1 (100) | 1 (100) |
aAn additional patient with pouchitis was treated with vedolizumab but did not have efficacy data.
The only variable predictive of a lack of response to vedolizumab was time from IPAA to pouchitis diagnosis. A shorter duration of pouchitis symptoms was associated with a poor response to vedolizumab. It is possible that many of these patients developed inflammation in the pouch related to complications of their surgery rather than CD of the pouch. For example, ischemia from surgery can lead to an ileoanal stricture and inflammation.4 Vedolizumab would likely not benefit these patients. Development of consensus diagnostic criteria for CD of the pouch, perhaps with duration from IPAA as a criterion, may be helpful in predicting which patients are likely to respond to vedolizumab. Such criteria would need to be evaluated in a prospective study.
Our finding that 71% had clinical improvement and over half had endoscopic improvement is encouraging considering that more than two thirds of our patient cohort had failed biologic therapies that have been reported to be effective in the management of pouchitis. For infliximab, Colombel et al described an 84% response rate in patients diagnosed with CD of the pouch.10 Haveran et al found that 54% of patients with fistulizing or stricturing disease responded to infliximab.11 Shen et al reported that adalimumab improved symptoms in 71% of patients with CD of the pouch.9 Another small study reported 100% response to infliximab.12
Our study is consistent with previous literature that vedolizumab seems safe. In the phase 3 clinical trial leading to approval of vedolizumab for CD, there was a higher incidence of nasopharyngitis and serious infection in the vedolizumab group compared with placebo,13 but subsequent studies suggest the risk of infection in vedolizumab is similar to placebo.26 It is reassuring that we observed only 3 serious infections, but further studies are required to compare the safety of vedolizumab to other agents for pouchitis.
It is important to point out that a majority of patients who responded to vedolizumab continued to require antibiotics, which might question the overall benefit of vedolizumab. Most patients, however, had an insufficient response to antibiotics and improved only after vedolizumab was added. Certainly, being able to discontinue antibiotics after responding to vedolizumab would be ideal. However, having improved symptoms with vedolizumab and antibiotics is probably a better outcome for patients than continuing with their symptoms without vedolizumab. Further research is needed to determine the consequences of long-term antibiotics in pouchitis. Of course, a prospective randomized placebo-controlled trial would be required to confirm vedolizumab’s efficacy in CD of the pouch and chronic pouchitis.
A major strength of this study is that this is the largest study of the clinical effectiveness of a therapy in treating pouch inflammation across multiple US academic centers with expertise in treating IBD in a patient population that is refractory to multiple nonbiologic and biologic therapies. We included patients with Crohn’s disease of the pouch in addition to chronic antibiotic-dependent/refractory pouchitis.
Many of the limitations of this study are due to its retrospective nature. We lacked an objective scoring system to define clinical and endoscopic response due to the limited details available in clinical care and endoscopy notes to calculate Pouchitis Disease Activity Index (PDAI) scores.27 We feel that our results are still informative because many clinicians do not use objective scoring systems in routine clinical practice. They rely on the simple question of whether a patient improved enough clinically and endoscopically for vedolizumab to be continued. Hence, we opted to use a strict definition of clinical remission to allow interpretation of the data even in the absence of correlating endoscopic data. There was also a lack of standardization of pouchoscopy reading across the various providers in the study or the use of central reading. However, we attempted to overcome this by using endoscopic findings of pouch ulceration that have been shown to have substantial reliability among central readers in the evaluation of endoscopic disease activity in pouchitis.28
Finally, there is no standard definition of CD of the pouch. We would expect vedolizumab to have some efficacy in CD of the pouch since it has been demonstrated to be efficacious in other CD phenotypes. Whether CADP or CARP share a similar pathogenesis to CD and thus could be expected to have some response to vedolizumab is unknown. We did not observe a difference in efficacy in patients with CD of the pouch or chronic pouchitis in this study. Further research is needed to define different phenotypes of pouch dysfunction to allow more personalized treatments.
In summary, in this multicenter, retrospective study, we found vedolizumab to be safe and effective in the management of CD of the pouch and CADP. Given the good safety profile observed in our study and others,21, 26 vedolizumab warrants consideration for the management of refractory pouchitis. Larger prospective studies are needed to evaluate its efficacy and safety in comparison with other drug classes and identify factors associated with response.
Supplementary Material
Acknowledgments
We would like to thank Billy Darren Nix for his assistance with this work.
Glossary
Abbreviations
- Anti-TNF
tumor necrosis factor-alpha inhibitor
- CADP
chronic antibiotic-dependent pouchitis
- UC
ulcerative colitis
- CD
Crohn’s disease
- IBD
inflammatory bowel disease
- IPAA
ileal pouch-anal anastomosis
- REDCap
Research Electronic Data Capture
Conflicts of Interest: MC is a member of the speakers bureau or has done consulting with UCB, AbbVie, Pfizer, and Takeda and has received grant support from Incyte, AbbVie, and Takeda. AG is a member of the speakers bureau or has done consulting with AbbVie and Janssen. PBP has received a speaker’s fee from Takeda. HHH has received consulting fees from Artizan, Alivio, Boehringer-Ingelheim, Celltrion, Finch, Lycera, Merck, Pfizer, and Seres and research support from Artizan and Pfizer. LER has received consulting fees from Ferring Pharmaceuticals and an honorarium paid to the Mayo Clinic. ELB has received consulting fees from Janssen. PD has received consulting fees from Janssen and Pfizer, speaker fees from Abbvie and research grant from Takeda. KNW, MHG, SP, GS, PH, SBH, DP, and GC have no disclosures or conflicts of interest relevant to this study.
Author Contributions: MG and PD were involved in involved in the study concept and design, acquisition of the data, analysis and interpretation of the data, and drafting and critical revision of the manuscript. PBP, KNW, MC, GS, PH, SBH, DP, GC, LER, HHH, and ELB were involved in study concept and design, acquisition of the data, analysis and interpretation of the data, and critical revision of the manuscript. All authors reviewed and approved the final draft of the manuscript submitted.
Supported by: This work was supported by the American College of Gastroenterology Junior Faculty Development Award (PD), the Nickolas Bunn Boddie, Sr., and Lucy Mayo Boddie Foundation (ELB and HH), and the Crohn’s and Colitis Foundation (ELB). This work was also supported by Clinical and Translational Science Award (CTSA, Grant UL1 TR000448) and Siteman Comprehensive Cancer Center and NCI Cancer Center Support Grant (P30 CA091842). Inflammatory bowel disease research at Washington University is supported by the philanthropic Givin’ It All for Guts Foundation (www.givinitallforguts.org). We also acknowledge additional support from the WUSM DDRCC funded through NIDDK P30 DK052574 in the conduct of the study.
REFERENCES
- 1. Ferrante M, Declerck S, De Hertogh G, et al. . Outcome after proctocolectomy with ileal pouch-anal anastomosis for ulcerative colitis. Inflamm Bowel Dis. 2008;14:20–28. [DOI] [PubMed] [Google Scholar]
- 2. Shen B. Pouchitis: what every gastroenterologist needs to know. Clin Gastroenterol Hepatol. 2013;11:1538–1549. [DOI] [PubMed] [Google Scholar]
- 3. Barnes EL, Herfarth HH, Sandler RS, et al. . Pouch-related symptoms and quality of life in patients with ileal pouch-anal anastomosis. Inflamm Bowel Dis. 2017;23:1218–1224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Lightner AL, Pemberton JH, Loftus EJ Jr. Crohn’s disease of the ileoanal pouch. Inflamm Bowel Dis. 2016;22:1502–1508. [DOI] [PubMed] [Google Scholar]
- 5. Madiba TE, Bartolo DC. Pouchitis following restorative proctocolectomy for ulcerative colitis: incidence and therapeutic outcome. J R Coll Surg Edinb. 2001;46:334–337. [PubMed] [Google Scholar]
- 6. Yu CS, Pemberton JH, Larson D. Ileal pouch-anal anastomosis in patients with indeterminate colitis: long-term results. Dis Colon Rectum. 2000;43:1487–1496. [DOI] [PubMed] [Google Scholar]
- 7. Shen B, Fazio VW, Remzi FH, et al. . Clinical features and quality of life in patients with different phenotypes of Crohn’s disease of the ileal pouch. Dis Colon Rectum. 2007;50:1450–1459. [DOI] [PubMed] [Google Scholar]
- 8. Gu J, Stocchi L, Kiran RP, et al. . Do clinical characteristics of de novo pouch Crohn’s disease after restorative proctocolectomy affect ileal pouch retention? Dis Colon Rectum. 2014;57:76–82. [DOI] [PubMed] [Google Scholar]
- 9. Shen B, Remzi FH, Lavery IC, et al. . Administration of adalimumab in the treatment of Crohn’s disease of the ileal pouch. Aliment Pharmacol Ther. 2009;29:519–526. [DOI] [PubMed] [Google Scholar]
- 10. Colombel JF, Ricart E, Loftus EV Jr, et al. . Management of Crohn’s disease of the ileoanal pouch with infliximab. Am J Gastroenterol. 2003;98:2239–2244. [DOI] [PubMed] [Google Scholar]
- 11. Haveran LA, Sehgal R, Poritz LS, et al. . Infliximab and/or azathioprine in the treatment of Crohn’s disease-like complications after IPAA. Dis Colon Rectum. 2011;54:15–20. [DOI] [PubMed] [Google Scholar]
- 12. Viscido A, Habib FI, Kohn A, et al. . Infliximab in refractory pouchitis complicated by fistulae following ileo-anal pouch for ulcerative colitis. Aliment Pharmacol Ther. 2003;17:1263–1271. [DOI] [PubMed] [Google Scholar]
- 13. Sandborn WJ, Feagan BG, Rutgeerts P, et al. ; GEMINI 2 Study Group Vedolizumab as induction and maintenance therapy for Crohn’s disease. N Engl J Med. 2013;369:711–721. [DOI] [PubMed] [Google Scholar]
- 14. Vivio EE, Kanuri N, Gilbertsen JJ, et al. . Vedolizumab effectiveness and safety over the first year of use in an IBD clinical practice. J Crohns Colitis. 2016;10:402–409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Philpott J, Ashburn J, Shen B. Efficacy of vedolizumab in patients with antibiotic and anti-tumor necrosis alpha refractory pouchitis. Inflamm Bowel Dis. 2017;23:E5–E6. [DOI] [PubMed] [Google Scholar]
- 16. Shelton E, Allegretti JR, Stevens B, et al. . Efficacy of vedolizumab as induction therapy in refractory IBD patients: a multicenter cohort. Inflamm Bowel Dis. 2015;21:2879–2885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Mir F, Yousef MH, Partyka EK, et al. . Successful treatment of chronic refractory pouchitis with vedolizumab. Int J Colorectal Dis. 2017;32:1517–1518. [DOI] [PubMed] [Google Scholar]
- 18. Schmid M, Frick JS, Malek N, et al. . Successful treatment of pouchitis with vedolizumab, but not fecal microbiota transfer (FMT), after proctocolectomy in ulcerative colitis. Int J Colorectal Dis. 2017;32:597–598. [DOI] [PubMed] [Google Scholar]
- 19. Coletta M, Paroni M, Caprioli F. Successful treatment with vedolizumab in a patient with chronic refractory pouchitis and primary sclerosing cholangitis. J Crohns Colitis. 2017;11:1507–1508. [DOI] [PubMed] [Google Scholar]
- 20. Bethge J, Meffert S, Ellrichmann M, et al. . Combination therapy with vedolizumab and etanercept in a patient with pouchitis and spondylarthritis. BMJ Open Gastroenterol. 2017;4:e000127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Bär F, Kühbacher T, Dietrich NA, et al. ; German IBD Study Group Vedolizumab in the treatment of chronic, antibiotic-dependent or refractory pouchitis. Aliment Pharmacol Ther. 2018;47:581–587. [DOI] [PubMed] [Google Scholar]
- 22. Harris PA, Taylor R, Thielke R, et al. . Research electronic data capture (redcap)–a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform. 2009;42: 377–381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Weaver KN, Gregory M, Syal G, et al. . Ustekinumab is effective for the treatment of Crohn’s disease of the pouch in a multicenter cohort. Gastrointest Endosc. 2018;88:360–369. [DOI] [PubMed] [Google Scholar]
- 24. Khan F, Gao XH, Singh A, et al. . Vedolizumab in the treatment of Crohn’s disease of the pouch. Gastroenterol Rep (Oxf). 2018;6:184–188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Dulai PS, Singh S, Jiang X, et al. . The real-world effectiveness and safety of vedolizumab for moderate-severe Crohn’s disease: results from the US VICTORY consortium. Am J Gastroenterol. 2016;111:1147–1155. [DOI] [PubMed] [Google Scholar]
- 26. Bye WA, Jairath V, Travis SPL. Systematic review: the safety of vedolizumab for the treatment of inflammatory bowel disease. Aliment Pharmacol Ther. 2017;46:3–15. [DOI] [PubMed] [Google Scholar]
- 27. Sandborn WJ, Tremaine WJ, Batts KP, et al. . Pouchitis after ileal pouch-anal anastomosis: a pouchitis disease activity index. Mayo Clin Proc. 1994;69:409–415. [DOI] [PubMed] [Google Scholar]
- 28. Samaan MA, Shen B, Mosli MH, et al. . Reliability among central readers in the evaluation of endoscopic disease activity in pouchitis. Gastrointest Endosc. 2018;88:360–369.e2. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.